Abstract
Background:
Younger women (age ≤40) with breast cancer undergoing NAC have higher rates of pCR. However, it is unknown whether axillary or breast downstaging rates differ by age. Here we compare pCR incidence, and surgical downstaging rates of the breast and axilla post-NAC, between patients age ≤40, 41–60, and ≥61.
Methods:
We identified 1383 women with stage I-III breast cancer treated with NAC and subsequent surgery from 11/2013–12/2018. pCR, and breast/axillary downstaging rates were assessed and compared across age groups.
Results:
Younger women were significantly more likely to have ductal histology, poorly differentiated tumors, and BRCA mutations; 35% of tumors were HR+/HER2–, 36% HER2+, and 29% TN, with similar subtype distribution across age groups (p=0.6). Overall, pCR rates did not differ by age. However, among patients with TN tumors (n=394), younger women had higher pCR rates (52% versus 35% among those 41–60, 29% among those ≥61; p=0.007) and were more likely to have tumors with high tumor-infiltrating lymphocyte (TIL) concentrations (p<0.001). Downstaging to breast-conserving surgery (BCS) eligibility post-NAC among initially BCS-ineligible patients was similar across age groups; younger women chose BCS less often (p<0.001). Among cN1 patients (n=813), 52% of women ≤40 avoided ALND with NAC, versus 39% and 37% in older groups (p<0.001).
Conclusions:
Younger women undergoing NAC for axillary downstaging were more likely to avoid ALND across all subtypes; however, overall pCR rates did not differ by age. Despite equivalent breast downstaging and BCS eligibility rates across age groups, younger women were less likely to undergo BCS.
INTRODUCTION
The incidence of early-onset breast cancer, defined as diagnosis at age ≤ 40 years, has steadily increased since the 1990s.1 Younger women often present with more advanced disease and have tumors with more aggressive features, such as higher nuclear grade, lymphovascular invasion, and human epidermal growth factor receptor 2 (HER2) overexpression or triple negative (TN) status (lacking HER2 and estrogen and progesterone receptor expression).2,3 Young women diagnosed with breast cancer have, on average, a higher risk of recurrence and death compared with older women.4,5 These biological differences and inferior survival outcomes highlight the importance of personalizing treatment for this high-risk population.
Despite overall worse outcomes, data from clinical trials suggest that younger age is associated with a higher likelihood of achieving a pathologic complete response (pCR) following neoadjuvant chemotherapy (NAC).6 NAC has become the standard of care for locally advanced and early-stage breast cancer among more aggressive subtypes, including TN and HER2 positive. NAC is also used to treat patients with large tumors or nodal disease to facilitate breast-conserving surgery (BCS) and less-extensive axillary surgery. While a pCR after NAC is associated with improved disease-free and overall survival7, the absence of pCR guides adjuvant treatment recommendations for patients with TN or HER2+ disease. In a pooled analysis of 8 neoadjuvant trials, women age < 40 years were more likely to obtain a pCR compared to women age 40–49 years and those age ≥ 50 years (21% versus 18% and 14%; p < 0.001). The superior pCR rate seen in young women was driven by higher rates in those with HER2 negative tumors (TN or hormone receptor [HR] positive/HER2 negative), suggesting that the influence of age on achieving a pCR may differ by biological subtype.
Whether women age ≤ 40 years, who often present with larger tumors and nodal disease, are more likely to achieve breast or axillary downstaging with NAC compared to older women, is unknown. We therefore compared rates of pCR, and of downstaging of the breast and axilla after modern NAC, between breast cancer patients age ≤ 40 years and those in older age groups.
METHODS
Study Population and Treatments
After institutional review board approval, consecutive patients with stage I–III breast cancer treated with NAC and subsequent surgery between November 2013 and December 2018 were identified from a prospectively maintained institutional database. Patients who had an indication for systemic chemotherapy because of tumor biology, receptor subtype, or clinical stage (nodal status, tumor size) were considered for NAC. Preoperative genomic testing was not used to select patients for NAC. We excluded male patients and those who received neoadjuvant endocrine therapy. The cohort was divided into three age groups: age ≤ 40 years, 41–60 years, and ≥ 61 years.
Clinicopathologic data were recorded. Clinical and pathologic stages were assigned according to the 8th edition of the American Joint Committee on Cancer staging system.8 HR positive status was defined by a threshold of > 1% staining in accordance with current guidelines.9 Patients were categorized as HR positive if they were estrogen receptor positive and/or progesterone receptor positive. HER2 overexpression was defined as 3+ by immunohistochemistry or gene amplification by fluorescence in situ hybridization.10 Based on these definitions, tumors were divided into three subtypes: HR positive/HER2 negative, HER2 positive, and HR negative/HER2 negative (TN).
Surgeons prospectively assessed eligibility for breast conservation prior to and at completion of NAC based on physical examination and imaging findings with no set size cutoff. Upon completion of NAC, patients underwent definitive breast surgery, except in cases of occult breast cancer, which we routinely manage with whole-breast radiotherapy.
Clinical nodal status was prospectively assessed by the surgeon before and after NAC. Patients with cN1 disease (defined as palpable and mobile ipsilateral axillary lymphadenopathy with biopsy-proven nodal metastasis) who converted to cN0 (defined as no palpable lymphadenopathy) on physical exam after NAC were eligible for sentinel lymph node biopsy (SLNB). SLNB was performed using dual lymphatic mapping. For cN1 patients with no residual palpable adenopathy after NAC, axillary lymph node dissection (ALND) was omitted if ≥ 3 sentinel lymph nodes (SLNs) were identified and all were pathologically negative, and ALND was performed for any positive SLN (including macrometastases, micrometastases, and isolated tumor cells). cT4 and cN2/3 patients were considered ineligible for SLNB irrespective of their response to NAC and underwent ALND, as the accuracy of SLNB in this setting is yet to be established.
For TN tumors, we evaluated the concentration of stromal tumor-infiltrating lymphocytes (TILs) in pre-NAC core biopsy specimens. Cases were scored by 2 dedicated breast pathologists on hematoxylin and eosin-stained sections according to the recommendations of the International TILs working group.11,12 TIL-high tumors were defined as those containing > 40% TILs.
Statistical Analysis
The primary outcome of interest was pCR. Overall pCR was defined as the absence of invasive carcinoma in the breast and axillary lymph nodes (ypT0/Tis, N0). The rate of breast pCR was calculated among patients presenting with cT1–T4 tumors, and nodal pCR was determined among all node-positive patients at presentation (cN1-N3). Rates of overall, breast, and nodal pCR were compared across age groups, in the overall cohort and within each tumor subtype.
Rates of breast and axillary downstaging were also assessed and compared across age groups. BCS-ineligible patients on presentation who became BCS-eligible after NAC were considered to have breast downstaging. We excluded patients with occult breast cancer or multicentric or cT4 disease at presentation, as they would be precluded from surgical downstaging. Patients who experienced axillary downstaging were those who initially presented with biopsy-proven cN1 disease and converted to cN0 after NAC. cT4 and cN2/3 patients were excluded, as the accuracy of SLNB in this setting is yet to be established.
Clinical and pathological characteristics were compared between patients age ≤ 40 years, 41–60 years, and ≥ 61 years using Student’s t-test or the Wilcoxon rank-sum test for continuous variables, and the chi-square or Fisher’s exact test for categorical variables. We compared the clinical and pathological characteristics of individuals who did and did not achieve overall pCR, including age group, histology, histological grade, lymphovascular invasion, cT stage, cN stage, receptor status, and NAC regimen. Factors significant at a type I error rate of 0.05 were included in a multivariable logistic regression analysis to account for confounding. To account for missing data on lymphovascular invasion (for 24% of individuals), we conducted a sensitivity analysis using multiple imputation through the mice package in R 3.6.3 (R Core Development Team, Vienna, Austria) assuming that data were missing at random. Multivariable analysis was conducted using the imputed data, and results were compared with the original analysis. Additional statistical analysis included univariate analyses of rates of pCR, overall and according to subtype, and breast and axillary downstaging after NAC stratified by age group.
RESULTS
Patient Characteristics
Among 1383 patients meeting inclusion criteria, 22% were age ≤ 40 years, 56% were age 41–60 years, and 22% were age ≥ 61 years. Table 1 details patient, tumor, and treatment characteristics of the study cohort by age group. Thirty-five percent of patients had HR positive/HER2 negative cancers, 36% had HER2 positive tumors, and 28.5% had TN, with similar subtype distribution across age groups (p = 0.6). Women age ≤ 40 years were significantly more likely to have ductal histology, poorly differentiated tumors, and BRCA mutations compared with older women.
TABLE 1.
Patient, tumor, and treatment characteristics of the study cohort by age group (categorical data presented as n (%), and continuous data presented as median [interquartile range])
| Characteristic | Entire cohort | Age ≤ 40 years | Age 41–60 years | Age ≥ 61 years | p |
|---|---|---|---|---|---|
| n = 1383 | n = 300 | n = 772 | n = 311 | ||
| Age, years | 50 (42–59) | 36 (32–38) | 50 (46–55) | 66 (63–70) | < 0.001 |
| Tumor size, cm | 4.0 (2.5–6.0) | 4.0 (3.0–6.0) | 4.0 (2.5–6.0) | 3.9 (2.8–6.0) | 0.80 |
| Race | |||||
| White | 849 (66.6%) | 168 (61%) | 467 (66%) | 214 (74%) | 0.005 |
| Black | 194 (15%) | 39 (14%) | 117 (16%) | 38 (13%) | |
| Asian/Pacific Islander | 149 (12%) | 39 (14%) | 82 (12%) | 28 (10%) | |
| Other | 83 (6.5%) | 28 (10%) | 46 (6%) | 9 (3%) | |
| Unknown | 108 | 26 | 60 | 22 | |
| Clinical tumor stage | < 0.001 | ||||
| T0 (Occult) | 11 (0.8%) | 2 (0.7%) | 5 (0.60%) | 4 (1.3%) | |
| T1 | 197 (14%) | 42 (14%) | 110 (14%) | 45 (14%) | |
| T2 | 766 (55%) | 167 (56%) | 423 (55%) | 176 (57%) | |
| T3 | 271 (20%) | 74 (25%) | 156 (20%) | 41 (13%) | |
| T4 | 136 (9.8%) | 14 (4.7%) | 78 (10%) | 44 (14%) | |
| Clinical nodal stage | > 0.90 | ||||
| N0 | 449 (32%) | 97 (32%) | 255 (33%) | 97 (31%) | |
| N1 | 813 (59%) | 180 (60%) | 447 (58%) | 186 (60%) | |
| N2-N3 | 121 (8.7%) | 23 (7.7%) | 70 (9.1%) | 28 (9.0%) | |
| Histology | 0.001 | ||||
| Ductal | 1244 (92%) | 287 (97%) | 687 (91%) | 270 (89%) | |
| Lobular or mixed | 99 (7.3%) | 7 (2.4%) | 63 (8.3%) | 29 (9.5%) | |
| Other | 16 (1.2%) | 2 (0.7%) | 8 (1.1%) | 6 (2.0%) | |
| Unknown | 24 | 4 | 14 | 6 | |
| Histologic grade | 0.034 | ||||
| Well differentiated | 21 (1.5%) | 0 (0%) | 14 (1.8%) | 7 (2.3%) | |
| Moderately differentiated | 391 (28%) | 78 (26%) | 216 (28%) | 97 (31%) | |
| Poorly differentiated | 964 (70%) | 221 (74%) | 536 (70%) | 207 (67%) | |
| Lymphovascular invasion | 406 (39%) | 96 (42%) | 226 (38%) | 84 (36%) | 0.40 |
| Unknown | 7 | 1 | 6 | 0 | |
| Tumor subtype | 0.60 | ||||
| HR+/HER2− | 487 (35%) | 106 (35%) | 271 (35%) | 110 (35%) | |
| HER2+ | 502 (36%) | 111 (37%) | 269 (35%) | 122 (40%) | |
| TN | 394 (28.5%) | 83 (28%) | 232 (30%) | 79 (25%) | |
| BRCA1/2 mutation status | < 0.001 | ||||
| Positive | 98 (11%) | 46 (18%) | 49 (9.8%) | 3 (2.6%) | |
| Negative | 672 (77%) | 207 (79%) | 401 (80%) | 64 (55%) | |
| Unknown result or VUS | 108 (12%) | 9 (3.4%) | 50 (10%) | 49 (42%) | |
| Not tested/missing | 505 | 38 | 272 | 195 | |
| Pathologic tumor stage | 0.57 | ||||
| T0 | 399 (29%) | 97 (33%) | 219 (29%) | 82 (27%) | |
| Tis | 99 (7.2%) | 21 (7.0%) | 58 (7.6%) | 19 (6.2%) | |
| T1 | 574 (42%) | 125 (42%) | 324 (42%) | 125 (41%) | |
| T2 | 232 (17%) | 44 (15%) | 124 (16%) | 64 (21%) | |
| T3 | 59 (4.3%) | 10 (3.4%) | 36 (4.7%) | 13 (4.2%) | |
| T4 | 11 (0.80%) | 1 (0.30%) | 6 (0.80%) | 4 (1.3%) | |
| Not assessed or occult | 11 | 2 | 5 | 4 | |
| Pathologic nodal stage | 0.20 | ||||
| N0 | 828 (60%) | 188 (63%) | 460 (60%) | 180 (58%) | |
| N1 | 344 (25%) | 81 (27%) | 191 (25%) | 72 (23%) | |
| N2-N3 | 208 (15%) | 30 10%) | 121 (16%) | 57 (18%) | |
| Neoadjuvant chemotherapy | < 0.001 | ||||
| ACT based | 1268 (92%) | 286 (95%) | 722 (94%) | 260 (84%) | |
| Taxane based | 95 (6.9%) | 14 (4.7%) | 42 (5.5%) | 39 (13%) | |
| CMF | 9 (0.7%) | 0 (0%) | 0 (0%) | 9 (2.9%) | |
| Other | 5 (0.4%) | 0 (0%) | 3 (0.4%) | 2 (0.6%) | |
| NAC included carboplatin | 172 (12%) | 47 (16%) | 98 (13%) | 27 (8.7%) | 0.029 |
| Final breast surgerya | < 0.001 | ||||
| BCS | 586 (43%) | 88 (30%) | 332 (43%) | 166 (54%) | |
| Unilateral mastectomy | 388 (28%) | 62 (21%) | 210 (27%) | 116 (38%) | |
| Bilateral mastectomy | 398 (29%) | 148 (50%) | 225 (29%) | 25 (8.1%) | |
| Final axillary surgery | |||||
| SLNB | 722 (52%) | 186 (62%) | 393 (51%) | 149 (48%) | < 0.001 |
| ALND | 661 (48%) | 114 (38%) | 379 (49%) | 162 (52%) | < 0.001 |
11 patients with occult primary breast cancer did not undergo primary breast surgery
HR hormone receptor, HER2 human epidermal growth factor receptor 2, TN triple-negative, BRCA1/2 breast cancer gene 1 or 2, VUS variant of unknown significance, ACT doxorubicin and cyclophosphamide followed by a taxane CMF cyclophosphamide, methotrexate, 5-fluorouracil, pCR pathologic complete response, NAC neoadjuvant chemotherapy, BCS breast-conserving surgery, SLNB sentinel lymph node biopsy, ALND axillary lymph node dissection
Most patients received both anthracycline and taxol (n = 1268, 92%), with younger women more often receiving this regimen (95% versus 94% in those age 41–60 years, and 84% in those age ≥ 61 years; p < 0.001). Carboplatin was included in a minority of NAC regimens (12%) and was most often used in the setting of TNBC (33% in women age ≤ 40 years, 27% in women age 41–60 years, and 16% in women age ≥ 61 years; p = 0.059). The majority of HER2 positive patients (99.9%) received neoadjuvant dual anti-HER2 therapy with trastuzumab and pertuzumab.
Rates of pCR
Thirty-four percent of all patients achieved a pCR after NAC. Compared with patients who had HR positive/HER2 negative tumors, patients with TN and HER2 positive tumors had higher rates of overall pCR (55% and 38%, respectively, versus 9.4% among HR positive/HER2 negative; p < 0.001), as well as breast and nodal pCR. Rates of overall, breast, and nodal pCR did not differ among age groups (Fig. 1a).
Fig. 1.
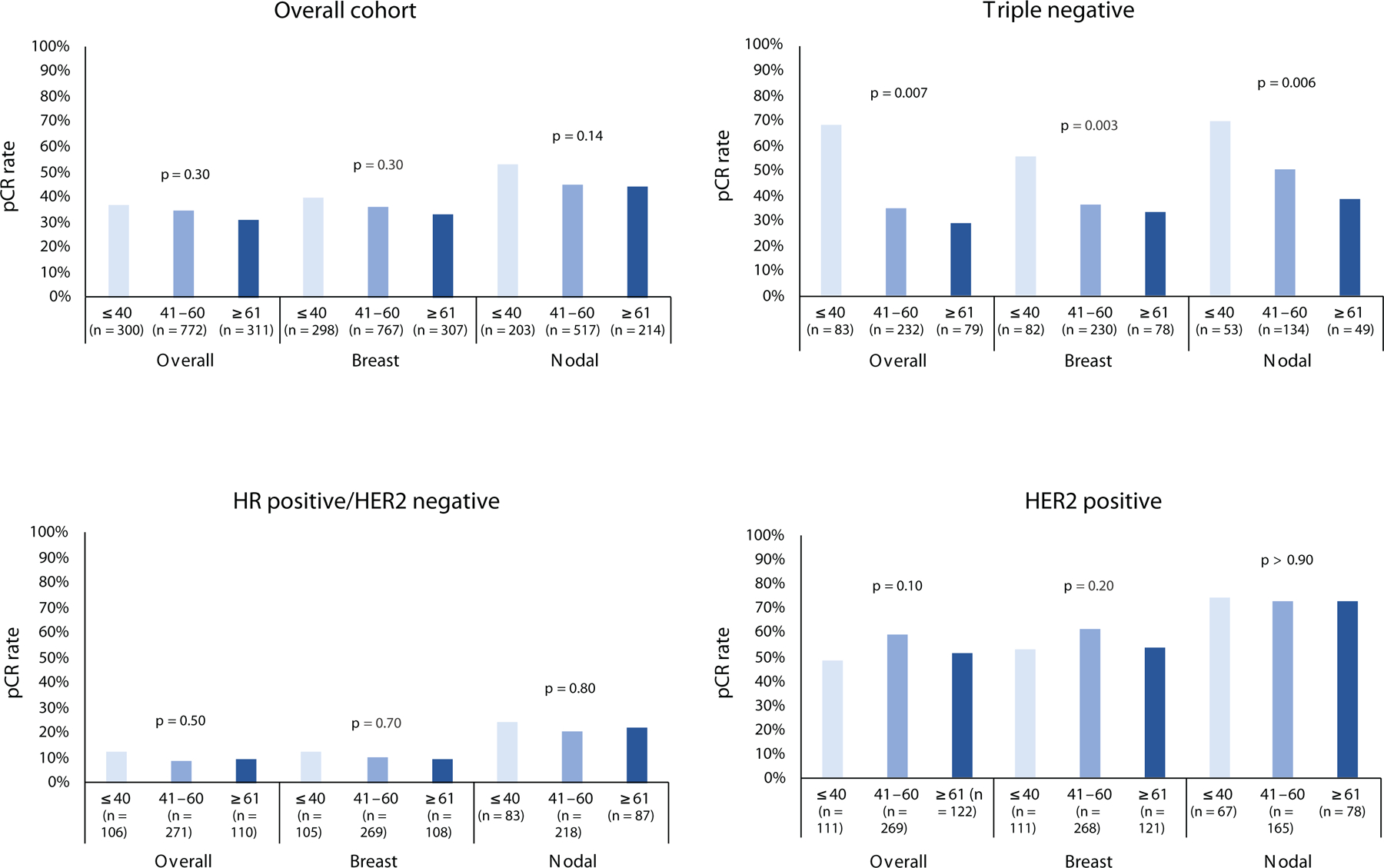
pCR rates by age group. a, overall cohort; b, triple negative; c, HR+/HER2−; d, HER2+. Top x-axis labels indicate age in years; lower labels indicate response type/location.
pCR pathologic complete response, HR hormone receptor
However, among patients with TN tumors (n = 394), women age ≤ 40 years more frequently achieved overall pCR (52% versus 35% of those age 41–60 years and 29% of those age ≥ 61 years; p =0.007), breast pCR (56% versus 37% of those age 41–60 years and 33% of those age ≥ 61 years; p = 0.003) and nodal pCR (70% versus 51% of those age 41–60 years and 39% of those age ≥ 61 years; p = 0.006)(Fig. 1b). No differences in rates of pCR by age were identified among other subtypes (Fig. 1c–d).
Factors Associated with pCR
On univariate analysis of factors associated with pCR in the entire cohort, ductal histology, poor differentiation, presence of lymphovascular invasion, lower clinical tumor stage, nodal stage at presentation, and TN or HER2 positive receptor status were associated with achievement of pCR (Table 2). There was no association between NAC regimen or age group and pCR. On multivariable analysis, poor differentiation (p < 0.001), lymphovascular invasion (p < 0.001), and receptor subtype (p < 0.001) remained independently associated with pCR. These results did not change substantially in a sensitivity analysis accounting for missing data.
TABLE 2.
Univariate and multivariable analysis of factors associated with pCR (T0/is N0) (data are n [%])
| Entire cohort (n = 1383) |
No pCR (n = 1017) |
pCR (n = 366) |
Univariate | Multivariable | |||
|---|---|---|---|---|---|---|---|
| p | OR | 95% CI | p | ||||
| Age category | 0.6 | 0.50 | |||||
| ≤ 40 years | 300 (22%) | 214 (21%) | 86 (23%) | ref | |||
| 41–60 years | 772 (56%) | 571 (56%) | 201 (55%) | 0.97 | 0.64–1.50 | ||
| ≥ 61 years | 311 (22%) | 232 (23%) | 79 (22%) | 0.77 | 0.46–1.30 | ||
| Histology | < 0.001 | 0.60 | |||||
| Ductal | 1244 (92%) | 906 (90%) | 338 (96%) | ref | |||
| Lobular/mixed | 99 (7.3%) | 89 (8.8%) | 10 (2.8%) | 0.67 | 0.23–1.66 | ||
| Other | 16 (1.2%) | 12 (1.2%) | 4 (1.1%) | 1.56 | 0.30–6.43 | ||
| Histologic grade | < 0.001 | < 0.001 | |||||
| Poorly differentiated | 964 (70%) | 638 (63%) | 326 (90%) | ref | |||
| Moderately differentiated | 391 (28%) | 354 (35%) | 37 (10%) | 0.31 | 0.18–0.50 | ||
| Well differentiated | 21 (1.5%) | 21 (2.1%) | 0 (0%) | 0.00 | |||
| Lymphovascular invasion | 406 (39%) | 361 (43%) | 45 (21%) | < 0.001 | 0.46 | 0.31–0.68 | < 0.001 |
| Clinical tumor stage | 0.017 | 0.081 | |||||
| T0-T1 | 210 (15%) | 145 (14%) | 65 (18%) | ref | |||
| T2 | 766 (55%) | 553 (54%) | 213 (58%) | 0.89 | 0.53–1.52 | ||
| T3 | 271 (20%) | 219 (22%) | 52 (14%) | 0.50 | 0.26–0.95 | ||
| T4 | 136 (9.8%) | 100 (9.8%) | 36 (9.8%) | 0.93 | 0.45–1.90 | ||
| Clinical nodal stage, n (%) | 0.001 | 0.40 | |||||
| N0 | 449 (32%) | 313 (31%) | 136 (37%) | ref | |||
| N1 | 813 (59%) | 626 (62%) | 187 (51%) | 0.86 | 0.60–1.26 | ||
| N2–3 | 121 (8.7%) | 78 (7.7%) | 43 (12%) | 1.30 | 0.69–2.40 | ||
| Tumor subtype, n (%) | < 0.001 | < 0.001 | |||||
| TN | 394 (28%) | 275 (27%) | 119 (33%) | ref | |||
| HR+ HER2− | 487 (35%) | 453 (45%) | 34 (9.3%) | 0.29 | 0.16–0.52 | ||
| HER2+ | 502 (36%) | 289 (28%) | 213 (58%) | 2.55 | 1.76–3.75 | ||
| NAC regimena, n (%) | 0.093 | - | |||||
| ACT-based | 1268 (92%) | 939 (93%) | 329 (91%) | - | - | - | |
| CMF-based | 9 (0.7%) | 9 (0.9%) | 0 (0%) | - | - | - | |
| Other | 5 (0.4%) | 3 (0.3%) | 2 (0.6%) | - | - | - | |
| Taxane-based | 95 (6.9%) | 64 (6.3%) | 31 (8.6%) | - | - | - | |
| NAC included carboplatin a | 172 (12%) | 116 (11%) | 56 (15%) | 0.057 | - | - | - |
NAC regimen was not included in the multivariable model, as it was not significant on univariate analysis
pCR pathologic complete response; OR odds ratio. CI confidence interval. TN triple-negative. HR hormone receptor. HER2 human epidermal growth factor receptor 2. NAC neoadjuvant chemotherapy. ACT doxorubicin and cyclophosphamide followed by a taxane. CMF cyclophosphamide, methotrexate, 5-fluorouracil
Breast and Axillary Downstaging Rates
Among 649 BCS-ineligible breast cancer patients with potential for downstaging as determined by their treating surgeon, 72% (n = 467) became BCS-eligible after NAC; the rate of conversion to BCS eligibility was similar across age groups (Fig. 2a). Among BCS-eligible patients post-NAC, patients age ≤ 40 years were less likely to choose BCS (45% versus 65% of those age 41–60 years, and 81% of those age ≥ 61 years; p < 0.001)(Fig. 2b).
Fig. 2.
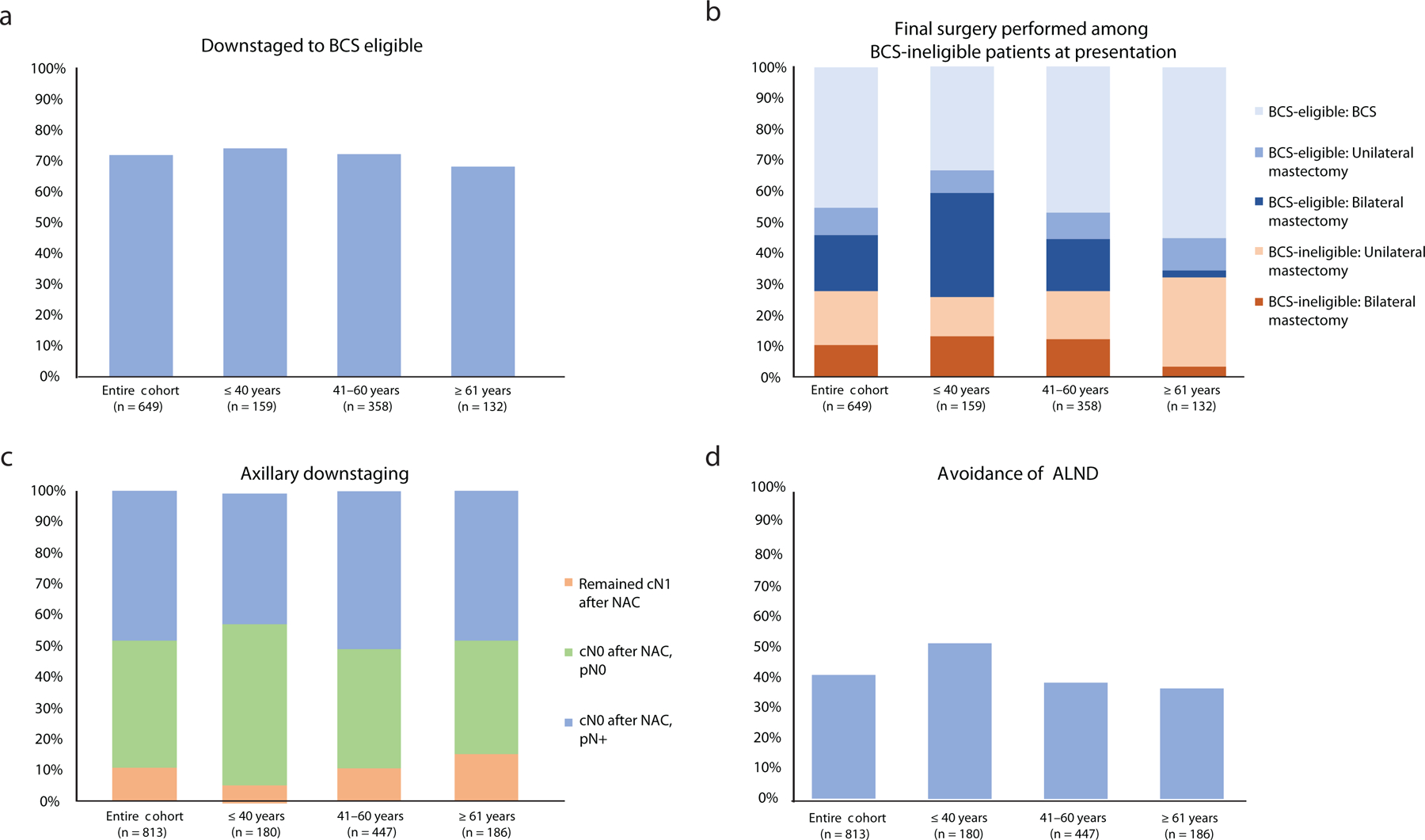
Downstaging and surgery by age. a, rate of downstaging to BCS eligibility; b, final surgery according to BCS eligibility; c, rate of axillary downstaging; d, percentage of patients who avoided ALND.
BCS breast-conserving surgery, NAC neoadjuvant chemotherapy, ALND axillary lymph node dissection
Among biopsy-proven cN1 patients at presentation (n =813), 94% of women age ≤ 40 years became cN0 after NAC and underwent SLNB, compared with 89% and 85% in older age groups (p = 0.02; Fig. 2c). In these patients (n = 726), rates of nodal pCR on SLNB were highest among women age ≤ 40 years (55% versus 43% of those age 41–60 years, and 43% of those age ≥ 61 years; p = 0.001). Fifty-two percent of women age ≤ 40 years who initially presented with cN1 disease avoided ALND after NAC compared with 38% and 37% in older age groups, respectively (p = 0.001)(Fig. 2d).
Association Between BRCA Mutation Status, Stromal TILs, and pCR Among Patients with TN Breast Cancer
Among women with TN breast cancer (TNBC) and known BRCA status (n = 312), women age ≤ 40 years were more likely to have a deleterious BRCA mutation compared with women in older age groups (29% versus 15% of those age 41–60 years, and 2.5% of those age ≥ 61 years; p = 0.001). Among BRCA carriers, women age ≤ 40 years achieved a pCR more often than older women (67% versus 44% of those 41–60 years, and 0% of those age ≥ 61 years; p = 0.058), although this analysis was carried out in a small sample, and the difference was not statistically significant.
Stromal TILs were measured in 341 women with TNBC (Table 3), of whom 16% (n=55) had tumors with TILs composing > 40% of the tumor stroma (i.e., “TIL-rich”). TIL-rich tumors were significantly more common among women age ≤ 40 years (25% versus 17% of those age 41–60 years, and 4.3% of those age ≥ 61 years; p=0.001). Within the entire cohort, pCR was significantly more frequent among patients with TIL-rich tumors (56% versus 35%; p = 0.005).
TABLE 3.
Effect of stromal TILs on pCR (ypT0/Tis, N0) in triple negative subtype
| Percent stromal TILs | Entire cohort | ≤ 40 years | 41–60 years | ≥ 61 years | p | |
|---|---|---|---|---|---|---|
| TILs ≤ 40% | n (% within age group) | 286 (84%) | 56 (75%) | 163 (83%) | 67 (96%) | 0.037 |
| pCR | 101 (35%) | 28 (50%) | 53 (33%) | 20 (30%) | ||
| TILs > 40% | n (% within age group) | 55 (16%) | 19 (25%) | 33 (17%) | 3 (4%) | 0.541 |
| pCR | 31 (56%) | 12 (63%) | 18 (55%) | 1 (33%) | ||
pCR pathologic complete response, TILs tumor-infiltrating lymphocytes
DISCUSSION
Motivated by prior studies indicating that younger patients are more likely to achieve pCR following NAC, we assessed the influence of age on rates of breast and axillary downstaging. In this large cohort of women with stage I–III breast cancer undergoing NAC, rates of axillary downstaging among patients with cN1 disease were significantly higher among women age ≤ 40 years compared with older women, allowing them to avoid ALND. While rates of pCR and downstaging to BCS-eligible did not differ by age in the overall cohort, younger women were less likely than older women to elect BCS when eligible.
Our findings emphasize the benefit of NAC for younger patients in achieving nodal pCR and de-escalating axillary surgery. The ability of NAC to support these outcomes in the general breast cancer population is well established by prospective trials.13–15 Among 630 cN1 patients in a recent study, 573 (91%) became cN0 and underwent SLNB, and 93% of these had successful mapping with identification of ≥ 3 SLNs; 41% of patients avoided ALND.16 In our cohort, women age ≤ 40 years who initially presented with cN1 disease were more likely than older women to have a clinically negative axilla after NAC and be eligible for SLNB. Among patients who became eligible for and underwent SLNB, younger women were most likely to have a nodal pCR and avoid ALND. This difference in axillary downstaging rates among age groups may relate to variations in NAC regimens, as younger women were more likely than older women to receive ACT (Adriamycin and cyclophosphamide, followed by Taxol)-based treatment. However, the type of NAC regimen was not significantly associated with overall pCR in our cohort. Among all patients with nodal disease on presentation (cN1-3), 47% achieved nodal pCR. Nodal pCR has been shown to be unrelated to nodal burden, but rather a function of tumor biology.17 This raises the question of whether SLNB can be considered in patients with cN2-3 disease with a complete clinical response after NAC, potentially sparing them from the morbidity of an ALND. The safety of this approach is currently under investigation.
The present findings do not confirm prior studies suggesting that women diagnosed with breast cancer at a young age have higher rates of pCR after NAC. The German Breast Group reported a higher rate of pCR of 21% in women age < 40 years (compared with 18% in those age 40–49 years and 14% in those age ≥ 50 years; p < 0.001) treated in 8 neoadjuvant trials.6 These results were echoed in a single-institution retrospective study in which 316 women age ≤ 40 years were more likely to achieve a pCR than women age > 40 years (37% versus 26%; p < 0.001).18 In the present study, achievement of pCR did not differ by age in the overall cohort, even after accounting for differences in tumor characteristics. This difference in results may reflect differences between study populations, as older women in our cohort had more-aggressive tumor characteristics than those in prior studies, and may reflect methodological differences, such as cutoffs used to define age groups for comparison. Similarly, Loibl et al.6 used a more restrictive definition of pCR, ypT0N0, and their patients were treated in the context of clinical trials, potentially including a selected patient population. In these prior studies6,18, the difference in rates of pCR by age was confined to women with TN and HR positive subtypes. Consistent with these findings, younger women with TN breast cancer in our cohort had significantly higher rates of pCR (52%) compared with older age groups (35% in those age 41–60 years and 29% in those age ≥ 61 years). No difference in pCR by age was seen among other subtypes.
As shown in the present study and supported by the literature6,18, younger women with TNBC more often achieve pCR than their older counterparts. In multiple clinical trials7,19,20, women with HER2 positive and TN tumors had the highest rates of pCR. We hypothesize that the higher pCR rates among TN patients reflect a higher proportion of BRCA mutation carriers and TIL-rich tumors among young women in this subgroup. Approximately 12% of breast cancer cases arising in women age ≤ 40 years are related to pathogenic mutations in BRCA1 or BRCA2 genes21,22, and these mutations are most common in TNBC.23 In our cohort, 29% of young women with TNBC had a deleterious BRCA mutation, compared with 17% and 6.3% among women in older age groups. Tumors in BRCA carriers are known to be particularly sensitive to chemotherapy, likely due to reduced capacity for DNA repair and higher tumor proliferation. Among TNBC patients specifically, BRCA carriers have a higher pCR rate than non-carriers.24–26 Similarly, higher concentrations of stromal TILs in TN tumors are associated with an increased likelihood of pCR27–29, and stromal TIL concentrations are higher in younger patients.29 Among women with TNBC in our study, 25% of women age ≤ 40 years had TIL-rich tumors, compared with only 17% and 4.3% of older women. Together, these data suggest that among TNBC patients, those age ≤ 40 years may be more likely to have chemosensitive tumors and achieve a pCR after NAC.
Approximately 72% of women in this study who were ineligible for BCS at presentation downstaged to BCS-eligible, within the published range of 42–75%.30–33 Variations in breast downstaging have been attributed to differences in cohort and study design, such as inclusion of T4 and multicentric disease, neoadjuvant systemic therapy regimen, tumor subtype distribution, and prospective versus retrospective assessment of eligibility.
Despite equivalent rates of downstaging in the breast across age groups, younger women were significantly less likely to choose and ultimately undergo BCS. Low acceptance of BCS after NAC has been previously described. In a prospective analysis of women with TNBC treated with NAC, Golshan et al. found that only 56% of BCS-eligible patients chose BCS.32 In a cohort of women age ≤ 40 years, 60% of 133 women eligible for BCS after neoadjuvant systemic therapy chose that approach.34 Patients may elect mastectomy because of personal preference, presence of a genetic mutation, family history, insurance coverage, and geographic variations.32–35 In our study, BCS-eligible younger women had uniformly lower rates of BCS compared to older women regardless of BRCA status, suggesting that the higher proportion of BRCA carriers among younger patients does not account for this difference. In light of recent data suggesting improved survival outcomes among women electing BCS compared to mastectomy36, our study suggests that shared decision making between clinicians and young patients with breast cancer is essential, and that further studies to understand surgical decision making and long-term patient-reported outcomes are needed.
Strengths of our study include the large consecutive cohort of patients with prospective determination of BCS eligibility before and after NAC, homogeneity in NAC regimens, and standardized pathologic assessment. Limitations of our study include the subjective bias of physician assessment in determination of BCS eligibility and its conduct at a single large-volume institution with highly specialized providers, which may limit generalizability. Nonetheless, the characteristics of our patient population are similar to previously published data and likely representative of women with breast cancer treated with NAC in the population at large. Furthermore, management decisions were concordant with national guidelines. Additionally, we were unable to evaluate for the successful completion of the recommended NAC regimen, which may be lower in older women who are more susceptible to toxicities. However, the similar rates of pCR across age groups suggest differential completion of NAC is unlikely to be a significant driver of differences in pCR in this cohort.
Conclusions
In a large cohort of women with stage I–III breast cancer treated with NAC, rates of pCR were similar across age groups. Among women with TNBC, women age ≤ 40 years most often achieved a pCR, likely owing to a higher proportion of BRCA carriers and TIL-rich tumors with enhanced chemosensitivity. Rates of axillary downstaging and avoidance of ALND were highest among young women compared with older age groups. Despite equivalent rates of downstaging to breast conservation, young women were less likely to elect BCS when eligible. This study supports the use of NAC in young women, particularly when node positive, with the goal of deescalating axillary surgery and avoiding mastectomy when desired. Further efforts to understand factors affecting surgical decision making and long-term patient-reported outcomes among young, BCS-eligible women are needed.
Supplementary Material
Synopsis.
Here we examine age-related pCR rate differences post-NAC in a large cohort of women with breast cancer. We find an association between NAC and a higher rate of axillary downstaging among young women presenting with clinical stage N1 disease.
ACKNOWLEDGMENTS
The preparation of this study was supported in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center, and this study was presented in virtual poster format at the 22nd Annual Meeting of the American Society of Breast Surgeons, April 29-May 2, 2021. Dr. Monica Morrow has received honoraria from Exact Sciences and Roche.
Footnotes
Disclosures: The preparation of this study was supported in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center, and this study was presented in virtual poster format at the 22nd Annual Meeting of the American Society of Breast Surgeons, April 29-May 2, 2021. Dr. Monica Morrow has received honoraria from Exact Sciences and Roche.
All other authors have no conflict of interest disclosures to report.
REFERENCES
- 1.Bouchardy C, Fioretta G, Verkooijen HM, et al. Recent increase of breast cancer incidence among women under the age of forty. Br J Cancer Jun 4 2007;96(11):1743–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colleoni M, Rotmensz N, Robertson C, et al. Very young women (<35 years) with operable breast cancer: features of disease at presentation. Ann Oncol Feb 2002;13(2):273–279. [DOI] [PubMed] [Google Scholar]
- 3.Kroman N, Jensen MB, Wohlfahrt J, Mouridsen HT, Andersen PK, Melbye M. Factors influencing the effect of age on prognosis in breast cancer: population based study. Bmj Feb 19 2000;320(7233):474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adami HO, Malker B, Holmberg L, Persson I, Stone B. The relation between survival and age at diagnosis in breast cancer. N Engl J Med Aug 28 1986;315(9):559–563. [DOI] [PubMed] [Google Scholar]
- 5.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol Jul 10 2008;26(20):3324–3330. [DOI] [PubMed] [Google Scholar]
- 6.Loibl S, Jackisch C, Lederer B, et al. Outcome after neoadjuvant chemotherapy in young breast cancer patients: a pooled analysis of individual patient data from eight prospectively randomized controlled trials. Breast Cancer Res Treat Jul 2015;152(2):377–387. [DOI] [PubMed] [Google Scholar]
- 7.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet Jul 12 2014;384(9938):164–172. [DOI] [PubMed] [Google Scholar]
- 8.Amin MB, Edge S, Greene F, et al. , eds. AJCC Cancer Staging Manual, 8th Edition. New York: Springer International Publishing; 2017. [Google Scholar]
- 9.Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch Pathol Lab Med May 2020;144(5):545–563. [DOI] [PubMed] [Google Scholar]
- 10.Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol Jul 10 2018;36(20):2105–2122. [DOI] [PubMed] [Google Scholar]
- 11.Dieci MV, Radosevic-Robin N, Fineberg S, et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol Oct 2018;52(Pt 2):16–25. [DOI] [PubMed] [Google Scholar]
- 12.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol Feb 2015;26(2):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol Jan 20 2015;33(3):258–264. [DOI] [PubMed] [Google Scholar]
- 14.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. Jama Oct 9 2013;310(14):1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol Jun 2013;14(7):609–618. [DOI] [PubMed] [Google Scholar]
- 16.Montagna G, Mamtani A, Knezevic A, Brogi E, Barrio AV, Morrow M. Selecting Node-Positive Patients for Axillary Downstaging with Neoadjuvant Chemotherapy. Ann Surg Oncol Oct 2020;27(11):4515–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gentile LF, Plitas G, Zabor EC, Stempel M, Morrow M, Barrio AV. Tumor Biology Predicts Pathologic Complete Response to Neoadjuvant Chemotherapy in Patients Presenting with Locally Advanced Breast Cancer. Ann Surg Oncol Dec 2017;24(13):3896–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villarreal-Garza C, Bargallo-Rocha JE, Soto-Perez-de-Celis E, et al. Real-world outcomes in young women with breast cancer treated with neoadjuvant chemotherapy. Breast Cancer Res Treat Jun 2016;157(2):385–394. [DOI] [PubMed] [Google Scholar]
- 19.Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg Oct 2014;260(4):608–614; discussion 614–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol May 20 2012;30(15):1796–1804. [DOI] [PubMed] [Google Scholar]
- 21.Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol Feb 2018;19(2):169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg SM, Ruddy KJ, Tamimi RM, et al. BRCA1 and BRCA2 Mutation Testing in Young Women With Breast Cancer. JAMA Oncol Jun 1 2016;2(6):730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couch FJ, Hart SN, Sharma P, et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol Feb 1 2015;33(4):304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fasching PA, Loibl S, Hu C, et al. BRCA1/2 Mutations and Bevacizumab in the Neoadjuvant Treatment of Breast Cancer: Response and Prognosis Results in Patients With Triple-Negative Breast Cancer From the GeparQuinto Study. J Clin Oncol Aug 1 2018;36(22):2281–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahnen E, Lederer B, Hauke J, et al. Germline Mutation Status, Pathological Complete Response, and Disease-Free Survival in Triple-Negative Breast Cancer: Secondary Analysis of the GeparSixto Randomized Clinical Trial. JAMA Oncol Oct 1 2017;3(10):1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wunderle M, Gass P, Häberle L, et al. BRCA mutations and their influence on pathological complete response and prognosis in a clinical cohort of neoadjuvantly treated breast cancer patients. Breast Cancer Res Treat Aug 2018;171(1):85–94. [DOI] [PubMed] [Google Scholar]
- 27.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol Jan 2018;19(1):40–50. [DOI] [PubMed] [Google Scholar]
- 28.Gao G, Wang Z, Qu X, Zhang Z. Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: a systematic review and meta-analysis. BMC Cancer Mar 4 2020;20(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loi S, Drubay D, Adams S, et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J Clin Oncol Mar 1 2019;37(7):559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg Sep 2015;262(3):434–439; discussion 438–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II-III HER2-positive breast cancer: surgical results of CALGB 40601 (Alliance). Breast Cancer Res Treat Nov 2016;160(2):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golshan M, Loibl S, Wong SM, et al. Breast Conservation After Neoadjuvant Chemotherapy for Triple-Negative Breast Cancer: Surgical Results From the BrighTNess Randomized Clinical Trial. JAMA Surg Mar 1 2020;155(3):e195410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petruolo O, Sevilimedu V, Montagna G, Le T, Morrow M, Barrio AV. How Often Does Modern Neoadjuvant Chemotherapy Downstage Patients to Breast-Conserving Surgery? Ann Surg Oncol Jan 2021;28(1):287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HJ, Dominici L, Rosenberg SM, et al. Surgical Treatment after Neoadjuvant Systemic Therapy in Young Women with Breast Cancer: Results from a Prospective Cohort Study. Ann Surg Dec 23 2020;Publish Ahead of Print. [DOI] [PubMed]
- 35.Criscitiello C, Curigliano G, Burstein HJ, et al. Breast conservation following neoadjuvant therapy for breast cancer in the modern era: Are we losing the opportunity? Eur J Surg Oncol Dec 2016;42(12):1780–1786. [DOI] [PubMed] [Google Scholar]
- 36.de Boniface J, Szulkin R, Johansson ALV. Survival After Breast Conservation vs Mastectomy Adjusted for Comorbidity and Socioeconomic Status: A Swedish National 6-Year Follow-up of 48 986 Women. JAMA Surg Jul 1 2021;156(7):628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


