Abstract
Objective
To further evaluate the connection between obesity and Parkinson's disease, we utilized A body shape index which normalizes waist circumference for Body mass index. Derived from the National Health and Nutrition Examination Survey.
Methods
Based on National Health and Nutrition Examination Survey data from 2005 to 2018, this study included 31,933 adult participants in total. First, all the participants were divided into the Parkinson's disease group and non-Parkinson's disease group, respectively. Next, according to their quartiles of A body shape index levels, they were further classified into Q1 group (0.058–0.077), Q2 group (0.078–0.081), Q3 group (0.082–0.084), and Q4 group (0.085–0.117). A body shape index was the primary exposure, while Parkinson's disease was the primary outcome. A body shape index is defined by waist circumference divided by Body mass index2/3 × height1/2, and the expected value of waist circumference based on height and weight derived empirically from National Health and Nutrition Examination Survey. Consequently, A body shape index and Parkinson's disease were analyzed through multifactor logistic regression.
Results
According to the unadjusted multivariate logistic analysis, the Q4 group had a greater likelihood of acquiring Parkinson's disease than the Q1 group [OR = 4.519, 95% CI: 3.094–6.600; P < 0.001]. After adjusting the demographic variables such as age, sex, and race, Q4 group was at a higher risk of Parkinson's disease acquisition than Q1 [OR (95% CI): 2.677 (1.774–4.038); P < 0.001]. Compared with Q1 group, the male participants were in a greater chance of getting Parkinson's disease than female participants in Q4 group, as shown by subgroup analysis by gender [male vs. female: OR = 6.563 (3.289–13.098) vs. OR = 3.827 (2.398–6.108); Interaction P-value<0.001].
Conclusions
There is a non-linear positive correlation between the adult A body shape index and the risk of Parkinson's disease. Adults are at a greater risk of getting Parkinson's disease as A body shape index rises, and the link is particularly strong among men aged 20 to 59.
Keywords: A body shape index, Parkinson's disease, Cross-sectional study, National health and nutrition examination survey
Highlights
-
•
ABSI positively associated with PD risk, especially in men and adults aged <60 years.
-
•
PD prevalence higher among adults with highest vs lowest ABSI quartile (4.519 odds).
-
•
Every 0.01 rise in ABSI tied to ∼2-fold greater PD risk after adjusting for confounders.
-
•
Non-linear dose-response: PD risk rises with increasing ABSI over 0.080 threshold.
-
•
High ABSI indicative of larger waist size and excess visceral fat which may impact PD risk.
1. Introduction
Parkinson's disease (PD) is the second most prevalent neurologic disease in the world, after Alzheimer's disease (AD), with up to 160 per 100,000 people over the age of 65 suffering from PD [1]. In addition to genetic factors, there are also some risk factors in our lifestyles that can increase the risk of developing PD, including decline in exercise performance [1].
The World Health Organization (WHO) estimates that more than 1 billion adults worldwide are overweight and 300 million are obese [2]. Obesity and weight problems contribute significantly to the development of a variety of disorders, including hypertension [3], cerebrovascular accidents [4], diabetes [5], cardiovascular disease [6], AD [7], and PD [8]. Interestingly, obesity has been linked to a number of diseases, and prior epidemiological research has demonstrated a complex and conflicting relationship between obesity and PD [[8], [9], [10]]. In several studies, polyunsaturated fatty acids were found to reduce PD risk, while saturated fatty acids increased it [11]. In a large prospective study, dietary fat intake was not associated with PD risk, except for a slight positive association between n-6 polyunsaturated fatty acids and linoleic acid [12]. Also, a cohort study found that obesity and lack of exercise may be associated with functional dependency as well as rapid progression during the early stages of PD [9].
Body mass index (BMI) is a measurement of weight corrected for height and is the most common indicator for assessing overweight and obesity [2]. BMI is defined as weight/height2 and as such does not directly measure body fat. Minimal correlation with height has been documented worldwide across genders, age span and ethnicity [13]. Statistics on obesity are mostly based on BMI, normal range from 18.5 to 24.9 overweight from 25 to 29.9, and 30+ obese. Interestingly in National Health and Nutrition Examination Survey (NHANES) the nadir BMI for 20-year mortality was 28 [13]. In addition, the correlation between BMI and the risk of various diseases is not linear [8]. Jeong et al. also reported that even after adjusting for fat distribution and body composition, as well as for smoking, alcohol, hypertension and dyslipidemia, those with a low BMI were more likely to acquire PD than those with normal or high BMI [14]. A Mendelian randomization study found a protective effect of BMI on PD incidence, which however was not statistically significant [15]. It is however, widely recognized that BMI alone may not adequately capture risk in many circumstances. A body shape index (ABSI) is defined by waist circumference (WC) divided by BMI2/3 × height1/2, which an approximation of the expected value of WC based on height and weight derived empirically from NHANES [16,17]. It is analogous to BMI which is weight divided by height2, an approximation of the expected value of weight based on height as derived by Quetelet in the 1800's. A body shape index (ABSI) standardizes WC by weight and height, similar to the standardization of body weight to height by BMI [18]. ABSI has been verified to reasonably reflect visceral fat [19]. It is currently believed that there is an association between PD and visceral obesity [8,9]. There are no large studies of a possible association between ABSI and PD.
Therefore, we identified subjects with PD and anthropometric measurements in NHANES a large population data base with free public access.
2. Methods
2.1. Participants
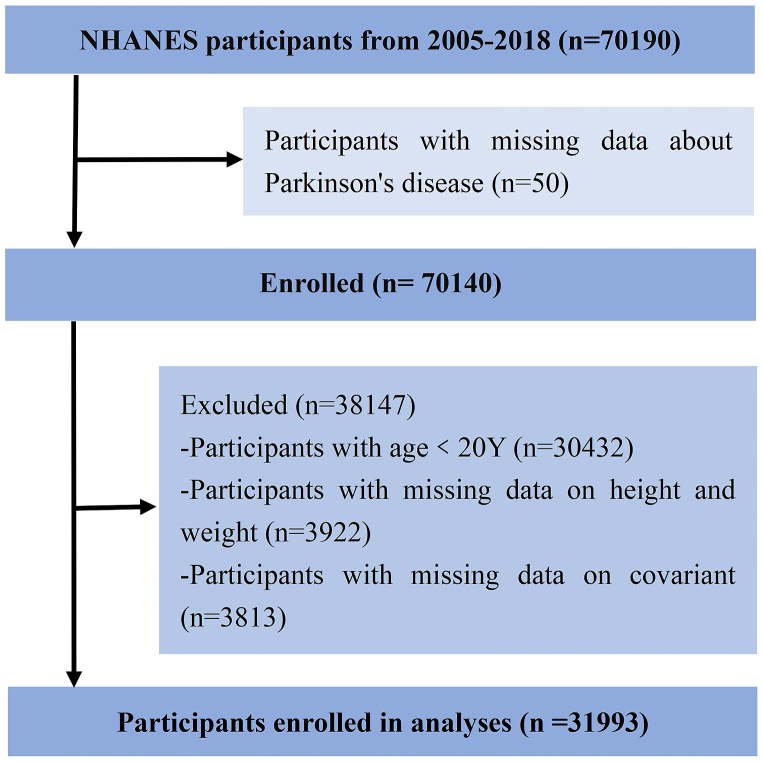
Fig. 1 depicts the inclusion procedure for this investigation. National Center for Health Statistics (NCHS) conducts a nationally representative cross-sectional survey known as NHANES. Participants were required to provide informed consent to participate in the survey before being interviewed or evaluated. This study utilized the NHANES public database from 2005 to 2018 and included 31,933 participants aged 20 or older (permission of an institutional review board and registration of clinical trials were not required).
Fig. 1.
Flow diagram of the screening and selection process. NHANES, National Health and Nutrition Examination Survey.
2.2. Exposure variables and data measurement
The primary exposure variable in this study was ABSI. Basic anthropometric measures were collected from participants, containing waist circumference in centimeters, height in meters, and weight (kg), BMI = weight/height2 and ABSI =WC/(BMI2/3 × height1/2) [20].
2.3. Outcome
This study's primary endpoint was PD. In the NHANES database, PD cases have been identified based on self-reported anti-PD medications [21]. PD is defined if individuals report using the PD drugs listed below, such as Benztropine, Methyldopa, Carbidopa, Levodopa, Entacapone, Amantadine and Ropinirole [22].
2.4. Potential covariates
Covariates were selected from those factors influencing PD that have been clearly reported previously. Age, gender, and race were demographic variables; marital status, education level, family income, and health insurance coverage were social factors; and smoking and alcohol intake were daily health-related behaviors. Finally, variables associated with medical comorbidities such as hypertension, diabetes and cancer were collected.
2.5. Statistical analysis
Empower Stats software (www.empowerstats.com) and R.4.0.5 (http://www.r-project.org) were used for all analyses. The sample size was determined using accessible data, and no prior sample size calculation was performed. For quantitative data analysis, The T-test or rank sum test was used to determine if cohort characteristics differed between exposure groups according to categorical variables. In order to determine whether exposure groups and outcome variables are associated, multifactor logistic regressions were used. Non-linear relationship curves between outcome variables and exposure factors were examined by using generalized additive model (GAM). Besides, curve inflexion points were determined by using two-stage linear regression model calculation. P-values of less than 0.05 (two-sided) were regarded as significant.
3. Results
3.1. Baseline information
We gathered survey information from a total of 70,190 respondents. After excluding data that lack outcome, exposure or covariate, 31,933 adult participants aged ≥20 years (313 people with PD and 31,680 without PD) accounted for in the study.
Table 1 shows how the characteristics of the cohort were spread out among the people who took part in the research. In the preliminary analysis, the ABSI of participants in the PD group was (0.084 ± 0.005) and the ABSI of the non-PD group was (0.082 ± 0.005), the PD group having considerably higher ABSI levels than the non-PD group (P < 0.001). Besides, in the PD group, the proportion of participants over 40 years old was higher than in the non-PD group (90.5% vs 66.1%; P < 0.001). Both groups had similar ratios of males to females, however (male: 49.6% vs 45.7%; P = 0.165). Similarly, regarding social factors, “Race", “Ratio of family income to poverty level" and “Marital status" statistically distinguishable between the two groups when compared (P < 0.001). In both groups, there was no statistically significant difference for “Education beyond high school" (53.2% vs 47.9%; P = 0.064). Furthermore, health insurance coverage was more prevalent in the non-PD group than in the PD group (93.6% vs. 78.7%; P < 0.001). Regarding phlebotomy, Estimated Glomerular Filtration Rate (eGFR), Aspartate aminotransferase (AST) and Alanine aminotransferase (ALT) were comparable between groups (P > 0.05), while non-PD patients had considerably higher Hemoglobin A1c (HbA1c) than PD patients. [(5.99 ± 1.12 vs (5.76 ± 1.06); P < 0.001]. Regarding lifestyle habits, the proportion of people in the PD group who smoked was noticeably higher than the proportion of smokers in non-PD (52.7% vs 45.0%; P = 0.007) but there was no noteworthy change between the two groups in alcohol consumption (75.7% vs 78.3%; P = 0.274). Similarly, in terms of comorbidities, the PD group had a larger number of individuals with BMI [(30.7 ± 7.1) vs (29.1 ± 6.8); P < 0.001], hypertension (63.9% vs 42.2%; P < 0.001), diabetes (32.9% vs 18.2%; P < 0.001), and cancer history (17.9% vs 9.4%; P < 0.001) compared to the non-PD population.
Table 1.
Characteristics of participants enrolled in study. Note. All values are displayed as n (%). χ2 analysis is used to test significance between groups for categorical variables. BMI, Body mass index; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin, glycosylated hemoglobin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ABSI, A body shape index.
| Characteristic | Non-Parkinson's disease (N = 31,680) | Parkinson's disease (N = 313) | P-value |
|---|---|---|---|
| Age (Y) | <0.001 | ||
| 20∼39 | 10,734 (33.9%) | 30 (9.6%) | |
| 40∼59 | 10,668 (33.7%) | 121 (38.7%) | |
| ≥60 | 10,278 (32.4%) | 162 (51.8%) | |
| Male | 15,723 (49.6%) | 143 (45.7%) | 0.165 |
| Race | <0.001 | ||
| Hispanic | 7701 (24.3%) | 54 (17.3%) | |
| Non-Hispanic white | 13,643 (43.1%) | 198 (63.3%) | |
| Non-Hispanic black | 6778 (21.4%) | 47 (15.0%) | |
| Other | 3558 (11.2%) | 14 (4.5%) | |
| Education beyond high school | 16,845 (53.2%) | 150 (47.9%) | 0.064 |
| Marital status | 0.003 | ||
| Never married | 5695 (18.0%) | 51 (16.3%) | |
| Married or living with partner | 19,028 (60.1) | 168 (53.7%) | |
| Divorced, separated, or widowed | 6957 (22.0%) | 94 (30.0%) | |
| Ratio of family income to poverty level | <0.001 | ||
| <1.0 | 6616 (20.9%) | 81 (25.9%) | |
| 1.0–2.9 | 13,316 (42.0%) | 150 (47.9%) | |
| ≥3 | 11,748 (37.1%) | 82 (26.2%) | |
| Health insurance coverage | 24,934 (78.7%) | 293 (93.6%) | <0.001 |
| eGFR, ml/min per 1.73 m2 | 85.0 ± 18.4 | 84.5 ± 19.0 | 0.110 |
| ALT (U/L) | 25.8 ± 20.4 | 24.6 ± 17.5 | 0.301 |
| AST (U/L) | 26.0 ± 17.1 | 26.6 ± 14.8 | 0.543 |
| HbA1c | 5.76 ± 1.06 | 5.99 ± 1.12 | <0.001 |
| Alcohol user | 24,800 (78.3%) | 237 (75.7%) | 0.274 |
| Smoker | 14,269 (45.0%) | 165 (52.7%) | 0.007 |
| Diabetes | 5760 (18.2%) | 103 (32.9%) | <0.001 |
| Hypertension | 13,364 (42.2%) | 200 (63.9) | <0.001 |
| Cancer | 2968 (9.4%) | 56 (17.9%) | <0.001 |
| BMI (kg/m2) | 29.1 ± 6.8 | 30.7 ± 7.1 | <0.001 |
| ABSI | 0.082 ± 0.005 | 0.084 ± 0.005 | <0.001 |
Note. All values are displayed as n (%). χ2 analysis is used to test significance between groups for categorical variables. BMI = body mass index; eGFR = estimated glomerular filtration rate; HbA1c = glycosylated Hemoglobin, type A1C; ALT = alanine aminotransferase; AST = aspartate aminotransferase; ABSI = a body shape index.
3.2. ABSI levels are greater in PD patients than in non-PD patients
Then, according to the quartile of the ABSI level of all the participants, four groups were divided into Q1 group (0.058–0.077), Q2 group (0.078–0.081), Q3 group (0.082–0.084), and Q4 group (0.085–0.117).
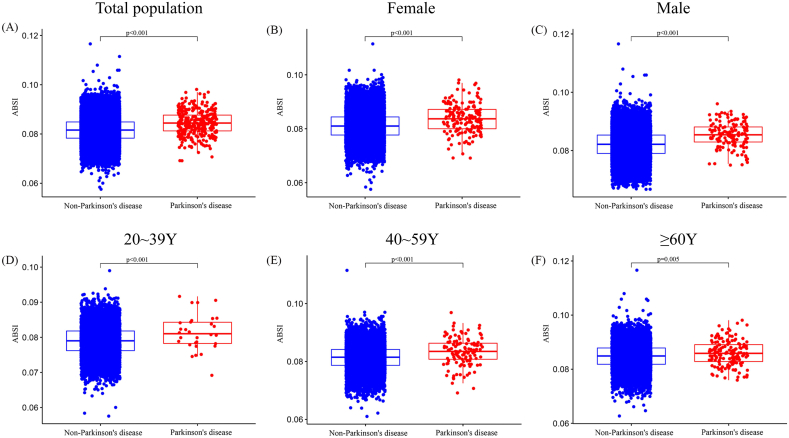
As seen in Fig. 2, the prevalence of PD varies across ABSI categories. In general, a significant difference was found between the PD group and the non-PD group in terms of ABSI levels (P < 0.001, Fig. 2A). In both male and female groups, the mean level of ABSI was considerably greater in the group with PD than in the group without PD (P < 0.001, Fig. 2B and C). For the whole population with different age stratification (20-39Y; 40-59Y; ≥60Y), we also found that the PD group has a drastically increased ABSI (P < 0.001, Fig. 2D–F).
Fig. 2.
The level of ABSI for different population in non-Parkinson's disease group and Parkinson's disease group. (A) Total population; (B) Female; (C) Male; (D) 20∼39Y; (E) 40∼59Y; (F) ≥60Y. ABSI, A body shape index.
3.3. Risk of PD increases with high ABSI
In an unadjusted multivariable logistic analysis, the probability of developing PD exceeded in the Q2 group than in the Q1 group [OR = 1.640, 95% CI (1.063–2.533); P < 0.05]. Besides, with the increase of ABSI, the risk of PD also demonstrated a rising tendency [OR = 2.408, 95% CI (1.602–3.619) in Q3 group; OR = 4.519, 95% CI (3.094–6.600) in Q4 group] (Table 2). After adjusting for age, sex, and race in model 1, the Q4 group inhabitants remained an increased risk of getting PD than the Q1 group populations [OR (95% CI) 2.677 (1.774–4.038); P < 0.001] (Table 2). Additionally, after adjusting for demographic, social, and comorbid factors (Model 3), the Q4 group populations remained at a greater likelihood of acquiring PD [OR (95% CI) 2.156 (1.418–3.276); P < 0.001] (Table 2). All of the above results suggest that a high ABSI can be a precursor to the development of PD.
Table 2.
Odds Ratios for associations between ABSI and Parkinson's disease (95% CI).
| Group | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|
| Un- adjusted | Ref. | 1.640 (1.063–2.533) * | 2.408 (1.602–3.619) ** | 4.519 (3.094–6.600) ** |
| Model 1 | Ref. | 1.365 (0.880–2.118) | 1.731 (1.136–2.639) * | 2.677 (1.774–4.038) ** |
| Model 2 | Ref. | 1.372 (0.883–2.131) | 1.693 (1.108–2.587) * | 2.390 (1.578–3.620) ** |
| Model 3 | Ref. | 1.333 (0.858–2.072) | 1.594 (1.042–2.439) * | 2.156 (1.418–3.276) ** |
Note. Model 1 was adjusted for age, sex, race.
Model 2 was adjusted for age, sex, race, education level, marital status, ratio of family income to poverty level, health insurance coverage.
Model 3 was adjusted for age, sex, race, education level, marital status, ratio of family income to poverty level, health insurance coverage, smoker, alcohol user, hypertension, cancer, diabetes.
*P < 0.05; **P < 0.001.
Besides that, the subgroup multifactorial logistic analysis by gender showed that the Q4 group populations are more likely to suffer from PD relative to the Q1 group populations regardless of their gender (P < 0.001). It also showed that men in the Q4 group are more likely to get PD than women [men vs women: OR = 6.563 (3.289–13.098) vs OR = 3.827 (2.398–6.108); Interaction P-value<0.001]. It indicated that this association is of significant gender difference. Thus, men with greater ABSI levels are more likely to develop PD.
Further, the subgroup multifactorial logistic analysis by age showed that in both the 20–39 Y and 40–59 Y groups, in Q4 group, the likelihood of developing PD is greater than Q1 (P < 0.05, Table 3). However, among those aged ≥60 Y, the risk of developing PD is not higher in the Q4 group [OR = 1.631, 95% CI (0.846–3.142); P > 0.05] (Table 3). This link between ABSI levels and the likelihood of PD varies statistically between age groups (Interaction P-value<0.001, Table 3).
Table 3.
Unadjusted Odds Ratios for subgroup analyses between ABSI and Parkinson's disease. Note. Model 1 was adjusted for age, sex, race. Model 2 was adjusted for age, sex, race, education level, marital status, ratio of family income to poverty level, health insurance coverage. Model 3 was adjusted for age, sex, race, education level, marital status, ratio of family income to poverty level, health insurance coverage, smoker, alcohol user, hypertension, cancer, diabetes. *P < 0.05; **P < 0.001. ABSI, A body shape index.
| Q1 | Q2 | Q3 | Q4 | Interaction P-value | |
|---|---|---|---|---|---|
| Age (Y) | <0.001 | ||||
| 20∼39 | Ref. | 1.629 (0.628–4.226) | 2.003 (0.725–5.530) | 4.468 (1.546–12.911) * | |
| 40∼59 | Ref. | 1.322 (0.696–2.513) | 1.778 (0.967–3.272) | 3.634 (2.026–6.516) ** | |
| ≥60 | Ref. | 1.042 (0.486–2.237) | 1.178 (0.585–2.374) | 1.631 (0.846–3.142) | |
| Sex | <0.001 | ||||
| Female | Ref. | 1.734 (1.033–2.911) * | 2.196 (1.327–3.632) * | 3.827 (2.398–6.108) ** | |
| Male | Ref. | 1.685 (0.756–3.756) | 3.220 (1.552–6.681) * | 6.563 (3.289–13.098) ** |
Note. *P < 0.05; **P < 0.001. ABSI, A body shape index.
3.4. ABSI and PD risk are nonlinear positive correlation
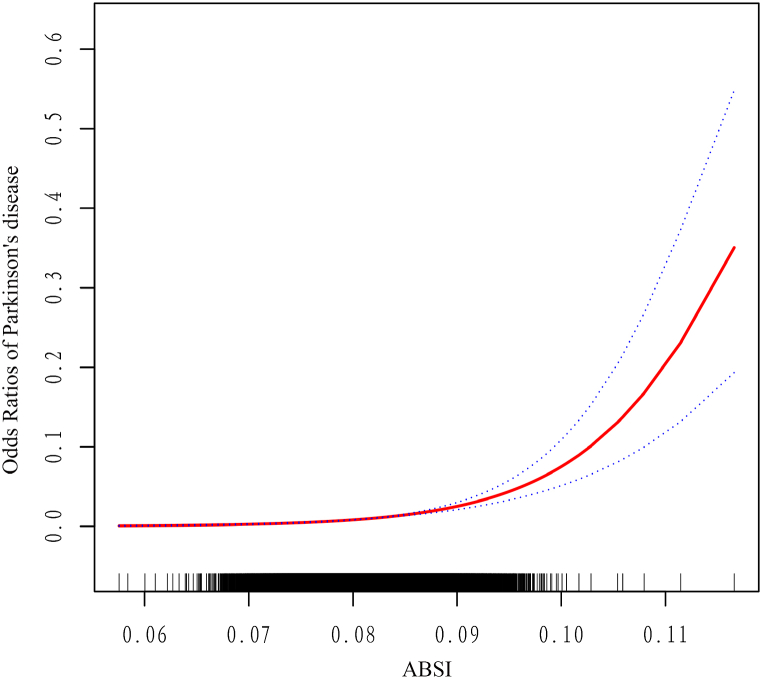
Fig. 3 illustrates ABSI dose-response curves and PD risk, based on the GAM. After adjusting various covariates such as demographic, social factors, and comorbidities, ABSI was positively related with the risk of PD in adults, and when ABSI increased, the overall risk of PD in adults tended to increase gradually (P < 0.05, Fig. 3). Besides, the inflexion point of the dose-response curve was identified by using a two-stage linear expression model, and the ABSI of this inflexion point was 0.080. When ABSI <0.080, the trend change in the curve was not significant (Fig. 3); when ABSI ≥0.080, the curve showed a significantly growing trend (Fig. 3). In the standardized linear model (Table 4), after controlling for age, sex, and race, each 0.01 unit increase in ABSI was related with1.918-fold higher adult PD risk [OR = 1.918, 95% CI (1.063–2.533); P < 0.05].
Fig. 3.
Dose-response association between ABSI and Odds Ratios of Parkinson's disease (Red: Odds Ratios; Blue dots: 95% CI). ABSI, A body shape index.
Table 4.
Threshold effect analysis of ABSI on Odds Ratios for Parkinson's disease using the two-piecewise linear regression model. ABSI, A body shape index.
| Adjusted β 95% CI) | p-value | |
|---|---|---|
| Fitting by the standard linear model | 1.918 (1.480–2.484) | <0.001 |
| Fitting by the two-piecewise linear model | ||
| Inflection point | 0.080 | |
| ABSI <0.080 | 1.796 (0.588–5.488) | 0.304 |
| ABSI ≥0.080 | 1.997 (1.412–2.826) | <0.001 |
Note. β represented increased value of Odds Ratios for Parkinson's disease when ABSI increased by 0.01 unit. All was adjusted for age, sex, race. ABSI, A body shape index.
When ABSI<0.080, there was no association between ABSI and the probability of adult PD [OR = 1.796, 95% CI (0.588–5.488); P = 0.304] (Table 4). When ABSI ≥0.080, each 0.01 unit rise in the adult ABSI was related with a 1.999-fold increase in the PD risk [OR = 1.997, 95% CI (1.412–2.826); P < 0.001] (Table 4). The above results are consistent with the trend of the curve fit plot in Fig. 3, suggesting the presence of the threshold saturation effect in the relationship between adult-onset PD risk and ABSI.
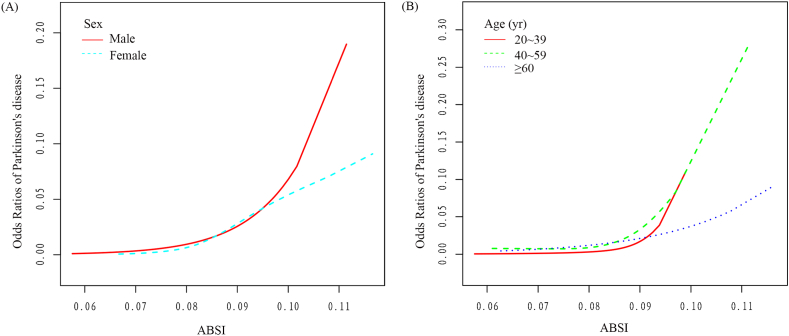
Moreover, GAM subgroup analysis was done, and the association between ABSI and the risk of PD in terms of dose was determined for several subgroups (Fig. 4). The ABSI and the risk of PD were statistically significantly positively correlated in both sexes. Besides, the total PD risk in adults seems to increase gradually with increasing ABSI, but the trend is much more pronounced in men than in women (Fig. 4A). In addition, ABSI is positively related with the risk of PD across a range of age groups, but the increasing trend is significantly stronger in those aged <60 years than in those aged ≥60 years (Fig. 4B).
Fig. 4.
Subgroup analysis of Dose-response association between ABSI and Odds Ratios of Parkinson's disease (Red: Odds Ratios; Blue dots: 95% CI).
4. Discussion
PD is a disease with lifelong risk and is diagnosed in 2% of men and 1.3% of women over the age of 40 in the United States (US) [23]. In general, PD prevalence is associated with race or ethnicity, and in the US, its incidence rate is higher in whites (0.74, 95% CI: 0.732–0.748) than in blacks (0.58, 95% CI: 0.575–0.581) [24]. Our study also found a significantly higher proportion of non-Hispanic whites than non-Hispanic blacks in the PD population (63.3% vs. 15.0%; P < 0.001).
Our study also found this similar trend that the proportion of the PD population aged ≥60 years (51.8%) is significantly higher than that of those aged 40–59 years (38.7%) and 20–39 years (9.6%). Besides, cancer can also increase the risk of PD, especially melanoma [25]. Previous research indicates that diabetes raises the likelihood of PD [26]. Prospective epidemiological studies also confirmed that the risk ratio for PD is 1.80 (95% CI:1.03–3.15) among all diabetic patients after adjusting the age factor [27]. Moreover, the risk of PD increases with the length and severity of increased fasting blood glucose levels [28]. We also found that the percentage of the PD population with diabetes is higher than of the non-PD population (32.9% vs. 18.2%; P < 0.001), which may be attributed to the fact that lower circulating insulin in diabetics affects their neuronal survival in the brain [29].
To sum up, the correlation of smoking and PD as well as the involved specific biological mechanisms or pathways needs to be further explored. Interestingly, a Mendelian study confirmed that there is no correlation that can be considered significant between alcohol consumption and PD (OR = 0.68; 95% Cl: 0.39–1.18; P = 0.17) [30]. We found that people with PD are not more likely to drink alcohol than people without PD, which supports this claim (75.7% vs. 78.3%; P = 0.274).
Numerous studies have proven that being overweight/obese may be associated with an increased risk of PD [[30], [31], [32]]. A diet that is high in fat, in particular one that includes an increased consumption of animal fat, is a risk factor for PD (OR = 5.3; 95%CI:1.8–15.5; P = 0.001) [33]. Chen et al. demonstrated that polyunsaturated fat replacement with saturated fat increased male risk of PD, but not female risk [34]. Moreover, two studies including Singaporeans and Dutch people also confirmed that the PD risk is inversely related to the consumption of monounsaturated fats [35,36]. However, in numerous prospective clinical investigations, no link between BMI and PD risk was discovered [[37], [38], [39]]. Only in a Finnish cohort study was it confirmed that BMI ≥27 is a significant PD risk factor compared to BMI <23, with a hazard ratio of 2.0 between groups [40]. Therefore, BMI is not necessarily an effective measure of individual fat content and cannot identify muscle and obesity. For instance, it cannot distinguish between “fake fat" and “real fat". Interestingly, according to the findings of a number of research, fat distribution may be more effective than overall body weight in assessing the risk of PD, particularly in persons who have a high waist-to-hip ratio or a high triceps skinfold thickness, both of which are associated with a considerably greater risk of PD [[40], [41], [42]]. In the non-smoking group, a waist circumference of 1.9 inches (95%CI: 1.0–3.4; P = 0.03) and a waist-to-hip ratio of 2.0 inches (95%CI: 1.1–3.6; P = 0.03) were substantially correlated with an increased PD risk [42]. According to these data, central obesity may lead to PD. ABSI correlates positively with visceral fat, regardless of muscle mass, and can provide a better indication of fat distribution than conventional BMI.
Our study also confirmed that ABSI is substantially higher among those with PD than among those without PD [(0.084 ± 0.005) vs. (0.082 ± 0.005); P < 0.001)]. Subsequent unadjusted or adjusted multifactorial logistic analyses also confirmed that a high ABSI is one of the factors that contribute to the development of PD and that the chance of having PD is increased in males with high ABSI compared to the female population with high ABSI. It indicates a greater connection between ABSI levels and the occurrence of PD in this male sample (Table 2, Table 3). Ultimately, the dose-response curves also revealed a substantial positive correlation between ABSI and the PD risk, with a steady increase in the overall risk of PD in adults with the progression of ABSI, which was more pronounced in men and those under the age of 60 (Fig. 4).
This study implies obesity is a PD risk factor. Obesity may increase the risk of PD because adipokines produced by adipose tissue upregulate systemic inflammation and create insulin resistance, which adds to the rapid course of the disease [43,44]. Obesity can also impair cognitive performance, as it disrupts cerebrovascular function, causes central inflammation, and induces oxidative stress in the brain [45]. In light of the fact that the number of overweight and obese people globally has skyrocketed in recent decades and has become one of the most significant health concerns among PD patients, we propose that PD patients should be encouraged to engage in regular physical activity [9], more emphasis needs to be placed on weight control and the reduction of fat intake to prevent the development of PD.
Nevertheless, our research is not without its flaws and restrictions. To begin, due to the fact that this inquiry is cross-sectional in nature, confounding variables from the NHANES that were not included may have influenced the findings. In addition, not all patients diagnosed with PD receive anti-PD medications. Therefore, there may be some underreporting of PD in the included population. Since the NHANES database currently includes only U.S. citizens, future studies that include populations from more countries. It is necessary to study more races in order to ascertain whether or not the correlation between ABSI and PD holds true for the overall population.
5. Conclusions
We analyzed a representative sample from the US, and found an ascending, nonlinear relationship between ABSI and the risk of developing PD. The association is strongest for males aged 20–59 years. ABSI accounts for height and weight (i.e. BMI) to quantify relative waist circumference/abdominal obesity. BMI and ABSI are complementary indices which on further study may prove useful to identify individuals at increased risk of PD.
Ethics approval and consent to participate
Review and/or approval by an ethics committee was not needed for this study because this study was reviewed and approved by National Center for Health Statistics (NCHS) Ethics Review Board (See Attachment).
Consent for publication
All authors agree to this submission.
Data availability statement
All data used in the generation of the results presented in this manuscript will be made available upon reasonable request from the corresponding author.
Funding
Not Applicable.
CRediT authorship contribution statement
Wei Huang: Writing – review & editing, Visualization, Conceptualization. Yingqi Xiao: Writing – original draft, Formal analysis. Li Zhang: Supervision, Project administration, Methodology. Hu Liu: Validation, Supervision, Resources, Methodology, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e26557.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Ascherio A., Schwarzschild M. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 2.Caballero B. Humans against obesity: who will win? Advances in nutrition (Bethesda, Md. 2019;10:S4–S9. doi: 10.1093/advances/nmy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seravalle G., Grassi G. Obesity and hypertension. Pharmacol. Res. 2017;122:1–7. doi: 10.1016/j.phrs.2017.05.013. https://10.1016/j.phrs.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 4.Kachur S., Lavie C., de Schutter A., Milani R., Ventura H. Obesity and cardiovascular diseases. Minerva Med. 2017;108(3):212–228. doi: 10.23736/S0026-4806.17.05022-4. [DOI] [PubMed] [Google Scholar]
- 5.Maggio C., Pi-Sunyer F. Obesity and type 2 diabetes. Endocrinol Metab. Clin. N. Am. 2003;32(4):805–822. doi: 10.1016/s0889-8529(03)00071-9. viii, [DOI] [PubMed] [Google Scholar]
- 6.Manson J., Colditz G., Stampfer M., Willett W., Rosner B., Monson R., Speizer F., Hennekens C. A prospective study of obesity and risk of coronary heart disease in women. N. Engl. J. Med. 1990;322(13):882–889. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 7.Ebrahimpour S., Zakeri M., Esmaeili A. Crosstalk between obesity, diabetes, and alzheimer's disease: introducing quercetin as an effective triple herbal medicine. Ageing Res. Rev. 2020;62 doi: 10.1016/j.arr.2020.101095. [DOI] [PubMed] [Google Scholar]
- 8.Chen J., Guan Z., Wang L., Song G., Ma B., Wang Y. Meta-analysis: overweight, obesity, and Parkinson's disease. International journal of endocrinology. 2014 doi: 10.1155/2014/203930. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim R., Jun J. Impact of overweight and obesity on functional and clinical outcomes of early Parkinson's disease. J. Am. Med. Dir. Assoc. 2020;21(5):697–700. doi: 10.1016/j.arr.2020.101095. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Jiménez C., Gaitán-Vaca D., Echeverria V., González J., Barreto G. Relationship between obesity, alzheimer's disease, and Parkinson's disease: an astrocentric view. Mol. Neurobiol. 2017;54(9):7096–7115. doi: 10.1007/s12035-016-0193-8. [DOI] [PubMed] [Google Scholar]
- 11.Kamel F., Goldman S., Umbach D., Chen H., Richardson G., Barber M., Meng C., Marras C., Korell M., Kasten M., Hoppin J., Comyns K., Chade A., Blair A., Bhudhikanok G., Webster Ross G., William Langston J., Sandler D., Tanner C. Dietary fat intake, pesticide use, and Parkinson's disease. Park. Relat. Disord. 2014;20(1):82–87. doi: 10.1016/j.parkreldis.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong J., Beard J., Umbach D., Park Y., Huang X., Blair A., Kamel F., Chen H. Dietary fat intake and risk for Parkinson's disease, Movement disorders. official journal of the Movement Disorder Society. 2014;29(13):1623–1630. doi: 10.1002/mds.26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roche A., Sievogel R., Chumlea W., Webb P. Grading body fatness from limited anthropometric data. Am. J. Clin. Nutr. 1981;34(12):2831–2838. doi: 10.1093/ajcn/34.12.2831. [DOI] [PubMed] [Google Scholar]
- 14.Kim H., Oh E., Lee J., et al. Relationship between changes of body mass index (BMI) and cognitive decline in Parkinson's disease (PD) Arch. Gerontol. Geriatr. 2012;55(1):70–72. doi: 10.1016/j.archger.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Heilbron K., Jensen M., Bandres-Ciga S., Fontanillas P., Blauwendraat C., Nalls M., Singleton A., Smith G., Cannon P., Noyce A. Unhealthy behaviours and risk of Parkinson's disease: a mendelian randomisation study. J. Parkinsons Dis. 2021;11(4):1981–1993. doi: 10.3233/JPD-202487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christakoudi S., Tsilidis K., Muller D., et al. A Body Shape Index (ABSI) achieves better mortality risk stratification than alternative indices of abdominal obesity: results from a large European cohort. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-71302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krakauer N., Krakauer J. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0039504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gažarová M., Galšneiderová M., Mečiarová L. Obesity diagnosis and mortality risk based on a body shape index (ABSI) and other indices and anthropometric parameters in university students. Rocz. Panstw. Zakl. Hig. 2019;70(3):267–275. doi: 10.32394/rpzh.2019.0077. [DOI] [PubMed] [Google Scholar]
- 19.Biolo G., Di Girolamo F., Breglia A., et al. Inverse relationship between "a body shape index" (ABSI) and fat-free mass in women and men: insights into mechanisms of sarcopenic obesity. Clin. Nutr. 2015;34(2):323–327. doi: 10.1016/j.clnu.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Christakoudi S., Tsilidis K.K., Evangelou E., Riboli E.J.C.M. 2021. A Body Shape Index (ABSI), Hip Index, and Risk of Cancer in the UK Biobank Cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Botelho J., Lyra P., Proena L.F.A., Godinho C., Machado V. Relationship between blood and standard biochemistry levels with periodontitis in Parkinson's disease patients: data from the NHANES 2011-2012. J. Personalized Med. 2020;10(69) doi: 10.3390/jpm10030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyra P., Machado V., Proena L., Mendes J.J., Botelho J. Tooth loss and blood pressure in Parkinson's disease patients: an exploratory study on NHANES data. MDPI AG. 2021;(9) doi: 10.3390/ijerph18095032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elbaz A., Bower J., Maraganore D., McDonnell S., Peterson B., Ahlskog J., Schaid D., Rocca W. Risk tables for parkinsonism and Parkinson's disease. J. Clin. Epidemiol. 2002;55(1):25–31. doi: 10.1016/s0895-4356(01)00425-5. [DOI] [PubMed] [Google Scholar]
- 24.Wright Willis A., Evanoff B., Lian M., Criswell S., Racette B. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 2010;34(3):143–151. doi: 10.1159/000275491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bose A., Petsko G., Eliezer D. Parkinson's disease and melanoma: Co-occurrence and mechanisms. J. Parkinsons Dis. 2018;8(3):385–398. doi: 10.3233/JPD-171263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foltynie T., Athauda D. Diabetes, BMI, and Parkinson's, movement disorders. official journal of the Movement Disorder Society. 2020;35(2):201–203. doi: 10.1002/mds.27941. [DOI] [PubMed] [Google Scholar]
- 27.Hu G., Jousilahti P., Bidel S., Antikainen R., Tuomilehto J. Type 2 diabetes and the risk of Parkinson's disease. Diabetes Care. 2007;30(4):842–847. doi: 10.2337/dc06-2011. [DOI] [PubMed] [Google Scholar]
- 28.Jeong S., Han K., Kim D., Rhee S., Jang W., Shin D. Body mass index, diabetes, and the risk of Parkinson's disease. Mov. Disord. 2020;35(2):236–244. doi: 10.1002/mds.27922. [DOI] [PubMed] [Google Scholar]
- 29.Athauda D., Foltynie T. Insulin resistance and Parkinson's disease: a new target for disease modification? Prog. Neurobiol. 2016:98–120. doi: 10.1016/j.pneurobio.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Domenighetti C., Sugier P., Sreelatha A., Schulte C., Grover S., et al. Mendelian randomisation study of smoking, alcohol, and coffee drinking in relation to Parkinson's disease. J. Parkinsons Dis. 2022;12(1):267–282. doi: 10.3233/JPD-212851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J., Liu L., Zhang L., Chen S., Chen Y., Cai C. Targeted fatty acid metabolomics to discover Parkinson's disease associated metabolic alteration. J. Mass Spectrom. : JMS. 2021;56(10) doi: 10.1002/jms.4781. [DOI] [PubMed] [Google Scholar]
- 32.Dong M., Wei Y., Hu L. Lipid metabolic dysregulation is involved in Parkinson's disease dementia. Metab. Brain Dis. 2021;36(3):463–470. doi: 10.1007/s11011-020-00665-5. [DOI] [PubMed] [Google Scholar]
- 33.Logroscino G., Marder K., Cote L., Tang M., Shea S., Mayeux R. Dietary lipids and antioxidants in Parkinson's disease: a population-based, case-control study. Ann. Neurol. 1996;39(1):89–94. doi: 10.1002/ana.410390113. [DOI] [PubMed] [Google Scholar]
- 34.Chen H., Zhang S., Hernán M., Willett W., Ascherio A. Dietary intakes of fat and risk of Parkinson's disease. Am. J. Epidemiol. 2003;157(11):1007–1014. doi: 10.1093/aje/kwg073. [DOI] [PubMed] [Google Scholar]
- 35.Tan L., Koh W., Yuan J., Wang R., Au W., Tan J., Tan E., Yu M. Differential effects of black versus green tea on risk of Parkinson's disease in the Singapore Chinese Health Study. Am. J. Epidemiol. 2008;167(5):553–560. doi: 10.1093/aje/kwm338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Lau L., Bornebroek M., Witteman J., Hofman A., Koudstaal P., Breteler M. Dietary fatty acids and the risk of Parkinson disease: the Rotterdam study. Neurology. 2005;64(12):2040–2045. doi: 10.1212/01.WNL.0000166038.67153.9F. [DOI] [PubMed] [Google Scholar]
- 37.Savica R., Grossardt B., Ahlskog J., Rocca W. Metabolic markers or conditions preceding Parkinson's disease: a case-control study. Mov. Disord. 2012;27(8):974–979. doi: 10.1002/mds.25016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palacios N., Gao X., McCullough M., Jacobs E., Patel A., Mayo T., Schwarzschild M., Ascherio A. Obesity, diabetes, and risk of Parkinson's disease. Mov. Disord. 2011;26(12):2253–2259. doi: 10.1002/mds.23855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sääksjärvi K., Knekt P., Männistö S., Lyytinen J., Jääskeläinen T., Kanerva N., Heliövaara M. Reduced risk of Parkinson's disease associated with lower body mass index and heavy leisure-time physical activity. Eur. J. Epidemiol. 2014;29(4):285–292. doi: 10.1007/s10654-014-9887-2. [DOI] [PubMed] [Google Scholar]
- 40.Hu G., Jousilahti P., Nissinen A., Antikainen R., Kivipelto M., Tuomilehto J. Body mass index and the risk of Parkinson disease. Neurology. 2006;67(11):1955–1959. doi: 10.1212/01.wnl.0000247052.18422.e5. [DOI] [PubMed] [Google Scholar]
- 41.Abbott R., Ross G., White L., Nelson J., Masaki K., Tanner C., Curb J., Blanchette P., Popper J., Petrovitch H. Midlife adiposity and the future risk of Parkinson's disease. Neurology. 2002;59(7):1051–1057. doi: 10.1212/wnl.59.7.1051. [DOI] [PubMed] [Google Scholar]
- 42.Chen H., Zhang S., Schwarzschild M., Hernán M., Willett W., Ascherio A. Obesity and the risk of Parkinson's disease. Am. J. Epidemiol. 2004;159(6):547–555. doi: 10.1093/aje/kwh059. [DOI] [PubMed] [Google Scholar]
- 43.Williams-Gray C., Wijeyekoon R., Yarnall A., Lawson R., Breen D., Evans J., Cummins G., Duncan G., Khoo T., Burn D., Barker R. Serum immune markers and disease progression in an incident Parkinson's disease cohort (ICICLE-PD) Mov. Disord. 2016;31(7):995–1003. doi: 10.1002/mds.26563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawton M., Baig F., Toulson G., Morovat A., Evetts S., Ben-Shlomo Y., Hu M. Blood biomarkers with Parkinson's disease clusters and prognosis: the oxford discovery cohort. Mov. Disord. 2020;35(2):279–287. doi: 10.1002/mds.27888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen J., Killcross A., Jenkins T. Obesity and cognitive decline: role of inflammation and vascular changes. Front. Neurosci. 2014;8:375. doi: 10.3389/fnins.2014.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in the generation of the results presented in this manuscript will be made available upon reasonable request from the corresponding author.