Abstract
Supportive care is the cornerstone of the poisoned patient’s treatment, waiting for eventual antidotes to act. We recently treated a case of a severe Ethylene Glycol intoxication with early-onset veno-arterial ECMO. The patient was taken to our Emergency Department with the suspicion of acute cerebrovascular accident, since he was found unconscious at home. The arterial blood gas and blood tests showed a severe metabolic acidosis with high serum lactates and creatinine levels. The cerebral Computed Tomography was negative. The rapid increase in serum lactates suggested Ethylene Glycol intoxication. Although the patient was not in shock yet, arterial and venous introducers were placed in to the femoral vessels so that when the patient showed the first signs of cardiogenic shock, veno-arterial ECMO could be initiated in a very short time. The hemodynamic state progressively improved and V-A ECMO was removed after 16 h of support with complete recovery.
Keywords: ethilene glycol, poisoning, veno-arterial ECMO, cardiogenic shock, intoxication supportive care
Introduction
Ethylene Glycol (EG) is a colorless, odorless and sweet tasting dihydroxyl polyalcohol, most commonly used as solvent for antifreeze solutions. When ingested, it can cause ethanol-like effects. Its toxicity is mediated by its metabolites (glycolic acid and oxalic acid) that produce metabolic acidosis, typically with rapid increase in serum lactate concentrations. Acute poisoning can cause serious symptoms like neurological impairment, acute renal failure and, ultimately, cardiopulmonary dysfunction and death.1–4 Treatment is based on supportive care and administration of the antidote fomepizole that inhibits the production of toxic metabolites. In the recent years Extra Corporeal Membrane Oxygenation (ECMO) has been increasingly used as the most aggressive supportive device in intoxication with good outcomes.5–8 As for other invasive therapies, the decisional process can be challenging and the correct timing is often unclear. We recently treated a case of EG poisoning with early-onset veno-arterial ECMO (V-A ECMO).
Case report
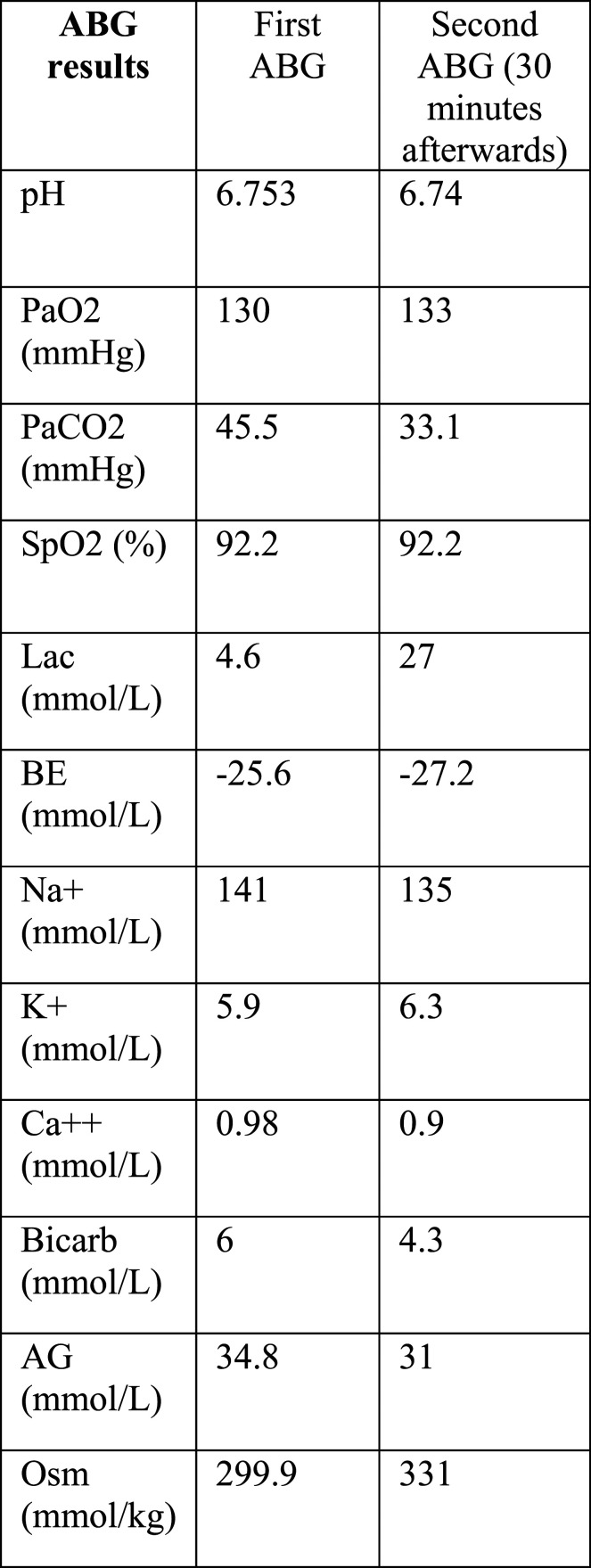
The patient was a 60 years-old caucasian man with a history of alcohol abuse, chronic liver disease and chronic kidney disease. He was regularly undergoing medical follow up and was due for an appointment when his wife found him unconscious at home. The emergency team found him tachypneic, with a Glasgow Coma Scale of 3. He was intubated and transported to our hospital with a suspected cerebrovascular accident. The first arterial blood gas (ABG) performed at the Emergency Department showed a severe metabolic acidosis with a mild increase in lactates, high anion gap and osmolal gap (Figure 1). The cerebral Computed Tomography was negative and an intoxication was then hypothesized. Blood samples for the determination of ethanol, methanol, ethylene glycol, benzodiazepines and opioids concentration were collected, together with complete blood tests. The latter showed a serum creatinine of 18 mg/dL. The patient was admitted to the Intensive Care Unit (ICU) and underwent immediate positioning of central line, coaxial catheter for hemodialysis and invasive arterial monitoring. A new ABG showed persistent acidosis with an abrupt and substantial increase in serum lactate concentration (Figure 1). With this data, together with the extremely high levels of serum creatinine, the local Poison Control Center supported our strong suspicion for ethylene glycol intoxication and promptly activated the delivery of fomepizole to our ICU. At the same time a dialysis machine was set up and the patient was given intravenous bicarbonates and crystalloids. Since ICU admission the patient started to be more hypotensive requiring noradrenaline up to 0.2 mcg/kg/min and soon after the ECG started to show QT prolongation and non-sustained bradycardia, so an infusion of magnesium and calcium was started. At this point, despite the ongoing renal replacement therapy, Noradrenaline requirements were progressively increasing, so we decided to electively insert arterial and venous femoral introducers for the potential need for V-A ECMO while alerting Cardiac Surgeon and Perfusionist. Soon after the diagnosis was confirmed by the laboratory with blood concentration of Ethylene Glycol of 32.2 mg/dL and urine concentration of 160.6 mg/dL. Around 15 min after the dialysis started the ECG worsened with persistent bradycardia and complete heart block requiring external pacing. The patient was then extremely hypotensive and the dialysis machine was unable to maintain a stable and sufficient blood flow. Cardiac Surgeon and Perfusionist were at the bedside at that point. An ECMO circuit type BE MECC 14-2000 (Getinge/Maquet GmbH, Rastatt, Germany) was set up and ready to run. We decided to start V-A ECMO. A 19 French (F) arterial cannula (Maquet) in left femoral artery and a 21F venous cannula (Maquet) in right femoral vein were positioned via the previously placed introducers. ECMO started in less than 10 min from the clinical decision. The patient’s hemodynamic state progressively stabilized, with just a brief phase of severe hypotension before ECMO started, but without cardiac arrest. Blood flow was kept between 2.5 and 3 L/min throughout the assistance (patient’s body surface area 1.94 m2).
Figure 1.
Sequence of arterial blood gases (ABGs), the first one at arrival in emergency department, the second one in ICU 30 min afterwards. The patient was intubated, ventilated in pressure control mode with 6 cm H2O of positive end-expiratory pressure (PEEP), 15 cm H2O of pressure control and 50% inspired fraction of oxygen (FiO2). Definitions: pH: acid-base balance; PaO2: partial pressure of oxygen; PaCO2: partial pressure of carbon dioxide; SpO2: oxygen saturation; Lac: lactate; BE: base excess; Na+: sodium; K+: potassium; Ca+: calcium; Bicarb: bicarbonate; AG: anion gap; Osm: serum osmolality.
In the following hours hemodynamics and ABG progressively improved and there was a reduction in EG plasma levels at the second determination 6 h after the first one (8.2 mg/dL). V-A ECMO was removed after 16 h of assistance. The patient then required 2 weeks of ICU and 10 days of ward stay for complete recovery and home discharge.
Discussion
The main discussion point in this case is the decision of placing the introducers in advance respect to a clear indication to ECMO.
In this context, thinking ECMO as an extreme support for severely shocked patients, as well as for patients in cardiac arrest, surely allows the selection of the sickest patients who could not have the chance to survive without this device. In these cases the decision of starting ECMO is somehow easy to take. On the other hand, choosing to assist a non-shocked patient with such an invasive tool, even if at risk of becoming shocked sooner or later could be a debatable decision.
Perhaps in some borderline cases as the one described here a good solution could be to facilitate the eventual need for an extracorporeal assistance, as we did by inserting the femoral introducers. In fact, one of the main issues when starting ECMO assistance in a severely shocked patient is the speed of cannulation. We were unable to find other EG intoxication cases requiring V-A ECMO in the literature.
Conclusion
V-A ECMO can be part of high level supportive care in EG intoxication. An early placement of the introducers can be considered in most severe and selected cases.
Acknowledgements
We thank Dr Valeria Petrolini and Dr Davide Lonati (Maugeri Clinical Research Institutes IRCCS, Pavia Poison Centre and National Toxicology Information Centre) for the help in clinical management of the present case.
Appendix
Abbreviation
- EG
Ethilene Glycol
- ECMO
Extra Corporeal Membrane Oxygenation
- V-A ECMO
veno-arterial Extra Corporeal Membrane Oxygenation
- ABG
acid base balance
- ICU
Intensive Care Unit
Footnotes
Author contributions: AO, FE, AE, and CM wrote the text. All the authors reviewed and approved the final manuscript before submission and participated in patient’s clinical management.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: FM received fees for lectures from GE Healthcare, Hamilton Medical, SEDA SpA. AO received fees for manuscript preparation from Hamilton Medical, outside the present work.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data availability: All clinical data are available as part of patient’s medical record.
Informed consent: Written informed consent was obtained for publication
ORCID iDs
Antonella Degani https://orcid.org/0000-0002-2754-5153
Costanza NJ Colombo https://orcid.org/0000-0003-1628-3642
Anita Orlando https://orcid.org/0000-0002-5804-8619
References
- 1.Hlozek T, Bursova M, Cabalaa R. Fast determination of ethylene glycol, 1,2-propylene glycol and glycolic acid in blood serum and urine for emergency and clinical toxicology by GC-FID. Talanta 2014; 130: 470–474. [DOI] [PubMed] [Google Scholar]
- 2.Ammar KA, Heckerling PS. Ethylene glycol poisoning with a normal anion gap caused by concurrent ethanol ingestion: importance of the osmolal gap. Am J Kidney Dis. 1996; 27: 130–133. [DOI] [PubMed] [Google Scholar]
- 3.Moreau CL, Kerns W, Tomaszewski CA, et al. Glycolate kinetics and hemodialysis clearance in ethylene glycol poisoning. META Study Group. J Toxicol Clin Toxicol 1998; 36: 659–666. [DOI] [PubMed] [Google Scholar]
- 4.Pernet P, Beneteau-Burnat B, Vaubourdolle M, et al. False elevation of blood lactate reveals ethylene glycol poisoning. Am J Emerg Med 2009; 27(1): 132.e1. [DOI] [PubMed] [Google Scholar]
- 5.Weiner L, Mazzeffi MA, Hines EQ, et al. Clinical utility of venoarterial-extracorporeal membrane oxygenation (VA-ECMO) in patients with drug-induced cardiogenic shock: a retrospective study of the Extracorporeal Life Support Organisations’ ECMO case registry. Clin Toxicol 2020; 58(7): 705–710. [DOI] [PubMed] [Google Scholar]
- 6.Lewis J, Zarate M, Tran S, et al. The recommendation and use of extracorporeal membrane oxygenation (ECMO) in cases reported to the California poison control system. J Med Toxicol 2019; 15: 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Lange DW, Skima MA, Meulenbelt J. Extracorporeal membrane oxygenation in the treatment of poisoned patients. Clin Toxicol 2013; 51: 385–393. [DOI] [PubMed] [Google Scholar]
- 8.Raleigh L, Ha R, Hill C. Extracorporeal membrane oxygenation applications in cardiac critical care. Semin Cardiothorac Vasc Anesth 2015; 194(4): 342–352. [DOI] [PubMed] [Google Scholar]