Abstract
Introduction
Atrial fibrillation (AF) ablation represents a safe and effective procedure to restore sinus rhythm. The idea that post-procedural AF episodes - during the blanking period - are not considered treatment failure has been increasingly challenged. The E-Patch, a single-use adhesive electrode, facilitates extended continuous ECG monitoring for 120 h. This pilot study aims to assess the effectiveness of this ambulatory monitoring device and investigate whether very-early AF recurrence correlates with delayed blanking period ablation outcomes.
Methods
We conducted a single-center, prospective, longitudinal study, including consecutive post-ablation patients monitored with the E-patch. The ability of the device to continuously record was analyzed, as well as the occurrence of AF episodes during external 7-day loop-recorder in the 2nd-month post-ablation.
Results
We included 40 patients, median age 62 years (IQR 56–70). E-Patch monitoring was obtained for a median of 118 h (IQR 112–120), with no discomfort nor interpretation artefacts. Very-early AF recurrence was detected in 11 (27.5 %) patients, with a median AF burden of 7 % (IQR 6 %–33 %). Late-blanking period AF was detected in 13 (33 %) of the external 7-day loop recordings. Of the 11 patients that had very-early AF recurrence, 10 (91 %) had late-blanking AF. Very-early AF detection showed 77 % (95 % CI 64 %-90 %) sensitivity and 96 % (95 % CI 90–100 %) specificity in predicting late-blanking AF, with a non-parametric ROC curve AUC of 0.903 (95 % 0.797––1.0).
Conclusion
The E-Patch was able to detect very-early AF during an extended period. Very-early AF detection emerges as a predictor of AF recurrence during the late blanking period post-ablation.
Keywords: Atrial fibrillation, Arrythmia, Blanking period, External loop recorder, Recurrence, Ablation
1. Introduction
Atrial fibrillation (AF) ablation through pulmonary vein isolation (PVI) is a safe and efficacious procedure for achieving sinus rhythm in patients with paroxysmal or persistent AF [1]. An early rhythm control strategy has shown clinical benefit and is currently the preferred pathway for most symptomatic patients with AF.[1], [2] When choosing the strategy for rhythm control, AF ablation has been shown to perform better than antiarrhythmics in improving quality of life and reducing symptoms and AF burden.[3] Additionally, in the EARLY-AF trial, patients with paroxysmal AF undergoing cryoballoon ablation were shown to have a lower rate of AF recurrence and therapy-related adverse outcomes.[4].
While being established as an efficient and beneficial therapeutic intervention, a significant proportion of patients experience AF recurrence after ablation.[1], [5] Risk factors associated with recurrence include non-paroxysmal AF, old age, left atrial (LA) size, and concomitant comorbidities - such as heart failure, hypertension, and obstructive sleep apnea (OSA).[6] Different scores have been developed to evaluate the risk of AF recurrence, but they have generally underperformed.[6] Of note, a relevant predictive value of early AF recurrence, has emerged as a strong individual predictor of late AF recurrence [6], [7], [8].
Early AF recurrence is defined as the occurrence of AF within 3 months following ablation. This period of early recurrence – the blanking period – was first described 20 years ago when it was observed that 15–49 % of patients that had a recurrence in this period ended up having delayed cure from AF on a longer follow-up.[9], [10], [11], [12] Concurrent pro and anti-arrhythmic factors compete in this early period.[12] Tissue ischemia and proliferation, transient local inflammation, cellular oxidative stress, nerve sprouting and tissue necrosis due to catheter ablation contribute to AF recurrence, whereas parasympathetic denervation, scar maturation and mechanical stabilization contribute as protective processes.[12] These multifactorial inputs lead to a strong predictor of late recurrence that is still a subject of active investigation, namely regarding the 3-month cutoff duration and the arrhythmic burden during the blanking period, with newer evidence pointing to a very high risk of AF at 12 months follow-up for patients with early AF recurrence[7], [13].
While until recently we have been using traditional ECG and Holter monitoring able to monitor AF recurrence, new smart gadgets and wearable devices are now available for heart rhythm monitoring. Single-use adhesive electrodes, such as the E-Patch (Bio Tel Heart), allow for continuous ECG monitoring for up to 120 h. This new monitoring modality makes it easier to follow heart rate and rhythm on an outpatient basis in the immediate period after PVI (Fig. 1).
Fig. 1.
The E-patch; A – The E-patch device; B – Application of the E-patch remote monitoring device on the chest wall of a patient.
In this pilot study, we set out to assess the ability of this continuous ambulatory monitoring wearable device to detect AF, and whether very-early AF recurrence correlated with late-blanking ablation outcomes.
2. Methods
2.1. Study design
We performed a prospective, single-centre, longitudinal study that included consecutive patients undergoing first AF ablation. Patients subjected to AF ablation consisted of adults with symptomatic paroxysmal or persistent AF. According to a previously published protocol, PVI was performed using radiofrequency or cryoablation techniques.[12]
The early monitoring approach consisted of applying the ePatch on the patient's chest during the first 24 h after PVI, at the moment of discharge, with a continuous recording for 5 days - a maximum of 120 h (Fig. 1). Subsequently, the occurrence of AF episodes was evaluated using an external 7-day loop recorder placed in the 2nd-month post-ablation (the late blanking period). In both devices heart rate and R-R variability plots were recorded and checked by the automated software algorithm. These tracings were then reviewed by one technician and validated by a cardiology specialist in electrophysiology. Antiarrhythmic drugs were continued throughout the blanking period with the same dosages as prior to ablation. AF recurrence was defined if a period of AF with > 30 s was detected in the ambulatory monitoring, evaluated at a 3-month follow-up appointment.[1]
The primary outcome of the study was the correlation between AF detection in the very-early (via the ePatch wearable) and the late blanking period, evaluated through the sensitivity, specificity and predictive ratios of very-early blanking AF detection as a predictor of late-blanking AF recurrence. Secondary outcomes included the evaluation of potential predictors of late AF recurrence obtained from ePatch data during the very-early blanking period, namely the assessment of statistical parameters of heart rate variability (HRV) – SDNN (NN standard deviation of all recording), SDANN (NN standard deviation in all 5-minute segments of the recording), RMSSD (square root of mean of sum of squares of differences between adjacent NN intervals) and pNN50 (percentage of differences between adjacent normal RR intervals that are greater than 50 ms computed from the entire recording). The ePatch tolerability and effectiveness in monitoring heart rhythm for the stipulated timeframe were also evaluated.
2.2. Statistical analysis
Relevant data regarding populational characteristics, co-morbidities and pharmacotherapy was collected before PVI. Continuous variables were reported as mean ± SD or median or IQR, depending on the data distribution pattern. Categorical data were depicted as frequencies and percentages. Differences between groups were calculated using Fisher’s exact test and the Wilcoxon rank-sum for categorical and continuous data, respectively. Evaluation of predictors of late-blanking AF recurrence was performed using univariate logistic regression models to assess for interactions between dependent and independent variables. Researchers opted not to pursue multivariate analysis given the low sample size and overall event rate.[14], [15]
Sensitivity was derived through the ratio of very-early recurrence and all late-blanking period recurrences. Specificity was calculated by dividing the number of no very-early recurrences by the number of no recurrences. Positive and negative predictive values (PPV/NPV) were derived from our cohort's estimates of sensitivity, specificity and disease prevalence. A receiver operator characteristic (ROC) curve analysis was performed to assess the correlation between the timing of very-early and late blanking period recurrences, with the calculation of the area under the curve (AUC) assuming a non-parametric model. Data were analyzed using Stata® Statistical Software Package, Version 17.0 (StataCorp LP, College Station, Texas, EUA).
2.3. Ethical considerations
The hospital ethics committee approved the study protocol (Ethics Committee approval number 974/2020). All participants provided written informed consent for data collection, and the study was conducted according to the Declaration of Helsinki guidelines.
3. Results
A total of 48 patients were screened for entry in this prospective study, with a total of 8 patients not accepting early monitoring with the E-patch. Our patient cohort consisted of a total of 40 sequential patients that were included from November 2021 to March 2022. The median patient age was 62 (IQR 56–70), with 16 (40 %) females, presenting with paroxysmal AF (n = 25, 62.5 %) and persistent AF (n = 15, 37.5 %). Cryoablation was used in 17 cases (42.5 %) and radiofrequency in the remaining participants. PVI was successful in all the patients, without periprocedural complications. There were no statistically significant differences in the use of antiarrhythmic drugs between patient groups. General cohort characteristics are presented in Table 1.
Table 1.
General patient characteristics stratified by late-blanking recurrence status.
| Characteristics | Total patients (N = 40) | With late blanking AF recurrence (N = 27) | Without late blanking AF recurrence (N = 13) | p-value |
|---|---|---|---|---|
| Age - yr [IQR] | 62 [70-56] | 59[70-57] | 66 [70-55] | 0.177 |
| Female sex - n (%) | 16 (40 %) | 10 (37 %) | 6 (46 %) | 0.581 |
| Cryoablation – n (%) | 17 (42.5 %) | 13 (48 %) | 4 (30 %) | 0.298 |
| BMI [IQR] | 28[32-24] | 28[31-24] | 29[33-25] | 0.678 |
| Paroxysmal AF - n (%) | 25 (62.5 %) | 15 (55 %) | 10 (77 %) | 0.191 |
| Persistent AF - n (%) | 15 (37.5 %) | 14 (52 %) | 1 (8 %) | 0.191 |
| Hypertension - n (%) | 22 (55 %) | 15 (55 %) | 7 (54 %) | 0.919 |
| Hypercholesterolemia - n (%) | 18 (45 %) | 14 (52 %) | 4 (31 %) | 0.207 |
| Diabetes - n (%) | 3 (7.5 %) | 1 (4 %) | 2 (16 %) | 0.189 |
| OSA - n (%) | 4 (10 %) | 2 (8 %) | 2 (26 %) | 0.431 |
| Smoker - n (%) | 6 (15 %) | 5 (19 %) | 1 (8 %) | 0.369 |
| CKD - n (%) | 4 (10 %) | 1 (4 %) | 3 (23 %) | 0.056 |
| Thyroid disease - n (%) | 9 (22.5 %) | 6 (22 %) | 3 (23 %) | 0.952 |
| Cancer - n (%) | 4 (10 %) | 1 (4 %) | 3 (23 %) | 0.056 |
| Heart Failure - n (%) | 9 (22.5 %) | 6 (22 %) | 3 (23 %) | 0.952 |
| Coronary disease - n (%) | 2 (5 %) | 1 (4 %) | 1 (8 %) | 0.588 |
| Ischemic stroke - (%) | 2 (5 %) | 1 (4 %) | 1 (8 %) | 0.588 |
| CHA2DS2-VASc [IQR] | 2[3-1] | 2[3-2] | 2[2-1] | 0.216 |
| Total medication [IQR] | 4 [5-3] | 4[5-3] | 4[6-3] | 0.923 |
| Beta-Blockers – n (%) | 32 (80 %) | 23 (85 %) | 9 (69 %) | 0.237 |
| Class I antyarrythmics – n (%) | 11 (27.5 %) | 9 (33 %) | 2 (16 %) | 0.234 |
| Class III antyarrythmics – n (%) | 5 (12.5 %) | 4 (15 %) | 1 (8 %) | 0.523 |
| Vitamin K antagonist – n (%) | 3 (7.5 %) | 2 (8 %) | 1 (8 %) | 0.432 |
| DOAC – n (%) | 37 (93 %) | 27 (93 %) | 10 (91 %) | 0.888 |
| ACE-Inhibitor – n (%) | 20 (50 %) | 15 (55 %) | 5 (38 %) | 0.311 |
| MRA – n (%) | 5 (12.5 %) | 4 (15 %) | 1 (8 %) | 0.505 |
AF – Atrial fibrillation AF; N – number; yr – years; SD – standard deviation; BMI – body mass index, OSA – obstructive sleep apnea; CKD – chronic kidney disease; DOAC – direct oral anticoagulant; ACE – angiotensin-converting enzyme; mineralocorticoid receptor antagonist.
Rhythm monitoring with the ePatch was obtained for a median of 118 (IQR 112–120) hours, with a minimum effective monitoring duration of 50 h. No discomfort complaints in the use of the device nor the appearance of interpretation artefacts were registered (Supplementary Fig. 1). Very-early AF recurrence was detected in 11 (27.5 %) patients, with a median AF burden of 7 % (IQR 6 %–33 %). Late blanking period AF was detected in 13 (33 %) of the external 7-day loop recordings. Out of the 11 patients that had very-early AF recurrence detected in the ePatch monitoring, 10 (91 %) maintained late-blanking periods of AF.
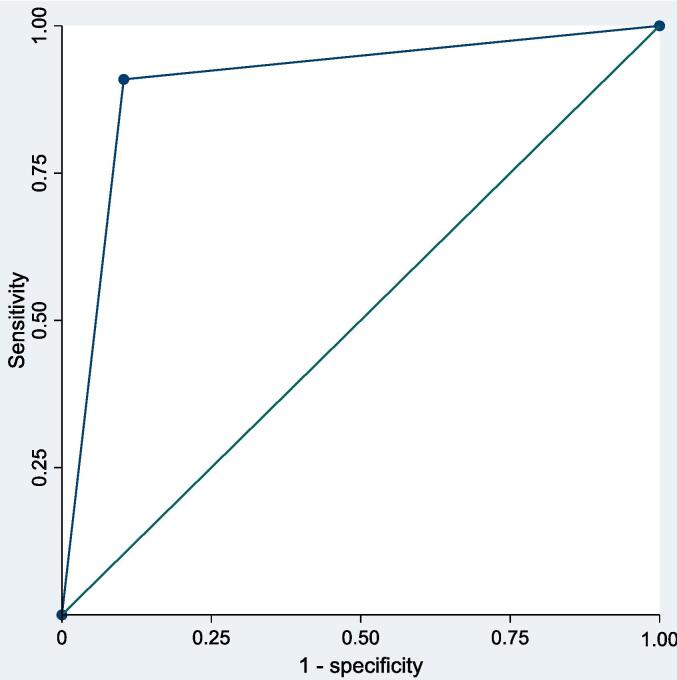
Very-early AF detection showed a sensitivity of 77 % (95 % CI 64 %-90 %) and a specificity of 96 % (95 % CI 90–100 %) in predicting late-blanking AF, with a PPV of 91 % (95 % CI 82 %-100 %) and a NPV of 90 % (95 % CI 80 %-100 %). A non-parametric ROC curve analysis derived an AUC of 0.903 (95 % 0.797––1.0) (Fig. 2). Additionally, a non-statistically significant trend for higher HRV parameters during the ePatch monitoring was observed in patients presenting with AF recurrence during the late blanking period, with the pNN50 variable (a marker reflecting the vagal tone) showing a significant value in the univariate logistic regression analysis (Table 2 and Supplementary Table 2).
Fig. 2.
Non-parametric ROC curve for the predictive effect of very-early blanking atrial fibrillation recurrence in predicting late blanking atrial fibrillation recurrence with a calculated AUC of 0.903.
Table 2.
Epatch monitoring data stratified by late-blanking recurrence of atrial fibrillation.
| Characteristics | Total patients (N = 40) | with late blanking AF recurrence (N = 27) | without late blanking AF recurrence (N = 13) | p-value |
|---|---|---|---|---|
| Monitoring time - hours [IQR] | 118[120-113] | 118[120-112] | 117[120-113] | 0.912 |
| Average HR - [IQR] | 66[72-60] | 65[71-58] | 70[75-60] | 0.219 |
| Maximum HR - [IQR] | 113[123-101] | 112[132-100] | 116[170-108] | 0.318 |
| Minimum HR - [IQR] | 49[55-38] | 49[55-42] | 49[53-34] | 0.410 |
| SVE – n [IQR] | 1544 [5666-181] | 1289[6089-132] | 3480[52431403] | 0.114 |
| Isolated SVE – n [IQR] | 1390 [5273] | 1089[6089-132] | 2535[5199-1397] | 0.144 |
| NN average - ms [IQR] | 917[975-838] | 922[1036-875] | 870[967-750] | 0.143 |
| SDNN - ms [IQR] | 121[165-97] | 121[142-98] | 160[228-85] | 0.405 |
| SDANN – ms [IQR] | 101[120-82] | 101[110-85] | 102[166-74] | 0.670 |
| RMSSD – ms [IQR] | 47[76-37] | 48[57-39] | 45[94-26] | 0.810 |
| pNN > 50 % - [IQR] | 8 %[21-4] | 7 % [12 %-4%] | 15 % [44 %-4%] | 0.285 |
AF – Atrial fibrillation AF; HR – heart rate; N – number; yr – years; SD – standard deviation; SVE – Supraventricular Ectopy; NN - normal to normal interval; SDNN – NN standard deviation in all recording; SDANN – NN standard deviation in all 5-minute segments of the recording; RMSDD - Square root of mean of sum of squares of differences between adjacent NN intervals; pNN50 - Percent of differences between adjacent normal RR intervals that are greater than 50 ms computed over the entire recording.
4. Discussion
Our work focused on the characterization of the very-early period following PVI through continuous ambulatory recording using a take-home wearable ECG-patch. In this pilot study, the ePatch was well tolerated among all patients and was able to detect very-early AF, which was highly predictive of late-blanking AF recurrence. While this finding should be interpreted in the context of an unblinded study with a small sample size, our results emphasize the importance of the occurrence of AF during the blanking in predicting later AF events.[6], [7], [13] The new data is the fact that very-early identification of AF after PVI has a high predictive power for late-blanking period AF recurrence.
As previously mentioned, there is currently an open debate regarding the duration of the blanking period and its relevance to patients and clinical outcomes.[7], [13] The median 7 % burden of AF during the very-early blanking period observed in this study corresponds to approximately 100 min of median daily AF, which represents a significant burden, five times higher than the 23-minute daily cutoff point, that represented a seven-fold increase in the risk of AF at one-year follow-up observed on a prior study from our group’[7]. The fact that most patients showing very-early AF recurrence had it with a significant arrhythmia burden may be interpreted as an indicator that an AF burden cutoff can be used in the future as a predictor of AF recurrence and clinical outcomes - but further studies are needed to validate these results.
Later detection of AF recurrence during the blanking period has been highly correlated with AF recurrence.[13], [14] A sub-analysis of the DECAAF-II trial has indicated that a higher burden of AF detection during the late-blanking period through an intermittent recording with a smartphone correlated with AF recurrence at 90 days.[14] Additionally, a sub-analysis of the ADVICE trial has showed that at 1-year follow up, comparing to 23 % without symptomatic AF during the blanking period, 92 % of patients that had symptomatic AF during the late blanking period had 1-year recurrence.[13] In both sub-analyses the detection of AF during the early blanking period also correlated – with less strength - with post-blanking AF recurrence. The fact that the very early blanking period was recorded continuously in our pilot study may explain the high rate of concomitant detection and concordance between very-early and late blanking periods. This very-early continuous characterization may lead to a better risk stratification for AF recurrence in the future.
Early studies on the blanking period were conducted in an era in which AF ablation technology through radiofrequency PVI and tridimensional mapping systems were less developed. In fact, a higher degree of substrate changes and inflammation caused by radiofrequency energy might have been produced, yielding a higher rate of early recurrences that were not sustained in time when compared to current findings.[12], [13] Albeit not being powered for this purpose, our study found no signal of a protective effect of cryoablation compared to radiofrequency in either early or late AF recurrence.
Sympathetic nervous system (SNS), particularly the parasympathetic activity modulation, has been considered as part of the postulated benefits of antral PVI – with the achievement of parasympathetic denervation in addition to PVI being suggested as a concurrent antiarrhythmic mechanism, increasing the success of AF ablation. [12], [15]
The indirect assessment of fluctuations in SNS inputs to the heart through statistical measures of HRV has been heavily linked to clinical outcomes, with higher values of SDNN, RMSSD, and pNN50 being all significantly associated with an increased risk of AF.[16], [17], [18]
In our study, higher HRV parameters, representing excessive autonomic fluctuations, appeared to be linked with AF recurrence after PVI. However, these findings represent a mere indicator for future studies.
4.1. Study limitations
The most important limitations of our study are connected to its unblinded nature, and the fact that it was a single-center observational study, albeit prospective. The study cohort consisted exclusively of patients undergoing PVI ablation, and submitted both to an early ePatch evaluation and late blanking period external loop recording. Therefore, it included a small selected population. Also, HRV parameters derived from the ePatch wearable device data might not be as validated as in the standard 24 h Holter recording. However, recently studies used the ePatch to successfully monitor HRV in different clinical settings.[19], [20]
5. Conclusions
The E-Patch was able to detect very-early AF during an extended period of time. The occurrence of AF in the very-early monitoring appears to be significantly predictive of late blanking period AF recurrence post-ablation. Also, higher HRV values, reflecting excessive autonomic fluctuations, may be linked to an increased risk of late blanking AF episodes. Our preliminary findings need to be validated in larger cohorts, preferably blinding to the early recording results.
Grant support.
This work did not receive any grants.
Ethical disclosures.
Protection of human and animal subjects.
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data.
The authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consent.
The authors declare that no patient data appear in this article.
CRediT authorship contribution statement
Miguel Marques Antunes: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Pedro Silva Cunha: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Bárbara Lacerda Teixeira: Writing – original draft, Validation, Software, Data curation. Guilherme Portugal: Writing – review & editing, Investigation, Formal analysis, Data curation, Conceptualization. Bruno Valente: Writing – original draft, Validation, Project administration, Formal analysis, Conceptualization. Ana Lousinha: Writing – review & editing, Project administration, Investigation, Formal analysis, Data curation. Ana Sofia Delgado: . Sandra Alves: Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation. Cátia Guerra: Validation, Software, Resources, Project administration, Formal analysis, Data curation. Rui Cruz Ferreira: Writing – review & editing, Visualization, Validation, Supervision, Resources, Methodology, Conceptualization. Mário Martins Oliveira: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Methodology, Investigation, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101369.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hindricks G., Potpara T., Dagres N., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2020;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 2.Kirchhof P., Camm A.J., Goette A., et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020;383(14):1305–1316. doi: 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]
- 3.Packer D.L., Mark D.B., Robb R.A., et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: The CABANA Randomized clinical trial. JAMA. 2019;321(13):1261–1274. doi: 10.1001/jama.2019.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade J.G., Wells G.A., Deyell M.W., et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N. Engl. J. Med. 2020;384(4):305–315. doi: 10.1056/NEJMoa2029980. [DOI] [PubMed] [Google Scholar]
- 5.Kis Z., Muka T., Franco O.H., et al. The Short and long-term efficacy of pulmonary vein isolation as a sole treatment strategy for paroxysmal atrial fibrillation: a systematic review and meta-analysis. Curr Cardiol Rev. 2017;13(3):199–208. doi: 10.2174/1573403x13666170117125124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kornej J., Schumacher K., Dinov B., et al. Prediction of electro-anatomical substrate and arrhythmia recurrences using APPLE, DR-FLASH and MB-LATER scores in patients with atrial fibrillation undergoing catheter ablation. Sci Rep. 2018;8(1):12686. doi: 10.1038/s41598-018-31133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva Cunha P., Portugal G., Laranjo S., et al. The atrial fibrillation burden during the blanking period is predictive of time to recurrence after catheter ablation. Int J Cardiol Heart Vasc. 2022;43 doi: 10.1016/j.ijcha.2022.101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim Y.G., Boo K.Y., Choi J.I., et al. Early Recurrence Is Reliable Predictor of Late Recurrence After Radiofrequency Catheter Ablation of Atrial Fibrillation. JACC Clin Electrophysiol. 2021;7(3):343–351. doi: 10.1016/j.jacep.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Oral H., Knight B.P., Özaydın M., et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J. Am. Coll. Cardiol. 2002;40(1):100–114. doi: 10.1016/S0735-1097(02)01939-3. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell D., Furniss S.S., Dunuwille A., et al. Delayed cure despite early recurrence after pulmonary vein isolation for atrial fibrillation. Am. J. Cardiol. 2003;91(1):83–85. doi: 10.1016/S0002-9149(02)03005-9. [DOI] [PubMed] [Google Scholar]
- 11.Cheema A., Vasamreddy C.R., Dalal D., et al. Long-term single procedure efficacy of catheter ablation of atrial fibrillation. J. Interv. Card. Electrophysiol. 2006;15(3):145–155. doi: 10.1007/s10840-006-9005-9. [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb L.A., Dekker L.R.C., Coronel R. The Blinding Period following Ablation Therapy for Atrial Fibrillation: Proarrhythmic and Antiarrhythmic Pathophysiological Mechanisms. 2021;7:416–430. doi: 10.1016/j.jacep.2021.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Willems S., Khairy P., Andrade J.G., et al. Redefining the blanking period after catheter ablation for paroxysmal atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2016;9(8):e003909. doi: 10.1161/CIRCEP.115.003909. [DOI] [PubMed] [Google Scholar]
- 14.Noujaim C., Lim C., Donnellan E., et al. Smartphone AF burden during the blanking period predicts catheter ablation outcomes: insights from DECAAF II. JACC: Clinical Electrophysiology. 2023;9(10):2085–2095. doi: 10.1016/j.jacep.2023.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Pachon-M J.C., Pachon-M E.I., Pachon C.T.C., et al. Long-Term evaluation of the vagal denervation by cardioneuroablation using holter and heart rate variability. Circ. Arrhythm. Electrophysiol. 2020;13(12):e008703. doi: 10.1161/CIRCEP.120.008703. [DOI] [PubMed] [Google Scholar]
- 16.Heart rate variability Standards of measurement, physiological interpretation, and clinical use. task force of the european society of cardiology and the north american society of pacing and electrophysiology. Eur. Heart J. 1996;17(3):354–381. [PubMed] [Google Scholar]
- 17.Shaffer F., Ginsberg J.P. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S.H., Lim K.R., Seo J.H., et al. Higher heart rate variability as a predictor of atrial fibrillation in patients with hypertension. Sci Rep. 2022;12(1):3702. doi: 10.1038/s41598-022-07783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frandsen M.N., Huang L., Petersen R.H., et al. Continuous perioperative heart rate variability monitoring in video-assisted thoracoscopic surgery lobectomy-a pilot study. J Clin Monit Comput. 2023;37(4):1071–1109. doi: 10.1007/s10877-023-01016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeppesen J., Christensen J., Johansen P., et al. Personalized seizure detection using logistic regression machine learning based on wearable ECG-monitoring device. Seizure. 2023;107:155–161. doi: 10.1016/j.seizure.2023.04.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.