Abstract
Entry into mitosis depends upon activation of the dual-specificity phosphatase Cdc25C, which dephosphorylates and activates the cyclin B-Cdc2 complex. Previous work has shown that the Xenopus polo-like kinase Plx1 can phosphorylate and activate Cdc25C in vitro. In the work presented here, we demonstrate that Plx1 is activated in vivo during oocyte maturation with the same kinetics as Cdc25C. Microinjection of wild-type Plx1 into Xenopus oocytes accelerated the rate of activation of Cdc25C and cyclin B-Cdc2. Conversely, microinjection of either an antibody against Plx1 or kinase-dead Plx1 significantly inhibited the activation of Cdc25C and cyclin B-Cdc2. This effect could be reversed by injection of active Cdc25C, indicating that Plx1 is upstream of Cdc25C. However, injection of Cdc25C, which directly activates cyclin B-Cdc2, also caused activation of Plx1, suggesting that a positive feedback loop exists in the Plx1 activation pathway. Other experiments show that injection of Plx1 antibody into early embryos, which do not require Cdc25C for the activation of cyclin B-Cdc2, resulted in an arrest of cleavage that was associated with monopolar spindles. These results demonstrate that in Xenopus laevis, Plx1 plays important roles both in the activation of Cdc25C at the initiation of mitosis and in spindle assembly at late stages of mitosis.
Progression through the eucaryotic cell cycle is controlled by the activation of several cyclin-dependent kinases (cdks) at specific points in the cycle. To maintain genomic stability, surveillance mechanisms known as checkpoints monitor the completion of essential events to prevent cell cycle progression if DNA is damaged, unreplicated, or improperly assembled on the mitotic spindle (reviewed in references 6 and 20). The biochemistry of cell cycle-dependent checkpoints is best characterized during G2 phase, when checkpoints determine whether a cell enters mitosis. The cdk that drives the G2/M transition, cyclin B-Cdc2, is not activated on schedule if DNA is damaged or if DNA replication is incomplete (2; reviewed in reference 7). Both the DNA damage and DNA replication checkpoints regulate cyclin B-Cdc2 activation in part through the phosphatase Cdc25C. Throughout late S and early G2 phases, cyclin B is synthesized and immediately complexes with Cdc2, which is kept catalytically inactive by phosphorylation of Tyr15 and Thr14 in the ATP-binding site (12, 16, 29). This phosphorylation and inactivation is catalyzed by the protein kinases Wee1 and Myt1 (42, 45), and dephosphorylation and activation of cyclin B-Cdc2 is catalyzed by the phosphatase Cdc25C (4, 13, 37).
Studies on vertebrate Cdc25C have shown that its ability to dephosphorylate cyclin B-Cdc2 and initiate mitosis is regulated by two distinct mechanisms. Activation of Cdc25C requires phosphorylation on specific serine and threonine sites (21, 25, 31), and this phosphorylation fails to occur if DNA synthesis is incomplete and the replication checkpoint is activated. It has also been suggested that the rate of phosphorylation of Cdc2 on tyrosine is increased when DNA replication is incomplete (52). The other Cdc25C regulatory mechanism is implicated in the G2 DNA damage checkpoint and involves activation of the kinase Chk1 to phosphorylate Cdc25C at a specific site, an event that promotes the binding of 14-3-3 proteins (10, 47, 51). Although this binding does not directly inhibit the phosphatase activity of Cdc25C in vitro, the proximity of the 14-3-3 binding site to a nuclear localization signal suggests that it may affect the colocalization of Cdc25C with its cdk substrate in the nucleus (10, 47, 51).
To better understand the DNA replication checkpoint, it is necessary to define the phosphorylation pathway by which Cdc25C becomes activated at the G2/M transition. Cyclin B-Cdc2 itself is able to phosphorylate Cdc25C at the activating sites, forming a positive feedback loop that contributes to the abrupt transition from G2 into M phase (23). However, a variety of evidence indicates that initial phosphorylation of Cdc25C at the G2/M transition occurs prior to cyclin B-Cdc2 activation, and full phosphorylation and activation of Cdc25C can be obtained in microcystin-treated egg extracts devoid of Cdc2 and Cdk2 (24). This has focused attention on the identification of other protein kinases that might function as “trigger” kinases for Cdc25C activation and might be subject to inhibition when the DNA replication checkpoint is activated. Recently, it was reported that the Xenopus polo-like kinase (plk), Plx1, can phosphorylate and activate Cdc25C in vitro (30). However, it is not known if Plx1 activity increases during the Xenopus cell cycle or if its activity is required for Cdc25C activation in vivo. Genetic studies with Drosophila melanogaster, Saccharomyces cerevisiae, and Schizosaccharomyces pombe implicate plk activity in centrosome functions, spindle assembly, and cytokinesis events, and mutations in plks usually correlate with defects in spindles or septum formation (39, 44; reviewed in references 14 and 33). These phenotypes are more consistent with a kinase that functions late in mitosis rather than during the initial stages of mitosis at the G2/M transition, and indeed, in D. melanogaster, polo activity peaks at the late-anaphase/telophase border, later than cyclin B-Cdc2 activity (8). In contrast, in mammalian cells, Plk1 activity peaks in concert with that of cyclin B-Cdc2 at the onset of mitosis (15, 17, 36). In these cells, Plk1 colocalizes and interacts with various components of the mitotic spindle apparatus, and moreover, microinjection of an antibody to Plk1 blocks cells in G2 or in a psuedomitotic state, with monopolar spindles (15, 32, 36). These considerations make it imperative to determine whether Plx1 activity is regulated during the Xenopus cell cycle and whether it is required for Cdc25C activation and spindle assembly events during mitosis.
MATERIALS AND METHODS
Preparation of oocytes and oocyte extracts.
Xenopus laevis females, obtained from Xenopus I (Ann Arbor, Mich.), were primed with 35 IU of pregnant mare’s serum gonadotropin 3 days prior to an experiment. Stage VI oocytes were dissected manually and cultured in modified Barth’s solution. Unless otherwise stated, maturation was induced by treatment with 3.2 μM progesterone. Samples were microinjected with a volume of 50 nl with a Medical Systems Corp. (Greenvale, N.Y.) PLI-100 apparatus. Oocytes were harvested at the indicated times, frozen in dry ice, and stored at −80°C until further analysis. For preparation of extracts, frozen oocytes were homogenized in 20 μl of extraction buffer per oocyte and centrifuged for 15 min in a microcentrifuge, and the supernatants were collected. Extraction buffer comprises 80 mM β-glycerophosphate (pH 7.4); 20 mM EDTA; 1 mM dithiothreitol (DTT); 0.1 mM sodium vanadate; 10 mM NaF; 10 μg each of pepstatin, chymostatin, and leupeptin per ml; 3 μM microcystin; and 1 mM phenylmethylsulfonyl fluoride.
Isolation of PLX1 cDNA.
On the basis of sequence information (30), a pair of primers (CGGGGTACCATGGCTCAAGTGGCCGGTA and GGAAGATCTTGCCGAGGCCTTTACGTGT; sequences not underlined are linker sequences) within the PLX1 coding sequence were synthesized and used in PCRs with a Xenopus oocyte cDNA library as the template. An expected 1.8-kb PCR product was cloned into a pOTV vector and identified as a full-length PLX1 cDNA by sequence analysis.
Generation of Plx1(N172A).
To prepare a catalytically inactive Plx1 mutant, codon 172 (AAC, coding for asparagine) was mutated to GCC (coding for alanine) (30) in the pOTV-PLX1 construct by PCR using oligonucleotides with the sequences CTCGGAGCCTTGTTCCTTAATGATGAAATG and GAACAAGGCTCCGAGCTTGAGGTCTCT.
Expression and purification of recombinant Plx1 in Sf9 cells.
Full-length PLX1 with C-terminal Flag and 6×His tags was subcloned into baculovirus transfer vector pVL1393 (Invitrogen, San Diego, Calif.). Insect Sf9 cells expressing Plx1 were harvested and lysed in buffer A (10 mM HEPES [pH 7.5], 150 mM NaCl) containing 1 mM EGTA, 0.5% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride. The clarified lysate was incubated with TALON metal affinity resin (Clontech, Palo Alto, Calif.) and washed twice with buffer A containing 1 mM EGTA and 0.5% Triton X-100, twice with buffer A containing 1 mM EGTA and 0.5% Nonidet P-40 (NP-40), and with buffer A containing 0.05% Brij 35. Plx1 was eluted with buffer A containing 250 mM imidazole, dialyzed extensively against 20 mM HEPES (pH 7.5)–88 mM NaCl–7.5 mM MgCl2–10 mM β-mercaptoethanol, and frozen in small aliquots at −80°C. The same procedure was used to obtain recombinant, kinase-dead Plx1(N172A).
Generation and affinity purification of rabbit polyclonal antibodies against Plx1.
Full-length PLX1 with C-terminal Flag and 6×His tags was subcloned into the bacterial expression vector pET-3d (Novagen, Madison, Wis.), and the resulting plasmid was transformed into BL21(DE3)pLysS. The bacteria were grown to an optical density at 600 nm (OD600) of 0.6 at 37°C in Luria-Bertani medium containing 100 μg of ampicillin per ml and 25 μg of chloramphenicol per ml. Expression was induced with 0.4 mM isopropyl-β-d-thiogalactopyranoside for 4 h. The bacteria were harvested and Plx1 was purified as described above, except that Plx1 bound to the TALON resin was used directly to immunize two rabbits. Plx1 antibodies were affinity purified as previously described (18) from immune sera on a column of Affi-gel 10 resin (Bio-Rad, Richmond, Calif.) coupled to recombinant Plx1 purified from Sf9 cells. Control immunoglobulin G (IgG) was prepared on a protein A-agarose (Sigma, St. Louis, Mo.) column from immune sera that had been depleted of all Plx1-specific antibodies with the Plx1 affinity column. The purified Plx1 antibodies and control IgG were concentrated with a Centricon 30 (Amicon, Beverly, Mass.) and dialyzed extensively against 20 mM HEPES (pH 7.5)–88 mM NaCl. Identical results were obtained with Plx1 antibodies from both rabbits.
Immunoblot analysis.
For immunoblot analysis, samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose. The membranes were blocked with 10% nonfat dry milk in 20 mM Tris-HCl (pH 7.5)–137 mM NaCl–0.1% Tween 20, incubated with primary antibody overnight at 4°C, washed, incubated with the appropriate secondary antibody (Jackson ImmunoResearch, West Grove, Pa.) for 1 h at ambient temperature, and washed again. For Cdc25C blots, the equivalent of one-half oocyte was loaded per lane, transfer was done with a semidry apparatus (Pharmacia/LKB, Piscataway, N.J.), and immunoreactive proteins were visualized by enhanced chemiluminescence (Amersham, Arlington Heights, Ill.). Preparation of antiserum against Cdc25C has been described previously (25). For Plx1 blots, the equivalent of one oocyte was loaded per lane, transfer was done with a Hoefer wet transfer apparatus (Pharmacia/LKB), and the blots were developed with an alkaline phosphatase system (Bio-Rad).
Immunoprecipitation and kinase assays.
For quantitation of histone H1 kinase activity, samples equivalent to one-fifth of an oocyte were incubated for 10 min at 30°C in a final volume of 30 μl of kinase buffer (20 mM HEPES [pH 7.2], 2 mM DTT, 10 mM MgCl2, 0.1 mM EGTA) containing 0.1 mg of bovine serum albumin per ml, 3.3 μg of the protein kinase A inhibitor protein per ml, 0.5 mg of histone H1 per ml, and 100 μM [γ-32P]ATP (1 to 5 cpm/fmol). The reactions were stopped by addition of 15 μl of glacial acetic acid. Samples of 35 μl were spotted onto P81 phosphocellulose squares, and after three washes with 15% acetic acid, incorporation of radiolabel was determined by counting Cerenkov radiation. At least 95% of the total histone H1 kinase activity in egg extracts is due to cyclin B-Cdc2 (34); therefore, assay of total histone H1 kinase activity reflects the activity of cyclin B-Cdc2.
For immunocomplex kinase assays of Plx1 activity, samples containing the equivalent of one oocyte were diluted into 200 μl of buffer B (50 mM Tris [pH 8.0], 0.5% NP-40, 120 mM NaCl, 20 mM EDTA, 1 mM DTT) containing proteinase inhibitors, 0.4 μg of anti-Plx1 antibodies, and 10 μl of protein A agarose beads and incubated at 4°C for 2 h. Samples were washed twice with 10 mM Tris (pH 7.0)–0.1% NP-40–1 M NaCl–1 mM DTT, twice with buffer B, and once with kinase buffer containing 0.01% Brij-35 and transferred to new tubes. The immunocomplexes were suspended in 30 μl of kinase buffer supplemented with 0.01% Brij-35–100 μM [γ-32P]ATP (5 to 10 cpm/fmol)–1 mg of α-casein (Sigma) per ml and incubated for 30 min at 30°C. The reactions were stopped by addition of 20 μl of four-times-concentrated sample buffer, the reaction mixtures were incubated at 95°C for 5 min, and 15-μl samples were subjected to SDS-PAGE. The casein bands were identified by staining, and incorporation of radiolabel was quantified by liquid scintillation spectrometry of the excised bands.
Microinjection and immunofluorescence of Xenopus embryos.
Embryos were obtained by in vitro fertilization, dejellied in 2% cysteine (pH 7.8), and cultured in 0.1× MMR as described previously (19). Embryos were microinjected at the two-cell stage with 25 nl of anti-Plx1 antibodies (1.5 mg/ml) or control IgG (1.5 mg/ml), cultured in 0.1× MMR for 4 to 5 h, and fixed in methanol. Embryos were photographed with a Wild Heerbrugg dissecting microscope equipped with a 35-mm camera. Immunofluorescence staining was performed as previously described (11). DNA was stained with SYTOX Green (Molecular Probes, Inc., Eugene, Oreg.), and α-tubulin was detected with an anti-α-tubulin monoclonal antibody (Sigma) and visualized by Lissamine rhodamine-conjugated donkey anti-mouse IgG antibodies (Jackson ImmunoResearch). Confocal microscopy was performed with an MRC-600 microscope (Bio-Rad). The confocal images presented here represent projections of Z-series scans.
RESULTS
Plx1 is activated during oocyte maturation.
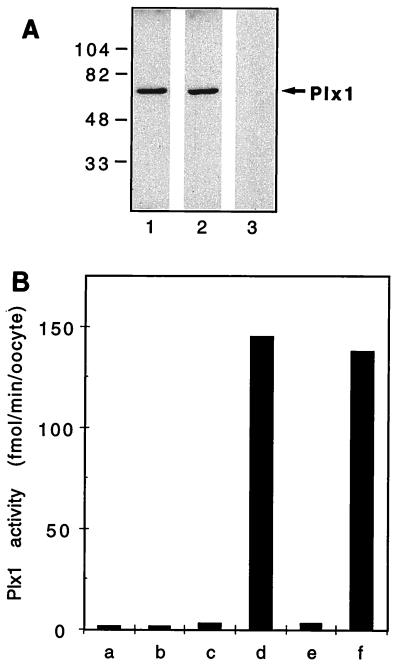
To analyze the activity of Plx1, antibodies against recombinant Plx1 purified from bacteria were raised and affinity purified as described in Materials and Methods. Control IgG was prepared from immune sera that had been depleted of all Plx1-specific antibodies. Affinity-purified Plx1 antibodies, but not the control IgG, detected a single polypeptide of 67 kDa in oocyte extracts by immunoblotting (Fig. 1A). Moreover, an increased amount of Plx1 protein could be detected by the Plx1 antibodies in extracts from oocytes that had been microinjected with PLX1 mRNA (data not shown). The plks are able to phosphorylate casein in vitro (8). Therefore, to compare Plx1 activity between resting (stage VI) oocytes in G2 phase and matured, progesterone-treated oocytes that had undergone germinal vesicle breakdown (GVBD) and entered M phase, Plx1 immunoprecipitates were used for casein kinase assays. Plx1 immunoprecipitates prepared from stage VI oocytes (G2 phase) had very low casein kinase activity, whereas immunoprecipitates prepared from matured oocytes that had entered M phase had at least a 30-fold increase in kinase activity toward casein (Fig. 1B). No casein kinase activity was observed in immunoprecipitates prepared with the control IgG. Casein kinase activity in the Plx1 immunoprecipitates was linear with time and amount of extract (data not shown).
FIG. 1.
Characterization of affinity-purified Plx1 antibodies. (A) An extract of stage VI oocytes was prepared and subjected to immunoblotting. Lanes: 1 and 2, anti-Plx1 antibodies from two different rabbits; 3, control IgG. Molecular masses (in kilodaltons) are indicated on the left. (B) Immunoprecipitates were assayed for casein phosphorylation. Bars: a, c, and e, stage VI oocytes; b, d, and f, mature oocytes 15 min after undergoing GVBD; a and b, control IgG; c and d and e and f, anti-Plx1 antibodies from two different rabbits.
Plx1 activation is concurrent with Cdc25C and cyclin B-Cdc2 activation.
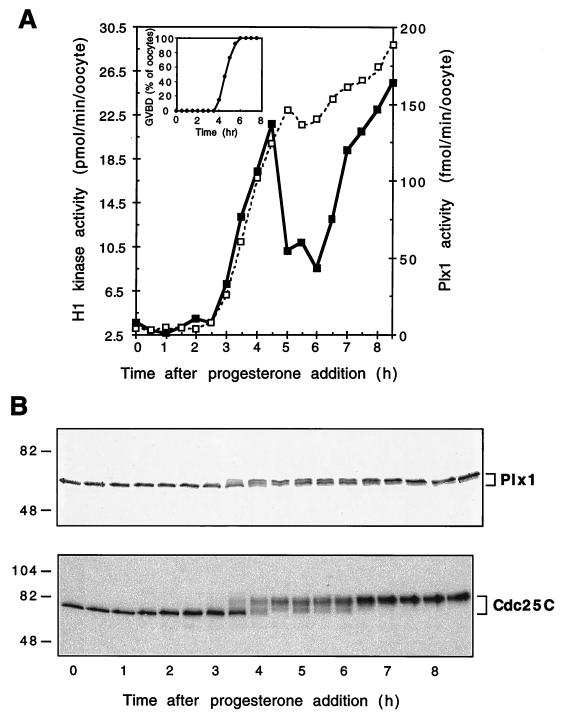
As described in the introduction, in different systems, plks have been implicated either in events at the G2/M transition or in anaphase-telophase events late in mitosis (14, 33). If Plx1 is involved in the G2/M transition in oocytes, its activity should correlate with the activity of other markers of that transition, such as Cdc25C and Cdc2-cyclin B. Therefore, a detailed analysis of Plx1 protein expression and activity was performed during oocyte maturation, a system in which the G2/M transition occurs synchronously after progesterone treatment. The level of Plx1 protein and its electrophoretic mobility were determined by immunoblot analysis, and Plx1 activity was determined by immunoprecipitation and casein kinase assays. Following progesterone addition, the level of Plx1 protein remained constant; however, its activity increased sharply approximately 1 h prior to GVBD in concert with increased histone H1 kinase activity (Fig. 2A and B). This indicates that changes in Plx1 activity are regulated posttranslationally. The increase in Plx1 activity correlated with the appearance of slower-migrating forms upon SDS-PAGE, similar to results reported for mammalian Plk (17) (Fig. 2B). To examine whether phosphorylation is responsible for the activation of Plx1 and its reduced mobility, immunoprecipitates from extracts of mature oocytes were treated with calf intestinal alkaline phosphatase and the effects of dephosphorylation on mobility and casein kinase activity were determined. Immunoprecipitated Plx1 not treated with phosphatase migrated as a slower-migrating form and had high casein kinase activity, whereas a substantial fraction of immunoprecipitated Plx1 treated with phosphatase was converted to the faster-migrating form and fivefold less kinase activity was evident (data not shown). Experiments with 32P-labeled oocytes demonstrated that phosphorylation of Plx1 was on serine and threonine residues. Therefore, in Xenopus oocytes, Plx1 is activated by phosphorylation, as has been reported for plks from other organisms (17, 55).
FIG. 2.
Activation of Plx1 during oocyte maturation. Oocytes were incubated in the presence of progesterone (3.2 μM), and groups of 10 oocytes were frozen at the times indicated. (A) Extracts were prepared, and histone H1 kinase (▪) and Plx1 (□) activities were determined. (Inset) The time of GVBD was established by examining oocytes for white spot formation with a dissecting microscope. (B) Samples of extracts were subjected to immunoblotting with anti-Plx1 or anti-Cdc25C antibodies (upper and lower panels, respectively). Molecular masses (in kilodaltons) are indicated on the left.
The time course of Plx1 activation during oocyte maturation was correlated with the activation of both Cdc25C and cyclin B-Cdc2. The activation of Cdc25C was evident in the appearance on SDS-PAGE of slower-migrating forms, which are known to reflect activation (21, 25, 31), and cyclin B-Cdc2 activity was determined by histone H1 kinase assays (Fig. 2A). As shown in Fig. 2A and B, Plx1 phosphorylation and activation occurred with the same kinetics as observed for the activation of Cdc25C and cyclin B-Cdc2 during meiosis I. However, cyclin B-Cdc2 activity declined during the transition between meiosis I and meiosis II and then rose again during meiosis II (Fig. 2A). In contrast, the activities of both Plx1 and Cdc25C remained elevated after GVBD. These results indicate that Plx1 activity is cell cycle regulated and is likely to play a role in early events at the G2/M transition, and its persistent activation suggests that it may also have a continued role in later events (see below).
Inhibition of Plx1 activation significantly delays both Cdc25C and cyclin B-Cdc2 activation in progesterone-treated oocytes.
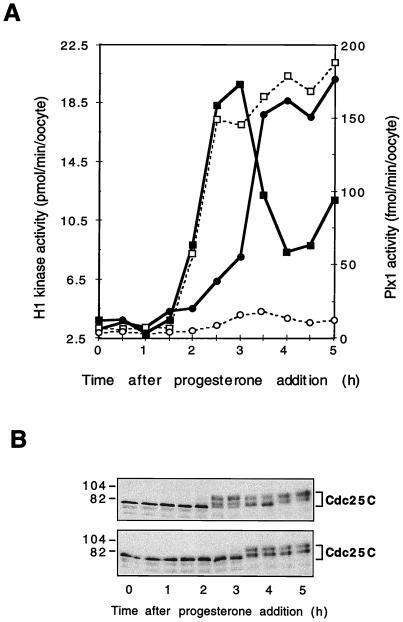
To determine whether activation of Plx1 is required for activation of Cdc25C and Cdc2, affinity-purified Plx1 antibody or control IgG was microinjected into stage VI oocytes. The oocytes were exposed to progesterone 2 h later, and the activities of Plx1, Cdc25C, and cyclin B-Cdc2 were monitored. As shown in Fig. 3A, the activation of Plx1 was inhibited almost completely by the Plx1 antibody but not by the control antibody. The inhibition of Plx1 activation was due to the inhibitory effect of Plx1 antibody on the phosphorylation of Plx1 as judged by the lack of a full electrophoretic shift in immunoblots (data not shown). In addition, the Plx1 antibody significantly delayed the activation of both Cdc25C and cyclin B-Cdc2 (Fig. 3A and B). Since Plx1 can phosphorylate and activate Cdc25C in vitro (30), these results support the hypothesis that Plx1 is a relevant Cdc25C-activating kinase involved in entry into mitosis.
FIG. 3.
Delay of cyclin B-Cdc2 and Cdc25C activation by Plx1 antibodies. Oocytes were microinjected with anti-Plx1 or control IgG (75 ng per oocyte) and then, after 2 h, incubated in the presence of progesterone (3.2 μM). Groups of six oocytes were frozen at the times indicated. (A) Extracts were prepared, and histone H1 kinase and Plx1 activities were determined. Symbols for histone H1 kinase activity, ▪, control IgG; •, anti-Plx1 IgG. Symbols for Plx1 activity: □, control IgG; ○, anti-Plx1 IgG. (B) Samples of extracts were immunoblotted for Cdc25C. Upper panel, control IgG injection; lower panel, anti-Plx1 IgG injection. Molecular masses (in kilodaltons) are indicated on the left.
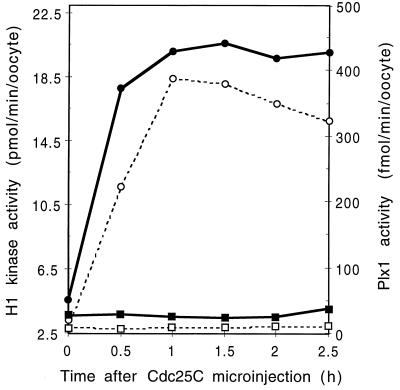
To confirm that the inhibitory effect of Plx1 antibody injection is due solely to its effect on Plx1, rescue experiments were performed. First, stage VI oocytes were injected with Plx1 antibody, and then, 2 h later, recombinant Plx1 or control buffer was injected. Following progesterone addition, the activities of Plx1, Cdc25C, and cyclin B-Cdc2 were determined for the two groups of oocytes. As shown in Fig. 4, recombinant Plx1 overcame the inhibitory effect of Plx1 antibody injection on the activation of both Cdc25C and cyclin B-Cdc2, indicating that the effect of Plx1 antibody is specifically due to its effect on Plx1.
FIG. 4.
Reversal of the inhibitory effect of Plx1 antibodies by Plx1. All oocytes were microinjected with anti-Plx1 IgG (75 ng per oocyte) and then, after 2 h, with either recombinant Plx1 (60 ng per oocyte) or buffer. They were then incubated in the presence of progesterone (3.2 μM), and groups of six oocytes were frozen at the indicated times. (A) Extracts were prepared, and histone H1 kinase and Plx1 activities were determined. Symbols for histone H1 kinase activity: ▪, buffer injection; •, Plx1 injection; Symbols for Plx1 activity: □, buffer injection; ○, Plx1 injection. (B) Samples of extracts were immunoblotted for Cdc25C. Top, buffer injection; bottom, Plx1 injection. Molecular masses (in kilodaltons) are indicated on the left.
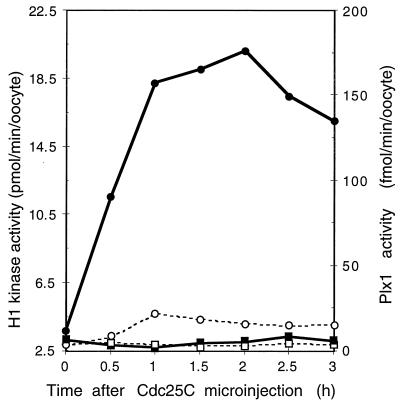
Cdc25C overcomes the inhibitory effect of Plx1 antibody on cyclin B-Cdc2 activation.
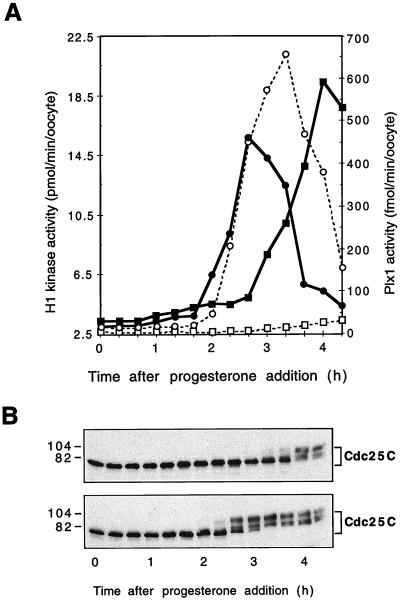
Previous studies in several laboratories have shown that injection of Cdc25C into stage VI oocytes leads to rapid activation of cyclin B-Cdc2 histone H1 kinase activity and oocyte maturation (reference 37; see Fig. 7). If Plx1 is a Cdc25C-activating kinase, injection of Cdc25C should overcome the inhibitory effect of Plx1 antibody injection on activation of cyclin B-Cdc2. Accordingly, oocytes were injected with Plx1 antibody and then 2 h later with recombinant Cdc25C (23), after which both cyclin B-Cdc2 and Plx1 activities were analyzed. As shown in Fig. 5, Cdc25C rapidly induced cyclin B-Cdc2 activation and, as expected, phosphatase-inactive Cdc25C(C457A) did not. Despite the similar kinetics of Plx1 and Cdc25C activation during progesterone-induced oocyte maturation (Fig. 2), this indicates that Plx1 functions upstream of Cdc25C and cyclin B-Cdc2. In antibody-injected oocytes, Plx1 remained inactive even when cyclin B-Cdc2 was activated following Cdc25C injection.
FIG. 7.
Activation of Plx1 in Cdc25C-injected oocytes. Oocytes were microinjected with either wild-type Cdc25C or phosphatase-inactive Cdc25C(C457A) (25 ng per oocyte), and groups of six oocytes were frozen at the indicated times. Extracts were prepared, and histone H1 kinase and Plx1 activities were determined. Symbols for histone H1 kinase activity: ▪, Cdc25C(C457A); •, Cdc25C. Symbols for Plx1 activity: □, Cdc25C(C457A); ○, Cdc25C.
FIG. 5.
Reversal of the inhibitory effect of Plx1 antibodies by Cdc25C. All oocytes were microinjected with anti-Plx1 IgG (75 ng per oocyte) and then, after 2 h, with either wild-type Cdc25C or phosphatase-inactive Cdc25C(C457A) (25 ng per oocyte), and groups of six oocytes were frozen at the indicated times. Extracts were prepared, and histone H1 kinase and Plx1 activities were determined. Symbols for histone H1 kinase activity: ▪, Cdc25C(C457A); •, Cdc25C; Symbols for Plx1 activity: □, Cdc25C(C457A); ○, Cdc25C.
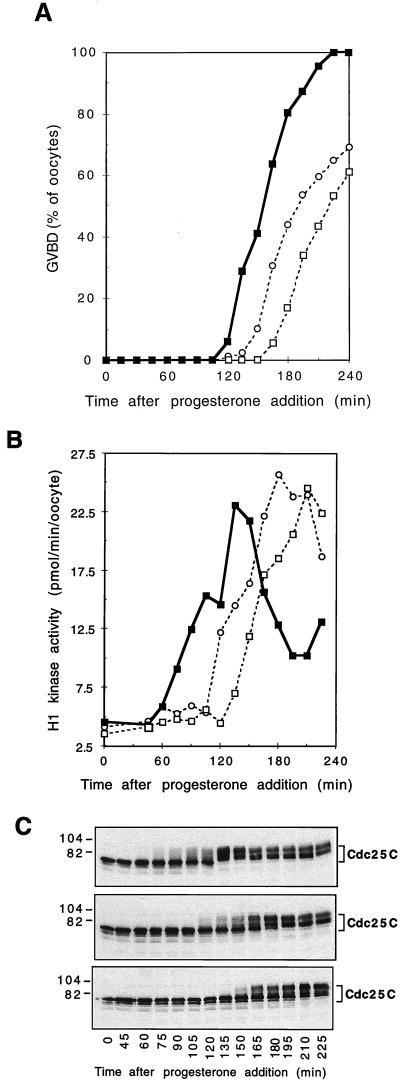
An elevated level of Plx1 accelerates Cdc25C and cyclin B-Cdc2 activation and oocyte maturation.
If Plx1 activates Cdc25C in vivo, an elevated level of Plx1 should accelerate Cdc25C activation. Stage VI oocytes were microinjected with recombinant wild-type Plx1, kinase-dead Plx1(N172A), or buffer and subsequently exposed to a threshold level of progesterone (48 nM) which is just sufficient to cause 100% GVBD. Under this condition, the elevated level of wild-type Plx1 accelerated Cdc25C and cyclin B-Cdc2 activation and also GVBD (Fig. 6). Interestingly, kinase-inactive Plx1(N172A) had a dominant-negative effect, delaying the activation of both Cdc25C and cyclin B-Cdc2 (Fig. 6). This result is consistent with the inhibitory effect of Plx1 antibody injection on these events (Fig. 3) and further supports an important role for Plx1 in the pathway of Cdc25C activation.
FIG. 6.
Acceleration of progesterone-induced maturation by Plx1. Oocytes were microinjected with either buffer, wild-type Plx1, or kinase-dead Plx1(N172A) (75 ng per oocyte) and then incubated in the presence of progesterone (48 nM). Groups of six oocytes were frozen at the indicated times. (A) Time course of maturation. Symbols: ▪, Plx1; ○, buffer; □, Plx1(N172A). (B) Extracts were prepared, and histone H1 kinase activity was determined. Symbols: ▪, Plx1; ○, buffer; □, Plx1(N172A). (C) Samples of extracts were immunoblotted for Cdc25C. Top, Plx1; middle, buffer; bottom, Plx1(N172A). Molecular masses (in kilodaltons) are indicated on the left.
Plx1 is activated during Cdc25C-induced oocyte maturation.
Many biological systems contain positive feedback loops. For example, a positive feedback loop exists between Cdc25C and cyclin B-Cdc2 (23) and between Mos synthesis and mitogen-activated protein kinase (40, 48). To investigate whether a positive feedback loop exists between Plx1 and downstream elements, oocytes were injected with recombinant Cdc25C, after which both cyclin B-Cdc2 and Plx1 activities were analyzed. As shown in Fig. 7, wild-type, but not phosphatase-inactive, Cdc25C rapidly induced the activation of both cyclin B-Cdc2 and Plx1, indicating that a positive feedback loop also exists between the Plx1 pathway and downstream components.
Plx1 function is required for mitotic spindle organization.
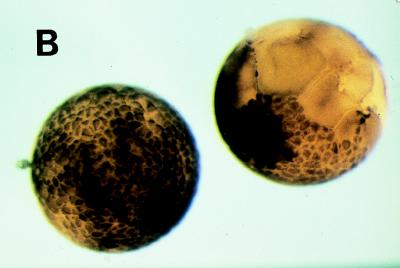
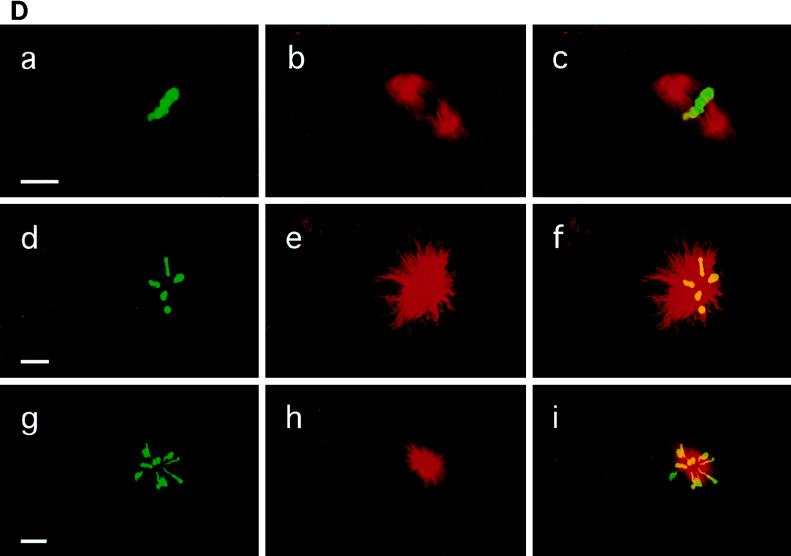
As described in Fig. 2, Plx1 activity remains high after full activation of cyclin B-Cdc2 at GVBD, suggesting that it might have additional functions besides activation of Cdc25C. To investigate this possibility, the effect of abrogation of Plx1 activity was investigated in early Xenopus embryos. In early embryonic cleavage cycles 2 to 12, cyclin B-Cdc2 is not tyrosine phosphorylated (9, 19), and thus, Cdc25C function is not required for cyclin B-Cdc2 activation. Instead, Cdc2 activation is controlled by the rate of translation of cyclin B (19, 43). Therefore, these cell cycles are well suited for investigating Plx1 functions distinct from Cdc25C activation. In early embryos, Plx1 is significantly deactivated at fertilization, increases dramatically at the first mitosis, and shows small oscillations in subsequent cell cycles (Fig. 8A). The decline in Plx1 activity at each cycle occurred later than the decline in H1 kinase activity. As shown in Fig. 3, injection of anti-Plx1 antibody into oocytes effectively inhibits the activation of Plx1. Therefore, to determine whether Plx1 function is important in early embryonic cell cycles, one blastomere of a two-cell embryo was injected with either anti-Plx1 IgG or control IgG. The uninjected blastomere served as an additional control. Injection of Plx1 antibody into the embryo caused cleavage arrest, whereas control IgG injection had no effect relative to uninjected controls (Fig. 8B). In various organisms, plks have been localized to spindles and implicated in mitotic spindle formation or centrosome replication or function. In addition, spindle abnormalities or altered cytokinesis events have been seen in other systems after perturbation of plk function (14, 33, 35, 39, 44). In embryonic Xenopus cells, Plx1 was found to be localized to normal mitotic spindles by confocal microscopy (Fig. 8C), similar to results obtained with mammalian cells (15, 36). As shown in Fig. 8C, early in prophase, Plx1 had a bipolar localization at the spindle poles and colocalized with α-tubulin. As shown in Fig. 8D, inhibition of Plx1 resulted in monopolar spindles with altered patterns of α-tubulin associated with a radial distribution of chromosomes around the pole. Monopolar spindles have also been described in Drosophila embryos with loss-of-function mutations in polo (39) and in mammalian cells after injection of anti-Plk1 antibodies (32). Together, these results indicate that Plx1 function is required for spindle organization, an event that occurs in late mitosis, and also for activation of Cdc25C, which occurs in the initial stages of mitosis.
FIG. 8.
Plx1 antibody arrests cleavage in early embryos. (A) Eggs were fertilized, and groups of 10 embryos were frozen at the indicated times. Extracts were prepared, and histone H1 kinase (▪) and Plx1 (□) activities were determined. (B) Plx1 antibody or control IgG was microinjected into one blastomere of a two-cell embryo, and embryonic development was monitored with a dissecting microscope. Plx1 antibody-injected blastomeres (right) ceased dividing one or two divisions after injection, whereas control antibody-injected cells continued to divide normally (left). (C) Cells in a late-blastula-stage embryo were fixed for confocal microscopy as described in Materials and Methods; a cell in early prophase is presented. a, Plx1 fluorescence; b, α-tubulin fluorescence of the same cell, staining spindle poles; c, merged image of a and b showing enrichment of Plx1 at the spindle poles. Bar, 15 μm. (D) Embryos were injected with either control IgG (a to c) or anti-Plx1 IgG (d to i) and processed for confocal microscopy as described in Materials and Methods. a, d, and g, DNA staining; b, e, and h, α-tubulin fluorescence staining; c, f, and i, merged images of a and b, d and e, and g and h, respectively. Bars, 15 μm.
DISCUSSION
The results reported here establish that the activity of Plx1 is cell cycle regulated and involved in the G2/M transition during Xenopus oocyte maturation. The inhibitory effects of Plx1 antibody injection, as well as the effects of wild-type and kinase-dead Plx1 on progesterone-induced Cdc25C activation, demonstrate that Plx1 functions in the Cdc25C activation pathway. Several lines of evidence support the hypothesis that these effects occur due to direct phosphorylation of Cdc25C by Plx1. First, Cdc25C is a substrate for Plx1 in vitro (30; our unpublished data). Second, activation of Plx1 correlates exactly with activation of Cdc25C during both meiosis I and meiosis II and Plx1 antibody or kinase-dead Plx1 significantly delays the phosphorylation and activation of Cdc25C. Third, Cdc25C itself can overcome the inhibitory effect of Plx1 antibody. These results support the hypothesis that Plx1 could be a trigger kinase, also termed kinase X (24), that initiates the positive feedback loop between Cdc25C and cyclin B-Cdc2. The existence of a trigger kinase had been predicted from studies in which initial activation of Cdc25C occurred prior to or in the absence of cyclin B-Cdc2 (24). The results shown here indicate that Plx1 may be a trigger kinase, but they do not exclude the possibility that other kinases also function as trigger kinases. Since injection of Plx1 antibody almost completely inhibited Plx1 activity yet did not completely block Cdc25C activation, it is likely that, in addition to Plx1 and cyclin B-Cdc2, other protein kinases also contribute to Cdc25C activation. Such a situation would not be unusual, since multiple protein kinases control the activity of the protein kinase Wee1 (26, 46, 54). It remains to be determined whether Plx1 can also phosphorylate and inhibit Wee1 or Myt1, which might further enhance a trigger kinase function governing entry into mitosis.
The results presented here indicate that the regulatory element upstream of Plx1 in the pathway that activates Cdc25C is a serine/threonine kinase. In both Drosophila and human cells, an electrophoretic shift of plk similar to that described here has been reported (17, 55), and a variety of evidence indicates that this shift is due to phosphorylation. This suggests that a general activation mechanism involving a protein kinase cascade, with a kinase that activates Plx1, has been conserved during evolution. Plx1 contains a single consensus site for phosphorylation by cyclin B-Cdc2, but phosphorylation in vitro by cyclin B-Cdc2 does not activate human Plk1 (17) or Plx1 (our unpublished data). At present, there are no genetic or biochemical candidates for a Plx1-activating kinase, but its eventual identification should contribute to further understanding of the pathway of Cdc25C activation and its role in the G2/M transition and the DNA replication checkpoint. In mammalian cells, changes in the abundance of Plk1 have been noted during the cell cycle and may also contribute to Plk1 activity (15, 17, 36), but in Xenopus oocyte and embryonic cell cycles, no change in the abundance of Plx1 was observed (Fig. 2 and data not shown).
Plks are members of a small subset of protein kinases that generate phosphoepitopes recognized by the MPM-2 antibody (3, 27, 53). This antibody recognizes a proline-directed epitope and reacts with a large number of proteins phosphorylated specifically at mitosis, which are located on several important mitotic structures, including centrosomes and kinetochores (56, 57). Cdc25C has been reported to exhibit MPM-2 immunoreactivity in mitosis, and both cyclin B-Cdc2 and Plx1 phosphorylate Cdc25C in vitro at MPM-2 epitopes (28, 30). Since Plx1 remains active during oocyte maturation even after entry into M phase, it seems likely that Plx1 also has other functions later in M phase which may involve MPM-2 immunoreactive components on centrosomes and spindles. Consistent with this idea, inhibition of Plx1 function in embryonic cleavage cycles disrupted bipolar-spindle formation and resulted in monopolar spindles displaying a radial array of chromosomes. Moreover, Plx1 activity remained high for a significant period after the metaphase/anaphase transition (Fig. 8A), consistent with a role in spindle assembly. Spindle abnormalities have been seen in other systems when plk function is impaired (32, 35, 39, 44), suggesting that plk may regulate centrosome or spindle pole body duplication or function, but the exact function of plk during spindle assembly has not yet been elucidated. In lower organisms, such as budding yeast and D. melanogaster, plk function has been evident only in late mitotic events involving spindle formation and cytokinesis (14, 33). As reported here, Plx1 also performs similar functions in X. laevis. In addition, Plx1 is important earlier in mitosis for the initial activation of Cdc25C, fulfilling the criteria expected for a trigger kinase. There are several reasons why this earlier effect may not have been observed in yeast and D. melanogaster. First, in budding yeast, cyclin B-Cdc2 activation is not normally controlled by Cdc25 activation and tyrosine dephosphorylation (1, 38). In fission yeast and D. melanogaster, Cdc25 activation is crucial but its activity level is controlled by transcription rather than by posttranslational modification (5, 41). In human cells, Cdc25C activity is controlled by phosphorylation, as in X. laevis (21, 25, 31), but since inhibition of plk function only delays mitosis, the plk loss-of-function phenotype was observed primarily as abnormalities of spindle organization in various cell lines (32). Interestingly, however, in nonimmortalized mammalian cells, Plk1 antibody injection increases the fraction of cells in G2, perhaps reflecting impairment of the Cdc25C activation pathway (32).
The finding here that Plx1 functions at two discrete points in mitosis is consistent with the pleiotropic action of other cell cycle-regulated protein kinases. For example, cyclin B-Cdc2 is required not only to initiate the G2/M transition but also to maintain metaphase (22). The Mos–mitogen-activated protein kinase cascade is required in X. laevis not only to cause cell cycle progression in meiosis I but also to cause metaphase arrest in meiosis II (49, 50). In the case of Plx1, the progesterone-treated oocyte is well suited for analysis of its role in Cdc25C activation, whereas embryos offer a different type of cell cycle, in which effects of Plx1 on spindle organization are evident. Further work using both of these systems should enhance elucidation of plk functions that are conserved during evolution.
ACKNOWLEDGMENTS
We thank Sang H. Kim for the purified Cdc25C preparations, Kyung S. Lee and R. L. Erikson for the gift of Plk1 constructs and helpful suggestions, and Jill C. Sible for helpful discussions and critical reading of the manuscript. The baculovirus-infected Sf9 cells were produced in the tissue culture/monoclonal antibody core facility at the University of Colorado Cancer Center.
This work was supported by a grant from the NIH (GM26743). Y.-W.Q. is an Associate and J.L.M. an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Amon A, Surana U, Muroff I, Nasmyth K. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. Nature. 1992;355:368–371. doi: 10.1038/355368a0. [DOI] [PubMed] [Google Scholar]
- 2.Dasso M, Newport J W. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell. 1990;61:811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- 3.Davis F M, Tsao T Y, Fowler S K, Rao P N. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci USA. 1983;80:2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunphy W G, Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;67:189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- 5.Edgar B A, Lehman D A, O’Farrell P H. Transcriptional regulation of string (cdc25): a link between developmental programming and the cell cycle. Development. 1994;120:3131–3143. doi: 10.1242/dev.120.11.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 7.Enoch T, Nurse P. Coupling M phase and S phase: controls maintaining the dependence of mitosis on chromosome replication. Cell. 1991;65:921–923. doi: 10.1016/0092-8674(91)90542-7. [DOI] [PubMed] [Google Scholar]
- 8.Fenton B, Glover D M. A conserved mitotic kinase active at late anaphase-telophase in syncytial Drosophila embryos. Nature. 1993;363:637–640. doi: 10.1038/363637a0. [DOI] [PubMed] [Google Scholar]
- 9.Ferrell J E, Jr, Wu M, Gerhart J C, Martin G S. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991;11:1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- 11.Gard D L, Hafezi S, Zhang T, Doxsey S J. Centrosome duplication continues in cycloheximide-treated Xenopus blastulae in the absence of a detectable cell cycle. J Cell Biol. 1990;110:2033–2042. doi: 10.1083/jcb.110.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautier J, Matsukawa T, Nurse P, Maller J. Dephosphorylation and activation of Xenopus p34cdc2 protein kinase during the cell cycle. Nature. 1989;339:626–629. doi: 10.1038/339626a0. [DOI] [PubMed] [Google Scholar]
- 13.Gautier J, Solomon M J, Booher R N, Bazan J F, Kirschner M W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- 14.Glover D M, Ohkura H, Tavares A. Polo kinase: the choreographer of the mitotic stage? J Cell Biol. 1996;135:1681–1684. doi: 10.1083/jcb.135.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golsteyn R M, Mundt K E, Fry A M, Nigg E A. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J Cell Biol. 1995;129:1617–1628. doi: 10.1083/jcb.129.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gould K L, Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989;342:39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- 17.Hamanaka R, Smith M R, O’Connor P M, Maloid S, Mihalic K, Spivak J L, Longo D L, Ferris D K. Polo-like kinase is a cell cycle-regulated kinase activated during mitosis. J Biol Chem. 1995;270:21086–21091. doi: 10.1074/jbc.270.36.21086. [DOI] [PubMed] [Google Scholar]
- 18.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 19.Hartley R S, Rempel R E, Maller J L. In vivo regulation of the early embryonic cell cycle in Xenopus. Dev Biol. 1996;173:408–419. doi: 10.1006/dbio.1996.0036. [DOI] [PubMed] [Google Scholar]
- 20.Hartwell L H, Weinert T A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann I, Clarke P R, Marcote M J, Karsenti E, Draetta G. Phosphorylation and activation of human cdc25-C by cdc2-cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 1993;12:53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holloway S L, Glotzer M, King R W, Murray A W. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell. 1993;73:1393–1402. doi: 10.1016/0092-8674(93)90364-v. [DOI] [PubMed] [Google Scholar]
- 23.Izumi T, Maller J L. Elimination of cdc2 phosphorylation sites in the cdc25 phosphatase blocks initiation of M-phase. Mol Biol Cell. 1993;4:1337–1350. doi: 10.1091/mbc.4.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izumi T, Maller J L. Phosphorylation and activation of the Xenopus Cdc25 phosphatase in the absence of Cdc2 and Cdk2 kinase activity. Mol Biol Cell. 1995;6:215–226. doi: 10.1091/mbc.6.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izumi T, Walker D H, Maller J L. Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kornbluth S, Sebastian B, Hunter T, Newport J. Membrane localization of the kinase which phosphorylates p34cdc2 on threonine 14. Mol Biol Cell. 1994;5:273–282. doi: 10.1091/mbc.5.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuang J, Ashorn C L. At least two kinases phosphorylate the MPM-2 epitope during Xenopus oocyte maturation. J Cell Biol. 1993;123:859–868. doi: 10.1083/jcb.123.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuang J, Ashorn C L, Gonzalez-Kuyvenhoven M, Penkala J E. cdc25 is one of the MPM-2 antigens involved in the activation of maturation-promoting factor. Mol Biol Cell. 1994;5:135–145. doi: 10.1091/mbc.5.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumagai A, Dunphy W G. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell. 1991;64:903–914. doi: 10.1016/0092-8674(91)90315-p. [DOI] [PubMed] [Google Scholar]
- 30.Kumagai A, Dunphy W G. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- 31.Kumagai A, Dunphy W G. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- 32.Lane H A, Nigg E A. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lane H A, Nigg E A. Cell-cycle control: POLO-like kinases join the outer circle. Trends Cell Biol. 1997;7:63–68. doi: 10.1016/S0962-8924(96)10051-9. [DOI] [PubMed] [Google Scholar]
- 34.Langan T A, Gautier J, Lohka M, Hollingsworth R, Moreno S, Nurse P, Maller J, Sclafani R A. Mammalian growth-associated H1 histone kinase: a homolog of cdc2+/CDC28 protein kinases controlling mitotic entry in yeast and frog cells. Mol Cell Biol. 1989;9:3860–3868. doi: 10.1128/mcb.9.9.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee K S, Erikson R L. Plk is a functional homolog of Saccharomyces cerevisiae Cdc5, and elevated Plk activity induces multiple septation structures. Mol Cell Biol. 1997;17:3408–3417. doi: 10.1128/mcb.17.6.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee K S, Yuan Y L, Kuriyama R, Erikson R L. Plk is an M-phase-specific protein kinase and interacts with a kinesin-like protein, CHO1/MKLP-1. Mol Cell Biol. 1995;15:7143–7151. doi: 10.1128/mcb.15.12.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee M S, Ogg S, Xu M, Parker L L, Donoghue D J, Maller J L, Piwnica-Worms H. cdc25+ encodes a protein phosphatase that dephosphorylates p34cdc2. Mol Biol Cell. 1992;3:73–84. doi: 10.1091/mbc.3.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lew D J, Reed S I. A cell cycle checkpoint monitors cell morphogenesis in budding yeast. J Cell Biol. 1995;129:739–749. doi: 10.1083/jcb.129.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Llamazares A, Moreira A, Tavares A, Girdam C, Spruce B A, Gonzales C, Karess R E, Glover D M, Sunkel C E. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 1991;5:2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- 40.Matten W T, Copeland T D, Ahn N G, Vande Woude G F. Positive feedback between MAP kinase and Mos during Xenopus oocyte maturation. Dev Biol. 1996;179:485–492. doi: 10.1006/dbio.1996.0277. [DOI] [PubMed] [Google Scholar]
- 41.Moreno S, Nurse P, Russell P. Regulation of mitosis by cyclic accumulation of p80Cdc25 mitotic inducer in fission yeast. Nature. 1990;344:549–552. doi: 10.1038/344549a0. [DOI] [PubMed] [Google Scholar]
- 42.Mueller P R, Coleman T R, Kumagai A, Dunphy W G. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 1995;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- 43.Murray A W, Solomon M J, Kirschner M W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- 44.Ohkura H, Hagan I M, Glover D M. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 1995;9:1059–1073. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- 45.Parker L L, Atherton-Fessler S, Piwnica-Worms H. p107wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc Natl Acad Sci USA. 1992;89:2917–2921. doi: 10.1073/pnas.89.7.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker L L, Walter S A, Young P G, Piwnica-Worms H. Phosphorylation and inactivation of the mitotic inhibitor Wee1 by the nim1/cdr1 kinase. Nature. 1993;363:736–738. doi: 10.1038/363736a0. [DOI] [PubMed] [Google Scholar]
- 47.Peng C Y, Graves P R, Thoma R S, Wu Z, Shaw A S, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 48.Roy L M, Haccard O, Izumi T, Lattes B G, Lewellyn A L, Maller J L. Mos proto-oncogene function during oocyte maturation in Xenopus. Oncogene. 1996;12:2203–2211. [PubMed] [Google Scholar]
- 49.Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude G F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988;335:519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- 50.Sagata N, Watanabe N, Vande Woude G F, Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989;342:512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez Y, Wong C, Thoma R S, Richman R, Wu Z, Piwnica-Worms H, Elledge S J. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 52.Smythe C, Newport J W. Coupling of mitosis to the completion of S phase in Xenopus occurs via modulation of the tyrosine kinase that phosphorylates p34cdc2. Cell. 1992;68:787–797. doi: 10.1016/0092-8674(92)90153-4. [DOI] [PubMed] [Google Scholar]
- 53.Taagepera S, Dent P, Her J H, Sturgill T W, Gorbsky G J. The MPM-2 antibody inhibits mitogen-activated protein kinase activity by binding to an epitope containing phosphothreonine-183. Mol Biol Cell. 1994;5:1243–1251. doi: 10.1091/mbc.5.11.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang Z, Coleman T R, Dunphy W G. Two distinct mechanisms for negative regulation of the Wee1 protein kinase. EMBO J. 1993;12:3427–3436. doi: 10.1002/j.1460-2075.1993.tb06017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tavares A A, Glover D M, Sunkel C E. The conserved mitotic kinase polo is regulated by phosphorylation and has preferred microtubule-associated substrates in Drosophila embryo extracts. EMBO J. 1996;15:4873–4883. [PMC free article] [PubMed] [Google Scholar]
- 56.Vandre D D, Davis F M, Rao P N, Borisy G G. Distribution of cytoskeletal proteins sharing a conserved phosphorylated epitope. Eur J Cell Biol. 1986;41:72–81. [PubMed] [Google Scholar]
- 57.Westendorf J M, Rao P N, Gerace L. Cloning of cDNAs for M-phase phosphoproteins recognized by the MPM2 monoclonal antibody and determination of the phosphorylated epitope. Proc Natl Acad Sci USA. 1994;91:714–718. doi: 10.1073/pnas.91.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]