Summary
Pathogenic mycobacteria orchestrate the complex cell populations known as granuloma that is the hallmark of tuberculosis. Foam cells, a lipid-rich cell-type, are considered critical for granuloma formation; however, the causative factor in foam cell formation remains unclear. Atherosclerosis is a chronic inflammatory disease characterized by the abundant accumulation of lipid-laden-macrophage-derived foam cells during which cholesterol 25-hydroxylase (CH25H) is crucial in foam cell formation. Here, we show that M. marinum (Mm), a relative of M. tuberculosis, induces foam cell formation, leading to granuloma development following CH25H upregulation. Moreover, the Mm-driven increase in CH25H expression is associated with the presence of phthiocerol dimycocerosate, a determinant for Mm virulence and integrity. CH25H-null mice showed decreased foam cell formation and attenuated pathology. Atorvastatin, a recommended first-line lipid-lowering drug, promoted the elimination of M. marinum and concomitantly reduced CH25H production. These results define a previously unknown role for CH25H in controlling macrophage-derived foam cell formation and Tuberculosis pathology.
Subject areas: Microbiology, Cell biology
Graphical abstract

Highlights
-
•
CH25H was induced and upregulated in pathogenic mycobacteria-infected macrophages
-
•
CH25H was a critical factor in modulating foam cell formation and expressed in granuloma
-
•
CH25H-null mice showed reduced foam cell formation and pathology upon Mm infection
-
•
CH25H may act as a player during statins-mediated adjuvant therapy against tuberculosis
Microbiology; Cell biology
Introduction
Tuberculosis, caused by Mycobacterium tuberculosis (Mtb), is the leading global cause of death from a bacterial infectious disease. As an intracellular bacterium, Mtb has developed numerous strategies to adapt to the host’s internal microenvironment to benefit its survival.1,2 Phthiocerol dimycocerosates (PDIM), an outer mycomembrane lipid of the cell wall, is ubiquitously distributed in all clinically and veterinary pathogenic mycobacterial species.3,4,5 Mycobacterium marinum (Mm), a close relative of Mtb that shares most evolutionary homologous genes and common virulence determinants,6 has been widely used to model the pathogenesis and underlying mechanisms of host-Mtb interactions.7,8,9 Importantly, Mm can establish infection in a murine model via intravenous inoculation and form granulomatous lesions, providing scope for new avenues of study to reveal fundamental mechanisms of mycobacterial pathogenesis.10
Granuloma, a hallmark of tuberculosis, is an architecture of various cell types and is traditionally considered necessary for restraining mycobacterial dissemination11,12; however, the significance of granuloma for tuberculosis has gradually changed with accumulating reports.7,11,12,13 Many studies have demonstrated that granulomas do not simply contain the bacilli but provide mycobacteria with a nutrient pool for survival.7,11,12,13,14 Foam cells, lipid-laden macrophages that ubiquitously express adipocyte differentiation-related protein (ADRP), were originally described in atherosclerosis,15,16 a condition that also forms during mycobacterial infection.14,17,18,19,20,21 Macrophage-derived foam cells with accumulated lipids provide a hospitable parasitic microenvironment and act as a reservoir for live Mtb within the host.21 Peroxisome proliferator-activated receptor gamma (PPARγ) is triggered by mycobacterial infection, leading to lipid droplet formation and reduced macrophage responses.17,18 CD36 plays a role in the formation of foam macrophages and provides a protective niche for mycobacteria,19 while targeting CD36 curtails Mtb survival by repressing foam cell formation.22 In Mm-infected zebrafish, foam cells are converted from macrophages and their formation is driven by the mycobacterial ESX1 locus.23 Recently, studies have revealed that lipid-lowering statin drugs inhibit foam cell formation and synergistically promote antimicrobial effects of tuberculosis treatments such as rifampicin and isoniazid in vivo and in vitro.24,25,26 Although cumulative evidence demonstrates that statins have potential as anti-tuberculosis therapeutics, how statins manipulate foam cell development and the underlying mechanism requires further investigation.
Cholesterol 25-hydroxylase (CH25H), which hydroxylates cholesterol to generate 25-hydroxycholesterol (25HC)27 and is highly expressed in activated macrophages28 as well as the lungs of mice from an Mtb aerosol-infected model,29 has been demonstrated to have not only broad antiviral activity,28,30 but also play a critical role in modulating inflammatory responses.31,32 Particularly, CH25H was proven to be a pivotal player in promoting macrophage differentiation into foam cells, mediating the accumulation of lipid droplets in macrophages to trigger the onset of atherogenesis.16,32,33 However, to our knowledge, no reports have investigated the significance and mechanism of CH25H on foam cell formation during mycobacterial infection.
Here, we show that M. marinum (Mm), manipulates foam cell development via CH25H induction. Moreover, increased CH25H expression in Mm-infected cells was induced by PDIM, a virulence determinant present in Mm.34,35 Knock-out of CH25H decreased foam cell formation and significantly attenuated pathology in Mm-infected mice. Atorvastatin, a recommended first-line statin, promoted the elimination of Mm in vivo and in vitro by reducing CH25H. Taken together, these results define a previously unknown role for CH25H in controlling macrophage-derived foam cell formation and tuberculosis pathology, demonstrating that CH25H-targeting may represent a promising adjuvant strategy for tuberculosis therapy in the future.
Results
WT_Mm induced foam cell formation and CH25H induction
Numerous foam cells were formed in the WT_Mm-challenged group at 1, 4, and 8 hpi, whereas this was dramatically decreased in the ΔPDIM-infected group (Figure 1A). Quantitation showed that WT_Mm indeed induced significantly more foam cells compared with ΔPDIM (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001) (Figure 1B). Subsequently, western blot revealed that CH25H was significantly upregulated in the WT_Mm-infected group compared with ΔPDIM-infected cells (Figures 1C and S1A). ADRP, the hallmark of foam cells, was also clearly increased in the WT_Mm-infected group, while reduced in ΔPDIM-challenged cells (Figures 1C and S1B). CH25H and 25HC were markedly upregulated in WT_Mm-infected macrophages (Figures 1C, S1C and S1D); however, they did not show significant changes in the ΔPDIM-infected groups (Figures 1C, S1C, and S1D). Moreover, the complementation of ΔPDIM mutant (ΔPDIM_Comp) not only restored the expression of CH25H and ADRP (Figures 1D and S1E–S1H), but also enhanced the formation of foam cells (Figures S1I and S1J). Collectively, these results suggest that pathogenic mycobacteria promote foam cell formation and CH25H expression.
Figure 1.
Pathogenic mycobacteria induce foam cell formation and CH25H upregulation
(A) In Mm-infected Raw264.7 macrophages (MOI = 5), wide-type (WT) Mycobacterium marinum (Mm) induced most of macrophages transdifferentiation into foam cells compared with a cell wall lipid PDIM mutant (ΔPDIM) at 1, 4, and 8 hpi. ox-LDL (50 μg/mL) treatment as a positive control (40× magnification).
(B) Quantitation of foam cell density demonstrated a significant difference between WT_Mm and ΔPDIM-treated groups at 1, 4, and 8 hpi (∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001). Data are representative of three biologically independent experiments.
(C) The cell lysates of WT_ Mm and/or ΔPDIM-treated cells were prepared and analyzed by western blot and demonstrated that CH25H was upregulated transparently in WT_Mm-infected Raw264.7 cells, while weakly expressed in ΔPDIM strain-challenged groups at matched time-points. Moreover, ADRP—a specific marker for foam cells, consistent with the CH25H expression at matched time-points between WT_Mm and ΔPDIM strain.
(D) Western blot detection demonstrated that complementation of ΔPDIM strain (ΔPDIM_Comp) restores the expression of CH25H and ADRP.
PDIM-replete mycobacteria upregulate CH25H expression during mycobacterial infection
PDIM-deficiency has been shown to decrease the uptake of mycobacteria by macrophages.36 Consistent with this previous report, we found fewer ΔPDIM bacteria were taken up by macrophages compared with the WT_Mm at a multiplicity of infection (MOI) 5 (Figure 2S). To exclude the possibility that decreased CH25H production was a result of fewer internalized ΔPDIM_Mm at MOI 5, we set up another group of ΔPDIM infections at MOI 20 to ensure intracellular mycobacteria numbers were equal to those occurring in WT_Mm infections at MOI 5. Despite the internalized bacteria in the ΔPDIM_Mm-infected group at MOI 20 being equivalent to WT_Mm infection at MOI 5 (Figure 2S), surprisingly, CH25H protein in the ΔPDIM group remained lower than that in WT_Mm-infected macrophages at MOI 5 (Figures 2A and 2B). Consistently, the foam cell marker ADRP was dramatically upregulated in the WT_Mm-infected group, but expressed at lower levels in macrophages infected with ΔPDIM at MOI 5 and 20 (Figures 2A and 2B). Staining for neutral lipids (Figures 2C and 2D) and immunofluorescence observation found that more lipid droplets and high expression of ADRP occurred in the WT_Mm-infected group (Figures 2E and 2F). These results indicate that mycobacteria replete with PDIM on their surface are required for the enhancement of expression of CH25H and ADRP during WT_Mm infection.
Figure 2.
Pathogenic mycobacteria induce CH25H expression via a virulence-determinant-dependent manner
(A and B) At MOI = 5, WT_Mm induced abundant CH25H and ADRP proteins compared with those in ΔPDIM-treated cells. While enhancing the ΔPDIM MOI at 20, the protein levels of CH25H and ADRP were still lower and equivalent to ΔPDIM MOI = 5 at indicated time-points (∗∗∗∗p < 0.0001). Data are representative of three biologically independent experiments.
(C and D) WT_Mm and ΔPDIM strain treated Raw264.7 macrophages for 4 h, stained for neutral lipids (BODIPY 493/503×) (C, 40× magnification) and quantitation between WT_ Mm and ΔPDIM treated groups, respectively (∗∗∗∗p < 0.0001). Data are representative of three biologically independent experiments.
(E and F) Fluorescent images show M. marinum (red), nuclear fluorescence (DAPI, blue), ADRP (green) and stained for the foam cell marker ADRP (merged) ((E): 100× magnification). Quantitation for ADRP at 4 hpi between WT- and ΔPDIM Mm-treated groups (∗∗∗∗p < 0.0001). Data are representative of three biologically independent experiments.
CH25H contributes to foam cell formation
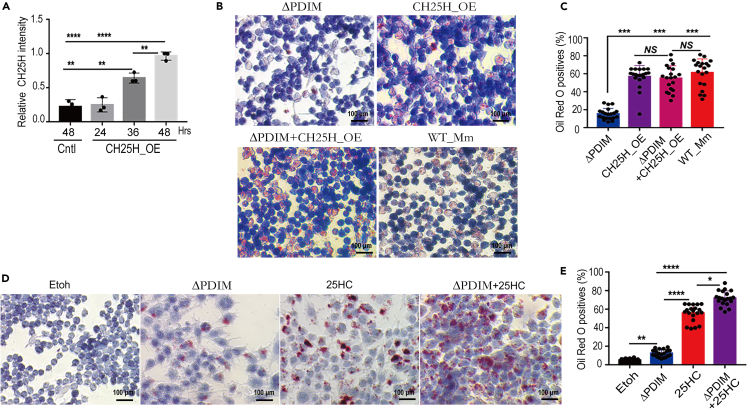
To assess whether CH25H contributes to foam cell formation upon WT_Mm infection, specific small interfering (si) RNAs against the Ch25h gene were designed (Table S1) and their efficacy was evaluated (Figure 3A). siRNA-2 demonstrated optimum inhibition of CH25H at both the mRNA and protein levels (termed as Ch25h_si2), and was thus chosen for subsequent experiments. The optimal Ch25h_si2 was transfected into Raw264.7 cells which were then infected with WT_Mm. At 4 hpi, cells were harvested and stained for foam cells. Reduced foam cell formation after CH25H silencing was evident following WT_Mm challenge (Figures 3B and 3C). Interestingly, a mouse CH25H overexpression plasmid was constructed and termed CH25H_OE (Figures S3A and S3B) and its efficiency in Raw264.7 cells was evaluated (Figure 4A). CH25H_OE was transinfected into Raw264.7 macrophages and oil red O staining found numerous foam cells was formed even in ΔPDIM group after CH25H overexpression (Figures 4B and 4C). Consistently, the addition of 25HC, the enzymatic product of CH25H, into ΔPDIM-infected groups significantly promoted foam cell formation (Figures 4D and 4E). Taken together, our findings indicate that pathogenic mycobacteria induce foam cell formation and CH25H contributes to this process.
Figure 3.
Silencing CH25H reduces the foam cell formation after WT_Mm infection
(A) Three siRNAs targeting the mouse Ch25h gene were designed and their efficiency was examined and demonstrated that siRNA-2 has an optimal inhibitory effect on the mouse Ch25h gene (termed as Ch25h_si2), which has been chosen for the continuous experiment.
(B) Ch25h_si2 was transfected into Raw264.7 cells and subsequently challenged with WT_Mm, then the cells were harvested and performed Oil Red O staining (40× magnification).
(C) Quantitation demonstrated that there was a significant difference with CH25H silencing in the WT_ Mm-infected group compared with without CH25H inhibition (∗∗∗∗p < 0.0001; NS, no significance). Data are representative of three biologically independent experiments.
Figure 4.
CH25H promotes the formation of foam cells
(A) CH25H protein level was enhanced after CH25H_OE was transfected into Raw264.7 macrophages and compared (∗∗p < 0.01; ∗∗∗∗p < 0.0001). Data are representative of three biologically independent experiments.
(B and C) CH25H_OE was transinfected into Raw264.7 and following infection with ΔPDIM_Mm, oil Red O staining found numerous foam cells were developed (∗∗∗p < 0.001; NS, no significance). Data are representative of three biologically independent experiments.
(D and E) 25HC markedly promoted the foam cell formation, especially supplementation into attenuated ΔPDIM_Mm-challenged groups (∗p < 0.05; ∗∗p < 0.01; ∗∗∗∗p < 0.0001). Data are representative of three biologically independent experiments.
Pathogenic mycobacteria induce CH25H primarily via Toll-like receptor 4 (TLR4) signaling
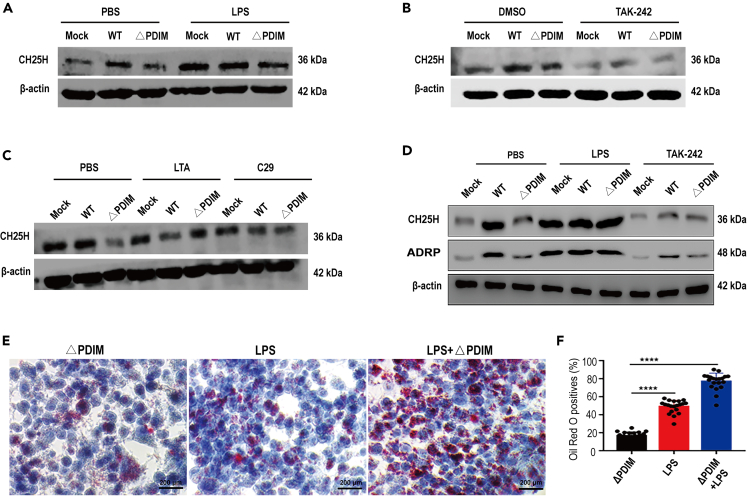
Previous studies demonstrated that expression of CH25H was markedly upregulated in macrophages stimulated with a TLR4-related agonist, but not lipoteichoic acid (LTA), a TLR2 agonist.37,38 In our study, we detected CH25H proteins in macrophages with a panel of activators and antagonists for TRL2 and TLR4, with or without WT- or ΔPDIM-Mm challenge. We found that the lower expression of CH25H in ΔPDIM-treated macrophages was significantly increased after exposure to LPS (Figures 5A and S4A). In contrast, CH25H was highly expressed in WT_Mm-infected macrophages but decreased dramatically after treatment with the TLR4 signaling inhibitor, TAK-242 (Figures 5B and S4B). However, neither LTA nor the TLR2 inhibitor, C29, impacted the expression trend of CH25H in macrophages following WT- or ΔPDIM-Mm infection (Figures 5C and S4C). Similarly, TAK-242 significantly reduced ADRP expression in WT_Mm-treated macrophages, while LPS enhanced ADRP in the ΔPDIM-infected group (Figures 5D, S4D, and S4E). Finally, oil red O staining demonstrated that LPS treatment rescued foam cell formation, resulting in increased foam cells in the LPS-treated ΔPDIM group (Figures 5E and 5F). Our results demonstrate that pathogenic mycobacteria promote foam cell formation by induction of CH25H expression via TLR4 signaling.
Figure 5.
Pathogenic mycobacteria promote foam cell formation via upregulated CH25H through TLR4 signaling activation
(A) LPS, a specific TLR4 agonist, greatly induced the upregulation of CH25H in ΔPDIM-infected macrophages (LPS 10 ng/mL).
(B) TAK-242, an antagonist for the TLR4 signal, markedly reduced the CH25H production even under WT_Mm challenging (TAK-242: 100 nM).
(C) Either LTA (10 μg/mL) or C29 (100 μM), the specific activator and inhibitor for TLR2 signal, has no significant effects on the CH25H expression.
(D) Either LPS or TAK-242 remarkably changed the expression profile of CH25H and ADRP under WT_Mm and/or ΔPDIM strain inoculation.
(E) Oil red O stain demonstrated that numerous foam cells were formed under LPS treatment and LPS co-incubated ΔPDIM_Mm (40× magnification).
(F) Quantitation of oil red O cells demonstrated that the LPS supplement promotes the foam cell formation in the ΔPDIM-infected group (∗∗∗∗p < 0.0001). Data are representative of three biologically independent experiments.
CH25H exacerbates morbidity in mice following mycobacterial infection
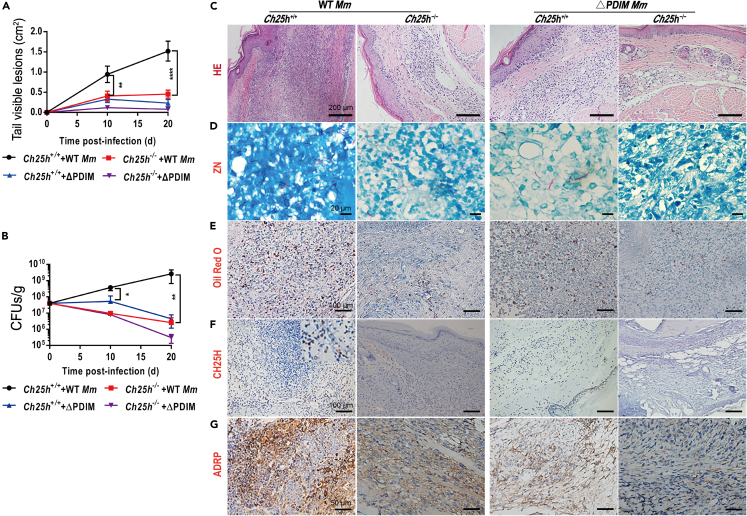
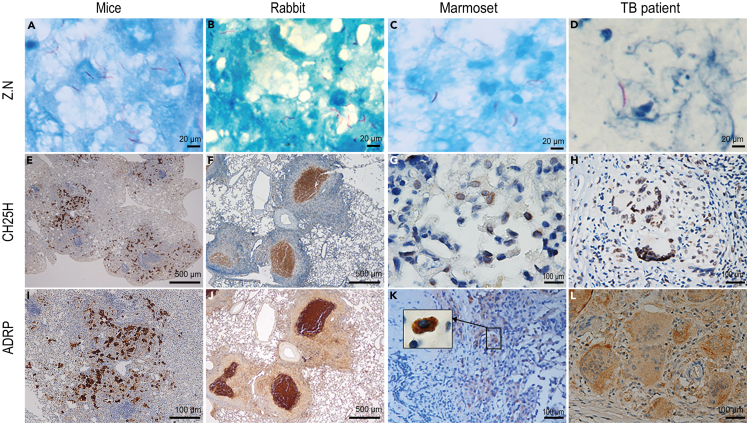
Next, we sought to determine the impact of CH25H on the outcome of mycobacterial infection in vivo. Genotyped Ch25h−/− mice (Figures S5A and S5B), and age- and sex-matched Ch25h+/+ siblings were intravenously injected with the same number of WT- or ΔPDIM-Mm.10 Surprisingly, Ch25h−/− mice were relatively protected following WT_Mm infection and only scattered abscesses were formed, while Ch25h+/+ counterparts demonstrated progressive larger-scale visible tail lesions (Figures 6A, S5C, and S5D). CFU counts demonstrated a decrease in the number of bacteria in WT_Mm-infected Ch25h−/− mice (Figure 6B). Histopathological evaluation revealed that there were numerous infiltrating inflammatory cells and granulomatous lesions in WT_Mm-infected Ch25h+/+ mice, whereas fewer inflammatory cells were observed in their Ch25h−/− siblings (Figure 6C). ZN staining found red rod bacteria distributed in tissues from WT_Mm-infected Ch25h+/+ and Ch25h−/− mice, but not in ΔPDIM-infected Ch25h−/− mice (Figure 6D). Oil red O staining showed that numerous foam cells were formed and extensively distributed in the granulomatous lesions of WT Mm-infected Ch25h+/+ mice (Figures 6E and S6A), while decreased foam cells were observed in matched Ch25h−/− siblings (Figures 6E and S6B). Immunohistochemistry staining demonstrated that CH25H was highly expressed within granulomatous lesions (Figure 6F). Consistent with foam cell dynamics, ADRP was highly expressed in WT_Mm-infected Ch25h+/+ mice, but not in their Ch25h−/− siblings (Figure 6G). Finally, CH25H and ADRP were also highly expressed in lung tissues from Mtb-challenged mice, rabbits, and marmosets as well as clinical tuberculosis patient specimens, distributed particularly within granulomas (Figures 7 and S7). Taken together, pathogenic mycobacteria could induce CH25H to aggravate the pathology and promote foam cell development during granuloma formation.
Figure 6.
CH25H exacerbates pathology in mice after mycobacterial infection via promoting foam cell formation
(A) Ch25h+/+ mice developed progressive and large inflammatory lesions compared to the reduced issues with their Ch25h−/− siblings after WT_Mm infection (n = 5).
(B) Host mycobacterial loading was gradually decreased in Ch25h−/− mice compared with the wide-type siblings after WT_Mm infection (n = 5).
(C) Histopathology examination demonstrated that typical granulomatous lesions formed in Ch25h+/+ mice after WT_Mm infection (20× magnification).
(D) ZN staining for detecting the mycobacterium marinum in mice tissues (100× magnification).
(E) Oil red O combined with hematoxylin staining found that there were plentiful foam cells in WT_Mm-challenged Ch25h+/+ mice, whereas the formation of foam cells markedly decreased in their Ch25h−/− siblings (32× magnification).
(F) Immunohistochemistry staining found that CH25H proteins were highly expressed and primarily distributed within granulomatous lesions (32× magnification).
(G) ADRP is obviously expressed in WT_Mm-challenged Ch25h+/+ mice compared to their Ch25h−/− siblings and disseminated within the granulomatous lesions (40× magnification).
Figure 7.
Profile of CH25H and ADRP in tissues from Mtb-challenged mice, rabbit and marmoset and clinical tuberculosis patient
(A–D) ZN staining showed that there were red-rod bacteria among different animal tissues including Mtb-infected lungs from mice, rabbits, and marmoset and clinical tuberculosis patients (100× magnification).
(E–H) CH25H is specifically expressed within the granulomatous lesions from Mtb-challenged mice, rabbit and marmoset, and TB patients ((E) and (F): 200× magnification; (G): 40× magnification; (H): 32× magnification).
(I–L) ADRP is expressed specifically within the granulomatous lesions of Mtb-challenged mice, rabbit and marmoset, and TB patients. In clinical TB patient samples, ADRP is markedly distributed within the multinuclear giant cells ((J): 200× magnification; (I), (K), and (L): 40× magnification).
Atorvastatin mitigates mycobacteria-mediated pathology by reducing foam cell formation via inhibition of CH25H
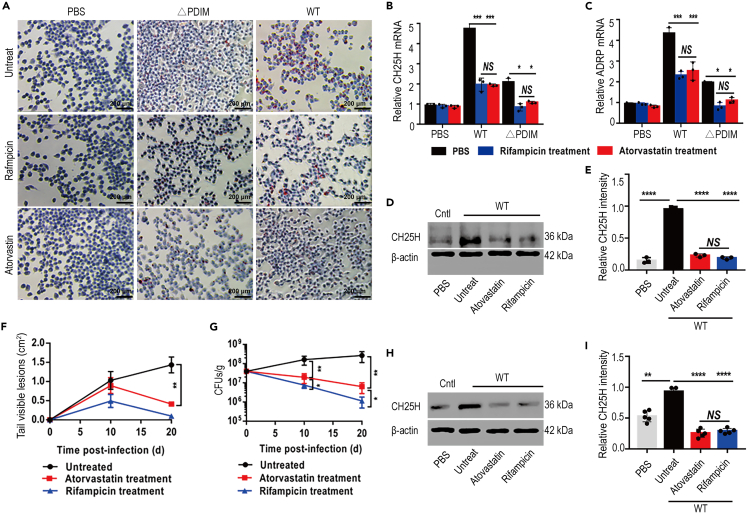
Atorvastatin administration markedly decreased in vitro foam cell formation following WT_Mm infection of Raw264.7 cells (Figures 8A and S8), and accordingly, the mRNA levels of CH25H and ADRP were also reduced (Figures 8B and 8C). Notably, CH25H protein was also dramatically decreased in atorvastatin-treated macrophages infected with WT_Mm (Figures 8D and 8E). The mouse infection model demonstrated smaller lesions in both atorvastatin- and rifampicin-treated animals, whereas progressive inflammatory lesions formed in untreated mice (Figures 8F and S9). CFU counts identified decreased bacterial numbers in mice following either atorvastatin or rifampicin treatment (Figure 8G). Western blot demonstrated that CH25H expression was significantly decreased in both atorvastatin- and rifampicin-treated WT_Mm-infected mice (Figures 8H and 8I). Interestingly, ultrahistopathology inspection demonstrated tight apposition between Mm-containing phagosomes and host lipid bodies (Figure S10A), in particular, the bacilli-containing phagosomes showed a tendency to be engulfed by lipid bodies (Figures S10B and S10C). Our data indicate that CH25H may act as a critical player during statin-mediated adjuvant therapy against Mtb.
Figure 8.
Atorvastatin administration decreased the CH25H expression and inhibited the formation of foam cells
(A) Atorvastatin administration inhibited the formation of foam cells even under WT_Mm infection (20× magnification).
(B and C) Atorvastatin treatment significantly reduced the mRNA expression level of CH25H and ADRP (∗p < 0.05; ∗∗∗p < 0.001). Data are representative of three biologically independent experiments.
(D and E) Atorvastatin treatment greatly decreased the protein level of CH25H in WT_Mm-infected cells (∗∗∗∗p < 0.0001). Data are representative of three biologically independent experiments.
(F and G) Atorvastatin administration remitted the tissue damages under WT_Mm infection (∗p < 0.05; ∗∗p < 0.01) (n = 5). Data are representative of three biologically independent experiments.
(H and I) Atorvastatin application dramatically reduced CH25H expression in WT_Mm-inoculated mice (∗∗p < 0.01; ∗∗∗∗p < 0.0001).
Discussion
Macrophages are not only the first line of host defense against Mtb, but also the primary parasitic niche for mycobacteria. For defense against macrophage-mediated immunosurveillance and killing, pathogenic mycobacteria induce granuloma development to facilitate bacterial survival and dissemination.7 Foam cells, originally described in atherosclerosis, are characterized by the formation of lipid-rich atheromatous lesions, which are not only thought to be a key driver of atherosclerotic diseases15,16 but have also emerged as a crucial setting during mycobacteria-induced granuloma formation.14,19,21,23,39,40,41,42 Mtb induces macrophage conversion into foam cells to reduce host cell capacity as well as make them a nutrient-rich reservoir for mycobacteria.21,42 Although reports have demonstrated that foam cells play an important role in sustaining persistent bacteria and contributing to granuloma formation, the critical factor regulating the transdifferentiation of macrophages into foam cells remains unclear.
Recently, there was a report demonstrating that Mtb-infected cells upregulate the oxysterol-producing enzyme CH25H on the circulating eosinophils in both mice and rhesus macaque models, and highly expressed CH25H in infected murine alveolar macrophages could promote the pulmonary recruitment of eosinophils during the early exposure to Mtb.43 In line with the CH25H upregulation in mice alveolar macrophages, our study demonstrated that CH25H is also upregulated in Raw264.7 macrophages and acts as an essential mediator for macrophage-derived foam cell formation during mycobacterial infection in vivo and in vitro. CH25H-null mice showed decreased foam cell formation and enhanced elimination of mycobacteria compared with Ch25h+/+ siblings. Abundant foam cells were formed and distributed within granulomatous lesions in WT_Mm-infected Ch25h+/+ mice, but not in Ch25h−/− siblings. Cumulative studies have demonstrated that CH25H and its enzymatic product, 25HC, act as a critical factor in modulating foam cell formation in a mouse model of diet-induced atherosclerosis.16 Consistent with this, we found that CH25H promotes the transdifferentiation of Raw264.7 macrophages into foam cells. Moreover, we also found that increased foam cells developed in LPS-activated Raw264.7 macrophages, while specific inhibition of TLR4 signaling repressed foam cell formation even following Mm infection.
Atorvastatin, a statin family member, markedly repressed mycobacterial viability and improved tissue damage when combined with rifampicin.44 Statin treatment markedly decreased the bacterial burden and reduced pathology in an Mtb-infected murine model.24 Recent studies demonstrated that simvastatin not only enhances the immune response against Mtb by promoting apoptosis,45 but also significantly strengthens the bactericidal activity of first-line drugs for tuberculosis in mice.25 Although increasing reports in the literature have demonstrated that statins have direct and/or indirect anti-mycobacterial effects and could act as an adjuvant for tuberculosis treatment, the critical player during this process remains to be identified. Consistent with previous reports, we found that atorvastatin inhibited the expression of CH25H, thereby decreasing foam cell formation and improving pathology during mycobacterial infection. Our data imply that inhibiting foam cell development may be an unrecognized anti-tuberculosis mechanism of statins, and CH25H plays an important role during this process.
We compared gross lesions and histopathology between Ch25h−/− mice and their Ch25h+/+ siblings after Mm infection at matched time-points. We found scattered small tail lesions and mild infiltration of inflammatory cells in Ch25h−/− mice, but progressive and granulomatous lesions in their Ch25h+/+ siblings. CH25H-null mice also exhibited powerful antimicrobial activity and reduced pathology compared with the increasing bacterial loading in their Ch25h+/+ siblings. These results are consistent with a previous report that CH25H-deficient mice showed a stronger capacity to repress Listeria monocytogenes growth in vivo.31 Moreover, deletion of CH25H demonstrated a stronger protective effect in a mouse model of influenza infection due to decreased inflammatory pathology.32 Our data suggest that in this murine infection model, Mm challenge upregulated CH25H to alter the inflammatory response, thereby amplifying inflammatory signaling.
In conclusion, to our knowledge, this study presents the first report of a previously unknown role of CH25H in modulating foam cell formation during mycobacterial infection, although further investigation of the detailed mechanism of mycobacterial regulation of CH25H expression and function remains necessary. Deletion of CH25H reduced the formation of foam cells and attenuated pathology in mice. CH25H also acts as an intrinsic effector linking statins and their anti-tuberculosis function. Thus, “host-directed therapy” targeting CH25H-mediated foam cell formation may lead to a more robust immune response against Mtb. Through this mechanism, the regulation of CH25H has the potential to be a viable means to shorten the overall duration of tuberculosis chemotherapies. Strategies targeting CH25H activity can be used as adjuvant therapy against Mtb and may avoid the development of drug resistance, thereby being conducive to the prevention and control of tuberculosis.
Limitations of the study
Although the present study provides interesting information on the role of CH25H-mediated foam cells for the formation of granuloma during mycobacterial infection, there are still some limitations that should be clarified in future studies. For instance, although CH25H was markedly up-regulated during mycobacterial in our study as well as other reports,29,43 considering the complex composition of mycobacteria and architecture of the mycobacteria cell wall and both structural and functional roles PDIM plays during infection,34,46 PDIM may not be the sole molecular component necessary for the upregulation of CH25H. Other bacterial components, in the absence of PDIM, may not present to the host cell in the same way, also influencing the host response to infection, whether they share a similar axis for the CH25H induction during mycobacterial infection. Although CH25H functionally produces 25HC, CH25H and 25HC may have different functions and affect various pathophysiological processes, experiments to identify the details and the potential mechanism of 25HC for granuloma formation will provide a deeper perspective of oxysterols during mycobacterial infection. Furthermore, a recent study revealed that CH25H indeed affects the eosinophil recruitment at the earliest response to Mtb-infection,43 and about the same time another report also demonstrated that some specific populations of eosinophils that expressed IL-4 and IL-13 within the granuloma,47 so whether CH25H drive granuloma formation by regulating eosinophil’s function and its interaction with macrophages during mycobacterial infection should be addressed in future studies.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| Wide-type Mycobacterium marinum (WT_Mm) | Obtained from Qian Gao (Fudan University) | ATCC BAA-535 |
| PDIM-deficient (ΔPDIM) Mycobacterium Marinum (ΔPDIM_Mm) |
Infect Immun, 2012, 80, 1381–1389. | |
| PDIM-deficient (ΔPDIM)_Complementary Mycobacterium marinum | Infect Immun, 2012, 80, 1381–1389. | |
| tdTomato-labeled WT_Mm | This study | N/A |
| tdTomato-labeled ΔPDIM_Mm | This study | N/A |
| E.coli DH5α | Takara | Cat#9057 |
| Antibodies | ||
| CH25H antibody (AA 1–100) (IHC-P) | Antibody-Online | Cat#AA 1-100 |
| Rabbit anti-ADRP | Abcam | Cat#ab108323; RRID:AB_10863476 |
| Mouse ADRP Antibody | Santa Cruz Biotechnology | sc-390169 |
| Mouse CH25H Antibody | Santa Cruz Biotechnology | sc-293256 |
| Goat Anti-Rabbit IgG H&L (HRP) | Abcam | Cat#ab205718; RRID:AB_2819160 |
| Goat Anti-Mouse IgG H&L (HRP) | Abcam | Cat#ab205719; RRID:AB_2755049 |
| Rabbit anti-Actin | Abcam | Cat#ab179467; RRID:AB_2737344 |
| Mouse anti-Actin | Abcam | Cat#ab8226; RRID:AB_306371 |
| Chemicals, peptides, and recombinant proteins | ||
| DMSO | Sigma-Aldrich | Cat#D8418 |
| Mayer’s Hematoxylin | Sigma-Aldrich | Cat#51275 |
| 25-hydroxycholesterol | Sigma-Aldrich | Cat#1015 |
| LPS (lipopolysaccharides) | Sigma-Aldrich | Cat#0111:B4 |
| C29 | MedChem Express | HY-100461 |
| TAK-242 | MedChem Express | HY-11109 |
| Lipoteichoic acid | Sigma-Aldrich | Cat#L3265 |
| Glutaraldehyde | Sigma | Cat#354400 |
| Critical commercial assays | ||
| Middlebrook 7H9 Broth | BD-Difco | Cat#27131 |
| Middlebrook 7H10 Broth | BD-Difco | Cat#262710 |
| BD BBL Middlebrook OADC Enrichment | BD-Difco | Cat#211886 |
| Hygromycin B | MedChemExpress | HY-B0490 |
| Kanamycin | MedChemExpress | HY-16566 |
| Ampicillin | MedChemExpress | HY-B0522 |
| Gentamycin | MedChemExpress | HY-A0276A |
| Bodipy493/503 | Sigma-Aldrich | Cat#790389 |
| Dulbecco’s modified Eagle medium | Gibco | Cat#12491015 |
| Mouse CH25H (Cholesterol 25-hydroxylase) ELISA KIT | Cloud-Clone CORP | Cat#SEG357Mu |
| Mouse 25 Hydroxycholesterol (25OHC) ELISA kit | MyBioSource | Cat#MBS7256104 |
| Dulbecco’s phosphate-buffered saline | Sigma-Aldrich | Cat#D5773 |
| 0.5% Red Oil O stock | Sigma-Aldrich | Cat#O1391 |
| PrimeSTAR® GXL Premix | Takara | Cat#R050A |
| NotI | New England Biolabs | Cat#R0189S |
| SalI | New England Biolabs | Cat#R0138S |
| In-Fusion | Takara | Cat#638947 |
| Lipomaster 2000 Transfection Reagent | Vazyme | Cat#TL201-01 |
| Triton X-100 | Sigma-Aldrich | Cat#T8787 |
| Atorvastatin | MedChemExpress | Cat#HY-B0589 |
| Rifampicin | MedChemExpress | Cat#HY-B0272 |
| 5-μm Filter | Pall | Cat#28144-095 |
| Ethanol absolute | Sinopharm | Cat#100092008 |
| BCA Protein Assay Kit | Beyotime Biotechnology | Cat#P0010S |
| Trizol | Invitrogen | Cat#15596018 |
| SYBR qPCR Master Mix | Vazyme Biotech, | Cat#Q712-02 |
| Experimental models: Cell lines | ||
| Raw264.7 macrophages | This study | ATCC TIB71 |
| Experimental models: Organisms/strains | ||
| Ch25h−/− mice | Jackson Laboratories | JAX: 16263 |
| Oligonucleotides | ||
| Primers are listed in Table S1 | This paper | N/A |
| Ch25h−/− mice genotyping primer | This paper | N/A |
| Ch25h_siRNA targeting Mouse Ch25h gene | This paper | N/A |
| Recombinant DNA | ||
| MSCV-IRES-Thy1.1 DEST | Addgene | Plasmid #17442 |
| Software and algorithms | ||
| GraphPad Prism 4.0 | Graphpad | https://www.graphpad.com |
| NIS-Elements Viewer | NIKON | www.microscope.healthcare.nikon.com/ |
| Other | ||
| Phosphate Buffer Saline | Solarbio Life Science | Cat#P1022 |
| Bovine Serum Albumin | Solarbio Life Science | Cat#A8010 |
| LB agar (powder) | Solarbio Life Science | Cat#L1015 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Decheng Wang (dcwang99@163.com).
Materials availability
All unique/stable reagents generated in this study are available from the lead contact with a completed Materials Transfer Agreement.
Data and code availability
-
•
Data reported in this paper will be shared by the lead contact upon request.
-
•
This study did not generate any unique datasets or code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Method details
Bacterial strains and growth conditions
Mycobacterium marinum (Mm) M strain (ATCC BAA-535) was used as the wild-type (WT) strain for this study,48 and a PDIM-deficient (ΔPDIM) strain derived from a fadD26 mutation was used as an attenuated control.48 All bacterial strains were grown at 32°C in 7H9 broth (Difco) supplemented with 10% oleic acid albumin dextrose-catalase (OADC enrichment), 0.5% glycerol, and 0.05% Tween 80 or on 7H10 agar with 10% OADC-0.5% glycerol. For fluorescence-labeled strains, 100 μL of 50 mg/mL hygromycin was added to 7H9-OADC per 100 mL.
Single-cell preparation of mm inocula
Briefly, the procedure of single-cell preparation of Mm inocula followed the established protocol.49 Firstly, the WT_Mm and ΔPDIM_Mm strains were cultured at 32°C for about 7 days until the OD600 reached 0.5–0.8 respectively, and then harvested by centrifugation for 15 min at 4000 g at room temperature. Discarded the supernatant and resuspended the pellet with 1 mL 7H9-OADC. After well mixed, the 1 mL resuspension was aliquoted to 200 μL into and transferred into five 1.5 mL microcentrifuge tubes, then each aliquoted solution was aspirated and ejected ten times by using a 27-gauge syringe. Add 1mL 7H9-OADC to each 200 μL aliquot and mix each aliquot gently. After centrifugation, the supernatant was collected and passed through a sterile 5-μm filter to obtain single-cell bacteria. The final bacterial concentration was measured by plating serial dilutions onto 7H10-OADC plate and CFU counting after 7–10 days of incubation at 32°C. 5 mL aliquots of WT_Mm and/or ΔPDIM_Mm were stored at −80°C. Before proceeding with the infection experiments, the CFU were assessed from aliquots frozen at −80°C and the exact number of viable bacteria by plate it onto 7H10-OADC for enumeration of CFU.
Raw264.7 macrophage infection with mm
The murine macrophage line Raw264.7 was seeded into six-well plates at a density of 5×105 cells/well and maintained at 37°C in 5% CO2 in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum for 24 h before infection. Then macrophages were infected with WT_Mm and ΔPDIM_Mm at an indicated multiplicity of infection (MOI) of 5 at 32°C for 3 h in 5% CO2. Cells were then gently washed three times with sterilized 1 × PBS buffer and incubated at 32°C in 5% CO2 for 1 h with fresh medium containing 1 mg/mL gentamycin to kill extracellular bacilli. Then cells were washed twice with PBS and continuously incubated at 32°C in 5% CO2 in fresh media with 20 μg/mL gentamycin. At different time points, the infected macrophage was collected and washed 3 times with PBS and the number of intracellular mycobacteria was confirmed by colony counting on 7H10-OADC plates according to an established protocol.50
Foam cell staining
Raw264.7 macrophages were infected with WT_Mm or ΔPDIM_Mm, with oxidized low-density lipoprotein (ox-LDL)-treatment as a positive control. Cells were harvested at 1, 4, 8 h post-infection (hpi) and washed twice with PBS. Remove PBS completely and fixed with 4% paraformaldehyde solution for 10 min at room temperature. Discard paraformaldehyde and add 3 mL fresh paraformaldehyde solution and then incubate for at least 1 h, or longer (cell sample can be kept in fixative for a couple of days before staining. Wrap with parafilm and cover with aluminum foil to prevent cells drying). Gently remove the paraformaldehyde solution with a pipette and wash the cells twice with ddH2O. Continuously washed the cells with 60% isopropanol at room temperature. Completely remove the isopropanol and add 3mL Oil Red O working solution (mix 60mL 0.5% oil red O stock with 40 mL ddH2O for 20 min and then filter) and incubate for 20 min. Rinsing cells immediately with ddH2O and then counterstained with Mayer’s Hematoxylin for 3 min. Wash cells three times with ddH2O and acquire the images under the microscope. For tissue oil red O staining, the mouse tail was sectioned and stained according to a published protocol.51
Western blot
Raw264.7 was seeded into six-well plates at a density of 5×105 cells/well and then infected with WT or ΔPDIM Mm as described above. The infected macrophages were harvested at indicated time-points and protein was extracted. Protein concentration was assessed by using the BCA Protein Assay Kit, according to the manufacturer’s instructions. Equal amounts of proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Nitrocellulose membranes were blocked in 5% bovine serum albumin (BSA) and subsequently probed with anti-CH25H (1:100, Santa Cruz Biotechnology, sc-293256) and anti-ADRP (1:1000, Abcam, ab108323) antibodies overnight. Indicated secondary antibodies conjugated to horseradish peroxidase were added, followed by visualization by using a ChemiQ system for imaging and analyzing gels. The results were confirmed by at least three independent experiments.
ELISA assay
Raw264.7 cells were seeded into six-well plates at a density of 5×105 cells/well and then infected with WT_Mm or ΔPDIM as described above. The cells of different groups at indicated time-points were collected and centrifuged for 15 min at 1000 g to remove cell debris and supernatants were harvested and performed different pre-processing according to exact detection objects including CH25H and 25HC. The contents of CH25H in the supernatant were measured immediately by using a commercial ELISA kit (Cloud-Clone CORP, China) according to the manufacturer’s instructions. 25HC contents were detected by using a Mouse 25 Hydroxycholesterol (25OHC) ELISA kit (MyBioSource, MBS7256104). Each sample was dispensed in triplicate, and the optical density of each well was determined at 450 nm in a microplate reader. This study was performed independently 3 times on different groups in triplicates.
Confocal microscopy
Raw264.7 macrophages were seeded on glass coverslips in six-well plates and infected with WT- or ΔPDIM-Mm. At 4 hpi, cells were washed with PBS and fixed in 4% paraformaldehyde for 30 min, followed by incubation with BODIPY 493/503 (Sigma Sku790389) for 30 min in the dark. After washing with PBS, coverslips were observed under confocal microscopy (Nikon AIR, Japan). For lipid-droplet quantitation, 40 fields of each coverslip were imaged by using confocal microscopy (1× zoom, 5–6 cells/field). A total of 200 cells were captured and BODIPY 493/503-positives in stacked confocal micrographs of each group were calculated. For ADRP examination, cells were permeabilized with 0.1% Triton X-100, washed thrice with PBS and incubated with 1:500 diluted anti-ADRP antibody (ab108323) at 4°C overnight. Cells were again washed thrice with PBS, incubated with optimally diluted secondary antibody for 1 h, washed thrice with PBS and then counterstained with DAPI for 5 min. After washing, micrographs of ADRP were acquired by confocal microscopy.
Effects of CH25H silencing and/or overexpression on foam cell formation
To further demonstrate the role of CH25H in modulating foam cell formation, three specific small interfering RNA (siRNA) (termed Ch25h_si1, Ch25_si2 and Ch25h_si3) against mouse Ch25h gene were designed, synthesized (Table S1) and their efficacy was evaluated in both mRNA and protein levels, and Ch25_si2 has the optimum efficiency for CH25H inhibition in both mRNA and protein levels. Subsequently, the Ch25_si2 was chosen for the continuous study to observe the effect of silencing CH25H on foam cell formation. On the contrary, a mouse Ch25h overexpression plasmid was constructed based on the backbone of MSCV-IRES-Thy1.1, then sequenced and finally termed as CH25H_OE. The CH25H_OE was transinfected into Raw264.7 macrophages and then infected with ΔPDIM_Mm at MOI = 5. At 8 hpi, all cells were harvested and stained with oil red O working solution as described above. Finally, 25HC—the major enzymatic product of CH25H, was dissolved with Etoh and administrated to detect the foam cell dynamics according to the published protocol.30
Raw264.7 macrophages treatment with TLR4 agonist LPS or antagonist TAK-242
To examine the induction of CH25H triggered from toll-like receptor 2 and/or 4 (TLR2/TLR4), Raw264.7 was seeded into six-well plates at a density of 5×105 cells/well and cultured. At the indicated time-points, the cells treated with TLR2 agonists lipoteichoic acid (LTA, Sigma)52 or agonist C29 (MedChem Express, HY-100461),53 or incubated with TLR4 agonist LPS (10 ng/mL) or antagonist TAK-242 (100 nM) as previously described.54
Generation of Ch25h−/− mice
Ch25h heterozygote mice (Ch25h+/−) generated on the C57BL/6 background were kindly provided by Dr. Li (Fudan University).55 Wide-type (WT) C57BL/6 mice were originally obtained from Shanghai SLAC Laboratory Animal Center and continuously bred in the Laboratory Animal Center of China Three Gorges University. Ch25h+/− mice were mated with WT C57BL/6 mice to generate more Ch25h+/− mice. WT (+/+) and homozygote (−/−) mice were obtained by intercrossing heterozygote (+/−) mice. The newborn mice were numbered and caged separately at 21 days, then a tail tip clipping of each mouse was obtained and treated with an animal genomic-DNA quick extraction kit (Beyotime Biotech) for DNA extraction. Finally, Ch25h homozygote (−/−) mice were identified and housed separately for use in subsequent experiments. All mice were housed in an SPF animal facility and provided with food and water ad libitum. The care and use of experimental animals complied with local animal welfare laws, guidelines, and policies. Ch25h homozygote (−/−) mice genotyping primer list as Table S2.
Infection of mice and harvesting of tails
Bacteria were cultured in 7H9 broth and harvested at the logarithmic growth phase (OD600 = 0.8–1.0) by centrifugation (1660×g, 10 min), and the final bacterial concentration was determined using a hemocytometer. Suspensions were diluted to 5×108 bacteria/mL. Female Ch25h+/+ mice and Ch25h−/− mice, 30 of each, aged 8–10 weeks, were housed under SPF conditions (five mice/cage) in the Laboratory Animal Center of China Three Gorges University. Mice were anesthetized by isoflurane and injected intravenously with indicated WT or ΔPDIM Mm in 100 μL of sterile PBS as previously described.10,56 At indicated time-points, five mice from each group were humanely euthanized. Tails were cut into 5 mm pieces, ground with a mortar and homogenized in 3 mL DMEM supplemented with 0.1% Triton X-. Tail suspensions were serially diluted and plated on 7H10 plates at 32°C and bacterial number was displayed as colony forming units (CFU)/g of tissue.10 For monitoring visible tail lesions, the length and width of individual visible lesions were measured at indicated time-points and the lesions areas were calculated.10 At day 10 and 20 post-infection, five mice from each group were sacrificed and tails were harvested and fixed with 4% paraformaldehyde for histopathology and immunohistochemical examination. The animal experiments were reviewed and approved by the Animal Care and Use Committee of China Three Gorges University (2019010I).
qRT-PCR
Samples from the different groups were harvested and immediately stored in a pre-cooled Trizol solution (Invitrogen, Cat#15596018). Total RNA was extracted by using a Trizol reagent according to the manufacturer’s instructions. Then RNA was reverse transcribed to cDNA using the PrimeScript RT reagent Kit with gDNA Eraser (Takara, RR047A). Subsequently, the quantitative real-time PCR was performed by using the indicated primers for target genes including Ch25h (F: 5′-CCAGCTCCTAAGTCACGTC-3’; R: 5′-CACGTCGAAGAAGGTCAG-3′), and Adrp (F: 5′-AGTATCCCTACCTGAAGTCTGTG-3’; R: 5′-CCCCTTACAGGCATAGGTATTG-3′). The Gapdh (F: 5′-AGTGTTTCCTCGTCCCGTAG-3’; R: 5′-GCCGTGAGTGGAGTCATACT-3′) (Table S3) was used as an internal control and reference gene. The qRT-PCR was performed in duplicate by using a StepOnePlus real-time PCR system (Applied Biosystems) and SYBR qPCR Master Mix (Vazyme Biotech, Cat#Q712-02). Fold changes were calculated by the 2−ΔΔCt quantification method and related to reference gene expression values.
Atorvastatin intervention
In vitro intervention
Raw264.7 cells were cultured and pretreated with atorvastatin (5 μmol/L) for 2 h, then inoculated with WT or ΔPDIM Mm. Rifampicin (5 μg/mL) treatment was used as a positive control. After 4 h infection, cells were harvested and subjected to oil red O staining, CH25H and ADRP detection.
In vivo intervention
Female C57BL/6 mice (6–8 weeks old), were purchased from the Laboratory Animal Center of China Three Gorges University and inoculated intravenously with WT_Mm in 100 μL of sterile PBS, then randomly divided into three groups: control (untreated), rifampicin-treated (10 mg/kg body. weight) and atorvastatin-treated (20 mg/kg body.weight) groups.57 At day 5 after WT_Mm-infection, rifampicin or atorvastatin were intraperitoneally injected every 2 days at the indicated dosage.
Expression of CH25H and ADRP in granulomatous lesions from Mtb-infected animals and clinical tuberculosis patients
Expression of CH25H and ADRP were detected in Mtb-infected mice, rabbits, marmosets, and clinical tuberculosis patients. The paraffin-embedded lung tissues of Mtb aerosol-infected mice, rabbits, and marmosets (under protocols LCIM-3, LCIM-4, and LCIM9) were obtained from the Tuberculosis Research Section of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD.58,59 Paraffin-embedded clinical tuberculosis patient samples were provided by the Third People’s Hospital of Yichang and approved by the Ethics Committee of the Third People’s Hospital of Yichang. Five-micrometer serial sections were made from all tissues and stained with Ziehl-Neelsen (Z.N), anti-CH25H and anti-ADRP antibodies, and images were captured by using the Cellsens imaging system (Olympus Life Sciences).
Transmission electron microscopy (TEM)
Tail tissue from WT_Mm-infected mice was collected and altered to small enough (about 3 mm in diameter) and immediately fixed with 2.5% glutaraldehyde solution for 3 h at 4°C. After rinsed with 0.1M phosphate buffer and post-fixed in 1% osmium tetroxide for 1 h. Subsequently, the tail tissues were dehydrated, embedded in epoxy resin, sectioned at 70 nm, and then performed staining with 2% uranyl acetate and 1% lead citrate. Finally, the ultrastructure of tail tissues especially the Mm-containing phagosomes and lipid bodies was inspected by using a Hitachi H-7500 transmission electron microscope (Hitachi Ltd., Tokyo, Japan).
Quantification and statistical analysis
All data are represented as mean ± standard deviation (SD) as indicated in the corresponding figure legends. Statistical analysis was performed with GraphPad Prism 4.0 (GraphPad Software, Inc., CA). All data are presented as the mean ± SD of at least 3 replicate analyses. For the comparison of two independent datasets, the Student’s t-tests were applied and p < 0.05 was considered statistically significant. For more than two samples, statistical significance was determined by one-way ANOVA or two-way ANOVA followed test with a statistical threshold of p < 0.05.
Acknowledgments
Decheng Wang is a special volunteer trainee of the Tuberculosis Research Section, LCIM, NIAID of National Institutes of Health. Material Analysis and Testing Center of China Three Gorges University provided the Confocal Microscope System. We thank Gillian Campbell, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript. This work was supported in part by the National Natural Science Foundation of China (grant nos. 31772709 and 31572485 to D.W.; 82272353to L.Z.), and the Open Foundation of Hubei Province Key Laboratory of Tumor Microenvironment and Immunotherapy (no. 2023KZL017), the new faculty startup research fund of China Three Gorges University (KJ2014B023 to D.W.), and in part by the intramural research program of NIAID, NIH (Bethesda MD, USA). We would like to dedicate this paper to the memory of our beloved Dr. Weifeng Huang, who passed away during the preparation and writing of this manuscript.
Author contributions
S.Z., L.Z., and D.C.W. designed the project; S.Z., D.Z., D.L., H.K.W., C.R.D., J.R.S., W.F.H., X.X., Z.W.Z., S.S.H., Z.J., B.Y., J.G., L.E.V., L.Z., and D.C.W. performed the experiments; L.Z. and D.C.W. analyzed the data and wrote the draft; L.E.V. did the critical reading for the draft; L.E.V. and D.C.W. revised the manuscript; D.C.W. re-submitted the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: February 12, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.109204.
Contributor Information
Lu Zhang, Email: zhanglu407@fudan.edu.cn.
Decheng Wang, Email: dcwang99@163.com.
Supplemental information
References
- 1.Russell D.G., Barry C.E., 3rd, Flynn J.L. Tuberculosis: what we don't know can, and does, hurt us. Science (New York, N.Y.) 2010;328:852–856. doi: 10.1126/science.1184784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awuh J.A., Flo T.H. Molecular basis of mycobacterial survival in macrophages. Cell. Mol. Life Sci. 2017;74:1625–1648. doi: 10.1007/s00018-016-2422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan P.J., Nikaido H. The envelope of mycobacteria. Annu. Rev. Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 4.Cox J.S., Chen B., McNeil M., Jacobs W.R., Jr. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature. 1999;402:79–83. doi: 10.1038/47042. [DOI] [PubMed] [Google Scholar]
- 5.Brennan P.J. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis. 2003;83:91–97. doi: 10.1016/s1472-9792(02)00089-6. [DOI] [PubMed] [Google Scholar]
- 6.Tobin D.M., Ramakrishnan L. Comparative pathogenesis of Mycobacterium marinum and Mycobacterium tuberculosis. Cell Microbiol. 2008;10:1027–1039. doi: 10.1111/j.1462-5822.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- 7.Davis J.M., Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oehlers S.H., Cronan M.R., Scott N.R., Thomas M.I., Okuda K.S., Walton E.M., Beerman R.W., Crosier P.S., Tobin D.M. Interception of host angiogenic signalling limits mycobacterial growth. Nature. 2015;517:612–615. doi: 10.1038/nature13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volkman H.E., Pozos T.C., Zheng J., Davis J.M., Rawls J.F., Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science (New York, N.Y.) 2010;327:466–469. doi: 10.1126/science.1179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlsson F., Kim J., Dumitru C., Barck K.H., Carano R.A.D., Sun M., Diehl L., Brown E.J. Host-detrimental role of Esx-1-mediated inflammasome activation in mycobacterial infection. PLoS Pathog. 2010;6:e1000895. doi: 10.1371/journal.ppat.1000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehlers S., Schaible U.E. The granuloma in tuberculosis: dynamics of a host-pathogen collusion. Front. Immunol. 2012;3:411. doi: 10.3389/fimmu.2012.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 2012;12:352–366. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 13.Rubin E.J. The granuloma in tuberculosis--friend or foe? N. Engl. J. Med. 2009;360:2471–2473. doi: 10.1056/NEJMcibr0902539. [DOI] [PubMed] [Google Scholar]
- 14.Russell D.G., Cardona P.J., Kim M.J., Allain S., Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat. Immunol. 2009;10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundberg B. Chemical composition and physical state of lipid deposits in atherosclerosis. Atherosclerosis. 1985;56:93–110. doi: 10.1016/0021-9150(85)90087-5. [DOI] [PubMed] [Google Scholar]
- 16.Gold E.S., Ramsey S.A., Sartain M.J., Selinummi J., Podolsky I., Rodriguez D.J., Moritz R.L., Aderem A. ATF3 protects against atherosclerosis by suppressing 25-hydroxycholesterol-induced lipid body formation. J. Exp. Med. 2012;209:807–817. doi: 10.1084/jem.20111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almeida P.E., Carneiro A.B., Silva A.R., Bozza P.T. PPARgamma Expression and Function in Mycobacterial Infection: Roles in Lipid Metabolism, Immunity, and Bacterial Killing. PPAR Res. 2012;2012:383829. doi: 10.1155/2012/383829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salamon H., Bruiners N., Lakehal K., Shi L., Ravi J., Yamaguchi K.D., Pine R., Gennaro M.L. Cutting edge: Vitamin D regulates lipid metabolism in Mycobacterium tuberculosis infection. J. Immunol. 2014;193:30–34. doi: 10.4049/jimmunol.1400736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genoula M., Marín Franco J.L., Dupont M., Kviatcovsky D., Milillo A., Schierloh P., Moraña E.J., Poggi S., Palmero D., Mata-Espinosa D., et al. Formation of Foamy Macrophages by Tuberculous Pleural Effusions Is Triggered by the Interleukin-10/Signal Transducer and Activator of Transcription 3 Axis through ACAT Upregulation. Front. Immunol. 2018;9:459. doi: 10.3389/fimmu.2018.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shim D., Kim H., Shin S.J. Mycobacterium tuberculosis Infection-Driven Foamy Macrophages and Their Implications in Tuberculosis Control as Targets for Host-Directed Therapy. Front. Immunol. 2020;11:910. doi: 10.3389/fimmu.2020.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peyron P., Vaubourgeix J., Poquet Y., Levillain F., Botanch C., Bardou F., Daffé M., Emile J.F., Marchou B., Cardona P.J., et al. Foamy macrophages from tuberculous patients' granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 2008;4:e1000204. doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahajan S., Chandra V., Dave S., Nanduri R., Gupta P. Stem bromelain-induced macrophage apoptosis and activation curtail Mycobacterium tuberculosis persistence. J. Infect. Dis. 2012;206:366–376. doi: 10.1093/infdis/jis354. [DOI] [PubMed] [Google Scholar]
- 23.Johansen M.D., Kasparian J.A., Hortle E., Britton W.J., Purdie A.C., Oehlers S.H. Mycobacterium marinum infection drives foam cell differentiation in zebrafish infection models. Dev. Comp. Immunol. 2018;88:169–172. doi: 10.1016/j.dci.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Parihar S.P., Guler R., Khutlang R., Lang D.M., Hurdayal R., Mhlanga M.M., Suzuki H., Marais A.D., Brombacher F. Statin therapy reduces the mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J. Infect. Dis. 2014;209:754–763. doi: 10.1093/infdis/jit550. [DOI] [PubMed] [Google Scholar]
- 25.Dutta N.K., Bruiners N., Pinn M.L., Zimmerman M.D., Prideaux B., Dartois V., Gennaro M.L., Karakousis P.C. Statin adjunctive therapy shortens the duration of TB treatment in mice. J. Antimicrob. Chemother. 2016;71:1570–1577. doi: 10.1093/jac/dkw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutta N.K., Bruiners N., Zimmerman M.D., Tan S., Dartois V., Gennaro M.L., Karakousis P.C. Adjunctive Host-Directed Therapy With Statins Improves Tuberculosis-Related Outcomes in Mice. J. Infect. Dis. 2020;221:1079–1087. doi: 10.1093/infdis/jiz517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 28.Liu S.Y., Aliyari R., Chikere K., Li G., Marsden M.D., Smith J.K., Pernet O., Guo H., Nusbaum R., Zack J.A., et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity. 2013;38:92–105. doi: 10.1016/j.immuni.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ngo M.D., Bartlett S., Bielefeldt-Ohmann H., Foo C.X., Sinha R., Arachchige B.J., Reed S., Mandrup-Poulsen T., Rosenkilde M.M., Ronacher K. A Blunted GPR183/Oxysterol Axis During Dysglycemia Results in Delayed Recruitment of Macrophages to the Lung During Mycobacterium tuberculosis Infection. J. Infect. Dis. 2022;225:2219–2228. doi: 10.1093/infdis/jiac102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C., Deng Y.Q., Wang S., Ma F., Aliyari R., Huang X.Y., Zhang N.N., Watanabe M., Dong H.L., Liu P., et al. 25-Hydroxycholesterol Protects Host against Zika Virus Infection and Its Associated Microcephaly in a Mouse Model. Immunity. 2017;46:446–456. doi: 10.1016/j.immuni.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reboldi A., Dang E.V., McDonald J.G., Liang G., Russell D.W., Cyster J.G. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science (New York, N.Y.) 2014;345:679–684. doi: 10.1126/science.1254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold E.S., Diercks A.H., Podolsky I., Podyminogin R.L., Askovich P.S., Treuting P.M., Aderem A. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc. Natl. Acad. Sci. USA. 2014;111:10666–10671. doi: 10.1073/pnas.1404271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z., Martin M., Zhang J., Huang H.Y., Bai L., Zhang J., Kang J., He M., Li J., Maurya M.R., et al. Krüppel-Like Factor 4 Regulation of Cholesterol-25-Hydroxylase and Liver X Receptor Mitigates Atherosclerosis Susceptibility. Circulation. 2017;136:1315–1330. doi: 10.1161/circulationaha.117.027462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cambier C.J., Takaki K.K., Larson R.P., Hernandez R.E., Tobin D.M., Urdahl K.B., Cosma C.L., Ramakrishnan L. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505:218–222. doi: 10.1038/nature12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q., Boshoff H.I.M., Harrison J.R., Ray P.C., Green S.R., Wyatt P.G., Barry C.E., 3rd PE/PPE proteins mediate nutrient transport across the outer membrane of Mycobacterium tuberculosis. Science (New York, N.Y.) 2020;367:1147–1151. doi: 10.1126/science.aav5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Astarie-Dequeker C., Le Guyader L., Malaga W., Seaphanh F.K., Chalut C., Lopez A., Guilhot C. Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids. PLoS Pathog. 2009;5:e1000289. doi: 10.1371/journal.ppat.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauman D.R., Bitmansour A.D., McDonald J.G., Thompson B.M., Liang G., Russell D.W. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc. Natl. Acad. Sci. USA. 2009;106:16764–16769. doi: 10.1073/pnas.0909142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diczfalusy U., Olofsson K.E., Carlsson A.M., Gong M., Golenbock D.T., Rooyackers O., Fläring U., Björkbacka H. Marked upregulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J. Lipid Res. 2009;50:2258–2264. doi: 10.1194/jlr.M900107-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lovewell R.R., Sassetti C.M., VanderVen B.C. Chewing the fat: lipid metabolism and homeostasis during M. tuberculosis infection. Curr. Opin. Microbiol. 2016;29:30–36. doi: 10.1016/j.mib.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Mahajan S., Dkhar H.K., Chandra V., Dave S., Nanduri R., Janmeja A.K., Agrewala J.N., Gupta P. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARγ and TR4 for survival. J. Immunol. 2012;188:5593–5603. doi: 10.4049/jimmunol.1103038. [DOI] [PubMed] [Google Scholar]
- 41.Guerrini V., Prideaux B., Blanc L., Bruiners N., Arrigucci R., Singh S., Ho-Liang H.P., Salamon H., Chen P.Y., Lakehal K., et al. Storage lipid studies in tuberculosis reveal that foam cell biogenesis is disease-specific. PLoS Pathog. 2018;14:e1007223. doi: 10.1371/journal.ppat.1007223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agarwal P., Combes T.W., Shojaee-Moradie F., Fielding B., Gordon S., Mizrahi V., Martinez F.O. Foam Cells Control Mycobacterium tuberculosis Infection. Front. Microbiol. 2020;11:1394. doi: 10.3389/fmicb.2020.01394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohrer A.C., Castro E., Tocheny C.E., Assmann M., Schwarz B., Bohrnsen E., Makiya M.A., Legrand F., Hilligan K.L., Baker P.J., et al. Rapid GPR183-mediated recruitment of eosinophils to the lung after Mycobacterium tuberculosis infection. Cell Rep. 2022;40:111144. doi: 10.1016/j.celrep.2022.111144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lobato L.S., Rosa P.S., Ferreira J.D.S., Neumann A.D.S., da Silva M.G., do Nascimento D.C., Soares C.T., Pedrini S.C.B., Oliveira D.S.L.D., Monteiro C.P., et al. Statins increase rifampin mycobactericidal effect. Antimicrob. Agents Chemother. 2014;58:5766–5774. doi: 10.1128/AAC.01826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerra-De-Blas P.D.C., Bobadilla-Del-Valle M., Sada-Ovalle I., Estrada-García I., Torres-González P., López-Saavedra A., Guzmán-Beltrán S., Ponce-de-León A., Sifuentes-Osornio J. Simvastatin Enhances the Immune Response Against Mycobacterium tuberculosis. Front. Microbiol. 2019;10:2097. doi: 10.3389/fmicb.2019.02097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cambier C.J., Banik S.M., Buonomo J.A., Bertozzi C.R. Spreading of a mycobacterial cell-surface lipid into host epithelial membranes promotes infectivity. Elife. 2020;9:e60648. doi: 10.7554/eLife.60648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cronan M.R., Hughes E.J., Brewer W.J., Viswanathan G., Hunt E.G., Singh B., Mehra S., Oehlers S.H., Gregory S.G., Kaushal D., Tobin D.M. A non-canonical type 2 immune response coordinates tuberculous granuloma formation and epithelialization. Cell. 2021;184:1757–1774.e14. doi: 10.1016/j.cell.2021.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu J., Tran V., Li M., Huang X., Niu C., Wang D., Zhu J., Wang J., Gao Q., Liu J. Both phthiocerol dimycocerosates and phenolic glycolipids are required for virulence of Mycobacterium marinum. Infect. Immun. 2012;80:1381–1389. doi: 10.1128/IAI.06370-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takaki K., Davis J.M., Winglee K., Ramakrishnan L. Evaluation of the pathogenesis and treatment of Mycobacterium marinum infection in zebrafish. Nat. Protoc. 2013;8:1114–1124. doi: 10.1038/nprot.2013.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Tang Y., Lin C., Zhang J., Mai J., Jiang J., Gao X., Li Y., Zhao G., Zhang L., Liu J. Crosstalk between the ancestral type VII secretion system ESX-4 and other T7SS in Mycobacterium marinum. iScience. 2022;25:103585. doi: 10.1016/j.isci.2021.103585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehlem A., Hagberg C.E., Muhl L., Eriksson U., Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 2013;8:1149–1154. doi: 10.1038/nprot.2013.055. [DOI] [PubMed] [Google Scholar]
- 52.Dai L., DeFee M.R., Cao Y., Wen J., Wen X., Noverr M.C., Qin Z. Lipoteichoic acid (LTA) and lipopolysaccharides (LPS) from periodontal pathogenic bacteria facilitate oncogenic herpesvirus infection within primary oral cells. PLoS One. 2014;9:e101326. doi: 10.1371/journal.pone.0101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang R., Tang L., Tian Y., Ji X., Hu Q., Zhou B., Zhenyu D., Heng X., Yang L. Cholesterol-modified DP7 enhances the effect of individualized cancer immunotherapy based on neoantigens. Biomaterials. 2020;241:119852. doi: 10.1016/j.biomaterials.2020.119852. [DOI] [PubMed] [Google Scholar]
- 54.Xiao L., Luo G., Guo X., Jiang C., Zeng H., Zhou F., Li Y., Yu J., Yao P. Macrophage iron retention aggravates atherosclerosis: Evidence for the role of autocrine formation of hepcidin in plaque macrophages. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids. 2020;1865:158531. doi: 10.1016/j.bbalip.2019.158531. [DOI] [PubMed] [Google Scholar]
- 55.Li J., Lu E., Yi T., Cyster J.G. EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature. 2016;533:110–114. doi: 10.1038/nature17947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song J., Chao J., Hu X., Wen X., Ding C., Li D., Zhang D., Han S., Yu X., Yan B., et al. E3 Ligase FBXW7 Facilitates Mycobacterium Immune Evasion by Modulating TNF-α Expression. Front. Cell. Infect. Microbiol. 2022;12:851197. doi: 10.3389/fcimb.2022.851197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parihar S.P., Guler R., Khutlang R., Lang D.M., Hurdayal R., Mhlanga M.M., Suzuki H., Marais A.D., Brombacher F. Statin therapy reduces the mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J. Infect. Dis. 2014;209:754–763. doi: 10.1093/infdis/jit550. [DOI] [PubMed] [Google Scholar]
- 58.Via L.E., Schimel D., Weiner D.M., Dartois V., Dayao E., Cai Y., Yoon Y.S., Dreher M.R., Kastenmayer R.J., Laymon C.M., et al. Infection dynamics and response to chemotherapy in a rabbit model of tuberculosis using [1⁸F]2-fluoro-deoxy-D-glucose positron emission tomography and computed tomography. Antimicrob. Agents Chemother. 2012;56:4391–4402. doi: 10.1128/aac.00531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Via L.E., England K., Weiner D.M., Schimel D., Zimmerman M.D., Dayao E., Chen R.Y., Dodd L.E., Richardson M., Robbins K.K., et al. A sterilizing tuberculosis treatment regimen is associated with faster clearance of bacteria in cavitary lesions in marmosets. Antimicrob. Agents Chemother. 2015;59:4181–4189. doi: 10.1128/aac.00115-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data reported in this paper will be shared by the lead contact upon request.
-
•
This study did not generate any unique datasets or code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.