Graphical abstract
Keywords: Volatile flavor compounds, Amino acids, Soluble sugars, Integrated freeze-drying, Conventional freeze-drying, Hot air drying
Highlights
-
•
Volatiles’ total content showed a first fast and then slow decrease during drying.
-
•
The IFD samples preserved higher content of volatiles, free amino acids, and soluble sugars.
-
•
Most volatile compounds showed negative correlations with amino acids.
-
•
Most volatile compounds showed positive correlations with soluble sugars.
Abstract
Durian contains rich flavor components that undergo complex changes during drying. In this study, durian was subjected to integrated freeze-drying (IFD), conventional freeze-drying (CFD), and hot air drying (AD). Compared with the fresh samples, those dried by IFD, CFD, and AD lost 11, 9, and 7 original volatile compounds, respectively, and generated 7, 6, and 8 new volatile compounds, respectively, and showed a rapid and then slow decreasing trend in the total content during drying. However, the types of amino acids and soluble sugars remained unchanged during each of the drying methods. Furthermore, volatile compounds showed a significant negative correlation with the majority of amino acids and a significant positive correlation with soluble sugars. The IFD samples had the highest content of volatile compounds, amino acids, and soluble sugars. Therefore, IFD is recommended as a preferable drying method for durian.
1. Introduction
Durian fruit has gained popularity because of its strong and unique odor (Ali et al., 2020, Husin et al., 2018). It is primarily cultivated in Southeast Asian countries (Ho, & Bhat, 2015). Durian is typically consumed fresh due to the challenges associated with transportation and storage (Bu et al., 2022, Niponsak et al., 2015). However, fresh durian has a pungent odor that necessitates processing to remove the unpleasant flavor while retaining its elegant aroma (Xu et al., 2022, Zhang et al., 2020, Raihana et al., 2015, Belgis et al., 2017). This processing aims to prolong its shelf life, improve product quality, and increase its overall value (Wongs-Aree, & Noichinda, 2022). In recent years, fresh durian has been transformed into snacks such as dried durian flakes and mixed fruit and vegetable crisps, which have become increasingly popular leisure foods (Dembitsky et al., 2011).
Drying is one of the most common durian processing methods, with hot air drying (AD) and freeze-drying (FD) being the two most widely used (Yi, Lyu, Bi, Zhou, & Zhou, 2017). The AD method is favored for its simplicity and low cost, whereas FD is considered the optimal method for drying fruits and vegetables (Jamradloedluk Nathakaranakule, Soponronnarit, & Prachayawarakorn, 2007). FD ensures the preservation of nutrients, flavor, and the original shape of the material, avoiding nutrient and color degradation caused by high-temperature heating (James et al., 2015, Harguindeguy and Fissore, 2020). FD can be further divided into conventional freeze-drying (CFD) and integrated freeze-drying (IFD), which have different pre-freezing methods. IFD employs the vacuum freezing (VF) method, which rapidly evaporates water from the material under vacuum conditions, effectively cooling and freezing it. This simplifies the CFD process, reduces energy consumption in large-scale production, and reduces production cost.
Chin et al. (2010) processed durian powder using FD and spray-drying and found that the drying method and maltodextrin affected the flavor compounds, sensory characteristics, and related physicochemical qualities of the dried product. Paengkanya et al. (2015) dried durian with microwave-vacuum combination drying (MWVC), microwave-hot air (MWHA) drying, and hot air (HA), and found that the MWVC method exhibited a higher drying rate with lower energy consumption. Lin et al. (2020) investigated the effects of high hydrostatic pressure (HHP) and Vacuum Freeze-Drying (VFD) on the flavor quality of durian fruit-vegetable mixed crisps and found that the combined HHP-VFD treatment preserved more volatile compounds and received the highest sensory evaluation. Harguindeguy et al. (2020) studied the effects of four FD processes on the nutritional characteristics of durian. Their findings revealed that VFD preserved vitamin C better, while microwave drying and infrared drying reduced processing time. Furthermore, atmospheric-pressure FD and VFD were effective in preserving phenolic compounds and total antioxidant ability.
Volatile flavor compounds, amino acids, and soluble sugars are important substances that influence the flavor quality of durian. Surprisingly, no studies have reported on the changes in these flavor compounds during the AD and FD of durian. In this experiment, Golden Pillow durian flesh was used for comparative experiments involving IFD, CFD, and AD. This study investigated the dynamic changes in volatile flavor compounds, amino acids, and soluble sugars in durian during the three drying processes, analyzed the interrelationships among these compounds, explored the action mechanisms of related flavor compounds in the freeze-dried durian, and determined the best method for durian drying. The primary objective of this study is to provide both theoretical and practical insights to aid in selecting appropriate durian drying methods and improving overall product quality.
2. Materials and methods
2.1. Materials
Fresh Golden Pillow durians that were free from mechanical damage, of uniform size, free from pests and diseases, and of the same maturity were purchased from the Nanjing Sugo Supermarket. Sucrose, fructose, glucose, maltose, other soluble sugar standards, 16 amino acid standards, and cyclohexanone were purchased from Sigma-Aldrich (Shanghai, China). O-phthalaldehyde (OPA), acetonitrile, methanol (chromatographically pure), 3-mercaptopropionic acid, boric acid, potassium phosphate (analytically pure), and sodium chloride (AR) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China).
2.1. Drying method
Fresh durian samples were made into 1 × 1 × 1 cm blocks and processed for IFD, CFD, and AD with drying times of 12 h, 12 h, and 9 h, respectively. After drying, the samples were removed from the vacuum freeze-dryer to determine the relevant indicators. The experiment of IFD, CFD and AD was all repeated three times and 50 unit samples were used for each drying experiment. The drying and sampling methods were as follows.
IFD: a vacuum freeze-dryer (SCIENTZ-50F; Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China) was used for freezing. The process involved opening the cold trap in advance until it reached −40 °C. The prepared sample was then placed on the material plate in the desiccator chamber. The vacuum pump was turned on, which underwent vacuuming and freezing for 0.5 h. Thereafter, the samples were conducted with the primary and secondary drying stages of VFD, in which the temperature rise programme was set to −10 °C for 1 h, 0 °C for 1 h, 10 °C for 2 h, 20 °C for 2 h, 30 °C for 2 h, 40 °C for 2 h, and 50 °C for 2 h. The schedule of the sampling time during IFD comprised vacuum freezing for 0.5 h (IF0.5 h), freeze-drying for 4 h (IFD4h), freeze-drying for 8 h (IFD8h), and freeze-drying for 12 h (IFD12h).
CFD: The samples were frozen at atmospheric pressure for 4 h in a −40 °C refrigerator and then freeze-dried. The heating procedure was the same as that used for IFD. The schedule of the sampling time during CFD comprised freezing for 4 h at atmospheric pressure (CF4h), freeze-drying for 4 h (CFD4h), freeze-drying for 8 h (CFD8h), and freeze-drying for 12 h (CFD12h).
AD: The samples were placed in a hot-air drying oven with the temperature set at 60 °C for 9 h. The schedule of the sampling time during AD comprised air drying for 3 h (AD3h), air drying for 6 h (AD6h), and air drying for 9 h (AD9h).
2.2. Determination of volatile flavor compounds by GC–MS
To measure the volatile compounds and subsequent indicators, we used 2.00 ± 0.01 g of the test sample. The volatile flavor compounds in the samples were extracted and separated using headspace solid-phase microextraction technology. The procedure involved fixing 2 g small sample pieces to 20 mL with distilled water and homogenizing (FJ200-SH; Shanghai Huxi Industrial Co., Ltd., Shanghai, China) at 10000 rpm for 2 min. A 5-mL homogenate, along with a solution of 1 g NaCl and 2 µL of 5 µL/mL cyclohexanone, was placed into a headspace vial for GC–MS quantification. A CAR/PDMS/DVB (50/30 µm divinylbenzene carboxene-poly (dimethylsiloxane), Supelco, Bellefonte, PA, USA) solid-phase microextraction needle was then inserted into the headspace of the sample vial, and the vial was kept in a water bath at 60 °C for 30 min. Thereafter, the extraction needle was removed, inserted into the GC–MS injector, and desorbed at 250 °C for 5 min, and the instrument began collecting data.
Volatile compounds were detected using GC–MS equipped with a DB-5MS capillary column (30 m × 250 µm, 0.25 um). The mass scanning range (mass-to-charge ratio, m/z) was set from 30 to 450, with a scanning rate of 5.27 times per second. The heating procedure involved an initial temperature of 40 °C for 2 min, followed by an increase to 120 °C at a rate of 5 °C/min for 3 min, and a final increase to 230 °C at 10 °C/min for 3 min. Volatile compounds were quantified by comparing the peak areas of the detected volatile compounds with those of the internal standard. The volatile components detected by GC–MS were matched qualitatively by a computer and the Wiley and NIST08 standard spectrum libraries, and the identification results were based on results with a similarity > 70, which were then compared with relevant reports and literature to determine the chemical components of the corresponding volatile flavor compounds. Fresh durian samples were used as controls. Each sample was tested three times each of the three drying methods. Test results are calculated as “mean ± standard deviation”.
The peak area normalization method was used for semi-quantitative analysis, and the formula was as follows:
where Ci was the content of the volatile compound (cyclohexanone equivalents), mg/g FW; Si was the peak area of the volatile compound, m2; S0 was the peak area of the internal standard substance added, m2;M0 was the mass of the internal standard substance added, mg; Md was the mass of the added test sample, g. Due to the decrease of moisture in the durian samples during drying, the mass of durian sample unit will change. In order to facilitate the comparison of volatile compounds, Md in the calculation formula is defined as the sample mass of fresh material unit (2 g), g FW.
2.3. Detection of the volatile compounds by electronic nose
Determination of volatile compounds in samples using the PEN3 electronic nose (Airsense, Schwerin, Germany) was carried out according to the method by Yang et al. (2023). The PEN3 electronic nose simulates the olfactory system of the human body and consists of 10 metal oxide sensors. The specific names and performance descriptions of these sensors are listed in Table S1.
2.4. Determination of free amino acids
Free amino acid content was determined according to the method of Xiao et al. (2022). The dried samples were ground in an ice bath and 10 mL of chloroform: methanol (15: 35) solution was added. After being left on ice for 30 min, 6 mL of distilled water was added, and the mixture was centrifuged at 10000 rpm for 15 min. The supernatant was removed and the residue was extracted twice with water. All the supernatants were combined and prepared.
Amino acid samples were pre-column derivatized with an OPA reagent solution. The derivatization reagent OPA was prepared by adding 1.5 mL of 0.4 mol/L borate buffer (pH 10.2) and 15 μL of 3-mercaptopropionic acid to 15 mg of OPA, which was then stored in a refrigerator at 4 °C. Subsequently, 10 μL of the amino acid samples were added to 50 μL of borate buffer and mixed with 10 μL of OPA and 640 μL of ultrapure water after 0.5 min. The solution was filtered through a 0.45-μm filter membrane to prepare derivatized solution samples. The amino acid composition and content of the derivatized solution samples were determined using a high performance liquid chromatograph (HPLC, HP1200) equipped with a ZORBAX Eclipse-AAA column (4.6 × 150 mm, 3.5 μm, Agilent, USA). The mobile phase ratio was acetonitrile: methanol: water (v/v/v) 45: 45: 10, the injection volume was 20 μL, and the column temperature was 40 °C. The results of free amino acid content were expressed in milligrams of free amino acids per gram (mg/g FW) of the original fresh sample unit. The measurements were repeated thrice for each sample using the three drying methods. The test results were calculated as “mean ± standard deviation”.
2.5. Determination of soluble sugars
The determination of soluble sugars was based on the national standard GB 5009.8-2016, the national food safety standard determination of fructose, glucose, sucrose, maltose, and lactose in foods (GB, 2016), and the method described by Xie et al. (2022) with slight modifications. One unit sample of durian was cut into small uniform pieces and transferred into a 50 mL centrifuge tube, 20 mL of water was added and centrifuged at 15000 rpm. Homogeneous extraction was conducted for 2 min, followed by a water bath at 70 °C for 20 min and centrifugation at 10,000 rpm for 15 min. The residue was added to 4 mL of distilled water and extracted twice. The extracts were combined, filtered through a 0.45 filter membrane, and tested. HP1200 high performance liquid chromatograph (Agilent, USA) equipped with a Series 200 amine-based column (250 × 4.6 mm, 5 µm) was used. The column temperature was 40 °C and mobile phase ratio was acetonitrile: water = 70:30 (v/v). The flow rate was 1.0 mL/min, injection volume was 20 μL, temperature of the refractive index detector was 40 °C, temperature of the drift tube of the evaporative light scattering detector was 80–90 °C, nitrogen pressure was 350 Kpa, and the impactor was closed. The results of soluble sugar content were expressed in milligrams of soluble sugars per gram (mg/g FW) of the original fresh sample unit.
2.6. Scanning electron microscopy analysis
The three dried samples were flash frozen using liquid nitrogen and then subjected to natural brittle fracture to obtain cross-sectional observation samples for scanning electron microscopy (SEM; EVO-LS10, Cambridge, Germany) analysis. The samples were fixed on the surface of the sample holder with double-sided adhesive tape. The samples were then coated with gold under vacuum and observed using a SEM microscope at 10 kV (Wang, Fu, Chen, Hu, & Xie,2018).
2.7. Data analysis
The experimental data were analyzed using ANOVA and Tukey’s HSD test using SPSS software (version 19.0; IBM, Chicago, IL, USA). Differences were considered statistically significant at p < 0.05. Principal component analysis (PCA), hierarchical cluster analysis (HCA), and Pearson’s correlation analysis of the volatile flavor compounds, free amino acids, and soluble sugars were carried out using Origin 2022 software (Origin Lab, MA, USA).
3. Results and discussion
3.1. Changes in volatile flavor compounds during the drying process
Volatile flavor compounds (odors) affect the human appetite and indirectly affect food intake and digestion. HS-SPME-GCMS was used to detect the types and contents of volatile flavoring compounds in durian subjected to the three drying methods, as shown in Table 1. A total of 51 compounds were detected in the fresh samples, comprising 36 esters, 5 alcohols, 4 aldehydes and ketones, 3 thioethers, and 3 alkanes. A total of 47 compounds were detected in the IFD-dried samples, including 33 esters, 4 alcohols, 2 aldehydes and ketones, 4 thioethers, and 4 alkanes. The following five esters were formed in the process: ethyl isovalerate; propyl 2-methylpropionate; 2-butenoic acid, butyl ester; ethyl 2-octenoate; and ethyl myristate, along with a thioether (ethyl methyl disulfide) and an alkane (heptadecane). The following eight esters were vaporized during the drying process: ethyl valerate, ethyl 2-methylpentanoate, propyl heptanoate, ethyl nonanoate, methyl n-caprate, ethyl trans-2-decenoate, ethyl (2E,4Z)-deca-2,4-dienoate, and capric acid propyl ester. Additionally, one alcohol (1-nonanol), two aldehydes and ketones (2-methylbutyraldehyde and heptaldehyde), disappeared. A total of 48 compounds were detected in the CFD samples, comprising 32 esters, 4 alcohols, 3 aldehydes and ketones, 5 thioethers and 4 alkanes. A total of six compounds were generated during the drying process. Of these, three were esters—ethyl isovalerate, propyl 2-methylpropionate, and 2-Butenoic acid, butylester —and 2 were thioethers—ethyl methyl disulfide and (ethylthio)ethene. Additionally, heptadecane was vaporized. Nine compounds were lost during the drying process, namely seven esters—ethyl 2-methylpentanoate, ethyl heptanoate, ethyl nonanoate, methyl n-caprate, ethyl trans-2-decenoate, ethyl (2E,4Z)-deca-2,4-dienoate, and capric acid propyl ester; one alcohol—1-nonanol; and one Aldehydes and ketones— heptaldehydes.
Table 1.
Volatile flavor compounds and their contents in the durian samples during drying / (µg/g FW).
| Compound Category | Retention Time/min | Code Number | Retention index | Name of compounds | IFD |
CFD |
AD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | IF 0.5 h | IFD 4 h | IFD 8 h | IFD 12 h | CF 4 h | CFD 4 h | CFD 8 h | CFD 12 h | AD 3 h | AD 6 h | AD 9 h | |||||
| Esters | 1.805 | 1 | 600 | Ethyl acetate | 8.63 ± 1.38a | 2.65 ± 0.20c | 1.97 ± 0.14 cd | 1.39 ± 0.07de | 0.30 ± 0.02ef | 7.61 ± 0.11b | 1.03 ± 0.10def | 0.36 ± 0.03ef | 0.20 ± 0.00f | 1.37 ± 0.01de | 1.21 ± 0.02def | 0.59 ± 0.06ef |
| 1.922 | 2 | 615.2 | Methyl propionate | 5.84 ± 1.13a | 1.43 ± 0.10c | 0.78 ± 0.01 cd | 0.37 ± 0.00d | 0.36 ± 0.03d | 4.02 ± 0.14b | 0.50 ± 0.03d | 0.29 ± 0.01d | 0.28 ± 0.02d | 1.02 ± 0.06 cd | 0.53 ± 0.00d | 0.20 ± 0.08d | |
| 2.767 | 3 | 678.95 | Ethyl propionate | 111.95 ± 2.15a | 31.41 ± 1.99c | 28.79 ± 1.07 cd | 13.49 ± 0.08 g | 9.74 ± 0.13ij | 65.04 ± 0.75b | 11.98 ± 0.11hi | 10.23 ± 0.04ij | 7.32 ± 0.23 k | 22.61 ± 0.06e | 17.66 ± 0.77f | 8.23 ± 0.23jk | |
| 2.954 | 4 | 697 | Methyl butyrate | 1.13 ± 0.04a | 0.87 ± 0.03b | 0.52 ± 0.01c | 0.22 ± 0.04de | 0.13 ± 0.00ef | 0.25 ± 0.03d | 0.20 ± 0.12def | 0.15 ± 0.00def | 0.10 ± 0.06 fg | NDg | NDg | NDg | |
| 3.583 | 5 | 744.6 | Ethyl isobutyrate | 3.53 ± 0.20a | 1.60 ± 0.11c | 1.49 ± 0.11 cd | 0.72 ± 0.01e | 0.53 ± 0.03ef | 2.93 ± 0.16b | 0.77 ± 0.02e | 0.74 ± 0.07e | 0.45 ± 0.02 fg | 1.35 ± 0.05d | 1.31 ± 0.04d | 0.27 ± 0.12 g | |
| 3.926 | 6 | 762 | Methyl 2-methylbutyrate | 11.46 ± 0.69a | 5.28 ± 0.36d | 3.56 ± 0.30f | 2.02 ± 0.11 g | 1.76 ± 0.03 g | 9.07 ± 0.30b | 2.45 ± 0.14 g | 2.31 ± 0.11 g | 2.09 ± 0.06 g | 7.51 ± 0.04c | 4.34 ± 0.39e | 0.58 ± 0.31 h | |
| 4.46 | 7 | 784 | Ethyl butyrate | 33.07 ± 1.98a | 15.05 ± 1.08b | 10.75 ± 0.80d | 5.18 ± 0.40ef | 4.46 ± 0.15efg | 13.11 ± 0.22c | 5.42 ± 0.20e | 3.40 ± 0.10 fg | 3.18 ± 0.09 g | 9.26 ± 0.02d | 5.52 ± 0.52e | 1.34 ± 0.55 h | |
| 4.66 | 8 | 792.6 | Propyl propionate | 53.19 ± 2.81a | 18.76 ± 0.85c | 10.67 ± 0.49d | 9.01 ± 0.08d | 4.07 ± 0.28e | 22.47 ± 0.90b | 8.87 ± 0.51d | 4.23 ± 0.18e | 2.41 ± 0.05e | 18.96 ± 0.04c | 10.61 ± 0.25d | 2.15 ± 0.02e | |
| 5.583 | 9 | 793 | Ethyl crotonate | 2.41 ± 0.10a | 2.16 ± 0.20b | 0.91 ± 0.05de | 0.76 ± 0.04e | 0.51 ± 0.01f | 1.25 ± 0.20c | 0.94 ± 0.05de | 0.46 ± 0.03f | 0.38 ± 0.01f | 1.10 ± 0.07 cd | 1.04 ± 0.04 cd | 1.01 ± 0.07 cd | |
| 5.676 | 10 | 833 | Ethyl 2-methylbutyrate | 214.09 ± 1.11a | 117.28 ± 1.70d | 105.25 ± 1.96e | 54.28 ± 0.87 g | 51.81 ± 2.17gh | 164.86 ± 0.69b | 77.54 ± 0.57f | 54.40 ± 1.35 g | 49.88 ± 1.47 h | 157.65 ± 0.05c | 117.34 ± 2.63d | 31.49 ± 0.82i | |
| 5.821 | 11 | 838.4 | Ethyl isovalerate | NDc | NDc | NDc | NDc | 0.42 ± 0.09a | NDc | 0.38 ± 0.11a | 0.25 ± 0.01b | 0.18 ± 0.06b | NDc | NDc | NDc | |
| 5.882 | 12 | 880 | Propyl 2-methylpropionate | NDg | 0.56 ± 0.00c | 0.50 ± 0.02 cd | 0.46 ± 0.00d | 0.26 ± 0.02f | NDg | 0.69 ± 0.05b | 0.37 ± 0.00e | 0.27 ± 0.02f | 0.78 ± 0.07a | 0.67 ± 0.00b | 0.67 ± 0.08b | |
| 7.064 | 13 | 884 | Propyl butyrate | 15.21 ± 1.03a | 8.37 ± 0.30b | 4.30 ± 0.33ef | 4.61 ± 0.20e | 1.68 ± 0.07 g | 4.96 ± 0.12e | 3.69 ± 0.15f | 1.81 ± 0.25 g | 0.99 ± 0.05 g | 7.04 ± 0.29c | 5.82 ± 0.04d | 1.88 ± 0.26 g | |
| 7.151 | 14 | 883 | Ethyl valerate | 4.00 ± 0.07a | 1.13 ± 0.06d | NDh | NDh | NDh | 1.83 ± 0.07b | 1.25 ± 0.02c | 0.87 ± 0.06f | 0.54 ± 0.02 g | 1.10 ± 0.05d | 0.99 ± 0.04e | 0.83 ± 0.00f | |
| 7.391 | 15 | 891.4 | Butyl propionate | 1.76 ± 0.19a | 1.17 ± 0.00bc | 0.93 ± 0.06c | 0.62 ± 0.10d | 0.43 ± 0.09def | 1.26 ± 0.26b | 0.32 ± 0.01f | 0.22 ± 0.01f | 0.15 ± 0.01f | 0.92 ± 0.01c | 0.52 ± 0.10de | 0.23 ± 0.02f | |
| 7.834 | 16 | 907 | Methyl hexanoate | 10.57 ± 0.63a | 3.35 ± 0.26c | 2.17 ± 0.03d | 0.67 ± 0.12gh | 0.32 ± 0.01hi | 2.01 ± 0.01d | 1.34 ± 0.06ef | 1.09 ± 0.06 fg | 0.52 ± 0.04hi | 7.36 ± 0.08b | 1.79 ± 0.13de | 0.04 ± 0.02i | |
| 8.273 | 17 | 924 | (E)-Ethyl-2-methyl-2-butenoate | 4.95 ± 0.22a | 3.28 ± 0.32c | 2.04 ± 0.17d | 1.10 ± 0.13 fg | 0.25 ± 0.02 h | 3.98 ± 0.14b | 1.74 ± 0.04de | 1.36 ± 0.11ef | 0.80 ± 0.02 g | 4.30 ± 0.08b | 4.13 ± 0.17b | 1.77 ± 0.30de | |
| 8.424 | 18 | 1017 | 2-Butenoic acid, butylester | NDg | NDg | 1.11 ± 0.09b | 0.20 ± 0.00f | 0.17 ± 0.00f | 2.45 ± 0.11a | 0.46 ± 0.04d | 0.35 ± 0.01e | 0.31 ± 0.00e | 1.05 ± 0.00b | 0.90 ± 0.04c | 0.82 ± 0.05c | |
| 8.48 | 19 | 954 | Propyl 2-methylbutanoate | 54.53 ± 1.41a | 28.48 ± 0.28d | 24.12 ± 1.57e | 12.36 ± 0.09 g | 12.72 ± 0.03 g | 39.40 ± 0.84c | 19.62 ± 0.17f | 12.64 ± 0.47 g | 7.47 ± 0.42 h | 44.19 ± 0.75b | 23.90 ± 0.67e | 5.07 ± 0.02i | |
| 9.247 | 20 | 958 | Isoamyl propionate | 2.20 ± 0.25a | 1.78 ± 0.04 cd | 1.27 ± 0.11e | 0.40 ± 0.01ij | 0.35 ± 0.04j | 2.10 ± 0.04ab | 0.90 ± 0.05f | 0.58 ± 0.04hi | 0.28 ± 0.02j | 1.93 ± 0.04bc | 1.72 ± 0.04d | 0.62 ± 0.10 g | |
| 9.305 | 21 | 980.5 | Propanoic acid, pentylester | 1.54 ± 0.05a | 1.47 ± 0.10a | 1.03 ± 0.05c | 0.58 ± 0.00d | 0.50 ± 0.04d | 1.09 ± 0.01c | 0.20 ± 0.01f | 0.64 ± 0.05d | 0.40 ± 0.02e | 1.27 ± 0.07b | 0.61 ± 0.06d | 0.07 ± 0.13f | |
| 10.075 | 22 | 980 | Propyl n-valerate | NDc | NDc | NDc | NDc | NDc | NDc | NDc | NDc | NDc | NDc | 2.03 ± 0.17a | 0.63 ± 0.00b | |
| 10.141 | 23 | 982.9 | Ethyl caproate | 255.67 ± 1.34a | 98.60 ± 1.90d | 55.13 ± 0.79e | 24.72 ± 1.4i | 15.11 ± 0.05j | 147.32 ± 0.56b | 52.34 ± 0.09f | 27.19 ± 0.40 h | 8.40 ± 0.05 k | 123.20 ± 0.08c | 39.37 ± 0.53 g | 4.36 ± 0.00 l | |
| 10.395 | 24 | 1006 | Ethyl 3-hexenoate | 1.97 ± 0.21a | 1.47 ± 0.08b | 1.11 ± 0.02c | 0.21 ± 0.04e | 0.19 ± 0.00e | 1.55 ± 0.10b | 0.28 ± 0.08e | 0.27 ± 0.01e | 0.24 ± 0.00e | 1.36 ± 0.00b | 0.82 ± 0.10d | 0.75 ± 0.00d | |
| 11.147 | 25 | 1141 | Ethyl 2-Methylpentanoate | 1.35 ± 0.15a | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb | |
| 11.235 | 26 | 1152 | Pentanoic acid,2-methyl-, propyl ester | 2.50 ± 0.39a | 1.27 ± 0.01c | 1.18 ± 0.10c | 0.39 ± 0.08d | 0.39 ± 0.06d | 2.41 ± 0.11a | 0.99 ± 0.05c | 0.54 ± 0.05d | 0.38 ± 0.01d | 2.31 ± 0.07ab | 2.06 ± 0.07b | 0.38 ± 0.10d | |
| 13.087 | 27 | 1019 | Propyl hexanoate | 77.77 ± 1.36a | 26.93 ± 0.78d | 11.32 ± 0.16f | 9.24 ± 0.34f | 3.86 ± 0.11 g | 35.63 ± 0.78c | 9.72 ± 1.79f | 4.23 ± 0.24 g | 1.34 ± 0.26 h | 39.32 ± 2.12b | 19.84 ± 0.06e | 1.32 ± 0.76 h | |
| 13.181 | 28 | 1083 | Ethyl heptanoate | 14.05 ± 1.04a | 4.68 ± 0.11c | 3.50 ± 0.21de | 1.58 ± 0.19f | 0.72 ± 0.11f | 6.79 ± 0.63b | 1.56 ± 0.08f | 0.73 ± 0.10f | 0.60 ± 0.04f | 3.70 ± 0.23d | 3.50 ± 0.04de | 2.63 ± 0.06e | |
| 13.973 | 29 | 1112 | Methyl octanoate | 8.09 ± 0.82a | 3.18 ± 0.20c | 1.52 ± 0.04de | 0.81 ± 0.21ef | 0.50 ± 0.04f | 1.61 ± 0.16d | 0.92 ± 0.03def | 0.60 ± 0.03f | 0.38 ± 0.02f | 4.85 ± 0.21b | 1.35 ± 0.05de | 0.36 ± 0.28f | |
| 16.032 | 30 | 1174 | Propyl heptanoate | 0.99 ± 0.00a | 0.72 ± 0.12b | 0.22 ± 0.08c | NDd | NDd | NDd | NDd | NDd | NDd | 0.66 ± 0.08b | 0.63 ± 0.10b | 0.35 ± 0.07c | |
| 16.133 | 31 | 1183 | Ethyl caprylate | 139.98 ± 0.99a | 61.07 ± 1.63b | 26.55 ± 1.71d | 17.31 ± 0.62e | 7.07 ± 0.19 g | 62.72 ± 0.65b | 18.22 ± 1.05e | 9.85 ± 0.65f | 3.73 ± 0.42 h | 49.98 ± 0.07c | 26.21 ± 1.38d | 2.93 ± 0.06 h | |
| 17.578 | 32 | 1231 | Ethyl 2-octenoate | NDc | NDc | 1.10 ± 0.08a | 1.00 ± 0.31a | 0.43 ± 0.27b | 1.10 ± 0.00a | 0.20 ± 0.03bc | NDc | NDc | NDc | NDc | NDc | |
| 18.902 | 33 | 1274 | Octanoic acid, propyl ester | 15.13 ± 1.57a | 3.64 ± 0.32b | 1.58 ± 0.19cde | 1.47 ± 0.13cde | 1.00 ± 0.20de | 2.18 ± 0.14bcd | 1.04 ± 0.06de | 0.30 ± 0.04e | 0.23 ± 0.02e | 2.90 ± 0.31bc | 2.59 ± 1.06bcd | 1.35 ± 0.52cde | |
| 19.029 | 34 | 1274 | Ethyl nonanoate | 1.81 ± 0.08a | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb | |
| 19.988 | 35 | 1307 | Methyl n-caprate | 2.13 ± 0.12a | 0.67 ± 0.00d | NDe | NDe | NDe | NDe | NDe | NDe | NDe | 1.59 ± 0.03b | 0.86 ± 0.12c | 0.68 ± 0.00d | |
| 22.55 | 36 | 1376 | Ethyl caprate | 30.83 ± 0.07a | 7.36 ± 0.51d | 2.84 ± 0.45 fg | 2.30 ± 0.11gh | 0.39 ± 0.00i | 3.14 ± 0.13e | 2.08 ± 0.07 h | 1.66 ± 0.02 h | 0.84 ± 0.12i | 12.22 ± 0.53b | 8.04 ± 0.07c | 0.32 ± 0.11i | |
| 23.88 | 37 | 1391 | Ethyl trans-2-decenoate | 1.42 ± 0.05a | NDc | NDc | NDc | NDc | NDc | NDc | NDc | NDc | 0.86 ± 0.09b | 0.77 ± 0.00b | 0.86 ± 0.09b | |
| 24.365 | 38 | 1447 | Ethyl (2E,4Z)-deca-2,4-dienoate | 1.63 ± 0.00a | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb | |
| 24.8 | 39 | 1473 | Capric acid propyl ester | 2.09 ± 0.30a | NDc | NDc | NDc | NDc | NDc | NDc | NDc | NDc | 0.87 ± 0.22b | 0.61 ± 0.00b | 0.87 ± 0.26b | |
| 26.609 | 40 | 1576 | Ethyl laurate | 7.75 ± 0.55a | 2.93 ± 0.30b | 1.76 ± 0.30c | 0.82 ± 0.08def | 0.30 ± 0.12f | 1.15 ± 0.17d | 0.47 ± 0.05ef | 0.56 ±.04ef | 0.43 ± 0.01ef | 2.77 ± 0.26b | 1.75 ± 0.02c | 1.01 ± 0.10de | |
| 29.276 | 41 | 1777 | Ethyl myristate | NDb | NDb | NDb | 0.19 ± 0.05a | 0.18 ± 0.08a | NDb | NDb | NDb | NDb | NDb | NDb | NDb | |
| 31.44 | 42 | 1976 | Palmitic acid ethyl ester | 2.14 ± 0.00a | 1.90 ± 0.12b | 1.20 ± 0.01d | 0.95 ± 0.00e | 0.50 ± 0.03f | 1.55 ± 0.17c | 0.98 ± 0.20e | 0.50 ± 0.06f | 0.21 ± 0.03 g | NDg | NDg | NDg | |
| Subtotal | 1107.36 ± 3.56a | 460.44 ± 2.45d | 311.06 ± 1.67e | 169.43 ± 1.43 g | 121.41 ± 1.14i | 616.84 ± 1.84b | 229.09 ± 1.05f | 143.18 ± 1.18 h | 94.98 ± 1.48j | 537.36 ± 1.36c | 311.04 ± 1.04e | 76.66 ± 1.66 k | ||||
| Alcohols | 1.545 | 43 | 561.2 | 1-Propanol | 2.67 ± 0.01a | 0.84 ± 0.05c | 0.82 ± 0.01c | 0.31 ± 0.00e | 0.08 ± 0.02f | 1.46 ± 0.00b | 0.40 ± 0.03de | 0.28 ± 0.02e | 0.09 ± 0.00f | 1.43 ± 0.10b | 0.52 ± 0.16d | 0.10 ± 0.03f |
| 2.235 | 44 | 674.4 | 1-Butanol | 1.24 ± 0.01a | 1.18 ± 0.04ab | 1.15 ± 0.00abc | 0.90 ± 0.20d | 0.29 ± 0.11ef | 1.04 ± 0.06bcd | 0.99 ± 0.02 cd | 0.39 ± 0.00e | 0.20 ± 0.00f | NDg | NDg | NDg | |
| 7.592 | 45 | 830 | 2,4-Dimethyl-3-pentanol | 1.62 ± 0.12a | 0.90 ± 0.00bc | 0.55 ± 0.05d | 0.25 ± 0.10e | 0.18 ± 0.00e | 1.47 ± 0.11a | 0.78 ± 0.02c | 0.49 ± 0.07d | 0.21 ± 0.03e | 1.01 ± 0.03b | 0.42 ± 0.01d | 0.12 ± 0.10e | |
| 12.394 | 46 | 1051 | 1-Octanol | 1.83 ± 0.13a | 1.73 ± 0.14a | 1.01 ± 0.01b | 0.77 ± 0.01c | 0.36 ± 0.17de | 1.70 ± 0.06a | 1.00 ± 0.06b | 0.41 ± 0.02d | 0.15 ± 0.07ef | 1.08 ± 0.01b | 0.42 ± 0.10d | 0.08 ± 0.12f | |
| 15.414 | 47 | 1157.2 | 1-nonanol | 2.37 ± 0.03a | 1.36 ± 0.00b | 1.20 ± 0.08b | 0.52 ± 0.01c | NDd | 1.20 ± 0.63b | 0.35 ± 0.00 cd | 0.27 ± 0.00 cd | NDd | 1.00 ± 0.08b | NDd | NDd | |
| Subtotal | 9.73 ± 0.33a | 6.01 ± 0.51c | 4.73 ± 0.23d | 2.75 ± 0.25f | 0.91 ± 0.21hi | 6.87 ± 0.37b | 3.52 ± 0.12e | 1.84 ± 0.34 g | 0.65 ± 0.15i | 4.52 ± 0.12d | 1.36 ± 0.16gh | 0.3 ± 0.15i | ||||
| Aldehydes and ketones | 2.102 | 48 | 627 | 2-Butenal | 6.33 ± 0.56b | 4.25 ± 0.32c | 2.52 ± 0.65d | 0.60 ± 0.20e | 0.20 ± 0.02e | 4.05 ± 0.20c | 2.05 ± 0.02d | 0.85 ± 0.06e | 0.35 ± 0.07e | 6.30 ± 0.04b | 6.52 ± 0.04b | 7.21 ± 0.28a |
| 3.288 | 49 | 650.03 | 2-Methyl butyraldehyde | 1.73 ± 0.02c | 0.88 ± 0.03de | 0.64 ± 0.08e | 0.30 ± 0.01f | NDg | 0.94 ± 0.08d | 0.68 ± 0.06e | 0.13 ± 0.00 fg | 0.05 ± 0.05 g | 1.68 ± 0.11c | 1.99 ± 0.04b | 2.81 ± 0.29a | |
| 7.159 | 50 | 851 | Heptaldehyde | 1.34 ± 0.07c | 0.48 ± 0.04de | 0.45 ± 0.00de | NDf | NDf | 0.66 ± 0.05d | 0.58 ± 0.00d | 0.36 ± 0.02e | NDf | 1.88 ± 0.05b | 1.92 ± 0.18b | 2.36 ± 0.20a | |
| 10.13 | 51 | 983 | Octanal | NDd | NDd | NDd | NDd | NDd | NDd | NDd | NDd | NDd | 0.33 ± 0.24c | 0.51 ± 0.01b | 0.99 ± 0.03a | |
| 13.391 | 52 | 1104 | 1-Nonanal | 4.09 ± 0.22b | 3.96 ± 0.06b | 1.11 ± 0.08e | 0.90 ± 0.11ef | 0.69 ± 0.07ef | 2.66 ± 0.45c | 1.84 ± 0.16d | 0.73 ± 0.01ef | 0.35 ± 0.01f | 4.31 ± 0.10b | 4.30 ± 0.51b | 5.06 ± 0.11a | |
| 24.897 | 53 | 1467.3 | 2-Tridecanone | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb | NDb | 0.75 ± 0.10a | 0.62 ± 0.07a | 0.02 ± 0.01b | |
| Subtotal | 13.49 ± 0.49c | 9.57 ± 0.17d | 4.72 ± 0.12f | 1.80 ± 0.2 g | 0.89 ± 0.19 h | 8.31 ± 0.31e | 5.15 ± 0.15f | 2.07 ± 0.07 g | 0.75 ± 0.15 h | 15.25 ± 0.25b | 15.59 ± 0.19b | 18.45 ± 0.45a | ||||
| Thioethers | 5.236 | 54 | 861 | Ethyl methyl disulfide | NDi | NDi | 7.30 ± 0.01a | 7.12 ± 0.02b | 1.20 ± 0.00 g | NDi | 5.30 ± 0.01c | 2.10 ± 0.03f | 1.01 ± 0.12 h | NDi | 3.28 ± 0.13e | 4.10 ± 0.01d |
| 7.686 | 55 | 935 | Ethyl disulfide | 7.08 ± 0.04c | 4.65 ± 0.07e | 4.00 ± 0.08 fg | 3.64 ± 0.13 g | 0.78 ± 0.15i | 5.75 ± 0.07d | 4.03 ± 0.08f | 3.64 ± 0.13 g | 1.78 ± 0.15 h | 7.32 ± 0.01c | 8.26 ± 0.29b | 8.80 ± 0.26a | |
| 10.534 | 56 | 695 | (Ethylthio)ethene | NDf | NDf | NDf | NDf | NDf | NDf | 4.10 ± 0.04a | 0.47 ± 0.00d | 0.10 ± 0.02f | 2.47 ± 0.21c | 2.65 ± 0.08b | 0.29 ± 0.00e | |
| 14.311 | 57 | 1101 | 3,5-Dimethyl-1,2,4-trithiolane | 5.53 ± 0.20a | 4.57 ± 0.05b | 3.79 ± 0.06d | 0.50 ± 0.08 h | 0.27 ± 0.02hi | 4.01 ± 0.02 cd | 3.33 ± 0.15e | 0.25 ± 0.08hi | 0.08 ± 0.06i | 4.22 ± 0.23c | 2.01 ± 0.05 g | 2.81 ± 0.29f | |
| 14.371 | 58 | 1157 | diethyl trisulphide | 8.45 ± 0.21bc | 7.50 ± 0.64de | 6.62 ± 0.55e | 4.76 ± 0.13f | 1.12 ± 0.06 g | 8.00 ± 0.40 cd | 6.62 ± 0.55e | 4.76 ± 0.13f | 0.92 ± 0.06 g | 8.50 ± 0.14bc | 9.20 ± 0.49b | 11.22 ± 0.06a | |
| 17.17 | 59 | 788 | Isopropyl sulfide | NDd | NDd | NDd | NDd | NDd | NDd | NDd | NDd | NDd | 3.44 ± 0.28c | 3.99 ± 0.01b | 6.11 ± 0.00a | |
| Subtotal | 21.06 ± 0.46e | 16.72 ± 0.52 fg | 21.71 ± 0.51e | 16.02 ± 0.22 g | 3.37 ± 0.37i | 17.51 ± 0.31f | 23.38 ± 0.38d | 11.22 ± 0.22 h | 3.89 ± 0.14i | 25.95 ± 0.45c | 29.39 ± 0.39b | 33.33 ± 0.33a | ||||
| Alkanes | 11.041 | 60 | 1044.39 | (D)-Limonene | 2.37 ± 0.10f | 2.65 ± 0.20f | 3.99 ± 0.07c | 4.41 ± 0.00b | 5.37 ± 0.00a | 2.5 ± 0.01f | 3.02 ± 0.01e | 3.55 ± 0.20d | 4.56 ± 0.10b | 1.24 ± 0.05 g | 0.87 ± 0.40 h | 0.25 ± 0.02i |
| 16.278 | 61 | 200.12 | Dodecane | 0.76 ± 0.00e | 1.08 ± 0.02e | 2.64 ± 0.11c | 3.69 ± 0.07a | 3.71 ± 0.03a | 1.01 ± 0.20e | 1.53 ± 0.21d | 2.36 ± 0.16c | 3.10 ± 0.13b | 0.30 ± 0.15f | 0.15 ± 0.09f | 0.06 ± 0.15f | |
| 22.736 | 62 | 236.21 | Tetradecane | 0.36 ± 0.87def | 0.87 ± 0.04cde | 1.10 ± 0.12 cd | 2.25 ± 0.28b | 3.62 ± 0.06a | 0.89 ± 0.30cde | 1.25 ± 0.30c | 2.67 ± 0.32b | 2.82 ± 0.20b | 0.27 ± 0.03def | 0.09 ± 0.14ef | 0.02 ± 0.00f | |
| 26.727 | 63 | 282.99 | Heptadecane | NDh | 0.50 ± 0.00d | 0.89 ± 0.02c | 1.01 ± 0.01b | 1.22 ± 0.02a | NDh | 0.16 ± 0.01 g | 0.20 ± 0.00f | 0.35 ± 0.05e | NDh | NDh | NDh | |
| Subtotal | 3.49 ± 0.51f | 5.10 ± 0.15de | 8.53 ± 0.33c | 11.36 ± 0.36b | 13.92 ± 0.92a | 4.40 ± 0.4ef | 5.96 ± 0.46d | 8.78 ± 0.22c | 10.83 ± 0.27b | 1.81 ± 0.09 g | 1.11 ± 0.04gh | 0.33 ± 0.07 h | ||||
| Total | 1155.13 ± 2.77a | 497.84 ± 1.26d | 350.75 ± 1.25f | 201.36 ± 1.64 h | 140.23 ± 1.23j | 653.93 ± 1.03b | 267.10 ± 0.25 g | 167.09 ± 1.01i | 111.10 ± 0.90 l | 584.89 ± 1.11c | 358.49 ± 1.49e | 129.07 ± 1.07 k | ||||
Note: “ND” indicates that is not detected or below detection limit. “FW” means fresh weight. Data points in the same row with different letters are significantly different (p < 0.05). IFD means integrated freeze-drying, CFD means conventional freeze-drying, AD means hot air drying.
A total of 52 compounds were detected in the AD-dried samples, namely 34 esters, 3 alcohols, 6 aldehydes and ketones, 6 thioethers, and 3 alkanes. Seven compounds were lost during the drying process, including five esters (methyl butyrate, ethyl 2-methylpentanoate, ethyl nonanoate, ethyl (2E,4Z)-deca-2,4-dienoate, and palmitic acid ethyl ester) and two alcohols (1-nonanol and 1-butanol). Eight compounds were newly generated, comprising three esters (propyl 2-methylpropionate, 2-butenoic acid, butylester, and propyl n-valerate) and two aldehydes and ketones (octanal and 2-tridecanone). The three thioethers used were ethyl methyl disulfide, (ethylthio)ethene, and isopropyl sulfide.
The total content of volatile compounds in the fresh samples was 1155.13 ± 2.77 μg/g. In IFD, CFD, and AD samples contained volatile compounds totaling 140.23 ± 1.23 μg/g, 111.10 ± 0.90 μg/g, and 129.07 ± 1.07 μg/g, respectively, with a loss ratio of 87.86, 90.38, and 88.83 %, respectively, compared to fresh samples, with significant differences (p < 0.05). As the three drying processes proceeded, there was a continuous decrease in the total volatile compound content, with both FD drying volatile flavor compound contents decreasing sharply, followed by a slow decrease. The rates of decrease in volatile flavor compounds from AD3h to AD6h and from AD6h to AD9h tended to be similar during the entire AD drying process. There was no significant difference in alcohol content among the three dried samples (p > 0.05). During IFD and CFD, the alkane content tended to increase, mainly due to the cleavage reaction or polymerization reaction of fatty acid alkoxyl radicals. Furthermore, the spatial structure of alkanes changed under low-temperature and vacuum conditions. Although alkanes do not significantly contribute to the overall flavor, they do have a moderating effect on the flavor compounds. Many studies have demonstrated that the volatile flavor compounds in fresh durian mainly consist of esters and sulfur-containing substances. Esters contribute a fruity aroma, while the sulfur-containing compounds contribute to the characteristic pungent durian odor (Aschariyaphotha et al., 2021, Belgis et al., 2017).
In terms of esters, the total content of IFD, CFD, and AD decreased during drying, with loss ratio of 89.06, 91.42, and 93.07 %, respectively, showing significant differences (p < 0.05). IFD retained more ester flavor compounds. In terms of thioethers, AD samples had the highest content of flavor compounds. Compared with the fresh samples, the thioether of the AD3h samples increased from three to five species, while from AD3h to the end of AD9h, the thioether species increased from five to six, with the addition of ethyl methyl disulfide. This may be due to the continuous dehydration of durian samples in the middle and late stages of AD, leading to chemical reactions such as the Maillard and the Strecker reactions, resulting in the production of new sulfur-containing compounds (Peng, 2019).
The characteristic aroma flavor in durian is not necessarily contributed by the highest content of volatile compounds. Durian is characteristiced by a flower-fruit fragrance and a strong pungent mixture odour similar to onion, garlic, and sulphur. Sulphur-containing compounds, especifically isopropyl sulphide and (Ethylthio)ethene, contribute to an oniony flavor. Ester compounds contribute to a fruit flavor. Ethyl caproate, ethyl 2-methylbutyrate, ethyl caprylate, ethyl propionate, propyl hexanoate, and propyl 2-methylbutanoate are the top six volatile flavor compounds in content in the fresh samples. After the three dying methods, the contents of the top six volatile flavor compounds all decreased. IFD contributed to the highest contents of the top six volatile flavor compounds in the dried samples, followed by CFD and finally AD. Therefore, the AD products had less fruity and more pungent odour (Peng, 2019).
Fig. S1 shows the radar plot of the response values of the volatile flavor compounds measured by the electronic nose sensor during drying. The main sensors affecting the volatile flavor substances of durian were W1W and W1S, indicating that the flavor compounds of durian are mainly sulfur-containing substances and methyl esters. Sulfur-containing compounds are responsible for durian’s odor, while esters constitutes the main source of the fruit aroma in durian. As shown in Fig. S1a and Fig. S1b, the volatile-compound response profile and trend in the IFD and CFD processes are basically the same, with the maximum response value of W1W occurring at 4 h, and the profile gradually diminishing as the drying process progressed. This indicates that the FD method reduces the formation of sulfur-containing compounds and pungent odor, which is conducive to the formation of a good flavor. However, the AD method did not show the same trend as the W1W in the two FD methods. As shown in Fig. S1c and Fig. S1d, the response value of the W1W sensor increased during the AD process and was eventually higher than that of the fresh samples, whereas the response value of W1S decreased continuously. This indicates that a large number of methyl esters were lost during AD drying and a large number of sulfur-containing compounds were produced, which was not conducive to the development of a favorable flavor. Combined with the results of volatile flavor compounds detected by GC–MS, the two types of FD are conducive to the formation of a good flavor, with IFD being the best, CFD being the second best, and AD being the last. The regular pattern of change in the overall flavor composition of the durian samples detected by electronic nose during the three drying processes was consistent with the results of the flavor components detected by GC–MS.
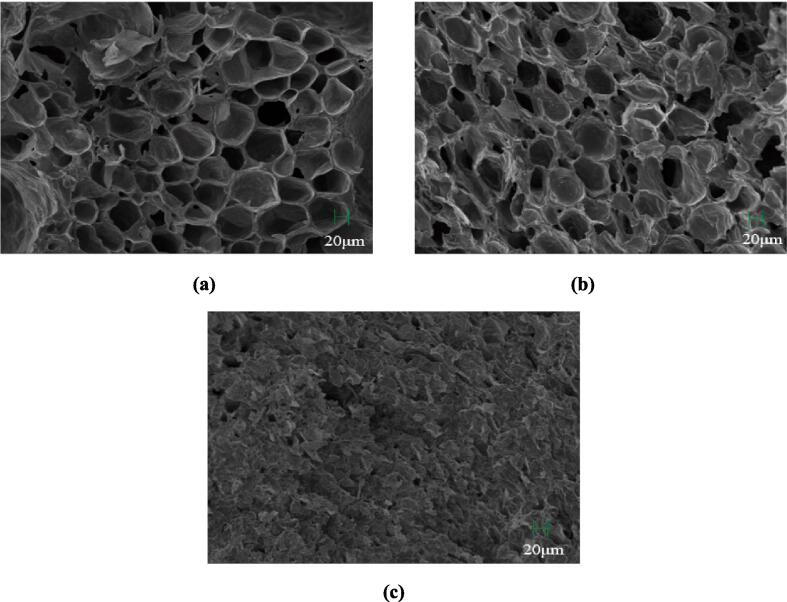
The main reasons for these differences were the principles of microzone capture and moisture migration. Fig. 1 shows the microstructures of the IFD, CFD, and AD samples. The IFD samples formed a relatively complete regular honeycomb pore structure, the CFD samples had some pore collapses, and the AD samples had collapsed and dried pores. The complete cellular structure facilitates the retention of volatile flavor compounds and hinders their spillage. These different results may be due to the different pre-freezing methods used for the two FD methods. Vacuum freezing is used by IFD, which causes the water in the material to evaporate rapidly, the material to freeze quickly, and the formation of smaller ice crystals, resulting in less damage to the cells (Prasetia et al., 2023). While CFD uses atmospheric pressure freezing, the freezing speed is slower, the formation of ice crystals is larger, and the degree of cellular damage is large, causing the volatile components to diffuse to the outside of the cell (Pupan et al., 2023). Based on the principle of microzone capture, the rate of loss of volatile components in CFD samples is higher than that of IFD (Krokida and Philippopoulos, 2006, Flink and Karel, 1970, Bangs and Reineccius, 1990, Voon et al., 2007). With the sublimation and evaporation of water during the drying process, the cell wall and pore chamber wall gradually become more solid, and the resistance of the microzone to the migration of volatile compounds increases; therefore, the loss of volatile compounds in the FD process is faster and then slower (Krokida and Philippopoulos, 2006, Flink and Karel, 1970, Bangs and Reineccius, 1990, Voon et al., 2007, Dehghannya et al., 2019).
Fig. 1.
The microstructure of the dried durian samples by integrated freeze-drying (a), conventional freeze-drying (b), and hot-air drying (c) image magnification is 200×.
3.2. Principal component, cluster, and Pearson’s correlation analyses of volatile flavor compounds
The effect of the drying process on the distribution of volatile compounds in durian was analyzed using principal component analysis. Fig. 2a shows that 77 % of the total volatile compounds were described by the first and second principal components (PC1: 56.7 %; PC2: 20.3 %). The PCA results in Fig. 2a show that the samples at different drying stages can be well differentiated based on the PC1 and PC2 scores. The fresh samples were well defined based on the larger positive values of PC1 and PC2. The IFD and CFD samples were clustered in the second quadrant, with IF0.5 h and CF4h grouped together. However, the AD samples were well distinguished based on the negative values of PC2. From the PCA results (Fig. 2b), it was possible to divide the 63 volatile compounds into several main clusters. These 63 compounds were distributed unevenly across the four quadrants. The volatile compounds in the samples were categorized according to their similarity (Fig. 2c). The samples with different drying stages in the horizontal direction were classified into four clusters: the first cluster comprised fresh samples, IF0.5 h, IFD4h, and CF4h; the second cluster comprised samples with IFD8h, IFD12h, CFD4h, CFD8h, and CFD12h; the third cluster included samples with AD3h and AD6h; and the fourth cluster included samples with AD9h. The 63 volatile compounds were vertically classified into six clusters, the first of which contained compounds ethyl acetate, methyl propionate, ethyl propionate, methyl butyrate, 1-Nonanal, ethyl isovalerate, palmitic acid ethyl ester, 1-Butanol, 3,5-Dimethyl-1,2,4-trithiolane, (E)-Ethyl-2-methyl-2-butenoate, 2-Butenal, ethyl disulfide, diethyl trisulphide, Ethyl crotonate, ethyl heptanoate, propyl butyrate, octanoic acid, propyl ester, Ethyl laurate, 2-Butenoic acid, butylester, ethyl 3-hexenoate, isoamyl propionate, and Pentanoic acid,2-methyl-, propyl ester. Compounds ethyl isobutyrate, methyl 2-methylbutyrate, ethyl 2-methylbutyrate, propyl propionate, ethyl caprylate, propyl hexanoate, propyl 2-methylbutanoate, ethyl caproate, 1-Propanol, ethyl butyrate, 2,4-Dimethyl-3-pentanol, methyl hexanoate, methyl octanoate, ethyl caprate, 1-Octanol, Butyl propionate, and propanoic acid, pentylester were contained by the second cluster. These clusters included compounds propyl 2-methylpropionate and Ethyl myristate. The fourth cluster consisted of compounds ethyl valerate, ethyl nonanoate, and ethyl (2E,4Z)-deca-2,4-dienoate. The fifth cluster comprised compounds propyl n-valerate, 2-Tridecanone, ethyl trans-2-decenoate, capric acid propyl ester, octanal, (D)-Limonene, dodecane, tetradecane, methyl n-caprate, and propyl heptanoate. Finally, the sixth cluster comprised compounds Ethyl 2-Methylpentanoate, ethyl methyl disulfide, ethyl 2-octenoate, 1-nonanol, heptadecane, isopropyl sulfide, 2-Methyl butyraldehyde, and (Ethylthio) ethene.
Fig. 2.
Principal component analysis, hierarchical cluster analysis, and Pearson’s correlation analysis based on the content of volatile flavor compounds in the samples during the three drying processes. Numbers 1–63 are the corresponding codes for the 63 kinds of volatile compounds in Table.1. (a): Score plot of principal component analysis of the different drying methods; (b): Score plot of principal component analysis of the volatile flavor compounds; (c): Heatmap of hierarchical cluster analysis; (d): Heatmap of Pearson’s correlation analysis.
The correlation coefficients of volatile compounds during the drying process were determined using Pearson's correlation analysis. The correlations among the 63 volatile compounds are shown in Fig. 2d. Red and blue represent positive and negative correlations, respectively, with darker colors indicating stronger correlations. Compounds 1–47 exhibited a strong positive correlation, except for ethyl isovalerate, propyl 2-methylpropionate, 2-Butenoic acid, butylester, propyl n-valerate, ethyl 2-octenoate, and ethyl myristate, which showed no significant correlation with other volatile compounds.
In contrast, compounds (D)-Limonene, dodecane, tetradecane, and heptadecane showed a significant negative correlation with many other compounds in compounds 48–63. The results of the above statistical analyses reflect, to a certain extent, the differences in volatile compounds in durians at different drying stages, providing basic information for the analysis of volatile compounds during the drying of durians.
3.3. Changes in amino acids and soluble sugars during the drying process
As shown in Table 2, the amino acids did not change after three types of drying. However, in terms of amino-acid content, complex changes in amino acids occurred during different drying processes, and different kinds of amino acid changes showed different trends. The total amino acid counts of fresh, IFD, CFD, and AD samples were 86.16 ± 0.18, 93.26 ± 1.32, 86.95 ± 1.33, and 76.80 ± 1.98, respectively, with significant differences (p < 0.05).
Table 2.
Contents of amino acids and soluble sugars in the durian samples during drying /(mg/g FW).
| Compound Category | Compound Name | Drying time |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | IF0.5 h | IFD 4 h | IFD 8 h | IFD 12 h | CF4h | CFD 4 h | CFD 8 h | CFD 12 h | AD 3 h | AD 6 h | AD 9 h | ||
| Amino acids | Aspartic acid (Asp) | 7.62 ± 0.10f | 1.84 ± 0.08j | 13.06 ± 0.38a | 10.31 ± 0.22 cd | 11.93 ± 0.44b | 5.01 ± 0.22i | 8.28 ± 0.11e | 10.68 ± 0.10c | 10.09 ± 0.11d | 6.88 ± 0.02 g | 6.70 ± 0.07 g | 6.02 ± 0.39 h |
| Glutamate (Glu) | 12.46 ± 0.03e | 14.27 ± 0.34d | 19.40 ± 0.34b | 21.74 ± 1.57a | 18.63 ± 0.47b | 11.92 ± 0.14ef | 14.81 ± 0.21d | 17.39 ± 0.12c | 15.27 ± 0.22d | 10.84 ± 0.22 fg | 9.99 ± 0.08 g | 7.37 ± 0.06 h | |
| Serine (Ser) | 4.06 ± 0.34i | 6.27 ± 0.21 fg | 10.33 ± 0.55c | 14.42 ± 0.19a | 12.00 ± 0.21b | 4.06 ± 0.10i | 6.10 ± 0.09 g | 6.76 ± 0.17f | 8.29 ± 0.08d | 6.05 ± 0.11 g | 7.54 ± 0.21e | 5.30 ± 0.23 h | |
| Histidine (His) | 11.70 ± 0.15d | 11.04 ± 0.28de | 5.99 ± 0.35 h | 4.67 ± 0.38i | 5.22 ± 0.33i | 9.59 ± 0.29 g | 12.69 ± 0.14c | 10.33 ± 0.20efg | 9.96 ± 0.19 fg | 15.41 ± 0.24b | 16.72 ± 0.60a | 10.64 ± 0.02ef | |
| Glycine (Gly) | 5.01 ± 0.11d | 1.30 ± 0.01hi | 6.77 ± 0.17a | 5.05 ± 0.29d | 5.57 ± 0.20c | 4.35 ± 0.05e | 6.03 ± 0.11b | 6.43 ± 0.11a | 2.70 ± 0.15f | 1.68 ± 0.11 g | 1.61 ± 0.22gh | 1.00 ± 0.00i | |
| Threonine (Thr) | 2.42 ± 0.16ab | 1.22 ± 0.05 g | 1.26 ± 0.06 fg | 0.73 ± 0.29 h | 1.54 ± 0.06ef | 1.77 ± 0.18de | 1.14 ± 0.06 g | 2.60 ± 0.01a | 2.29 ± 0.12bc | 2.02 ± 0.00 cd | 2.49 ± 0.15ab | 0.95 ± 0.01gh | |
| Arginine (Arg) | 3.74 ± 0.37 cd | 4.95 ± 0.09a | 4.13 ± 0.06bc | 4.84 ± 0.22a | 4.03 ± 0.17bc | 2.21 ± 0.01e | 2.06 ± 0.08e | 3.55 ± 0.14d | 3.97 ± 0.19bc | 4.16 ± 0.16bc | 4.40 ± 0.12b | 3.45 ± 0.07d | |
| Alanine (Ala) | 2.85 ± 0.06e | 2.69 ± 0.10e | 4.07 ± 0.27c | 4.56 ± 0.13b | 3.76 ± 0.02c | 2.09 ± 0.10f | 2.14 ± 0.05f | 3.17 ± 0.08d | 5.81 ± 0.02a | 1.60 ± 0.07 g | 1.00 ± 0.28 h | 0.71 ± 0.10 h | |
| Tyrosine (Tyr) | 1.12 ± 0.02i | 2.33 ± 0.04e | 3.12 ± 0.04c | 3.65 ± 0.24b | 4.23 ± 0.17a | 1.29 ± 0.03hi | 1.98 ± 0.08f | 1.51 ± 0.01gh | 1.70 ± 0.10 g | 2.29 ± 0.08e | 2.62 ± 0.21d | 2.84 ± 0.04d | |
| Cystine (Cys) | 15.53 ± 0.26c | 7.65 ± 0.11e | 1.88 ± 0.06 g | 1.44 ± 0.06 g | 1.53 ± 0.12 g | 9.57 ± 0.32d | 7.65 ± 0.17e | 7.24 ± 0.09e | 5.90 ± 0.09f | 20.40 ± 0.26a | 20.12 ± 0.05a | 19.34 ± 0.28b | |
| Valine (Val) | 4.67 ± 0.10b | 1.87 ± 0.18 g | 1.60 ± 0.02gh | 1.37 ± 0.06 h | 0.88 ± 0.10i | 3.01 ± 0.07f | 3.31 ± 0.07e | 5.98 ± 0.09a | 1.62 ± 0.20gh | 4.35 ± 0.13c | 4.52 ± 0.16bc | 3.64 ± 0.18d | |
| Methionine (Met) | 2.67 ± 0.11 g | 4.07 ± 0.05e | 5.95 ± 0.16c | 6.99 ± 0.51b | 7.54 ± 0.20a | 2.52 ± 0.04 g | 3.54 ± 0.10f | 4.58 ± 0.08d | 5.74 ± 0.17c | 2.74 ± 0.04 g | 3.65 ± 0.03ef | 3.32 ± 0.10f | |
| Phenylalanine (Phe) | 2.28 ± 0.12i | 4.76 ± 0.12 g | 6.32 ± 0.10d | 7.86 ± 0.46b | 8.59 ± 0.21a | 2.84 ± 0.11 h | 4.45 ± 0.20 g | 5.67 ± 0.30ef | 7.17 ± 0.10c | 5.31 ± 0.02f | 5.57 ± 0.12f | 6.08 ± 0.08de | |
| Isoleucine (Ile) | 1.24 ± 0.16e | 1.02 ± 0.08ef | 0.71 ± 0.02 g | 0.92 ± 0.19 fg | 1.04 ± 0.04ef | 2.09 ± 0.09c | 5.39 ± 0.05a | 2.72 ± 0.06b | 0.87 ± 0.03 fg | 1.66 ± 0.09d | 1.72 ± 0.14d | 0.68 ± 0.08 g | |
| Leucine (Leu) | 6.46 ± 0.08 cd | 7.57 ± 0.56ab | 7.66 ± 0.99a | 6.57 ± 0.44bcd | 5.99 ± 0.28d | 6.83 ± 0.01abcd | 6.07 ± 0.02d | 2.44 ± 0.09f | 3.25 ± 0.25f | 7.00 ± 0.09abcd | 7.23 ± 0.06abc | 4.58 ± 0.23e | |
| Lysine (Lys) | 2.33 ± 0.31b | 1.91 ± 0.10c | 1.62 ± 0.10c | 1.21 ± 0.06d | 0.78 ± 0.06f | 2.94 ± 0.07a | 2.71 ± 0.05a | 2.32 ± 0.13b | 2.32 ± 0.05b | 1.64 ± 0.03c | 1.15 ± 0.02de | 0.88 ± 0.01ef | |
| Subtotal | 86.16 ± 0.18d | 74.76 ± 1.10e | 93.87 ± 0.41bc | 96.33 ± 1.21ab | 93.26 ± 1.32c | 72.09 ± 1.60f | 88.35 ± 1.30d | 93.37 ± 0.56c | 86.95 ± 1.33d | 94.30 ± 0.43abc | 97.03 ± 0.53a | 76.80 ± 1.98e | |
| Fructose (Fru) | 3.32 ± 0.03a | 2.70 ± 0.01c | 3.21 ± 0.04ab | 2.12 ± 0.03d | 1.40 ± 0.36e | 2.81 ± 0.05bc | 2.92 ± 0.16abc | 1.71 ± 0.06e | 0.96 ± 0.03f | 3.04 ± 0.15abc | 2.80 ± 0.21bc | 1.57 ± 0.06e | |
| Glucose (Gluc) | 4.83 ± 0.09a | 4.30 ± 0.08b | 4.15 ± 0.02bcd | 4.30 ± 0.04b | 3.94 ± 0.07de | 4.27 ± 0.20bc | 3.95 ± 0.06de | 3.87 ± 0.01de | 3.74 ± 0.20de | 4.32 ± 0.05b | 3.97 ± 0.25cde | 3.18 ± 0.08f | |
| Sucrose (Suc) | 31.23 ± 0.32a | 29.17 ± 0.23b | 28.65 ± 0.24b | 21.69 ± 0.73c | 18.12 ± 0.22d | 29.57 ± 1.12b | 29.14 ± 0.21b | 21.50 ± 1.23c | 16.25 ± 0.48e | 21.07 ± 0.19c | 18.65 ± 0.35d | 13.15 ± 0.42f | |
| Soluble sugars | Maltose (Malt) | 2.35 ± 0.42a | 0.35 ± 0.07bc | 0.16 ± 0.06bc | 0.12 ± 0.04bc | 0.06 ± 0.00bc | 0.42 ± 0.09b | 0.21 ± 0.02bc | 0.06 ± 0.02bc | 0.03 ± 0.01c | 0.31 ± 0.06bc | 0.15 ± 0.02bc | 0.06 ± 0.02bc |
| Subtotal | 41.73 ± 0.63a | 36.51 ± 0.11b | 36.00 ± 0.21b | 28.23 ± 1.21c | 23.52 ± 1.30e | 37.08 ± 0.33b | 36.22 ± 1.02b | 27.14 ± 1.36 cd | 20.98 ± 0.41f | 28.75 ± 0.96c | 25.57 ± 1.00d | 17.96 ± 0.29 g | |
| Total | 127.89 ± 0.56b | 111.27 ± 0.26 g | 129.87 ± 0.77a | 124.56 ± 0.44c | 116.78 ± 0.57f | 109.17 ± 0.33 h | 124.57 ± 0.67c | 120.51 ± 0.54e | 107.93 ± 0.77 h | 123.05 ± 0.78d | 122.60 ± 0.27d | 94.77 ± 0.13i | |
Note: “ND” indicates that is not detected or below detection limit. “FW” means fresh weight. Data points in the same row with different letters are significantly different (p < 0.05). IFD means integrated freeze-drying, CFD means conventional freeze-drying, AD means hot air drying.
The main three amino acids from highest to lowest in fresh samples were glutamic acid, histidine, and cystine, with the first three amino acids in the AD samples being the same as those in the fresh samples. However, after the entire drying process of CF0.5 h, IFD4h, IFD8h, and IFD12h, the three amino acids with the highest content were aspartic acid, glutamic acid, and serine. The first three amino acids did not change in CF4h; however, after CFD4h, until the end of drying, the first three amino acids changed to aspartic acid, glutamic acid, and histidine. Changes in the amino acid content of IFD decreased at IF0.5 h, reached a maximum at IFD8h, and then decreased gradually. During CFD, these changes decreased at CF4h, reached a maximum at IFD8h, and then decreased gradually. The highest total amino acid content was found for AD6h. The total amino acid content for all three drying methods initially increased and then decreased. The reason for these changes may be due to the fact that, during the drying process, with the prolongation of time and the increase in temperature, the protein is decomposed into amino acids under the action of a protease, and concurrently, the amino acids themselves are also continuously released. At the late stage of drying, the Maillard reaction occurs between free amino acids and reducing sugars (Maninang et al., 2011, Tan et al., 2020, Zeng et al., 2022). The total amount of free amino acids in the fresh and three dried samples, in descending order, was IFD > fresh > CFD > AD, with the AD sample having a significantly lower (p < 0.05) amount of free amino acids than the fresh sample, due to Strecker degradation and the Maillard reaction occurring at this stage (Tan et al., 2020, Zeng et al., 2022).
The soluble sugar contents of fresh, IFD, CFD, and AD samples were 41.73 ± 0.63, 23.52 ± 1.30, 20.98 ± 0.41, and 17.97 ± 0.29, respectively, with significant differences (p < 0.05). Excluding the AD method, where the soluble sugar content first increased and then decreased, the soluble sugar content of the other two FD methods continuously decreased during the drying process. The significant (p < 0.05) decrease in the soluble sugar content of durian may be due to the gradual increase in the concentration of soluble sugars in the cell tissues as FD progresses, which may undergo decomposition and transformation or participate in the reaction of other substances under the combined effect of sugar metabolism-related enzyme activities and environmental temperatures, which reduces the content of soluble sugars in durian.
3.4. Principal component, cluster, and Pearson’s correlation analyses of amino acids and soluble sugars
As seen in Fig. 3a, results can be divided into the following four quadrants: the first quadrant comprises IFD4h and IFD8h samples; the second quadrant contains fresh, CF4h, IF0.5 h, CFD4h, and CFD8h samples; the third quadrant comprises AD3h, AD6h, and AD9h samples; and the fourth quadrant comprises IFD12h and CFD12h samples. Fig. 3b illustrates the principal component analysis of the 16 amino acids and four soluble sugars, which were roughly divided into four clusters based on the PC1 and PC2 scores, with clusters similar to those in Fig. 3c.
Fig. 3.
Principal component analysis, hierarchical cluster analysis, and Pearson’s correlation analysis based on the contents of the amino acids and the soluble sugars in the samples during the three drying processes. The abbreviated words represent the nonvolatile flavor compounds in Table.2. (a): Score plot of principal component analysis of the different drying methods; (b): Score plot of principal component analysis of the amino acids and the soluble sugars; (c): Heatmap of hierarchical cluster analysis; (d): Heatmap of Pearson’s correlation analysis.
According to Fig. 3c, the horizontal direction can be divided into three clusters: the first cluster includes fresh samples; the second cluster contains IF0.5 h, AD3h, AD6h, AD9h, CF4h, and CFD4h and IFD4h, IFD8h, IFD12h, CFD8h, and CFD12h are included in the third cluster. The vertical direction was divided into four main clusters: the first cluster contained Asp, Glu, Ala, Gly, Ser, Met, Phe, Tyr, and Arg; the second cluster included His, Cys, Val, Thr, Ile, and Lys; and the fourth cluster contained Leu, Fru, Malt, Gluc, and Suc.
According to Fig. 3d, which shows the results of the Pearson's correlation analysis between amino acids and soluble sugars, it is possible to illustrate the positive and negative correlations between the two amino acids based on the correlation pairs. As shown in Fig. 3d, Asp was significantly and positive correlated with Glu, Ser, Gly, Ala, Met, and Phe (p < 0.05). Glu showed a significant positive correlation with Ser, Gly, Ala, and Met, as well as a significant negative correlation with His and Cys (p < 0.05). Ser showed a significant positive correlation with Ala, Tyr, Met, and Phe, and a significant negative correlation with His, Cys, and Val (p < 0.05). His, Thr, Ala, Tyr, Cys, Met, Leu, Lys, Fru, and Gluc showed significant positive correlations with the 1–2 types of amino acids (p < 0.05). His, Gly, Arg, Ala, Tyr, Val, and Phe levels negatively correlated with amino acids of 1–2 types. The above results show that amino acids and soluble sugars in durian fruit change during the drying process, and thus, we can categorize them according to their correlation to show that there are differences. This will provide a theoretical basis for durian processing.
3.5. Pearson’s correlation analysis of volatile compounds with amino acids and soluble sugars
As shown in Fig. 4, among the 16 amino acids, Asp, Glu, Ser, His, Gly, Ala, Tyr, Cys, Val, Met, and Phe showed strong negative correlations with 6, 10, 11, 6, 6, 8, 4, 5, 3, 21, and 33 types of volatile compounds, respectively. Among them, Met and Phe were negatively correlated with many compounds, indicating that the more amino acids produced, the less favorable the formation of these flavor compounds. The four soluble sugars showed significant positive correlations with most volatile compounds (p < 0.05). Therefore, we speculated that a number of reactions related to the above mentioned compounds occurred during the drying process, thus explaining the chemical mechanism of volatile profile formation in our experiments. The significant correlations of volatile flavor compounds with amino acids or soluble sugars can be explained by two reasons. Firstly, the amino acids and the soluble sugars facilitate the formation of the volatile flavor compounds or their precursors. Secondly, the chemical bonds formed by the degradation of amino acids or soluble sugars have a great influence on the formation of volatile flavor compounds (Maninang et al., 2011, Tan et al., 2020). However, the reasons for the changes in the various volatile compounds, amino acids and soluble sugars during drying are very complex. This is because they interact with each other as well as other precursors involved in Maillard reactions, thermal degradation, and oxidative reactions with heating to form a combined flavor in the final product. (Tan et al., 2020, Zeng et al., 2022). The interrelationships among volatile compounds, amino acids, and soluble sugars illustrate the tendency of various flavor compounds to interact with each other during the drying process, which provides theoretical support for the study of flavor compounds.
Fig. 4.
Pearson’s correlation analysis based on the contents of volatile and the amino acids and soluble sugars in the samples during the three drying processes. Numbers 1–63 are the corresponding codes for the 63 kinds of volatile compounds in Table.1. The abbreviated words represent the amino acids and soluble sugars in Table 2.
4. Conclusion
In this study, we investigated the dynamics and correlations of volatile flavor compounds, amino acids, and soluble sugars in durian during IFD, CFD, and AD. The fresh, IFD, CFD, and AD samples contained 51, 47, 48, and 52 volatile compounds, respectively. The total contents of volatile compounds all showed a trend of decreasing quickly and then slowly during IFD, CFD, and AD, resulting in 140.23 ± 1.23 μg/g, 111.10 ± 0.90 μg/g, and 129.07 ± 1.07 μg/g, respectively, in the total content of the dried samples, with significant differences (p < 0.05). The IFD samples had the highest content of volatile flavor compounds (particularly esters) and fewer sulfur-containing compounds. The total content of amino acids in IFD, CFD, and AD samples were 93.26 ± 1.32 mg/g, 86.95 ± 1.33 mg/g, and 76.80 ± 1.98 mg/g, respectively, and the total content of soluble sugars were 23.52 ± 1.30, 20.98 ± 0.41, and 17.97 ± 0.29 mg/g, with significant differences (p < 0.05). Correlation analysis showed that volatile flavor compounds presented significant negative correlations with a variety of amino acids and significant positive correlations with soluble sugars, indicating that most amino acids and soluble sugars in durian were closely related to variations in volatile compounds (p < 0.05). Based on these results, it can be concluded that the IFD method is optimal for the drying of durian.
CRediT authorship contribution statement
Feifei Yang: Writing – original draft, Methodology, Investigation. Qianju Wang: Methodology, Investigation, Data curation. Wuyi Liu: Writing – review & editing, Methodology. Hongwei Xiao: Writing – review & editing, Conceptualization. Jiaqi Hu: Writing – review & editing, Investigation. Xiaojie Duan: Investigation, Data curation. Xiyun Sun: Methodology, Funding acquisition, Conceptualization. Chunju Liu: Methodology, Data curation. Haiou Wang: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Natural Science Foundations of China (No. 31872901), Key Projects from Education Department of Liaoning Province (No. LJKZZ20220089), and Excellent Scientific and Technological Innovation Team of Colleges and Universities of Jiangsu Province, China (SUJIAOKE〔2021〕No.1). The authors would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101238.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Ali M.M., Hashim N., Abd Aziz S., Lasekan O. Exploring the chemical composition, emerging applications, potential uses, and health benefits of durian: A review. Food Control. 2020;113 doi: 10.1016/j.foodcont.2020.107189. [DOI] [Google Scholar]
- Aschariyaphotha W., Wongs-Aree C., Bodhipadma K., Noichinda S. Fruit volatile fingerprints characterized among four commercial cultivars of thai durian (Durio zibethinus) Journal of Food Quality. 2021;2021:1–12. doi: 10.1155/2021/1383927. [DOI] [Google Scholar]
- Bangs W.E., Reineccius G.A. Prediction of flavor retention during spray drying: An empirical approach. Journal of food science. 1990;55(6):1683–1685. doi: 10.1111/j.1365-2621.1990.tb03600.x. [DOI] [Google Scholar]
- Belgis M., Wijaya C.H., Apriyantono A., Kusbiantoro B., Yuliana N.D. Volatiles and aroma characterization of several lai (durio kutejensis) and durian (Durio zibethinus) cultivars grown in Indonesia. Scientia Horticulturae. 2017;220(16):291–298. doi: 10.1016/j.scienta.2017.03.041. [DOI] [Google Scholar]
- Bu Z., Luo W., Wei J., Peng J., Wu J., Xu Y., Yu Y., Li L. Impacts of thermal processing, high pressure, and CO2-assisted high pressure on quality characteristics and shelf life of durian fruit puree. Foods. 2022;11(17):2717. doi: 10.3390/foods11172717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghannya J., Kadkhodaei S., Heshmati M.K., Ghanbarzadeh B. Ultrasound-assisted intensification of a hybrid intermittent microwave-hot air drying process of potato: Quality aspects and energy consumption. Ultrasonics. 2019;96:104–122. doi: 10.1016/j.ultras.2019.02.005Get. rights and content. [DOI] [PubMed] [Google Scholar]
- Dembitsky V.M., Poovarodom S., Leontowicz H., Leontowicz M., Vearasilp S., Trakhtenberg S., Gorinstein S. The multiple nutrition properties of some exotic fruits: Biological activity and active metabolites. Food research international. 2011;44(7):1671–1701. doi: 10.1016/j.foodres.2011.03.003. [DOI] [Google Scholar]
- Flink J., Karel M. Retention of organic volatiles in freeze-dried solutions of carbohydrates. Journal of Agricultural and Food Chemistry. 1970;18(2):295–297. doi: 10.1021/jf60168a014. [DOI] [Google Scholar]
- GB 5009.8-2016. (2016). National food safety standard: Determination of fructose, glucose, sucrose, maltose, and lactose in food. Beijing, China: Standards Press of China. (In Chinese).
- Harguindeguy M., Fissore D. On the effects of freeze-drying processes on the nutritional properties of foodstuff: A review. Drying Technology. 2020;38(7):846–868. doi: 10.1080/07373937.2019.1599905. [DOI] [Google Scholar]
- Ho L.H., Bhat R. Exploring the potential nutraceutical values of durian (Durio zibethinus l.)–an exotic tropical fruit. Food Chemistry. 2015;168(1):80–89. doi: 10.1016/j.foodchem.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Husin N.A., Rahman S., Karunakaran R., Bhore S.J. A review on the nutritional, medicinal, molecular and genome attributes of durian (Durio zibethinus L.), the king of fruits in Malaysia. Bioinformation. 2018;14(6):265. doi: 10.6026/97320630014265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C., Purnell G., James S.J. A review of novel and innovative food freezing technologies. Food and Bioprocess Technology. 2015;8:1616–1634. doi: 10.1007/s11947-015-1542-8. [DOI] [Google Scholar]
- Jamradloedluk J., Nathakaranakule A., Soponronnarit S., Prachayawarakorn S. Influences of drying medium and temperature on drying kinetics and quality attributes of durian chip. Journal of Food Engineering. 2007;78(1):198–205. doi: 10.1016/j.jfoodeng.2005.09.017. [DOI] [Google Scholar]
- Krokida M.K., Philippopoulos C. Volatility of apples during air and freeze drying. Journal of Food Engineering. 2006;73(2):135–141. doi: 10.1016/j.jfoodeng.2005.01.012. [DOI] [Google Scholar]
- Lin N., Liu B., Liu Z., Qi T. Effects of different drying methods on the structures and functional properties of phosphorylated antarctic krill protein. Journal of food science. 2020;85(11):3690–3699. doi: 10.1111/1750-3841.15503. [DOI] [PubMed] [Google Scholar]
- Maninang J.S., Wongs-Aree C., Kanlayanarat S., Sugaya S., Gemma H. Influence of maturity and postharvest treatment on the volatile profile and physiological properties of the durian (Durio zibethinus Murray) fruit. International Food Research Journal. 2011;18(3):1067–1075. [Google Scholar]
- Niponsak A., Laohakunjit N., Kerdchoechuen O. Contribution to volatile fingerprinting and physico-chemical qualities of minimally processed durian cv. ‘monthong’ during storage: Identification of a novel chemical ripeness marker. Food and Bioprocess Technology. 2015;8:1229–1243. doi: 10.1007/s11947-015-1486-z. [DOI] [Google Scholar]
- Paengkanya S., Soponronnarit S., Nathakaranakule A. Application of microwaves for drying of durian chips. Food and bioproducts processing. 2015;96:1–11. doi: 10.1016/j.fbp.2015.06.001. [DOI] [Google Scholar]
- Peng J.S.M. Volatile esters and sulfur compounds in durians & a suggested approach to enhancing economic value of durians. Malaysian Journal of Sustainable Agriculture. 2019;3(2):5–15. doi: 10.26480/mjsa.02.2019.0515. [DOI] [Google Scholar]
- Prasetia H.A., Budiawan S., Syahputra A., Umiarsih R., Pangastuweni R., Fauzidanty M.R., Harahap I.S., Setyabudi D.A., Affandi D.M.U., Sutian W. Effects of freezing time on degradation of durian (durio zibethinus murr.) fruit’s attributes during the frozen storage. Tropical life sciences research. 2023;34(1):19. doi: 10.21315/tlsr2023.34.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupan N., Dhamvithee P., Jangchud A., Boonbumrung S. Influences of different freezing and thawing methods on the physico-chemical, flavor, and sensory properties of durian puree (cv. monthong) Journal of Food Processing and Preservation. 2018;42(7):e13669. [Google Scholar]
- Raihana A.N., Marikkar J.M.N., Amin I., Shuhaimi M. A review on food values of selected tropical fruits’ seeds. International Journal of Food Properties. 2015;18(11):2380–2392. doi: 10.1080/10942912.2014.980946. [DOI] [Google Scholar]
- Chin S.T., Nazimah S.A.H., Quek S.Y., Man Y.B.C., Rahman R.A., Hashim D.M. Effect of thermal processing and storage condition on the flavour stability of spray-dried durian powder. LWT-Food Science and Technology. 2010;43(6):856–861. doi: 10.1016/j.lwt.2010.01.001. [DOI] [Google Scholar]
- Tan X.Y., Misran A., Cheong K.W., Daim L.D.J., Ding P., Dek M.S.P. Postharvest quality indices of different durian clones at ripening stage and their volatile organic compounds. Scientia Horticulturae. 2020;264 doi: 10.1016/j.scienta.2019.109169. [DOI] [Google Scholar]
- Voon Y.Y., Hamid N.S.A., Rusul G., Osman A., Quek S.Y. Volatile flavour compounds and sensory properties of minimally processed durian (Durio zibethinus cv. D24) fruit during storage at 4 °C. Postharvest biology and technology. 2007;46(1):76–85. doi: 10.1016/j.postharvbio.2007.04.004. [DOI] [Google Scholar]
- Wang H.O., Fu Q.Q., Chen S.J., Hu Z.C., Xie H.X. Effect of hot-water blanching pretreatment on drying characteristics and product qualities for the novel integrated freeze-drying of apple slices. Journal of Food Quality. 2018;2018:1347513. doi: 10.1155/2018/1347513. [DOI] [Google Scholar]
- Wongs-Aree C., Noichinda S. Postharvest Handling. Academic Press; 2022. Postharvest quality properties of potential tropical fruits related to their unique structural characters; pp. 277–316. [DOI] [Google Scholar]
- Xiao Z., Niu M., Niu Y. Comparative study on volatile compounds and taste components of different durian cultivars based on GC-MS, UHPLC, HPAEC-PAD, e-tongue and e-nose. Molecules. 2022;27(4):1264. doi: 10.3390/molecules27041264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H., Zhao R., Liu C., Wu Y., Duan X., Hu J., Yang F., Wang H. Dynamic changes in volatile flavor compounds, amino acids, organic acids, and soluble sugars in lemon juice vesicles during freeze-drying and hot-air drying. Foods. 2022;11(18):2862. doi: 10.3390/foods11182862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Zang E., Sun S., Li M. Main flavor compounds and molecular regulation mechanisms in fruits and vegetables. Critical Reviews in Food Science and Nutrition. 2022;1–21 doi: 10.1080/10408398.2022.2097195. [DOI] [PubMed] [Google Scholar]
- Yang F., Sun X., Hu J., Cai H., Xiao H., Wu X., Liu C., Hu J., Wang H. Edible gum addition improves the quality of freeze-dried restructured strawberry blocks. Food Chemistry: X. 2023;18(30) doi: 10.1016/j.fochx.2023.100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J.Y., Lyu J., Bi J.F., Zhou L.Y., Zhou M. Hot air drying and freeze drying pre-treatments coupled to explosion puffing drying in terms of quality attributes of mango, pitaya, and papaya fruit chips. Journal of Food Processing and Preservation. 2017;41(6):e13300. [Google Scholar]
- Zeng Z., Wang J., Wen X., Wang Y., Li X., Liu D., Geng F. Metabolomic analysis provides insights into the mechanism of color and taste changes in dictyophora indusiata fruiting bodies under different drying processes. Food Research International. 2022;162 doi: 10.1016/j.foodres.2022.112090. [DOI] [PubMed] [Google Scholar]
- Zhang C., Chen X., Zhang J., Kilmartin P.A., Quek S.Y. Exploring the effects of microencapsulation on odour retention of fermented noni juice. Journal of Food Engineering. 2020;273 doi: 10.1016/j.jfoodeng.2019.109892. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.