Abstract
Odontogenic pain can be debilitating, and nonopioid analgesic options are limited. This randomized placebo-controlled clinical trial aimed to assess the effectiveness and safety of cannabidiol (CBD) as an analgesic for patients with emergency acute dental pain. Sixty-one patients with moderate to severe toothache were randomized into 3 groups: CBD10 (CBD 10 mg/kg), CBD20 (CBD 20 mg/kg), and placebo. We administered a single dose of respective oral solution and monitored the subjects for 3 h. The primary outcome measure was the numerical pain differences using a visual analog scale (VAS) from baseline within and among the groups. Secondary outcome measures included ordinal pain intensity differences, the onset of significant pain relief, maximum pain relief, changes in bite force within and among the groups, psychoactive effects, mood changes, and other adverse events. Both CBD groups resulted in significant VAS pain reduction compared to their baseline and the placebo group, with a maximum median VAS pain reduction of 73% from baseline pain at the 180-min time point (P < 0.05). CBD20 experienced a faster onset of significant pain relief than CBD10 (15 versus 30 min after drug administration), and both groups reached maximum pain relief at 180-min. Number needed to treat was 3.1 for CBD10 and 2.4 for CBD20. Intragroup comparisons showed a significant increase in bite forces in both CBD groups (P < 0.05) but not in the placebo group (P > 0.05). CBD20 resulted in a significant difference in mean percent bite force change in the 90- and 180-min time points compared to the placebo group (P < 0.05). Compared to placebo, sedation, diarrhea, and abdominal pain were significantly associated with the CBD groups (P < 0.05). There were no other significant psychoactive or mood change effects. This randomized trial provides the first clinical evidence that oral CBD can be an effective and safe analgesic for dental pain.
Keywords: clinical trial, endodontics, analgesics, non-narcotic, toothache, pain measurement
Introduction
Dental pain is a prevalent and often incapacitating health concern (Lipton et al. 1993; Pau et al. 2003). Toothache represents the number one most “avoidable” emergency department (ED) visit concern, defined as a visit that did not receive care and patients were discharged home (Hsia and Niedzwiecki 2017). Nonetheless, this type of pain is primarily acute. When it is mild to moderate in strength, it can be managed with nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen, or acetaminophen (APAP), with success (Moore and Hersh 2013). However, certain patients with health limitations cannot take NSAIDs or acetaminophen, or these analgesics may not be as effective in specific patient cohorts (Harirforoosh et al. 2013; Bruno et al. 2014; Hartling et al. 2016; Major et al. 2016).
In patients who require alternatives to NSAIDs and APAP, synthetic opioids (e.g., hydrocodone, oxycodone) are typically the following line of defense (Moore and Hersh 2013). In a study, dentists were among the top 10 prescribers of opioids, often prescribed for emergency pain and pain persisting after dental procedures (Levy et al. 2015; Steinmetz et al. 2017). Other data suggest a 30% chance of getting opioids after a standard dental procedure such as tooth extraction or root canal treatment (Steinmetz et al. 2017). Furthermore, 40% to 42% of patients will fill an opioid prescription after a dental pain-related ED visit (Roberts et al. 2020). To minimize the opioid crisis, health care providers need access to alternative nonopioid analgesics to manage dental pain.
Cannabinoids could be promising opioid alternatives. Indeed, states with medical and adult-use marijuana laws have demonstrated a 5% to 8% reduction in opioid prescriptions for Medicaid enrollees (Bradford and Bradford 2017). Clinical trials on cancer and chronic and neuropathic pain have used tetrahydrocannabinol (THC) combined with cannabidiol (CBD), the 2 primary compounds of cannabis, with successful outcomes (Nurmikko et al. 2007; Lynch et al. 2014). Nonetheless, their combined use as analgesics is still limited as THC is psychoactive and illicit per federal law.
Conversely, CBD is nonpsychoactive and nonaddictive and has shown promising results as an analgesic alternative (Babalonis et al. 2017). In preclinical models, CBD has demonstrated analgesic and anti-inflammatory action (Schuelert and McDougall 2011; Ward et al. 2014; De Gregorio et al. 2019). Limited clinical evidence suggests CBD’s analgesic efficacy against peripheral neuropathy and chronic pain (Notcutt et al. 2004; Capano et al. 2020; Xu et al. 2020). More than 100 clinical trials actively pursue CBD as an analgesic alternative for various pain disorders (clinicaltrials.gov). To our knowledge, there are no published clinical data using CBD as an analgesic for acute dental pain. This study aims to assess the effectiveness and safety of a Food and Drug Administration (FDA)–approved CBD drug against emergency dental pain in a phase IIA proof-of-principle study. We hypothesized that CBD would provide a minimum of 30% pain reduction from the preoperative measurements for patients with an emergency toothache. This effect size is comparable to 400 mg ibuprofen for acute odontogenic pain (Taggar et al. 2017).
Materials and Methods
Study Design
This study was an investigator-initiated triple-arm, phase IIA, randomized placebo-controlled trial with double masking (participant, outcomes assessor). The study population consisted of adult patients 18 to 75 y old, presenting at the UT Health School of Dentistry, San Antonio, Texas, with moderate to severe odontogenic pain, defined as ≥30 mm on a 100-mm visual analog scale (VAS). Table 1 presents the inclusion and exclusion criteria. The institutional review board approved the study (IRB HSC20200305H, ClinicalTrials.gov NCT04642404). Appendix Figure 1 represents the Consolidated Standards of Reporting Trials (CONSORT) checklist. All eligible subjects signed informed consent before the initiation of the study.
Table 1.
Inclusion/Exclusion Criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Healthy adults 18–75 y old, ASA class I or II | ASA class III or IV, patients with hepatic impairment, pregnant a or lactating women |
| Permanent tooth with moderate to severe odontogenic pain (i.e., ≥30 on a 100-mm VAS) | Patients on drugs metabolized by enzymes that also metabolize CBD (e.g., clobazam, diazepam, topiramate, warfarin) b |
| Clinical pulpal diagnosis of irreversible pulpitis or pulpal necrosis and periapical diagnosis of symptomatic apical periodontitis | Self-reported prior experience inhaling cannabis (either via smoking or vaporization) |
| Test negative for recent cannabis use and/or other drugs of abuse including alcohol (urine tests collected at screening visit) c | Use of opioids in the month prior to screening/treatment visit and/or NSAIDS or acetaminophen 6 h prior to treatment |
| Participant able to understand the forms (English or Spanish) and provide informed written consent | Unwilling to participate |
ASA, American Society of Anesthesiologists; CBD, cannabidiol; NSAID, nonsteroidal anti-inflammatory drug; VAS, visual analog scale.
Pregnancy test will be performed at the screening visit.
Epidiolex is metabolized by CYP3A4 and CYP2C19 and has the potential to inhibit CYP2C8, CYP2C9, and CYP2C19 at clinically relevant concentrations; therefore, we chose to avoid potential drug interactions.
Cannabis trials often present with high placebo effect. Cannabis-naive subjects are thus proposed to minimize this effect as well the possibility of tachyphylaxis.
Intervention Drug
Epidiolex is an FDA-approved CBD oral solution derived from the cannabis plant, approved for the treatment of epileptic seizures in specific rare and severe syndromes in patients 2 y of age and older (Devinsky et al. 2016; Devinsky et al. 2017). The drug comes as an oil-based oral solution in a 100-mg/mL bottle (100 mL) with a maximum recommended dose of 20 mg/kg/d (Greenwich Biosciences 2018). The use of this drug in our trial met all the requirements for an exemption from an FDA Investigational New Drug approval (Holbein 2009). The pharmaceutical company GW had no participation in the design and conduction of this trial, nor did it provide any funding or material support.
Study Arms
Subjects had to test negative for 12 controlled substances to proceed with enrollment (urinalysis; DrugConfirm, Confirm BioSciences). Female subjects also submitted a negative pregnancy test. Patients who satisfied all eligibility criteria were randomly assigned to one of the following groups:
Group CBD10: CBD (drug: Epidiolex, 100 mg/mL) 10-mg/kg single dose
Group CBD20: CBD (drug Epidiolex, 100 mg/mL) 20-mg/kg single dose
Group placebo: placebo (drug: placebo)
The 20-mg/kg dose is the maximum recommended daily dose from the manufacturer (Greenwich Biosciences) and the FDA. Placebo drug was a 10-mL 1:1 compounded mix of commercial sesame oil and ORA Sweet solution (Perrigo) to produce a similar taste, texture, and color as the drug.
Randomization and Blinding
A block randomization design using the R software was performed with blocks of 6 to allow 2 permutations of each intervention within the block, and the treatment allocation ratio was 1:1:1 among the groups. We then imported the randomization sequence into the REDCap software (Vanderbilt University), an institution-based secure electronic data capture software for the data collection (Harris et al. 2021). The groups were entered as A (i.e., CBD10), B (i.e., CBD20), and C (i.e., placebo) in REDCap, keeping the subject and the outcome assessor blinded to group allocation. Each subject received a random allocation letter at the time of baseline data collection before the intervention. The provider, who was not blinded to the treatment group, prepared the allocated drug into a measuring cap in a different treatment room, away from the subject, and then had the subject drink the medicine and a cup of water. The moment of drug administration was defined as time 0 min.
Intervention and Data Collection
Study data were collected before drug administration (baseline [BL]) and at 7 subsequent time points (15, 30, 45, 60, 90, 120, and 180 min) after drug administration (time 0 min) during a 3-h total observation period (Appendix Fig. 2). The 3 h was selected based on the reported Tmax (2.5 h) for Epidiolex (Greenwich Biosciences). Ibuprofen 600 mg (or acetaminophen/codeine 650/60 mg, if a contraindication for ibuprofen existed) was provided in the 3 h as a rescue medication if needed, and it was recorded. Subjects were encouraged to wait at least 60 min after administration of the drug study before consuming any rescue medication (Daniels et al. 2018).
Subjects were recalled within 1 wk (7-d time point) to capture any additional side effects.
Patient demographics (age, sex, race, tooth number, weight, and body mass index) were collected. The data collection instruments included the VAS (0–100 mm), a pain intensity assessment questionnaire, a bite transducer to measure the bite force (Newton), psychoactive and mood change questionnaires, and a questionnaire on adverse events (AEs). The VAS was a nonnumbered scale from 0 to 100 mm, with 0 defined as “no pain” and 100 defined as “worst pain ever had.” For pain intensity, the subjects were asked to answer the following question: “Compared to your pain levels before you entered the study, how would you rate your tooth pain level at the moment?” The responses were categorized as toothache “reduced,” “similar,” or “increased.” The digital bite transducer is a previously validated instrument used to assess changes in the bite forces (N) before and after the intervention (Alelyani et al. 2020). The study included the Bowdle questionnaire (13 questions assessing drug high, alterations in internal perception, and alterations in external perception) to depict psychoactive changes (van de Donk et al. 2019). The Bond and Lader mood scale (16 scales assessing alertness, contentment, and calmness) was used to assess mood changes (Zuurman et al. 2008). Finally, subjects answered a questionnaire on common AEs and reported any other side effects that were not listed. Common side effects from much higher doses (1,500–6,000 mg single dose) included diarrhea, nausea, headache, dizziness, and somnolence (Taylor et al. 2018).
Outcomes
The primary outcome measure was the VAS pain differences from baseline within and among the groups at each time point. Secondary outcome measures included pain intensity differences, the onset of significant pain relief (intragroup analyses of time points compared to baseline, P < 0.05), the maximum pain relief, changes in bite force within and among the groups, psychoactive effects, mood changes, and other adverse events. Subjects reported self-reported pain relief duration and time-to-next analgesic at the follow-up visit.
Sample Size and Data Analysis
Based on available literature, we considered that a minimum 30% reduction of pain would be clinically relevant (Farrar et al. 2000; Cepeda et al. 2003). In order to achieve 80% power to detect 30% reduction in pain (99% control vs. 69% test group participants with pain ≥30/100 on a VAS scale), we used a 2-sided z test at a = 0.025 (adjusted for multiple comparisons to control) and we calculated the sample size at 20/group.
Mixed-model analyses were used for intragroup comparisons for the numerical variables among the different time points (VAS, bite force, Bowdle, Bond/Lader questionnaires), the onset of significant pain relief, and the time of maximum analgesia. The Bowdle and Bond/Lader questions were first analyzed individually. Then data from questions were pooled for each effect category (i.e., “drug high,” “internal perception,” “external perception,” “calmness,” “mood,” and “alertness”). Following testing of the interaction term between time and group in the mixed-model analysis, intergroup comparisons between the placebo and the experimental groups were performed when appropriate using parametric and nonparametric post hoc tests (Holms–Bonferroni adjustment) following the assessment of data normality (Wilk–Shapiro normality tests). Numbers needed to treat (NNTs) were calculated for both CBD doses—namely, the number of patients needing treatment before 1 patient experiences a minimum of 50% pain relief. Categorical variables (pain intensity and AEs) were analyzed with χ2 tests. JMP software (JMP) was used for the statistical analysis.
Results
We assessed 130 subjects for eligibility and enrolled 64 participants in the study (Appendix Fig. 3). Most reasons for exclusion included previous opiate or cannabinoid use and unwillingness to participate. Of the 64 participants, 3 participants were excluded from the data analysis due to unrealistic VAS results (n = 2) and <30/100 BL VAS pain (n = 1). VAS results were deemed “unrealistic” when subjects reported complete pain relief (VAS = 0) within the first 15 min of the study. The final sample size per group was as follows: CBD10, n = 20; CBD20, n = 20; and placebo, n = 21 (Appendix Fig. 3). The average age (mean, SD) of the participants was 44 ± 13.7, with 65.5% females and 34.5% males. Hispanic/Latinos corresponded to most of the study population (68%), followed by 11% of Whites/Caucasians. Age, sex, race, tooth type, weight, and body mass index (BMI) were equally distributed among the 3 groups (Table 2, P > 0.05). All subjects completed the 3-h observation period without requesting rescue pain medication.
Table 2.
Patient Demographics.
| Characteristic | Group | P Value | ||
|---|---|---|---|---|
| CBD10 | CBD20 | Placebo | ||
| N | 20 | 20 | 21 | |
| Age, y | >0.05 | |||
| Mean | 44.6 | 44.75 | 43.1 | |
| SD | 12.66 | 11.61 | 16.78 | |
| Sex, n | >0.05 | |||
| Female | 13 | 16 | 11 | |
| Male | 7 | 4 | 10 | |
| Race, n | >0.05 | |||
| Black | 4 | 2 | 0 | |
| Hispanic | 11 | 16 | 16 | |
| Hispanic/Native | 0 | 0 | 1 | |
| White | 5 | 2 | 4 | |
| Tooth type, n | >0.05 | |||
| Anterior | 3 | 1 | 0 | |
| Molar | 12 | 15 | 16 | |
| Premolar | 5 | 4 | 5 | |
| Weight, kg | >0.05 | |||
| Mean | 94.37 | 87.71 | 92.43 | |
| SD | 21.04 | 22.21 | 22.7 | |
| Body mass index | >0.05 | |||
| Mean | 33.5 | 33.32 | 32.32 | |
| SD | 6.42 | 6.63 | 5.5 | |
There were no significant differences among the groups (analysis of variance and χ2 tests, P values).
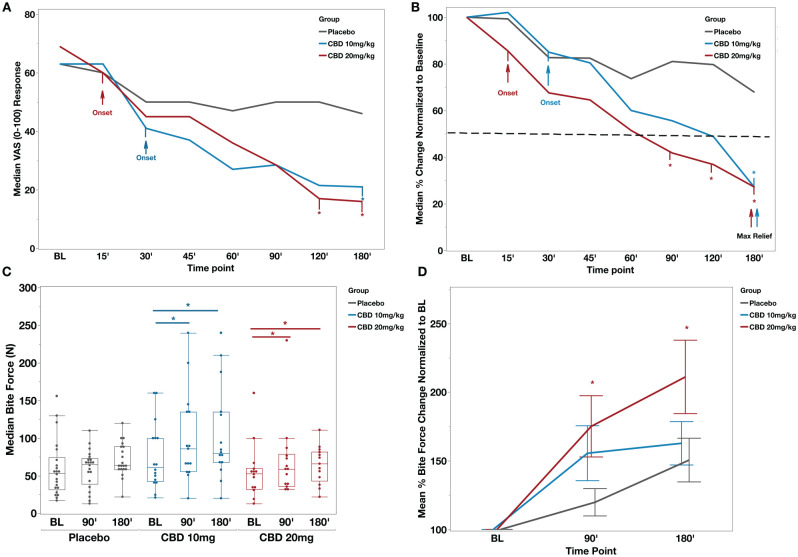
Median (interquartile range [IQR]) VAS pain scores at BL were 63 (51.5, 79.5) for CBD10, 69 (59, 76.25) for CBD20, and 63 (51.7, 73.5) for the placebo (Fig. 1A). At the 180-min time point, VAS pain scores (median; IQR) were CBD10 (21; 1, 38), CBD20 (20; 4.5, 39.2), and placebo (48; 13, 58.7) (Fig. 1A).
Figure 1.
CBD reduced the dental pain and increased the bite force in patients presented with emergency toothache. (A) Median visual analog scale (VAS) pain scores per time point for all groups. Arrows indicate the onset of significant pain score differences from baseline (BL) for the cannabidiol (CBD) groups. Asterisks depict significant differences from the placebo group. Mixed-model analysis, “time point” (P < 0.001), “Group * Time Point” (P = 0.0013), and “Group” (P = 0.55). (B) Median percent change from BL. The dotted line represents a 50% reduction in BL pain. Maximum pain relief occurred at 180 min after CBD administration in both CBD groups, significantly different from the placebo. Placebo also experienced pain relief with a maximum of 33% median pain reduction from BL pain. Asterisks depict significant differences from the placebo group. Wilcoxon test for intergroup comparisons, P < 0.05. (C) Box plots depicting median bite force (Newton) scores per time point for all groups. Both CBD groups noted a significant increase in bite force at 90 and 180 min compared to BL, while placebo group changes were not significant. Mixed-model analysis, “time point” (P < 0.001), “Group * Time Point” (P = 0.28), and “Group” (P = 0.19). (D) Mean percent bite force change normalized to baseline. Asterisks depict significant change in CBD 20 mg/kg compared to the placebo group (t test each pair per time point, P < 0.05).
CBD10 experienced the onset of significant pain relief in 30 min after drug administration, and CBD20 experienced the onset of significant pain relief in 15 min after drug administration compared to their BL VAS measurements (Fig. 1A, P < 0.05). The pain continued to decline in both CBD10 and CBD20 groups, reaching a 50% reduction at the 60-min time point for CBD20 and 120-min time point for CBD10 (Fig. 1B). Both CBD groups experienced a maximum median pain reduction of 73% from the BL at the 180-min time point, with CBD10 median pain being 27% (IQR, 1.26%, 47.5%) of the BL pain and CBD20 median pain to be 27% (IQR, 10%, 58%) of the BL VAS pain (Fig. 1B). The placebo group experienced a maximum of 33% pain reduction from BL (P < 0.05), with a median VAS pain of 67% (41%, 91%) of BL at the 180-min time point. Intergroup comparisons showed that both CBD groups produced significant pain relief compared to the placebo group (P < 0.05) at 180 min for CBD10 and 120- and 180-min time points for CBD20 (P < 0.05). Finally, a subset of subjects (n = 42/61) responded to the questions regarding pain relief duration after the observation period and the time they took their next analgesic during their follow-up visit, with no significant differences among the groups (P > 0.05, Appendix Table 1).
For CBD10, bite force (N) (median; IQR) was significantly increased from BL (61; 42.5, 100), to the 90-min time point (86; 56, 135), to the 180-min time point (80; 68, 135) (P < 0.05, Fig. 1C). For CBD20, bite force (N) (median; IQR) was significantly increased from BL (53; 32.5, 60), to the 90-min time point (59; 35.7, 78.7), to the 180-min time point (66; 43, 82) (P < 0.05, Fig. 1C). There were no significant differences in bite force between the time points in the placebo group. When bite forces were normalized to BL measurements for each group (percent change from BL), intergroup comparisons showed a significant difference in CBD20 mean percent bite force change in the 90- and 180-min time points compared to the placebo group (P < 0.05, Fig. 1D).
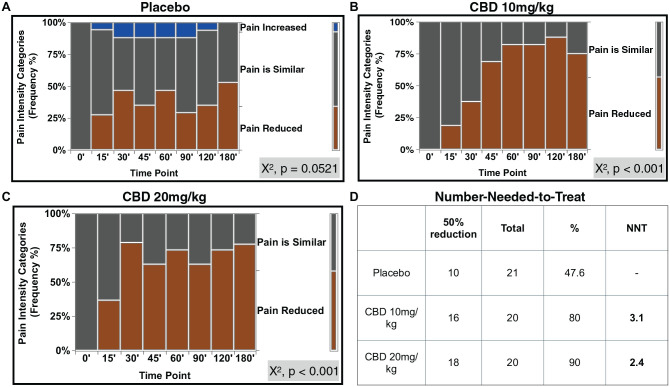
Pain intensity assessment showed that an increase in the “pain reduced” category in the CBD groups was significantly associated with an increase in time (P < 0.001). In contrast, there was no significant association between pain intensity and time points for the placebo group (P = 0.0521, Fig. 2A–C). NNT was 3.1 for CBD10 and 2.4 for CBD20 (Fig. 2D). Importantly, VAS numerical scale was significantly associated with the pain intensity categorical variable (simple logistic regression, P < 0.001).
Figure 2.
The frequency of “Pain Reduced” category significantly increased with time in both CBD groups. Pain intensity assessment for (A) placebo, (B) CBD 10 mg/kg, and (C) CBD 20 mg/kg. Pain categories compared to baseline (BL) pain: “pain increased,” “pain similar,” and “pain reduced,” χ2 tests, P < 0.05. (D) Number needed to treat (NNT) for a 50% reduction in BL pain for the experimental groups. CBD, cannabidiol.
Regarding the safety outcomes, there were no statistically significant differences between and within the groups for psychoactive (Bowdle questions) or mood change effects (Bond/Lader questions) (P > 0.05, Appendix Table 2). Analysis of the AE questionnaire showed that CBD10 was 14 times more likely to experience sedation (i.e., calmness, relaxation, or sleepiness) compared to the placebo group within the 3 h (P < 0.05). The CBD20 group was 10 times more likely to experience diarrhea and abdominal pain and 8 times more likely to experience sedation than the placebo group within the 3 h (P < 0.05, Table 3). Abdominal pain was also significantly associated with CBD20 after the 3-h observation period but resolved within the same day (P < 0.05, Table 3).
Table 3.
Adverse Events Reported within the 3-h Observation Period and after the Observation Period at the 7-d Follow-up Visit.
| Side Effects | CBD10, n (%) | CBD20, n (%) | Placebo, n (%) | χ2, P Value | CBD10, n (%) | CBD20, n (%) | Placebo, n (%) | χ2, P Value |
|---|---|---|---|---|---|---|---|---|
| n | 21 | 20 | 23 | 18 | 18 | 22 | ||
| Time Point | 3 h | 3 h < t < 7 d | ||||||
| CNS disorders | ||||||||
| Somnolence | 3 (14.2) | 3 (15) | 1 (4.3) | >0.05 | 1 (5) | 0 | 1 (4.5) | >0.05 |
| Convulsion (seizure) | 0 | 0 | 0 | >0.05 | 0 | 0 | 0 | >0.05 |
| Ataxia (impaired coordination) | 0 | 0 | 0 | >0.05 | 0 | 0 | 0 | >0.05 |
| Pyrexia (fever) | 0 | 0 | 0 | >0.05 | 0 | 0 | 0 | >0.05 |
| Sedation | 6 (28.5) | 3 (15) | 0 | 0.01 | 1 (5) | 0 | 0 | >0.05 |
| Abnormal behavior | 0 | 0 | 0 | >0.05 | 0 | 0 | 1 (4.5) | >0.05 |
| Headache | 2 (9.5) | 1 | 0 | >0.05 | 0 | 0 | 0 | >0.05 |
| Psychomotor hyperactivity | 0 | 0 | 0 | >0.05 | 0 | 0 | 0 | >0.05 |
| Gastrointestinal disorders | ||||||||
| Decreased appetite | 2 (9.5) | 1 (5) | 0 | >0.05 | 0 | 0 | 0 | >0.05 |
| Nausea | 1 (4.7) | 0 | 0 | >0.05 | 1 (5) | 0 | 0 | >0.05 |
| Diarrhea | 1 (4.7) | 4 (20) | 0 | 0.03 | 2 (11) | 3 (16.6) | 0 | >0.05 |
| Vomiting | 0 | 1 (5) | 0 | >0.05 | 0 | 2 (11) | 0 | >0.05 |
| Abdominal pain | 1 (4.7) | 4 (20) | 0 | 0.03 | 0 | 4 (22.2) | 0 | 0.006 |
| Respiratory disorders | ||||||||
| Nasopharyngitis | 1 (4.7) | 0 | 0 | >0.05 | 0 | 0 | 0 | >0.05 |
| Pneumonia | 0 | 0 | 0 | >0.05 | 0 | 0 | 0 | >0.05 |
| Infectious disorders | ||||||||
| Viral infection | 0 | 0 | 0 | >0.05 | 0 | 0 | 0 | >0.05 |
| Pharyngitis streptococcal | 0 | 0 | 0 | >0.05 | 0 | 0 | 0 | >0.05 |
| Gastroenteritis viral | 0 | 0 | 0 | >0.05 | 0 | 0 | 0 | >0.05 |
| Rash | 0 | 0 | 0 | >0.05 | 0 | 0 | 0 | >0.05 |
| Fatigue | 3 (14.2) | 4 (20) | 1 (4.3) | >0.05 | 1 (5) | 2 (11) | 0 | >0.05 |
| Other | ||||||||
| Dry mouth | 1 (4.7) | 0 | 1 | >0.05 | 0 | 0 | 0 | >0.05 |
| Anxiety | 0 | 0 | 0 | >0.05 | 0 | 0 | 0 | >0.05 |
| Paresthesia (tingling) | 1 (4.7) | 0 | 1 (4.3) | >0.05 | 0 | 0 | 1 (4.5) | >0.05 |
| Hot flashes | 1 (4.7) | 0 | 0 | >0.05 | 0 | 0 | 1 (4.5) | >0.05 |
| Angina (chest tightness) | 1 (4.7) | 0 | 0 | >0.05 | 0 | 0 | 0 | >0.05 |
| Double vision | 0 | 0 | 1 (4.3) | >0.05 | 0 | 0 | 1 (4.5) | >0.05 |
χ2 tests, P < 0.05.
CNS, central nervous system.
Discussion
The results from this randomized clinical trial demonstrate the analgesic effectiveness of a pure CBD drug (Epidiolex, GW) for managing acute toothache. To our knowledge, this is the first randomized clinical trial testing CBD for managing emergency dental pain. Importantly, Epidiolex (the only FDA-approved CBD compound) has been recently descheduled, allowing more accessible prescriptions for patients in the US, although it is not currently approved for use in dental pain management.
CBD10 and CBD20 had a similar analgesic profile, with a fasted onset of significant pain relief shown in the higher dose. Interestingly, CBD10 appears to achieve lower raw VAS scores for the time points of 30 to 90 min compared to CBD20, possibly due to the small sample size and the high variability among the subjects (Fig. 1A). When the raw VAS scores were normalized to the baseline scores in Figure 1B, the percent pain reduction from BL shows the CBD dose effect more clearly, with the CBD20 achieving a higher, although not significant, percent pain reduction for the first 120 min. Another observation is that the female/male ratio was 2:1, which agrees with the findings that women experience increased pain sensitivity (Bartley and Fillingim 2013). Although sex was not significant among the groups, a more balanced sex distribution was observed in the placebo group compared to the CBD groups, and it is possible that inflated pain relief was observed in the placebo group.
Dental patients who cannot receive NSAIDs or acetaminophen due to underlying medical conditions or allergies have no alternatives to avoid opioid prescriptions to achieve pain relief. Reported NNT for ibuprofen 400 to 600 mg was 2.5 to 2.7 (95% confidence interval [CI], 2.0–4.2), and ibuprofen 200 mg/APAP 500 mg was 1.6 (95% CI, 1.5–1.8) (Derry et al. 2009; Moore et al. 2015). Furthermore, the reported NNT of oxycodone 10 mg/APAP 650 mg, a standard dental prescription, was 2.3 (95% CI, 2.0–6.4) (Moore et al. 2015). Notably, NNT was 3.1 for the CBD 10 mg/kg and 2.4 for the CBD 20 mg/kg, which falls in the reported range of NNT for ibuprofen and oxycodone/APAP combination for acute dental pain. Our results indicate that a single dose of CBD is as potent as current analgesic regimens and can manage emergency dental pain effectively.
Reduced bite force can negatively affect masticatory efficiency and compromise a patient’s nutrition and quality of life (Fujimoto et al. 2020). Patients with pulpal and periapical disease will likely experience reduced biting threshold and mechanical allodynia on the affected tooth and the contralateral side (Alelyani et al. 2020). In a previous study, researchers measured the bite force on patients who underwent third molar extractions and received ibuprofen or placebo postsurgery using a numerical scale to record the pain provoked by biting on the ipsilateral side. They found that ibuprofen significantly increased biting forces compared to placebo (Norholt et al. 1998). In contrast, a more recent placebo-controlled study using the same bite force transducer used in our study showed that ibuprofen did not significantly change the mechanical thresholds of teeth presenting with apical periodontitis and that the effect was similar to placebo (Read et al. 2014). Our study showed that both CBD doses led to significant intragroup differences in bite forces. CBD 20 mg/kg had a significant overall effect compared to placebo, suggesting that CBD can improve tooth function when mechanical allodynia is present.
Last, a single dose of CBD did not produce any significant psychoactive or mood effects. Sedation was associated with both doses, a side effect that was previously reported, and it is on the Epidiolex label (Greenwich Biosciences 2018). The incidence of sedation was high in epilepsy studies initially but started to diminish with subsequent doses as patients became more used to the drug (Devinsky et al. 2017). Furthermore, reported sedation is associated with calming, anxiolytic, and not drowsy effects (Shannon et al. 2019). This is justified by CBD’s strong affinity for the serotonin (5-HT)1A receptor and limited affinity to cannabinoid receptors (De Gregorio et al. 2019). Such findings support the safe use of Epidiolex for dental patients, as more than 1 dose of analgesics is typically required to manage dental pain. Abdominal pain and mild diarrhea are commonly reported AEs on the drug label. In our study, after the first few incidents of diarrhea, we started giving participants loperamide (Imodium; J&J), which alleviated their symptoms.
Important limitations due to the small sample size include the inability to explore age- and sex-related differences. Also, factors known to affect pain perception, like preexisting chronic pain and other social and psychological factors, were not assessed but will be considered in our next steps toward developing a larger-scale phase III clinical trial.
This study showed for the first time that pure CBD could provide more than 70% analgesia to patients with emergency dental pain and increase their bite force during the analgesic effect while maintaining a safe drug profile with minimal side effects. This novel study can catalyze the use of CBD as an alternative analgesic to opioids for acute inflammatory pain conditions, which could ultimately help to address the opioid epidemic.
Author Contributions
V. Chrepa, contributed to conception and design, data acquisition, analysis and interpretation, drafted and critically revised manuscript; S. Villasenor, A. Mauney, contributed to data acquisition, drafted the manuscript; G. Kotsakis, contributed to data analysis and interpretation, critically revised the manuscript; L. Macpherson, contributed to data interpretation, critically revised the manuscript. All authors gave their final approval and agreed to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345231200814 for Cannabidiol as an Alternative Analgesic for Acute Dental Pain by V. Chrepa, S. Villasenor, A. Mauney, G. Kotsakis and L. Macpherson in Journal of Dental Research
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the American Association of Endodontists Foundation (AAEF #44096). All authors decline any conflict of interest associated with this study.
ORCID iDs: V. Chrepa  https://orcid.org/0000-0003-3136-378X
https://orcid.org/0000-0003-3136-378X
A. Mauney  https://orcid.org/0009-0009-8865-2970
https://orcid.org/0009-0009-8865-2970
A supplemental appendix to this article is available online.
References
- Alelyani AA, Azar PS, Khan AA, Chrepa V, Diogenes A. 2020. Quantitative assessment of mechanical allodynia and central sensitization in endodontic patients. J Endod. 46(12):1841–1848. [DOI] [PubMed] [Google Scholar]
- Babalonis S, Haney M, Malcolm RJ, Lofwall MR, Votaw VR, Sparenborg S, Walsh SL. 2017. Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers. Drug Alcohol Depend. 172:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley EJ, Fillingim RB. 2013. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 111(1):52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford AC, Bradford WD. 2017. Medical marijuana laws may be associated with a decline in the number of prescriptions for Medicaid enrollees. Health Aff (Millwood). 36(5):945–951. [DOI] [PubMed] [Google Scholar]
- Bruno A, Tacconelli S, Patrignani P. 2014. Variability in the response to non-steroidal anti-inflammatory drugs: mechanisms and perspectives. Basic Clin Pharmacol Toxicol. 114(1):56–63. [DOI] [PubMed] [Google Scholar]
- Capano A, Weaver R, Burkman E. 2020. Evaluation of the effects of CBD hemp extract on opioid use and quality of life indicators in chronic pain patients: a prospective cohort study. Postgrad Med. 132(1):56–61. [DOI] [PubMed] [Google Scholar]
- Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB. 2003. What decline in pain intensity is meaningful to patients with acute pain? Pain. 105(1–2):151–157. [DOI] [PubMed] [Google Scholar]
- Daniels SE, Atkinson HC, Stanescu I, Frampton C. 2018. Analgesic efficacy of an acetaminophen/ibuprofen fixed-dose combination in moderate to severe postoperative dental pain: a randomized, double-blind, parallel-group, placebo-controlled trial. Clin Ther. 40(10):1765–1776.e5. [DOI] [PubMed] [Google Scholar]
- De Gregorio D, McLaughlin RJ, Posa L, Ochoa-Sanchez R, Enns J, Lopez-Canul M, Aboud M, Maione S, Comai S, Gobbi G. 2019. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 160(1):136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry C, Derry S, Moore RA, McQuay HJ. 2009. Single dose oral ibuprofen for acute postoperative pain in adults. Cochrane Database Syst Rev. 2009(3):CD001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, Scheffer IE, Thiele EA, Wright S; Cannabidiol in Dravet Syndrome Study Group. 2017. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 376(21):2011–2020. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, Miller I, Flamini R, Wilfong A, Filloux F, et al. 2016. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 15(3):270–278. [DOI] [PubMed] [Google Scholar]
- Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. 2000. Defining the clinically important difference in pain outcome measures. Pain. 88(3):287–294. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Suito H, Nagao K, Ichikawa T. 2020. Does masticatory ability contribute to nutritional status in older individuals? Int J Environ Res Public Health. 17(20):7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwich Biosciences. 2018. Highlights of prescribing information. Carlsbad, CA, USA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf [Google Scholar]
- Harirforoosh S, Asghar W, Jamali F. 2013. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 16(5):821–847. [DOI] [PubMed] [Google Scholar]
- Harris PA, Delacqua G, Taylor R, Pearson S, Fernandez M, Duda SN. 2021. The REDCap mobile application: a data collection platform for research in regions or situations with internet scarcity. JAMIA Open. 4(3):ooab078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartling L, Ali S, Dryden DM, Chordiya P, Johnson DW, Plint AC, Stang A, McGrath PJ, Drendel AL. 2016. How safe are common analgesics for the treatment of acute pain for children? A systematic review. Pain Res Manag. 2016:5346819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein MB. 2009. Understanding FDA regulatory requirements for investigational new drug applications for sponsor-investigators. J Investig Med. 57(6):688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia RY, Niedzwiecki M. 2017. Avoidable emergency department visits: a starting point. Int J Qual Health Care. 29(5):642–645. [DOI] [PubMed] [Google Scholar]
- Levy B, Paulozzi L, Mack KA, Jones CM. 2015. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007–2012. Am J Prev Med. 49(3):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton JA, Ship JA, Larach-Robinson D. 1993. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 124(10):115–121. [DOI] [PubMed] [Google Scholar]
- Lynch ME, Cesar-Rittenberg P, Hohmann AG. 2014. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 47(1):166–173. [DOI] [PubMed] [Google Scholar]
- Major JM, Zhou EH, Wong HL, Trinidad JP, Pham TM, Mehta H, Ding Y, Staffa JA, Iyasu S, Wang C, et al. 2016. Trends in rates of acetaminophen-related adverse events in the United States. Pharmacoepidemiol Drug Saf. 25(5):590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PA, Hersh EV. 2013. Combining ibuprofen and acetaminophen for acute pain management after third-molar extractions: translating clinical research to dental practice. J Am Dent Assoc. 144(8):898–908. [DOI] [PubMed] [Google Scholar]
- Moore RA, Derry S, Aldington D, Wiffen PJ. 2015. Single dose oral analgesics for acute postoperative pain in adults—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2015(9):CD008659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norholt SE, Aagaard E, Svensson P, Sindet-Pedersen S. 1998. Evaluation of trismus, bite force, and pressure algometry after third molar surgery: a placebo-controlled study of ibuprofen. J Oral Maxillofac Surg. 56(4):420–427; discussion 427–429. [DOI] [PubMed] [Google Scholar]
- Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, Sansom C. 2004. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 ‘N of 1’ studies. Anaesthesia. 59(5):440–452. [DOI] [PubMed] [Google Scholar]
- Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. 2007. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain. 133(1-3):210–220. [DOI] [PubMed] [Google Scholar]
- Pau AK, Croucher R, Marcenes W. 2003. Prevalence estimates and associated factors for dental pain: a review. Oral Health Prev Dent. 1(3):209–220. [PubMed] [Google Scholar]
- Read JK, McClanahan SB, Khan AA, Lunos S, Bowles WR. 2014. Effect of ibuprofen on masking endodontic diagnosis. J Endod. 40(8):1058–1062. [DOI] [PubMed] [Google Scholar]
- Roberts RM, Bohm MK, Bartoces MG, Fleming-Dutra KE, Hicks LA, Chalmers NI. 2020. Antibiotic and opioid prescribing for dental-related conditions in emergency departments: United States, 2012 through 2014. J Am Dent Assoc. 151(3):174–181.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuelert N, McDougall JJ. 2011. The abnormal cannabidiol analogue o-1602 reduces nociception in a rat model of acute arthritis via the putative cannabinoid receptor GPR55. Neurosci Lett. 500(1):72–76. [DOI] [PubMed] [Google Scholar]
- Shannon S, Lewis N, Lee H, Hughes S. 2019. Cannabidiol in anxiety and sleep: a large case series. Perm J. 23:18-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz CN, Zheng C, Okunseri E, Szabo A, Okunseri C. 2017. Opioid analgesic prescribing practices of dental professionals in the united states. JDR Clin Trans Res. 2(3):241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggar T, Wu D, Khan AA. 2017. A randomized clinical trial comparing 2 ibuprofen formulations in patients with acute odontogenic pain. J Endod. 43(5):674–678. [DOI] [PubMed] [Google Scholar]
- Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. 2018. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 32(11):1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Donk T, Niesters M, Kowal MA, Olofsen E, Dahan A, van Velzen M. 2019. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain. 160(4):860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, Walker EA. 2014. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol. 171(3):636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DH, Cullen BD, Tang M, Fang Y. 2020. The effectiveness of topical cannabidiol oil in symptomatic relief of peripheral neuropathy of the lower extremities. Curr Pharm Biotechnol. 21(5):390–402. [DOI] [PubMed] [Google Scholar]
- Zuurman L, Roy C, Schoemaker RC, Hazekamp A, den Hartigh J, Bender JC, Verpoorte R, Pinquier JL, Cohen AF, van Gerven JM. 2008. Effect of intrapulmonary tetrahydrocannabinol administration in humans. J Psychopharmacol. 22(7):707–716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345231200814 for Cannabidiol as an Alternative Analgesic for Acute Dental Pain by V. Chrepa, S. Villasenor, A. Mauney, G. Kotsakis and L. Macpherson in Journal of Dental Research