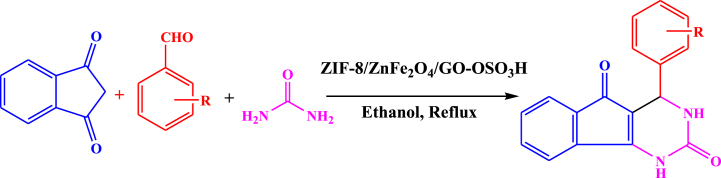
Abstract
In this report, we synthesized some pyrimidine derivatives by multi-component reaction of urea, benzaldehydes, and 1,3-indandione in the presence of ZIF-8/ZnFe2O4/GO-OSO3H nanocomposite under reflux conditions. Initially, graphene oxide was prepared from graphite, and then it was sulfonated using ClOSO3H. Next, GO-OSO3H nanosheets were used to support ZIF-8/ZnFe2O4 nanostructure. The construction of the synthesized structure was established using different spectral techniques such as X-ray crystallography (XRD), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX/Mapping), Fourier transform infrared (FTIR), thermal gravimetric analysis (TGA), vibrating sample magnetometer (VSM), and Brunauer-Emmett-Teller (BET). The present method provides various benefits including the efficiency of outcomes, easy separation of the catalyst, and excellent yield of the products within short reaction times. Moreover, the antibacterial activities of pyrimidine derivatives were investigated via the agar-well diffusion method on gram-negative (Escherichia coli) and gram-positive (Staphylococcus aureus) bacteria and the obtained results illustrated reasonable effects.
Keywords: Graphene oxide, ZIF-8/ZnFe2O4/GO-OSO3H, Multi-component reactions, Heterogeneous catalyst, Heterocycle, Metal-organic frameworks, Pyrimidine
1. Introduction
Heterocyclic compounds are widely distributed in nature and have biological and industrial importance. Today, many drugs contain heterocycles that are not extracted from natural sources, but synthesized in the laboratory [1]. Heterocyclic compounds are of interest to researchers due to their valuable biological actions, as well as antiviral [2], antibacterial [3], anti-inflammatory [4], antitumor [5], and antihypertensive [6]. Meanwhile, clinical properties such as antimicrobial [7], antihistamine, and antiasthmatic [8] have been described for the pyrimidine derivatives.
Metal-organic frameworks (MOFs) have captivated the consciousness of many investigators which are rapidly developing and are characterized by high porosity and abundant properties. MOFs are generally composed of metal clusters with open crystal lattices which are permanently assembled through strong cross-links [9].
Newly, utilizing MOFs as catalysts has become a new research field [10]. The diversity of MOF topologies (i.e. pores shapes and sizes) makes MOFs attractive for a broad range of applications. The most crucial characteristic of MOFs is their high surface area. Integration of metal clusters has led to significant recovery of MOFs in porosity and stability [11]. A wide variety of MOF's structures are engineered synergistically between metal nodes, practical linkage, enclosed layers, or nanoparticles for numerous and choosy heterogeneity interplays and activates in these MOF-based nanocatalysts. The most commonly used MOFs are HKUST-1, MIL-53, Fe-BTC, UiO-66, and ZIF-8. Consequently, these materials were broadly utilized in diverse fields including drug delivery, sensing, catalyst, etc. [12,13].
In recent years, MIL structures have been used as catalyst in the Groebke-Blackburn-Bienaymé reaction (GBB reaction) [14], the Hantzsch synthesis [15,16], and the Biginelli reaction [17]. Moreover, the application of UiO-66 MOFs has been studied in Gewald condensation [18], pyrimido [4,5-d] pyrimidine synthesis [19,20], spirooxindoles synthesis [21], and dihydro-2-oxopyrroles synthesis [22].
One of the crucial subgroups of MOFs is the zeolitic imidazolate framework (ZIF) in which maximum series contain Co or Zn as the center of metal and imidazole imitative as connectors. ZIF-8 is the greater reviewed in the ZIFs toolbox massed from 2-methylimidazolen and Zn2+ [23]. ZIF structures have also played catalytic role in organic transmutations, like Friedel-Crafts acylation [24], Knoevenagel condensation [25], the synthesis of quinazolines [26], and the reduction of acetylenes [27]. In addition, the use of ZIFs has been expanded in industrial applications such as water purification from heavy metals and organic dyes [28,29], as well as the selective filtration of gaseous pollutants [30].
Magnetic nanoparticles, especially iron oxide, can rapidly bulk up. Therefore, covering the surface and supporting it through porous polymers, graphene derivatives, supramolecules, or even via other metals (neutral or noble) and oxides are the maximum common ways to protect gathering [31]. Magnetic nanoparticles are an important substrate for linking inorganic and organic catalysts. This can lead to major advances in the growth of different nanocatalytic systems via immobilizing homogeneous catalysts on magnetic nanoparticles. Magnetic nanoparticles can be also considered attached to organometallic frameworks, which provide the superiority of enhanced surface area and growth feedback rate [32].
One of the oxidized derivatives of graphene is graphene oxide (GO) which has a wide range of oxygen-containing useful groups including hydroxyl, carboxyl, and epoxy groups. Mixing GO with MOFs can exchange the distance among GO layers to have a variety of applications in GO/MOF catalysis, which can take advantage of the advisable assets of both kinds of materials and at the same time boost their physical properties. These composites increase the surface stability of MOFs and lead to new applications in various fields [33]. Pd–ZIF-8/rGO which have been used to catalyze the Knoevenagel condensation and reduce the resulting imine product [34]. Moreover, GO/Fe3O4/UiO-66-NH2 was exploited in the optimal synthesis of chromene polycycles [35].
Recently, various nanostructures including rGO@Fe3O4@Ni [36], Fe3O4@GlcA@Ni-MOF [37], Cu-BTC@Fe3O4 [38], Fe3O4@P4VP@metal-organic framework [39], and MOF-5@SiO2@Fe3O4 [40] have been used as catalyst in organic reactions. Although these reported nanocomposites have efficient catalytic activity and enjoy high stability, high surface area, high yields, and easy long-term reusability, however, intrinsic functionalities of substrates can be exploited as easy anchoring sites for nanocomponents.
Gram-negative and Gram-positive bacteria are classified based on their cell wall structure and response to Gram staining. Gram-negative bacteria such as Escherichia coli have a thin layer of peptidoglycan in their cell wall that is surrounded by an outer membrane containing lipopolysaccharides, which makes them more resistant to some antibiotics. When stained with a warm stain, they appear pink or red under the microscope. On the other hand, Gram-positive bacteria such as Staphylococcus aureus have a thick layer of peptidoglycan in their cell wall, but they lack an outer membrane, which makes them more sensitive to antibiotics, and antibiotics easily penetrate the cell wall. When stained with warm stain, they appear purple or blue under the microscope [41,42].
The incorporation of the merits of MOFs and GO to construct a novel class of composites with both enhanced functionality and large surface area is of great significance and interest. Recently, nanotechnology with a wide diversity of nanomaterials has created a new revolution in science and especially in chemical fields [[43], [44], [45], [46], [47], [48]].
This study, considering the importance of heterogeneous catalysts based on graphene oxide, ZIF-8, and magnetic nanoparticles, is intended to produce ZIF-8/ZnFe2O4/GO-OSO3H nanocomposite as a robust and reusable catalyst for the production of pyrimidine derivatives (Scheme 1). In addition, the antibacterial activities of pyrimidine derivatives were investigated via agar-well diffusion method on gram-negative (Escherichia coli) and gram-positive (Staphylococcus aureus) bacteria that showed satisfactory consequences.
Scheme 1.
Synthesis of pyrimidines using ZIF-8/ZnFe2O4/GO-OSO3H as catalyst.
2. Experimental
2.1. Materials and analysis
The high-purity chemicals were bought from Sigma-Aldrich and Merck. The substances with the commercial reagent grades were utilized without further purification. The melting point was unmodified and defined in a capillary tube over a melting point microscope (Boetius). 1H NMR and 13C NMR spectra were attained on Bruker 250 MHz spectrometer with CDCl3 as a solvent and TMS as an internal standard. Recording FT-IR spectra was performed on Magna-IR, spectrometer 550. Powder XRD (X-ray diffraction) was performed on a Philips diffractometer (X'pert Co.) with Cu Kα mono chromatized radiation (λ = 1.5406 Å). The microscopic morphology of the products was observed through SEM (LEO, 1455VP). The energy dispersive analysis of X-ray was used to perform compositional analysis (EDX, Kevex, Delta Class 1). A Mettler Toledo TGA was considered to perform thermogravimetric analysis (TGA) under argon, and heating was performed to 825 °C from room temperature. A Belsorp mini automatic adsorption tool was used to measure nitrogen adsorption-desorption isotherms at 196 °C followed by degassing the specimens for 5 h at 150 °C. The sample weight was estimated at 10 mg in the TG test with heating at 10 °C per minute. To analyze the magnetometer, the vibrating sample (VSM) was examined using a device (MDKFD) at room temperature.
2.2. Synthesis of graphene
In this study, graphene was prepared using graphite, KMNO4, and H2SO4 via the modified Hummer's method [49].
2.3. Preparation of graphene oxide
GO was made via the modified Hummer's method, as follows: sodium nitrate (2.5 g) and graphene (5 g) were put in sulfuric acid (115 mL, 98%), and the solution was set down on a magnetic stirrer provided with a condenser put in an ice bath. In continuation, KMnO4 (15 g) was added steadily over 120 min. The reaction mixture was then put in a water bath (35 °C) and mixed for 30 min. Then, 230 mL of deionized water was slowly poured into the vessel, and the mixture was kept at 98 °C for 15 min. To terminate the reaction, 700 mL of deionized water and H2O2 (50 mL, 30%) were consequently poured into the solution. When the reaction was complete, the residue was cleaned with deionized water and HCl (5%) three times. The gained GO was then dried at 60 °C for 12 h [50].
2.4. Synthesis of sulfonated GO
GO (1 g) was distributed in chloroform for 1 h and then chlorosulfonic acid was poured into the dispersed solution. The solution was then refluxed for 4 h at 60 °C in a round-bottomed flask via a cold-water condenser. Finally, the cooled suspension was filtered and washed with excess ethanol to afford sulfonated graphene oxide (Step 1, Scheme 2) [51].
Scheme 2.
The preparation steps of ZIF-8/ZnFe2O4/GO-OSO3H nanocatalyst.
2.5. Preparation of ZnFe2O4 nanoparticles
Initially, FeCl3.6H2O (0.2 M) and ZnCl2.6H2O (0.1 M) were dissolved individually in 75 mL of distilled water. Next, NaOH (2 M) was poured dropwise into the FeCl3 solution till the pH of the solution reached 10. Then, the ZnCl2 solution was added to another mixture. Next, the temperature of the reaction was increased to 80 °C for 3 h to produce a brown residue. The earned residue was washed several times with distilled water and ethanol via centrifugation, and then kept at room temperature for 24 h. Finally, ZnFe2O4 nanoparticles were obtained in the furnace at 500 °C for 5 h (Step 2, Scheme 2) [52].
2.6. Preparation of ZIF-8
6.5 g of 2-methylimidazole was dispersed in 80 mL of methanol. Then, Zn(NO3)2.6H2O solution (0.25 M) was added to 2-methylimidazole solution under vigorous stirring at room temperature for 24 h. The obtained solid was then collected via centrifugation and washed with methanol for five times. Eventually, the achieved product dried at 75 °C under vacuum (Step 3, Scheme 2) [53].
2.7. Synthesis of ZIF-8/ZnFe2O4
A mixture of ZIF-8 (0.2 g) and ZnFe2O4 (0.1 g) was mixed in methanol (20 mL), and the mixture was placed in an autoclave at 100 °C for 24 h. The final precipitate was washed twice with DMF and methanol and then dried overnight at room temperature (Step 4, Scheme 2) [54].
2.8. Preparation of ZIF-8/ZnFe2O4/GO-OSO3H
Initially, sulfonated GO (0.5 g) and ZIF-8/ZnFe2O4 (1.1 g) were dispersed in 30 mL of DMF. Then, it was placed in an autoclave at a temperature of 120 °C for 12 h. Afterward, the obtained sediment was washed twice with DMF and methanol. Finally, the produced ZIF-8/ZnFe2O4/GO-OSO3H was dried at 40 °C for 24 h (Step 5, Scheme 2) [55].
The preparation steps of ZIF-8/ZnFe2O4/GO-OSO3H nanocatalyst are shown in Scheme 2.
2.9. The preparation method for the synthesis of pyrimidine derivatives
To synthesize pyrimidine derivatives, a mixture of 1,3-indanedione (0.5 mmol), urea (1.5 mmol), arylaldehyde (0.5 mmol), and ZIF-8/ZnFe2O4/GO-OSO3H (0.005 g) was stirred in ethanol as solvent (7 mL) for about 30 min. The reaction progress was monitored by TLC (n-hexane: ethyl acetate 5: 1). After completion of the reaction, the heterogeneous catalyst was separated via a magnet, and then the resultant precipitate was filtered and recrystallized from ethanol and dried for 12 h. The spectral data of new products are shown below.
4- Cyano -3,4-dihydro-1H-indeno [1,2-d] pyrimidine-2,5-dione (4i): C18H11N202:1H NMR (300 MHz, CDCl3) δ: 5.49 (s, 1H, –CH), 7.19 (s, 1H, NH), 8.44 ppm (s, 1H, NH),7.2–8.4 (m, 8H, HAr); 13C NMR (62.9 MHz, CDCl3) δ: 41.22, 115.28, 118.26, 123.66, 123.70, 131.83, 132.2, 132.90, 133.72, 135.88, 135.99, 136.71, 140.19, 142.55, 143.31, 188.49, 189.21; FT-IR (KBr):1589 (C C), 1693 (C O), 1724 (C O), 2225 (CN), 3437 (NH) cm−1.
2- Methoxy -3,4-dihydro-1H-indeno [1,2-d] pyrimidine-2,5-dione (4j): C18H14N203: 1H NMR (300 MHz, CDCl3) δ: 3.07 ppm (s, 3H, OCH3), 5.13 (s, 1H, CH), 7.74 (s,1H, NH), 8.44 (s, 1H, NH), 6.96–8.89 (m,8H, HAr); 13CNMR (62.9 MHz, CDCl3) δ: 38.89, 55.79, 105.02, 110.69, 120.42, 122.05, 123.22, 128.29, 133.99, 135.05, 135.71, 135.42, 140.11, 141.43, 142.42, 160.56, 189.33, 190.68; FT-IR (KBr): 1684 (C O), 1705 (C O), 3425 (NH) cm1.
2-Hydroxy, 4-Fluoro-3,4-dihydro-1H-indeno [1,2-d]pyrimidine-2,5-dione (4k): C17H11N202F: 1H NMR (300 MHz, CDCl3) δ: 5.75 ppm (s,1H, CH), 7.20–7.83 (m,7H, HAr), 9.00 (s, 1H, NH), 9.20 (1H, NH), 10.20 (1H,OH); 13CNMR (62.9 MHz, CDCl3) δ: 37.7, 38.3, 112.1, 114.9, 117.1, 126.0, 129.6, 129.7, 131.2, 135.0, 137.4, 138.9, 139.3, 151.5, 154.5, 186.0; FT-IR (KBr): 1590 (C O), 1701 (C O), 3170 (NH), 3407 (OH) cm−1.
2- Hydroxy, 4-Bromo-3,4-dihydro-1H-indeno [1,2-d]pyrimidine-2,5-dione (4l): C17H11N202Br: 1H NMR (300 MHz, CDCl3) δ: 5.70 ppm (s, 1H, CH), 9.00 (s, 1H, NH), 9.20 (1H, NH), 10.0 (s, 1H,OH), 7.20–7.83 (m,7H, HAr); 13CNMR (62.9 MHz, CDCl3) δ: 37.6, 37.7, 112.1115.5, 117.1, 126.0, 129.6, 129.7, 131.2, 131.8, 134.2, 137.4, 138.9, 140.01, 154.9, 156.8, 187.0; FT-IR (KBr): 1600 (C O), 1715 (C O), 2950 (NH), 3402 (OH) cm−1.
2.10. Evaluation of antibacterial activity
The antibacterial activity of the prepared products was evaluated using well diffusion method on Mueller-Hinton Agar (MHA). Inhibitory zones were reported in millimeters. S. aureus (ATCC 25923) and E. Coli (ATCC 25922) were used as references for the antibacterial assay of the products. In addition, gentamicin was used as a positive standard. Briefly, MHA plates were inoculated with a bacterial strain under aseptic conditions. All the compounds were prepared with a concentration of 512 μg/mL in the solvent and antibacterial tests of the compounds were performed according to CLSI standards [56], and incubated at 37 °C for 24 h. After the incubation period, the diameter of growth inhibition zones was measured.
3. Results and discussion
3.1. FT-IR spectroscopy
The structure of ZIF-8/ZnFe2O4/GO-OSO3H was considered via FT-IR spectroscopy (Fig. 1). As shown, the peak at 3428 cm−1 is due to the stretching vibration of OH groups in the structure [57]. The peak at 2923 cm−1 is due to the C–H stretch of imidazole groups [58], and at 2853 cm−1 stretching vibration of the C–H bond appears [59]. Moreover, the peak at 1632 cm−1 corresponds to the tensile vibration of the groups of carbonyl located at the edging of GO, while the adsorption band at 1484 cm−1 is due to the C N bond in the imidazole ring. In addition, the peaks at 1384 cm−1, 1156 cm−1 are related to the stretching vibration of C–O and S O bonds, respectively [60]. Moreover, the peaks at 757 cm−1, and 616 cm−1 are relevant to the Zn–N and Zn–O bonds [61], and the peak at 458 cm−1 is relevant to the Fe–O.
Fig. 1.
FT-IR spectrum of ZIF-8/ZnFe2O4/GO-OSO3H.
3.2. SEM analysis
Field emission scanning electron microscopy (SEM) is a useful technique for determining the size distribution of particles and porosity. The morphology and size of the ZIF-8/ZnFe2O4/GO-OSO3H particles were determined using SEM are shown in Fig. 2. The results indicate that the prepared nanostructure exhibits a uniform particle shape with a particle size ranging from 71 to 85 nm (Fig. 2a and b).
Fig. 2.
The SEM images of ZIF-8/ZnFe2O4/GO-OSO3H (Fig. 2a and b) with various magnifications.
3.3. BET analysis
Brunauer-Emmett-Teller (BET) analysis (Fig. 3a), adsorption/desorption (Fig. 3b), and BJH analysis (Fig. 3c) of the ZIF-8/ZnFe2O4/GO-OSO3H nanocomposite are illustrated in Fig. 3. The average diameter of the pores of the nanocatalyst is 1.21 nm, which shows the presence of porosity on the nanoscale. The analysis isotherm for ZIF-8/ZnFe2O4/GO-OSO3H is more consistent with the type I adsorption isotherm, which confirms the pore volume distribution in the fine range. Also, the pore volume of the cavities in the ZIF-8/ZnFe2O4/GO-OSO3H was calculated to be 1.8928 cm−3g−1, which was constant through the literature [62].
Fig. 3.
BET analysis (a), adsorption/desorption (b), and BJH analysis (c) of ZIF-8/ZnFe2O4/GO-OSO3H nanocomposite.
3.4. EDX analysis
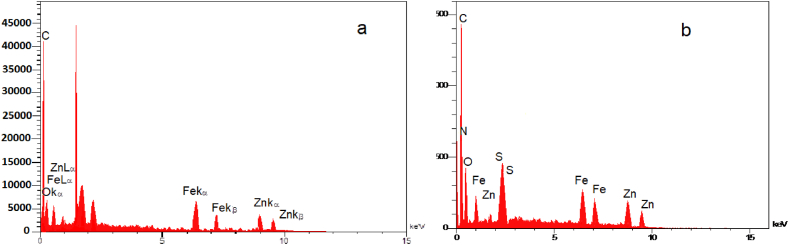
The chemical Purity of the samples experimented with utilizing energy-dispersive X-ray spectroscopy (EDX). Fig. 4a–b shows the EDX spectra of ZIF-8/ZnFe2O4 and ZIF-8/ZnFe2O4/GO-OSO3H, respectively. As illustrated, ZIF-8/ZnFe2O4 has only O, Zn, C, and Fe elements, and in Fig. 4b the elements including Fe, Zn, O, S, and C were observed for ZIF-8/ZnFe2O4/GO-OSO3H. In addition, EDX examination has been accomplished in an elemental mapping manner on ZIF-8/ZnFe2O4/GO-OSO3H nanocomposite (Fig. 5). The highly dispersed distribution of elements confirmed that there were no impurities in the produced nanocomposite. It was concluded that all of the elements including Zn (Fig. 5a), C (Fig. 5b), Fe (Fig. 5c), S (Fig. 5d), N (Fig. 5e), O (Fig. 5f), and all elements (Fig. 5g) were verified by EDX-mapping analysis. The obtained results exhibit superb purity and homogeneous dispersion. Fig. 5g illustrates the homogeneous arrangement of components all-round the structure.
Fig. 4.
EDX spectra of ZIF-8/ZnFe2O4 (a), and ZIF-8/ZnFe2O4/GO-OSO3H (b).
Fig. 5.
EDX-Mapping of ZIF-8/ZnFe2O4/GO-OSO3H; Zn (Fig. 5a), C (Fig. 5b), Fe (Fig. 5c), S (Fig. 5d), N (Fig. 5e), O (Fig. 5f), and all elements (Fig. 5g).
3.5. XRD analysis
The analysis of X-ray diffraction (XRD) for ZIF-8/ZnFe2O4/GO-OSO3H is shown in Fig. 6. Diffraction peaks at 33°, 35°, and 62° are related to the crystalline structure of ZnFe2O4 [63]. According to the literature, the peaks at 12.5°, 18° are correspond to ZIF-8, and the related peak of GO-OSO3H at 14° overlaps with very strong peaks related to Zn. Moreover, the presence of OSO3H groups prevents GO peaks from appearing well. It can also be assumed that GO-OSO3H nanosheets are grouped by ZnFe2O4 nanoparticles in the 20–30° section [64].
Fig. 6.
The XRD pattern of ZIF-8/ZnFe2O4/GO-OSO3H.
3.6. TGA analysis
Thermogravimetric analysis (TGA) shows that the ZIF-8/ZnFe2O4/GO-OSO3H sample has three weight loss regions at 100–800 °C, indicating the organometallic nature of the structure (Fig. 7). The first one at 0–100 °C is connected to the deprivation of solvents from the frame. Loss weight for the second stage is in the range of 200–500 °C, illustrating the decomposition of the structure of ZIF-8 because of the decomposition of the metal-organic framework [65]. Finally, the observed degradation at 500–800 °C can be attributed to the destruction of GO-OSO3H [[66], [67], [68], [69]].
Fig. 7.
TGA analysis of ZIF-8/ZnFe2O4/GO-OSO3H.
3.7. VSM analysis
Vibrating sample magnetometers (VSM) for ZIF-8/ZnFe2O4 and ZIF-8/ZnFe2O4/GO-OSO3H samples are shown in Fig. 8. As observed, the saturation magnetization of ZIF-8/ZnFe2O4 is about 0.07 emu/g (Fig. 8a), and the saturation magnetization of ZIF-8/ZnFe2O4/GO-OSO3H is 0.05 emu/g (Fig. 8b). It has been concluded that GO-OSO3H is non-magnetic, and when it is added, the magnetic property decreases.
Fig. 8.
VSM analysis of ZIF-8/ZnFe2O4 (a), ZIF-8/ZnFe2O4/GO –OSO3H (b).
3.8. Optimization of reaction conditions
In this research, by selecting the model reaction using 1,3-Indandione, 4 cyano-benzaldehyde, and urea, different conditions including solvents, temperatures, and catalysts were investigated. As shown in Table 1, different solvents such as H2O, EtOH, H2O/EtOH (1:1), DMF, CH3CN, and PhCH3 along with solvents-free conditions at different temperatures using ZIF-8/ZnFe2O4/GO-OSO3H were evaluated. As illustrated, the best results were obtained in the attendance of ethanol as solvent under reflux conditions (Table 1).
Table 1.
The model reaction optimization in different solvents and temperatures using ZIF-8/ZnFe2O4/GO-OSO3H nanocatalyst.
| Entry | solvents | Temp. (°C) | Time (min) | Yielda (%) |
|---|---|---|---|---|
| 1 | – | 25 | 240 | 50 |
| 2 | – | 70 | 180 | 60 |
| 3 | – | 80 | 180 | 60 |
| 4 | – | 100 | 180 | 70 |
| 5 | H2O | 70 | 180 | 65 |
| 6 | CH3CN | Reflux | 160 | 60 |
| 7 | H2O | Reflux | 180 | 60 |
| 8 | EtOH | Reflux | 45 | 98 |
| 9 | DMF | Reflux | 60 | 90 |
| 10 | PhCH3 | Reflux | 360 | 45 |
Isolated Yield.
After optimizing the reaction conditions, to compare our prepared catalyst with other catalysts, the model reaction was conducted by diverse catalysts that had acidic or basic properties. The results are shown in Fig. 9. In the investigation of different catalysts, 0.01 g of each catalyst was used, and the accomplishment of the reaction was checked via TLC. The best performance corresponded to the ZIF-8/ZnFe2O4/GO-OSO3H catalyst, which has several acidic sites in its structure.
Fig. 9.
The effect of various catalysts and the absence of a catalyst on the model reaction.
To evaluate the effectiveness of the catalyst, a comparison was made in the model reaction in the presence or the absence of the ZIF-8/ZnFe2O4/GO-OSO3H catalyst with different amounts, including 0–0.007 g. As illustrated, the proper amount of the catalyst was 0.005 g with an efficiency of 98% yield in 45 min (Fig. 10).
Fig. 10.
The optimization of the catalyst amount for the synthesis of the corresponding pyrimidine.
In the continuation of our study, we decided to examine diverse benzaldehydes to evaluate the catalytic activity of ZIF-8/ZnFe2O4/GO-OSO3H in the synthesis of pyrimidine derivatives. As expected, aryl aldehydes with electron-withdrawing groups give rise to higher yields and shorter reaction times than aldehydes with electron-donating groups (Table 2). The advantages of this research are include high efficiency, mild reaction conditions, the catalyst's reusability, and a short duration.
Table 2.
Synthesis of pyrimidine derivatives with benzaldehyde derivatives, using ZIF-8/ZnFe2O4/GO-OSO3H as a catalyst
*Compounds 4i, 4j, 4k, and 4l are new products.
3.9. Reusing and recycling the catalyst
The recyclability of the ZIF-8/ZnFe2O4/GO-OSO3H catalyst was investigated under the optimal conditions of the model study. As shown in Fig. 11, due to the heterogeneity and magnetic properties of the catalyst, it was simply divided from the crude via an external magnet and used for six cycles.
Fig. 11.
The reusability of ZIF-8/ZnFe2O4/GO-OSO3H catalyst.
3.10. The proposed reaction mechanism
The proposed mechanism, based on the present study and previous literature [70], for the synthesis of pyrimidine derivatives catalyzed by ZIF-8/ZnFe2O4/GO-OSO3H is shown in Scheme 3. It is presumed that ZnFe2O4 and ZIF-8 act as Lewis acids which increase the electrophilicity of the carbonyl groups of the aldehyde and 1,3-indanedione through a strong coordination bond [71,72]. The reaction proceeds via condensation of 1,3-indanedione 1 via aryl aldehyde 2 to yield intermediate I. Dehydration of the intermediate I, followed by the Michael addition of urea, led to the formation of intermediate II. In the end, the intermediate III goes through a process called intramolecular cycloaddition to make product 4.
Scheme 3.
Proposed mechanism for the synthesis of Pyrimidine derivatives using ZIF-8/ZnFe2O4/GO-OSO3H.
3.11. Results of antimicrobial activities
The inhibition zone diameter was evaluated via evaluation of antimicrobial activities (Fig. 12). Antibacterial test results were included for compounds (4a-4j) by Staphylococcus aureus bacterium (Fig. 12a), and also for compounds (4a-4j) using Escherichia coli bacterium (Fig. 12b). The diameter of the inhibition zone for the synthesized pyrimidines against Staphylococcus aureus and Escherichia coli bacteria is depicted in Table 3.
Fig. 12.
The antibacterial test results including Staphylococcus aureus (a), and Escherichia coli (b) bacteria.
Table 3.
The antibacterial activities of pyrimidine derivatives.
| Product |
S. aureus (mm) (ATTCC-25923) |
E. coli (mm) (ATTCC-25922) |
|---|---|---|
| 4a | - | - |
| 4b | 36 | – |
| 4c | – | – |
| 4d | - | 24 |
| 4e | 40 | - |
| 4f | 13 | - |
| 4 g | 13 | 23 |
| 4h | – | – |
| 4i | 15 | 25 |
| 4j | 15 | - |
4. Conclusion
In this study, a reasonable synthesis method for the preparation of pyrimidine compounds using ZIF-8/ZnFe2O4/GO-OSO3H as an efficient catalyst is reported. The acidic nature of the catalyst makes it more efficient and less time-consuming, with excellent yields in comparison with other catalysts. The properties of the produced heterogeneous catalyst were examined via different methods, including EDS, SEM, BET, TGA, VSM, XRD, and FT-IR analysis. The catalyst was simply retrieved and reused for six cycles without any notable loss of its catalytic activity. The antibacterial attributes of pyrimidines were considered via the agar-well diffusion technique on gram-negative (E.mcoli) and gram-positive (S. aureus) bacteria which demonstrated appropriate results. Utilization of this catalyst is suggested as a green and reasonable method for the synthesis of pyrimidine derivatives under green reaction conditions.
CRediT authorship contribution statement
Maryam Mahdavi: Methodology, Investigation, Data curation. Mohammad Ali Ghasemzadeh: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Funding acquisition, Formal analysis, Conceptualization. Ali Javadi: Visualization, Supervision, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The Financial Support was provided by the Research Affairs Office of the Islamic Azad University, Qom Branch, Qom, I. R. Iran [grant number 2019-2898].
References
- 1.Czaja A.U., Trukhan N., Müller U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009;38:1284–1293. doi: 10.1039/b804680h. [DOI] [PubMed] [Google Scholar]
- 2.Kappe C.O. Highly versatile Solid phase synthesis of biofunctional 4-aryl-3,4-dihydropyrimidines using resin-bound isothiourea building blocks and multidirectional resin cleavage. Bioorg. Med. Chem. Lett. 2000;10:49–51. doi: 10.1016/s0960-894x(99)00572-7. [DOI] [PubMed] [Google Scholar]
- 3.Patil A.D., V Kumar N., C Kokke W., Bean M.F., Freyer A.J., D Brosse C., Carte B. Novel alkaloids from the sponge batzella sp.: inhibitors of HIV gp120-human CD4 binding. J. Org. Chem. 1995;60:1182–1188. [Google Scholar]
- 4.Palaska E., Şahin G., Kelicen P., Durlu N.T., Altinok G. Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides. 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. Il Farmaco. 2002:101–107. doi: 10.1016/s0014-827x(01)01176-4. [DOI] [PubMed] [Google Scholar]
- 5.Grover Gary J., Dzwonczyk Steven, McMullen Diane M., Normandin Diane E., Parham Charles S., Sleph Paul G., Moreland Suzanne. Pharmacologic profile of the dihydropyrimidine calcium channel blockers SQ 32,547 and SQ 32,946. J. Cardiovasc. Pharmacol. 1995;26:289–294. doi: 10.1097/00005344-199508000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Oliver Kappe C. 100 years of the biginelli dihydropyrimidine synthesis. Tetrahedron. 1993;49:6937–6963. [Google Scholar]
- 7.Ghasemzadeh M.A., Safaei-Ghomi J. An efficient, one-pot synthesis of polyfunctionalised dihydropyridines catalysed by AgI nanoparticles. J. Chem. Res. 2014;38:313–316. [Google Scholar]
- 8.Marinescu M. Biginelli reaction mediated synthesis of antimicrobial pyrimidine derivatives and their therapeutic properties. Molecules. 2021;26:6022. doi: 10.3390/molecules26196022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim K.C. Design strategies for metal-organic frameworks selectively capturing harmful gases. J. Organomet. Chem. 2018;854:94–105. [Google Scholar]
- 10.Jiao L., Wang Y., Jiang H.L., Xu Q. Metal-organic frameworks as platforms for catalytic appliations. Adv. Mater. 2017;30:37. doi: 10.1002/adma.201703663. [DOI] [PubMed] [Google Scholar]
- 11.Denny M.S., Moreton J.C., Benz L., Cohen S.M. Metal–organic frameworks for membrane-based separations. Nat. Rev. Mater. 2016;1:12. [Google Scholar]
- 12.Howarth A.J., Peters A.W., Vermeulen N.A., C Wang T., Hupp J.T., Farha O.K. “Best practices for the synthesis, activation, and characterization of metal–organic frameworks. Chem. Mater. 2016;29:26–39. [Google Scholar]
- 13.Bibi S., Pervaiz E., Ali M. Synthesis and applications of metal oxide derivatives of ZIF-67: a mini-review. Chem. Pap. 2021;75:2253–2275. [Google Scholar]
- 14.Shaabani A., Sepahvand H., Amini M.M., Hashemzadeh A., Borjian Boroujeni M., Badali E. Tandem oxidative isocyanide-based cycloaddition reactions in the presence of MIL-101(Cr) as a reusable solid catalyst. Tetrahedron. 2018;74:1832–1837. [Google Scholar]
- 15.Rostamnia S., Alamgholiloo H., Jafari M. Ethylene diamine post‐synthesis modification on open metal site Cr‐MOF to access efficient bifunctional catalyst for the Hantzsch condensation reaction. Appl. Organomet. Chem. 2018;32:4370–4379. [Google Scholar]
- 16.Babaee S., Zarei M., Sepehrmansourie H., Zolfigol M.A., Rostamnia S. Synthesis of metal–organic frameworks MIL-101(Cr)-NH2 containing phosphorous acid functional groups: application for the synthesis of N-Amino-2-pyridone and pyrano [2,3-c]pyrazole derivatives via a cooperative vinylogous anomeric-based oxidation. ACS Omega. 2020;5:6240–6249. doi: 10.1021/acsomega.9b02133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sepehrmansouri H., Zarei M., Zolfigol M.A., Moosavi-Zare A.R., Rostamnia S., Moradi S. Multilinker phosphorous acid anchored En/MIL-100(Cr) as a novel nanoporous catalyst for the synthesis of new N-heterocyclic pyrimido[4,5-b]quinolines. Mol. Catal. 2020;481:110303–110320. [Google Scholar]
- 18.Erfaninia N., Tayebee R., Dusek M., Amini M.M. Ethylene diamine grafted nanoporous UiO‐66 as an efficient basic catalyst in the multi‐component synthesis of 2‐aminithiophenes. Appl. Organomet. Chem. 2018;32:4307–4317. [Google Scholar]
- 19.Ghorbani-Vaghei R., Davood Azarifar D.A., Daliran S., Oveisi A.R. The UiO-66-OOSO3H metal–organic framework as a green catalyst for the facile synthesis of dihydro-2-oxypyrrole derivatives. RSC Adv. 2016;6:29182–29189. [Google Scholar]
- 20.Mahmoudi Z., Ghasemzadeh M.A., Kabiri-Fard H. Fabrication of UiO-66 nanocages confined brønsted ionic liquids as an efficient catalyst for the synthesis of dihydropyrazolo[4′,3’:5,6]pyrano[2,3-d]pyrimidines. J. Mol. Struct. 2019;1194:1–10. [Google Scholar]
- 21.MirhosseiniEshkevari B., Ghasemzadeh M.A., Esnaashari M. Highly efficient and green approach for the synthesis of spirooxindole derivatives in the presence of novel Brønsted acidic ionic liquids incorporated in UiO‐66 nanocages. Appl. Organomet. Chem. 2019;33:5027–5040. [Google Scholar]
- 22.Shaabani A., Mohammadian R., Hooshmand S.E., Hashemzadeh A., Amini M.M. Zirconium metal-organic framework (UiO-66) as a robust catalyst toward OSOlvent-free synthesis of remarkable heterocyclic rings. ChemistrySelect. 2017;2:11906–11911. [Google Scholar]
- 23.Wang Q., Astruc D. State of the art and prospects in metal–organic framework (MOF)-Based and MOF-derived nanocatalysis. Chem. Rev. 2019;120:1438–1511. doi: 10.1021/acs.chemrev.9b00223. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen Ltl, Le kka, Phan Nts. A zeolite imidazolate framework ZIF-8 catalyst for friedel-crafts acylation. Chin. J. Catal. 2012;33:688–696. [Google Scholar]
- 25.TranK U.P., Le K., Phan N.T. Expanding applications of metal− organic frameworks: zeolite imidazolate framework ZIF-8 as an efficient heterogeneous catalyst for the knoevenagel reaction. ACS Catal. 2011;1:120–127. [Google Scholar]
- 26.Truong T., Hoang T.M., Nguyen C.K., Huynh Q.T., Phan N.T. Expanding applications of zeolite imidazolate frameworks in catalysis: synthesis of quinazolines using ZIF-67 as an efficient heterogeneous catalyst. RSC Adv. 2015;5:24769–24776. [Google Scholar]
- 27.Hu M., Zhao S., Liu S., Chen C., Chen W., Zhu W., Li Y. MOF‐Confined sub‐2 nm atomically ordered intermetallic PdZn nanoparticles as high‐performance catalysts for selective hydrogenation of acetylene. Adv. Mater. 2018;30 doi: 10.1002/adma.201801878. [DOI] [PubMed] [Google Scholar]
- 28.Omer A.M., Abd El-Monaem E.M., Abd El-Latif M.M., El-Subruiti G.M., Eltaweil A.S. Facile fabrication of novel magnetic ZIF-67 MOF@aminated chitosan composite beads for the adsorptive removal of Cr(VI) from aqueous solutions. Carbohydr. Polym. 2021;265 doi: 10.1016/j.carbpol.2021.118084. [DOI] [PubMed] [Google Scholar]
- 29.Qiao Y., He N., ZhangX X., Zhao, Zhao X., Li W., Li C. In situ growth of MOF crystals to synthesize a graphene oxide/ZIF-7 gel with enhanced adsorption capacity for methylene blue. New J. Chem. 2022;46:14103–14111. [Google Scholar]
- 30.Valencia L., Abdelhamid H.N. Nanocellulose leaf-like zeolitic imidazolate framework (ZIF-L) foams for selective capture of carbon dioxide. Carbohydr. Polym. 2019;213:338–345. doi: 10.1016/j.carbpol.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Ghasemzadeh M.A. Synthesis and characterization of Fe3O4@SiO2 NPs as an effective catalyst for the synthesis of tetrahydrobenzo [a] xanthen-11-ones. Acta Chim. Slov. 2015;62:977–985. doi: 10.17344/acsi.2015.1501. [DOI] [PubMed] [Google Scholar]
- 32.Ghasemzadeh M.A., Mirhosseini-Eshkevari B., Abdollahi-Basir M.H. Rapid and efficient one-pot synthesis of 3,4-dihydroquinoxalin-2-amine derivatives catalyzed by Co3O4@SiO2 core-shell nanoparticles under ultrasound irradiation. Comb. Chem. High Throughput Screen. 2016;19:592–601. doi: 10.2174/1386207319666160524141831. [DOI] [PubMed] [Google Scholar]
- 33.Govan J., Gun’ko Y. Recent advances in the application of magnetic nanoparticles as a support for homogeneous catalysts. Nanomaterials. 2014;4:222–241. doi: 10.3390/nano4020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H., Wang Y., Jia A., Wang C., Wu L., Yang Y., Wang Y. A novel bifunctional Pd–ZIF-8/rGO catalyst with spatially separated active sites for the tandem Knoevenagel condensation–reduction reaction. Catal. Sci. Technol. 2017;7:5572–5584. [Google Scholar]
- 35.Abdollahi-Basir M.H., Shirini F., Tajik H., Ghasemzadeh M.A. One-pot synthesis of chromenes in the presence of magnetic nanocomposite based on NH2-UiO-66 (Zr), graphene oxide and Fe3O4. J. Mol. Struct. 2022;1263 [Google Scholar]
- 36.Esmati M., Zeynizadeh B. Synthesis of GO and rGO@Fe3O4@Ni as remarkable nanocatalyst systems for solvent-free and chemoselective coupling reactions of dimedone with aromatic aldehydes. Appl. Organomet. Chem. 2021;35:e6321–e6338. [Google Scholar]
- 37.Ghorbani-Choghamarani A., Taherinia Z., Mohammadi M. Facile synthesis of Fe3O4@GlcA@Ni-MOF composites as environmentally green catalyst in organic reactions. Environ. Technol. Innov. 2021;24:102050–102061. [Google Scholar]
- 38.Wang L., Yang S., Chen L., Yuan S., Chen Q., He M.Y., Zhang Z.H. Magnetically recyclable Cu-BTC@Fe3O4 composite-catalyzed C(aryl)-S-P bond formation using aniline, P(O)H compounds and sulfur powder. Catal. Sci. Technol. 2017;7:2356–2361. [Google Scholar]
- 39.Miao Z., Shu X., Ramella D. Synthesis of a Fe3O4@P4VP@metal-organic framework core-shell structure and studies of its aerobic oxidation reactivity. RSC Adv. 2017;(7):2773–2779. doi: 10.1039/d4ra90003k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q., Jiang S., Ji S., Shi D., Li H. Synthesis of magnetically recyclable MOF-5@SiO2@Fe3O4 catalysts and their catalytic performance of Friedel-Crafts alkylation. J. Porous Mater. 2015;(22):1205–1214. [Google Scholar]
- 41.Flatamico P., Smith J. In: Food Infection and Intoxication. Riemann H., Cliver D., editors. Elsevier Inc.; 2006. Escherichia coli infections; pp. 205–239. [Google Scholar]
- 42.Schmitt M., Schuler-Schmid U., Scmidt-Lorenz W. Temperature limits of growth, TNase and enterotoxin production of Staphylococcus aureus strains isolated from foods. Int. J. Food Microbiol. 1990;(11):1–19. doi: 10.1016/0168-1605(90)90036-5. [DOI] [PubMed] [Google Scholar]
- 43.Rostamnia S., Doustkhah E., Zeynizadeh B. Exfoliation effect of PEG-type surfactant on Pd supported GO (SE-Pd(nanoparticle)/GO) in cascade synthesis of amides: a comparison with Pd(nanoparticle)/rGO. J. Mol. Catal. Chem. 2016;416:88–95. [Google Scholar]
- 44.Rostamnia S., Zeynizadeh B., Doustkhah E., Golchin Hosseini H. Exfoliated Pd decorated graphene oxide nanosheets (PdNP-GO/P123): non-toxic, ligandless and recyclable in greener Hiyama cross-coupling reaction. J. Colloid Interface Sci. 2015;451:46–52. doi: 10.1016/j.jcis.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 45.Moradi L., Sadeghi H. Efficient pathway for the synthesis of amido alkyl derivatives using KCC-1/PMA immobilized on magnetic MnO2 nanowires as recyclable solid acid catalyst. J. Mol. Struct. 2023;1274:134477–134478. [Google Scholar]
- 46.Karimi-Maleh H., Liu Y., Li Z., Darabi R., Orooji Y., Karaman C., Karimi F., Baghayeri M., Rouhi J., Fu L., Rostamnia S., Rajendran S., Sanati A.L., Sadeghifar H., Ghalkhani M. Calf thymus ds-DNA intercalation with pendimethalin herbicide at the surface of ZIF-8/Co/rGO/C3N4/ds-DNA/SPCE; A bio-sensing approach for pendimethalin quantification confirmed by molecular docking study. Chemosphere. 2023;332:138815–138825. doi: 10.1016/j.chemosphere.2023.138815. [DOI] [PubMed] [Google Scholar]
- 47.Sadeghi S., Neamani H., Moradi L. Immobilization of CdCl2on filamentous silica nanoparticles as an efficient catalyst for the solvent free synthesis of some amidoalkyl derivatives. Polycycl. Aromat. Comp. 2022;43:1957–1973. [Google Scholar]
- 48.Rostamnia S., Lamei K. Diketene-based neat four-component synthesis of the dihydropyrimidinones and dihydropyridine backbones using silica sulfuric acid (SSA) Chin. Chem. Lett. 2012;23:930–932. [Google Scholar]
- 49.Upare P.P., Hong D.-Y., Kwak J., Lee M., Chitale S.K., Chang J.-S., Hwang D.W., K Hwang Y. Direct chemical conversion of xylan into furfural over sulfonated graphene oxide. Catal. Today. 2019;324:66–72. [Google Scholar]
- 50.Hummers W.S., Jr., Offeman R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958;80 1339-1339. [Google Scholar]
- 51.Sanaei-Rad S., Ghasemzadeh M.A., Aghaei S. Synthesis and structure elucidation of ZnFe2O4/IRMOF-3/GO for the drug delivery of tetracycline and evaluation of their antibacterial activities. J. Organomet. Chem. 2022;960:122–221. [Google Scholar]
- 52.Peizhi Guo P., Cui Lijun, Wang Yiqian, Lv Meng, Wang Baoyan, Zhao X.S. Facile synthesis of ZnFe2O4 nanoparticles with tunable magnetic and sensing properties. Langmuir. 2013;29:899–9003. doi: 10.1021/la401627x. [DOI] [PubMed] [Google Scholar]
- 53.Vinosha P.A., Mely L.A., Jeronsia J.E., Krishnan S., Das S.J. Synthesis and properties of spinel ZnFe2O4 nanoparticles by facile co-precipitation route. Optik. 2017;134:99–108. [Google Scholar]
- 54.Shi J., Zhang L., Sun N., Hu D., Shen Q., Mao F., Gao Q., Wei W. Facile and rapid preparation of Ag@ZIF-8 for carboxylation of terminal alkynes with CO2 in mild conditions. ACS Appl. Mater. Interfaces. 2019;11:28858–28867. doi: 10.1021/acsami.9b07991. [DOI] [PubMed] [Google Scholar]
- 55.Adhikari C., Das A., ChakrabortyZeolitic A. Imidazole framework (ZIF) nanospheres for easy encapsulation and controlled release of an anticancer drug doxorubicin under different external stimuli: a way toward smart drug delivery system. Mol. Pharm. 2015;12:3158–3166. doi: 10.1021/acs.molpharmaceut.5b00043. [DOI] [PubMed] [Google Scholar]
- 56.El-Bindary A.A., Toson E.A., Shoueir K.R., Aljohani H.A., Abo-Ser M.M. Metal-organic frameworks as efficient materials for drug delivery: synthesis, characterization, antioxidant, anticancer, antibacterial and molecular docking investigation. Appl. Organomet. Chem. 2020;34:e5905–e5919. [Google Scholar]
- 57.Hasanzadeh M., Shadjou N. (Fe3O4)-Graphene oxide-OSO3H as a new magnetic nanocatalyst for electro-oxidation and determination of selected parabens. J. Nanosci. Nanotechnol. 2013;13:4909–4916. doi: 10.1166/jnn.2013.7605. [DOI] [PubMed] [Google Scholar]
- 58.Wei Y., Hao Z., Zhang F., Li H. A functionalized graphene oxide and nano-zeolitic imidazolate framework composite as a highly active and reusable catalyst for [3 + 3] formal cycloaddition reactions. J. Mater. Chem. 2015;3:14779–14785. [Google Scholar]
- 59.Huang D., Xin Q., Ni Y., Shuai Y., Wang S., Li Y., Ye H., Lin X L., Ding, Zhang Y. Synergistic effects of zeolite imidazole framework@graphene oxide composites in humidified mixed matrix membranes on CO2 separation. RSC Adv. 2018;8:6099–6109. doi: 10.1039/c7ra09794h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanaei-Rad S., Ghasemzadeh M.A., Razavian S.M.H. Synthesis of a novel ternary ZIF-8/GO/MgFe2O4 nanocomposite and its application in drug delivery. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-98133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abbasian A.R., Shafiee Afarani M. One-step solution combustion synthesis and characterization of ZnFe2O4 and ZnFe1.6O4 nanoparticles. Appl. Phys. 2019;125:721. [Google Scholar]
- 62.Shuai C., Xu Y., Feng P., Wang G., Xiong S., Peng S. Antibacterial polymer scaffold based on meOSOporous bioactive glass loaded with in situ grown silver. Chem. Eng. J. 2019;374:304–315. [Google Scholar]
- 63.Dreyer D.R., Park S., Bielawski C.W., Ruoff R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010;39:228–240. doi: 10.1039/b917103g. [DOI] [PubMed] [Google Scholar]
- 64.Doustkhah E., Rostamnia S. Covalently bonded sulfonic acid magnetic graphene oxide: Fe3O4@GO-Pr-OSO3H as a powerful hybrid catalyst for synthesis of indazolophthalazinetriones. J. Colloid Interface Sci. 2016;478:280–287. doi: 10.1016/j.jcis.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 65.Su H., Li Z., Huo Q., Guan J., Kan Q. Immobilization of transition metal (Fe2+, Co2+, VO2+ or Cu2+) Schiff base complexes onto graphene oxide as efficient and recyclable catalysts for epoxidation of styrene. RSC Adv. 2014;4:9990–9996. [Google Scholar]
- 66.Uregen N., Pehlivanoğlu K., Ozdemir Y., Devrim Y. Development of polybenzimidazole/graphene oxide composite membranes for high-temperature PEM fuel cells. Int. J. Hydrogen Energy. 2018;42:2636–2647. [Google Scholar]
- 67.Esmaeili N., Mohammadi P., Abbaszadeh M., Sheibani H. Au nanoparticles decorated on magnetic nanocomposite (GO-Fe3O4/Dop/Au) as a recoverable catalyst for degradation of methylene blue and methyl orange in water. Int. J. Hydrogen Energy. 2019;44:23002–23009. [Google Scholar]
- 68.Warekar P.P., Kolekar G.B., Deshmukh M.B., Anbhule P.V. An efficient and modified biginelli-type synthesis of 3,4-dihydro-1H-indeno[1,2-d]pyrimidine-2,5-dione using phosphorous pentoxide. Synth. Commun. 2014;44:3594–3601. [Google Scholar]
- 69.Aswin K., Mansoor S.S., Logaiya K., Sudhan P.N., Ahmed R.N. Facile synthesis of 3,4-dihydropyrimidin-2(1H)-ones and -thiones and indeno[1,2-d]pyrimidines catalyzed byp-dodecylbenzenesulfonic acid. J. Taibah Univ. Sci. 2014;8:236–247. [Google Scholar]
- 70.Dabholkar V.V., Patil S.R., Pandey R.V. Design, synthesis, characterization, and antimicrobial activity of biginelli products of indandione. J. Heterocycl. Chem. 2012;49:929–932. [Google Scholar]
- 71.Park J., An K., Hwang Y., Park J.G., Noh H.J., J Y., Kim J.H., Park N.M., Hwang T. Hyeon. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004;3:891–895. doi: 10.1038/nmat1251. [DOI] [PubMed] [Google Scholar]
- 72.Aguirre-Díaz M., Gándara L., Iglesias F., Snejko M., Gutiérrez-Puebla N., Ángeles Monge E. Tunable catalytic activity of solid solution metal-organic frameworks in one-pot multicomponent reactions. J. Am. Chem. Soc. 2015;137 doi: 10.1021/jacs.5b02313. 6132-1635. [DOI] [PubMed] [Google Scholar]