Abstract
BACKGROUND:
Surgical treatment of complex perianal fistula is technically challenging, associated with risk of failure, and may require multiple procedures. In recent years, several biologic agents have been developed for permanently eradicating anal fistulous disease with variable success. In this study, the treatment is an autologous whole-blood product created from the patients’ blood. It forms a provisional matrix that was found to be safe and effective in healing acute and chronic cutaneous wounds.
OBJECTIVE:
The study aimed to assess the efficacy and safety of an autologous blood clot product as a treatment for transsphincteric perianal fistulas.
DESIGN:
A prospective single-arm study.
SETTINGS:
A single tertiary medical center.
PATIENTS:
Patients with simple or complex transsphincteric fistulas confirmed by MRI were included in the study. Cause was either cryptoglandular or Crohn’s disease related (in the absence of active luminal bowel disease).
INTERVENTION:
The outpatient procedure was performed under general anesthesia and consisted of: 1) physical debridement and cleansing of the fistula tract; 2) suture closure of the internal opening; and 3) instillation of the autologous blood clot product into the entire tract.
MAIN OUTCOME MEASURES:
Safety and efficacy at 6- and 12-months after surgery.
RESULTS:
Fifty-three patients (77% men) with a median age of 42 (20–72) years were included in the study. Three patients withdrew consent, and 1 patient was lost to follow-up. At the time of this interim analysis, 49 and 33 patients completed the 6- and 12-month follow-up period. Thirty-four of the 49 patients achieved complete healing (69%) at 6 months, but 20 of the 33 patients (60%) achieved healing after 1 year. All patients who achieved healing at 6 months remained healed at the 1-year mark. In a subgroup analysis of patients with Crohn’s disease, 7 of 9 patients completed 1-year follow-up, with 5 patients (71%) achieving clinical remission. No major side effects or postoperative complications were noted, but 2 adverse events occurred (admission for pain control and coronavirus 2019 infection).
LIMITATIONS:
Noncomparative single-arm pilot study.
CONCLUSIONS:
Treatment with an autologous blood clot product in perianal fistular disease was found to be feasible and safe, with an acceptable healing rate in both cryptoglandular and Crohn’s disease fistula-in-ano. Further comparative assessment is required to determine its potential role in the treatment paradigm of fistula-in-ano. See Video Abstract.
BRAZO PARA EVALUAR LA SEGURIDAD Y EFICACIA DE RD2-VER.02, UN COÁGULO DE SANGRE AUTÓLOGO, EN EL TRATAMIENTO DE LA FÍSTULA ANAL
ANTECEDENTES:
El tratamiento quirúrgico de la fístula perianal compleja es técnicamente desafiante, se asocia con riesgo de fracaso y puede requerir múltiples procedimientos. En los últimos años, se han desarrollado varios agentes biológicos con el fin de erradicar permanentemente la enfermedad fistulosa anal con éxito variable. El tratamiento RD2-Ver.02 es un producto de sangre total autólogo creado a partir de la sangre de los pacientes, que forma una matriz provisional que resultó segura y eficaz para curar heridas cutáneas agudas y crónicas.
OBJETIVO:
Evaluar la eficacia y seguridad de RD2-Ver.02 como tratamiento para las fístulas perianales transesfinterianas.
DISEÑO:
Un estudio prospectivo de un solo brazo.
LUGARES:
Un único centro médico terciario.
PACIENTES:
Se incluyeron en el estudio pacientes con fístulas transesfinterianas simples o complejas confirmadas mediante resonancia magnética. La etiología fue criptoglandular o relacionada con la enfermedad de Crohn (en ausencia de enfermedad intestinal luminal activa).
INTERVENCIÓN:
El procedimiento ambulatorio se realizó bajo anestesia general y consistió en: 1) desbridamiento físico y limpieza del trayecto fistuloso; 2) cierre con sutura de la abertura interna; y 3) instilación de RD2-Ver.02 en todo el tracto.
PRINCIPALES MEDIDAS DE VALORACIÓN:
Seguridad y eficacia a los 6 y 12 meses después de la cirugía.
RESULTADOS:
Se incluyeron en el estudio 53 pacientes (77% varones) con una mediana de edad de 42 (20-72) años. Tres pacientes retiraron su consentimiento y un paciente se perdió durante el seguimiento. En el momento de este análisis intermedio, 49 y 33 pacientes completaron el período de seguimiento de 6 y 12 meses, respectivamente. Treinta y cuatro (34) pacientes lograron una curación completa (69%) a los 6 meses, mientras que 20 de 33 pacientes (60%) lograron una curación después de un año. Todos los pacientes que lograron la curación a los 6 meses permanecieron curados al año. En un análisis de subgrupos de pacientes con enfermedad de Crohn, 7/9 pacientes completaron un seguimiento de un año y 5 pacientes (71%) alcanzaron la remisión clínica. No se observaron efectos secundarios importantes ni complicaciones postoperatorias, mientras que ocurrieron 2 eventos adversos (ingreso para control del dolor e infección por COVID-19).
LIMITACIONES:
Estudio piloto no comparativo de un solo brazo.
CONCLUSIONES:
Se encontró que el tratamiento con RD2-Ver.02 en la enfermedad fístula perianal es factible y seguro, con una tasa de curación aceptable tanto en la fístula criptoglandular como en la de Crohn en el ano. Se requiere una evaluación comparativa adicional para determinar su papel potencial en el paradigma de tratamiento de la fístula anal. (Pre-proofed version)
Keywords: Autologous blood clot, Crohn’s disease, Perianal fistula, Surgical outcomes
Video Abstract
Video Abstract.
Anal fistula (AF) is a common and frequently disabling medical disorder that affects millions of people worldwide. It occurs when aberrant drainage tracts connect the anal canal and rectum with the skin around the anus. AF, which is classified on the basis of its anatomic location and the relation to the external sphincter muscles, can be simple or complex.1 Transsphincteric fistulas, which are the second most common type of perianal fistulas, can also be classified as complex if they involve more than 30% of the external sphincter muscle, high fistulas, and fistulas associated with IBD, among other parameters.1–5
The main factors affecting the ability to successfully manage AF are fistulas with multiple tracts, persistence of inflammation due to untreated sepsis or granulation tissue, inability to accurately locate and close internal openings, and active Crohn’s disease (CD). Despite multiple attempts at refining surgical management, recurrence rates remain unacceptably high for both cryptoglandular and CD-related AF.6
Surgical treatment remains the standard of care for perianal fistular disease.7 A fistulotomy is highly successful for simple transsphincteric fistulas; however, even this procedure risks some damage to the external anal sphincter and subsequent incontinence. Complex AF by definition involves more than 30% of the external sphincter muscle, rendering treatment far more challenging in terms of both successful eradication and fecal continence.8 The proliferation of surgical procedures and technologies is evidence of these challenges; however, both recurrence rates and incontinence rates have contributed to the lack of a universally accepted surgical approach.9 This is particularly true in CD-related AF, where success is even lower.10
Recently, efforts have shifted to various biologic products, including fibrin glue and mesenchymal and adipose-derived stem cells with the hope of avoiding anal sphincter injury while permanently occluding the fistula tract.11 However, robust clinical trials have produced only moderate success rates despite significant treatment costs. The result is that these biologic interventions are often restricted to very specific patient populations.11
RD2 ver.02 (RedDress Ltd, Pardes Hannah-Karkur, Israel) is an autologous whole-blood-clot treatment created from the patient’s peripheral blood at the time of the procedure.12 The treatment was found to be safe and effective in acute and chronic soft tissue wounds.13–17 The blood clot product is applied to the entire wound surface, thus creating a temporary scaffold and matrix to facilitate tissue healing.18
In this study, we aimed to explore the safety and efficacy of RD2 ver.02 treatment for closure of simple and complex transsphincteric anal fistula.
MATERIALS AND METHODS
Study Design
This report is an interim analysis of an open label, prospective pilot study, which aimed to examine the safety and efficacy of RD2 ver.02 in patients suffering from transsphincteric anal fistula. The study was conducted in a large tertiary medical center in Israel (Sheba Medical Center). The study was initiated in April 2021 and is currently ongoing. The study was approved by the hospital’s Ethics Committee (approval no.: 7731-20). All of the patients enrolled in the study provided informed consent.
Study Participants
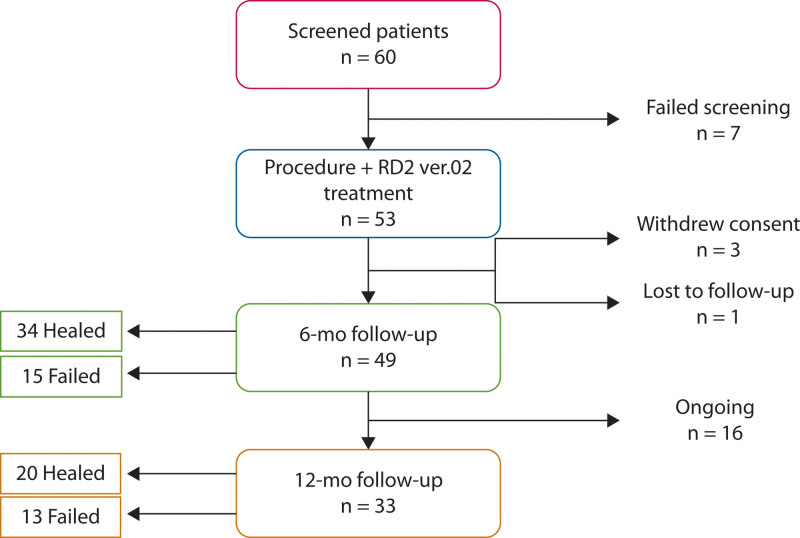
The study population consisted of patients aged 18 years or older with transsphincteric perianal fistula confirmed by a preoperative MRI. Sixty patients were screened for the study. Seven patients were excluded from the study after MRI determined the presence of an intersphincteric fistula amenable to fistulotomy (Fig. 1). Fifty-three patients who met the inclusion criteria were enrolled in the study and received the study procedure and RD2 ver.02 treatment. Inclusion criteria included patients aged 18 years or older with transsphincteric cryptoglandular or CD-related anal fistula confirmed by MRI. Patients on antiplatelet or oral anticoagulants were eligible. Patients with prior failed attempts at fistula closure were also allowed. Exclusion criteria included patients with active luminal IBD involving any portion of the GI tract, any malignancy, life expectancy of less than 24 months, prior medical history of coagulation disorders and/or abnormal platelet function, prolonged PT/PTT, and systemic steroids. Patients with IBD were excluded if they were suffering from any abdominal/extraintestinal symptoms or were actively treated for these symptoms by their gastroenterologist.
FIGURE 1.
Study flow chart.
Simple fistulas were categorized as transsphincteric AF with ≤30% of the external sphincter, and complex fistulas were defined as AF with more than 30% of the external sphincter, high transsphincteric fistulas (with or without a high blind tract), or horseshoe fistulas, based on the 2022 American Society of Colon and Rectal Surgeons guidelines for treatment of fistula-in-ano.7,19
The Procedure
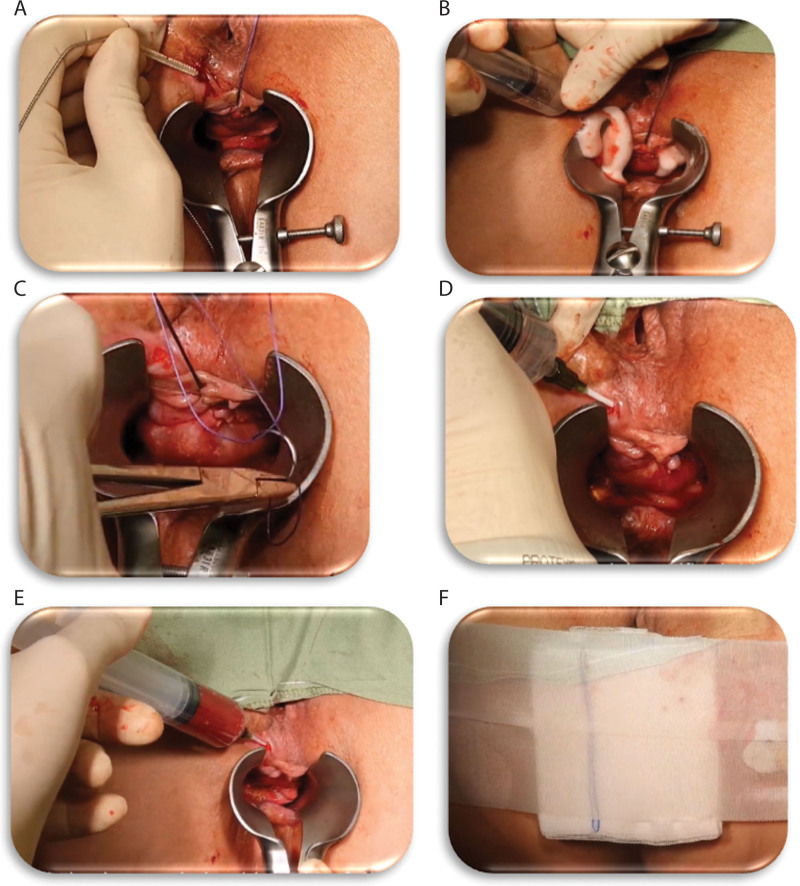
The procedure in the study was performed in an outpatient setting. Patients without a prior colonoscopy within 2 years from a surgical intervention were recommended to undergo a screening colonoscopy before surgery. The study procedure was performed under general anesthesia and the surgical steps were as follows: 1) debridement and cleansing of the fistula using a fistula brush and hydrogen peroxide followed by saline rinsing to remove any remains of hydrogen peroxide; 2) suture closure of the internal fistula opening, confirmed by water leak test; and 3) instillation of the RD2 ver.02 preparation.
Eighteen milliliters of blood was drawn from the patient for the RD2 ver.02 preparation. The blood was collected in acid citrate dextrose adenine vacuum tubes and was transferred to the activation mold containing calcium gluconate (50 mg) and kaolin (28 mg) powders (RedDress, Pardes Hannah-Karkur, Israel) for activation ex vivo. The RD2-Ver.02 preparation was instilled during withdrawal from the fistula tract of a 14G Angiocath IV catheter from the closed internal opening to the external fistula opening. The operative field was observed for approximately 5 minutes to assure coagulation of the RD2 ver.02 preparation within the fistula tract (Fig. 2). A video of the procedure can be seen in the supplement files (see Supplemental Video 1).
FIGURE 2.
Detailed description of the surgical and RD2 ver.02 instillation procedures. A, Fistula debridement with a fistula brush. B, Fistula cleaning with hydrogen peroxide. C, Suturing of the fistula internal opening. D, Water leak test. E, Preparation of RD2 ver.02 treatment and application to the fistula cavity. F, Blockage of the outer opening with an adhesive dressing to allow the blood to coagulate inside the fistula tract. Dress the fistula and anus using the nonadherent dressing and secure it with a Medi-strip.
Video 1.
Postoperatively, patients were given oral antibiotics (ciprofloxacin 500 mg twice daily + metronidazole 500 mg three times daily for 7 days) and were prescribed stool softeners (sodium picosulfate 30 g daily for 1 week), both based on the surgeon’s discretion.
Patients followed up at the clinic at 1 week, 2 weeks, 1 month, 2 months, 3 months, and 6 months after the procedure. Long-term follow-up is to be conducted 12 months after the procedure.
Healing/Remission Assessment of the Fistula
Clinical efficacy was assessed using fistula clinical symptoms compared with baseline. Fistula clinical symptoms included discharge from the fistula, healing/remission of the fistula tract, and external opening, as determined by the investigator at the follow-up visits.
The symptoms were graded on a scale of deterioration, no improvement, minor improvement, medium improvement, and significant improvement. Complete healing/remission was defined as the absence of any anal symptoms, including pain and discharge from the fistula, and a clinically confirmed closed external opening, which were validated with a postoperative MRI. Postoperative MRI was performed at 6 months in all patients. Healing at 12 months was evaluated clinically.
Study Outcomes
The primary end point was healing rate by 6 months of AF treated with RD2 ver.02, defined as the absence of any anal symptom, with no discharge from the fistula and a closed external opening confirmed on clinical evaluation and a postoperative MRI, which was performed at 6 months in all evaluated patients.
Safety end point was evaluated by the overall incidence and severity of adverse events (AEs), based on Common Terminology Criteria for Adverse Events, version 5.5
RESULTS
A total of 53 patients met the inclusion criteria and underwent the study procedure. Four (4) patients did not complete follow-up, 3 withdrew informed consent before the follow-up portion of the study, and 1 was lost to follow-up (Fig. 2). All patients who underwent the study procedure were included in the safety analysis. However, the data presented in the interim efficacy data at 6 months are presented as per protocol for those who participated in the postoperative follow-up regimen and as intent to treat for all patients, regardless of their adherence to the follow-up regimen. The study demographic and clinical data are demonstrated in Table 1. The majority of patients were men with a median age of 42 (range, 20–72) years and had cryptoglandular AF (n = 44; 83%). MRI findings diagnosed 19 patients (36%) as having simple and 34 patients (64%) as having complex AF. Twenty-five patients (47%) had a seton present for at least 6 to 8 weeks before the procedure. The seton was removed at the time of the procedure. Forty percent of patients had failed other interventions, ranging from 2 to 6 prior treatments before the procedure, including fistulectomy (n = 7; 13%) or ligation of the intersphincteric fistula tract (n = 4; 8%). The mean operative time was 25 ± 3 minutes.
TABLE 1.
Patients demographic and clinical characteristics
| Parameter | n (%) |
|---|---|
| Age, y, median (range) | 42 (20–72) |
| Sex | |
| Male | 41 (77.4%) |
| Female | 12 (22.6%) |
| Causes | |
| Cryptoglandular disease | 44 (83.0%) |
| Crohn’s disease | 9 (16.9%) |
| Comorbidities | |
| Smoking | 13 (24.5%) |
| Type 2 diabetes mellitus | 2 (3.7%) |
| Type of fistula (preoperative MRI) | |
| Simple | 19 (35.8%) |
| Complex | 34 (64.2%) |
| Multiple tracts (preoperative MRI) | 20 (37.7%) |
| Multiple openings | 13 (24.5%) |
| Prior fistula treatment | |
| Fistulotomy and fistulectomy | 7 (13.2%) |
| LIFT | 4 (7.5%) |
| Other surgical procedures | 10 (18.8%) |
LIFT = ligation of intersphincteric fistula tract.
Interim Results Analysis
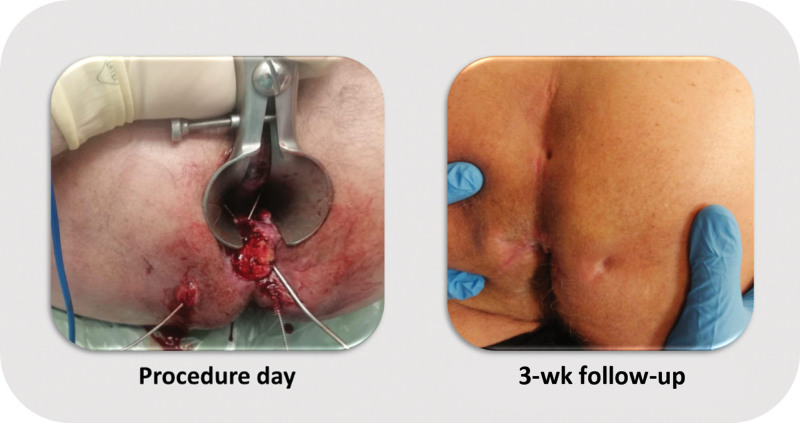
At the time of this interim analysis, 49 patients had reached the 6-month follow-up and 33 had reached 1-year follow-up (Fig. 2). At 6-month follow-up, 34 patients (69%) were healed after RD2 ver.02 treatment. At 1 year, 20 subjects reached complete healing (61%). All the patients who achieved healing at 6 months remained healed at 1 year. A total of 20 patients presented with multiple tracts, and 13 patients had multiple openings and multiple tracts at the time of the procedure. Among the patients with multiple tracts, 13 of 20 (65%) healed by 6 months. As for the group of patients with both multiple openings and tracts, 9 of 13 (69%) healed by the 6-month time frame. Stratifying for the complexity of the fistula demonstrated that 23 of 34 (68%) complex AFs were healed after 6 months, similar to the outcome for simple AF (11/19; 58%). An example of a patient with a complex fistula who achieved fistula healing can be seen in Figure 3. Kaplan–Meier analysis demonstrated that the median duration of success was 379 days (95% CI, 354.8–403.1).
FIGURE 3.
Example of a patient who presented with a complex transsphincteric fistula with 4 external openings and was treated with RD ver. 02.
Patients With CD
Nine patients with perianal CD were enrolled in the study. At the time of enrollment, 8 patients were on maintenance treatment with biological therapy for their CD without evidence of active GI disease. At the time of this analysis, all 9 patients reached the 6-month follow-up, but only 7 of them have reached the 12-month follow-up time point.
At the 6-month follow-up, 6 of 9 patients (67%) were healed, 1 patient showed progression toward healing, and 2 patients were considered initial treatment failures. At the 1-year mark, 5 of 6 patients ultimately remained healed (83%) and 1 patient failed at 1 year.
Safety
There were no device-related adverse events. There were a total of 15 AEs, of which 2 were serious adverse events (SAEs). The first SAE was hospitalization because of coronavirus 2019 and was not related to the device or the study. The second SAE was hospitalization under observation for pain with no evidence of perianal sepsis. The patient was treated with morphine and discharged less than 24 hours after admission.
DISCUSSION
In this study, we performed the first-in-human assessment of RD2 ver.02 for the management of simple and complex transsphincteric AF. Interim analysis demonstrated that healing at 6 and 12 months was 69% and 60%. In addition, we found that all patients who were healed at 6 months remained healed at the 1-year follow-up mark. These findings were originally presented at the annual meeting of the American Society of Colon and Rectal Surgeons.19
In recent years, several attempts have been made to create an injectable material capable of effectively and permanently closing an anal fistula tract. These included fibrin glue, plugs, and mesenchymal and adipose-derived stem cells.20,21 However, overall success rates with these injectables have been modest, with some improvement with fistular drainage, but with a healing rate of 41.7% at 12 months.22 Outcomes for the management of complex AF with these products have been somewhat less successful with rates of 14% to 50%.23 A different strategy was the anal fistula plug, which was the merger of lyophilized porcine intestinal submucosa to serve as a bioabsorbable xenograft and a scaffolding material, creating a physical occlusion device for fistula closure.24 Although initial results were promising, long-term analysis indicates much more moderate rates of healing of 40% to 50%, especially in complex cases.25,26 Finally, stem cell therapy has recently gained traction in perianal CD. Long-term results were published for the ADMIRE-CD trial, which evaluated adipose-derived mesenchymal stem cell therapy for perianal CD, with remission rates of 56% compared with 40% in the control group.27 However, the indications for stem cell therapy are fairly limited and there is little evidence on healing rates in non-CD patients. The limited success associated with these prior biologic materials coupled with the significant product-related costs has degraded any evidence for cost-effective benefits for anal fistula treatment.28–30 Conversely, the data suggest that RD2 ver.02 may be effective for both cryptoglandular and CD-associated transsphincteric fistula-in-ano, regardless of whether a previous attempt at fistula closure had been attempted. This therapy is potentially less risky compared with other treatments because the patient’s whole blood is the principal therapeutic agent.
RD2 ver.02 is a novel whole-blood clot product that offers a functional extracellular matrix to protect and facilitate tissue healing.31 Coagulation agents are mixed with the patient’s blood to accelerate clot formation, and the resulting mixture is applied into wound cavities, allowing the coagulation process to occur inside the cavity, providing a fibrin scaffold and facilitating tissue healing.14 Because the final coagulation steps occur inside the cavity, the clot and its beneficial factors come into contact with the entire surface of the treated cavity such as an anal fistula. It was proposed that the clots serve as a provisional extracellular matrix and promote wound re-epithelialization.32 Furthermore, as the coagulation process occurs, the blood clot attaches to the exposed surface of the cavity and contracts, causing the surface to draw closer together as it forms a scaffold.
Previous studies have demonstrated the safety and efficacy of this technology in treating challenging nonhealing cutaneous wounds12–17 and complex surgical wounds.33 Using autologous blood clots, which provide a transient scaffold and recruit surrounding cells to activate wound healing,32 presents a promising strategy to treat open and chronic wounds.
Study Limitations
This study shows the efficacy and safety of RD2 ver.02 in transsphincteric perianal fistulas. The study has several limitations, mainly due to the fairly small sample of patients inherent in a pilot study. The lack of randomization and a control group limits the generalization of the results as well as any assessment of comparative effectiveness for RD2 ver.02. Although we acknowledge the limitation of the lack of randomization and a control group in our study, it is important to discuss this issue in more detail to provide a comprehensive understanding of its implications. The absence of control patients raises the possibility that the positive outcomes observed may not be solely attributable to our RD2 ver.02 product but could also be influenced by the inherent benefits of the curettage and internal opening closure procedure itself. This raises the need for a more nuanced interpretation of our findings. In addition, the study is ongoing and a subset of patients still needs to be fully assessed at 1 year.
Despite these limitations, a healing rate of 69% in a mixed population, including patients with CD and with more than half of the patients presenting with a complex fistula, is fairly impressive. The vast inclusion criteria for the study highlight the advantages of RD2 ver.02, which can be used in patients with different causes for perianal fistular disease, is easy to use, and does not require any specialized expertise from the operating surgeon for the use of RD2 ver.02.
CONCLUSIONS
RD2 ver.02 treatment was found to be safe and effective in achieving healing in transsphincteric AF of both cryptoglandular and CD-related origin within 6 months of treatment. Once healed, the closure seems very durable with minimal risk of recurrence. Prior therapeutic intervention does not interfere with the application of RD2 ver.02 and potentially successful fistula closure.
Further comparative studies are needed to confirm the potential benefits of RD2 ver.02, which represents a safe biologic alternative for the closure of transphincteric AF.
ACKNOWLEDGMENTS
The authors express their sincere gratitude to Dr Anthony J. Senagore for his invaluable assistance and contributions to this scientific study.
Footnotes
Funding/Support: None reported.
Financial Disclosure: Dr Ram is a consultant at RedDress Ltd.
Disclaimer: The RD2 ver.02 product was supplied by the company free of charge for this study.
Presented at the annual scientific meeting of the American Society of Colon and Rectal Surgeons, Seattle, WA, June 3 to 6, 2023.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML and PDF versions of this article on the journal’s website (www.dcrjournal.com).
Contributor Information
Edward Ram, Email: edwardrm@netvision.net.il.
Yaniv Zager, Email: yaniv039@gmail.com.
Dan Carter, Email: dr.dancarter@gmail.com.
Olga Saukhat, Email: Olga.Saukhat@sheba.health.gov.il.
Roi Anteby, Email: anteby.roi@gmail.com.
Ido Nachmany, Email: ido.nachmany@sheba.health.gov.il.
REFERENCES
- 1.Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1–12. [DOI] [PubMed] [Google Scholar]
- 2.Carr S, Velasco AL. Fistula-in-Ano. Treasure Island, FL: StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK557517/. [PubMed] [Google Scholar]
- 3.Vogel JD, Johnson EK, Morris AM, et al. Clinical practice guideline for the management of anorectal abscess, fistula-in-ano, and rectovaginal fistula. Dis Colon Rectum. 2016;59:1117–1133. [DOI] [PubMed] [Google Scholar]
- 4.Steele SR, Kumar R, Feingold DL, Rafferty JL, Buie WD; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of perianal abscess and fistula-in-ano. Dis Colon Rectum. 2011;54:1465–1474. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed October 12, 2023.
- 6.Garg P, Sodhi SS, Garg N. Management of complex cryptoglandular anal fistula: challenges and solutions. Clin Exp Gastroenterol. 2020;13:555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaertner WB, Burgess PL, Davids JS, et al. ; Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of anorectal abscess, fistula-in-ano, and rectovaginal fistula. Dis Colon Rectum. 2022;65:964–985. [DOI] [PubMed] [Google Scholar]
- 8.Bubbers EJ, Cologne KG. Management of complex anal fistulas. Clin Colon Rectal Surg. 2016;29:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Göttgens KWA, Smeets RR, Stassen LPS, Beets G, Breukink SO. Systematic review and meta-analysis of surgical interventions for high cryptoglandular perianal fistula. Int J Colorectal Dis. 2015;30:583–593. [DOI] [PubMed] [Google Scholar]
- 10.Lee JL, Yoon YS, Yu CS. Treatment strategy for perianal fistulas in Crohn disease patients: the surgeon’s point of view. Ann Coloproctol. 2021;37:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-Olmo D, Gómez-Barrera M, de la Portilla F. Surgical management of complex perianal fistula revisited in a systematic review: a critical view of available scientific evidence. BMC Surg. 2023;23:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kushnir I, Kushnir A, Serena T, Garfinkel D. Efficacy and safety of a novel autologous wound matrix in the management of complicated, chronic wounds: a pilot study. Wounds. 2016;28:317–327. [PubMed] [Google Scholar]
- 13.Naude L, Idensohn P, Bruwer F, et al. An observational pilot study to collect safety and efficacy data on wound care using whole blood clot technology on hard-to-heal wounds. Wounds Int J. 2021;12:42–53. [Google Scholar]
- 14.Snyder R, Kasper M, Patel K, et al. Safety and efficacy of an autologous blood clot product in the management of Texas 1A or 2A neuropathic diabetic foot ulcers: a prospective, multicenter, open label pilot study. Wounds. 2018;30:84–89. [PubMed] [Google Scholar]
- 15.Gurevich M, Wahab N, Wachuku C, Ead KJ, Snyder RJ. ActiGraft treatment in complex wounds with exposed structure—a case series. Ann Rev Resear. 2021;7:555701. [Google Scholar]
- 16.Williams M, Davidson D, Wahab N, Hawkins J, Wachuku CD, Snyder R. Innovative treatment utilizing an autologous blood clot for diabetic foot ulcers. Wounds. 2022;34:195–200. [PubMed] [Google Scholar]
- 17.Landau Z, Whitacre KL, Leewood C, Hawkins J, Wachuku CD. Utilization of a topical autologous blood clot for treatment of pressure ulcers. Int Wound J. 2023;20:806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rausch MK, Parekh SH, Dortdivanlioglu B, Rosales AM. Synthetic hydrogels as blood clot mimicking wound healing materials. Prog Biomed Eng (Bristol). 2021;3:042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ram E, Zager Y, Carter D, et al. An autologous whole blood clot treatment in patients with anal fistula [ASCRS abstract S11]. Dis Colon Rectum. 2023;66:e361. [Google Scholar]
- 20.Ji L, Zhang Y, Xu L, Wei J, Weng L, Jiang J. Advances in the treatment of anal fistula: a mini-review of recent five-year clinical studies. Front Surg. 2021;7:586891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hjortrup A, Moesgaard F, Kjærgård J. Fibrin adhesive in the treatment of perineal fistulas. Dis Colon Rectum. 1991;34:752–754. [DOI] [PubMed] [Google Scholar]
- 22.de la Portilla F, Muñoz-Cruzado MVD, Maestre MV, et al. Platelet-rich plasma (PRP) versus fibrin glue in cryptogenic fistula-in-ano: a phase III single-center, randomized, double-blind trial. Int J Colorectal Dis. 2019;34:1113–1119. [DOI] [PubMed] [Google Scholar]
- 23.Swinscoe MT, Ventakasubramaniam AK, Jayne DG. Fibrin glue for fistula-in-ano: the evidence reviewed. Tech Coloproctol. 2005;9:89–94. [DOI] [PubMed] [Google Scholar]
- 24.Van Koperen PJ, Bemelman WA, Bossuyt PM, et al. The anal fistula plug versus the mucosal advancement flap for the treatment of anorectal fistula (PLUG trial). BMC Surg. 2008;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen MS, Kjær ML, Andersen J. Efficacy of plug treatment for complex anorectal fistulae: long-term Danish results. Ann Coloproctol. 2019;35:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almeida IS, Wickramasinghe D, Weerakkody P, Samarasekera DN. Treatment of fistula in-ano with fistula plug: experience of a tertiary care centre in South Asia and comparison of results with the West. BMC Res Notes. 2018;11:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Olmo D, Gilaberte I, Binek M, et al. Follow-up study to evaluate the long-term safety and efficacy of darvadstrocel (mesenchymal stem cell treatment) in patients with perianal fistulizing Crohn’s disease: ADMIRE-CD phase 3 randomized controlled trial. Dis Colon Rectum. 2022;65:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayne DG, Scholefield J, Tolan D, et al. ; FIAT Trial Collaborative Group. A multicenter randomized controlled trial comparing safety, efficacy, and cost-effectiveness of the surgisis anal fistula plug versus surgeon’s preference for transsphincteric fistula-in-ano: the FIAT trial. Ann Surg. 2021;273:433–441. [DOI] [PubMed] [Google Scholar]
- 29.Eberspacher C, Mascagni D, Ferent IC, et al. Mesenchymal stem cells for cryptoglandular anal fistula: current state of art. Front Surg. 2022;9:815504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng F, Huang Z, Li Z. Efficacy and safety of mesenchymal stem cells in treatment of complex perianal fistulas: a meta-analysis. Stem Cells Int. 2020;2020:8816737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder RJ, Schultz G, Wachuku C, Rashid AM, Ead JK. Proposed mechanism of action of topically applied autologous blood clot tissue. J Am Podiatr Med Assoc. 2023;113:20–140. [DOI] [PubMed] [Google Scholar]
- 32.Serena TE, Kushnir I, Kushnir A, Yaakov RA, Eckert KA. The safety of an autologous whole blood clot product applied to full thickness dermal wounds in a porcine model for up to 18 days. Chronic Wound Care Manag Res. 2019; 6:39–49. [Google Scholar]
- 33.Gurevich M, Heinz SM, Fridman R, Hawkins J, Wachuku CD. Use of autologous whole blood clot in the treatment of complex surgical wounds: a case series. J Wound Care. 2023;32(Suppl 2):S4–S9. [DOI] [PubMed] [Google Scholar]