Abstract
BACKGROUND
Acute embolic occlusion of the common carotid artery (CCA) alone is rare. However, once it occurs, recanalization is challenging due to the large volume of the clot, larger diameter of the CCA, and risk of procedure-related distal embolism into the intracranial arteries.
OBSERVATIONS
The authors report two cases of acute embolic occlusion of CCA alone, caused by a cardiac embolus trapped at the proximal end of a preexisting atherosclerotic plaque at the cervical carotid bifurcation. In both cases, the CCA was successfully recanalized using retrograde thrombectomy in a hybrid operating room. In case 1, a 78-year-old male with acute right CCA occlusion underwent retrograde thrombectomy, where the cervical carotid bifurcation was exposed and incised, and the entire embolus was retrieved with forceps. Despite successful revascularization, massive bleeding from the CCA just after the retrieval remained a concern. In case 2, a 79-year-old female with acute right CCA occlusion underwent retrograde thrombectomy in the same manner. Because manual retrieval failed, a Fogarty balloon catheter inserted from the arteriotomy successfully retrieved the entire thrombus with minimal blood loss.
LESSONS
Retrograde thrombectomy through the arteriotomy of the cervical carotid bifurcation safely and effectively recanalizes acute embolic occlusion of the CCA alone.
Keywords: common carotid artery occlusion, retrograde thrombectomy, hybrid operating room, cardiac embolism, carotid endarterectomy
ABBREVIATIONS: CCA = common carotid artery, CT = computed tomography, ECA = external carotid artery, ICA = internal carotid artery, MRI = magnetic resonance imaging, NIHSS = National Institutes of Health Stroke Scale, SPECT = single photon emission computed tomography
Acute embolic occlusion of the common carotid artery (CCA) alone is rarely observed because most emboli pass through the CCA and reach the carotid bifurcation or intracranial internal carotid artery (ICA).1–3 However, once it occurs, normal anterograde endovascular thrombectomy is not necessarily safe or effective, depending on the volume and stability of the emboli, hemodynamic status of the cerebral hemisphere, and remaining collateral pathways. For instance, catheter aspiration is less effective for massive emboli occupying long distances, and stent retrieval may cause distal migration of the emboli when crossing the lesion, which deteriorates cerebral perfusion by blocking residual collateral flow.4,5
In this article, we report two cases of acute embolic occlusion of CCA alone. The patients presented with moderate neurological deficit associated with ipsilateral hemodynamic compromise. A mobile thrombus was found stuck at the atherosclerotic irregular wall of the CCA, and collateral flow from the external carotid artery (ECA) to ICA was patent. In a hybrid operating room, we exposed and opened the cervical carotid bifurcation and retrieved the emboli from the CCA in a retrograde fashion. We discuss indication, strategy, procedural techniques, and appropriate devices to obtain favorable outcomes of acute embolic occlusion of CCA alone.
Illustrative Cases
Case 1
A 79-year-old male was awaiting coronary artery bypass grafting during hospitalization. He had atrial fibrillation but the anticoagulant was discontinued for open chest surgery. He presented with sudden left-sided weakness and dysarthria and was referred to the stroke department. He was alert and oriented, and his National Institutes of Health Stroke Scale (NIHSS) score was 4. Head magnetic resonance imaging (MRI) showed watershed acute ischemic stroke in his right cerebral hemisphere on diffusion-weighted imaging (Fig. 1A), and decreased signal intensity of the right ICA to the middle cerebral artery on time-of-flight magnetic resonance angiography (Fig. 1B). Cervical computed tomography (CT) angiography showed a defect of contrast media from the origin of the right CCA to the carotid bifurcation, with patent collateral flow from ECA to ICA (Fig. 1C and D). Approximately 20% hemodynamic compromise of the right cerebral hemisphere was observed by single photon emission computed tomography (SPECT) (123I-IMP SPECT, ARG method) (Fig. 1E). Carotid ultrasonography revealed that a hypoechoic thrombus, which was mobile according to the pulse, was stuck at the atherosclerotic static plaque in the CCA (Video 1, Fig. 1F). Carotid ultrasonography screening performed 17 days previously already showed moderate atherosclerotic stenosis at the right carotid bifurcation. Based on these findings, we diagnosed acute CCA occlusion caused by a fragile cardiac embolus trapped by an atherosclerotic plaque. To prevent neurological worsening due to prolonged hemodynamic compromise and further distal migration of the embolus, we planned revascularization of the occluded CCA. Because normal anterograde catheter thrombectomy through the femoral or brachial artery puncture was considered less effective or riskier, we performed retrograde thrombectomy through the surgically exposed cervical carotid bifurcation.
FIG. 1.
Case 1. Images obtained in a 79-year-old male who presented with left motor weakness and dysarthria during hospitalization. A: Diffusion-weighted MRI shows multiple acute ischemic lesions in the right corona radiata. B: Reconstructed time-of-flight magnetic resonance angiography shows decreased signal intensity of the right ICA to the middle cerebral artery (arrow). C: Three-dimensional reconstruction of the cervical CT angiogram shows a defect of contrast media from the origin of the right CCA to the carotid bifurcation (arrowheads), with patent collateral flow from ECA to ICA (arrow). D: Maximum intensity projection of the cervical CT shows a thrombotic occlusion (arrowheads) and a stump (arrow) of the right CCA. E: SPECT (123I-IMP SPECT, ARG method) shows approximately 20% hemodynamic compromise of the right cerebral hemisphere. F: Carotid ultrasonography reveals a hypoechoic thrombus (arrow) stuck at the atherosclerotic plaque (dotted circles) in the CCA.
VIDEO 1. Video clip showing the preoperative right carotid ultrasonography of case 1. Left and right indicate ICA and CCA, respectively. A hypoechoic mobile thrombus stuck at the static atherosclerotic plaque is observed. Click here to view
Two days later, surgery was performed under general anesthesia in a hybrid operating room (Infinix-Celeve, Canon). A 5-French sheath introducer was inserted in the right femoral artery for intraoperative angiography. After the carotid bifurcation was exposed, the ICA and the ECA were clamped. The CCA was not clamped to prevent fragmentation of the embolus. Arterial incision revealed a fragile thrombus stuck in the atherosclerotic plaque (Fig. 2A). We caught and pulled the thrombus with forceps, resulting in removal of the entire thrombus and recanalization of CCA, with massive blood loss from the arteriotomy for several seconds until the CCA was clamped (Fig. 2B and C). Retrieval of the entire embolus was confirmed by intravascular ultrasound (Fig. 2D). Then, carotid endarterectomy to remove the atherosclerotic plaque was performed in the usual manner using an internal carotid shunt. Recanalization of the CCA and the ICA was confirmed by intraoperative angiography (Fig. 2E and F). The estimated blood loss was 250 ml. Schematic illustrations of thrombectomy in this case are shown in (Fig. 2G).
FIG. 2.
Case 1. Intraoperative findings of retrograde thrombectomy. A: Intraoperative photograph shows a fragile red thrombus (arrow) is trapped by a whitish atherosclerotic plaque (arrowheads). B: A large clot (arrow) is retrieved by manual traction with forceps. C: Immediately after the clot is removed, massive bleeding (arrow) occurs from the arteriotomy by recanalization of the CCA. D: Intravascular ultrasound reveals no residual clot in the CCA. E and F: Intraoperative angiograms show recanalization of the ICA and CCA. G: Illustration of retrograde thrombectomy. The fragile mobile embolus and atherosclerotic plaque are depicted in purple and yellow, respectively. Massive bleeding from the arteriotomy occurs for several seconds between clot removal and CCA clamping.
The postoperative course of the patient was uneventful without neurological deterioration. The coronary artery bypass grafting surgery was postponed, and he was discharged to a rehabilitation facility with mild left motor weakness on postoperative day 35.
Case 2
A 78-year-old female underwent a mastectomy for right-sided breast cancer. She had paroxysmal atrial fibrillation but the anticoagulant was discontinued during the perioperative period. Five days later, she presented with acute in-hospital ischemic stroke with left motor weakness and left hemispatial neglect, and was referred to the stroke department. She was alert and oriented, and her NIHSS score was 6. Head MRI showed watershed acute ischemic stroke in her right cerebral hemisphere (Fig. 3A), and CT angiography showed a defect of contrast media from the origin of the right CCA to the carotid bifurcation, with an opening ECA-to-ICA collateral pathway (Fig. 3B and C). Approximately 10% hemodynamic compromise of the right cerebral hemisphere was observed by SPECT (123I-IMP SPECT, ARG method) (Fig. 3D). Carotid ultrasonography revealed calcified atherosclerotic plaque and occlusion of the CCA by massive mobile thrombus (Fig. 3E). We planned retrograde thrombectomy as safe and effective recanalization of the occluded CCA.
FIG. 3.
Case 2. Images obtained in a 78-year-old female who presented with left motor weakness and spatial neglect during hospitalization. A: Diffusion-weighted MRI shows multiple acute ischemic lesions in the right parietal cortex and watershed subcortical regions. B: Three-dimensional reconstruction of the cervical CT angiogram shows a defect of contrast media from the origin of the right CCA to the carotid bifurcation (arrowheads), with patent collateral flow from ECA to ICA (arrow). C: Maximum intensity projection of the cervical CT shows thrombotic occlusion (arrowheads) of the right CCA. Calcification of the carotid bifurcation (arrow) suggests preexisting atherosclerotic change. D: SPECT (123I-IMP SPECT, ARG method) shows approximately 10% hemodynamic compromise of the right cerebral hemisphere. E: Carotid ultrasonography reveals a hypoechoic thrombus (arrow) stuck at the atherosclerotic plaque (dotted circles) in the CCA.
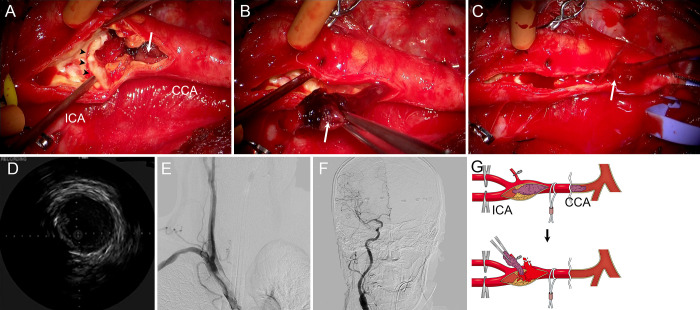
Five days later, surgery was performed under general anesthesia in a hybrid operating room. A 5-French sheath introducer was inserted in the right femoral artery for intraoperative angiography. After the carotid bifurcation was exposed, the ICA and the ECA were clamped. Arterial incision revealed a fragile thrombus stuck in the atherosclerotic plaque. We caught and pulled the thrombus with forceps, but only removed small pieces and most of the thrombus remained in the CCA (Fig. 4A). Subsequently, we inserted a 4-French Fogarty catheter (Edwards Lifesciences) with the aid of a 0.035-inch guidewire from the arteriotomy. The tip of the Fogarty catheter was advanced to the origin of the CCA across the thrombus. Then, the balloon was inflated, and the catheter was pulled distally under fluoroscopy (Fig. 4B and C). Just before the balloon came out of the arteriotomy, the CCA was clamped by squeezing a tourniquet. An approximately 35-mm-long thrombus retrieved by the Fogarty catheter is shown in Fig. 4D. Removal of the entire thrombus and recanalization of the CCA was also confirmed by intraoperative angiography (Fig. 4E and F) and intravascular ultrasound. Carotid endarterectomy to remove the atherosclerotic plaque was performed in the usual manner using an internal carotid shunt. The estimated blood loss was 80 mL. Schematic illustrations of thrombectomy in this case are shown in (Fig. 4G).
FIG. 4.
Case 2. Intraoperative findings of retrograde thrombectomy. A: Intraoperative photograph shows a small fragment of the red thrombus (arrow) is removed by forceps, failing to recanalize the occluded CCA. B: Intraoperative roadmap fluoroscopy shows that the balloon of the Fogarty catheter (arrow) is inflated at the origin of the right CCA. C: The inflated Fogarty balloon catheter is retrieved distally (arrow) to reach the site of arteriotomy. D: Photograph of the large (35 mm) clot retrieved by the Fogarty catheter. E and F: Intraoperative angiograms show recanalization of the ICA and CCA. G: Illustration of retrograde thrombectomy. The fragile mobile embolus and the atherosclerotic plaque are depicted in purple and yellow, respectively. The CCA is clamped just before the balloon comes out of the arteriotomy, preventing blood loss from the recanalized CCA.
The postoperative course of the patient was uneventful without neurological deterioration. She was discharged to a rehabilitation facility with mild left motor weakness on postoperative day 16.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
Acute embolic occlusion of the CCA alone is rare. Because of the large diameter of the CCA, and the even larger diameter of the cervical carotid bifurcation, cardiac emboli generally pass through the carotid bifurcation to reach ICA or its peripheral intracranial branches with smaller diameters. Thus, acute embolic occlusion of the CCA alone occurs only when the embolus is large enough to occlude the CCA of large diameter and the CCA has a specific pathological or anatomical condition to retain the embolus, such as atherosclerotic stenosis, arthritis, or severe kinking.1–3 In addition, normal anterograde mechanical thrombectomy, the validity of which has been well established for acute ICA occlusion to date, is more challenging for acute CCA occlusion for several reasons. First, even if successfully captured in the aspiration catheter, the large thrombus may occlude the guiding catheter or may be fragmented and cause embolism in the arteries of other organs during retrieval.1,3 Piece-by-piece aspiration of a large thrombus may be effective, but recanalization takes more time. Second, in the case of CCA occlusion, ipsilateral cerebral blood flow is partially compensated by collateral pathways through the circle of Willis and the backflow of the ECA at the cervical carotid bifurcation.6 Crossing the lesion to use a stent retriever without distal ICA protection may cause distal embolism or plaque shift to occlude the potential collateral pathways, resulting in symptom deterioration.7 Also, the maximum diameter of the commercially available stent retriever is 6 mm, which is not large enough to retrieve a clot from the CCA with an 8- to 10-mm diameter.1,8 Thus, the revascularization strategy for acute CCA occlusion must be individualized according to the size of the emboli, collateral blood flow, and neurological condition of the patient.
In this article, we successfully retrieved the entire thrombus in the CCA without causing distal embolism of the intracranial vessels. Surgical exposure of the carotid bifurcation allowed early and secure clamping of the ICA, preventing intraprocedural distal embolism. Arteriotomy from ICA to CCA was large enough to retrieve a large thrombus and enabled endarterectomy of the concomitant atherosclerotic plaque. Because a thrombus in the CCA sometimes extends proximally, methodology and confirmation of complete retrieval without residual thrombi is another concern. In case 1, manual traction of the visible part by forceps fortunately removed the entire embolus. However, the patient experienced massive blood loss because we could not predict how much embolus would be retrieved by manual traction. To prevent fragmentation of the embolus, we could not clamp the CCA in advance (Fig. 2G). In case 2, because manual traction failed, we recognized the validity of a Fogarty catheter in retrograde thrombectomy. A Fogarty catheter retrieves the thrombus by pulling back the inflated balloon placed beyond the thrombus. Because physiological blood flow was against the Fogarty catheter and ICA was clamped, a distal embolism during lesion crossing and thrombus retrieval was less likely to occur. Fogarty catheters have been used in the same manner, retrieving proximal thrombi by a distally introduced catheter, in thrombectomy of subclavian and femoral arteries, for example.9 Under fluoroscopy, the balloon of the Fogarty catheter was placed just proximal to the thrombus (contrast defect), and thus we were sure that we captured the entire thrombus. Just before the balloon was pulled out of the arteriotomy, the CCA was clamped to prevent blood loss from the arteriotomy (Fig. 4G). Intraoperative angiography and intravascular ultrasound were helpful to confirm complete retrieval of the thrombus.
Indication of retrograde thrombectomy for acute CCA occlusion must be determined carefully by multimodal examinations. If the patient lacks a mobile plaque, medical treatment at the acute stage followed by bypass reconstruction may be another option.6,10 In addition, the use of a Fogarty catheter in the CCA risks vessel dissection, although such a risk is considered equivalent to that of subclavian and femoral arteries. However, to the best of our knowledge, this is the first description of the validity and detailed technical aspects of retrograde thrombectomy for acute occlusion of the CCA alone, from the perspective of preventing intraoperative distal embolism, complete retrieval of a large thrombus, and simultaneous endarterectomy of the concomitant atherosclerotic plaque.
Lessons
These cases suggest that acute CCA occlusion, which was generated by a cardiac embolus trapped by a preexisting atherosclerotic plaque, could be successfully recanalized by retrograde thrombectomy accessed from arteriotomy of the cervical carotid bifurcation. We hope that the one-stage multimodality as in the present cases will be also effectively applied to other complex disorders of the craniocervical vessels.11,12
Author Contributions
Conception and design: Fukuda, Okune, Matsuoka, Ueba. Acquisition of data: Okune, Matsuoka, Hayashi, Ueba. Analysis and interpretation of data: Fukuda, Okune, Fukui. Drafting the article: Fukuda, Okune, Ueba. Critically revising the article: Fukuda, Fukui. Reviewed submitted version of manuscript: Fukuda, Nishimoto, Fukui. Approved the final version of the manuscript on behalf of all authors: Fukuda. Administrative/technical/material support: Matsuoka, Hayashi. Study supervision: Fukui, Ueba.
Supplemental Information
Videos
Video 1. https://vimeo.com/899241760.
References
- 1. Ideguchi M, Kim K, Suzuki M, et al. Mechanical thrombectomy for acute common carotid artery occlusion. Neurol Med Chir (Tokyo) 2023;63(2):73–79. doi: 10.2176/jns-nmc.2022-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hino T, Sato M, Hayakawa M, et al. A case of acute embolic occlusion of the common carotid artery in which a giant thrombus was retrieved using the parallel stent retriever technique. J Neuroendovasc Ther. 2022;16(2):87–92. doi: 10.5797/jnet.cr.2020-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rigatelli G, Martire G, Gentile M, Michielan F, Amistà P. Combined use of export catheter and penumbra vacuum thromboaspiration in a challenging case of acute common carotid artery occlusion. Cardiovasc Revasc Med. 2016;17(7):468–469. doi: 10.1016/j.carrev.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 4. Riegler C, Siebert E, Kleine JF, Nolte CH. Thrombus migration in ischemic stroke due to large vessel occlusion: a question of time. J Neurointerv Surg. 2023;15(e2):e216–e222. doi: 10.1136/jnis-2022-019365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belkin M, Mackey WC, Pessin MS, Caplan LR, O’Donnell TF. Common carotid artery occlusion with patent internal and external carotid arteries: diagnosis and surgical management. J Vasc Surg. 1993;17(6):1019–1028. [PubMed] [Google Scholar]
- 6. Riles TS, Imparato AM, Posner MP, Eikelboom BC. Common carotid occlusion. Assessment of the distal vessels. Ann Surg. 1984;199(3):363–366. doi: 10.1097/00000658-198403000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riegler C, von Rennenberg G, Bollwerg K, et al. Endovascular therapy in patients with internal carotid artery occlusion and patent circle of Willis. J Neurointerv Surg. 2023. 10.1136/jnis-2023-020556 [DOI] [PMC free article] [PubMed]
- 8. Baek JH, Yoo J, Song D, et al. Predictive value of thrombus volume for recanalization in stent retriever thrombectomy. Sci Rep. 2017;7(1):15938. doi: 10.1038/s41598-017-16274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teodoro C, Bertanha M, Girard FPCM, et al. Results of treatment of acute occlusions of limb arteries at a university hospital - retrospective study. J Vasc Bras. 2020;19:e20200031. doi: 10.1590/1677-5449.200031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogata T, Yasaka M, Wakugawa Y, Kitazono T, Okada Y. Morphological classification of mobile plaques and their association with early recurrence of stroke. Cerebrovasc Dis. 2010;30(6):606–611. doi: 10.1159/000319889. [DOI] [PubMed] [Google Scholar]
- 11. Macierewicz J, Armon MP, Cleveland TJ, Gaines PA, Beard JD. Carotid endarterectomy combined with proximal stenting for multilevel disease. Eur J Vasc Endovasc Surg. 2000;20(6):572–575. doi: 10.1053/ejvs.2000.1241. [DOI] [PubMed] [Google Scholar]
- 12. Iihara K, Satow T, Matsushige T, et al. Hybrid operating room for the treatment of complex neurovascular and brachiocephalic lesions. J Stroke Cerebrovasc Dis. 2013;22(8):e277–e285. doi: 10.1016/j.jstrokecerebrovasdis.2012.07.014. [DOI] [PubMed] [Google Scholar]