ABSTRACT
Background
Nitrate (NO3−) has been suggested as a prebiotic for oral health. Evidence indicates dietary nitrate and nitrate supplements can increase the proportion of bacterial genera associated with positive oral health whilst reducing bacteria implicated in oral disease(s). In contrast, chlorhexidine-containing mouthwashes, which are commonly used to treat oral infections, promote dysbiosis of the natural microflora and may induce antimicrobial resistance.
Methods
A systematic review of the literature was undertaken, surrounding the effects of nitrate on the oral microbiota.
Results
Overall, n = 12 in vivo and in vitro studies found acute and chronic nitrate exposure increased (representatives of) health-associated Neisseria and Rothia (67% and 58% of studies, respectively) whilst reducing periodontal disease-associated Prevotella (33%). Additionally, caries-associated Veillonella and Streptococcus decreased (25% for both genera). Nitrate also altered oral microbiome metabolism, causing an increase in pH levels (n = 5), which is beneficial to limit caries development. Secondary findings highlighted the benefits of nitrate for systemic health (n = 5).
Conclusions
More clinical trials are required to confirm the impact of nitrate on oral communities. However, these findings support the hypothesis that nitrate could be used as an oral health prebiotic. Future studies should investigate whether chlorhexidine-containing mouthwashes could be replaced or complemented by a nitrate-rich diet or nitrate supplementation.
KEYWORDS: Nitrate, nitrite, nitric oxide, periodontal disease, chlorhexidine, antimicrobial resistance
Introduction
Dietary NO3− is a polyatomic ion that naturally occurs in vegetables, particularly leafy greens (lettuce, kale and spinach) and beetroots [1–6]. Additionally, NO3− is commonly added to processed meats for flavouring, preservation and antimicrobial purposes [2]. Evidence suggests NO3− added to processed meats can lead to the formation of carcinogenic nitrosamines [4]. However, ≈ 80% of dietary nitrate is obtained from vegetables which are considered anticarcinogenic [4,7]. Moreover, plant-based NO3− exerts positive effects on oral and systemic health [1,2,4]. NO3− consumption leads to the production of nitric oxide (NO), a bioactive molecule associated with host defence, neuronal communication, improved vascular and metabolic health, and improved exercise performance [1,4–7]. Importantly, NO is a potent vasodilator that regulates blood pressure (BP) and blood flow within tissues and organs. High NO3− diets are associated with a reduced risk of cardiovascular disease (CVD), diabetes and cognitive impairment, as increased NO bioavailability enhances the delivery of oxygen and nutrients to body systems [4,6]. Interestingly, NO can also be produced endogenously from L-arginine and NADPH in an oxygen-dependent reaction involving NO synthase enzymes (NOS), of which there are three main types (endothelial NOS (eNOS), inducible NOS (iNOS) and neuronal NOS (nNOS)) [8]. Unfortunately, endogenous NO production becomes less efficient with age [3–5]. However, dietary NO3− is reduced to NO via the nitrate-nitrite-nitric oxide pathway (NO3−-NO2−-NO) (Figure 1) [1,3,4,6]. This process is facilitated by nitrate-reducing bacteria (NRB) inside the oral cavity, including Rothia and Neisseria, which are associated with positive oral health [7,8]. High-nitrate diets have been shown to increase health-associated genera and decrease the number of disease-associated bacteria [7,8].
Figure 1.
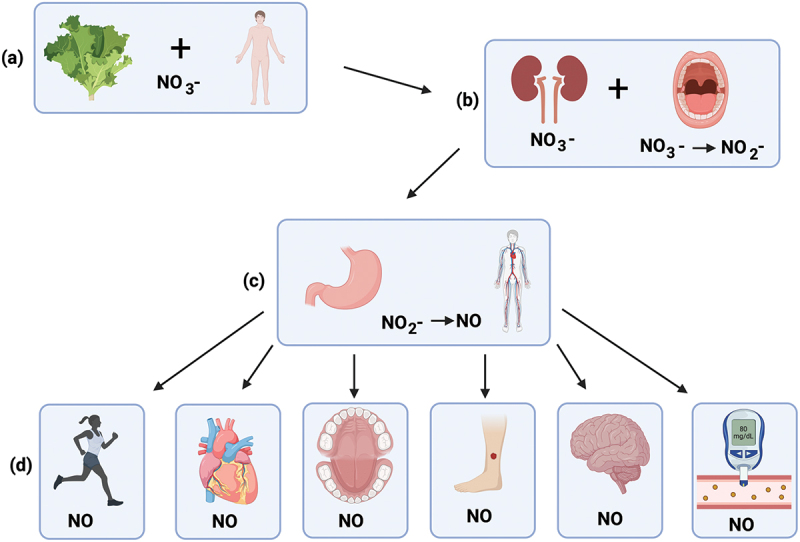
Schematic representation of the NO3−-NO2−-NO pathway (a) Nitrate-rich vegetables are consumed, NO3− enters the GI tract and is absorbed into the bloodstream (b) about 75% of NO3− is excreted in urine, whilst approximately 25% is concentrated by the salivary glands (facilitated by the transporter protein sialin) and is reduced to NO2− by bacteria inside the oral cavity (c) NO2− is then swallowed and reduced to NO in the stomach and/or other body tissues after entering the systemic circulation (d) NO induces health benefits, including improvements to exercise performance, cardiovascular health, oral health, wound healing, cognitive function and blood glucose levels (created with BioRender.com).
In contrast, some bacteria are linked to the development of dental caries (e.g. Lactobacillus and cariogenic representatives of Streptococcus, Veillonella and Actinomyces), whilst others are linked to the development of halitosis and periodontitis (e.g. Porphyromonas, Fusobacterium and Prevotella) [7,8]. Cariogenic bacteria ferment carbohydrates (e.g. glucose and sucrose) into organic acids (e.g. lactic acid) which can decrease oral pH and cause demineralisation of tooth enamel [6,7]. Studies have shown NO3− limits oral acidification via lactic acid/proton consumption during denitrification and nitrite reduction to ammonium by oral bacteria, which can prevent caries development [4,6–8]. Additionally, periodontal disease (PD) is a chronic oral infection that affects 20–50% of the global population [9–11]. PD is characterised by chronic inflammation, bleeding gums and destruction of the periodontium; the supporting tissue of the teeth [11]. Although the condition is multifactorial, complex biofilms (dental plaque) containing a large diversity of bacterial species contribute to PD manifestation and progression [7,9,10]. Interestingly, some studies have found PD patients have high concentrations of NO in their saliva and gingival crevicular fluid, potentially due to excessive iNOS activation driven by proinflammatory cytokines which can contribute to tissue damage [8,11]. In contrast, dietary NO3− appears to induce a controlled, low-level production of NO that is beneficial for oral health [7,8].
Treatment for oral disease(s) typically involves mechanical plaque removal and the use of chlorhexidine-containing mouthwashes (CHX) to reduce gingival inflammation [9,10]. Unfortunately, prolonged use of CHX mouthwash can disrupts the oral microbiota, displacing NO3− reducing species and lowering oral pH, in addition to negatively affecting the expected BP reduction following an NO3− dose [4,8]. Moreover, evidence suggests that antimicrobial resistance (AMR) is developing against antibacterial mouthwash, thus promoting cross-resistance to antibiotics [4,10,12]. Worryingly, annual deaths related to AMR are expected to reach 10 million yearly by 2050 [13]. Thus, the emergence of resistant strains can induce a life-threatening infection inside a compromised host and/or spread environmentally [10,13,14]. In terms of periodontitis, CHX resistance would enable disease-causing bacteria to increase and exacerbate oral disease progression [7,10].
Several studies have suggested that dietary NO3− should be investigated as a prebiotic for oral health, as a mechanism to prevent or treat oral disease(s) [4,7,15–18]. Prebiotics are food components that promote the growth of beneficial microorganisms [7]. Using NO3− as a prebiotic could reduce the overreliance on CHX mouthwashes by giving health-associated species a growth advantage and potentially limiting the growth of disease-associated species [4,7]. To address this hypothesis, a systematic review of the literature was performed with the scope to answer the following research question: What are the effects of nitrate on the oral microbiota?
Methodology
Overview
Evidence presented in this review to satisfy the research aim was derived from clinical and in vitro studies. A narrative synthesis will highlight the effects of NO3− on the oral microbiome.
Search strategy
This review was conducted following PRISMA (2020) guidelines [19]. Four databases were accessed (Table 1). All relevant papers came from peer-reviewed journal articles. The search criteria narrowly focused on the effects of NO3− on oral microorganisms. A date restriction was applied (2012–2022) to find the most up to date information relevant to the topic. Key words combined with Boolean operators and search strings were used to confine search results to the topic of interest (Table 1). Mendeley and Microsoft Excel were used for record keeping. A PRISMA diagram is shown in the Results section.
Table 1.
Search strategy used during systematic review.
| Main Concept | Keywords/key terms | Boolean Operators | Databases |
|---|---|---|---|
| Search Strategy | |||
| The effects of nitrate on oral microbiome composition and activity | nitrate effects impact “oral microbiome” “oral microbiota” |
AND OR |
Scopus NCBI Science Direct Google Scholar |
| Search string 1: (effects OR impact) AND (nitrate) AND (“oral microbiome” OR “oral microbiota”) | |||
Inclusion and exclusion criteria
Material to be included in the review: 1) Peer-reviewed in vitro and in vivo studies analysing the effect of NO3− on oral community composition and activity 2) Studies published between 2012–2022 3) no restrictions for country, participant gender, age and race.
Material to be excluded from the review: 1) Studies that did not analyse complex oral microbiota samples (saliva, tongue samples or dental plaque) 2) duplicate research papers 3) non-relevant articles and/or articles containing insufficient information to answer the research question 4) non-English language articles 5) book chapters, conference notes, reviews and theses 6) articles with no full-text access.
Quality assessment checks
Relevant studies were analysed against a Scottish Intercollegiate Guidelines Network flowchart (2022) to determine study type [20,21] (Appendix A). Studies were established to be randomised control trials (RCTs), Quasi-experiments (non-randomised control studies (non-RCS) and pre-post interventions), cross-sectional studies, and in vitro analyses. All studies were quality assessed against critical appraisal tools to determine validity and suitability.
Each RCT was quality assessed against two critical appraisal tools provided by the Center for evidence-Based Management and Critical Appraisal Skills Programme [22,23]. Quasi-experimental studies were critically appraised against tools provided by the British Medical Journal and The Joanna Briggs Institute [24,25]. Similarly, cross sectional studies were quality assessed against two appraisal tools provided by the Center for evidence-Based Management and STROBE [26,27]. Lastly, in vitro studies were critically appraised using a checklist provided by the University of Exeter and The QUIN tool [28,29] (See appendix B-I). All tools had a checklist format and contained key questions highlighted in Table 2.
Table 2.
Common quality assessment questions for RCTs, quasi-experiments, cross-sectional studies and in vitro analyses.
| Typical critical appraisal questions |
|---|
| Did the study have a focused research question? |
| Was the study design valid? |
| Was the sample size adequate and target population representative? |
| Was there a clear method of selection/randomisation and/or a control group? |
| Was the intervention/exposure clearly reported? |
| Were outcomes measured reliably and are results clear and unbiased? |
| Have confounders been identified? |
| Will the results help locally/target populations? |
Most tools consisted of optional answers, including ‘yes’, ‘no’, ‘unclear’ or ‘can’t tell”. Good quality studies should return a majority of ‘yes’ answers. If appraisal results caused uncertainty regarding inclusion or exclusion, the project team were consulted for a second opinion. Overall, selected papers had a good critical appraisal result.
Data collection and narrative synthesis
A summary table was created in Microsoft Word to store key information from each study (Table 3). Key trends, similarities, and differences between studies were explored and an overall conclusion relating to the research question was reached.
Table 3.
Studies assessing NO3− supplementation and oral ecology (2012–2022).
| First author, Year and journal | Study Type and Location | Aim/participant information | Laboratory investigation(s) | Results/Findings | Author’s Conclusions |
|---|---|---|---|---|---|
|
In vivo: randomised, double-blind, placebo-controlled parallel study (UK) |
The effects of dietary NO3− on vascular and platelet function, and the oral microbiome n = 67 hypercholesterolemic M and F participants Age: 18–80 yrs old BMI: 18.5–40 kg/m2 |
BR juice vs PL (6-wk, once-daily) Analyses: Vascular and platelet function, BP, saliva, urine and plasma NO3−/NO2− levels and oral microbiome (saliva) Methods: FMD, aPWV, portable BP device, flow cytometry, chemiluminescence and16s rRNA gene Illumina Sequencing |
In the treatment group: FMD and aPWV improved (p < 0.001 and p = 0.06, respectively); platelet monocyte aggregates significantly reduced (p < 0.004); Rothia mucilaginosa increased and Neisseria flavescens significantly increased (p < 0.01) |
Chronic dietary NO3− consumption improves vascular function in hypercholesterolemic individuals; changes are related to alterations in the oral microbiome/increased levels of NRB |
|
In vitro study (Netherlands) |
Assessing the effects of dietary NO3− on oral ecology and biochemistry MAM biofilm model inoculated with stimulated saliva from a healthy M and F donor Age: 20–30 yrs old |
n=4 microcosms received NO3−, n = 4 microcosms acted as control; all received weekly NO3− dose and sucrose pulse (4-wk exp) Analyses: NO3−, and NO2− concentrations, oral pH, short chain fatty acid production and bacterial composition Methods: Capillary electrophoresis, pH meter and 16s rRNA gene Illumina Sequencing |
In one donor, NO3− supplementation significantly increased Neisseria on a genus-level. In both donors, Neisseria OTUs were associated with NO3− supplementation and in one donor, Veillonella OTUs. Butyrate levels were lower after nitrate addition in NO3− receiving microcosms (p < 0.05) |
NO3− affects the composition/biochemistry of oral microbiomes, but changes vary depending on the inoculum (donor) |
|
In vivo: randomised, double-blind, cross-over trial (UK) |
Examining relationships between oral ecology and physiological indices of NO bioavailability, and assessing the impact of NO3− supplementation on these variables n = 18 normotensive M and F Age: 19–22 yrs old; 70–79 yrs old |
BR juice vs PL (10 days, twice-daily) Analyses: oral ecology (tongue and saliva), plasma, NO3− and NO2− concentrations, BP and arterial stiffness Methods: 16s rRNA gene Illumina Sequencing, chemiluminescence, automated sphygmomanometer MAP measurements and PWV |
NO3− supplementation increased NO3−/NO2− plasma concentrations (p < 0.05); Rothia/Neisseria genera increased and Prevotella/Veillonella decreased vs PL (p < 0.05). SBP and MAP were significantly lower in the older age group vs PL (p < 0.05) |
Dietary NO3− altered the oral microbiota and changes in oral ecology positively impacted NO homeostasis and vascular health |
|
In vivo: non-randomised, single blinded, cross-over trial (UK) | Evaluating dietary NO3− intake in vegetarians vs omnivores and the impact of AM on NO3− NO2 levels, RMR, BP and the oral microbiome. Healthy M and F participants n = 22 vegetarians n = 19 omnivores Age: 18–45 yrs old |
PL vs Corsodyl (2-wk trial) Analyses: RMR, BP, plasma and salivary NO3/NO2− concentrations, saliva pH, blood glucose and lactate levels, and oral ecology (saliva) Methods: RMR calculations, oscillometric device, chemiluminescence, biochemistry analyser, digital pH meter and 16s rRNA gene Illumina Sequencing |
No significant differences in NO3−/NO2− concentrations, NRC, and oral ecology between groups following PL (p > 0.05). AM decreased NO2− levels (p<0.05), NRC and oral pH, and increased salivary lactate and glucose levels in both groups (all p < 0.001) (Prevotella, Actinomyces, Rothia and Fusobacterium genera decreased (p < 0.05)) |
Dietary NO3− consumption in vegetarians vs omnivores was not statistically different. |
|
In vivo placebo-controlled, single-blind randomised cross over trial (UK) |
Analysing the effects of dietary NO3− on oral microbiome composition and NRC, and assessing vascular responses to acute NO3− ingestion. n = 11 healthy M Age: 30 ± 7 yrs old BM: 86.9 ± 14.1kg |
BR juice vs PL (7 days, twice daily) Analyses: plasma and salivary NO3−/NO2− concentrations, microbiome (tongue), oral pH, HR, BP and endothelial functioning Methods: chemiluminescence, 16s rRNA gene Illumina Sequencing, pH-meter, FMD, automated BP monitor, telemetry and MAP measurements |
Plasma/saliva NO3− and NO2− levels and salivary pH increased post NO3− supplementation (p<0.05). Prevotella, Actinomyces and Streptococcus genera decreased in the BR group; Neisseria subflava increased in both groups compared to baseline, but this increase was most significant for the BR group compared to PL (p = 0.001) |
Dietary NO3− altered the oral microbiome in favour of oral health, but changes did not enhance NRC or vascular responses to acute NO3− ingestion |
|
In vivo: before-and-after study (UK) | Analysing CVB, CVA and CD for plasma and saliva NO3−/NO2− concentrations pre and post-ingestion of BR juice, and assessing the CVB of NR bacteria on the tongue n = 10 Recreationally active M Age: 25 ± 5 yrs old BM: 81 ± 11 kg |
BR juice (3 identical trials, 6–10 days apart) Analyses: salivary, plasma and urine NO2− and NO3− concentrations, BP, MAP, microbiome (tongue) Methods: chemiluminescence, oscillometric device, MAP measurements and16s rRNA gene Illumina Sequencing |
Profound CVB variations in saliva and plasma NO markers, and NR bacteria at baseline vs post BR ingestion. Neisseria subflava was negatively associated with peak saliva/plasma NO2− levels; Rothia mucilaginosa and Haemophilus parainfluenzae were positively and negatively associated with between-trial peak saliva/plasma NO2− levels (all p < 0.05). BR ingestion reduced BP |
There is profound CVB in oral NR bacterial abundance on the tongue and NO metabolites in plasma/saliva pre and post-ingestion of BR |
|
In vitro study (Spain) |
Establishing the short-term effect of a growth medium containing NO3− dose on oral biofilm growth, composition and biochemistry n = 12 saliva samples from healthy donors |
Samples were incubated in nutrient-rich media with or without NO3− and examined at 5h and 9h Analyses: NO3−/NO2− levels, ammonium, lactate and pH measurements, protein and DNA quantification Methods: Reflectoquant reflectometer, Bradford protein assay and 16s rRNA gene Illumina Sequencing |
NO3− increased ammonium and pH levels, and decreased lactate production (all p<0.01) NO3− increased abundance of Neisseria and Rothia genera after 5h (p<0.01) and reduced Streptococcus, Oribacterium, Veillonella, Fusobacterium, Leptotrichia, Prevotella, Alloprevotella and Porphyromonas after 5 and/or 9h (p<0.05) |
NO3− significantly altered the oral environment to benefit the host and could serve as a prebiotic for oral health |
|
In vitro study (Spain) |
Isolating oral NR bacteria and assessing their in vitro probiotic capabilities Probiotic isolation: n = 5 healthy M and F donors Age: 23–32 yrs old Saliva donors: n = 9 healthy M and F donors Age: 23–45 yrs old |
6 Rothia sp. underwent in vitro biofilm tests (saliva biofilms). Analyses: NRC, bacterial identification; NO3−/NO2− levels, ammonium, lactate and pH measurements Methods: cell culture, Griess reaction, 16s rRNA gene Illumina Sequencing, Reflectoquant reflectometer and the Nessler method |
Biofilm NRC increased when adding isolates compared to controls (p<0.05). Adding NO3− or NO3− + isolates caused a smaller decrease in pH (p<0.05) and some isolates in combination with NO3−increased lactate consumption. A positive correlation between NRC and ammonium production was observed, even in samples without NO3− (p<0.01) |
Oral communities with reduced NRC could benefit from a NR probiotic or symbiotic combination (NO3− and NR probiotic) |
|
In vivo: randomised, placebo-controlled cross-over trial (UK) |
Assessing relationships between cognitive and physiological traits and clusters of co-occurring oral bacteria n = 30 elderly M and F Age: 70–80 yrs old |
BR juice vs PL (10 day trial) Analyses: plasma NO3−/NO2− levels, microbiome (saliva), cognitive function, BP, arterial stiffness, exercise assessments, muscle function, brain matter Methods: chemiluminescence, 16s rRNA gene Illumina Sequencing, WGCNA, cognitive function tests, BP measurement, PWV, MAP measurements, VO2 max, p-MRS of quadriceps and H-MRS of brain |
Rothia-Streptococcus microbiome module increased after NO3− supplementation and showed stable associations with cardiovascular indices of health; Neisseria-Haemophilus module increased and showed stable associations with cognitive indices of health. Co-occurring Prevotella-Veillonella diminished after NO3− ingestion | NO3− sensitive oral microbiome modules could be potential pre- and probiotic targets to modify age-associated cardiovascular and cognitive impairments |
|
In vivo: randomised control trial (USA) | Analysing the effects of NO3−/NO2− processed meat and nitrate-enriched drinking water on salivary microbial populations n = 63 healthy M and F volunteers Age: 18–70 yrs old BMI: 18–25 kg/m2 |
2 groups: 3 meat intervention phases and 1 meat intervention phase + nitrate-enriched water (7-week trial) Analyses: urinary NO3−/creatinine levels, microbiome (saliva) Methods: ion chromatography and mass spectrometry, Beckman clinical analyser (colorimetric method) and 16s rRNA gene Illumina Sequencing |
Urinary NO3− levels were reduced during the phytochemical-enriched-low- NO2− meat diet and poultry diet compared to baseline (p < 0.009). NO3− rich water induced changes to salivary microbiome (p < 0.001). Neisseria, Kingella, Lautropia, Alloprevotella and Haemophilus genera increased; Streptococcus decreased (FDR < 0.05) | Drinking NO3− enriched water increases oral NR bacteria abundance, which can subsequently increase NOC production |
|
In vivo: placebo-controlled, blinded, cross-over trial (study 1) and before-and-after study (study 2 & 3) (Spain) |
Evaluating the ability of NO3− to reduce oral acidification caused by sugar fermentation in vivo Study 1: n = 12 healthy M and F participants, age: 25–60 yrs old Study 2: n = 6 healthy M and F participants, age:24–46 yrs old Study 3: n = 6 healthy M and F participants, age: 25–33 yrs old |
Sucrose rinses 1, 2 and 4h after NO3− ingestion (BR vs placebo) Analyses: salivary NO3−/NO2− concentrations, microbiome (saliva), pH levels, lactate and ammonium measurements Methods: Reflectoquant reflectometer, 16s rRNA gene Illumina Sequencing, the Nessler method and lactate colorimetric assay |
Sucrose rinse 4hrs: NO3−supplementation reduced lactate production (p < 0.05), increased pH and Rothia genera abundance (p < 0.05). Relative abundances of Neisseria and Rothia negatively correlated with lactate production, and Neisseria abundance positively correlated with pH post sucrose intake (p < 0.05) |
NO3− can limit oral acidification induced by sugar fermentation, predominantly due to lactate usage by NR bacteria |
| In vivo: randomised control trial (Germany) | Evaluating the impact of a NO3−-rich diet on NO3− fermenting bacterial abundance within the pocket microbiota n = 37 periodontal recall patients with gingival inflammation and reduced periodontium |
lettuce juice vs PL (14 days, three times daily) Analyses: subgingival microbial samples (pre and post plaque removal) and salivary NO3−/NO2− levels Methods: anion exchange chromatography and16s rRNA gene Illumnia Sequencing |
No baseline differences in bacterial diversity between the BR and PL group (p > 0.05) NO3−supplementation induced significant increases in Rothia and Neisseria genera (p < 0.05) and significantly altered alpha- and beta- diversity (p < 0.05) compared to PL group |
NO3− supplementation in periodontal recall patients reduced gingival inflammation and induced significant changes to subgingival microbial populations; NO3− could be used as an adjunct therapy to plaque removal to control gingival inflammation |
M = male, F = female, BMI = body mass index, BR = beetroot, PL = placebo, wk = week, FMD = flow-mediated dilation, aPWV = aortic pulse wave velocity, BP=blood pressure, rRNA = ribosomal ribonucleic acid, NRB = nitrate reducing bacteria, MAM = multiplaque artificial mouth, exp = experiment, MAP = mean arterial pressure, PWV = pulse wave velocity, SBP = systolic blood pressure, AM = antibacterial mouthwash, RMR = resting metabolic rate, NRC = nitrate reducing capacity, HR = heart rate, CVB= within-person biological variation, CVA = analytical imprecision, CD = critical difference, NR = nitrate reducing, WGCNA = weighted gene co-expression network analysis, VO2 max = maximal oxygen consumption, p-MRS = phosphorus magnetic resonance spectroscopy, H-MRS = proton magnetic resonance spectroscopy, NOC = N-nitroso compounds, OTU = operational taxonomic unit.
Results
Prisma diagram and summary table
Applying the search strategy outlined in Section 2.3 returned 3133 potential articles (Figure 2). This total was reduced to 1943 after removing 1190 duplicates. Thereafter, title and abstract screening removed a further 1745 non-related articles, leaving 198 articles to be fully screened. Subsequently, a further 188 papers met the exclusion criteria (section 2.4). Thus, 10 articles satisfied the inclusion guidelines and featured in the review. Additionally, 2 further papers were found through screening the reference lists of eligible articles. Overall, 12 studies satisfied the inclusion criteria (Table 3).
Figure 2.
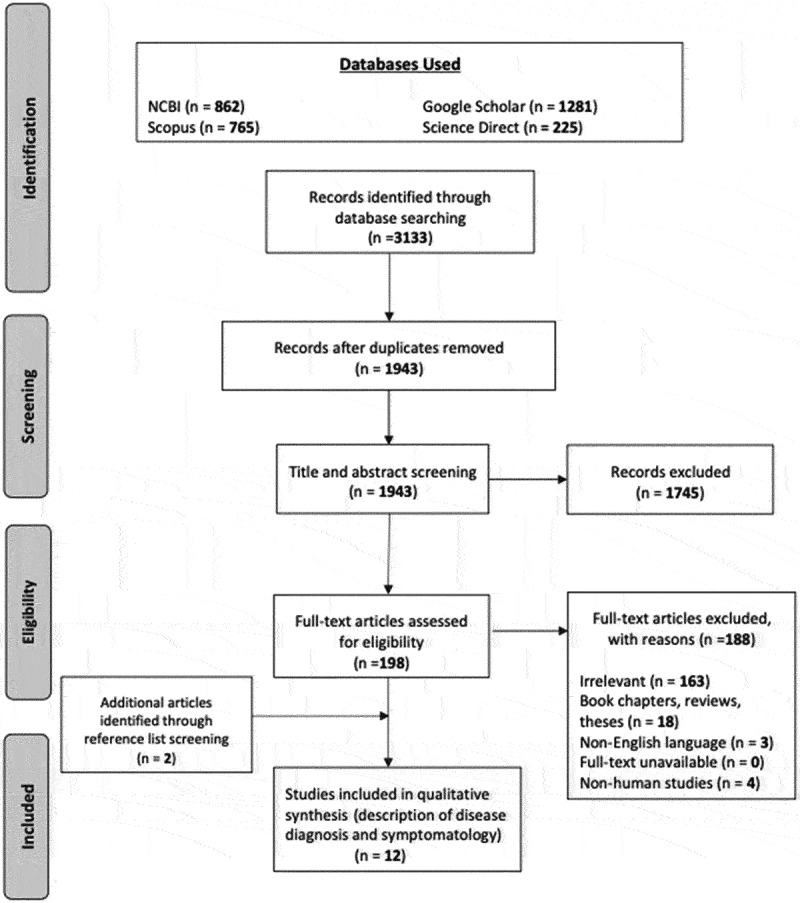
PRISMA flow diagram.
Study characteristics
Several study types were identified from this systematic approach: 7 were randomised control trials (RCTs), 3 were in vitro analyses and 2 demonstrated a Quasi experimental design (Table 4). Moreover, studies were highly variable by location. Additionally, 11 of the studies reported that NO3− induced oral microbiome changes, with 8 emphasising NO3− had a positive impact on oral health. Lastly, several studies (n=6) reported NO3−-associated systemic benefits, predominantly improvements to cardiovascular health.
Table 4.
Summary of study characteristic (n = 12).
| Study type RCTs in vitro Quasi exp (non-RCTs and pre-post interventions) |
7 3 2 |
| Location UK Spain USA Netherlands Germany |
6 3 1 1 1 |
| NO3− altered the oral microbiome Yes |
11 |
| impact on oral health Positive *Unspecified |
8 4 |
| NO3− and other health benefits CV health Cognitive health |
5 1 |
*studies were investigating the impact of NO3− induced oral microbiome changes on other parameters (e.g. cardiovascular health), and did not directly analyse the positive or negative impact of NO3− on oral health.
Laboratory investigations
The most common laboratory procedures for NO3−/NO2− quantification included chemiluminescence, chromatography, the Griess colorimetric method and the RQflex® reflectometer. Additionally, 16s rRNA gene Illumnia Sequencing was predominantly used for bacteria identification/quantification. Typically, the V3-V4 region of the 16s rRNA gene was amplified, using, the 341F and 806R or 805R primer set (Table 5).
Table 5.
Most common laboratory procedures across all studies.
| NO3− and NO2− Quantification | Bacterial ID/quantification (primers) |
|---|---|
| Chemiluminescence, The Griess colorimetric method, chromatography and Reflectoquant RQflex® | 16s rRNA gene Illumnia Sequencing (V3-V4 region) |
| 341F (forward primer): 5’-CCTACGGGNGGCWGCAG-3’ | |
| 805R (reverse primer): 5’-GACTACHVGGGTATCTAATCC-3’ | |
| 806R (reverse primer): 5’-GGACTACHVGGGTWTCTAAT-3’ |
Bacteria constituents
Several bacterial genera were identified across all studies, including gram-positive and gram-negative genera, with and without nitrate producing capacity (NPC). Moreover, some genera contained spp. that are associated with oral disease and dental caries (Table 6).
Table 6.
Bacterial taxa identified across all studies.
| Genus | Species | NPC | Disease-associated* |
|---|---|---|---|
| Gram positive | |||
|
Lactobacillus Streptococcus Rothia Actinomyces Oribacterium |
mucilaginosa | Yes Yes Yes |
Dental caries Dental caries Dental caries |
| Gram negative | |||
|
Neisseria Veillonella Prevotella Fusobacterium Haemophilus Leptotrichia Alloprevotella Porphyromonas Kingella Lautropia |
flavescens/subflava parainfluenzae |
Yes Yes Yes Yes Yes |
Dental caries Periodontitis Periodontitis Periodontitis Periodontitis Periodontitis |
A search was performed on Bacterial Feature Finder (http://csbg.cnb.csic.es/BaFF) to determine microbiological characteristics [39]. NPC, and disease status were determined based on recent findings in the literature [4,6–8,17,38]. NPC = nitrite-production capacity, meaning bacteria have been found to produce nitrite in the presence of nitrate [6]. *Disease-associated on a genus level. Note that some genera are clearly disease-associated (i.e. they generally increase in caries or periodontitis) but can contain health-associated species and strains, thus, future studies should test oral microbiome changes to at least species-level.
Nitrate supplementation induced modifications in oral communities
Post NO3− supplementation, there is an increase in bacterial spp. associated with good oral health, whilst a decrease is observed in genera linked to oral disease (Table 7). For instance, Neisseria and Rothia increased the most, at 67% and 58%, respectively (Table 6). In terms of percentage decreases, Prevotella and Veillonella decreased the most across all studies, at 33% and 25%, respectively.
Table 7.
Bacterial population increase versus decrease post NO3− supplementation.
| Bacterial genera that increase | Percent of studies (n = 12) |
|---|---|
| Neisseria (incl. flavescens and subflava spp.) | 67% |
| Rothia (incl. mucilaginosa sp.) | 58% |
| Haemophilus | 17% |
| Alloprevotella/Prevotella | 8% |
| Veillonella | 8% |
| Kingella | 8% |
| Lautropia | 8% |
| Streptococcus | 8% |
| Bacterial genera that decrease | Percent of studies (n = 12) |
| Alloprevotella/Prevotella | 33% |
| Veillonella | 25% |
| Streptococcus | 25% |
| Fusobacterium | 8% |
| Oribacterium | 8% |
| Porphyromonas | 8% |
| Leptotrichia | 8% |
| Actinomyces | 8% |
| Haemophilus parainfluenza | 8% |
| Neisseria subflava | 8% |
Additionally, eleven studies reported an increase in NRB associated with eubiosis, whilst five studies reported a decrease in bacterial species associated dysbiosis (Table 8). Interestingly, five studies reported both an increase in NRB associated with eubiosis and a decrease in bacterial genera associated with dysbiosis. Lastly, five studies reported oral pH changes post NO3− treatment.
Table 8.
NO3− supplementation and oral community composition/activity.
| Impact of NO3− supplementation on oral health | Studies (n = 12) | Ref. |
|---|---|---|
| Studies reporting an increase in NRB associated with eubiosis | 11 | [7,15,17,30–32,34–38] |
| Studies reporting a decrease in bacterial species associated with dysbiosis | 5 | [7,15,32,36,37] |
| Studies reporting an increase in NRB associated with eubiosis and decrease in bacterial species associated with dysbiosis | 5 | [7,15,32,36,37] |
| Studies reporting an increase in salivary pH levels and/or lactate consumption | 5 | [7,15,31,35,38] |
Additional findings
Five studies reported other in vivo health benefits, including improvements to cardiovascular and cognitive health (Table 9). Moreover, one study emphasised that NO3− supplementation could be used for plaque control or to treat periodontitis. Lastly, a study discussed the detrimental impact of antiseptic mouthwash on oral communities (Table 9).
Table 9.
NO3− supplementation and other health outcomes.
Discussion
NO3− promotes eubiosis inside the oral cavity
NO3− supplementation increased oral health-associated NRB, particularly Rothia and Neisseria, whilst reducing generally disease-associated Prevotella, Veillonella and Streptococcus (Table 7). Collectively, most studies (n=11) reported an increase in NRB associated with eubiosis and/or a decrease in bacterial spp. associated with dysbiosis, post NO3− supplementation (Table 8). Eubiosis refers to a balanced symbiotic relationship between oral microbes [40–42]. In contrast, dysbiosis occurs when insults to oral ecology, such as sugar consumption, smoking, poor dental hygiene or antimicrobial mouthwash, disrupt this commensal relationship, which causes disease-associated bacteria to increase [41–46]. In PD, dysbiosis activates an excessive inflammatory response that destroys host tissues, while in dental caries, dysbiosis can contribute to acidification and enamel demineralisation [11,41]. Overall, NO2− and NO production via the NO3−-NO2−-NO pathway promotes microbial homeostasis by exerting antibacterial effects against certain disease-associated bacteria, potentially maintaining a balanced microenvironment that lowers oral disease risk [8]. Thus, findings from this review indicate NO3− can alter the oral microbiota in favour of oral health, highlighting that it may have a protective role against the development of oral disease, or the potential to be used as a therapeutic intervention.
Additionally, studies that measured oral pH (n=5) found acute and chronic NO3− consumption beneficially altered oral biochemistry, by increasing salivary pH levels and/or enhancing lactic acid/lactate consumption [7,15,31,35,38]. These findings coincide with other studies which have shown acute increases in oral pH post NO3− consumption [18,47]. An increase in pH combined with a decrease in Veillonella and Streptococcus is a positive change from a caries perspective; Streptococcus is a carbohydrate fermenting genus whilst Veillonella consumes the lactic acid produced by Streptococci, thus causing a consistent increase in both genera in the supragingival plaque of individuals with caries [48]. Nevertheless, these genera also contain health-associated species (e.g. S. dentisani) that can decrease during caries development [49]. Future studies should explore the effects of NO3− on the oral microbiota at species level. Overall, oral bacteria consume protons and lactic acid during denitrification and nitrite reduction to ammonium which increases pH levels, limits oral acidification and inhibits the growth of cariogenic representatives [7,35,38].
Importantly, one study in this review emphasised that NO3− could be used as a therapeutic intervention for plaque control or periodontal treatment [17]. Jockel-Schneider et al., [16,17] found lettuce juice consumption, coupled with gingival debridement, in PD patients reduced gingival inflammation and significantly altered the subgingival microbiome to benefit oral health; NR Rothia and Neisseria genera significantly increased. Changes were associated with a significant increase in mean salivary NO3− levels. Thus, NO3− supplementation should be explored as an adjunct therapy to treat periodontitis [17]. This idea is supported by a recent study in which subgingival plaque of PD patients was grown in vitro and NO3− decreased biofilm formation, the levels of periodontitis-associated species and the dysbiosis index [50]. Overall, NO3− alters the oral environment to benefit the host, which makes it a strong candidate as a prebiotic for oral health (|Figure 3, left side) [7,8,16,17].
Figure 3.
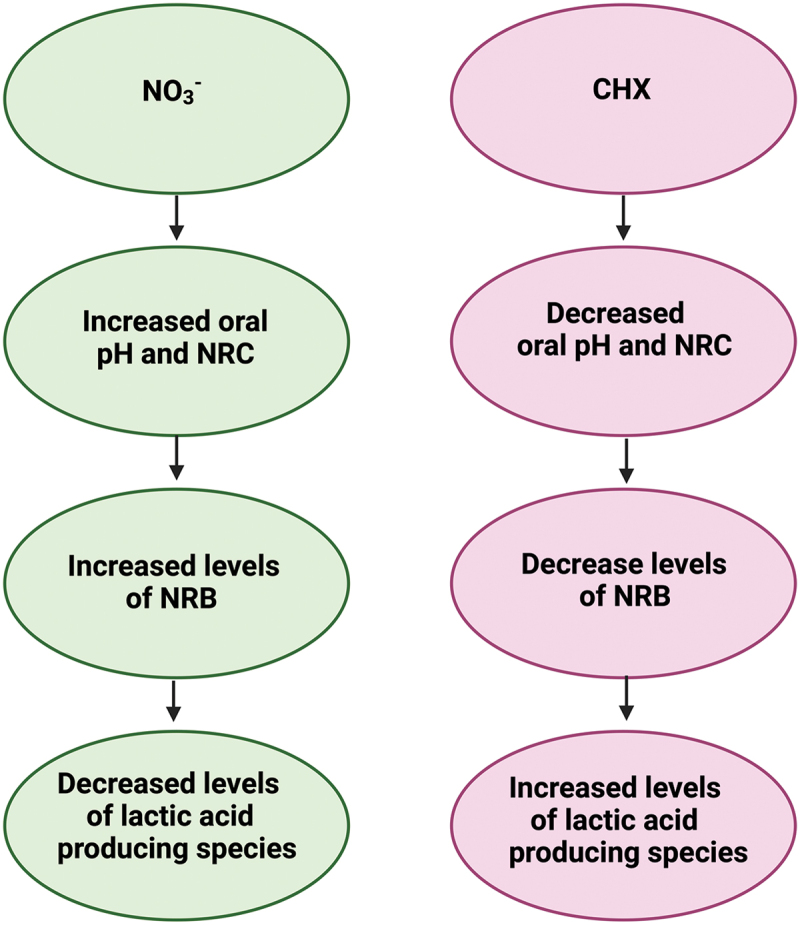
The effects of NO3− vs CHX on the oral environment: (left) summary based on findings in this review (right) summary based on (limited) current literature examining the effects of CHX on the oral environment (created with BioRender.com).
Advantages of NO3− supplementation over the use of chlorhexidine mouthwash
CHX inhibits plaque formation and exerts antibacterial action against various gram-positive and gram-negative bacteria [45,51,52]. In this review, Ashworth et al., [33] showed CHX mouthwash decreased NRC and oral pH, whilst significantly increasing salivary lactate and glucose levels. Other studies have reported similar findings (Figure 3, right side). Chatzigiannidou et al., [53] found that prolonged use of CHX-containing mouthwash altered salivary pH and increased lactic-acid producing species. Moreover, Bescos et al., [45] showed 7-day use of a CHX mouth rinse increased Streptococcus spp. abundance and reduced levels of Actinomyces spp., whilst also decreasing salivary pH and increasing lactate/glucose levels. Additionally, studies have shown CHX-containing mouth rinses are inducing resistance. Kulik et al., [54] detected two- to fourfold increases in minimum inhibitory concentrations (MIC’s) of P. gingivalis against sub-inhibitory concentrations of CHX after ≈ 30 passages. Wang et al., [55] found CHX induced one to two-fold MIC increases in F. nucleatum and P. gingivalis. Thus, CHX-containing mouthwash can induce adaptation in oral bacteria and can also facilitate the development of cross-resistance to other antiseptics, including antibiotics, thus exacerbating AMR occurrence [10,12,52]. In contrast, NO3− exerts antibacterial action through NO2− and NO production, and resistance has not yet been observed [56]. Moreover, NO is significantly less toxic to human gingival fibroblasts than clinical concentrations of CHX [56,57]. Figure 3 compares and contrasts the effects of NO3− and CHX on the oral environment.
NO3− and secondary findings
As discussed, NO3− reduction improves both oral and systemic health [1,4,6]. NO is a well-known vasodilator that decreases arterial stiffness and blood pressure [5,58–61]. The positive effects of NO on blood pressure have been well documented [1,3,4,40,62–64]. Webb et al., [62] showed a dose of beetroot juice (500ml) could significantly reduce systolic blood pressure (SBP) and diastolic blood pressure (DBP) by ≈ 10 mm Hg and ≈ 8 mm Hg, respectively. Moreover, Kenjale et al., [63] showed beetroot juice could significantly reduce DBP in subjects with peripheral arterial disease vs placebo. Similarly, studies have shown NO has positive effects on vascular function. Endothelial dysfunction is associated with arterial stiffness and impaired blood flow [61,64]. Acute (2–6 hours; 68–583 mg) and chronic (7–42 days; 300–650 mg/d) NO3− consumption can significantly reduce arterial stiffness [59]. Additionally, another study showed consuming spinach soup for 7 days significantly reduced arterial stiffness and BP [65]. In contrast, CHX mouthwash has been shown to negate the BP lowering effects of NO3− [4].
In this review, five studies emphasised that oral microbiome changes were associated with cardiovascular health improvements, namely improvements to BP, mean arterial pressure (MAP), flow-mediated dilation (FMD), pulse wave velocity (PWV) and platelet aggregation [15,30,32,34,36]. Vanhatalo et al., [32] showed that dietary NO3− supplementation reduced BP and improved MAP in older participants, whilst another clinical study suggested that NO3− sensitive microbial modules could serve as pre and probiotic targets to alter age-associated cardiovascular impairments [32,36]. A lowering of 5 mmHg in SBP can reduce the risk of CVD death caused by stroke and heart disease by 14% and 9%, respectively [5]. Furthermore, two studies reported improvements to FMD and PWV following NO3− consumption, whilst another trial recorded BP lowering effects [15,30,34]. Additionally, one study reported NO3− supplementation significantly lowered platelet aggregation [30]. Thus, evidence suggests dietary NO3− has cardioprotective benefits which are associated with oral microbiome-related changes post NO3− ingestion.
As previously highlighted, non-plant-based sources of NO3− and NO2− (processed meats and drinking water) can induce carcinogenesis and, in infants, increase the risk of methemoglobinemia [2,66]. A study in this review emphasised that organic sources of NO3− and NO2− can cause the formation of carcinogenic N-nitroso compounds (NOCs) linked to colorectal cancer [37]. However, high levels of antioxidants in vegetables counteract nitrosation [4]. Furthermore, an individual’s daily NO3− intake is mostly derived from fruits and vegetables and generally exceeds acceptable daily intake levels (ADI’s) (3.7mg NO3−/kg/day), as established by The World Health Organisation [2,66]. Given the positive systemic and oral health benefits of inorganic NO3−, perhaps ADI’s should be revaluated to reflect NO3−/NO2− source.
NO3−as a prebiotic for oral health
Throughout, connections between dietary NO3− consumption and positive oral health have been highlighted. In total, n=11 studies showed NO3− supplementation increased NR health-associated bacteria and/or reduced levels of common oral pathogens, thus establishing eubiosis and reducing oral disease risk [7,15,17,30–32,34–38]. Additionally, n=5 studies showed NO3− consumption modified oral biochemistry by increasing pH levels and reducing lactate production, thus also minimising dysbiosis [7,15,31,35,38]. Lastly, one study showed NO3− decreased gingival inflammation in patients with chronic gingivitis [16,17].
In contrast, evidence suggests CHX-containing mouthwashes used for periodontal treatment could stimulate dysbiosis and caries development, and could be exacerbating AMR [10,12,51,54,55]. Given the positive effects of vegetable-based sources of NO3− and the low risk of emerging NO resistance in oral pathogens [56], evidence in this review indicates NO3− could be used as an effective prebiotic to promote oral health. Overall, NO3− could serve as a preventative strategy against PD in high-risk groups or as a therapeutic intervention in individuals with PD (Figure 4). Although more studies are required to confirm the effects of NO3− and secondary metabolites on oral commensals, evidence provided in this review strongly indicates that NO3− could be used to simulate eubiosis and oral health.
Figure 4.
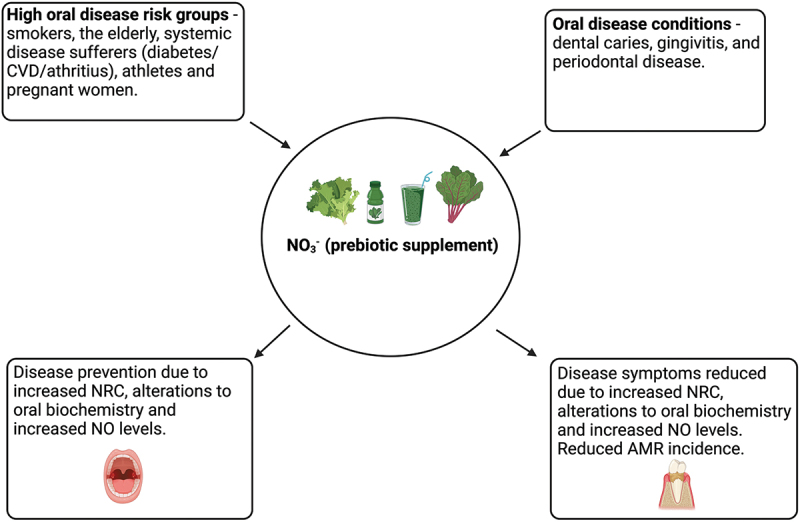
NO3− as a prebiotic for oral health. NO3− supplementation could be used in high risk groups to prevent oral disease (left side) or be used as a treatment method for individuals with oral disease(s) (right side) (created with BioRender.com).
Conclusion
This review has evaluated the impact of NO3− on the oral microbiome. The findings indicate that dietary NO3− increases oral health-associated bacterial genera, including Rothia and Neisseria, whilst reducing bacteria implicated in oral disease, including Prevotella and Veillonella spp. NO3− also beneficially alters the biochemical environment, by increasing oral pH and reducing dental caries risk. Additionally, the link between oral and systemic benefits was highlighted, particularly in relation to NO3− associated cardioprotective benefits. Further in vivo studies are required to uncover the mechanisms underlying the beneficial effects of NO3− on the oral microbiome; however, the evidence presented in this review indicates dietary NO3− could be used as a prebiotic for oral health.
Supplementary Material
Funding Statement
This research received no external funding.
Highlights
This systematic review evaluated the effects of nitrate on the oral microbiome.
Dietary nitrate and nitrate supplements increased oral health-associated genera, particularly Rothia and Neisseria.
The genus Prevotella, which is normally associated with periodontal disease and halitosis, decreased post-nitrate consumption.
Nitrate also increased oral pH, while decreasing the levels of Streptococcus and Veillonella, which is a positive change from a caries perspective.
Oral health benefits were linked to systemic benefits, particularly improvements to markers of cardiovascular disease risk.
Overall, nitrate could be considered as a prebiotic for oral health.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20002297.2024.2322228
References
- [1].Shannon OM, Easton C, Shepherd AI, et al. Dietary nitrate and population health: a narrative review of the translational potential of existing laboratory studies. BMC Sports Sci Med Rehabil. 2021;13(1):1–14. doi: 10.1186/s13102-021-00292-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Martin K. Dietary nitrates, nitrites, and food safety: Risks versus benefits. Act Scie Nutr. 2021;5(6):65–76. doi: 10.31080/ASNH.2020.05.0884 [DOI] [Google Scholar]
- [3].Stanaway L, Rutherfurd-Markwick K, Page R, et al. Performance and health benefits of dietary nitrate supplementation in older adults: a systematic review. Nutrients. 2017;9(11):1171. doi: 10.3390/nu9111171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bryan NS, Burleigh MC, Easton C. The oral microbiome, nitric oxide and exercise performance. Nitric Oxide. 2022;125-126:23–30. doi: 10.1016/j.niox.2022.05.004 [DOI] [PubMed] [Google Scholar]
- [5].McDonagh STJ, Wylie LJ, Thompson C, et al. Potential benefits of dietary nitrate ingestion in healthy and clinical populations: a brief review. Eur J Sport Sci. 2019;19(1):15–29. doi: 10.1080/17461391.2018.1445298 [DOI] [PubMed] [Google Scholar]
- [6].Rosier BT, Takahashi N, Zaura E, et al. The importance of nitrate reduction for oral health. J Dent Res. 2022;101(8):887–897. doi: 10.1177/00220345221080982 [DOI] [PubMed] [Google Scholar]
- [7].Rosier BT, Buetas E, Moya-Gonzalvez EM, et al. Nitrate as a potential prebiotic for the oral microbiome. Sci Rep. 2020;10(1). doi: 10.1038/s41598-020-69931-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morou-Bermúdez E, Torres-Colón JE, Bermúdez NS, et al. Pathways linking oral bacteria, nitric oxide metabolism, and health. J Dent Res. 2022;101(6):623–631. doi: 10.1177/00220345211064571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim). 2017;11(2):72–80. [PMC free article] [PubMed] [Google Scholar]
- [10].Saleem HG, Seers CA, Sabri AN, et al. Dental plaque bacteria with reduced susceptibility to chlorhexidine are multidrug resistant. BMC Microbiol. 2016;16(1):1–9. doi: 10.1186/s12866-016-0833-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Paul O, Arora P, Mayer M, et al. Inflammation in periodontal disease: possible link to vascular disease. Front Physiol. 2021;11. doi: 10.3389/fphys.2020.609614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Verspecht T, Rodriguez Herrero E, Khodaparast L, et al. Development of antiseptic adaptation and cross-adaptation in selected oral pathogens in vitro. Sci Rep. 2019;9(1). doi: 10.1038/s41598-019-44822-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Murray CJL, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet (London, England). 2022;399(10325):629–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309–318. doi: 10.1179/2047773215Y.0000000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Burleigh M, Liddle L, Muggeridge DJ, et al. Dietary nitrate supplementation alters the oral microbiome but does not improve the vascular responses to an acute nitrate dose. Nitric Oxide. 2019;89:54–63. doi: 10.1016/j.niox.2019.04.010 [DOI] [PubMed] [Google Scholar]
- [16].Jockel-Schneider Y, Goßner SK, Petersen N, et al. Stimulation of the nitrate-nitrite-NO-metabolism by repeated lettuce juice consumption decreases gingival inflammation in periodontal recall patients: a randomized, double-blinded, placebo-controlled clinical trial. J Clinic Periodontol. 2016;43(7):603–608. doi: 10.1111/jcpe.12542 [DOI] [PubMed] [Google Scholar]
- [17].Jockel-Schneider Y, Schlagenhauf U, Stölzel P, et al. Nitrate-rich diet alters the composition of the oral microbiota in periodontal recall patients. J Periodontol. 2021;92(11):1536–1545. doi: 10.1002/JPER.20-0778 [DOI] [PubMed] [Google Scholar]
- [18].Hohensinn B, Haselgrübler R, Müller U, et al. Sustaining elevated levels of nitrite in the oral cavity through consumption of nitrate-rich beetroot juice in young healthy adults reduces salivary pH. Nitric Oxide. 2016;60:10–15. doi: 10.1016/j.niox.2016.08.006 [DOI] [PubMed] [Google Scholar]
- [19].PRISMA . PRISMA 2020 checklist; 2021. [cited 2022 Nov 1]. Available from: http://www.prisma-statement.org
- [20].Scottish Intercollegiate Guidelines Network . Algorithm for classifying study design for questions of effectiveness; 2021. [cited 2022 Nov 2022]. Available from: https://www.sign.ac.uk/assets/study_design.pdf
- [21].University of Strathclyde . Systematic Review Steps; 2022. [cited 2022 Nov 1]. Available from: https://guides.lib.strath.ac.uk/systematic/steps
- [22].Centre for Evidence-Based Management . Critical Appraisal Of a Controlled Study; 2019. [cited 2022 Nov 2]. Available from: https://cebma.org/resources-and-tools/what-is-critical-appraisal/
- [23].Critical Appraisal Skills Programme . CASP Randomised Controlled Trial Checklist; 2022. [cited 2022 Nov 2]. Available from: https://casp-uk.net/casp-tools-checklists/
- [24].Greenhalgh T, Robert G, Bate P, et al. Diffusion of innovations in health service organisations a systematic literature review: appendix 2, critical appraisal checklists. London: BMJ. 2005. [Google Scholar]
- [25].The Joanna Briggs Institute . Checklist For Quasi-Experimental Studies; 2022. [cited 2022 Nov 2]. Available from: https://jbi.global/critical-appraisal-tools
- [26].Centre for Evidence-Based Management . Critical Appraisal Checklist For Cross-Sectional Study; 2014. [cited 2022 Nov 2]. Available from: https://cebma.org/wp-content/uploads/Critical-Appraisal-Questions-for-a-Cross-Sectional-Study-July-2014-1.pdf
- [27].Strengthening the reporting of observational studies in epidemiology. STROBE Checklist For Cross-Sectional Studies; 2022. [cited 2022 Nov 2]. Available from: https://www.strobe-statement.org/checklists/
- [28].University of Exeter Library . Checklist For In Vitro Studies; 2022. [cited 2022 Nov 2]. Available from: https://libguides.exeter.ac.uk/c.php?g=660317&p=4708597
- [29].Sheth VH, Shah NP, Jain R, et al. Development and validation of a risk-of-bias tool for assessing in vitro studies conducted in dentistry: the QUIN. J Prosthet Dent. 2022. doi: 10.1016/j.prosdent.2022.05.019 [DOI] [PubMed] [Google Scholar]
- [30].Velmurugan S, Gan JM, Rathod KS, et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr. 2016;103(1):25–38. doi: 10.3945/ajcn.115.116244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Koopman JE, Buijs MJ, Brandt BW, et al. Nitrate and the origin of saliva influence composition and short chain fatty acid production of oral microcosms. Microb Ecol. 2016;72(2):479–492. doi: 10.1007/s00248-016-0775-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vanhatalo A, Blackwell JR, L’Heureux JE, et al. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic Biol Med. 2018;124:21–30. doi: 10.1016/j.freeradbiomed.2018.05.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ashworth A, Cutler C, Farnham G, et al. Dietary intake of inorganic nitrate in vegetarians and omnivores and its impact on blood pressure, resting metabolic rate and the oral microbiome. Free Radic Biol Med. 2019;138:63–72. doi: 10.1016/j.freeradbiomed.2019.05.010 [DOI] [PubMed] [Google Scholar]
- [34].Liddle L, Burleigh MC, Monaghan C, et al. Variability in nitrate-reducing oral bacteria and nitric oxide metabolites in biological fluids following dietary nitrate administration: An assessment of the critical difference. Nitric Oxide. 2019;83:1–10. doi: 10.1016/j.niox.2018.12.003 [DOI] [PubMed] [Google Scholar]
- [35].Rosier BT, Moya-Gonzalvez EM, Corell-Escuin P, et al. Isolation and Characterization of Nitrate-Reducing Bacteria as Potential Probiotics for Oral and Systemic Health. Front Microbiol. 11. doi: 10.3389/fmicb.2020.555465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vanhatalo A, L’Heureux JE, Kelly J, et al. Network analysis of nitrate-sensitive oral microbiome reveals interactions with cognitive function and cardiovascular health across dietary interventions. Redox Biol. 2021;41:101933. doi: 10.1016/j.redox.2021.101933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sinha R, Zhao N, Goedert JJ, et al. Effects of processed meat and drinking water nitrate on oral and fecal microbial populations in a controlled feeding study. Environ Res. 2021;197:111084. doi: 10.1016/j.envres.2021.111084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rosier BT, Palazón C, García-Esteban S, et al. A single dose of nitrate increases resilience against acidification derived from sugar fermentation by the oral microbiome. Front Cell Infect Microbiol. 2021;11:692883. doi: 10.3389/fcimb.2021.692883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].López-Ibáñez J, Martín LT, Chagoyen M, et al. Bacterial feature finder (BaFF)-a system for extracting features overrepresented in sets of prokaryotic organisms. Bioinformatics (Oxford Academia). 2019;35(18):3482–3483. doi: 10.1093/bioinformatics/btz099 [DOI] [PubMed] [Google Scholar]
- [40].Pignatelli P, Fabietti G, Ricci A, et al. How periodontal disease and presence of nitric oxide reducing oral bacteria can affect blood pressure. Int J Mol Sci. 2020;21(20):7538. doi: 10.3390/ijms21207538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Radaic A, Kapila YL. The oralome and its dysbiosis: new insights into oral microbiome-host interactions. Computat Struct Biotechnol J. 2021;19:1335–1360. doi: 10.1016/j.csbj.2021.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Miyoshi T, Oge S, Nakata S, et al. Gemella haemolysans inhibits the growth of the periodontal pathogen porphyromonas gingivalis. Sci Rep. 2021;11(1). doi: 10.1038/s41598-021-91267-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhou P, Manoil D, Belibasakis GN, et al. Veillonellae: beyond bridging species in oral biofilm ecology. Front Oral Health. 2021;2. doi: 10.3389/froh.2021.774115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Acar B, Çağlayan F, İnkaya AÇ, et al. Actinomyces-associated lesions located in the gingiva: case report of rare gingival lesions. Contemp Clin Dent. 2017;8(1):182–184. doi: 10.4103/0976-237X.205067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bescos R, Ashworth A, Cutler C, et al. Effects of chlorhexidine mouthwash on the oral microbiome. Sci Rep. 2020;10(1). doi: 10.1038/s41598-020-61912-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liu T, Chen Y-C, Jeng S-L, et al. Short-term effects of chlorhexidine mouthwash and Listerine on oral microbiome in hospitalized patients. Front Cell Infect Microbiol. 2023;13:13. doi: 10.3389/fcimb.2023.1056534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Burleigh MC, Sculthorpe N, Henriquez FL, et al. Nitrate-rich beetroot juice offsets salivary acidity following carbohydrate ingestion before and after endurance exercise in healthy male runners. PLoS One. 2020;15(12):e0243755. doi: 10.1371/journal.pone.0243755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mashima I, Nakazawa F, O’Toole GA. The interaction between streptococcus spp. And veillonella tobetsuensis in the early stages of oral biofilm formation. J Bacteriol. 2015;197(13):2104–2111. doi: 10.1128/JB.02512-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].López-Santacruz HD, López-López A, Revilla-Guarinos A, et al. Streptococcus dentisani is a common inhabitant of the oral microbiota worldwide and is found at higher levels in caries-free individuals. Int Microbiol. 2021;24(4):619–629. doi: 10.1007/s10123-021-00222-9 [DOI] [PubMed] [Google Scholar]
- [50].Mazurel D, Carda-Diéguez M, Langenburg T, et al. Nitrate and a nitrate-reducing Rothia aeria strain as potential prebiotic or synbiotic treatments for periodontitis. NPJ Biofilms Microbiomes. 2023;9(1):40. doi: 10.1038/s41522-023-00406-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pałka Ł, Nowakowska-Toporowska A, Dalewski B. Is chlorhexidine in Dentistry an Ally or a foe? A narrative review. Healthcare. 2022;10(5):764. doi: 10.3390/healthcare10050764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Brookes ZLS, Belfield LA, Ashworth A, et al. Effects of chlorhexidine mouthwash on the oral microbiome. J Dent. 2021;113:103768. doi: 10.1016/j.jdent.2021.103768 [DOI] [PubMed] [Google Scholar]
- [53].Chatzigiannidou I, Teughels W, Van de Wiele T, et al. Oral biofilms exposure to chlorhexidine results in altered microbial composition and metabolic profile. NPJ Biofilms Microbiomes. 2020;6(1):13. doi: 10.1038/s41522-020-0124-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kulik EM, Waltimo T, Weiger R, et al. Development of resistance of mutans streptococci and porphyromonas gingivalis to chlorhexidine digluconate and amine fluoride/stannous fluoride-containing mouthrinses, in vitro. Clin Oral Invest. 2015;19(6):1547–1553. doi: 10.1007/s00784-014-1379-y [DOI] [PubMed] [Google Scholar]
- [55].Wang S, Wang H, Ren B, et al. Do quaternary ammonium monomers induce drug resistance in cariogenic, endodontic and periodontal bacterial species? Dental Mater. 2017;33(10):1127–1138. doi: 10.1016/j.dental.2017.07.001 [DOI] [PubMed] [Google Scholar]
- [56].Nambu T, Wang D, Mashimo C, et al. Nitric Oxide Donor Modulates a Multispecies Oral Bacterial Community—An In Vitro Study. Microorganisms. 2019;7(9):353. doi: 10.3390/microorganisms7090353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Backlund CJ, Sergesketter AR, Offenbacher S, et al. Antibacterial efficacy of exogenous nitric oxide on periodontal pathogens. J Dent Res. 2014;93(11):1089–1094. doi: 10.1177/0022034514529974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspect Med. 2005;26(1–2):33–65. doi: 10.1016/j.mam.2004.09.003 [DOI] [PubMed] [Google Scholar]
- [59].Bondonno CP, Blekkenhorst LC, Liu AH, et al. Vegetable-derived bioactive nitrate and cardiovascular health. Mol Aspect Med. 2018;61:83–91. doi: 10.1016/j.mam.2017.08.001 [DOI] [PubMed] [Google Scholar]
- [60].Fadel PJ. Nitric oxide and cardiovascular regulation. Hypertension. 2017;69(5):778–779. doi: 10.1161/HYPERTENSIONAHA.117.08999 [DOI] [PubMed] [Google Scholar]
- [61].Infante T, Costa D, Napoli C. Novel insights regarding nitric oxide and cardiovascular diseases. Angiology. 2021;72(5):411–425. doi: 10.1177/0003319720979243 [DOI] [PubMed] [Google Scholar]
- [62].Webb AJ, Patel N, Loukogeorgakis S, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51(3):784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kenjale AA, Ham KL, Stabler T, et al. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol. 2011;110(6):1582–1591. doi: 10.1152/japplphysiol.00071.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ivy JL. Inorganic Nitrate Supplementation for Cardiovascular Health. Methodist DeBakey Cardiovasc J. 2019;15(3):200–206. doi: 10.14797/mdcj-15-3-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jovanovski E, Bosco L, Khan K, et al. Effect of spinach, a high dietary nitrate source, on arterial stiffness and related hemodynamic measures: a randomized, controlled trial in healthy adults. Clin Nutr Res. 2015;4(3):160–167. doi: 10.7762/cnr.2015.4.3.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bedale W, Sindelar JJ, Milkowski AL. Dietary nitrate and nitrite: Benefits, risks, and evolving perceptions. Meat Sci. 2016;120:85–92. doi: 10.1016/j.meatsci.2016.03.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


