Abstract
Background:
To construct an effective prognostic index to predict overall survival (OS) and triplet regimen efficacy for advanced gastric cancer (AGC) patients treated with platinum-based and fluorouracil-based chemotherapy.
Objectives:
Between 2011 and 2021, 679 patients from two randomized phase III trials and one phase II trial were enrolled.
Designs:
We collected 11 baseline clinicopathological and 14 hematological parameters to establish a prognostic index.
Methods:
Univariate and multivariate Cox analyses were used to screen prognostic factors, and a prognostic index nomogram was conducted.
Results:
Seven prognostic factors were identified: primary tumor site in the non-proximal gastric area, signet-ring cell carcinoma (SRCC)/mucinous carcinoma, peritoneal metastasis, neutrophil count higher than the upper limit of normal value (ULN), lymphocyte count lower than the lower limit of normal value, lactate dehydrogenase level higher than the ULN, and alkaline phosphatase level higher than the ULN as significant for prognosis. A prognostic nomogram named the Fudan advanced gastric cancer prognostic risk score (FARS) index was constructed, and patients in the high-risk group had significantly shorter OS than those in the low-risk group (median OS, 15.5 versus 8.0 months, p < 0.001). The areas under the curve of the FARS index for 1-, 2-, and 3-year OS were 0.70, 0.72, and 0.77, respectively. A validation and external cohort verified the prognostic value of the FARS index. Moreover, three triplet regimen efficacy parameters were identified: SRCC/mucinous adenocarcinoma, primary tumor location in the non-proximal gastric area, and peripheral neutrophil count higher than the ULN; a TRIS index was subsequently conducted. In patients with any two of the three parameters, the triplet regimen showed significantly longer OS than the doublet regimen (p = 0.018).
Conclusion:
The constructed FARS index to predict the OS of AGC patients and the TRIS index to screen out the dominant population for triplet regimens can be used to aid clinical decision-making and individual risk stratification.
Keywords: advanced gastric cancer, chemotherapy, overall survival, prognostic index, triplet regimen efficacy predictive index
Plain language summary
A prognostic index in locally advanced and metastatic gastric cancer
To date, no recognized systematic prognostic score has been established for advanced gastric cancer (AGC). Our research aims to construct an effective prognostic index to predict overall survival (OS) for AGC patients to aid clinical decision-making and individual risk stratification. In our research, seven prognostic factors were identified: primary tumor site in the non-proximal gastric area, signet-ring cell carcinoma (SRCC)/mucinous carcinoma, peritoneal metastasis, neutrophil count higher than the upper limit of normal value (ULN), lymphocyte count lower than the lower limit of normal value, lactate dehydrogenase level higher than the ULN, and alkaline phosphatase level higher than the ULN as significant for prognosis. A prognostic index named the Fudan advanced gastric cancer prognostic risk score (FARS) index was constructed, and patients in the high-risk group had significantly shorter OS than those in low-risk group (median OS, 15.5 months vs. 8.0 months, P < 0.001). Moreover, three triplet regimen efficacy parameters were identified: SRCC/mucinous adenocarcinoma, primary tumor location in the non-proximal gastric area, and peripheral neutrophil count higher than the ULN; a TRIS index was subsequently conducted. In patients with any two of the three parameters, the triplet regimen showed significantly longer OS than the doublet regimen (P = 0.018).
Introduction
Gastric cancer is the fourth most common cancer worldwide and ranks second in terms of mortality. In China, 84% of gastric cancer cases progress to the advanced stage. Over the past few decades, chemotherapy has been the primary treatment for advanced gastric cancer (AGC). Patients receiving the best supportive care for AGC have a median survival of 3–4 months; however, combination chemotherapy significantly prolongs their median overall survival (mOS) to 7–11 months and enhances their quality of life.1,2 In the past 2 years, programmed cell death protein-1 (PD-1) antibody plus chemotherapy has been approved as the first-line treatment of AGC by the Food and Drug Administration and National Medical Products Administration. However, the Checkmate-649 clinical trial showed that compared with the chemotherapy group, the mOS of the nivolumab plus chemotherapy group was prolonged by only 2.2 months in all randomized populations. 3 Therefore, chemotherapy remains the cornerstone of AGC treatment.
To date, no recognized systematic prognostic score has been established for AGC. In 2004, a prognostic index for AGC named the Japan Clinical Oncology Group (JCOG) prognostic index 4 was established based on three well-known clinical trials from 1992 to 2001 in Europe.5–7 In the 1990s, the recommended chemotherapy regimens for AGC were epirubicin, cisplatin, and fluorouracil (ECF); methotrexate, doxorubicin, and fluorouracil (FAMTX); and methotrexate, cisplatin, and fluorouracil. In the 21st century, next-generation cytotoxic drugs (capecitabine, S-1, and oxaliplatin) including epirubicin, oxaliplatin, and capecitabine (EOX); epirubicin, oxaliplatin, and fluorouracil (EOF); oxaliplatin and capecitabine (XELOX); and oxaliplatin and S-1 (SOX) became important components of the first-line chemotherapy regimen for AGC. Previous trials demonstrated that the substitution of oxaliplatin for cisplatin or capecitabine for 5-fluorouracil, oxaliplatin, or capecitabine was, at minimum, non-inferior to cisplatin or 5-fluorouracil, respectively; or resulted in a trend toward longer median progression-free survival or mOS.8,9 In our previous EXELOX clinical trial, the XELOX doublet regimen is non-inferior to the EOX triplet regimen as the first-line treatment of AGC. 10 Therefore, the JCOG prognostic index is no longer appropriate for AGC since the updated iteration of a chemotherapy regimen. In the past two decades, many studies have been conducted on prognostic factors for AGC; however, studies developing a widely recognized systemic prognostic index remain lacking.
In the late 20th century and early 21st century, the ECF triplet regimen was widely used.5,6 The REAL-2 study then demonstrated that capecitabine and oxaliplatin had comparable efficacy to fluorouracil and cisplatin: thus, EOX, EOF, and ECX (epirubicin, oxaliplatin, and capecitabine) regimens were subsequently recommended by the guidelines. 11 However, the status of epirubicin AGC guidelines gradually declined. Until recently, the triplet regimen still exists in NCCN guidelines as a recommended regimen for first-line treatment in AGC. 12 However, at present, the doublet regimen is the preferred chemotherapy regimen in most phase III clinical trials such as the checkmate-649 and Keynote-859. Our previous large-sample randomized phase III trial EXELOX also showed that the XELOX doublet regimen was not inferior to the EOX triplet regimen. 10 However, subgroup analysis in this study showed that some patients with adverse prognostic factors receiving the triplet regimen had longer survival than those receiving the doublet regimen. The objective response rate was higher in the triplet regimen group than in the doublet regimen group. Therefore, retrospective analysis of large sample data may screen out the dominant population for the triplet regimen in AGC treatment.
In the present study, we enrolled patients from three clinical trials in our center: the EXELOX trial (NCT02395640), 10 EOF trial (NCT00767377), 13 and the TXE trial (NCT01963702). 14 In these trials, the patients received next-generation chemotherapy regimens such as XELOX and EOX. First, we aimed to assess the prognostic significance of clinical, pathological, hematological, and biochemical parameters and screen out the meaningful prognostic factors to establish a systemic prognostic index. Second, we aimed to explore the predictive factors for the triplet regimen to screen out the dominant population.
Methods
Patients’ enrollment
The retrospective study was conducted at the Fudan University Shanghai Cancer Centre, covering data from November 2011 to August 2020. The inclusion criteria for the present study were patients (i) with unresectable, locally advanced or metastatic gastric or gastroesophageal junction cancer, (ii) with histologically confirmed adenocarcinoma, and (iii) who participated in first-line AGC clinical trials conducted in our site including EXELOX trial (NCT02395640), EOF trial (NCT00767377), and TXE trial (NCT01963702).
In total, 679 patients were enrolled, which was identified as the training cohort. Subsequently, we established a validation cohort of 50 metastatic gastric cancer (MGC) patients randomly selected from the patients in our center. Moreover, 37 patients from Shanxi Cancer Hospital and Anhui Cancer Hospital in the EXELOX trial were identified as external validation cohorts. The patients were regularly followed up after treatment, and the treatment efficacy was evaluated every 6 weeks.
Inclusion of clinical parameters
Our study aimed to establish a prognostic model for MGC; thus, we included 11 and 14 baseline clinicopathological and hematological parameters, respectively. These parameters are reportedly prognostic factors for gastric cancer as well as other tumors.
The 11 baseline clinicopathological parameters comprised ECOG (Eastern Cooperative Oncology Group Performance Status) (0–2), sex (male or female), age (years), histological grade, pathological type (adenocarcinoma or mucinous adenocarcinoma/signet-ring cell carcinoma [SRCC]), primary tumor location (proximal or non-proximal), liver metastasis (yes or no), lung metastasis (yes or no), peritoneal metastasis (yes or no), ovary metastasis (yes or no), and a number of metastatic sites at random (1–3). The 14 baseline hematological parameters included peripheral hemoglobin (Hb) count (g/L), white blood cell (WBC) count (×109/L), neutrophil count (×109/L), lymphocyte count (×109/L), monocyte count (×109/L), eosinophil count (×109/L), basophil count (×109/L), platelet count (*109/L), albumin (g/L), lactate dehydrogenase (LDH) (U/L), alkaline phosphatase (ALP) (U/L), γ-glutamyl transferase (γ-GGT) (U/L), CEA (U/L), and CA199 (U/L).
Univariate Cox analysis was performed to screen out each prognostic factor. Because the hematological parameters were all continuous variables, we deem it inconvenient for clinical practice and wide utilization. Thus, we transformed them into categorical variables according to the upper limit of normal (ULN) or lower limit of normal (LLN). Accordingly, lymphocyte count and albumin were transformed into categorical variables according to their LLN, whereas the remaining parameters were transformed into categorical variables according to their ULN.
The nomogram establishing
Prognostic factors screened out using the univariate Cox analysis were included in the multivariate Cox analysis. The factors contained in the nomogram are similar to those selected using multivariate Cox analysis. The nomogram is established through the ‘RMS’ package. The detail of ‘RMS’ package is available at http://cran.r-project.org/web/packages/rms. We then established a risk score system according to the nomogram system. The risk score was calculated as the summation of the product of each prognostic factor and the corresponding risk index. To visualize the prognosis of patients with different risk scores, the nomogram also lists 1-year, 2-year, and 3-year survival rates at different risk levels.
The cutoff value of the risk score
To better determine the threshold of high-risk patients after calculating the risk score, the ‘Surv_cutpoint’ function of the ‘Survminer’ package was used to determine the optimal cutoff point. The ‘Survminer’ package is a visual survival analysis package commonly used in the R language. The ‘Surv_cutpoint’ function of the ‘Survminer’ package used a maximally selected rank statistical method to determine the optimal cutoff value. This is an outcome-oriented method providing a cutoff value that corresponds to the most significant relationship with the overall survival (OS) time. The cutoff value was determined using OS data of the training cohort. To verify the generality and accuracy of our risk score system, the formula for its calculation and cutoff value was used in the validation and external cohort to determine the high-risk group.
Statistical analysis
All statistical analyses were performed using R version 4.0.3 (http://cran.r-project.org) and Stata statistical software, version 14.0 (StataCorp, College Station, TX, USA). All p values were two-sided, and statistical significance was set at p < 0.05 if not mentioned. All confidence intervals (CIs) were stated at the 95% confidence level.
The reporting of this study conforms to the Reporting recommendations for tumor marker prognostic studies (REMARK) statement. 15
Results
Patient’s clinical characteristics and correlation with prognosis
In total, 679 patients were diagnosed with AGC in the Fudan University Shanghai Cancer Centre; of them, 397 were from the EXELOX trial (NCT02395640), 149 from the EOF trial (NCT00767377), and 133 from the TXE trial (NCT01963702). The EXELOX trial was a multi-center clinical trial enrolling 448 patients; of them, 397 were treated in our center, and 51 were from 6 other centers. The 397 patients from our center were subsequently enrolled in the training cohort of the present study. The EOF and TXE trials reported 150 and 134 patients who could be evaluated, respectively. In both studies, one patient was excluded because of unavailable baseline hematological parameters. For the first-line treatment, 264 patients received the XELOX regimen, 197 received the EOX regimen, 149 received the EOF regimen, and 69 received the TX (docetaxel and capecitabine) regimen. No significant difference in overall survival was observed among these patients (p = 0.653). Univariate analysis was performed to evaluate the prognostic value of clinical characteristics including ECOG, sex, age, histologic grade, pathologic type, liver metastasis, lung metastasis, peritoneal metastasis, ovary metastasis, and number of metastatic sites at random; the results are shown in Table 1. Patients with adenocarcinoma had significantly longer OS than those with mucinous carcinoma/SRCC (p = 0.000). Patients whose primary tumor location was in the proximal gastric area exhibited longer OS than those whose primary tumor location was in the non-proximal gastric area (p = 0.001). Patients with peritoneal metastasis had significantly shorter OS than those without (p = 0.000). Patients with lung metastasis exhibited significantly longer OS than those without (p = 0.032), which did not correspond with clinical practice. We observed that the rate of peritoneal metastasis in patients with lung metastasis was 14% (9/62), significantly lower than that in patients without lung metastasis, 26% (161/617). Hence, we excluded lung metastasis from further analysis.
Table 1.
Univariate analysis of the association between clinicopathologic parameters and survival in the training cohort.
| Characteristics | No. (%) | Median OS (months) | p |
|---|---|---|---|
| ECOG | 0.260 | ||
| 0 | 67 (9.9) | 13.1 | |
| 1–2 | 612 (90.1) | 12.0 | |
| Sex | 0.405 | ||
| Male | 437 (65.4) | 12.0 | |
| Female | 242 (34.6) | 12.0 | |
| Age | 0.522 | ||
| 65 | 160 (23.6) | 12.6 | |
| ⩽65 | 519 (76.4) | 12.0 | |
| Histologic grade | 0.084 | ||
| Well/moderately differentiated | 95 (14.0) | 19.0 | |
| Poorly differentiated | 458 (67.5) | 11.0 | |
| Unknown | 126 (18.5) | 13.5 | |
| Pathologic type | 0.000 | ||
| Adenocarcinoma | 547 (80.6) | 13.5 | |
| SRCC/mucinous adenocarcinoma | 132 (19.4) | 9.0 | |
| Primary tumor location | 0.001 | ||
| Proximal gastric | 171 (25.2) | 16.0 | |
| Non-proximal gastric | 508 (74.8) | 11.5 | |
| Liver metastasis | 0.243 | ||
| Yes | 264 (38.9) | 12.0 | |
| No | 415 (61.1) | 12.5 | |
| Lung metastasis | 0.032 | ||
| Yes | 62 (9.1) | 12.0 | |
| No | 617 (90.9) | 18.0 | |
| Peritoneal metastasis | 0.000 | ||
| Yes | 170 (25.0) | 13.5 | |
| No | 509 (75.0) | 10.0 | |
| Ovary metastasis | 0.768 | ||
| Yes | 170 (25.0) | 12.0 | |
| No | 509 (75.0) | 14.5 | |
| Number of metastatic sites at random | 0.237 | ||
| 1 | 179 (26.4) | 14.6 | |
| 2 | 185 (27.2) | 11.0 | |
| 3 | 315 (46.4) | 11.6 | |
| First-line regimen | 0.240 | ||
| XELOX | 264 (38.9) | 12.0 | |
| EOX | 197 (29.0) | 12.0 | |
| EOF | 149 (21.9) | 12.6 | |
| TX | 69 (10.2) | 13.1 | |
EOF, epirubicin, oxaliplatin, and fluorouracil; EOX, epirubicin, oxaliplatin, and capecitabine; SRCC, signet-ring cell carcinoma; XELOX, oxaliplatin and capecitabine.
Baseline hematological parameters and correlation with prognosis
In this study, we analyzed 14 baseline hematological parameters: peripheral Hb level, WBC, neutrophil, lymphocyte, monocyte, eosinophil, basophil, platelet count, albumin, LDH, ALP, γ-GGT, CEA, and CA199 levels (Table 2). The univariate Cox analysis demonstrated significantly longer OS in patients whose following parameters were higher than ULN values than in patients whose following parameters were within normal values: WBC count (p = 0.011), neutrophil count (p = 0.000), monocyte count (p = 0.004), LDH level (p = 0.001), ALP level (p = 0.000), γ-GGT level (p = 0.000), and CA199 level (p = 0.013). Furthermore, patients whose lymphocyte level was lower than the LLN value had significantly longer OS than those whose lymphocyte level was within the normal value (p = 0.000).
Table 2.
Univariate analysis of the association of baseline hematological parameters and survival in the training cohort.
| Characteristics | No. (%) | Median OS | p |
|---|---|---|---|
| Hemoglobin | 0.180 | ||
| <LLD | 395 (58.2) | 11.6 | |
| Normal | 284 (41.8) | 12.9 | |
| White blood cell | 0.011 | ||
| >ULD | 76 (11.2) | 10.0 | |
| Normal | 603 (88.8) | 13.0 | |
| Neutrophil | 0.000 | ||
| >ULD | 112 (16.5) | 10.0 | |
| Normal | 567 (83.5) | 13.0 | |
| Lymphocyte | 0.000 | ||
| <LLD | 147 (21.6) | 9.6 | |
| Normal | 532 (78.4) | 14.0 | |
| Monocyte | 0.004 | ||
| >ULD | 117 (17.2) | 10.0 | |
| Normal | 562 (72.8) | 12.6 | |
| Eosinophil | 0.710 | ||
| >ULD | 19 (2.8) | 11.0 | |
| Normal | 660 (97.2) | 12.0 | |
| Basophil | 0.168 | ||
| >ULD | 28 (4.1) | 11.0 | |
| Normal | 651 (95.9) | 12.0 | |
| Platelet | 0.123 | ||
| >ULD | 105 (15.5) | 10.1 | |
| Normal | 574 (84.5) | 13.0 | |
| Albumin | 0.114 | ||
| ⩽LLD | 334 (49.2) | 11.3 | |
| Normal | 345 (50.8) | 13.0 | |
| Lactate dehydrogenase | 0.001 | ||
| >ULD | 187 (27.5) | 10.0 | |
| Normal | 492 (72.5) | 13.0 | |
| γ-glutamyl transferase | 0.000 | ||
| >ULD | 195 (28.7) | 11.5 | |
| Normal | 484 (71.3) | 13.0 | |
| Alkaline phosphatase | 0.000 | ||
| >ULD | 128 (18.9) | 8.8 | |
| Normal | 551 (81.1) | 13.4 | |
| CEA | 0.054 | ||
| >ULD | 360 (53.0) | 11.5 | |
| Normal | 319 (47.0) | 13.5 | |
| CA199 | 0.013 | ||
| >ULD | 333 (49.0) | 11.0 | |
| Normal | 346 (51.0) | 13.5 |
LLD, lower limit of normal; ULD, upper limit of normal.
Prognostic model of seven risk factors
According to the univariate analysis, three clinical characteristics (including pathological type, primary tumor location, and peritoneal metastasis) and seven baseline hematological parameters (including WBC, neutrophil, lymphocyte, and monocyte count and LDH, ALP, γ-GGT, and CA199 levels) were significantly associated with OS and eventually enrolled in the multivariate Cox regression model (Table 3). The multivariate Cox regression demonstrated that primary tumor location [proximal gastric versus non-proximal gastric, hazard ratio (HR) = 1.287, 95% CI = 1.040–1.592; p = 0.021], pathological type (adenocarcinoma versus mucinous carcinoma/SRCC, HR = 1.858, 95% CI = 1.493–2.313; p = 0.000), peritoneal metastasis (yes versus no, HR = 1.350, 95% CI = 1.100–1.657; p = 0.004), neutrophil count (normal versus higher than ULN, HR = 1.504, 95% CI = 1.060–2.134; p = 0.022), lymphocyte count (normal versus lower than LLN, HR = 1.656, 95% CI = 1.343–2.043; p = 0.000), LDH level (normal versus higher than ULN, HR = 1.276, 95% CI = 1.034–1.574; p = 0.023), and ALP level (normal versus higher than ULN, HR = 1.424, 95% CI = 1.103–1.838; p = 0.007) were significantly associated with OS.
Table 3.
Multivariate Cox regression analysis of prognostic factors for overall survival in the training cohort.
| Characteristics | Overall survival | |
|---|---|---|
| Hazard ratio (95% CI) | p Value | |
| Non-proximal gastric | 1.287 (1.040–1.592) | 0.021 |
| SRCC/mucinous adenocarcinoma | 1.858 (1.493–2.313) | 0.000 |
| Peritoneal metastasis | 1.350 (1.100–1.657) | 0.004 |
| Leucocyte > ULN | 0.765 (0.503–1.166) | 0.213 |
| Neutrophil > ULN | 1.504 (1.060–2.134) | 0.022 |
| Lymphocyte < ULN | 1.656 (1.343–2.043) | 0.000 |
| Monocyte > ULN | 1.031 (0.795–1.338) | 0.816 |
| Alkaline phosphatase > ULN | 1.424 (1.103–1.838) | 0.007 |
| Lactate dehydrogenase > ULN | 1.276 (1.034–1.575) | 0.023 |
| γ-glutamyl transferase > ULN | 1.144 (0.911–1.437) | 0.246 |
| CA199 > ULN | 1.185 (0.994–1.411) | 0.058 |
SRCC, signet-ring cell carcinoma; ULN, upper limit of normal value.
Next, we constructed a risk score system of the seven risk factors using a nomogram, which can more elaboratively show the impact of different risk factors on prognosis [Figure 1(a)]. Accordingly, the risk score was calculated using the following formula: risk score (RS) = (4.25) × primary tumor location + (10.00) × pathological type + (4.75) × peritoneal metastasis + (4.25) × neutrophil + (8.00) × lymphocyte + (4.00) × LDH + (6.75) × ALP. The risk score index was named the Fudan Advanced Gastric Cancer Prognostic Risk Score (FARS) index.
Figure 1.
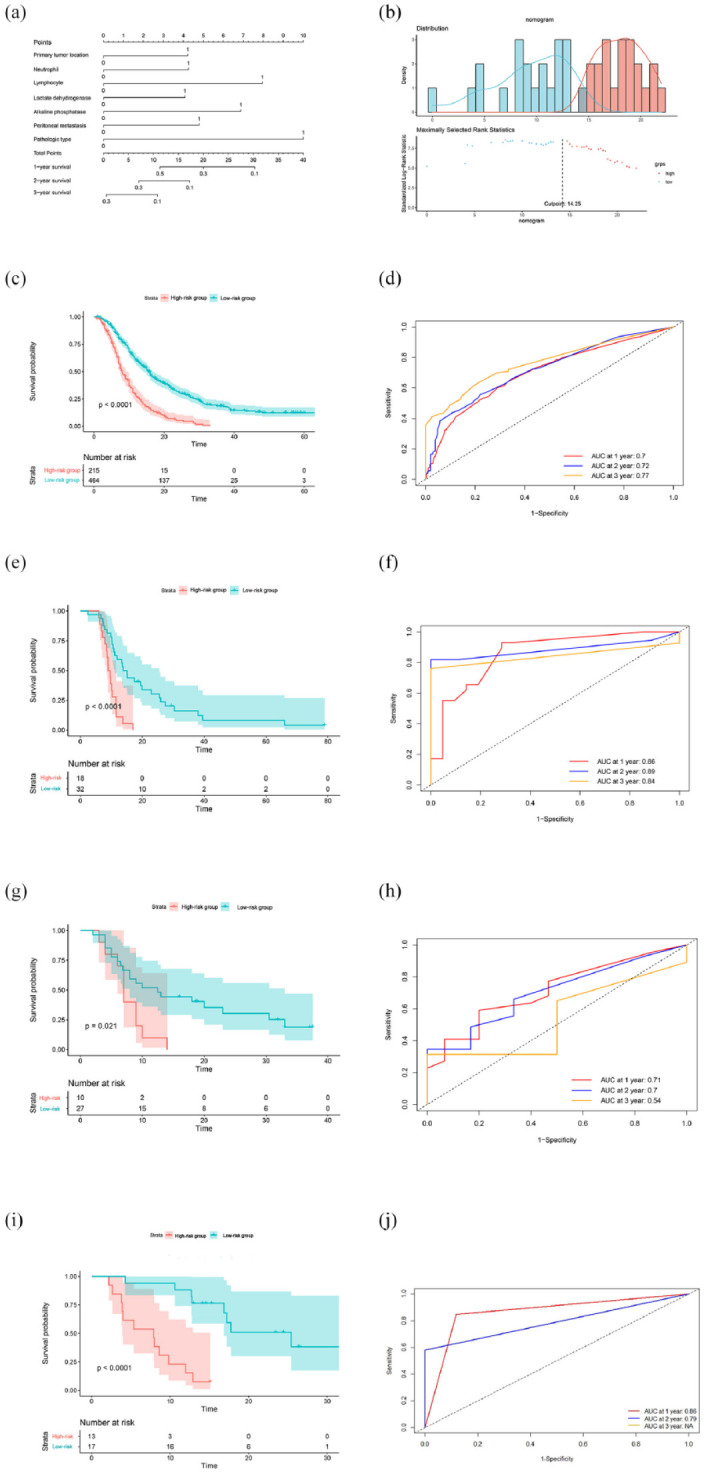
(a) A risk score system of the seven risk factors using a nomogram. (b) The optimal cutoff value for the risk score was evaluated using the ‘Surv_cutpoint’. (c) In the training cohort, the Kaplan–Meier curve showed that patients in the high-risk group had significantly shorter OS than those in the low-risk group (p < 0.001). (d) The areas under the curve in the FARS index for 1-, 2-, and 3-year OS were 0.70, 0.72, and 0.77, respectively. (e) In the validation cohort, patients in the high-risk group had significantly shorter OS than those in the low-risk group (p < 0.001). (f) In the training cohort, the areas under the curve in the FARS index for 1-, 2-, and 3-year OS were 0.86, 0.89, and 0.84, respectively. (g) In the external cohort, patients in the high-risk group had significantly shorter OS than those in the low-risk group (p = 0.019). (h) In the external cohort, the areas under the curve in the FARS index for 1-, 2-, and 3-year OS were 0.71, 0.70, and 0.54, respectively. (i) In the exploration cohort, patients in the high-risk group had significantly shorter OS than those in the low-risk group (p < 0.001). (j) In the external cohort, the areas under the curve in the FARS index for 1-, 2-, and 3-year OS were 0.86, 0.79, and not available, respectively.
FARS, Fudan Advanced Gastric Cancer Prognostic Risk Score; OS, overall survival.
FARS index for AGC patients
The risk score for each patient was calculated using the FARS index, and the optimal cutoff value for the risk score was evaluated using the ‘Surv_cutpoint’ function; the cutoff value was finally set at 14.25 [Figure 1(b)]. According to the cutoff value, 679 patients in the training cohort were divided into high- and low-risk groups. Kaplan–Meier (KM) curve showed that patients in the high-risk group had significantly shorter OS than those in the low-risk group (mOS, 15.5 versus 8.0 p < 0.001) [Figure 1(c)]. We also calculated the area under the curve (AUC) of the FARS index. We used time-dependent receiver operator characteristic curve curves to estimate the 1-, 2-, and 3-year OS rates, resulting in AUCs of 0.70, 0.72, and 0.77, respectively [Figure 1(d)].
Validation cohort and external cohort
To further evaluate the prognostic value of the FARS index, we validated the index using a validation cohort of 50 MGC patients from our center and an external cohort of 37 MGC patients from other centers. We randomly extracted 50 AGC patients to establish a validation cohort from the case bank of our department, including cases from 2013 to 2018. Patients in the external cohort were all participants in the EXELO trial. The risk score of each patient was assessed using the FARS index; subsequently, the patients were divided into high- and low-risk groups according to the cutoff value of 14.25. In the validation cohort, the KM curve showed that patients in the high-risk group had significantly shorter OS than those in the low-risk group [p < 0.001, Figure 1(e)]. The AUCs of the FARS index for 1-, 2-, and 3-year OS were 0.86, 0.89, and 0.84, respectively [Figure 1(f)]. In the external cohort, patients in the high-risk group had significantly shorter OS than those in the low-risk group [p = 0.021, Figure 1(g)]. The AUCs of the FARS index for 1-, 2-, and 3-year OS were 0.71, 0.70, and 0.54, respectively [Figure 1(h)].
Exploration cohort
In the past 2 years, PD-1 antibody plus chemotherapy has been approved as the first-line treatment of AGC; thus, an exploration cohort of 30 MGC patients treated with PD-1 antibody and chemotherapy as the first-line treatment was established. The risk score of each patient was assessed using the FARS index, and the patients were then divided into high- and low-risk groups according to the cutoff value of 14.25. In the exploration cohort, the KM curve showed that patients in the high-risk group had significantly shorter OS than those in the low-risk group [p < 0.001, Figure 1(i)]. The AUCs of the FARS index for 1- and 2-year OS were 0.86 and 0.79, respectively [Figure 1(j)], whereas the AUC for the 3-year OS was not available.
Prediction model of triplet regimen
To investigate the patient population who can benefit from the triplet regimen more than the doublet regimen, we performed a subgroup analysis of 11 baseline clinicopathological parameters (Figure 2) and 14 baseline hematological parameters (Figure 3) to compare the OS in the patients who received doublet regimen with the OS of those who received triplet regimen. The 11 baseline clinicopathological parameters included ECOG, sex, age, histological grade, pathological type, liver metastasis, lung metastasis, peritoneal metastasis, ovary metastasis, and the number of metastatic sites at random. The 14 baseline hematological parameters comprised peripheral Hb level, WBC count, neutrophil count, lymphocyte count, monocyte count, eosinophil count, basophil count, platelet count, albumin level, LDH level, ALP level, γ-GGT level, CEA level, and CA199 level.
Figure 2.
Forest plot of overall survival of patients with advanced gastric cancer in different subgroups of baseline clinicopathologic parameters in the training cohort.
Figure 3.
Forest plot of overall survival of patients with advanced gastric cancer in different subgroups of baseline hematological parameters in the training cohort.
The results showed no significant survival difference between the doublet- and triplet-regimen groups in the subgroup analysis. Subsequently, we identified HRs < 1.1 as the standard to enroll the parameters for further analysis. The following four parameters were screened out: sex as male, pathological type mucinous adenocarcinoma/SRCC, primary tumor location at the non-proximal gastric area, and peripheral neutrophil count higher than the ULN. Because sex is not an independent prognostic factor in the entire population, we excluded the parameter sex as male from further analysis.
Finally, the pathological type mucinous adenocarcinoma/SRCC, primary tumor location at the non-proximal gastric area, and peripheral neutrophil count higher than the ULN were identified as the triplet regimen efficacy parameters. The KM curve showed that in patients with any two of the three parameters (159 patients), patients who received the triplet regimen had significantly longer OS than those who received the doublet regimen [p = 0.018, Figure 4(a)]. In patients with one or without any of the three parameters (500 patients), the KM curve showed no difference in OS between patients who received the triplet regimen and patients who received the doublet regimen [p = 0.800, Figure 4(b)]. Therefore, we screened out the dominant population of triplet regimens and named the triplet regimen efficacy predictive system as the TRIS index.
Figure 4.
(a) In the patients with any two of the three parameters (159 patients), patients who received the triplet regimen had significantly longer OS than patients who received the doublet regimen p = 0.018). (b) In the patients with one parameter or without any parameter (500 patients), there was no statistical difference in OS between patients who received a triplet regimen and patients who received a doublet regimen (p = 0.799).
OS, overall survival.
Discussion
In the present study, we enrolled 679 patients with pathologically confirmed AGC. We analyzed 11 baseline clinicopathological parameters and 14 baseline hematological parameters to screen out the prognostic factors. Subsequently, we established a new prognostic index named the FARS index comprising seven prognostic factors, categorizing patients into two different risk groups using this index demonstrated significantly differing OS between the groups. A triplet regimen efficacy predictive system called the TRIS index comprising three predictive factors was constructed to screen out patients who can benefit from the triplet regimen. The seven prognostic and three predictive factors are readily available to physicians before the choice of treatment. Thus, the two indices are convenient, reliable, and repeatable, which would aid in making clinical decisions and stratifying risk levels for AGC patients.
The strength of our study lies in the data, which were derived from three registered and published clinical trials of AGC patients, ensuring the availability of complete and reliable clinical, pathological, and survival data. In the training cohort, more than 90% of patients died; thus, the survival data are mature. The FARS index identified two groups with significantly different clinical outcomes; patients in the low-risk group had nearly double the median OS than those in the high-risk group (mOS, 15.5 versus 8.0 months). Moreover, the accuracy and repeatability of the FARS index were confirmed by an internal and an external validation cohort. Patients of the internal validation cohort were randomly extracted from the case bank of our center; therefore, the cohort is representative. The external cohort represents the extensibility of the FARS index. The AUC values for the FARS index of the training cohort were between 0.7 and 0.8, whereas the AUC values of the training and validation cohorts were between 0.8 and 0.9. However, the AUC value for the 3-year OS of the external validation cohort was 0.54. The mOS of the training cohort was 12.0 months, whereas the mOS of the external validation cohort was 9.0 months. We expect that the OS difference between the training and external validation cohorts might be the main reason for the low AUC value for the 3-year OS of the external validation cohort.
The FARS index includes three clinicopathological prognostic factors. First, patients with primary tumors located in the proximal gastric area had significantly longer OS than those with non-proximal tumor locations. Second, peritoneal metastasis is a prognostic factor in the FARS index. Peritoneal metastasis is a widely recognized poor prognostic factor for MGC, 16 occurring in approximately one-third of MGC patients. 17 Owing to the presence of a peritoneal-plasma barrier and poor vascularity, the response to systemic therapy of peritoneal metastasis is poor. 18 Intraperitoneal treatment, such as hyperthermic intraperitoneal chemotherapy and pressurized intraperitoneal aerosol chemotherapy, is a research hotspot of peritoneal metastasis. Peritoneal metastasis is also identified as a strategic factor for several global clinical trials on gastric cancer. Third, patients with SRCC/mucinous adenocarcinoma had poorer outcomes than patients with adenocarcinoma. SRCC is a highly malignant pathological type.
Furthermore, the FARS index included four hematological parameters. First, serum LDH level correlated with prognosis in many solid and hematological malignant tumor types and is associated with tumor burden.19–21 Second, serum ALP level is also identified as a prognostic factor in different tumor types, including colorectal, 22 prostate, 23 and hepatic carcinoma, 24 which is also a factor in the JCOG prognostic index. 4 Third, inflammation is one of the six biological capabilities of tumor development and a hallmark of cancer, and leucocyte count is an important marker of inflammation.25,26 Fourth, the lymphocytes are an important part of the immune system. Low levels of lymphocyte counts may be related to the body’s poor immune response to tumors.
The FARS index was established in AGC patients treated with chemotherapy without PD-1 antibody. In the past 2 years, PD-1 antibody plus chemotherapy has been approved as the first-line treatment of AGC. PD-L1 combined positive score (CPS) is an important efficacy predictive factor. In Checkmate-649 and Oriental-16 trials, two trials with a relatively high proportion of patients with CPS ⩾ 5, the median OS of patients in the PD-1 antibody-plus-chemotherapy group was prolonged by 2.2 months(13.8 versus 11.6 months) and 2.9 months and (15.2 versus 12.3 months), respectively, than those in the chemotherapy group. However, in the Attraction-4 trial, patients in the PD-1 antibody-plus-chemotherapy group had similar OS with those in the chemotherapy group (17.45 versus 17.15 months), and the proportion of patients with a tumor proportion score > 1% is only 16%. Hence, the expression of PD-L1 is significantly related to the response to PD-1 antibody, and the efficacy of PD-1 antibody on patients with low or negative expression of PD-L1 is uncertain.
In the real-world setting, patients with low or negative expression of PD-L1 occupy a large proportion of AGC patients; therefore, exclusive chemotherapy remains an important and indispensable part of the first-line treatment of AGC patients. Moreover, in the exploration cohort enrolling 30 MGC patients treated with PD-1 antibody and chemotherapy as first-line treatment, the FARS index identified two groups with significantly different clinical outcomes. This result supports our plan to conduct a study on MGC patients treated with PD-1 antibody and chemotherapy as first-line treatment with a larger sample size in the future. To sum up, the FARS index is a valuable prognostic system in the current era.
Our study also identified patients who could potentially benefit from a triplet regimen and established the TRIS index. This index comprised three important factors: SRCC/mucinous adenocarcinoma, primary tumor location in the non-proximal gastric area, and neutrophil count higher than the ULN. The three factors are all adverse prognostic factors, which indicates that patients with adverse prognostic factors may have a higher probability of benefitting from a triplet regimen.
We have mentioned earlier that PD-1 antibody combined with chemotherapy is the first-line treatment of AGC, and the regimen is the currently recommended guideline. Currently, the chemotherapy regimens of several large-sample phase III clinical trials on AGC are oxaliplatin-based and fluorouracil-based doublet regimens. Although the immune checkpoint inhibitor itself has a certain tumor-reducing ability, it mainly depends on the tail effect to prolong the OS. Therefore, the efficacy of chemotherapy in the early stage of disease treatment is very important. Particularly for patients with high malignant types and tumor burden, high-intensity chemotherapy should be used to control tumor growth as soon as possible. Our study established a model for predicting the efficacy of the triplet regimen and demonstrated that the triplet regimen was more likely to prolong survival than the doublet regimen in gastric cancer patients with adverse prognostic factors. This result screened out the dominant population favorable to the triplet regimen and also provided a new basis for the selection of chemotherapy regimens for this patient population using the TRIS index in future phase III clinical studies.
Conclusion
The FARS and TRIS indices comprise readily available clinical parameters that are convenient, practical, and repeatable. The FARS index can be used for clinical outcome assessment and individual risk stratification, whereas the TRIS index helps screen out the dominant population for triplet regimens.
Supplemental Material
Supplemental material, sj-pdf-1-tam-10.1177_17588359241233982 for Multivariate prognostic index and triplet regimen efficacy predictive index in locally advanced and metastatic gastric cancer: pooled analysis from three clinical trials using individual patient data by Wan-Jing Feng, Xiao-Ying Zhao, Yi-Fu He, Ming-Zhu Huang, Zhi-Yu Chen, Yu-Sheng Wang, Xiao-Dong Zhu and Wei-Jian Guo in Therapeutic Advances in Medical Oncology
Footnotes
ORCID iD: Wan-Jing Feng  https://orcid.org/0000-0002-6597-4362
https://orcid.org/0000-0002-6597-4362
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Wan-Jing Feng, Department of Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai, P. R. China; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, P. R. China.
Xiao-Ying Zhao, Department of Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai, P. R. China; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, P. R. China.
Yi-Fu He, Department of Medical Oncology, The First Affiliated Hospital, Division of Life Science and Medicine, University of Science and Technology of China, Hefei, Anhui, P. R. China.
Ming-Zhu Huang, Department of Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai, P. R. China; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, P. R. China.
Zhi-Yu Chen, Department of Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai, P. R. China; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, P. R. China.
Yu-Sheng Wang, Department of Gastroenterology, Shanxi Cancer Hospital, Taiyuan, Shanxi 030013, P. R. China.
Xiao-Dong Zhu, Department of Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai 200032, P. R. China; Department of Oncology, Shanghai Medical College, Fudan University, 270 Dong’An Road, Shanghai 200032, P. R. China.
Wei-Jian Guo, Department of Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai 200032, P. R. China; Department of Oncology, Shanghai Medical College, Fudan University, 270 Dong’An Road, Shanghai 200032, P. R. China.
Declarations
Ethics approval and consent to participate: The patients enrolled in this research were from three clinical trials approved by the Ethics Committee of Fudan University Shanghai Cancer, as No. 1503144-8, No. 1204109-14, and No. IRB50-15. All patients signed an informed consent form before enrollment. A further retrospective analysis was included in the informed consent.
Consent for publication: Our research did not contain patients’ individual images or data. Patients enrolled in this research all signed the informed consent for analysis and publication.
Author contributions: Wan-Jing Feng: Conceptualization; Funding acquisition; Writing – original draft.
Xiao-Ying Zhao: Data curation; Resources.
Yi-Fu He: Data curation; Resources.
Ming-Zhu Huang: Data curation; Resources.
Zhi-Yu Chen: Data curation; Resources.
Yu-Sheng Wang: Data curation; Resources.
Xiao-Dong Zhu: Conceptualization; Data curation; Resources.
Wei-Jian Guo: Conceptualization; Resources; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was supported by Shanghai AntiCancer Association EYAS Project (No. SACA-CY22B06) and Shanghai Anti-Cancer Association and Shanghai Cancer Center Joint Fund (No. YJQN202211).
The authors declare that there is no conflict of interest.
Availability of data and materials: The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Wöhrer SS, Raderer M, Hejna M .Palliative chemotherapy for advanced gastric cancer. Ann Oncol 2004; 15: 1585–1595. [DOI] [PubMed] [Google Scholar]
- 2. Rizzo A, Mollica V, Ricci AD, et al. Third- and later-line treatment in advanced or metastatic gastric cancer: a systematic review and meta-analysis. Future Oncol 2020; 16: 4409–4418. [DOI] [PubMed] [Google Scholar]
- 3. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021; 398: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chau I, Norman AR, Cunningham D, et al. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer—pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol 2004; 22: 2395–2403. [DOI] [PubMed] [Google Scholar]
- 5. Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997; 15: 261–267. [DOI] [PubMed] [Google Scholar]
- 6. Ross P, Nicolson M, Cunningham D, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 2002; 20: 1996–2004. [DOI] [PubMed] [Google Scholar]
- 7. Tebbutt NC, Norman A, Cunningham D, et al. A multicentre, randomised phase III trial comparing protracted venous infusion (PVI) 5-fluorouracil (5-FU) with PVI 5-FU plus mitomycin C in patients with inoperable oesophago-gastric cancer. Ann Oncol 2002; 13: 1568–1575. [DOI] [PubMed] [Google Scholar]
- 8. Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 2009; 20: 666–673. [DOI] [PubMed] [Google Scholar]
- 9. Al-Batran SE, Hartmann JT, Probst S, et al.; Arbeitsgemeinschaft Internistische Onkologie. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 2008; 26: 1435–1442. [DOI] [PubMed] [Google Scholar]
- 10. Zhu X, Huang M, Wang Y, et al. XELOX doublet regimen versus EOX triplet regimen as first-line treatment for advanced gastric cancer: an open-labeled, multicenter, randomized, prospective phase III trial (EXELOX). Cancer Commun 2022; 42: 314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cunningham D, Starling N, Rao S, et al. Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United, Capecitabine and oxaliplatin for advanced esophagogastric cancer. New Engl J Med 2008; 358: 36–46. [DOI] [PubMed] [Google Scholar]
- 12. Ajani JA, D'Amico TA, Bentrem DJ, et al. Gastric Cancer, version 2.2022. NCCN Clinical Practice Guidelines in Oncology, J Natl Compr Canc Netw 2022; 20: 167–192. [DOI] [PubMed] [Google Scholar]
- 13. Zhu X, Zhao X, Peng W, et al. Epirubicin combined with oxaliplatin and 5-day continuous infusion of 5-fluorouracil as a first-line treatment for metastatic gastric cancer: treatment outcomes and analysis of prognostic factors. J Cancer Res Clin Oncol 2015; 141: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao XY, Liu X, Li WH, et al. Randomized phase II study of TX followed by XELOX versus the reverse sequence for chemo-naive patients with metastatic gastric cancer. Front Oncol 2022; 12: 911160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McShane LM, Altman DG, Sauerbrei W, et al.; N.C.I.E.W.G.o.C.D. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005; 97: 1180–1184. [DOI] [PubMed] [Google Scholar]
- 16. Tan HL, Chia CS, Tan GH, et al. Gastric peritoneal carcinomatosis – a retrospective review. World J Gastrointest Oncol 2017; 9: 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koemans WJ, Lurvink RJ, Grootscholten C, et al. Synchronous peritoneal metastases of gastric cancer origin: incidence, treatment and survival of a nationwide Dutch cohort. Gastric Cancer 2021; 24: 800–809. [DOI] [PubMed] [Google Scholar]
- 18. Sugarbaker PH, Van der Speeten K, Stuart OA .Pharmacologic rationale for treatments of peritoneal surface malignancy from colorectal cancer. World J Gastrointest Oncol 2010; 2: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. García R, Hernández JM, Caballero MD, et al. Serum lactate dehydrogenase level as a prognostic factor in Hodgkin’s disease. Br J Cancer 1993; 68: 1227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schneider RJ, Seibert K, Passe S, et al. Prognostic significance of serum lactate dehydrogenase in malignant lymphoma. Cancer 1980; 46: 139–143. [DOI] [PubMed] [Google Scholar]
- 21. Zhang X, Guo M, Fan J, et al. Prognostic significance of serum LDH in small cell lung cancer: a systematic review with meta-analysis. Cancer Biomark 2016; 16: 415–423. [DOI] [PubMed] [Google Scholar]
- 22. Köhne CH, Cunningham D, Di Costanzo F, et al. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol 2002; 13: 308–317. [DOI] [PubMed] [Google Scholar]
- 23. Tsuzuki S, Kawano S, Fukuokaya W, et al. Prognostic model with alkaline phosphatase, lactate dehydrogenase and presence of Gleason pattern 5 for worse overall survival in low-risk metastatic hormone-sensitive prostate cancer. Jpn J Clin Oncol 2021; 51: 1665–1671. [DOI] [PubMed] [Google Scholar]
- 24. Su K, Huang W, Li X, et al. Evaluation of lactate dehydrogenase and alkaline phosphatase as predictive biomarkers in the prognosis of hepatocellular carcinoma and development of a new nomogram. J Hepatocell Carcinoma 2023; 10: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hanahan D, Weinberg RA .Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 26. Grivennikov SI, Greten FR, Karin M .Immunity, inflammation, and cancer. Cell 2010; 140: 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tam-10.1177_17588359241233982 for Multivariate prognostic index and triplet regimen efficacy predictive index in locally advanced and metastatic gastric cancer: pooled analysis from three clinical trials using individual patient data by Wan-Jing Feng, Xiao-Ying Zhao, Yi-Fu He, Ming-Zhu Huang, Zhi-Yu Chen, Yu-Sheng Wang, Xiao-Dong Zhu and Wei-Jian Guo in Therapeutic Advances in Medical Oncology





