Abstract
Introduction:
Odontoblasts produce dentin throughout life and in response to trauma. The purpose of this study was to identify the roles of endogenous Wnt signaling in regulating the rate of dentin accumulation.
Methods:
Histology, immunohistochemistry, vital dye labeling and histomorphometric assays were used to quantify the rate of dentin accumulation as a function of age. Two strains of Wnt reporter mice were employed to identify and follow the distribution and number of Wnt-responsive odontoblasts as a function of age. To demonstrate a causal relationship between dentin secretion and Wnt signaling, dentin accumulation was monitored in a strain of mice in which Wnt signaling was aberrantly elevated.
Results:
Dentin deposition occurs throughout life, but the rate of accumulation slows with age. This decline in dentin secretion correlates with a decrease in endogenous Wnt signaling. In a genetically modified strain of mice, instead of tubular dentin, aberrantly elevated Wnt signaling resulted in accumulation of reparative dentin or osteodentin, secreted from predontoblasts.
Conclusions:
Wnt signaling regulates dentin secretion by odontoblasts, and the formation of reparative or osteodentin is the direct consequence of elevated Wnt signaling. These preclinical data have therapeutic implications for the development of a biologically based pulp-capping medicant.
Keywords: Wnt signal, pulp, aging, odontoblasts
Introduction
Bone turnover is maintained by osteoclasts that resorb bone matrix and osteoblasts that deposit new matrix. With aging, the balance between osteoblast and osteoclast function is lost, and the result is osteoporosis, a low bone mass disease (1, 2). The dental pulp, on the other hand, only houses dentin-producing odontoblasts; consequently, dentin accumulates with age (3).
In response to activities of daily living including exercise that can cause micro-cracks, osteoclasts resorb mineralized matrix and osteoblasts deposit new osteoid. This remodeling activity restores the structural integrity of the skeleton. The dental pulp is also capable of mounting a response to sub-acute trauma e.g., extreme temperature changes, or acid production by bacteria- but this is distinctive from bone because there is no remodeling activity. Instead, odontoblasts can only increase their rate of dentin secretion and in doing so, create a thicker protective barrier between the insult and the pulp (4). In acute trauma, other cells in the pulp, e.g., pre-odontoblasts can be recruited to rapidly secrete a mineralized matrix that protects the pulp. This mineralized tissue does not have a tubular structure because the cells producing it lack processes (5, 6) and is called osteoid-like dentin, or osteodentin (7).
A key regulator of the balance between osteoblast and osteoclast function is the Wnt pathway (8–10). Wnt signals operate at multiple levels in the programs of osteoblastogenesis and osteoclastogenesis, but in general, the effects of this pathway can be summarized as having a pro-osteogenic effect (11). For example, the genetic gain in Wnt function mutations cause high bone mass conditions (12, 13), whereas loss of Wnt function leads to low bone mass diseases including osteoporosis (14). Others and we have demonstrated that the Wnt pathway is also operational in the dental pulp (15–20).
Here, we sought to better understand the role of Wnt signaling in age-related changes to the pulp, and specifically how these changes might contribute to the ability of the pulp to respond to sub-acute traumas resulting from the activities of daily living. We employed three transgenic mouse strains: in the Axin2LacZ/+ strain of mice, the LacZ gene is under control of the promoter for the Wnt dependent target gene Axin2 (21); consequently, Wnt-responsive cells can be identified by visualization of Xgal staining (15). In the Axin2CreERT2/+; R26RmTmG/+ strain of mice, the Cre gene is under control of the Axin2 promoter; when presented with tamoxifen, cells expressing Cre/Axin2 undergo a recombination event, and then become permanently labeled with GFP (16, 19). Thus, Wnt-responsive cells and all their progeny express GFP (15). Finally, in the DMP1–8kb-Cre+/−; Catnblox(ex3)/lox(ex3) (also referred to as daβcatOt) mice, the Cre gene is under control of the DMP1 promoter; DMP1 is expressed in odontoblasts (22); therefore, in daβcatOt mice, βcatenin is stabilized, leading to elevated Wnt signaling in DMP1-expressing cells.
Collectively, analyses of tissues from these mice demonstrated that 1) odontoblasts are sensitive to an endogenous Wnt signal; 2) endogenous Wnt signaling in the pulp decreases with age, along with rate of dentin secretion; and 3) genetically amplifying Wnt signaling in the pulp leads to a massive and rapid accumulation of dentin that, because of its rate of accrual, resembles osteodentin.
Materials and Methods
Animals
Experimental procedures were approved by the Stanford Committee on Animal Research (#13146) and the Institutional Animal Care and Use Committee of Indiana University School of Medicine. Four strains of transgenic mice were used: Axin2LacZ/+, Axin2CreERT2/+; R26RmTmG/+, DMP1–8kb-Cre+/−; Catnblox(ex3)/lox(ex3) (daβcatOt), and DMP1GFP. For detailed information on the individual strains as well as breeding, please see the Supplemental Materials and Methods.
Vital dye labeling and quantification of mineral apposition rate
Vital dye labeling was previously described (23). Mice were intraperitoneally injected with 20 mg/kg of Calcein (Sigma-Aldrich) and 30 mg/kg of Alizarin Red (Sigma-Aldrich) with a 10-day interval. Two days after Alizarin Red injection, mice were sacrificed and harvested. Samples were fixed in 4% PFA overnight, dehydrated with 30% sucrose overnight, and then processed for hard tissue embedding and sectioning, using Kawamoto’s method (24). Mineral apposition rates (MAR) were calculated by measuring the distance between the Calcein labeled bone (green line) and Alizarin Red labeled bone (red line); this measurement was made at eight distinct, randomly chosen sites around the maxillary first molars, in three separate mice. MAR was then calculated as the distance in μm/interval of time between when the labels were injected.
Sample preparation, processing, histology, Xgal staining, and immunohistochemistry assays
Maxillae were harvested, fixed, sectioned at a thickness of 8 μm, and processed using established procedures (18). Aniline blue and Movat’s pentachrome staining were performed as described before (18, 25). For aniline blue staining, sections were submerged in 1% aniline blue (B8563, Sigma-Aldrich) for 3 minutes until a dark blue color developed. Slides were then washed in 1% acetic acid (A38–212, Fisher) for 5 minutes and repeated twice until appropriately discolored. The Movat’s pentachrome method employs alcian blue, (A3157, Sigma-Aldrich), sodium thiosulfate (14518, Alfa Aesar), crocein-scarlet (210757, Sigma-Aldrich) acid-fuchisn (F8129, Sigma-Aldrich), phosphotungstic acid (P4006, Sigma-Aldrich), acetic acid, and alcoholic saffron (3801, Sigma-Aldrich). Movat’s pentachrome staining results in nuclei that are black, collagen and reticulin that are yellow, glycosaminoglycans are light blue, and muscle, cytoplasm and keratin in red (26). Dentin and alveolar bone were stained green/yellow while the PDL and dental pulp stain red.
To detect β-galactosidase activity, X-gal staining was performed (Supplemental Materials and Methods). Immunostaining was performed using standard procedures (25). For the information of primary and secondary antibodies, please see the Supplemental Materials and Methods.
Statistical analyses
All data were presented as mean ± standard deviation of each group. One-way analysis of variance (ANOVA) was used to quantify differences, using Prism 7 (GraphPad Software, USA). A p-value ≤0.05 was considered significant.
Results
Dentin deposition rate declines with age
Similar to humans, we found that mice exhibited an accumulation of dentin throughout life and as a consequence, the volume of the pulp cavity was gradually reduced (Fig. 1A–C, quantified in D). This increase in dentin volume occurred even as the rate of dentin accumulation slowed in older mice (Fig. 1D): for example, the average rate of dentin deposition in 1-month-old mice was between 2.1~8.4 μm/day (Fig. 1E) while in 6-month-old mice, the rate of deposition was below the limit of resolution (Fig. 1F).
Figure 1. Dentin deposition rate declines with age.
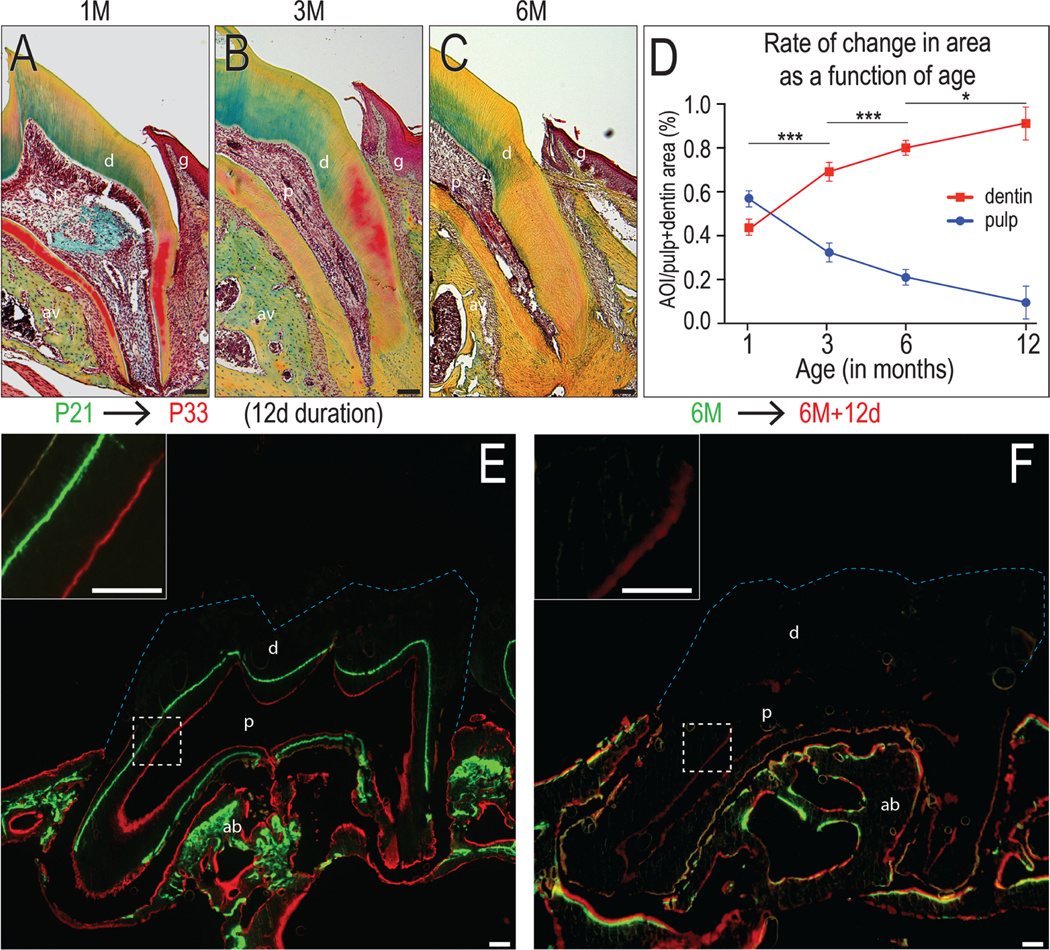
Representative Movat’s pentachrome stained maxillary molar sections showing the dentin thickness and pulp volume in (A) 1M, (B) 3M to (C) 6M groups. In Movat’s pentachrome, dentin and alveolar bone stain green to yellow, and PDL and dental pulp stain red. (D) Quantification of the pulp area and dentin area over the total pulp + dentin area from 1M, 3M, 6M and 12M mice (N=3 for each group). Vital dye labeling showing the new dentin formation in mice around (E) 1M and (F) 6M old in a 10-day interval. Calcein (green) was first injected, Alizarin red (red) was injected 10 days later, and mice were harvest 2 days after the last injection. Blue dotted lines indicate the crown. Abbreviations: d: dentin; p: pulp; ab: alveolar bone; g: gingiva; scale bars: 100 μm. Data are expressed as mean ± standard deviation; *p < 0.05; ***p < 0.0001.
The decline in dentin deposition is paralleled by reduced Wnt responsiveness
Does Wnt signaling regulate the rate of dentin secretion? In Axin2LacZ/+ reporter mice, cells that are responsive to an endogenous Wnt signal can be identified by their Xgal+ve status. In 1-month-old mice, abundant Xgal+ve cells lined the dentin/pulp interface (Fig. 2A). In 3-month-old mice, a single layer of Xgal+ve Wnt-responsive odontoblasts was evident (Fig. 2B). In 6-month-old mice, only a small portion of odontoblasts remained Xgal+ve (Fig. 2C). Thus, within the pulp, there was a significant decline in the number of Xgal+ve cells as a function of age (Fig. 2D).
Figure 2. The decline in dentin deposition is paralleled by a decline in the population of Wnt-responsive odontoblasts.
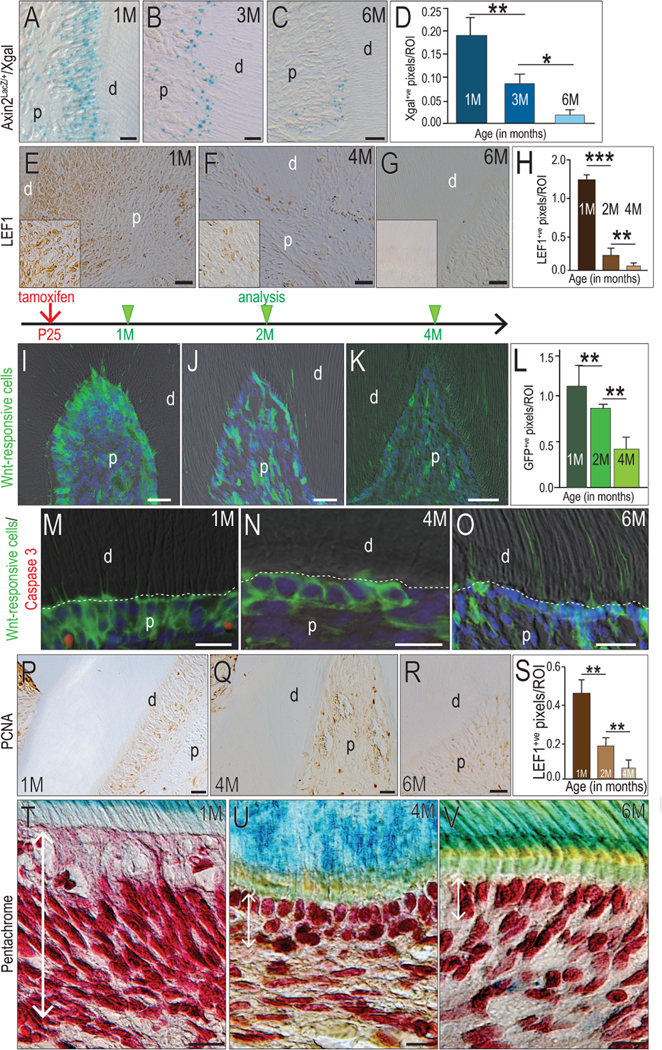
Xgal+ve, Wnt-responsive cells in the maxillary first molars at the age of (A) 1M, (B) 3M and (C) 6M. (D) Quantification of Xgal+ve pixels in the pulp by age. Microscopy images showing Lef1 expression in odontoblasts in (E) 1M, (F) 4M and (G) 6M groups. (H) Quantification of Lef1+ve pixels in the pulp by age. (I) GFP+ve, Wnt-responsive cells in the maxillary first molar dental pulp of 1M-old mice, 5 days after tamoxifen treatment. GFP+ve, Wnt-responsive cells were examined with (J) 1-month tracing and (K) 3-month tracing. (L) Quantification of GFP+ve, Wnt-responsive cells in the pulp area. Co-Immunostaining of apoptosis marker Caspase 3 (Red) with Wnt-responsive cells (Green) in odontoblasts of (M) 1M, (N) 4M and (O) 6M mice. Immunostaining for the proliferation marker PCNA in odontoblasts in (P) 1M, (Q) 4M and (R) 6M mice. (S) Quantification of PCNA+ve pixels in the pulp by age. Pentachrome staining showing the odontoblasts in the maxillary first molar in (T) 1M, (U) 4M and (V) 6M mice. Double head arrows indicate the odontoblast layer. Abbreviations: d, dentin; p, pulp; scale bars: 25 μm. Data are expressed as mean ± standard deviation; *p < 0.05; **p < 0.01; *** p < 0.0001.
We used a second method to determine if endogenous Wnt signaling declined with age. In 1-month-old mice, cells at the dentin/pulp interface abundantly expressed the Wnt transcription factor, Lef1 (Fig. 2E). Lef1 immunostaining was reduced in 4-month-old mice (Fig. 2F), and by 6 months of age, Lef1+ve cells were no longer detectable in the pulp (Fig. 2G), demonstrating a progressive decline in Wnt signaling in the pulp as a function of age (quantified in 2H).
The gradual loss in Wnt-responsive odontoblasts was confirmed using a lineage tracing strain Axin2CreERT2/+; R26RmTmG/+ mice. The Wnt-responsive population and its descendants was identifiable by virtue of its GFP expression. In 1-month-old mice, most odontoblasts were GFP+ve (Fig. 2I), indicating that they were responsive to an endogenous Wnt signal. In 2-month-old mice, the number of GFP+ve cells had declined significantly (Fig. 2J), indicating that descendants of the initial Wnt-responsive population had been lost. In 4-month-old mice, GFP+ve descendants of the initial Wnt-responsive population had diminished still further (Fig. 2K; quantified in L).
How were the GFP+ve cells lost? Co-immunostaining of GFP and the apoptosis marker, Caspase 3, was performed at the 1, 4, and 6-month time points. Only the 1-month old mice showed evidence of GFP/Caspase 3 co-expression (Fig. 2M); in 4- and 6-month old mice, Caspase 3 expression was undetectable (Fig. 2N,O). Therefore, some reduction in the Wnt-responsive population appeared to be due to apoptosis. Coupled with the gradual reduction in mitotic activity as shown by PCNA immunostaining (Fig. 2P–S), it became readily apparent that the density of Wnt-responsive cells at the dentin-pulp interface was reduced as a function of age (Fig. 2T–V).
Wnt signaling regulates the rate of dentin secretion
Data thus far demonstrated a correlation between Wnt signaling and the rate of dentin deposition. To establish a causal relationship between Wnt signaling and the rate of dentin deposition, we genetically engineered odontoblasts to always express a stabilized form the Wnt pathway intermediate, beta catenin. To achieve this we used a strain of mice in which Cre was expressed in odontoblasts. Odontoblasts and pulp cells expressed DMP1 (Fig. 3A), suggesting that the DMP1-Cre strain of mice could serve as a means to activate Wnt signaling in odontoblasts.
Figure 3. Wnt signaling regulates the rate of dentin secretion.
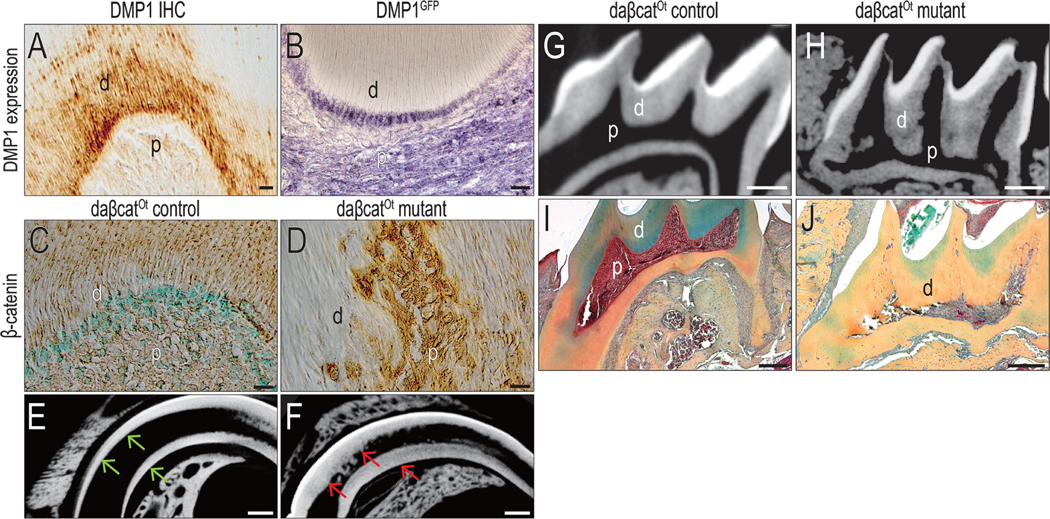
(A) Immunohistochemistry identifies DMP1 expression (brown) in a maxillary first molar. (B) Immunohistochemistry confirmed the GFP expression (purple) in a maxillary first molar in DMP1GFP mice. Immunohistochemistry for β-catenin (brown) in odontoblasts in (C) control and (D) daβcatOt mutant mice (42-d-old). The sections were counterstained with fast green. Representative micro-CT sagittal-sectional reconstructed images of incisor from (E) control and (F) daβcatOt mutant mice (42-d-old). Representative micro-CT sagittal-sectional reconstructed images of the maxillary first molar from (G) control and (H) daβcatOt mutant mice (42-d-old). Pentachrome staining of the maxillary first molar from (I) control and (J) daβcatOt mutant mice (42-d-old). Dentin stains green to yellow, with the more mature dentin staining yellow. Abbreviations: d, dentin; p, pulp. Scale bars: 25 μm (A-D), and 200 μm (E-J).
We confirmed that DMP1 was expressed in odontoblasts and pulp cells by following the expression of GFP in DMP1GFP reporter mice. In these transgenic mice, GFP is under control of the DMP1 promoter and as expected, odontoblasts were positively labeled (Fig. 3B). We then proceeded with crossing DMP1-Cre mice with a strain of mice expressing the stabilized form of β-catenin. These mice, referred to as daβcatOt, exhibited amplified Wnt/β-catenin signaling in DMP-expressing cells (see Fig. 3C,D and reference (27)). Compared with age-matched littermates (Fig. 3E), significantly more dentin accumulated in daβcatOt mutant teeth, in both the incisors (Fig. 3F) and the molars (compare Fig. 3G with H). The dentin accumulation nearly eliminated the pulp cavity in the crown region (Fig. 3G, H). Histologic analyses confirmed μCT imaging (compare Fig. 3I,J). Cumulatively, these data demonstrated that amplified Wnt signaling in odontoblasts led to a dramatic increase in dentin accumulation.
Accelerating deposition converts a tubular dentin into osteodentin
The nature of the dentin was examined in daβcatOt mutant mice. Whereas in control animals, the tubular structure of dentin was obvious and the pulp cavity was densely cellular (Fig. 4A), the dentin of daβcatOt mutant had no tubular arrangement and cells were trapped within the mineralized matrix (Fig. 4B). In controls, aniline blue staining highlighted the ordered nature of the odontoblasts and the tubular dentin (Fig. 4C), in daβcatOt mutants, the odontoblasts were disorganized, and the dentin had an osteoid-like appearance (Fig. 4D).
Figure 4. Accelerating deposition converts a tubular dentin into osteodentin.
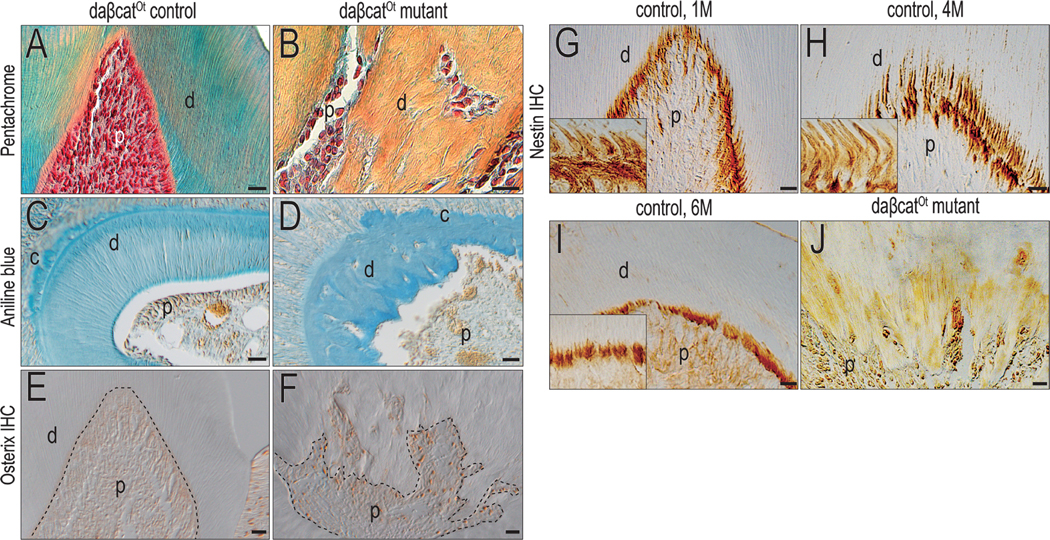
Representative Pentachrome staining of a maxillary first molar in (A) control and (B) daβcatOt mutant mice. Representative cross-section of Aniline Blue staining of the maxillary first molar in (C) control and (D) daβcatOt mutant mice. Immunostaining of Osterix of the maxillary first molar in (E) control and (F) daβcatOt mutant mice. Immunostaining of Nestin in (G) 1M, (H) 4M and (I) 6M control mice. (J) Immunostaining of Nestin in daβcatOt mutant mice. Abbreviations: d, dentin, p, pulp. Scale bar: 25 μm.
Osterix expression was undetectable in the adult pulp of control mice (Fig. 4E). In contrast, Osterix was strongly expressed in the adult pulp of daβcatOt mutant mice (Fig. 4F), indicating the pre-odontoblasts, instead of odontoblasts, were presented in daβcatOt mutant. Nestin is expressed by mature, fully differentiated odontoblasts as shown by analyses of 1-, 4-, and 6-month-old pulps (Fig. 4G–I and see (28, 29)). In daβcatOt mutants, Nestin expression was abolished, and in its place were only pre-odontoblasts secreting atubular osteodentin (Fig. 4J). These data supported our hypothesis that aberrantly elevated Wnt signaling triggered osteodentin production by pre-odontoblasts.
Discussion
Wnt signals maintain dentin secretion
Here we provide evidence that the survival of odontoblasts and their continued production of dentin is ensured in part by endogenous Wnt signaling. This interpretation is strongly supported by the literature (reviewed in (30)). For example, using a loss-of-function approach, Han and collogues demonstrated that β-catenin was required for odontoblast differentiation (31) and in a murine pre-clinical study, Yoshioka and colleagues demonstrated that Wnt signaling and β-catenin are both up-regulated in response to sub-acute pulp trauma (32). These data and others (19, 33) firmly establish the Wnt/β-catenin pathway as a critical regulator of odontoblast function. We extend these observations by demonstrating that the age-related decline in dentin production is mirrored by a decline in Wnt signaling (Fig. 1) and a diminishment in the population of Wnt-responsive cells in the pulp (Fig. 2).
Not all published data comport with our model. For example, Berdahl and colleagues showed that rather than activation, inhibition of Wnt/β-catenin signaling by Msx2 controls formation of osteodentin (34). It is not clear at this time how these data can be reconciled with findings shown here. Others have shown using in vitro analyses that Msx2 enhances Wnt signaling and promotes mineralization (35). Some of these differences may be due to timing or the duration of the Wnt signal. Indeed, constitutive activation or constitutive repression of the Wnt/β-catenin pathway can lead to similar outcomes in mineralized tissues.
The repair response of the pulp is orchestrated by Wnt signals
In patients and in animal models, the pulp demonstrates an extraordinarily vigorous repair response to trauma. Variously referred to as reactionary, reparative, and tertiary dentin, the mineralized matrix produced in response to injury tends to be atubular and rather than being secreted by fully differentiated odontoblasts, it is produced by pre-odontoblastic progenitor cells in the pulp ((36) and reviewed by (6)). This “back-up” system appears to be ideally suited for a relatively quiescent tissue that must survive for an entire lifetime. How is this quiescent status maintained, and how is it reversed upon injury is still open for debate. In tissues that do not turn over, adult stem cells exist in a mitotically arrested state with a low metabolic demand (37). In response to injury, however, this stem cell pool is capable of proliferating and then differentiating to aid in tissue repair (37). Applying this theory to the pulp seems reasonable: it is a tissue with very low turnover rate, but fully capable of mounting a robust repair response to trauma. In many tissues, Wnt signals maintain adult stem cells in a quiescent state (38) and we propose the pathway plays a similar role in the adult pulp.
Clinical implications
If primary odontoblasts respond to trauma and secrete copious amounts of new dentin, then the pulp generally retains its vitality. When that repair response is attenuated with age, the result is typically chronic inflammation followed by pulp necrosis, which necessitates a root canal. The vast majority of endodontic cases begin with sub-acute, chronic trauma to the pulp, e.g., chronic pulpitis that is not adequately repaired by the pulp’s natural defense strategy. Our goal is to devise new biologically based strategies to enhance this natural repair response that has become attenuated as a result of aging. We envision a therapeutic strategy to stimulate the repair potential of an aged pulp by enhancing Wnt signaling via delivery of a liposomal formulation of WNT protein. We demonstrated the feasibility of such an approach (15), with one important caveat. In our studies, the dentin injury was produced in an otherwise healthy tooth. This characteristic does not match with most endodontic pulp cases, where the pulp is inflamed and potentially infected at the time of treatment. Consequently, it will be critically important to test a WNT-based strategy to enhance dentin secretion in a model that replicates this clinical scenario.
Supplementary Material
Highlights:
Dentin deposition occurs throughout life, but the rate of accumulation slows with age.
Dentin secration rate correlates with endogenous Wnt signaling.
Elevated Wnt signaling in odontoblasts leads to osteodentin formation.
Acknowledgements
The authors deny any conflicts of interest. We thank Ustun Serdar Tulu for his help in microCT analysis and Joseph Grauer for his insight and revision of the manuscript. This research project was supported by grants from the NIH (R01DE024000-11) to JAH, and National Natural Science Foundation of China (81500835) and the Fundamental Research Funds for the Central Universities (lzujbky-2018-99) to YZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- 1.Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol 2011;6:121–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet 2011;377(9773):1276–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta P, Kaur H, Shankari GSM, Jawanda MK, Sahi N. Human age estimation from tooth cementum and dentin. J Clin Diagn Res 2014;8(4):ZC07–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith AJ, Cassidy N, Perry H, Begue-Kirn C, Ruch JV, Lesot H. Reactionary dentinogenesis. Int J Dev Biol 1995;39(1):273–280. [PubMed] [Google Scholar]
- 5.Tomes CR. On the Structure and Development of Vascular Dentine. Philosophical Transactions of the Royal Society of London 1878;169:25–47. [Google Scholar]
- 6.Goldberg M, Kulkarni AB, Young M, Boskey A. Dentin: structure, composition and mineralization. Front Biosci (Elite Ed) 2011;3:711–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguiar MC, Arana-Chavez VE. Ultrastructural and immunocytochemical analyses of osteopontin in reactionary and reparative dentine formed after extrusion of upper rat incisors. J Anat 2007;210(4):418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron R, Rawadi G. Wnt signaling and the regulation of bone mass. Curr Osteoporos Rep 2007;5(2):73–80. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest 2006;116(5):1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong Z, Ethen NJ, Williams BO. WNT signaling in bone development and homeostasis. Wiley Interdiscip Rev Dev Biol 2014;3(6):489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodine PV, Komm BS. Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord 2006;7(12):33–39. [DOI] [PubMed] [Google Scholar]
- 12.Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, Robinson DR, et al. Lrp5 functions in bone to regulate bone mass. Nat Med 2011;17(6):684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Wesenbeeck L, Cleiren E, Gram J, Beals RK, Benichou O, Scopelliti D, et al. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet 2003;72(3):763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa AG, Bilezikian JP. Sclerostin: therapeutic horizons based upon its actions. Curr Osteoporos Rep 2012;10(1):64–72. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Yuan X, Liu B, Tulu US, Helms JA. Wnt-Responsive Odontoblasts Secrete New Dentin after Superficial Tooth Injury. J Dent Res 2018;97(9):1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lohi M, Tucker AS, Sharpe PT. Expression of Axin2 indicates a role for canonical Wnt signaling in development of the crown and root during pre- and postnatal tooth development. Dev Dyn 2010;239(1):160–167. [DOI] [PubMed] [Google Scholar]
- 17.Kim TH, Bae CH, Lee JC, Ko SO, Yang X, Jiang R, et al. beta-catenin is required in odontoblasts for tooth root formation. J Dent Res 2013;92(3):215–221. [DOI] [PubMed] [Google Scholar]
- 18.Lim WH, Liu B, Cheng D, Hunter DJ, Zhong Z, Ramos DM, et al. Wnt signaling regulates pulp volume and dentin thickness. J Bone Miner Res 2014;29(4):892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter DJ, Bardet C, Mouraret S, Liu B, Singh G, Sadoine J, et al. Wnt Acts as a Prosurvival Signal to Enhance Dentin Regeneration. J Bone Miner Res 2015;30(7):1150–1159. [DOI] [PubMed] [Google Scholar]
- 20.Ishimoto K, Hayano S, Yanagita T, Kurosaka H, Kawanabe N, Itoh S, et al. Topical application of lithium chloride on the pulp induces dentin regeneration. PLoS One 2015;10(3):e0121938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol 2002;22(4):1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, Feng JQ. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res 2007;86(4):320–325. [DOI] [PubMed] [Google Scholar]
- 23.Rauch DA, Hurchla MA, Harding JC, Deng H, Shea LK, Eagleton MC, et al. The ARF tumor suppressor regulates bone remodeling and osteosarcoma development in mice. PLoS One 2010;5(12):e15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Q, Yuan X, Zhang X, Chen J, Shi Y, Brunski JB, et al. Mechanoadaptive Responses in the Periodontium Are Coordinated by Wnt. J Dent Res 2019;98(6):689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minear S, Leucht P, Jiang J, Liu B, Zeng A, Fuerer C, et al. Wnt proteins promote bone regeneration. Science translational medicine 2010;2(29):29ra30. [DOI] [PubMed] [Google Scholar]
- 26.Doello K. A new pentachrome method for the simultaneous staining of collagen and sulfated mucopolysaccharides. Yale J Biol Med 2014;87(3):341–347. [PMC free article] [PubMed] [Google Scholar]
- 27.Tu X, Delgado-Calle J, Condon KW, Maycas M, Zhang H, Carlesso N, et al. Osteocytes mediate the anabolic actions of canonical Wnt/beta-catenin signaling in bone. Proc Natl Acad Sci U S A 2015;112(5):E478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita S, Hideshima K, Ikeda T. Nestin expression in odontoblasts and odontogenic ectomesenchymal tissue of odontogenic tumours. Journal of clinical pathology 2006;59(3):240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyazaki T, Kanatani N, Rokutanda S, Yoshida C, Toyosawa S, Nakamura R, et al. Inhibition of the terminal differentiation of odontoblasts and their transdifferentiation into osteoblasts in Runx2 transgenic mice. Arch Histol Cytol 2008;71(2):131–146. [DOI] [PubMed] [Google Scholar]
- 30.da Rosa WLO, Piva E, da Silva AF. Disclosing the physiology of pulp tissue for vital pulp therapy. Int Endod J 2018;51(8):829–846. [DOI] [PubMed] [Google Scholar]
- 31.Han N, Zheng Y, Li R, Li X, Zhou M, Niu Y, et al. beta-catenin enhances odontoblastic differentiation of dental pulp cells through activation of Runx2. PLoS One 2014;9(2):e88890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshioka S, Takahashi Y, Abe M, Michikami I, Imazato S, Wakisaka S, et al. Activation of the Wnt/beta-catenin pathway and tissue inhibitor of metalloprotease 1 during tertiary dentinogenesis. Journal of biochemistry 2013;153(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aurrekoetxea M, Irastorza I, Garcia-Gallastegui P, Jimenez-Rojo L, Nakamura T, Yamada Y, et al. Wnt/beta-Catenin Regulates the Activity of Epiprofin/Sp6, SHH, FGF, and BMP to Coordinate the Stages of Odontogenesis. Front Cell Dev Biol 2016;4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amri N, Djole SX, Petit S, Babajko S, Coudert AE, Castaneda B, et al. Distorted Patterns of Dentinogenesis and Eruption in Msx2 Null Mutants: Involvement of Sost/Sclerostin. The American journal of pathology 2016;186(10):2577–2587. [DOI] [PubMed] [Google Scholar]
- 35.Cheng SL, Shao JS, Cai J, Sierra OL, Towler DA. Msx2 exerts bone anabolism via canonical Wnt signaling. J Biol Chem 2008;283(29):20505–20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon S, Cooper P, Smith A, Picard B, Ifi CN, Berdal A. Evaluation of a new laboratory model for pulp healing: preliminary study. Int Endod J 2008;41(9):781–790. [DOI] [PubMed] [Google Scholar]
- 37.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol 2013;14(6):329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science 2010;327(5965):542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


