Abstract
Thrombopoietin (TPO) is a hematopoietic growth factor that plays fundamental roles is both megakaryopoiesis and thrombopoiesis through binding to its receptor, c-mpl. Although TPO has been shown to activate various types of intracellular signaling molecules, such as the Janus family of protein tyrosine kinases, signal transducers and activators of transcription (STATs), and ras, the precise mechanisms underlying TPO-induced proliferation and differentiation remain unknown. In an effort to clarify the mechanisms of TPO-induced proliferation and differentiation, c-mpl was introduced into F-36P, a human interleukin-3 (IL-3)-dependent erythroleukemia cell line, and the effects of TPO on the c-mpl-transfected F-36P (F-36P-mpl) cells were investigated. F-36P-mpl cells were found to proliferate and differentiate at a high rate into mature megakaryocytes in response to TPO. Dominant-negative (dn) forms of STAT1, STAT3, STAT5, and ras were inducibly expressed in F-36P-mpl cells, and their effects on TPO-induced proliferation and megakaryocytic differentiation were analyzed. Among these dn molecules, both dn ras and dn STAT5 reduced TPO- or IL-3-induced proliferation of F-36P-mpl cells by ∼30%, and only dn ras could inhibit TPO-induced megakaryocytic differentiation. In accord with this result, overexpression of activated ras (H-rasG12V) for 5 days led to megakaryocytic differentiation of F-36P-mpl cells. In a time course analysis on H-rasG12V-induced differentiation, activation of the ras pathway for 24 to 28 h was required and sufficient to induce megakaryocytic differentiation. Consistent with this result, the treatment of F-36P-mpl cells with TPO was able to induce prolonged activation of ras for more than 24 h, whereas IL-3 had only a transient effect. These results suggest that prolonged ras activation may be involved in TPO-induced megakaryocytic differentiation.
Thrombopoietin (TPO) is a ligand for the c-mpl proto-oncogene that is a member of the hematopoietin receptor superfamily with high sequence similarity to the receptors for erythropoietin (EPO) and granulocyte colony-stimulating factor. c-mpl is expressed in hematopoietic tissues, particularly in CD34+ hematopoietic progenitor cells, megakaryocytes, and platelets, while its ligand TPO is detected primarily in the liver, kidneys, and smooth muscle, with lesser amounts present in the spleen and bone marrow (for a review, see reference 18). A number of experiments have shown that TPO stimulates proliferation and differentiation of megakaryocytic progenitor cells in vitro and in vivo (9, 43). Furthermore, c-mpl- or TPO-deficient mice generated by gene targeting were reported to exhibit a striking decrease in the number of platelets and megakaryocytic progenitor cells (5, 12). These findings indicate that the TPO/c-mpl system is a physiological regulator of platelet and megakaryocyte production.
Like the receptors for EPO, granulocyte colony-stimulating factor, and interleukin-3 (IL-3), the TPO receptor c-mpl does not contain any recognized kinase domain or enzymatic motif in the cytoplasmic domain. Upon ligand binding, however, c-mpl has been shown to transmit a series of biochemical events, including tyrosine phosphorylation and activation of the Janus family of protein tyrosine kinases (JAKs), signal transducers and activators of transcription (STATs), phosphatidylinositol 3 kinase, and Shc (8, 27, 29, 32). Furthermore, recent studies done by using mutational analysis of the c-mpl cytoplasmic domain have provided some insights into the mechanisms of TPO-induced proliferation and differentiation. It was demonstrated that the membrane-proximal region including box 1 and box 2 motifs is required for both TPO-induced proliferation and activation of the JAK-STAT signal transduction pathway (13). It was also reported that the membrane-proximal region is necessary for both TPO-induced proliferation and differentiation and that the distal region can transmit differentiation signals (28, 34). Moreover, tyrosine-599 of c-mpl was found to be required for Shc phosphorylation and for inducing terminal differentiation of murine M1-mpl and WEHI3B-D+-mpl cells into macrophages (1). However, little is known about which signaling molecules are directly involved in TPO-induced proliferation and megakaryocytic differentiation.
In this study, therefore, we investigated the molecular mechanism of TPO-induced proliferation and megakaryocytic differentiation in a c-mpl-transfected, IL-3-dependent, human erythroleukemia cell line, F-36P-mpl, that can proliferate and then differentiate at a high rate into mature megakaryocytes in response to TPO. By using dominant-negative (dn) forms of STATs and ras, we show here that prolonged activation of ras is required for TPO-induced megakaryocytic differentiation, whereas both the STAT5 and ras pathways are involved in TPO-induced proliferation.
MATERIALS AND METHODS
Reagents and antibodies.
Highly purified recombinant human TPO (rhTPO) and rhIL-3 were provided by the Kirin Brewery Company Ltd. (Tokyo, Japan). Monoclonal antibody (MAb) AP2 (anti-human GP IIb/IIIa complex) was generously provided by T. Kunicki (Scripps Research Institute, La Jolla, Calif.) (33). Rabbit anti-c-Mpl serum was supplied by the Kirin Brewery Company Ltd. A murine antiphosphotyrosine MAb was supplied by B. Drucker (Oregon Health Science University, Portland, Oreg.). Rabbit anti-STAT1, STAT3, and STAT5b polyclonal antibodies (Abs) were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, Calif.). A murine anti-pan-Ras MAb and an antihemagglutinin (HA) MAb (12CA5) were purchased from Oncogene Research Products (Cambridge, Mass.) and Boehringer GmbH (Mannheim, Germany), respectively.
Plasmid construct and cDNAs.
The dn forms of HA-tagged STAT1 (HA-STAT1F), STAT3 (HA-STAT3D), and STAT5 (HA-STAT5694F) used were described previously (31, 41). The dn (S17N) and dominant-active (G12V) mutant forms of human c-H-ras cDNAs used were kindly provided by T. Satoh (Tokyo Institute of Technology, Yokohama, Japan) (40). A c-mpl expression vector, Humplpas12, was provided by the Kirin Brewery Company Ltd. These cDNAs were subcloned into the NotI site of pRSVICAT (Stratagene, La Jolla, Calif.) instead of the chloramphenicol acetyltransferase gene by using NotI linkers.
Cell lines and cultures.
F-36P, a human IL-3-dependent erythroleukemia cell line (2), was obtained from the Riken Cell Bank (Tsukuba, Japan). F-36P cells were cultured in RPMI 1640 (Nakarai Tesque) supplemented with 10% fetal calf serum (Flow, North Ryde, Australia) in the presence of 10-ng/ml rhIL-3.
Cell proliferation assay.
To quantitate the proliferation of the cells, a [3H]thymidine incorporation assay was used as previously described (39).
Morphological analysis.
The morphological characteristics of the cultured cells were determined by staining cytospin preparations (Shandon, Pittsburgh, Pa.) with May-Grunwald-Giemsa.
Flow cytometry.
The surface phenotypes of cells were examined by the indirect immunofluorescence method on a FACSort apparatus (Becton Dickinson, Oxnard, Calif.). The DNA content of cultured cells was quantitated by staining with propidium iodide (PI) and analysis on a FACSort apparatus.
Northern blot analysis.
The isolation of total cellular RNA and the method used for Northern blot analysis were described previously (23).
Immunoprecipitation and immunoblotting.
Preparation of cell lysates, immunoprecipitation, gel electrophoresis, and immunoblotting were performed as described previously (24). Immunoreactive proteins were visualized with the enhanced-chemiluminescence detection system (DuPont NEN, Boston, Mass.).
Lac-inducible system.
To express a target cDNA, we used a LacSwitch II inducible-expression system (Stratagene). In short, F-36P cells were initially cotransfected with Lac repressor (Lac-R), pCMV-LacI, and c-mpl expression vectors by electroporation (250 V, 960 μF; Bio-Rad Laboratories, Richmond, Calif.). The transfected cells were screened by culture with hygromycin (Sigma, St. Louis, Mo.) at 0.5 mg/ml. Of several hygromycin-resistant clones, one (designated F-36P-mpl) was further transfected with pOPRSVI containing HA-dn-STAT1, HA-dn-STAT3, HA-dn-STAT5, dn-ras, or H-rasG12V. The pOPRSVI expression vector contains the Rous sarcoma virus promoter linked to the Escherichia coli lactose operon, and expression of target cDNA is suppressed by Lac-R through the lactose operon. When isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the culture medium, Lac-R was released from the lactose operon and transcription of the target cDNA was initiated. After selection with G418 (Sigma) at 1.5 mg/ml, the induction levels of the target proteins in each clone were examined before and after treatment with IPTG (0.5 mM) by Western blot analyses.
Luciferase assay.
Four types of luciferase constructs with potential STAT- or AP1-binding sequences were used as reporter genes. The details of 4× APRE-Lu and 3× AP1-Lu were described previously (30). In addition, two types of double-stranded oligonucleotides were subcloned into the KpnI-SalI site just upstream of the minimal murine JunB promoter (−42 to +136) linked to the firefly luciferase gene, and their sequences were as follows: three tandem repeats of ISRE from the GBP gene (3× ISRE-Lu), 5′-TCGACACTTTCAGTTTCATCATGGTAC-3′ (the recognition site is underlined); three tandem repeats of GAS from the β-casein gene (3× β-Cas-Lu), 5′-TCGAAGATTTCTAGGAATTCAAATCGTAC-3′ (the recognition site is underlined). A luciferase assay was performed by using the Dual-Luciferase Reporter System (Promega, Madison, Wis.), in which transfection efficiency was monitored by using cotransfected pRL-CMV-Rluc, a renilla luciferase expression vector. The cultured cells were electroporated with 30 μg of the reporter gene together with 30 μg of pRL-CMV-Rluc. The transfected cells were serum and IL-3 starved for 12 h and then stimulated with rhTPO (30 ng/ml) or rhIL-3 (10 ng/ml) for 5 h. To examine the effects of dn mutants, the cells were pretreated with 0.5 mM IPTG for 24 h before electroporation and cultured with IPTG during the assay. The cells were lysed in lysis buffer supplied by the manufacturer, and then firefly and the renilla luciferase activities were measured on an LB96P luminometer (Berthold Japan, Tokyo, Japan). Relative firefly luciferase activities were calculated by normalizing transfection efficiency to the renilla luciferase activities. The experiments were performed in triplicate, and similar results were obtained from at least three independent experiments.
Detection of an active, GTP-bound form of ras.
An active, GTP-bound form of ras was detected as described by de Rooij and Bos (4). Briefly, cDNA of the minimal ras-binding domain (RBD) of Raf1 (amino acids 51 to 131) was subcloned into the BamHI-EcoRI site of pGEX-2T in frame. Glutathione S-transferase (GST)-RBD fusion protein production was induced in E. coli by IPTG treatment. After sonication, the crude extract was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and GST-RBD fusion protein induction was confirmed by staining with Coomassie blue. A GTP-bound form of ras was pulled from the total cellular lysate obtained from 107 cultured cells and suspended in 500 μl of radioimmunoprecipitation assay buffer by incubation with the GST-RBD protein precoupled with glutathione-Sepharose beads (Pharmacia AB, Uppsala, Sweden) for 1 h at 4°C. The beads were collected by centrifugation, washed with radioimmunoprecipitation assay buffer three times, boiled, and subjected to SDS-PAGE. Immunoblotting was performed with a murine anti-ras MAb (17) as described previously (24). Immunoreactive proteins were visualized with the enhanced-chemiluminescence detection system.
RESULTS
F-36P cells expressing c-mpl show proliferation and megakaryocytic differentiation in response to TPO.
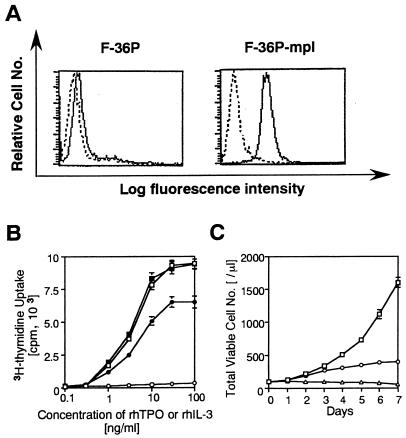
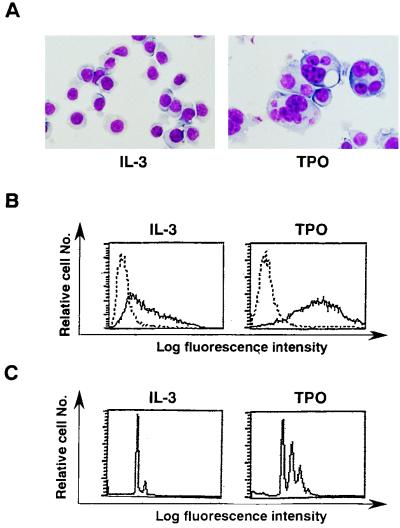
F-36P is a human IL-3-dependent erythroleukemia cell line that undergoes erythroid differentiation in response to EPO (2). On the hypothesis that this cell line might possess a potential to differentiate into mature megakaryocytes in response to TPO, we introduced a human c-mpl expression vector together with a Lac-R expression vector. After culture with hygromycin for 4 weeks, several hygromycin-resistant cells were obtained and then cloned. Of these clones, one (designated F-36P-mpl) was subjected to further analyses, because this clone showed the most intensive expression of Lac-R mRNA and could proliferate and differentiate at a high rate into mature megakaryocytes in response to rhTPO. First, cell surface expression of c-Mpl (the c-mpl product) in parental F-36P and F-36P-mpl cells was examined by flow cytometric analysis. In contrast to a barely detectable level of c-Mpl expression in parental F-36P cells, considerable c-Mpl expression was observed in F-36P-mpl cells (Fig. 1A). Next, we examined dose-dependent effects of rhIL-3 and rhTPO on short-term (48 h) growth of parental F-36P and F-36P-mpl cells by a [3H]thymidine incorporation assay. Parental F-36P cells showed dose-dependent proliferation in response to rhIL-3 over a range of 0.1 to 100 ng/ml but not in response to rhTPO (Fig. 1B). In contrast, proliferation of F-36P-mpl cells was stimulated by both rhIL-3 and rhTPO in a dose-dependent manner over ranges of 0.1 to 100 and 0.1 to 30 ng/ml, respectively (Fig. 1B). When F-36P-mpl cells were cultured with rhTPO (30 ng/ml) for 7 days, their proliferation gradually declined after 4 days and almost stopped after 7 days, whereas they showed continuous growth when cultured with rhIL-3 (10 ng/ml) (Fig. 1C). To examine the effects of rhTPO in the long-term (>4-day) culture, we examined the changes in morphological characteristics, surface phenotypes, and DNA content of F-36P-mpl cells after a 5-day culture with rhTPO. As shown in cytospin preparations, a substantial fraction (∼65%) of F-36P-mpl cells showed morphological alterations indicative of megakaryocytic maturation after the culture with rhTPO, whereas F-36P-mpl cells cultured with rhIL-3 were absolutely composed of small, undifferentiated blast cells (Fig. 2A). Next, we examined changes in the surface expression of megakaryocytic lineage-specific surface marker GPIIb/IIIa, which is a complex of integrins αIIb and β3, after a 5-day culture with rhTPO by flow cytometric analysis with anti-GP IIb/IIIa MAb AP2 (33). As shown in Fig. 2B, GPIIb/IIIa expression significantly increased after the culture with rhTPO compared with that in the culture with rhIL-3. DNA content analysis revealed that 57.8% of F-36P-mpl cells cultured with rhIL-3 were in G0/G1 (2N), 34% were in S phase, and 8.2% were in G2/M (4N) (Fig. 2C). By contrast, a 5-day culture with rhTPO led to polyploid formation: there was a striking increase in the 4N fraction to 38% in addition to the appearance of 8N (28%) and 16N fractions (4%) (Fig. 2C). These results suggested that the growth arrest observed in F-36P-mpl cells after long-term culture with rhTPO was due to megakaryocytic maturation.
FIG. 1.
(A) Flow cytometric analysis of F-36P and F-36P-mpl cells. The expression of c-Mpl was examined by staining with rabbit anti-c-Mpl serum (——) or preimmune rabbit serum (–––). (B) Dose response of F-36P and F-36P-mpl to rhIL-3 and rhTPO. Triplicate aliquots of cells were cultured in serum-free medium with various concentration of rhIL-3 or rhTPO for 48 h, and then cell proliferation was measured by a [3H]thymidine incorporation assay (F-36P; □, IL-3; ○, TPO; F-36P-mpl; ▪, IL-3; •, TPO). (C) Changes in the total viable cell number during culture with or without rhIL-3 or rhTPO. F-36P-mpl cells were resuspended in 10% fetal calf serum–RPMI containing 10-ng/ml rhIL-3 or 30-ng/ml rhTPO at a cell density of 100/μl, and the total viable cell number was determined by the trypan blue dye exclusion method at the time indicated (□, IL-3; ○, TPO; ▵, free of growth factor). The results shown are the means ± the standard deviations of triplicate cultures.
FIG. 2.
(A) Light micrograph of F-36P-mpl cells after a 5-day culture with rhIL-3 or rhTPO. A cytocentrifugation preparation from each culture was stained with May-Grunwald-Giemsa (original magnification, ×100). (B and C) Flow cytometric analyses of F-36P-mpl cells. Expression of GPIIb/IIIa was examined by staining with MAb AP2 (——) and a control Ab of the same isotype (–––) (B). The DNA content of F-36P-mpl cells was examined by staining with PI solution and analysis on a FACSort apparatus (C).
TPO-mediated STAT phosphorylation in F-36P-c-mpl cells.
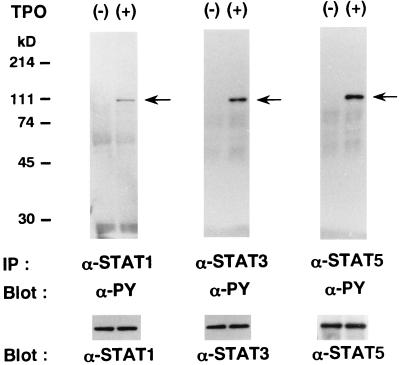
It has previously been reported that STATs are activated by a variety of cytokines, such as TPO, EPO, and IL-6, and act as key regulators in cytokine-induced proliferation and differentiation (for reviews, see references 14 and 15). Since TPO was reported to activate various combinations of STAT1, STAT3, and STAT5 according to cell types (8, 27, 29, 32), we examined the effects of rhTPO on tyrosine phosphorylation of STAT proteins in F-36P-mpl cells. As shown in Fig. 3, stimulation with rhTPO for 15 min was found to induce tyrosine phosphorylation of STAT1, STAT3, and STAT5 in F-36P-c-mpl cells. Furthermore, the rhTPO-induced tyrosine phosphorylation of STAT1, STAT3, and STAT5 was also observed in cells that underwent megakaryocytic differentiation after 5 days of culture with rhTPO (data not shown).
FIG. 3.
TPO-induced tyrosine phosphorylation of STAT proteins in F-36P-mpl cells. F-36P-mpl cells were serum and factor starved for 12 h and then either left unstimulated or stimulated with rhTPO for 15 min. Total cell lysates were immunoprecipitated with an anti-STAT1, an anti-STAT3, or an anti-STAT5b Ab. The blots were probed with an antiphosphotyrosine (α-PY) MAb. The filters were then stripped and reprobed with anti-STAT1, anti-STAT3, and anti-STAT5b Abs, respectively.
Inducible expression of dn STATs and dn ras.
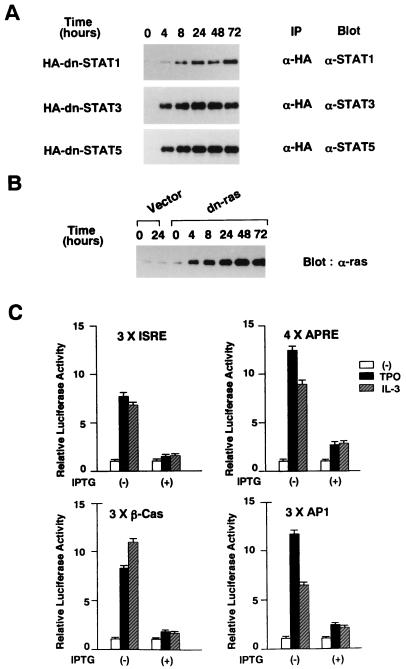
To characterize the mechanism underlying TPO-induced proliferation and megakaryocytic differentiation of F-36P-mpl cells, we prepared several stable clones that can inducibly express dn forms of STAT1 (HA-STAT1F), STAT3 (HA-STAT3D), STAT5 (HA-STAT5694F), and ras (H-rasS17N) (31, 40, 41). As shown in a representative Western blot in Fig. 4A, addition of 0.5 mM IPTG to the culture medium led to induction of dn STAT1, dn STAT3, and dn STAT5 as early as 4 h, and their expressions reached a peak at ∼24 h that was maintained for up to 72 h in the HA-immunoprecipitated proteins. Furthermore, Western blot analysis of the whole-cell lysates revealed that treatment with IPTG led to induction of dn ras in a pattern almost similar to that of dn STATs (Fig. 4B). In addition, the induced dn ras was found to be far more abundant than was endogenous ras (Fig. 4B). To evaluate the inhibitory effects of these mutants in each signalling pathway, we performed luciferase assays with reporter plasmids that included 3× ISRE (transactivated by STAT1), 4× APRE (transactivated by STAT3), 3× β-Cas (transactivated by STAT5), and 3× AP1 (transactivated by ras-mediated AP1). In the absence of IPTG, treatment with rhTPO stimulated the luciferase activity driven by 3× ISRE 7.7-fold, that driven by 4× APRE 12.4-fold, that driven by 3× β-Cas 8.2-fold, and that driven by 3× AP1 11.7-fold (Fig. 4C). Similarly, rhIL-3 stimulated the luciferase activity driven by 3× ISRE 6.8-fold, that driven by 4× APRE 8.5-fold, that driven by 3× β-Cas 11.1-fold, and that driven by 3× AP1 6.4-fold in the absence of IPTG (Fig. 4C). By contrast, when dn mutant protein expression was induced by pretreatment with IPTG, both rhTPO- and rhIL-3-induced luciferase activities were severely reduced in all of the reporter plasmids. The rhTPO-induced activity reductions were as follows: 3× ISRE, 1.5-fold; 4× APRE, 2.7-fold; 3× β-Cas, 1.8-fold; 3× AP1, 2.4-fold. The rhIL-3-induced activity reductions were as follows: 3× ISRE, 1.6-fold; 4× APRE, 2.8-fold; 3× β-Cas, 1.6-fold; 3× AP1, 2.1-fold (Fig. 4C). Furthermore, in F-36P-mpl cells transfected with an empty, IPTG-inducible vector, rhTPO and rhIL-3 were able to activate 3× ISRE, 4× APRE, 3× β-Cas, and 3× AP1 nearly the same way under both conditions with or without IPTG pretreatment, suggesting that IPTG does not affect rhTPO- or rhIL-3-induced reporter gene activities. Taken together, all of the dn mutants were considerably effective in impairing each signaling pathway.
FIG. 4.
(A and B) Inducible expression of dn STATs and dn ras. Each clone was treated with 0.5 mM IPTG for the times indicated. HA-immunoprecipitated proteins (A) or total cell lysates (B) were subjected to SDS-PAGE. The blots were probed with anti-STAT1, anti-STAT3, anti-STAT5b, and anti-ras Abs. (C) Effects of dn mutants on each signaling pathway. Four types of reporter plasmids, each containing 3× ISRE, 4× APRE, 3× β-Cas, and 3× AP1, were used as reporter genes. F-36P-mpl cells were electroporated with 30 μg of the reporter gene together with 30 μg of pRL-CMV-Rluc. The cells were serum and IL-3 starved for 12 h and then stimulated with rhTPO (30 ng/ml) or rhIL-3 (100 ng/ml) for 5 h. To examine the effects of dn mutants, the cells were pretreated with 0.5 mM IPTG for 24 h before electroporation and cultured with IPTG during the assay. Relative firefly luciferase activities were calculated by normalizing transfection efficiency to renilla luciferase activities. The results shown are means ± the standard deviations of triplicate experiments. Open bars, unstimulated.
Effects of dn mutants on TPO- and IL-3-induced proliferation.
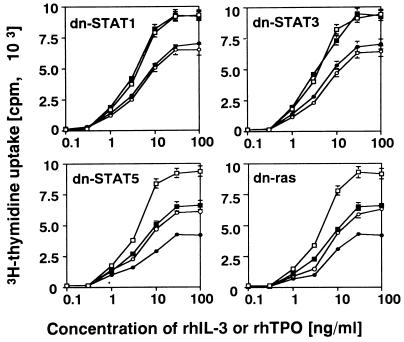
We examined the effects of these dn mutants on rhTPO- and rhIL-3-induced proliferation. The cells of each clone were IL-3 starved for 24 h in the presence or absence of IPTG, cultured with various concentrations of rhIL-3 or rhTPO with or without IPTG for 48 h, and then subjected to a [3H]thymidine incorporation assay. As shown in Fig. 5, dn STAT1 or dn STAT3 did not affect rhTPO- or rhIL-3-induced proliferation. In contrast, dn STAT5 inhibited rhTPO- and rhIL-3-dependent proliferation by 29% (at an rhTPO concentration of 30 ng/ml) and 31% (at an rhIL-3 concentration of 100 ng/ml), respectively (Fig. 5). In addition, dn ras also inhibited rhTPO- and rhIL-3-induced proliferation by 26% (at an rhTPO concentration of 30 ng/ml) and 28% (at an rhIL-3 concentration of 100 ng/ml), respectively (Fig. 5).
FIG. 5.
Effects of dn STATs and dn ras on rhTPO- and rhIL-3-induced proliferation. Cells of each clone were IL-3 starved for 24 h and then cultured for 48 h with rhTPO or rhIL-3 at the concentrations indicated. The cells were left untreated or treated with 0.5 mM IPTG during the 24-h starvation period and the 48-h culture period. Cell proliferation was quantitated with a [3H]thymidine incorporation assay. The results shown are means ± the standard deviations of triplicate experiments. □, IL-3 without IPTG; ▪, IL-3 with IPTG; ○, TPO without IPTG; •, TPO with IPTG.
Effects of dn mutants on TPO-induced megakaryocytic differentiation.
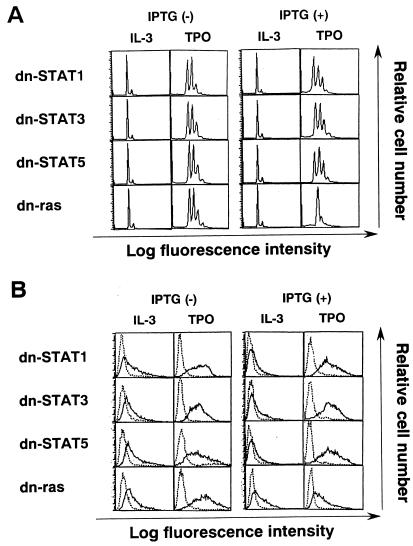
Next, we examined the effects of dn mutants on TPO-induced megakaryocytic differentiation. Morphological analysis demonstrated that in the absence of IPTG, all of the dn STAT1, dn STAT3, dn STAT5, and dn ras clones showed megakaryocytic differentiation after a 5-day culture with rhTPO (30 ng/ml) but not with rhIL-3 (10 ng/ml) as in the case of the parental F-36P-mpl clone (Fig. 6). In the presence of IPTG, by contrast, rhTPO-induced megakaryocytic differentiation was found to be effectively suppressed in a dn ras clone but not in a dn-STAT1, dn-STAT3, or dn-STAT5 clone (Fig. 6). In agreement with data on the morphological analysis, furthermore, flow cytometric analyses revealed that both polyploid formation and GPIIb/IIIa expression induced by rhTPO were blocked by dn ras in the presence of IPTG, whereas dn STAT1, dn STAT3, or dn STAT5 had little or no effect (Fig. 7A and B).
FIG. 6.
Light micrograph of F-36P-mpl cells. Clones were cultured for 5 days under the conditions indicated. To examine the effects of dn mutants, the cells were pretreated with 0.5 mM IPTG for 24 h and then cultured in the presence of 0.5 mM IPTG for 5 days. A cytocentrifugation preparation from each culture was stained with May-Grunwald-Giemsa (original magnification, ×100).
FIG. 7.
Flow cytometric analyses of dn mutant clones after a 5-day culture (as described in the legend to Fig. 6) with rhTPO. DNA content was examined by staining with PI solution and analysis on a FACSort apparatus (A). Expression of GPIIb/IIIa was examined by staining with MAb AP2 (——) and a control Ab of the same isotype (–––) (B).
Effects of activated ras on megakaryocytic differentiation of F-36P-mpl cells.
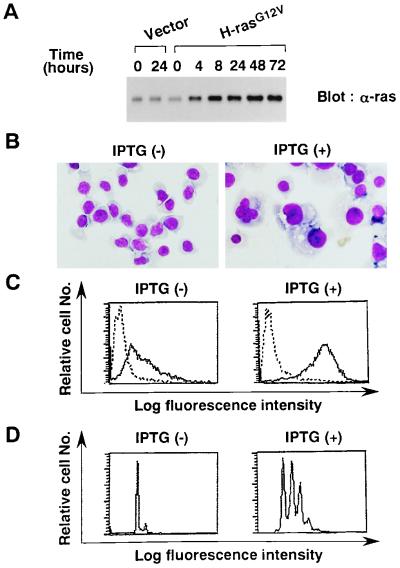
To further determine if the ras pathway is directly involved in megakaryocytic differentiation, we prepared a clone (designated F-36P-H-rasG12V) that could inducibly express activated c-H-ras (H-rasG12V) (40). Expression of the H-rasG12V protein was examined by Western blot analysis with anti-ras antibody before and after treatment with 0.5 mM IPTG (Fig. 8A). Compared with cells transfected with the vector alone, F-36P-H-rasG12V cells were found to express significantly larger amount of ras protein after IPTG treatment for more than 4 h, indicating that the rasG12V protein was effectively induced in F-36P-H-rasG12V cells. When F-36P-H-rasG12V cells were cultured with rhIL-3 in the absence of IPTG, the cells were exclusively composed of undifferentiated blast cells (Fig. 8B, left panel). In contrast, treatment with IPTG for 5 days in a medium containing rhIL-3 led to marked morphological changes indicative of megakaryocytic differentiation in about 75% of the cultured cells (Fig. 8B, right panel), although most of the differentiated cells possessed one giant nucleus rather than the multilobular nuclei that were observed after treatment with rhTPO (Fig. 2A and 6). Surface phenotypic and DNA content analyses revealed that the 5-day culture with IPTG resulted in increased expression of GPIIb/IIIa (Fig. 8C) and in polyploid formation (Fig. 8D). Similar results were also observed in a culture medium that did not contain rhIL-3 (data not shown). These results suggested that activated ras could confer megakaryocytic differentiation of F-36P-mpl cells.
FIG. 8.
Effects of H-rasG12V on megakaryocytic differentiation. (A) Inducible expression of H-rasG12V. F-36P-H-rasG12V and F-36P-Vector (transfected with an empty vector) cells were treated with 0.5 mM IPTG for the times indicated, and expression of H-rasG12V was examined by Western blot analysis. (B) Light micrograph of F-36P-H-rasG12V after a 5-day culture with or without IPTG (original magnification, ×100). (C and D) Flow cytometric analyses of GPIIb/IIIa expression (C) and DNA content (D) after a 5-day culture with or without IPTG.
Sustained ras activation by TPO.
In addition to TPO, various types of hematopoietic growth factors, including EPO, IL-3, IL-6, and SCF, are known to activate ras pathways, although these cytokines are not capable of inducing megakaryocytic differentiation. To determine the difference between TPO and other hematopoietic growth factors in ras activation, we transfected 3× AP1-Lu into F-36P-mpl cells together with pSV2neo and performed a luciferase assay. Several of the G418-resistant clones (one of which was designated F-36P-AP1-Lu) were selected for further analysis, since in these clones rhTPO and rhIL-3 stimulated AP-1-driven luciferase activity in about the same way as in the transient assay whose results are shown in Fig. 4C. After serum and rhIL-3 starvation for 24 h, F-36P-AP1-Lu cells were cultured with rhTPO or rhIL-3 for up to 24 h, and expression of luciferase mRNA was examined by Northern blot analysis. Both rhTPO and rhIL-3 treatments led to similarly rapid induction of luciferase mRNA as early as 2 h (Fig. 9A). In accord with the results in Fig. 4C, however, rhTPO induced more intensive expression of luciferase mRNA than did rhIL-3. Furthermore, rhTPO had a more durable effect on luciferase mRNA expression than did rhIL-3: rhTPO induced luciferase mRNA expression for more than 24 h, while rhIL-3 did so for up to 8 h (Fig. 9A). Similar results were obtained with four independent clones other than F-36P-AP1-Lu cells (data not shown). These results suggested that ras pathway activation by TPO may be more effective in both intensity and duration than that of IL-3 in F-36P cells.
FIG. 9.
(A) Northern blot analysis of luciferase mRNA. F-36P-AP1-Lu cells were serum and rhIL-3 starved for 24 h and then stimulated with rhTPO (30 ng/ml) or rhIL-3 (10 ng/ml) for the times indicated. Expression of luciferase mRNA was examined by Northern blot analysis. (B) Detection of an active, GTP-bound form of ras during treatment with rhTPO or rhIL-3. F-36P-mpl cells were serum and rhIL-3 starved for 24 h and then stimulated with rhTPO (30 ng/ml) or rhIL-3 (10 ng/ml) for the times indicated. GTP-bound ras was pulled from total cell lysates by incubation with a GST-RBD fusion protein precoupled with glutathione-Sepharose beads. The beads were collected and subjected to SDS-PAGE. Immunoblotting was performed with a murine anti-ras MAb (17). The total amount of ras was estimated by immunoblotting with an anti-ras MAb using total cell lysates.
To further examine the changes in the activation state of ras, we measured the amount of an active, GTP-bound form of ras during treatment with rhTPO or rhIL-3. F36P-mpl cells were serum and IL-3 starved for 24 h and then stimulated with rhTPO or rhIL-3 for up to 24 h. Active, GTP-bound ras was pulled from total cell lysates by a GST-RBD fusion protein which binds to GTP-bound ras but not to inactive, GDP-bound ras (4). After serum and rhIL-3 starvation, the active, GTP-bound form decreased to an undetectable level. Treatment with rhTPO led to induction of GTP-bound ras at 1 h, and the elevation of ras-GTP levels was sustained for more than 24 h, whereas rhIL-3 showed only a transient effect on induction of GTP-bound ras (from 1 to 6 h) (Fig. 9B). In contrast, Western blot analysis using total cell lysates revealed that the total amounts of ras did not change during both treatments. These findings coincided with the data on AP-1-driven luciferase mRNA (Fig. 9A) and suggested that TPO is capable of inducing sustained ras activation.
Time-dependent effects of H-rasG12V on megakaryocytic differentiation.
We next examined the time-dependent effects of H-rasG12V on megakaryocytic differentiation. After treatment of F-36P-H-rasG12V cells with 0.5 mM IPTG for 24 h, the cells were washed and resuspended in IPTG-free medium, and then changes in the expression levels of H-rasG12V were examined by Western blot analysis for up to 12 h. The expression of F-36P-H-rasG12V significantly decreased 8 h after IPTG deprivation and became undetectable after 10 h, because expression of total ras protein returned to the basal level at 10 h (Fig. 10A). To examine time-dependent effects of H-rasG12V, F-36P-H-rasG12V cells were initially treated with IPTG for the various periods indicated, washed, and cultured in an IPTG-free medium 120 h after the initiation of IPTG treatment. The morphological characteristics, surface expression of GPIIb/IIIa, and DNA content of each sample were then analyzed. The treatment with IPTG for 8 h was not able to induce surface phenotypic change or polyploid formation (Fig. 10B). The treatment for 10 and 12 h also failed to induce megakaryocytic differentiation in most of the cultured cells, although only a small proportion of the cells (10-h treatment, ∼2%; 12-h treatment, ∼10%) revealed megakaryocytic differentiation in cytospin preparations (data not shown). By contrast, morphological analysis demonstrated that treatment for 14 and 16 h led to megakaryocytic differentiation in about 30 and 50% of cultured cells, respectively (data not shown). These results were largely consistent with those of DNA content analysis showing a marked increase in the 4N fraction and the appearance of an 8N fraction after 16 h (Fig. 10B). The treatment for 20 h was found to be about the same as that for 120 h in inducing polyploid formation and GPIIb/IIIa expression (Fig. 10B). Since expression of the H-rasG12V protein was found to continue for about 8 h after IPTG deprivation (Fig. 10A), activation of the ras pathway for 24 to 28 h was supposed to be required and sufficient for inducing megakaryocytic differentiation.
FIG. 10.
(A) Changes in H-rasG12V expression after IPTG deprivation. F-36P-H-rasG12V cells were treated with IPTG for 24 h, washed, and then cultured in IPTG-free medium for up to 12 h. Changes in H-rasG12V expression were examined by Western blot analysis. (B) Time-dependent effects of H-rasG12V on megakaryocytic differentiation. F-36P-H-rasG12V cells were treated with IPTG for the times indicated, washed, and then resuspended in IPTG-free culture medium. At 120 h after the addition of IPTG, GPIIb/IIIa expression and DNA content were determined by flow cytometric analyses.
DISCUSSION
TPO is known to regulate growth and megakaryocytic differentiation of normal hematopoietic stem/progenitor cells (9, 43). In addition, we and others have reported that TPO is capable of inducing proliferation and, albeit to a limited degree, differentiation of human leukemia cell lines, as well as acute myeloblastic leukemia cells (22–24, 28, 34). However, the precise mechanisms underlying TPO-induced proliferation and megakaryocytic differentiation remain to be determined, largely due to the difficulty in preparing a sufficient number of normal megakaryocytic progenitor cells and also the lack of useful cell lines that show growth and megakaryocytic differentiation in a TPO-dependent manner. In this study, by generating F-36P-mpl cells that were unique in their ability to proliferate and differentiate at a high rate into mature megakaryocytes in response to rhTPO, we investigated the molecular mechanisms responsible for the TPO-dependent proliferation and megakaryocytic differentiation.
After binding of TPO to c-mpl, JAKs are known to be rapidly phosphorylated on tyrosine and activated as tyrosine kinases; the activated JAKs, in turn, induce the phosphorylation of latent cytoplasmic proteins called STATs (8, 13, 28, 32). Upon phosphorylation, STATs dimerize, translocate to the nucleus, and participate in transcriptional regulation by binding to specific DNA sequences (for reviews, see references 14 and 15). In F-36P-mpl cells, Western blot analysis and luciferase assays demonstrated that all of STAT1, STAT3, and STAT5 were phosphorylated on tyrosine and activated in response to TPO. However, only dn STAT5, not dn STAT1 or dn STAT3, could inhibit rhIL-3- and rhTPO-induced proliferation, by about 30%, suggesting that STAT5 is involved, at least partially, in the cell proliferation induced by TPO and IL-3. In mice, STAT5 is encoded by two highly related, chromosomally linked genes, those for STAT5A and STAT5B, and disruption of the STAT5A gene alone was shown to be sufficient to inhibit STAT5 activity (10). It has recently been reported that although STAT5A-targeted mice undergo normal development without apparent hematopoietic abnormalities (21), macrophages obtained from the bone marrow of STAT5A-targeted mice showed a 33% decreased proliferative response to rh granulocyte-macrophage colony-stimulating factor compared with that of those from normal mice (10). These findings, including ours, suggest that STAT5 is one of the signaling molecules used by hematopoietic growth factors to promote cell proliferation, although the growth-promoting activity of STAT5 could be replaced by other signaling molecules, such as other STATs or ras, in vivo.
Several lines of evidence suggest that STATs are involved in some aspects of cellular differentiation, as well as cell proliferation. It has recently been reported that STAT5 is involved in EPO-induced erythroid differentiation of the murine erythroleukemia cell lines ELM-I-1 and SKT6 (16, 41), while the opposite result was obtained with the human IL-3-dependent erythrocytic leukemia cell line TF-1 (3). Furthermore, STAT3 has also been revealed to participate in IL-6-induced macrophage differentiation and in antiapoptotic signal transmission from IL-6 (11, 31). In the case of F-36P-mpl cells, neither dn STAT5, dn STAT1, nor dn STAT3 could inhibit TPO-induced megakaryocytic differentiation, indicating that each type of STAT may not be involved at least in megakaryocytic differentiation of F-36P-mpl cells. This finding seems to be at variance with our previous results showing that STAT5 could play a role in TPO-induced megakaryocytic differentiation of CMK megakaryoblastic leukemia cells through induction of p21WAF1 (17). However, it is possible that STATs are associated with cellular differentiation in a manner dependent on the cell type or stage of differentiation. Since TPO is not capable of inducing p21WAF1 expression in F-36P-mpl cells (unpublished observation), it would be important to investigate the role of STAT5 in p21WAF1 induction in normal megakaryocyte progenitor cells. Furthermore, additional information about the target genes of each STAT would be particularly helpful in understanding the biological significance of STATs in cellular differentiation.
A number of previous studies have indicated that ras plays a crucial role in mitogenic signaling of various growth factors: the extracellular signals are transmitted from the cell surface receptor to a protein kinase cascade consisting of raf, MEK (mitogen-activated protein kinase [MAPK]-activating enzymes, also called MAPKK), and MAPKs through the mediation of a membrane-bound-form of ras (for a review, see reference 25). Activated ras has also been reported to drive the cells to leave the quiescent state (G0) and to pass through the G1/S transition of the cell cycle through the induction of cyclin D1 (7, 20). In accord with the previous findings, we show here that dn ras inhibits TPO- and IL-3-induced proliferation of F-36P-mpl cells by about 30%, indicating that the ras pathway is involved, at least partially, in TPO-induced proliferation. Furthermore, we provide unique evidence that prolonged activation (>24 h) of the ras pathway is essential for TPO-induced megakaryocytic differentiation of F-36P-mpl cells. This observation is in contrast to the previous findings that overexpression of constitutively activated ras led, in most cases, to a differentiation block in myoblasts, keratinocytes, B lymphoblasts, and 32D c13 myeloblastic cells (6, 19, 37, 38). However, ectopic overexpression of constitutive activated N-ras was shown, exceptionally, to result in the induced neuronal differentiation of PC12 cells, a rat pheochromocytoma line (35). In PC12 cells, treatment with nerve growth factor or fibroblast growth factor was shown to induce a sustained elevation of ras and ERK (extracellular signal-related kinase; MAPK) activities, leading to neuronal differentiation; epidermal growth factor, which did not cause neuronal differentiation, was found to stimulate only transient activation of ras and ERK (35). Recently, it was reported that constitutive activated ERK induced megakaryocytic differentiation of K562 erythroleukemia cells and CMK megakaryoblastic leukemia cells, both of which can differentiate into megakaryocytes after treatment with phorbol esters (26, 42). However, it was also reported that overexpression of constitutively activated MEK did not affect the growth and maturation stage of UT7-mpl cells that are capable of megakaryocytic differentiation in response to TPO (36). In the case of F-36P-mpl cells, the MEK inhibitor PD98059 induced partial abrogation of megakaryocytic differentiation in both TPO-stimulated F-36P-mpl cells and F-36P-H-rasG12V cells (TPO-stimulated F-36P-mpl cells, ∼35%; F-36P-H-rasG12V cells, ∼60% [unpublished observation]). These results suggest that although MEK activation may be one of the downstream targets of ras for megakaryocytic differentiation, an additional signal(s) mediated through prolonged ras activation may be required to facilitate TPO-mediated megakaryocytic differentiation. Furthermore, since most of the megakaryocytes developed after introduction of constitutively activated H-rasG12V possessed one giant nucleus but not multilobular nuclei that were observed after treatment with TPO, it was also suggested that a c-mpl-mediated signaling cascade other than the ras pathway may also be involved in TPO-induced megakaryocytic differentiation. Further studies using this TPO/F36-P-mpl system will enable us to elucidate more precisely the mechanisms regulating megakaryopoiesis.
ACKNOWLEDGMENTS
We thank T. Satoh for providing c-H-rasS17N and c-H-rasG12V cDNAs. We thank Y. Fujitani for thoughtful discussion.
This work was supported in part by grants from the Ministry of Education, Science and Culture; the Inamori Foundation; the Senri Life Science Foundation; and the Mochida Memorial Foundation.
REFERENCES
- 1.Alexander W S, Maurer A B, Novak U, Harrison-Smith M. Tyrosine-599 of the c-Mpl receptor is required for Shc phosphorylation and the induction of cellular differentiation. EMBO J. 1996;15:6531–6540. [PMC free article] [PubMed] [Google Scholar]
- 2.Chiba S, Takaku F, Tange T, Shibuya K, Misawa C, Sasaki K, Miyagawa K, Yazaki Y, Hirai H. Establishment and erythroid differentiation of a cytokine-dependent human leukemia cell line F-36P: a parental line requiring granulocyte-macrophage colony-stimulating factor or interleukin-3, and a subline requiring erythropoietin. Blood. 1991;78:2261–2268. [PubMed] [Google Scholar]
- 3.Chretien S, Varlet P, Verdier F, Gobert S, Cartron J-P, Gisselbrecht S, Mayeux P, Lacombe C. Erythropoietin-induced erythroid differentiation of the human erythroleukemia cell line TF-1 correlates with impaired STAT5 activation. EMBO J. 1996;15:4174–4181. [PMC free article] [PubMed] [Google Scholar]
- 4.de Rooij J, Bos J L. Minimal ras-binding domain of raf1 can be used as an activation-specific probe for ras. Oncogene. 1997;14:623–625. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]
- 5.de Sauvage F J, Carver-Moore K, Luoh S M, Ryan A, Dowd M, Eaton D L, Moore M W. Physiological regulation of early and late stages of megakaryopoiesis by thrombopoietin. J Exp Med. 1996;183:651–656. doi: 10.1084/jem.183.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dlugosz A A, Cheng C, Williams E K, Dharia A G, Denning M F, Yuspa S H. Alterations in murine keratinocyte differentiation induced by activated Ha-ras genes are mediated by protein kinase C-α. Cancer Res. 1994;54:6413–6420. [PubMed] [Google Scholar]
- 7.Dobrowolski S, Harter M, Stacey D W. Cellular ras activity is required for passage through multiple points of the G0/G1 phase in BALB/c 3T3 cells. Mol Cell Biol. 1994;14:5441–5449. doi: 10.1128/mcb.14.8.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drachman J G, Kaushansky K. Dissecting the thrombopoietin receptor: functional elements of the Mpl cytoplasmic domain. Proc Natl Acad Sci USA. 1997;94:2350–2355. doi: 10.1073/pnas.94.6.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farese A M, Hunt P, Boone T, MacVittie T J. Recombinant human megakaryocyte growth and development factor stimulates thrombopoiesis in normal nonhuman primates. Blood. 1995;85:54–59. [PubMed] [Google Scholar]
- 10.Feldman G M, Rosenthal L A, Liu X, Hayes M P, Wynshow-Boris A, Leonard W J, Hennighausen L, Finbloom D S. STAT5A-deficient mice demonstrate a defect in granulocyte-macrophage colony-stimulating factor-dependent proliferation and gene expression. Blood. 1997;90:1768–1776. [PubMed] [Google Scholar]
- 11.Fukada T, Hibi M, Yamanaka Y, Takahashi M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity. 1996;5:449–460. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- 12.Gurney A L, Carver-Moore K, Sauvage F J, Moore M W. Thrombocytopenia in c-mpl-deficient mice. Science. 1994;265:1445–1447. doi: 10.1126/science.8073287. [DOI] [PubMed] [Google Scholar]
- 13.Gurney A L, Wong S C, Henzel W J, Sauvage F J. Distinct regions of c-mpl cytoplasmic domain are coupled to the JAK-STAT signal transduction pathway and Shc phosphorylation. Proc Natl Acad Sci USA. 1995;92:5292–5296. doi: 10.1073/pnas.92.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ihle J N. Cytokine receptor signaling. Nature (London) 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 15.Ihle J N. Signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 16.Iwatsuki K, Endo T, Misawa H, Yokouchi M, Matsumoto A, Ohtsubo M, Mori K J, Yoshimura A. STAT5 activation correlates with erythropoietin receptor-mediated erythroid differentiation of an erythroleukemia cell line. J Biol Chem. 1997;272:8149–8152. doi: 10.1074/jbc.272.13.8149. [DOI] [PubMed] [Google Scholar]
- 17.Kanai T, Hirohashi S, Noguchi M, Shimoyama Y, Shimosato Y, Noguchi S, Nishimura S, Abe O. Monoclonal antibody highly sensitive for the detection of ras p21 in immunoblotting analysis. Jpn J Cancer Res. 1987;78:1314–1318. [PubMed] [Google Scholar]
- 18.Kaushansky K. Thrombopoietin: the primary regulator of platelet production. Blood. 1995;86:419–431. [PubMed] [Google Scholar]
- 19.Kreider B L, Rovera G. The immediate early gene response to a differentiative stimulus is disrupted by the v-abl and v-ras oncogenes. Oncogene. 1992;7:135–140. [PubMed] [Google Scholar]
- 20.Liu J-J, Chao J-R, Jiang M-C, Ng S-Y, Yen J J-Y, Yang-Yen H-F. Ras transformation results in an elevated level of cyclin D1 and acceleration of G1 progression in NIH 3T3 cells. Mol Cell Biol. 1995;15:3654–3663. doi: 10.1128/mcb.15.7.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Robinson G W, Wagner K-W, Garrett L, Wynshow-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura I, Kanakura Y, Kato T, Ikeda H, Ishikawa J, Horikawa Y, Hashimoto K, Moriyama Y, Tsujimura T, Nishiura T, Miyazaki H, Matsuzawa Y. Growth response of acute myeloblastic leukemia cells to recombinant human thrombopoietin. Blood. 1995;86:703–709. [PubMed] [Google Scholar]
- 23.Matsumura I, Kanakura Y, Kato T, Ikeda H, Horikawa Y, Ishikawa J, Kitayama H, Nishiura T, Tomiyama Y, Miyazaki H, Matsuzawa Y. The biological properties of recombinant human thrombopoietin in the proliferation and megakaryocytic differentiation of acute myeloblastic leukemia cells. Blood. 1996;88:3074–3082. [PubMed] [Google Scholar]
- 24.Matsumura I, Ishikawa J, Nakajima K, Oritani K, Tomiyama Y, Miyagawa J-I, Kato T, Miyazaki H, Matsuzawa Y, Kanakura Y. Thrombopoietin-induced differentiation of a human megakaryoblastic leukemia cell line, CMK, involves transcriptional activation of p21WAF1/Cip1 by STAT5. Mol Cell Biol. 1997;17:2933–2943. doi: 10.1128/mcb.17.5.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormick F. Activators and effectors of ras p21 protein. Curr Opin Genet Dev. 1994;4:71–76. doi: 10.1016/0959-437x(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 26.Melemed A S, Ryder J W, Vik T A. Activation of the mitogen-activated protein kinase pathway is involved in and sufficient for megakaryocytic differentiation of CMK cells. Blood. 1997;90:3462–3470. [PubMed] [Google Scholar]
- 27.Morella K K, Bruno E, Kumaki S, Lai C, Fu J, Wang H, Murray L, Hoffman R, Timour M, Benit L, Gisselbrecht S, Zhuang H, Wojchowski D M, Baumann H, Gearing D P. Signal transduction by the receptors for thrombopoietin (c-mpl) and interleukin-3 in hematopoietic and nonhematopoietic cells. Blood. 1995;86:557–571. [PubMed] [Google Scholar]
- 28.Morita H, Tahara T, Matsumoto A, Kato T, Miyazaki H, Ohashi H. Functional analysis of the cytoplasmic domain of the human Mpl receptor for tyrosine-phosphorylation of the signaling molecules, proliferation and differentiation. FEBS Lett. 1996;395:228–234. doi: 10.1016/0014-5793(96)01047-2. [DOI] [PubMed] [Google Scholar]
- 29.Mu S X, Xia M, Elliott G, Bogenberger J, Swift S, Bennett L, Lappinga D L, Hecht R, Lee R, Saris C J M. Megakaryocyte growth and development factor and interleukin-3 induce patterns of protein-tyrosine phosphorylation that correlate with dominant differentiation over proliferation of mpl-transfected 32D cells. Blood. 1995;86:4532–4543. [PubMed] [Google Scholar]
- 30.Nakajima K, Kusafuka T, Takeda T, Fujitani Y, Nakae K, Hirano T. Identification of a novel interleukin-6 response element containing an Ets-binding site and a CRE-like site in the junB promoter. Mol Cell Biol. 1993;13:3027–3041. doi: 10.1128/mcb.13.5.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakajima K, Yamanaka Y, Nakae K, Kojima H, Ichiba M, Kiuchi N, Kitaoka T, Fukada T, Hibi M, Hirano T. A central role for Stat3 in IL-6-induced regulation of growth and differentiation in M1 leukemia cells. EMBO J. 1996;15:3651–3658. [PMC free article] [PubMed] [Google Scholar]
- 32.Pallard C, Gouileux F, Benit L, Cocault L, Souyri M, Levy D, Groner B, Gisselbrecht S, Dusanter-Fourt I. Thrombopoietin activates a STAT5-like factor in hematopoietic cells. EMBO J. 1995;14:2847–2856. doi: 10.1002/j.1460-2075.1995.tb07284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pidard D, Montgomery R R, Bennet J S, Kunicki T J. Interaction of AP2, a monoclonal antibody specific for the human platelet glycoprotein IIb/IIIa complex, with intact platelets. J Biol Chem. 1983;258:12582–12586. [PubMed] [Google Scholar]
- 34.Porteu F, Rouyez M, Cocault L, Benit L, Charon M, Picaro F, Gisselbrecht S, Souyri M, Dusanter-Fourt I. Functional regions of the mouse thrombopoietin receptor cytoplasmic domain: evidence for a critical region which is involved in differentiation and can be complemented by erythropoietin. Mol Cell Biol. 1996;16:2473–2482. doi: 10.1128/mcb.16.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qui M S, Green S H. PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron. 1992;9:705–717. doi: 10.1016/0896-6273(92)90033-a. [DOI] [PubMed] [Google Scholar]
- 36.Rouyez M-C, Boucheron C, Gisselbrecht S, Dusanter-Fourt I, Porteu F. Control of thrombopoietin-induced megakaryocytic differentiation by the mitogen-activated protein kinase pathway. Mol Cell Biol. 1997;17:4991–5000. doi: 10.1128/mcb.17.9.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russo S, Tato F, Grossi M. Transcriptional down-regulation of myogenin expression is associated with v-ras-induced block of myogenin in unestablished quail muscle cells. Oncogene. 1997;14:63–73. doi: 10.1038/sj.onc.1200805. [DOI] [PubMed] [Google Scholar]
- 38.Sirinian M I, Marchetti A, Di-Rocco G, Starace G, Jucker R, Nasi S. Ras oncogene transformation of a human B lymphoblast is associated with lymphocyte activation and with a block of differentiation. Oncogene. 1993;8:157–163. [PubMed] [Google Scholar]
- 39.Sugahara H, Kanakura Y, Furitsu T, Ishihara K, Oritani K, Ikeda H, Kitayama H, Ishikawa J, Hashimoto K, Kanayama Y, Matsuzawa Y. Induction of programmed cell death in human hematopoietic cell lines by fibronectin via its interaction with very late antigen 5. J Exp Med. 1994;179:1757–1766. doi: 10.1084/jem.179.6.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terada K, Kaziro Y, Satoh T. Ras is not required for the interleukin 3-induced proliferation of a mouse pro-B cell line BaF3. J Biol Chem. 1995;46:27880–27886. doi: 10.1074/jbc.270.46.27880. [DOI] [PubMed] [Google Scholar]
- 41.Wakao H, Chida D, Damen J E, Krystal G, Miyajima A. A possible involvement of Stat5 in erythropoietin-induced hemoglobin synthesis. Biochem Biophys Res Commun. 1997;234:198–205. doi: 10.1006/bbrc.1997.6486. [DOI] [PubMed] [Google Scholar]
- 42.Whalen A N, Galasinski S C, Shapiro P S, Nahreini T S, Ahn N G. Megakaryocytic differentiation induced by constitutive activation of mitogen-activated protein kinase kinase. Mol Cell Biol. 1997;17:1947–1958. doi: 10.1128/mcb.17.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeigler F C, Sauvage F, Widmer H R, Keller G A, Donhue C, Schreiber R D, Malloy B, Hass P, Eaton D, Matthews W. In vitro megakaryopoietic and thrombopoietic activity of c-mpl ligand (TPO) on purified murine hematopoietic stem cells. Blood. 1994;84:4045–4052. [PubMed] [Google Scholar]