Abstract
N-MYC (encoded by MYCN) is a critical regulator of hematopoietic stem cell function. While the role of N-MYC deregulation is well established in neuroblastoma, the importance of N-MYC deregulation in leukemogenesis remains elusive. Here, we demonstrate that N-MYC is overexpressed in acute myeloid leukemia (AML) cells with chromosome inversion inv(16) and contributes to the survival and maintenance of inv(16) leukemia. We identified a previously unknown MYCN enhancer, active in multiple AML subtypes, essential for MYCN mRNA levels and survival in inv(16) AML cells. We also identified eukaryotic translation initiation factor 4 gamma 1 (eIF4G1) as a key N-MYC target that sustains leukemic survival in inv(16) AML cells. The oncogenic role of eIF4G1 in AML has not been reported before. Our results reveal a mechanism whereby N-MYC drives a leukemic transcriptional program and provides a rationale for the therapeutic targeting of the N-MYC/eIF4G1 axis in myeloid leukemia.
N-MYC and EIF4G1 are overexpressed in acute myeloid leukemia and play a major role in leukemic survival and maintenance.
INTRODUCTION
The core binding factor (CBF) is a heterodimeric transcription factor complex composed of DNA binding RUNX proteins (encoded by one of three genes: RUNX1, RUNX2, and RUNX3) and the non–DNA binding CBFβ protein. RUNX1 and CBFβ play essential roles in an overlapping and differentiation stage–specific manner in embryonic and adult hematopoiesis (1, 2). The inv(16)(p13q22) results in the formation of a chimeric gene consisting of the 5′ portions of CBFB fused to the 3′ portion of the smooth muscle myosin heavy chain gene, MYH11, which encodes CBFβ-SMMHC fusion protein. Inv(16) is reported in 5 to 8% of acute myeloid leukemia (AML) patients. CBFβ-SMMHC has a higher affinity for RUNX1 than the native CBFβ (3, 4). CBFβ-SMMHC–expressing embryos die at mid-gestation due to block in definitive hematopoiesis (5), which is very similar to the phenotype from Runx1 and Cbfb knockout embryos (1, 2), suggesting that the CBFβ-SMMHC fusion protein is a dominant repressor of RUNX1. Studies using conditional knock-in mouse models demonstrated that CBFβ-SMMHC deregulates hematopoietic stem cell (HSC) differentiation in adult hematopoiesis by expanding short-term HSCs and myeloid progenitor cells (6–8). These aberrant myeloid progenitors with pre-leukemic potential upon acquisition of secondary cooperating mutations induce AML (9–11). Recent attempts to inhibit protein-protein interaction between RUNX1 and CBFβ-SMMHC via small-molecule inhibitor AI-10-49 demonstrated promising results in selectively inducing apoptosis in primary human inv(16) AML cells and a genetic mouse model for inv(16) (12, 13). Further, we demonstrated that AI-10-49–induced cell death is partly mediated by repression of c-MYC expression in inv(16) AML cells (14).
The MYC family genes include three closely related genes: MYC (encoding c-MYC), MYCN (encoding N-MYC), and MYCL (encoding L-MYC) (15). While c-MYC function is widely investigated in hematological malignancies, the function of other MYC family members in inv(16) AML, and AML in general, remains poorly understood. Here, we demonstrate that AI-10-49 treatment in inv(16) AML cells down-regulated MYCN transcript levels. Using primary human inv(16) AML cells and the patient-derived xenograft (PDX) model, we demonstrate that N-MYC is required for the maintenance of inv(16) AML survival. Furthermore, by comparing N-MYC transcriptional targets with gene expression changes in human inv(16) AML, we identified eukaryotic translation initiation factor 4 gamma 1 (eIF4G1) as a key N-MYC target in leukemic survival. Collectively, our results illustrate that the oncogenic N-MYC/eIF4G1 axis is instrumental in inv(16) AML leukemogenesis.
RESULTS
MYCN is up-regulated in inv(16) AML
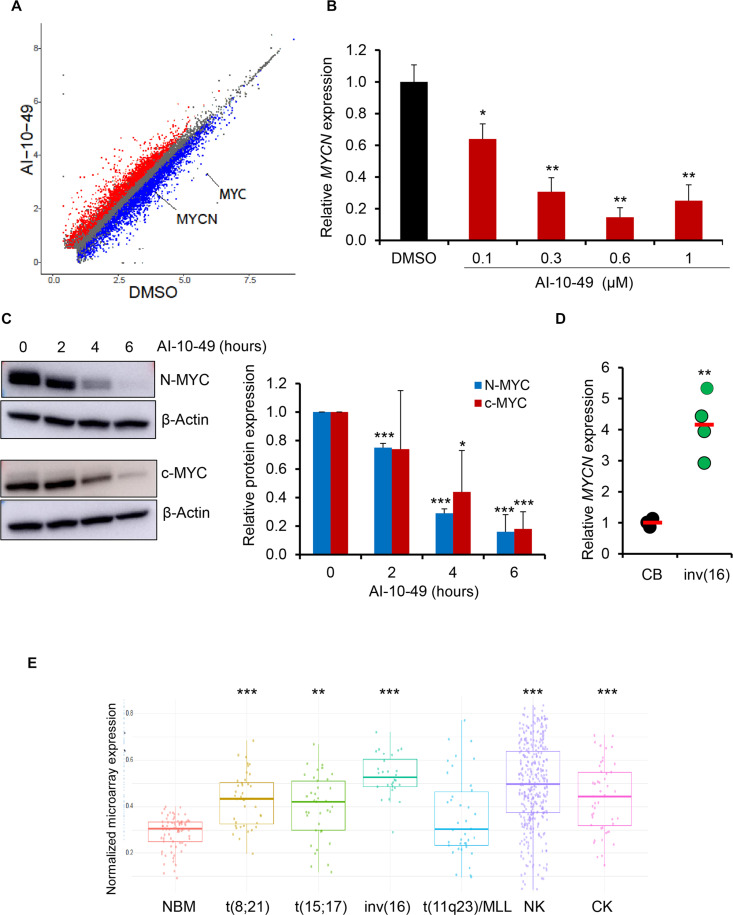
AI-10-49 treatment results in transcriptional changes, including c-MYC repression, and triggers apoptosis in inv(16) AML cells (14). To understand the potential role of other MYC family members, we re-analyzed previous RNA-sequencing (RNA-seq) data (14). We found that the MYCN transcript is down-regulated with AI-10-49 treatment in human inv(16) AML cell line ME-1 (Fig. 1, A and B, and fig. S1A) (16). The MYCL transcript is not expressed in ME-1 cells. Similar to the repression of MYCN and MYC at transcript levels, we also observed the down-regulation of N-MYC and c-MYC protein levels with AI-10-49 in inv(16) AML cells (Fig. 1C and fig. S1B). AI-10-49 treatment did not display any changes in MYCN transcript levels in non-inv(16) AML cell lines (fig. S1C). Down-regulation of MYCN expression by AI-10-49 suggested that MYCN overexpression could be a critical step in inv(16) leukemogenesis. To test this, we analyzed MYCN transcript levels in human AML patient samples. Our data demonstrated that MYCN transcript levels were up-regulated in primary inv(16) AML patient samples compared to healthy control samples (Fig. 1, D and E). Bromodomain inhibitors have been reported to repress MYC and MYCN in cancer cells (17, 18). We have previously shown that bromodomain inhibitor JQ1 down-regulates MYC transcript and synergizes with AI-10-49 in inducing apoptosis in inv(16) leukemic cells. We found that JQ1 treatment in inv(16) AML cells induces repression of MYCN transcription (fig. S1D). This suggests that in addition to c-MYC, N-MYC may have important roles in apoptosis induction by JQ1 in inv(16) AML cells in the previous study (14). Genomic deletion of CBFB-MYH11 resulted in the down-regulation of MYCN transcript levels (fig. S1, E and F). RUNX1 is required for CBFβ-SMMHC–induced leukemogenesis (19). We found that RUNX1 inhibition with small-molecule inhibitor AI-14-91 (20) resulted in the down-regulation of MYCN transcript levels (fig. S1G). Together, our results demonstrate that MYCN is one of the major downstream targets of CBFβ-SMMHC in inv(16) AML.
Fig. 1. MYCN is up-regulated in inv(16) AML.
(A) Scatterplot of differentially expressed genes in RNA-seq analysis between DMSO- and AI-10-49–treated ME-1 cells (>2-fold change; FDR < 0.01). Genes that significantly changed are colored in red and blue for up-regulated and down-regulated, respectively. MYC and MYCN are highlighted. (B) MYCN transcript levels in DMSO/AI-10-49–treated (6 hours) ME-1 cells by real-time RT-PCR. Histogram representative of triplicate experiments. (C) N-MYC and c-MYC protein levels in AI-10-49–treated (1 μM) ME-1 cells by Western blot. The quantification of three independent experiments is shown on the right. (D) MYCN transcript levels in human cord blood CD34+ cells (CB) and primary human inv(16) AML CD34+ cells [inv(16)]. Each symbol represents the average of a triplicate experiment from one sample, and the average value of the group is shown in red. (E) Normalized expression of MYCN transcript levels in normal bone marrow (NBM) and AML samples in the Haferlach dataset extracted from the Leukemia Gene Atlas (expression array) database. P values between NBM and different AML subtypes are shown. MLL, mixed lineage leukemia; NK, normal karyotype; CK, complex karyotype. Error bars represent the SD. Significance was calculated using an unpaired t test. *P < 0.05, **P < 0.005, ***P < 0.0005.
N-MYC is required for inv(16) AML cell survival
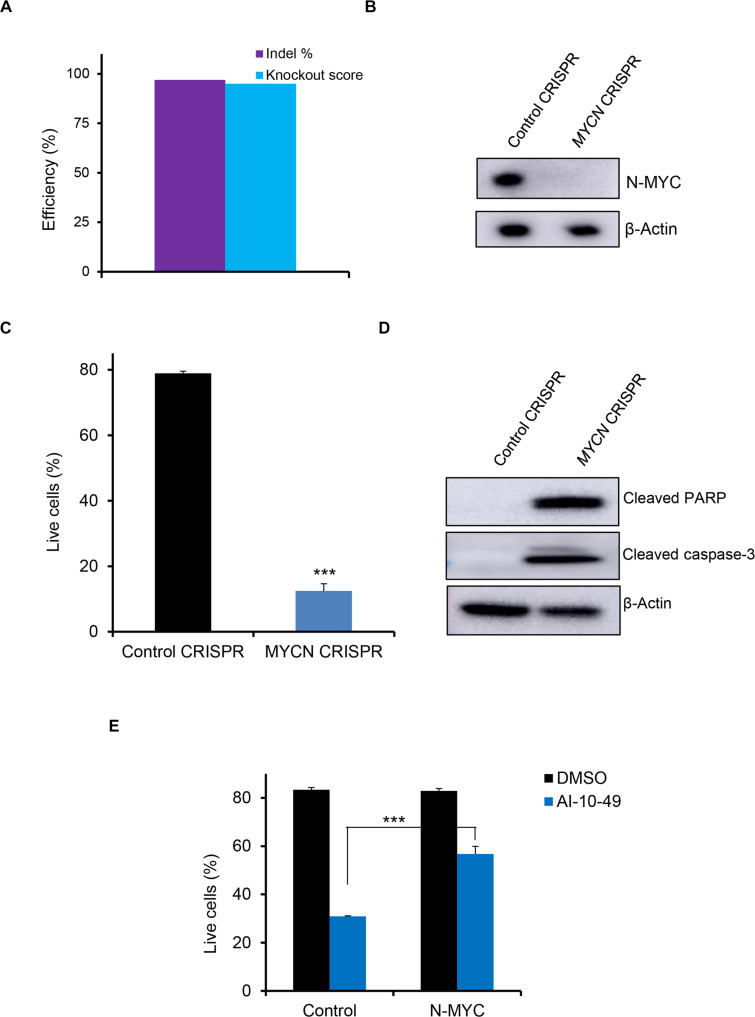
To understand the functional role of N-MYC in inv(16) AML cells, we assessed whether the deletion of MYCN affects the survival of inv(16) AML cells. We applied CRISPR/Cas9 technology using a ribonucleoprotein (RNP) complex approach to delete MYCN in inv(16) AML cells. ME-1 cells transfected with Cas9 protein and three MYCN chemically modified guide RNAs (CM-gRNAs) produced efficient deletion of MYCN genomic regions (Fig. 2A and fig. S2, A and B), resulting in a 95% reduction in N-MYC protein levels (Fig. 2B). MYCN deletion in ME-1 cells substantially reduced cell viability due to apoptosis (Fig. 2C). The onset of apoptosis was accompanied by cleavage of caspase-3 and poly(adenosine diphosphate–ribose) polymerase (PARP) proteins in ME-1 cells (Fig. 2D). Granulocyte differentiation markers such as CD11b, CD15, neutrophil elastase, and CSF1-R were not changed during MYCN deletion (fig. S2, C to F), suggesting that MYCN deletion induces apoptosis and not granulocytic differentiation in inv(16) AML cells. Ectopic N-MYC overexpression partially rescued AI-10-49–induced apoptosis in ME-1 cells (Fig. 2E). Together, these results indicate that N-MYC contributes to the survival of inv(16) AML cells. MYCN deletion in four non-inv(16) AML cell lines demonstrated that N-MYC is important in the survival of leukemic cells in an AML subtype–specific manner (fig. S2, G and H). In contrast, normal human hematopoietic stem/progenitor cells (HSPCs) were not affected in survival or differentiation during MYCN deletion (fig. S2, I to K). These data suggest that N-MYC selectively regulates the survival of leukemic cells in specific AML subtypes without affecting normal hematopoiesis.
Fig. 2. N-MYC is required for inv(16) AML cell survival.
(A) ME-1 cells were transfected with Cas9 and control gRNA/pool of three MYCN gRNAs (Synthego Gene Knockout Kit V2) by RNP approach, and editing efficiency was analyzed by inference of CRISPR editing (ICE). (B) N-MYC protein levels in control/MYCN-edited ME-1 cells by Western blot. (C) Cell survival analysis in control/MYCN-edited ME-1 cells by annexin V/7AAD assay. Histogram representative of triplicate experiments. (D) Cleaved PARP and cleaved caspase-3 protein levels in control/MYCN-edited ME-1 cells by Western blot. (E) Cell viability analysis (annexin V/7AAD assay) in ME-1 cells expressing N-MYC treated with DMSO or AI-10-49. Histogram representative of triplicate experiments. Error bars represent the SD. Significance was calculated using an unpaired t test. **P < 0.005.
N-MYC silencing effectively reduces AML burden and extends survival in the NSGS xenograft model
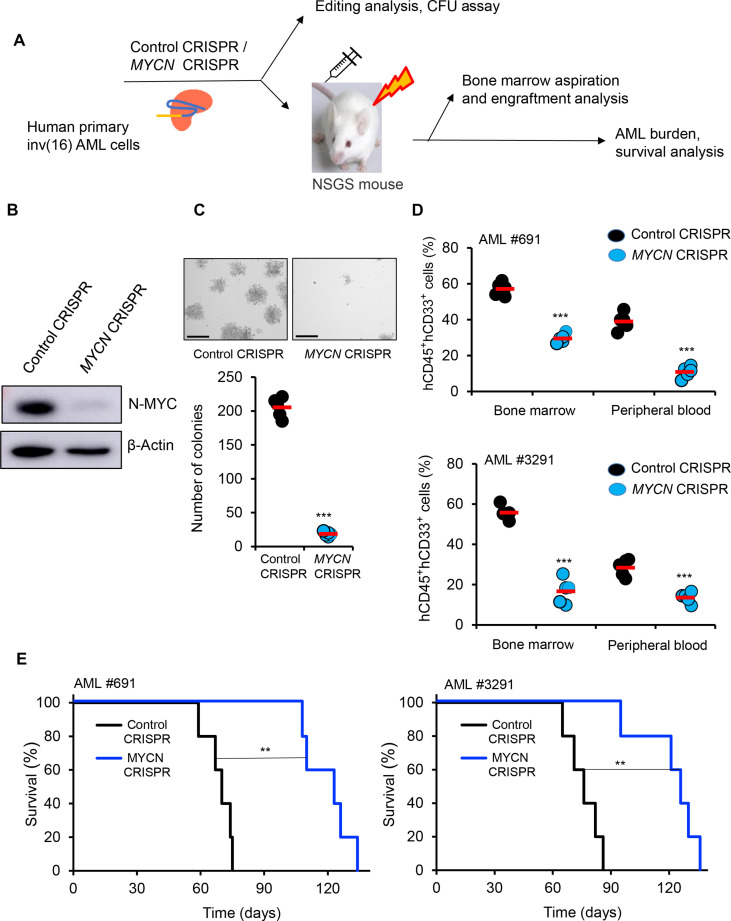
To further evaluate the role of N-MYC in inv(16) AML cell survival, we conducted MYCN genomic editing in primary human inv(16) AML cells. T cell–depleted primary inv(16) AML cells were transfected with Cas9 protein and three MYCN CM-gRNAs (Fig. 3A), resulting in a 90% reduction in N-MYC protein levels (Fig. 3B). MYCN deletion substantially reduced the clonogenic potential of primary inv(16) AML cells, as reflected by a marked decrease in colony number (Fig. 3C). The requirement of N-MYC in inv(16) leukemia in vivo was tested by transplanting the edited leukemic cells into irradiated (280 cGy) NSGS (NOD/SCID-IL2RG-SGM3) mice (21, 22) via tail vein injection. Engraftment efficiency of control and MYCN-edited leukemic cells in the bone marrow 5 days after transplantation was similar between groups (fig. S3, A and B). AML burden estimated by the frequency of hCD45+ hCD33+ AML stem/progenitor cells was markedly reduced in the bone marrow and peripheral blood of mice transplanted with MYCN-deleted inv(16) AML cells compared to control mice (Fig. 3D and fig. S3, C and D), suggesting that N-MYC is required for the maintenance of inv(16) leukemic cells. In addition, the median leukemic latency in mice transplanted with MYCN-deleted inv(16) AML cells was substantially extended (Fig. 3E). Collectively, these results demonstrate that N-MYC is important for the survival and maintenance of inv(16) leukemia.
Fig. 3. N-MYC silencing effectively reduces AML burden and extends survival in the NSGS xenograft model.
(A) Schematic of MYCN editing by CRISPR/Cas9 RNP approach in primary human inv(16) AML cells and transplantation in NSGS mouse model. (B) N-MYC protein levels in control/MYCN-edited primary human inv(16) AML cells by Western blot. (C) Colony counts for methylcellulose colony-forming assay performed upon 12 days of control/MYCN-edited primary inv(16) AML cells. Data are representative of three replicates. The average value of each group is shown in red. Scale bar, 275 μm. (D) Flow cytometric quantification of hCD45+ hCD33+ cells in NSGS mice transplanted with control/MYCN-edited primary inv(16) AML cells 5 weeks after transplantation. Each symbol represents a mouse. The average value of each group is shown in red. (E) Kaplan-Meier survival curve of NSGS mice transplanted with control/MYCN-edited primary inv(16) AML cells (n = 5 per group). Error bars represent the SD. Significance was calculated using an unpaired t test [(C) and (D)] and log-rank test (E). **P < 0.005, ***P < 0.0005.
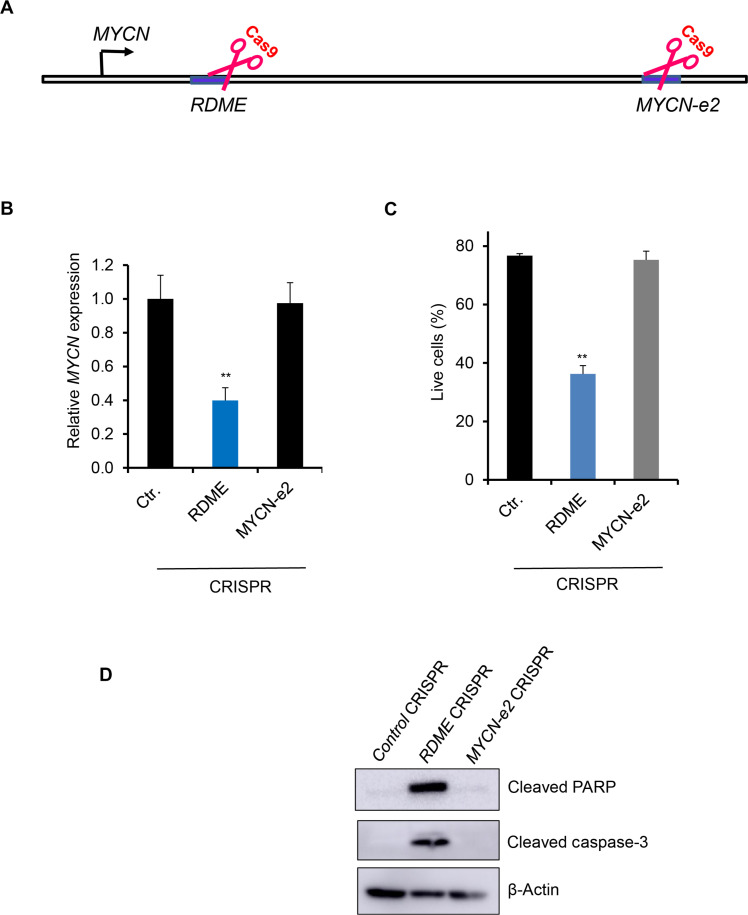
RUNX1 binds to an MYCN distal enhancer transcriptionally active in inv(16) AML cells
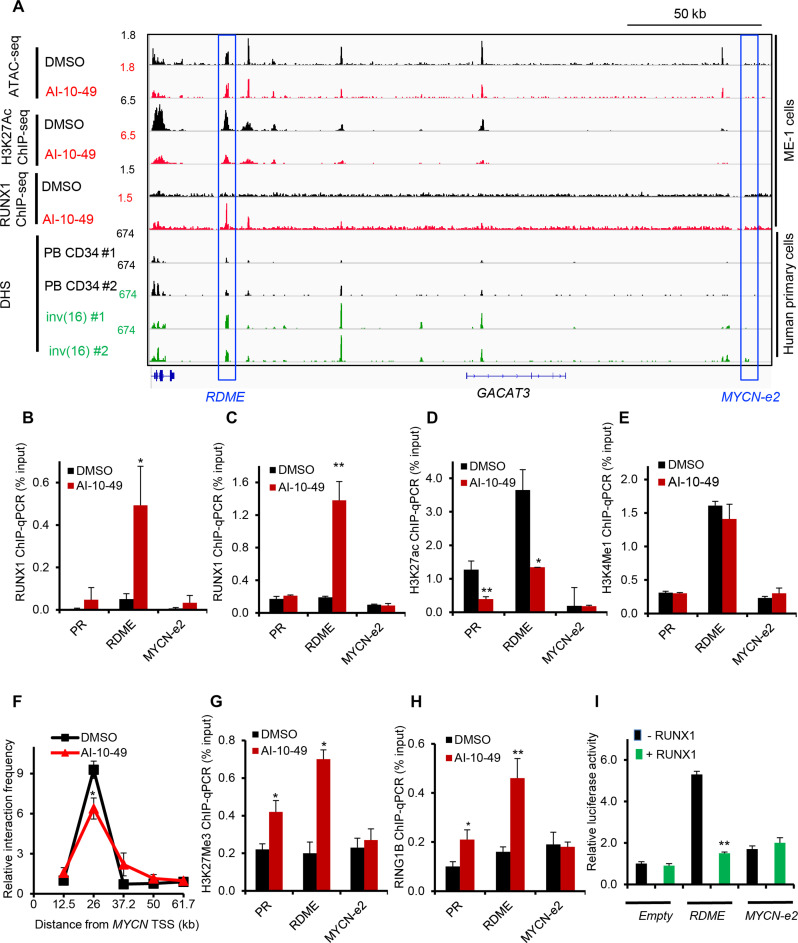
Distal enhancers regulate target gene transcription through chromatin loops that link enhancers with target gene promoters (23). We have previously shown that AI-10-49 induces genome-wide RUNX1 occupancy in inv(16) AML cells (14). We have demonstrated that RUNX1 binding at MYC distal enhancers plays a critical role in c-MYC expression and survival in inv(16) AML cells (14). We hypothesized that AI-10-49–mediated MYCN repression is due to direct RUNX1 binding at MYCN cis-regulatory elements, such as enhancers. To understand RUNX1 regulation of MYCN, we analyzed RUNX1 DNA binding profile and chromatin accessibility at the MYCN locus, previously reported in ME-1 cells (14). Analysis of RUNX1 chromatin immunoprecipitation–sequencing (ChIP-seq) peaks in AI-10-49–treated cells identified a genomic element located 26 kb downstream of MYCN transcription start site (TSS), named RDME (RUNX1-dependent MYCN enhancer) with increased RUNX1 binding (Fig. 4A). Analysis of the genomic sequence of RDME identified a consensus RUNX binding site (TGYGGT). Previous studies have identified multiple enhancer elements (e1 to e5) within 1.5 Mb downstream of MYCN TSS regulating MYCN expression in brain tumor cells (24). Enhancer element e2 (MYCN-e2) contains a consensus RUNX binding site. We did not find any RUNX1 binding at MYCN-e2 with AI-10-49 treatment in inv(16) AML cells (Fig. 4A). Enhanced RUNX1 binding at RDME, but not at MYCN-e2 and the MYCN promoter, was validated by conducting ChIP-qPCR (quantitative polymerase chain reaction) in ME-1 cells (Fig. 4B) and primary human inv(16) AML cells (Fig. 4C). H3K27ac, a histone mark representing transcriptionally active regions, was enriched at RDME and MYCN promoter (PR) and markedly reduced with AI-10-49 (Fig. 4 A and D). Analysis of chromatin accessibility by assay for transposase-accessible chromatin with high-throughput sequencing(ATAC-seq) demonstrated that RDME is a highly accessible chromatin element (Fig. 4A). On the basis of the ATAC-seq and H3K27ac ChIP-seq, MYCN-e2 is transcriptionally inactive in inv(16) AML cells. H3 Lys4 mono-methylation (H3K4me1) histone mark, which represents active enhancers, is enriched at RDME, suggesting that RDME functions as a bona fide enhancer (Fig. 4E). In support of this hypothesis, chromosome conformation capture (3C) analysis demonstrated that RDME associates with the MYCN proximal promoter and this interaction was inhibited with AI-10-49 treatment (Fig. 4F). Having known that RUNX1 recruits polycomb repressive complex 1 (PRC1) component RING1B to chromatin during hematopoietic differentiation (25) and in inv(16) AML cells (14), we hypothesized that during AI-10-49 treatment RUNX1 is repressing MYCN by recruiting PRC1 complex to RDME. Our results indicate that AI-10-49 treatment results in an enhanced repressive histone mark, H3K27Me3 at RDME, associated with increased enrichment of PRC1 complex member RING1B (Fig. 4, G and H). CBFB-MYH11 genomic editing in ME-1 cells displayed enhanced RUNX1 binding (fig. S4A) and a repressive chromatin state at RDME (fig. S4B). In luciferase reporter assays in ME-1 cells, RDME was found to have the strongest activity, which was suppressed by RUNX1 overexpression (Fig. 4I). In contrast, MYCN-e2 had no detectable enhancer activity (Fig. 4I). Collectively, these data demonstrate that during AI-10-49 treatment in inv(16) AML cells, RUNX1 released from CBFβ-SMMHC binds to the MYCN enhancer and recruits the PRC1 complex, resulting in MYCN repression.
Fig. 4. RUNX1 binds to MYCN distal enhancer transcriptionally active in inv(16).
(A) Representative examples of Integrative Genome Viewer (IGV) tracks of ATAC-seq, ChIP-seq, and DNase I–seq analysis in ME-1 cells, healthy peripheral blood CD34+ cells, and purified primary human AML cells. PB CD34, mobilized CD34+ cells in peripheral blood from healthy individuals; inv(16), CD34+ cells from human primary AML samples with inv(16). (B and C) ChIP-qPCR analysis for RUNX1 in DMSO- or AI-10-49–treated ME-1 cells (B) and primary human inv(16) AML cells (C). (D and E) ChIP-qPCR analysis for H3K27ac (D) and H3K4me1 (E) in DMSO- or AI-10-49–treated ME-1 cells. (F) Interaction frequency measured by chromosome conformation capture (3C) assays in ME-1 cells treated with DMSO/AI-10-49 for 6 hours. The 3C “anchor” primer targets the MYCN promoter region, and the 3C “bait” primers target the noncoding regions 3′ to MYCN. Asterisk indicates a statistically significant reduction in interaction frequency in AI-10-49 sample compared to DMSO. Data are representative of triplicate experiments. (G and H) ChIP-qPCR analysis for H3K27Me3 (G) and RING1B (H) in DMSO- or AI-10-49–treated ME-1 cells. (I) Luciferase reporter activity and response to RUNX1 overexpression in ME-1 cells with pGL3 plasmid without any enhancer (“Empty”), with RDME enhancer (“RDME”), or with MYCN-e2 enhancer (MYCN-e2). Histogram representative of triplicate experiments. Error bars represent the SD. Significance was calculated using an unpaired t test. *P < 0.05, **P < 0.005.
To further understand the role of RDME in inv(16) AML, we re-analyzed deoxyribonuclease (DNase) I hypersensitive site (DHS) analysis by DNase I–seq, previously reported in primary AML cells (26). We found that RDME is a highly chromatin-accessible region in primary inv(16) AML cells compared to peripheral blood CD34+ cells from healthy individuals (Fig. 4A). Furthermore, in addition to inv(16) AML, RDME acts as a chromatin-accessible region in multiple AML subtypes such as t(8:21), RUNX1 mutant AML, and biallelic CEBPA mutant (fig. S4C). RDME is accessible in AML subtypes with RUNX1 inactivation [t(8:21), RUNX1 mutant AML], suggesting that RUNX1 inactivation may result in MYCN overexpression by removing the RDME-mediated repression. Alternatively, it is possible that other myeloid transcription factors (e.g., C/EBPα) also bind to RDME and act as a MYCN repressor. Together, RDME acts as an oncogenic enhancer in regulating MYCN expression in specific AML subtypes, including inv(16).
RDME regulates MYCN transcription and cell survival
To investigate the functional role of RDME in inv(16) AML cells, we determined whether deletion of RDME by CRISPR/Cas9 approach can affect MYCN expression. ME-1 cells were transfected with plasmids expressing Cas9 and two gRNAs for RDME and MYCN-e2 to produce deletions surrounding the RUNX1 binding site in these regions. Our data show efficient deletion of RDME and MYCN-e2 in ME-1 cells (fig. S5, A to F). RDME deletion resulted in a 60% reduction in MYCN transcript levels (Fig. 5, A and B) and a 50% reduction in cell viability due to apoptosis (Fig. 5C). RDME-deleted ME-1 cells displayed an increase in the cleaved caspase-3 and cleaved PARP proteins, confirming that RDME deletion induced apoptosis in ME-1 cells (Fig. 5D). Meanwhile, the deletion of MYCN-e2 did not affect MYCN transcript levels or cell viability in ME-1 cells (Fig. 5, B to D). In contrast, normal human HSPCs were not affected in survival or differentiation during RDME deletion (fig. S5, G and H). These data suggest that RDME selectively regulates the survival of inv(16) AML cells without affecting normal hematopoiesis. Collectively, our data suggest that RDME functions as an oncogenic enhancer to regulate MYCN expression and the viability of inv(16) AML cells.
Fig. 5. RDME regulates MYCN transcription and inv(16) AML cell survival.
(A) Schematic of CRISPR/Cas9-mediated deletion of MYCN enhancer elements. (B) N-MYC transcript levels in MYCN enhancer–deleted ME-1 cells by real-time RT-PCR. (C) Cell survival analysis in control/MYCN enhancer–deleted ME-1 cells by annexin V/7AAD assay. (D) Cleaved PARP and cleaved caspase-3 protein levels in control/MYCN enhancer–deleted ME-1 cells by Western blot. Histogram representative of triplicate experiments. Error bars represent the SD. Significance was calculated using an unpaired t test. **P < 0.005.
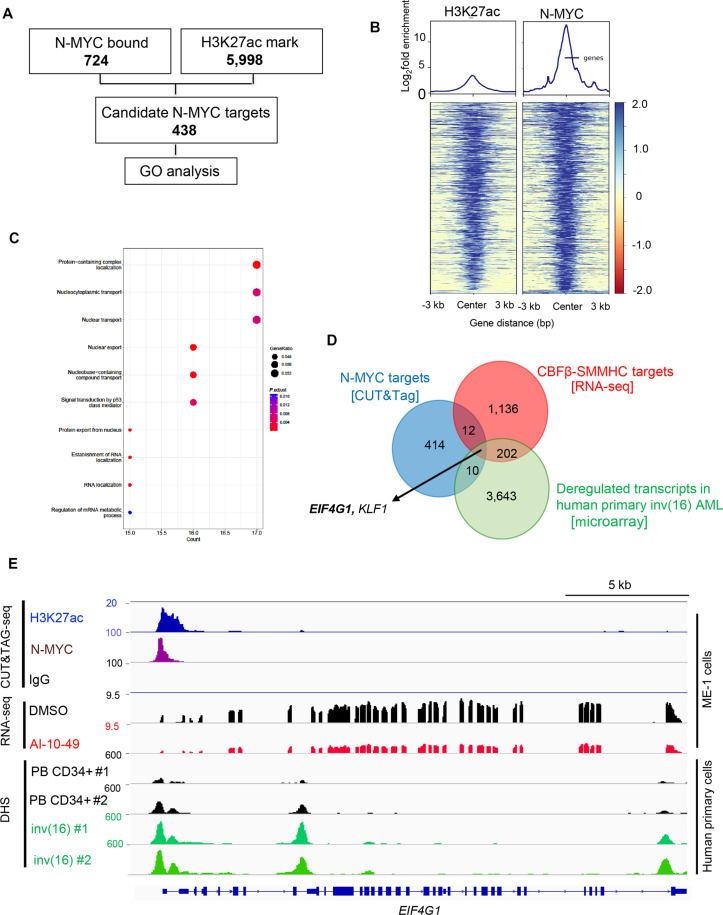
EIF4G1 is a key N-MYC target in inv(16) AML
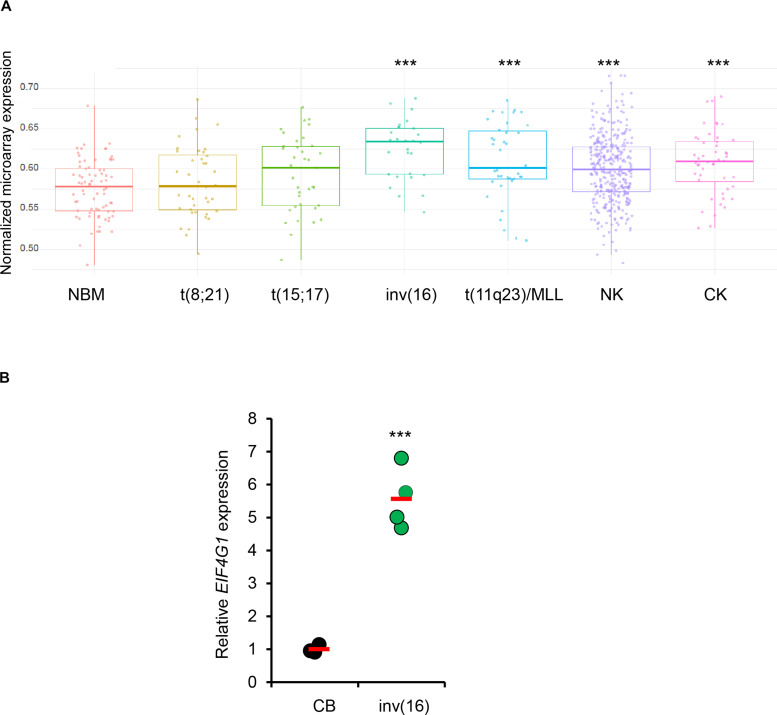
To identify how N-MYC regulates survival in inv(16) AML cells, we performed CUT&Tag-seq (cleavage under targets and tagmentation sequencing) in ME-1 cells using N-MYC and H3K27ac antibodies (Fig. 6A). Motif analysis demonstrated that N-MYC–occupied genomic elements were highly enriched for N-MYC (fig. S6A). Comparison of N-MYC–occupied genomic regions to RUNX1 occupied regions in ME-1 cells (14) revealed minimal overlap, suggesting that N-MYC and RUNX1 regulate distinct genomic regions (fig. S6B). Comparison of H3K27ac peaks centered on N-MYC peak center yielded a strong correlation of N-MYC peak intensity with H3K27ac marks (Fig. 6B and tables S3 and S4). N-MYC transcriptional targets identified by N-MYC binding and H3K27ac peaks were used to perform gene ontology (GO) analysis. This analysis revealed that N-MYC binding is associated with several pathways, including mRNA metabolism, mRNA splicing, nuclear transport, and chromatin modification (Fig. 6C and fig. S6C). Comparison of N-MYC targets identified by CUT&Tag-seq with previously identified CBFβ-SMMHC transcriptional targets (14) and transcripts substantially deregulated in primary human inv(16) AML samples identified EIF4G1 (encoding eIF4G1) and Kruppel-like factor 1 (KLF1) as potential N-MYC transcriptional targets in inv(16) AML (Fig. 6D). Of these, only EIF4G1 transcripts were up-regulated in primary human inv(16) AML samples and down-regulated with AI-10-49 treatment. EIF4G1 was one of the regulators of mRNA metabolic process signature genes identified (Fig. 6C). N-MYC binds to the EIF4G1 promoter and is associated with a transcriptionally active H3K27ac histone mark (Fig. 6E and fig. S6D). We validated N-MYC binding at EIF4G1 by conducting ChIP-qPCR (fig. S6E). DHS analysis (26) revealed that the EIF4G1 promoter is a highly accessible chromatin region in primary inv(16) AML cells compared to peripheral blood CD34+ cells from healthy individuals (Fig. 6E). Genomic deletion of MYCN in ME-1 cells by CRISPR/Cas9 RNPs resulted in a marked reduction of eIF4G1 protein levels (fig. S6F). We observed down-regulation of eIF4G1 at mRNA and protein levels with AI-10-49 in inv(16) AML cells (fig. S6, G and H). AI-10-49 treatment did not display any changes in EIF4G1 transcript levels in non-inv(16) AML cell lines (fig. S6I). Genomic deletion of CBFB-MYH11 resulted in the down-regulation of EIF4G1 transcript levels (fig. S6 J). RUNX1 inhibition with small-molecule inhibitor AI-14-91 resulted in the down-regulation of EIF4G1 transcript levels (fig. S6K). EIF4G1 transcript levels were up-regulated in primary inv(16) AML patient samples (Fig. 7, A and B). Together, EIF4G1 is a key N-MYC target with potential roles in leukemic cell survival in inv(16) AML.
Fig. 6. EIF4G1 is a key N-MYC target in inv(16) AML.
(A) Filtering scheme for identifying genome-wide N-MYC targets by CUT&Tag analysis in inv(16) AML. N-MYC–bound regions (P < 0.05) were intersected with the H3K27ac histone mark (P < 0.05). Bold numbers indicate the number of genomic regions identified in each analysis/filtering step. Candidate MYCN targets identified were used for GO enrichment analysis. (B) Distribution of H3K27ac peaks centered on the N-MYC peak center in CUT&Tag-seq. (C) Dot plot of GO enrichment analysis of N-MYC targets identified in inv(16) AML. The diameter indicates the number of genes overlapping the GO term, and the color indicates the enrichment P value. Top 10 pathways were displayed. (D) Venn diagram showing the overlap between significant differentially expressed genes in AI-10-49–treated ME-1 cells (±2-fold change; adjusted P < 0.05) identified in the RNA-seq, transcripts significantly deregulated in human inv(16) AML CD34+ cells compared to NBM CD34+ cells in Haferlach dataset extracted from Leukemia Gene Atlas (expression array) database, and peaks called in N-MYC CUT&Tag. (E) Representative examples of IGV tracks of CUT&Tag-seq, RNA-seq, and DNase I–seq analysis in ME-1 cells, healthy peripheral blood CD34+ cells, and purified human primary AML cells.
Fig. 7. EIF4G1 is up-regulated in inv(16) AML.
(A) Normalized expression of EIF4G1 transcript levels in NBM and AML samples in the Haferlach dataset extracted from the Leukemia Gene Atlas (expression array) database. P values between NBM and different AML subtypes are shown. (B) eIF4G1 transcript levels in human cord blood CD34+ cells (CB) and human primary inv(16) AML CD34+ [inv(16)] cells. Each symbol represents the average of a triplicate experiment from one sample, and the average value of the group is shown in red. Error bars represent the SD. Significance was calculated using an unpaired t test. ***P < 0.0005.
eIF4G1 regulates inv(16) AML maintenance
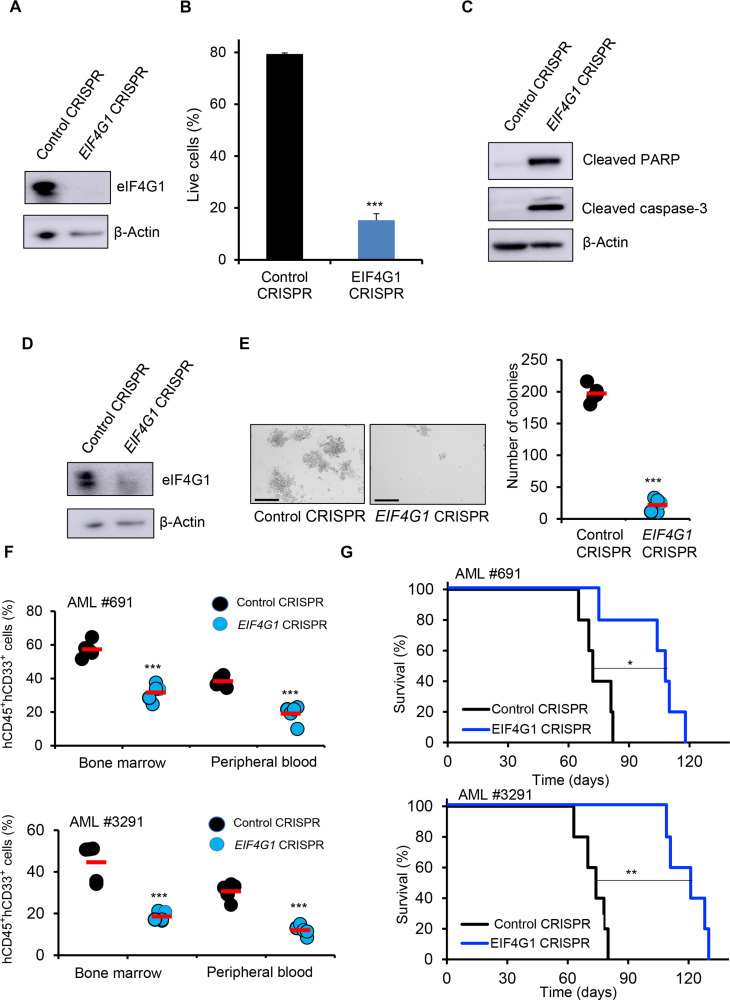
Since N-MYC plays an essential role in inv(16) leukemia maintenance and EIF4G1 is a major target of N-MYC, we reasoned that eIF4G1 would be instrumental in regulating inv(16) AML survival. To test this, we applied CRISPR/Cas9 technology using RNPs to delete EIF4G1 in inv(16) AML cells. ME-1 cells transfected with Cas9 protein and three EIF4G1 CM-gRNAs produced efficient deletion of EIF4G1 genomic regions (fig. S7, A to C), resulting in a 95% reduction in eIF4G1 protein levels (Fig. 8A). EIF4G1 deletion in ME-1 cells substantially lowered cell viability due to apoptosis (Fig. 8B). The onset of apoptosis was accompanied by the cleavage of caspase-3 and cleaved PARP proteins in ME-1 cells (Fig. 8C). EIF4G1 deletion in four non-inv(16) AML cell lines demonstrated that EIF4G1 is important in the survival of leukemic cells in addition to inv(16) AML (fig. S7, D and E). In contrast, normal human HSPCs were not affected in survival or differentiation during EIF4G1 deletion (fig. S7, F to H). These data suggest that eIF4G1 specifically regulates the survival of leukemic cells, including inv(16) AML cells.
Fig. 8. eIF4G1 regulates inv(16) AML maintenance.
(A) ME-1 cells were transfected with Cas9 and control gRNA/pool of three EIF4G1 gRNAs by RNP approach, and eIF4G1 protein levels were analyzed by Western blot. (B) Cell survival analysis in control/EIF4G1-edited ME-1 cells by annexin V/7AAD assay. Histogram representative of triplicate experiments. (C) Cleaved PARP and cleaved caspase-3 protein levels in control/EIF4G1-edited ME-1 cells by Western blot. (D) Human primary inv(16) AML cells were transfected with Cas9 and control gRNA/pool of three EIF4G1 gRNAs by RNP approach, and eIF4G1 protein levels were analyzed by Western blot. (E) Colony counts for methylcellulose colony-forming assay performed upon 12 days of control/EIF4G1-edited human primary inv(16) AML cells. Data are representative of three replicates. The average value of each group is shown in red. Scale bar, 275 μm. (F) Flow cytometric quantification of hCD45+ hCD33+ cells in NSGS mice transplanted with control/EIF4G1-edited primary inv(16) AML cells 5 weeks after transplantation. Each symbol represents a mouse. The average value of each group is shown in red. (G) Kaplan-Meier survival curve of NSGS mice transplanted with control/EIF4G1-edited primary inv(16) AML cells (n = 5 per group). Error bars represent the SD. Significance was calculated using an unpaired t test [(B), (E), and (F)] and log-rank test (G). *P < 0.05, **P < 0.005, ***P < 0.0005.
To further assess the role of eIF4G1 in inv(16) AML cell survival, we conducted EIF4G1 genomic editing in primary human inv(16) AML cells, as described in Fig. 3A. T cell–depleted primary inv(16) AML cells were transfected with Cas9 protein and three EIF4G1 gRNAs, resulting in an 85% reduction in eIF4G1 protein levels (Fig. 8D). EIF4G1 deletion substantially reduced the clonogenic potential of primary inv(16) AML cells, as reflected by a marked decrease in colony number (Fig. 8E). The requirement of eIF4G1 in inv(16) leukemia in vivo was tested by transplanting the edited leukemic cells into irradiated (280 cGy) NSGS mice via tail vein injection. Engraftment efficiency of control and EIF4G1-edited leukemic cells in the bone marrow 5 days after transplantation was similar between groups (fig. S7, I and J). AML burden estimated by the frequency of hCD45+ hCD33+ AML stem/progenitor cells was markedly reduced in the bone marrow and peripheral blood of mice transplanted with EIF4G1-edited AML cells compared to control mice (Fig. 8F and fig. S7, K and L), suggesting that eIF4G1 is required for the maintenance of inv(16) leukemic cells. In addition, the median leukemic latency in mice transplanted with EIF4G1 deleted AML cells was substantially extended (Fig. 8G). Collectively, these results demonstrate that EIF4G1 is a critical N-MYC target instrumental in inv(16) AML maintenance.
DISCUSSION
Both c-Myc and N-Myc are essential for normal development, as shown by embryonic lethality at mid-gestation due to the lack of either gene in mouse models (27–29). N-Myc is vital for fetal HSC proliferation (30). Deletion of c-Myc in adult mouse hematopoiesis results in the accumulation of HSCs that are defective in further differentiation (31, 32). Meanwhile, deletion of N-Myc in adult mouse hematopoiesis did not display any phenotype (33). Combined deletion of both c-Myc and N-Myc in adult mice results in pancytopenia and rapid lethality, demonstrating that both c-Myc and N-Myc are required for HSC proliferation, self-renewal, and survival (33). Thus, c-Myc and N-Myc proteins have redundant functions in normal hematopoiesis. The oncogenic role of c-MYC is well established in AML (14, 34, 35). While the requirement of c-MYC and the pathways orchestrated by c-MYC are widely investigated in hematological malignancies, the role of N-MYC in leukemia remains largely unexplored.
Here, using primary human inv(16) AML samples and a PDX model for inv(16) AML, we revealed a previously unidentified role of N-MYC in leukemogenesis. The oncogenic function of N-MYC has been widely studied in neuroblastoma (36–38), and emerging studies show its involvement in prostate cancer, pancreatic cancer, and retinoblastoma (39–42). MYCN amplification is reported in AML and lymphoma (43, 44). N-MYC overexpression is reported in pediatric AML, erythroleukemia, and Burkitt lymphoma (45–47). Overexpression of Mycn in mouse bone marrow cells results in enhanced proliferation and self-renewal of myeloid progenitors, resulting in AML development (48). T cell progenitor–specific N-Myc overexpression in mice induces peripheral T cell lymphoma (PTCL) (49). Here, we demonstrate that CBFβ-SMMHC oncogene in inv(16) AML cells modulates MYCN transcript levels, and N-MYC is a critical regulator of inv(16) AML cell survival.
Both c-Myc and N-Myc are expressed in HSCs (33, 50). No compensatory up-regulation of c-Myc is observed upon loss of N-Myc, suggesting that when N-Myc is deleted, the unchanged c-Myc transcript levels can support HSC survival and proliferation (33). Having shown that c-MYC is required for inv(16) AML cell survival (14), how can we explain that the loss of N-MYC also regulates the survival of inv(16) AML cells? Multiple explanations can be made to describe the oncogenic dependency of N-MYC in inv(16) AML cells. First, although the N-Myc protein functions very similarly to c-Myc (51), there are major differences in the expression pattern of c-Myc and N-Myc proteins in mouse hematopoietic compartments. While N-Myc is expressed at high levels in self-renewing and quiescent HSCs, c-Myc is expressed in multiple progenitor compartments (50). In addition, N-Myc overexpression can reprogram mature blood cells to HSCs (52), suggesting that N-Myc can induce stem cell properties in mature blood cells. Thus, the unique expression pattern of c-Myc and N-Myc could have distinct roles in AML cell survival depending on the cell of origin. Second, c-Myc and N-Myc proteins may have different binding partners, thereby exerting distinct transcriptional programs in AML cells. c-Myc and N-Myc proteins differ in MIZ1 binding and have distinct functions in medulloblastoma (53). c-Myc and N-Myc protein-protein interactions in AML cells remain largely unknown. Thus, N-MYC and c-MYC may have overlapping and distinct transcriptional targets in AML. Third, MYCN mRNA may also have an oncogenic role in AML independent of N-MYC protein. In neuroblastoma cells, MYCN mRNA acts as a competing endogenous RNA (ceRNA) for miRNA let-7, which targets MYCN mRNA (54). Although it is known that let-7 is down-regulated in inv(16) AML (55), whether let-7 and/or other mRNAs are targeted by MYCN mRNA awaits further studies.
We identified RDME as a previously unknown MYCN enhancer, essential for MYCN mRNA levels and survival in inv(16) AML cells. By contrast, normal human HSPCs do not require RDME for survival, suggesting that RDME is specifically active in inv(16) AML cells. RDME is a chromatin-accessible region in inv(16) AML cells and is bound by RUNX1 during AI-10-49 treatment. We found several chromatin-accessible regions downstream of RDME in inv(16) AML cells without RUNX1 binding during AI-10-49 treatment (Fig. 4A). While there were no substantial changes in chromatin accessibility at RDME during AI-10-49 treatment, there were marked changes in accessibility at multiple regulatory elements downstream of RDME (Fig. 4A). This suggests that CBFβ-SMMHC potentially regulates multiple regulatory elements at the MYCN locus, in addition to RDME in inv(16) AML cells. The current study was limited in investigating the role of transcription factors other than RUNX1 regulating MYCN expression in inv(16) AML cells. Further studies are needed to understand what potential proteins bind to the MYCN locus to maintain chromatin accessibility; thereby providing key insight into the mechanism of action of CBFβ-SMMHC in inv(16) AML.
We identified EIF4G1 as a key N-MYC target regulating inv(16) AML cell survival. eIF4G1 plays a vital role in translation initiation by acting as an adapter that enhances the assembly of the eIF4F complex by recruiting eIF4E and eIF4A. Cancer cells are highly dependent on increased eIF4F activity compared to normal cells (56, 57). eIF4E is overexpressed and known to have oncogenic potential in M4 and M5 subtypes of AML (58, 59). eIF4A is overexpressed in AML, and its inhibition can induce anti-leukemic effects in FLT3-ITD AML (60). eIF4G1 expression is highly activated in breast, prostate, and squamous cell lung cancers and is associated with metastatic progression and inferior survival (61–63). Neuroblastoma patients with MYCN amplification present elevated eIF4G1 transcript levels and are associated with lower survival (64). The oncogenic function of eIF4G1 in AML has not been investigated before. Since eIF4G1 is a global translation initiation regulator, how can we attribute an oncogenic role for eIF4G1 in inv(16) AML? Emerging studies suggest that eIF4G1 can regulate translation in an mRNA-specific manner in cancer. eIF4G1 can regulate the translation of a subset of mRNAs important for survival in breast cancer (65). In non–small cell lung cancer, eIF4GI controls the expression of specific immunoregulatory proteins (66). Future studies addressing whether eIF4G1 regulates translation in an mRNA-specific manner, resulting in the rewiring of the AML proteome, warrant key insights.
Our results on the oncogenic role of the N-MYC/eIF4G1 axis provide key knowledge for developing better therapeutic strategies for patients with inv(16) AML. The development of bromodomain inhibitors has gained wide attention in indirectly inhibiting c-MYC and N-MYC in cancer (17, 18). However, recent clinical trials revealed that most of these inhibitors had limited clinical utility due to unexpected toxicities (67). So, a promising alternative is to target pathways downstream of c-MYC and N-MYC in AML. Since c-MYC and N-MYC play a major role in normal hematopoiesis, targeting pathways activated by c-MYC and N-MYC in AML cells will have major therapeutic benefits. The recent development of SBI-756, an eIF4G1 small-molecule inhibitor, has demonstrated promising efficacy in multiple cancer types, including melanoma, B-acute lymphocytic leukemia, and lymphoma (68–70). Future studies using combinations of AI-10-49 with SBI-756 should lead to greater therapeutic responses for inv(16) AML. In addition, since our data suggest that the N-MYC/eIF4G1 axis is also important in the survival of non-inv(16) AML subtypes, targeting N-MYC and eIF4G1 may provide a better clinical outcome for AML subtypes with N-MYC and eIF4G1 overexpression.
In conclusion, we have discovered N-MYC as a major leukemic driver in inv(16) AML. Furthermore, we also identified eIF4G1 as a key N-MYC transcriptional target in AML survival, providing insights into developing improved treatment avenues for patients with inv(16) AML.
MATERIALS AND METHODS
Mice
All animal experiments were performed in accordance with a protocol reviewed and approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. NSGS (NOD/SCID-IL2RG-SGM3) mice (The Jackson Laboratory, #013062) have been previously described (21, 22) and maintained at the Medical College of Wisconsin animal facility.
Cell line and primary hematopoietic cell cultures
Human inv(16) AML ME-1 cells [Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ), #ACC-537] were cultured in RPMI 1640 with 20% fetal bovine serum (FBS), 25 mM Hepes, penicillin (100 U/ml), streptomycin (100 μg/ml), and plasmocin (1 μl/ml). Kasumi-1 [American Type Culture Collection (ATCC), #CRL-2724], NB-4 (DSMZ, #ACC-207), THP-1 (ATCC, #TIB-202), K562 (AATCC, #CCL-243), U937 (ATCC, #CRL-1593.2), and Kasumi-6 (DSMZ, #ACC-686) were cultured in RPMI 1640 with 10% FBS, 25 mM Hepes, penicillin (100 U/ml), and streptomycin (100 μg/ml). Human cord blood samples were collected from the Medical College of Wisconsin Tissue Bank, and CD34+ cells were isolated using the CD34 Microbead kit (Miltenyi Biotec, #130-046-702). The use of the cord blood samples for research purposes was approved by the Ethics Committee of the Medical College of Wisconsin. Human AML samples were received from AML banks by M. Carroll (University of Pennsylvania) and Carsten Mueller Tidow (University Hospital, Heidelberg, Germany). Details of the AML samples are provided in table S2. Hematopoietic CD34+ cells were isolated from human AML bone marrow samples using the CD34 Microbead kit. All patients gave written consent for the use of their samples. Personal information from AML and cord blood samples was unavailable as the samples were anonymized. Human cord blood CD34+ cells, as well as human primary leukemic cells, were cultured in StemSpan SFEM II (STEMCELL Technologies, #09605), penicillin (100 U/ml), and streptomycin (100 μg/ml) supplemented with human recombinant TPO (10 ng/ml), human recombinant FLT3L (10 ng/ml), human recombinant stem cell factor (SCF) (100 ng/ml), human recombinant interleukin-3 (IL-3) (10 ng/ml), and human recombinant IL-6 (20 ng/ml). For granulocyte differentiation experiments, cord blood CD34+ cells were seeded in StemSpan SFEM II and then cultured for 16 days in the presence of a cytokine cocktail: days 1 to 5: recombinant human SCF (50 ng/ml), fms-like tyrosine kinase 3 (100 ng/ml), IL-3 (5 ng/ml), granulocyte-macrophage colony-stimulating factor (GM-CSF) (5 ng/ml), and G-CSF (30 ng/ml); days 5 to 8: IL-3 (5 ng/ml) and G-CSF (30 ng/ml); and days 8 to 16: G-CSF (30 ng/ml). Cell cultures were routinely tested for mycoplasma contamination using Universal Mycoplasma Detection Kit (ATCC, #30-1012K) and PlasmoTest Mycoplasma Detection Kit (InvivoGen, #rep-pt1).
Small-molecule inhibitors and cytokines
AI-10-49 and JQ1 were purchased from ApexBio. AI-4-88 and AI-14-91 were provided by J. H. Bushweller. All cytokines were purchased from PeproTech.
CRISPR/Cas9 genomic editing of MYCN enhancers
The sgRNAs specific for 5′ to the region of interest were cloned in pLentiCRISPRv2 (Addgene, #52961). sgRNAs corresponding to 3′ to the region of interest were cloned in pDecko-mCherry (Addgene, #78534). The puromycin resistance cassette in pLentiCRISPRv2 was replaced by a green fluorescent protein (GFP) gene using standard cloning techniques. Oligonucleotide sequences are listed in table S1. ME-1 cells (2 × 106) were nucleofected with CRISPR/Cas9 plasmids (2 μg each) using Nucleofector Technology (Lonza Biologics) with the program X-01 and Amaxa Cell Line Nucleofector Kit V. Samples were sorted by flow cytometry 24 hours later. Cells were cultured overnight, and dead cells were eliminated by dead cell removal kit (Miltenyi Biotec, #130-090-101).
CRISPR/Cas9 genomic editing of MYCN, EIF4G1, and CBFB-MYH11
Gene editing experiments were conducted using the RNP complex. Gene Knockout Kit v2 (Synthego) consisting of three CM-gRNAs was used to target MYCN and EIF4G1 in ME-1 cells, cord blood CD34+ cells, and primary human AML CD34+ cells. Negative Control Scrambled sgRNA and SpCas9 protein were purchased from Synthego.
To target CBFB-MYH11, two CM-gRNAs (Synthego) targeting the MYH11 region were used. To assemble the RNP complex, sgRNAs were combined with Cas9 protein and incubated for 10 min at room temperature.
Primary human inv(16) AML CD34+ cells were T cell–depleted using CD3 Microbeads (Miltenyi Biotec, #130-050-101). Primary human inv(16) AML CD34+ cells and cord blood CD34+ cells were nucleofected with the RNP complex, composed of 100 pmol of Cas9 and 300 pmol of CM-sgRNA, using the P3 Primary Cell 4D-Nucleofector X Kit for Amaxa 4-D device (Lonza, #V4XP-3032). Cells (2 × 105 per condition) were nucleofected in separated strip wells using program EO-100. Cell viability was assessed by flow cytometry 24 hours after nucleofection, and gene editing efficacy was evaluated 72 hours after nucleofection by inference of CRISPR editing (ICE) and Western blot analysis.
CRISPR/Cas9-induced gene editing analysis by ICE
Genomic DNA was isolated from edited cells 72 hours after nucleofection. The edited region was PCR-amplified, and the PCR product was sequenced by the Sanger method. The chromatograms were analyzed by ICE (https://ice.synthego.com). The percentage of editing was calculated according to the frameshift produced in the edited chromatogram compared to the control sequence. Primers used in these PCRs are listed in table S1.
Colony-forming unit assay
Twenty-four hours after nucleofection of AML inv(16) CD34+ cells with the RNP complex, cells were resuspended in MethoCult Express (STEMCELL Technologies, #04437), plated at 3000 cells/ml on a 12-well plate, and cultured for 12 days to form colonies.
Transplantation of gene-edited AML cells in NSGS mice
Twenty-four hours after nucleofection of AML inv(16) CD34+ cells with the RNP complex, 1 × 106 cells were transplanted into sublethally (280 rads) irradiated 6- to 8-week-old immunodeficient NSGS mice (n = 5 per group) by tail vein injection. Successful engraftment of edited AML cells was evaluated by flow cytometry of bone marrow aspirates 5 days later. The leukemic burden was analyzed by flow cytometry of peripheral blood and bone marrow aspirates 5 weeks later. Mice were sacrificed after visible characteristics of AML, including reduced motility and grooming activity, hunched back, and pale paws (anemia).
Flow cytometry
For flow cytometry, cells were washed twice with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), stained for 20 to 30 min at 4°C in the dark, and analyzed with a BD LSRII flow cytometer.
Flow cytometry analysis was performed using FlowJo software. The following antibodies were used: human CD33 monoclonal antibody (eBioscience, #12-0338-42), human CD45 monoclonal antibody (BD Biosciences, #555485), CD11b monoclonal antibody (BD Biosciences, #555388), and CD15 monoclonal antibody (BD Biosciences, #555401).
Annexin V assay
For testing the role of MYCN and eIF4G1 silencing in inv(16) cell survival, ME-1 cells were transfected with control/MYCN/EIF4G1 CM-sgRNAs and live cells (7AAD− annexin V−) were assessed by annexin V assay. Annexin assay was conducted 21 days (ME-1) or 14 days (Kasumi-1, NB4, THP-1, Kasumi-6) or 16 days (cord blood CD34+) after nucleofection. For testing the role of MYCN enhancer deletion in inv(16) AML cell survival, ME-1 cells were transfected with empty vector/Cas9 (Ctr.) or MYCN enhancer sgRNA/Cas9 constructs and sorted 24 hours later, and live cells (7AAD− annexin V−) were assessed by annexin V assay 14 days later. For evaluating whether N-MYC overexpression can rescue AI-10-49–mediated cell death, ME-1 cells were transduced with control plasmid (Addgene, #120426) or N-MYC plasmid (Addgene, #120463). Selection with hygromycin (50 μg/ml) began 48 hours after transduction and lasted 8 days. Cells were treated with dimethyl sulfoxide (DMSO) or AI-10-49 (1 μM) for 24 hours, and live cells (7AAD− annexin V−) were assessed by annexin V assay. For the detection of apoptotic cell death, Annexin V Apoptosis Detection Kit I (BD Biosciences, #559763) was used as per the manufacturer’s instructions. Briefly, 5 μl of annexin-phycoerythrin (PE) antibody and 10 μl of 7-amino-actinomycin D (7AAD) were added, and cells were centrifuged at 2000 rpm for 10 min, resuspended in 100 μl of 1× annexin V binding buffer, and incubated for 15 min at room temperature in the dark, followed by addition of 500 μl of 1× annexin binding buffer. Cell viability was determined as the percent of 7AAD−/annexin V− cells with a BD LSRII flow cytometer.
Quantitative RT-PCR analysis
To evaluate MYC, MYCN, and EIF4G1 transcriptional regulation by AI-10-49 and JQ1 in inv(16) cells, ME-1 cells were treated with AI-10-49/JQ1 for corresponding time points.
To evaluate MYCN and EIF4G1 transcriptional regulation by AI-4-88 and AI-14-91 in inv(16) AML cells, ME-1 cells were treated with these inhibitors (20 μM) for 24 hours. mRNA was isolated from three independent experiments, and quantitative reverse transcription PCR (qRT-PCR) was conducted. For testing the role of MYCN enhancer deletion in MYCN transcriptional regulation in inv(16) cells, ME-1 cells were transfected with empty vector/Cas9 (Ctr.) or MYCN enhancer sgRNA/Cas9 and sorted 48 hours later, and mRNA was isolated from three independent experiments 24 hours later. MYCN expression was estimated by qRT-PCR analysis. Total mRNA was isolated with a PureLink RNA Mini Kit (Life Technologies), and cDNA synthesis was performed with a SuperScript III kit (Life Technologies), as per the manufacturers’ instructions. Quantitative PCR analysis was conducted on an Applied Biosystems QuantStudio 6 Flex Real-Time PCR System with Power SYBR Green PCR Master Mix (Applied Biosystems). Expression levels were determined with the ΔCT method and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Sequences of primers are provided in table S1.
Luciferase reporter assays
The pGL3 promoter luciferase reporter system (Promega, #E1910) was used. The enhancer regions were cloned in pGL3-Promoter Vector (Promega, #E1761) using Nhe I and Xho I restriction enzyme sites. The enhancer luciferase constructs were then cotransfected with a control Renilla luciferase construct into cells using Lonza nucleofection. The luciferase signal was first normalized to the Renilla luciferase signal and then normalized to the signal from the empty pGL3 plasmid. Primers used for cloning are listed in table S1. RUNX1 plasmid (Addgene, #14585) was used in luciferase assays.
Chromatin immunoprecipitation–qPCR
For ChIP-qPCR experiments, ME-1 cells were treated with DMSO or AI-10-49 (1 μM) for 6 hours. For conducting ChIP-qPCR after CBFB-MYH11 genomic editing, cells were used 4 days after nucleofection. Cross-linking of proteins to DNA was accomplished by adding 1% formaldehyde for 10 min to cultured cells at room temperature. After neutralization with glycine, cells were lysed in a lysis buffer with protease inhibitors, and samples were sonicated to an average DNA length of 200 to 400 base pairs (bp) with a bioruptor (Diagenode). After sonication, the chromatin was immunoprecipitated with 5 to 10 μg of the antibody of interest at 4°C overnight. Antibody-bound complexes were isolated with Dynabeads (Life Technologies). DNA was purified using the phenol–chloroform–isoamyl alcohol method. Immunoprecipitated DNA was analyzed by qPCR on a QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems) with Power SYBR Green PCR Master Mix and calculated as % of input. For ChIP qPCR in human primary AML sample with inv(16), CD34+ cells were enriched using CD34 MicroBead Kit (Miltenyi Biotec, #130-046-702) and cultured overnight, followed by dead cell removal by dead cell removal kit (Miltenyi Biotec, #130-090-101). Cells were treated with DMSO/AI-10-49 (5 μM) for 8 hours, followed by the ChIP procedure mentioned above. Details of the sequences of primers are provided in table S1. The following antibodies were used: RUNX1 polyclonal antibody (Abcam, #ab23980), H3K4Me1 polyclonal antibody (Abcam, #ab8895), H3K27ac polyclonal antibody (Abcam, #ab4729), H3K27me3 monoclonal antibody (Abcam, #ab6002), N-Myc (D1V2A) monoclonal antibody (Cell Signaling Technology, #84406), RING1B (Cell Signaling Technology, #5694), and normal rabbit immunoglobulin G (IgG) (Cell Signaling Technology, #2729).
Cleavage under targets and tagmentation
CUT&Tag was performed according to the protocol reported by the Henikoff laboratory (71). ME-1 cells were harvested and centrifuged at 600g for 3 min at room temperature. Aliquots of cells (0.5 × 106) were washed once in 1.5 ml of wash buffer [20 mM Hepes (pH 7.5), 150 mM NaCl, 0.5 mM spermidine, 1× protease inhibitor cocktail] by gentle pipetting and resuspended in 100 μl of wash buffer. Bio-Mag Plus Concanavalin A–coated beads (Polysciences) were prepared by washing twice in 1.5 ml of binding buffer [20 mM Hepes (pH 7.9), 10 mM KCl, 1 mM CaCl2, and 1 mM MnCl2] and resuspended in binding buffer (10 μl per sample). Activated beads (10 μl) were added per sample with gentle vortexing and placed on an end-over-end rotator for 10 min at room temperature. The unbound supernatant was removed by placing the tube on the magnetic stand, and bead-bound cells were resuspended in 50 μl of ice-cold DIG Wash buffer [20 mM Hepes (pH 7.5), 150 mM NaCl, 0.5 mM spermidine, 1× protease inhibitor cocktail, 0.05% digitonin] containing 2 mM EDTA, 0.1% BSA, and a 1:50 dilution of the appropriate primary antibody (N-Myc, H3K27Ac, or IgG). After overnight incubation at 4°C, the primary antibody was removed, and the cells were resuspended in 100 μl of DIG Wash buffer containing 1:100 dilution secondary antibody. Cells were incubated at room temperature for 45 min and washed twice in 1 ml of DIG Wash buffer to remove unbound antibodies. The pA-Tn5 adapter complex containing 2.5 μl of 20× CUTANA pAG-Tn5 preloaded adapter complex (EpiCypher, #15-1017) was prepared in 50 μl of Dig-300 buffer [20 mM Hepes (pH 7.5), 300 mM NaCl, 0.5 mM spermidine, 1× protease inhibitor cocktail, 0.01% digitonin]. After removing the supernatant, 50 μl of pA-Tn5 adapter complex was added to the cells with gentle vortexing and incubated for 1 hour at room temperature. Cells were washed twice in 1 ml of Dig-300 buffer to remove unbound pA-Tn5 protein. Cells were then resuspended in 300 μl of tagmentation buffer (10 mM MgCl2 in Dig-300 buffer) and incubated at 37°C for 1 hour. To stop tagmentation and reverse cross-link DNA, 10 μl of 0.5 M EDTA, 3 μl of 10% SDS, and 2.5 μl of proteinase K (20 mg/ml) were added to each sample and incubated at 55°C for 1 hour. To extract DNA, 300 μl of phenol–chloroform–isoamyl alcohol was added to each sample and vortexed at full speed for ~2 s. The whole mixture was then transferred to a phase-lock tub and centrifuged at 16,000g for 3 min at room temperature. Chloroform (300 μl) was added to the same phase-lock tube, inverted ~10×, and centrifuged at 16,000g for 3 min at room temperature. The aqueous layer was collected in a fresh 1.5-ml tube containing 750 μl of 100% ethanol, chilled on ice, and centrifuged at 16,000g for 10 min at 4°C. The pellet was washed again in 100% ethanol and air-dried. Finally, the DNA pellet was dissolved in 25 μl of 1 mM tris-HCl (pH 8) and 0.1 mM EDTA. The following antibodies were used for CUT&Tag: guinea pig anti-rabbit secondary antibody (Antibodies-Online.com, #ABIN101961), H3K27ac polyclonal antibody (Abcam, t#ab4729), N-Myc (D1V2A) monoclonal antibody (Cell Signaling Technology, #84406), and normal rabbit IgG (Cell Signaling Technology, #2729).
The DNA libraries were prepared by mixing 21 μl of DNA with 2 μl of a universal i5 primer and 2 μl of a uniquely barcoded i7 primer (72). NEBNext HiFi 2× PCR Master mix (25 μl) was added to the DNA primer mix and PCR amplification was performed using the following PCR cycling conditions: 72°C for 5 min (gap filling); 98°C for 30 s; 13 cycles of 98°C for 10 s and 63°C for 10 s; final extension at 72°C for 1 min and hold at 8°C. Post-PCR cleanup of the libraries was performed by adding 1.3× volume of Ampure XP beads (Beckman Counter) and incubated for 10 min at room temperature. The tube was placed on the magnetic stand, and the liquid was carefully withdrawn without disturbing the beads. Beads were gently washed twice in 200 μl of 80% ethanol and resuspended in 25 μl of 10 mM tris (pH 8.0) by vortexing at full speed. After 5 min, the tube was placed on the magnetic stand and the liquid was carefully withdrawn into a fresh tube. The quality of the libraries was determined by checking the size distribution and concentration of libraries on an Agilent 4150 TapeStation with D1000 reagents.
CUT&Tag data analysis
Sample sequence alignment and peak calling were conducted using previously described specific parameters (71). CUT&Tag data were aligned to the hg38 human genome using Bowtie2 (73), and peaks were called using SEACR v1.3 (74) from each sample using IgG controls in stringent peak calling mode. Visualization was created using deepTools (75). Peaks were tested for differential expression between controls and samples with DESeq2 v1.24.0 (76). DESeq2 Wald tests were used to determine whether fold changes were significantly different from zero. Preranked gene set enrichment analysis (GSEA) was conducted using shrunken fold changes and clusterProfiler v3.12.0 (77). GO database was used for GSEA (78). The Benjamini-Hochberg method was used to adjust P values for false discovery in both differential expression and GSEA analyses (79).
Chromosome conformation capture
3C assays were performed as previously described (80). Briefly, 10 million ME-1 cells were fixed in a 1% formaldehyde/10% FBS solution for 10 min. Nuclei were isolated and digested with Dpn II at 37°C. DNA was ligated with 7000 U of T4 DNA ligase for 4 hours at 16°C. Samples were then de-cross-linked overnight at 65°C. DNA was extracted using phenol/chloroform method. 3C ligation products were quantified by SYBR Green–based PCR. Bacterial artificial chromosome (BAC) library (RP11-480N14)–containing DNA fragments covering the tested regions were used as template controls for normalizing digestion, ligation, and primer efficiency. Template and BAC libraries were normalized by subtracting CT of the internal primers from CT of the test primers, yielding ΔCT. ΔCT from the BAC was subtracted from ΔCT of the template to avoid PCR biases and random interactions from the test primers. Interaction frequency is calculated as the value of 2−(ΔCT of template − ΔCT of BAC). 3C primer sequences are provided in table S1.
Immunoblotting
For immunoblotting, cells were lysed in modified radioimmunoprecipitation assay (RIPA) buffer [50 mM tris (pH 7.5), 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, and 1 mM EDTA] with phosphatase inhibitor (Sigma) and protease inhibitors (Millipore) for 30 min in ice followed by centrifugation. Protein concentrations were determined with the Bio-Rad Protein Assay (Bio-Rad). Proteins were separated on precast Novex 10% Tris-Glycine gel/NuPAGE 4-12% Bis-Tris gel at 100 to 160 V using the Mini Gel Tank (Invitrogen) and were blotted onto PVDF membrane at 20 V for 90 min. The following antibodies were used: N-Myc (D1V2A) rabbit monoclonal antibody (Cell Signaling Technology, #84406), c-MYC (Cell Signaling Technology, #18583), CBFβ (Cell Signaling Technology, #62184), eIF4G1 (Cell Signaling Technology, #2858), cleaved caspase-3 (Asp175) antibody (Cell Signaling Technology, #9661), cleaved PARP (Asp214) (D64E10) antibody (Cell Signaling Technology, #5625), and β-actin (13E5) rabbit monoclonal antibody–horseradish peroxidase (HRP) conjugate (Cell Signaling Technology, #5125S).
Genome-wide datasets
All CUT&Tag data are available in Gene Expression Omnibus (GSE227930).
Publicly available datasets
Data from the following publicly available datasets were processed: GSE101790 (ATAC-seq in ME-1 cells), GSE101789 (ChIP-seq in ME-1 cells), GSE101788 (RNA-seq in ME-1 cells), and GSE108316 (AML patient DNase-seq).
Statistical analysis
For mouse leukemia survival analysis, the leukemia latency and P values were estimated using GraphPad Prism (version 8.2.1). The P value between groups was calculated using the log-rank test. For calculating the P value between AML subtypes (Leukemia Gene Atlas), P values were adjusted with Benjamini-Hochberg’s false discovery rate (FDR) correction. For the rest of the analysis, the P value was calculated using a two-tailed t test.
Acknowledgments
We thank S. A. Wolfe for help with genome editing using CRISPR/CAS9 RNP approach and B. Bonacci for assistance with flow cytometry. We also thank the University of Pennsylvania Stem Cell and Xenograft Core (RRID: SCR_010035) for providing human AML samples.
Funding: The Pulikkan laboratory is supported by American Cancer Society Institutional Research Grant from Medical College of Wisconsin Cancer Center and Versiti Blood Research Institute.
Author contributions: J.A.P. and P.S.P. were responsible for conceptualization; J.A.P., P.S.P., and S.S. were responsible for investigations; A.J.V., S.Z., and R.B. conducted bioinformatics analysis; N.Z., S.R., and J.H.B. provided comments on the manuscript; C.M.-T. and J.H.B. provided resources; J.A.P. was responsible for project administration and acquisition of funding; and J.A.P. wrote the original draft.
Competing interests: J.A.P. holds a patent for AI-10-49 (US2019/033889). J.H.B. holds patents for AI-10-49 (US2019/033889 and US8748618B2). The other authors declare that they have no competing interests.
Data and materials availability: The raw files for our next-generation sequencing data have been deposited in Gene Expression Omnibus (GSE227930). AI-4-88 and AI-14-91 can be provided by J.H.B., pending scientific review and a completed material transfer agreement. Requests for AI-4-88 and AI-14-91 should be submitted to: J.H.B. (jhb4v@virginia.edu). All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S7
Legends for tables S1 to S4
Other Supplementary Material for this manuscript includes the following:
Tables S1 to S4
REFERENCES AND NOTES
- 1.Okuda T., van Deursen J., Hiebert S. W., Grosveld G., Downing J. R., AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84, 321–330 (1996). [DOI] [PubMed] [Google Scholar]
- 2.Wang Q., Stacy T., Miller J. D., Lewis A. F., Gu T. L., Huang X., Bushweller J. H., Bories J. C., Alt F. W., Ryan G., Liu P. P., Wynshaw-Boris A., Binder M., Marin-Padilla M., Sharpe A. H., Speck N. A., The CBFbeta subunit is essential for CBFalpha2 (AML1) function in vivo. Cell 87, 697–708 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Cao W., Adya N., Britos-Bray M., Liu P. P., Friedman A. D., The core binding factor (CBF) alpha interaction domain and the smooth muscle myosin heavy chain (SMMHC) segment of CBFbeta-SMMHC are both required to slow cell proliferation. J. Biol. Chem. 273, 31534–31540 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Lukasik S. M., Zhang L., Corpora T., Tomanicek S., Li Y., Kundu M., Hartman K., Liu P. P., Laue T. M., Biltonen R. L., Speck N. A., Bushweller J. H., Altered affinity of CBF beta-SMMHC for Runx1 explains its role in leukemogenesis. Nat. Struct. Biol. 9, 674–679 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Castilla L. H., Wijmenga C., Wang Q., Stacy T., Speck N. A., Eckhaus M., Marin-Padilla M., Collins F. S., Wynshaw-Boris A., Liu P. P., Failure of embryonic hematopoiesis and lethal hemorrhages in mouse embryos heterozygous for a knocked-in leukemia gene CBFB-MYH11. Cell 87, 687–696 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Castilla L. H., Garrett L., Adya N., Orlic D., Dutra A., Anderson S., Owens J., Eckhaus M., Bodine D., Liu P. P., The fusion gene Cbfb-MYH11 blocks myeloid differentiation and predisposes mice to acute myelomonocytic leukaemia. Nat. Genet. 23, 144–146 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Kuo Y. H., Landrette S. F., Heilman S. A., Perrat P. N., Garrett L., Liu P. P., Le Beau M. M., Kogan S. C., Castilla L. H., Cbf beta-SMMHC induces distinct abnormal myeloid progenitors able to develop acute myeloid leukemia. Cancer Cell 9, 57–68 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Kamikubo Y., Zhao L., Wunderlich M., Corpora T., Hyde R. K., Paul T. A., Kundu M., Garrett L., Compton S., Huang G., Wolff L., Ito Y., Bushweller J., Mulloy J. C., Liu P. P., Accelerated leukemogenesis by truncated CBF beta-SMMHC defective in high-affinity binding with RUNX1. Cancer Cell 17, 455–468 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue L., Pulikkan J. A., Valk P. J., Castilla L. H., NrasG12D oncoprotein inhibits apoptosis of preleukemic cells expressing Cbfβ-SMMHC via activation of MEK/ERK axis. Blood 124, 426–436 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao L., Melenhorst J. J., Alemu L., Kirby M., Anderson S., Kench M., Hoogstraten-Miller S., Brinster L., Kamikubo Y., Gilliland D. G., Liu P. P., KIT with D816 mutations cooperates with CBFB-MYH11 for leukemogenesis in mice. Blood 119, 1511–1521 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H. G., Kojima K., Swindle C. S., Cotta C. V., Huo Y., Reddy V., Klug C. A., FLT3-ITD cooperates with inv(16) to promote progression to acute myeloid leukemia. Blood 111, 1567–1574 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Illendula A., Pulikkan J. A., Zong H., Grembecka J., Xue L., Sen S., Zhou Y., Boulton A., Kuntimaddi A., Gao Y., Rajewski R. A., Guzman M. L., Castilla L. H., Bushweller J. H., A small-molecule inhibitor of the aberrant transcription factor CBFβ-SMMHC delays leukemia in mice. Science 347, 779–784 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Surapally S., Tenen D. G., Pulikkan J. A., Emerging therapies for inv(16) AML. Blood 137, 2579–2584 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pulikkan J. A., Hegde M., Ahmad H. M., Belaghzal H., Illendula A., Yu J., O'Hagan K., Ou J., Muller-Tidow C., Wolfe S. A., Zhu L. J., Dekker J., Bushweller J. H., Castilla L. H., CBFβ-SMMHC inhibition triggers apoptosis by disrupting MYC chromatin dynamics in acute myeloid leukemia. Cell 174, 172–186.e21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DePinho R., Mitsock L., Hatton K., Ferrier P., Zimmerman K., Legouy E., Tesfaye A., Collum R., Yancopoulos G., Nisen P., Kriz R., Alt F., Myc family of cellular oncogenes. J. Cell. Biochem. 33, 257–266 (1987). [DOI] [PubMed] [Google Scholar]
- 16.Yanagisawa K., Horiuchi T., Fujita S., Establishment and characterization of a new human leukemia cell line derived from M4E0. Blood 78, 451–457 (1991). [PubMed] [Google Scholar]
- 17.Delmore J. E., Issa G. C., Lemieux M. E., Rahl P. B., Shi J., Jacobs H. M., Kastritis E., Gilpatrick T., Paranal R. M., Qi J., Chesi M., Schinzel A. C., McKeown M. R., Heffernan T. P., Vakoc C. R., Bergsagel P. L., Ghobrial I. M., Richardson P. G., Young R. A., Hahn W. C., Anderson K. C., Kung A. L., Bradner J. E., Mitsiades C. S., BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puissant A., Frumm S. M., Alexe G., Bassil C. F., Qi J., Chanthery Y. H., Nekritz E. A., Zeid R., Gustafson W. C., Greninger P., Garnett M. J., McDermott U., Benes C. H., Kung A. L., Weiss W. A., Bradner J. E., Stegmaier K., Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov. 3, 308–323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhen T., Cao Y., Ren G., Zhao L., Hyde R. K., Lopez G., Feng D., Alemu L., Zhao K., Liu P. P., RUNX1 and CBFβ-SMMHC transactivate target genes together in abnormal myeloid progenitors for leukemia development. Blood 136, 2373–2385 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Illendula A., Gilmour J., Grembecka J., Tirumala V. S. S., Boulton A., Kuntimaddi A., Schmidt C., Wang L., Pulikkan J. A., Zong H., Parlak M., Kuscu C., Pickin A., Zhou Y., Gao Y., Mishra L., Adli M., Castilla L. H., Rajewski R. A., Janes K. A., Guzman M. L., Bonifer C., Bushweller J. H., Small molecule inhibitor of CBFβ-RUNX binding for RUNX transcription factor driven cancers. EBioMedicine 8, 117–131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krevvata M., Shan X., Zhou C., Dos Santos C., Habineza Ndikuyeze G., Secreto A., Glover J., Trotman W., Brake-Silla G., Nunez-Cruz S., Wertheim G., Ra H. J., Griffiths E., Papachristou C., Danet-Desnoyers G., Carroll M., Cytokines increase engraftment of human acute myeloid leukemia cells in immunocompromised mice but not engraftment of human myelodysplastic syndrome cells. Haematologica 103, 959–971 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wunderlich M., Chou F. S., Link K. A., Mizukawa B., Perry R. L., Carroll M., Mulloy J. C., AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia 24, 1785–1788 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng W., Lee J., Wang H., Miller J., Reik A., Gregory P. D., Dean A., Blobel G. A., Controlling long-range genomic interactions at a native locus by targeted tethering of a looping factor. Cell 149, 1233–1244 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sin-Chan P., Mumal I., Suwal T., Ho B., Fan X., Singh I., Du Y., Lu M., Patel N., Torchia J., Popovski D., Fouladi M., Guilhamon P., Hansford J. R., Leary S., Hoffman L. M., Mulcahy Levy J. M., Lassaletta A., Solano-Paez P., Rivas E., Reddy A., Gillespie G. Y., Gupta N., Van Meter T. E., Nakamura H., Wong T. T., Ra Y. S., Kim S. K., Massimi L., Grundy R. G., Fangusaro J., Johnston D., Chan J., Lafay-Cousin L., Hwang E. I., Wang Y., Catchpoole D., Michaud J., Ellezam B., Ramanujachar R., Lindsay H., Taylor M. D., Hawkins C. E., Bouffet E., Jabado N., Singh S. K., Kleinman C. L., Barsyte-Lovejoy D., Li X. N., Dirks P. B., Lin C. Y., Mack S. C., Rich J. N., Huang A., A C19MC-LIN28A-MYCN oncogenic circuit driven by hijacked super-enhancers is a distinct therapeutic vulnerability in ETMRs: A lethal brain tumor. Cancer Cell 36, 51–67.e7 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Yu M., Mazor T., Huang H., Huang H. T., Kathrein K. L., Woo A. J., Chouinard C. R., Labadorf A., Akie T. E., Moran T. B., Xie H., Zacharek S., Taniuchi I., Roeder R. G., Kim C. F., Zon L. I., Fraenkel E., Cantor A. B., Direct recruitment of polycomb repressive complex 1 to chromatin by core binding transcription factors. Mol. Cell 45, 330–343 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assi S. A., Imperato M. R., Coleman D. J. L., Pickin A., Potluri S., Ptasinska A., Chin P. S., Blair H., Cauchy P., James S. R., Zacarias-Cabeza J., Gilding L. N., Beggs A., Clokie S., Loke J. C., Jenkin P., Uddin A., Delwel R., Richards S. J., Raghavan M., Griffiths M. J., Heidenreich O., Cockerill P. N., Bonifer C., Subtype-specific regulatory network rewiring in acute myeloid leukemia. Nat. Genet. 51, 151–162 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charron J., Malynn B. A., Fisher P., Stewart V., Jeannotte L., Goff S. P., Robertson E. J., Alt F. W., Embryonic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes Dev. 6, 2248–2257 (1992). [DOI] [PubMed] [Google Scholar]
- 28.Stanton B. R., Perkins A. S., Tessarollo L., Sassoon D. A., Parada L. F., Loss of N-myc function results in embryonic lethality and failure of the epithelial component of the embryo to develop. Genes Dev. 6, 2235–2247 (1992). [DOI] [PubMed] [Google Scholar]
- 29.Davis A. C., Wims M., Spotts G. D., Hann S. R., Bradley A., A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 7, 671–682 (1993). [DOI] [PubMed] [Google Scholar]
- 30.Ye M., Zhang H., Amabile G., Yang H., Staber P. B., Zhang P., Levantini E., Alberich-Jorda M., Zhang J., Kawasaki A., Tenen D. G., C/EBPa controls acquisition and maintenance of adult haematopoietic stem cell quiescence. Nat. Cell Biol. 15, 385–394 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson A., Murphy M. J., Oskarsson T., Kaloulis K., Bettess M. D., Oser G. M., Pasche A. C., Knabenhans C., Macdonald H. R., Trumpp A., c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 18, 2747–2763 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baena E., Ortiz M., Martinez A. C., de Alboran I. M., c-Myc is essential for hematopoietic stem cell differentiation and regulates Lin(−)Sca-1(+)c-Kit(−) cell generation through p21. Exp. Hematol. 35, 1333–1343 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Laurenti E., Varnum-Finney B., Wilson A., Ferrero I., Blanco-Bose W. E., Ehninger A., Knoepfler P. S., Cheng P. F., MacDonald H. R., Eisenman R. N., Bernstein I. D., Trumpp A., Hematopoietic stem cell function and survival depend on c-Myc and N-Myc activity. Cell Stem Cell 3, 611–624 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi J., Whyte W. A., Zepeda-Mendoza C. J., Milazzo J. P., Shen C., Roe J. S., Minder J. L., Mercan F., Wang E., Eckersley-Maslin M. A., Campbell A. E., Kawaoka S., Shareef S., Zhu Z., Kendall J., Muhar M., Haslinger C., Yu M., Roeder R. G., Wigler M. H., Blobel G. A., Zuber J., Spector D. L., Young R. A., Vakoc C. R., Role of SWI/SNF in acute leukemia maintenance and enhancer-mediated Myc regulation. Genes Dev. 27, 2648–2662 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delgado M. D., Leon J., Myc roles in hematopoiesis and leukemia. Genes Cancer 1, 605–616 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M., Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 224, 1121–1124 (1984). [DOI] [PubMed] [Google Scholar]
- 37.Schwab M., Varmus H. E., Bishop J. M., Grzeschik K. H., Naylor S. L., Sakaguchi A. Y., Brodeur G., Trent J., Chromosome localization in normal human cells and neuroblastomas of a gene related to c-myc. Nature 308, 288–291 (1984). [DOI] [PubMed] [Google Scholar]
- 38.Weiss W. A., Aldape K., Mohapatra G., Feuerstein B. G., Bishop J. M., Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 16, 2985–2995 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rickman D. S., Schulte J. H., Eilers M., The expanding world of N-MYC-driven tumors. Cancer Discov. 8, 150–163 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Dardenne E., Beltran H., Benelli M., Gayvert K., Berger A., Puca L., Cyrta J., Sboner A., Noorzad Z., MacDonald T., Cheung C., Yuen K. S., Gao D., Chen Y., Eilers M., Mosquera J. M., Robinson B. D., Elemento O., Rubin M. A., Demichelis F., Rickman D. S., N-Myc Induces an EZH2-mediated transcriptional program driving neuroendocrine prostate cancer. Cancer Cell 30, 563–577 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fielitz K., Althoff K., De Preter K., Nonnekens J., Ohli J., Elges S., Hartmann W., Kloppel G., Knosel T., Schulte M., Klein-Hitpass L., Beisser D., Reis H., Eyking A., Cario E., Schulte J. H., Schramm A., Schuller U., Characterization of pancreatic glucagon-producing tumors and pituitary gland tumors in transgenic mice overexpressing MYCN in hGFAP-positive cells. Oncotarget 7, 74415–74426 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee W. H., Murphree A. L., Benedict W. F., Expression and amplification of the N-myc gene in primary retinoblastoma. Nature 309, 458–460 (1984). [DOI] [PubMed] [Google Scholar]
- 43.Hirvonen H., Hukkanen V., Salmi T. T., Pelliniemi T. T., Alitalo R., L-mycand N-mycin hematopoietic malignancies. Leuk. Lymphoma 11, 197–205 (1993). [DOI] [PubMed] [Google Scholar]
- 44.Kawagoe H., Grosveld G. C., Conditional MN1-TEL knock-in mice develop acute myeloid leukemia in conjunction with overexpression of HOXA9. Blood 106, 4269–4277 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukuda Y., Wang Y., Lian S., Lynch J., Nagai S., Fanshawe B., Kandilci A., Janke L. J., Neale G., Fan Y., Sorrentino B. P., Roussel M. F., Grosveld G., Schuetz J. D., Upregulated heme biosynthesis, an exploitable vulnerability in MYCN-driven leukemogenesis. JCI Insight 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L., Xu F., Chang C. K., He Q., Wu L. Y., Zhang Z., Li X., MYCN contributes to the malignant characteristics of erythroleukemia through EZH2-mediated epigenetic repression of p21. Cell Death Dis. 8, e3126 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mundo L., Ambrosio M. R., Raimondi F., Del Porro L., Guazzo R., Mancini V., Granai M., Jim Rocca B., Lopez C., Bens S., Onyango N., Nyagol J., Abinya N., Navari M., Ndede I., Patel K., Paolo Piccaluga P., Bob R., de Santi M. M., Russell R. B., Lazzi S., Siebert R., Stein H., Leoncini L., Molecular switch from MYC to MYCN expression in MYC protein negative Burkitt lymphoma cases. Blood Cancer J. 9, 91 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawagoe H., Kandilci A., Kranenburg T. A., Grosveld G. C., Overexpression of N-Myc rapidly causes acute myeloid leukemia in mice. Cancer Res. 67, 10677–10685 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Vanden Bempt M., Debackere K., Demeyer S., Van Thillo Q., Meeuws N., Prieto C., Provost S., Mentens N., Jacobs K., Gielen O., Nittner D., Ogawa S., Kataoka K., Graux C., Tousseyn T., Cools J., Dierickx D., Aberrant MYCN expression drives oncogenic hijacking of EZH2 as a transcriptional activator in peripheral T-cell lymphoma. Blood 140, 2463–2476 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.King B., Boccalatte F., Moran-Crusio K., Wolf E., Wang J., Kayembe C., Lazaris C., Yu X., Aranda-Orgilles B., Lasorella A., Aifantis I., The ubiquitin ligase Huwe1 regulates the maintenance and lymphoid commitment of hematopoietic stem cells. Nat. Immunol. 17, 1312–1321 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malynn B. A., de Alboran I. M., O'Hagan R. C., Bronson R., Davidson L., DePinho R. A., Alt F. W., N-myc can functionally replace c-myc in murine development, cellular growth, and differentiation. Genes Dev. 14, 1390–1399 (2000). [PMC free article] [PubMed] [Google Scholar]
- 52.Riddell J., Gazit R., Garrison B. S., Guo G., Saadatpour A., Mandal P. K., Ebina W., Volchkov P., Yuan G. C., Orkin S. H., Rossi D. J., Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell 157, 549–564 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vo B. T., Wolf E., Kawauchi D., Gebhardt A., Rehg J. E., Finkelstein D., Walz S., Murphy B. L., Youn Y. H., Han Y. G., Eilers M., Roussel M. F., The interaction of Myc with Miz1 defines medulloblastoma subgroup identity. Cancer Cell 29, 5–16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Powers J. T., Tsanov K. M., Pearson D. S., Roels F., Spina C. S., Ebright R., Seligson M., de Soysa Y., Cahan P., Theissen J., Tu H. C., Han A., Kurek K. C., LaPier G. S., Osborne J. K., Ross S. J., Cesana M., Collins J. J., Berthold F., Daley G. Q., Multiple mechanisms disrupt the let-7 microRNA family in neuroblastoma. Nature 535, 246–251 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jongen-Lavrencic M., Sun S. M., Dijkstra M. K., Valk P. J., Lowenberg B., MicroRNA expression profiling in relation to the genetic heterogeneity of acute myeloid leukemia. Blood 111, 5078–5085 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Bhat M., Robichaud N., Hulea L., Sonenberg N., Pelletier J., Topisirovic I., Targeting the translation machinery in cancer. Nat. Rev. Drug Discov. 14, 261–278 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Malka-Mahieu H., Newman M., Desaubry L., Robert C., Vagner S., Molecular pathways: The eIF4F translation initiation complex-new opportunities for cancer treatment. Clin. Cancer Res. 23, 21–25 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Tamburini J., Green A. S., Bardet V., Chapuis N., Park S., Willems L., Uzunov M., Ifrah N., Dreyfus F., Lacombe C., Mayeux P., Bouscary D., Protein synthesis is resistant to rapamycin and constitutes a promising therapeutic target in acute myeloid leukemia. Blood 114, 1618–1627 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Topisirovic I., Guzman M. L., McConnell M. J., Licht J. D., Culjkovic B., Neering S. J., Jordan C. T., Borden K. L., Aberrant eukaryotic translation initiation factor 4E-dependent mRNA transport impedes hematopoietic differentiation and contributes to leukemogenesis. Mol. Cell. Biol. 23, 8992–9002 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishida Y., Zhao R., Heese L. E., Akiyama H., Patel S., Jaeger A. M., Jacamo R. O., Kojima K., Ma M. C. J., Ruvolo V. R., Chachad D., Devine W., Lindquist S., Davis R. E., Porco J. A. Jr., Whitesell L., Andreeff M., Ishizawa J., Inhibition of translation initiation factor eIF4a inactivates heat shock factor 1 (HSF1) and exerts anti-leukemia activity in AML. Leukemia 35, 2469–2481 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silvera D., Arju R., Darvishian F., Levine P. H., Zolfaghari L., Goldberg J., Hochman T., Formenti S. C., Schneider R. J., Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat. Cell Biol. 11, 903–908 (2009). [DOI] [PubMed] [Google Scholar]
- 62.Jaiswal P. K., Koul S., Shanmugam P. S. T., Koul H. K., Eukaryotic translation initiation factor 4 gamma 1 (eIF4G1) is upregulated during prostate cancer progression and modulates cell growth and metastasis. Sci. Rep. 8, 7459 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Comtesse N., Keller A., Diesinger I., Bauer C., Kayser K., Huwer H., Lenhof H. P., Meese E., Frequent overexpression of the genes FXR1, CLAPM1 and EIF4G located on amplicon 3q26-27 in squamous cell carcinoma of the lung. Int. J. Cancer 120, 2538–2544 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Wang H., Wang X., Xu L., Zhang J., Cao H., Prognostic significance of MYCN related genes in pediatric neuroblastoma: A study based on TARGET and GEO datasets. BMC Pediatr. 20, 314 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Badura M., Braunstein S., Zavadil J., Schneider R. J., DNA damage and eIF4G1 in breast cancer cells reprogram translation for survival and DNA repair mRNAs. Proc. Natl. Acad. Sci. U.S.A. 109, 18767–18772 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Del Valle L., Dai L., Lin H. Y., Lin Z., Chen J., Post S. R., Qin Z., Role of EIF4G1 network in non-small cell lung cancers (NSCLC) cell survival and disease progression. J. Cell. Mol. Med. 25, 2795–2805 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shorstova T., Foulkes W. D., Witcher M., Achieving clinical success with BET inhibitors as anti-cancer agents. Br. J. Cancer 124, 1478–1490 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feng Y., Pinkerton A. B., Hulea L., Zhang T., Davies M. A., Grotegut S., Cheli Y., Yin H., Lau E., Kim H., De S. K., Barile E., Pellecchia M., Bosenberg M., Li J. L., James B., Hassig C. A., Brown K. M., Topisirovic I., Ronai Z. A., SBI-0640756 attenuates the growth of clinically unresponsive melanomas by disrupting the eIF4F translation initiation complex. Cancer Res. 75, 5211–5218 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herzog L. O., Walters B., Buono R., Lee J. S., Mallya S., Fung A., Chiu H., Nguyen N., Li B., Pinkerton A. B., Jackson M. R., Schneider R. J., Ronai Z. A., Fruman D. A., Targeting eIF4F translation initiation complex with SBI-756 sensitises B lymphoma cells to venetoclax. Br. J. Cancer 124, 1098–1109 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vo T. T., Herzog L. O., Buono R., Lee J. S., Mallya S., Duong M. R., Thao J., Gotesman M., Fruman D. A., Targeting eIF4F translation complex sensitizes B-ALL cells to tyrosine kinase inhibition. Sci. Rep. 11, 21689 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaya-Okur H. S., Wu S. J., Codomo C. A., Pledger E. S., Bryson T. D., Henikoff J. G., Ahmad K., Henikoff S., CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 10, 1930 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buenrostro J. D., Wu B., Litzenburger U. M., Ruff D., Gonzales M. L., Snyder M. P., Chang H. Y., Greenleaf W. J., Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 523, 486–490 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Langmead B., Salzberg S. L., Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meers M. P., Tenenbaum D., Henikoff S., Peak calling by Sparse Enrichment Analysis for CUT&RUN chromatin profiling. Epigenetics Chromatin 12, 42 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramirez F., Ryan D. P., Gruning B., Bhardwaj V., Kilpert F., Richter A. S., Heyne S., Dundar F., Manke T., deepTools2: A next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu G., Wang L. G., Han Y., He Q. Y., clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ashburner M., Ball C. A., Blake J. A., Botstein D., Butler H., Cherry J. M., Davis A. P., Dolinski K., Dwight S. S., Eppig J. T., Harris M. A., Hill D. P., Issel-Tarver L., Kasarskis A., Lewis S., Matese J. C., Richardson J. E., Ringwald M., Rubin G. M., Sherlock G., Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25, 25–29 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benjamini Y. H., Y., Controlling the false discovery rate: A practical and powerful Approach to multiple testing. J. R. Stat. Soc. 57, 289–300 (1995). [Google Scholar]
- 80.Hagege H., Klous P., Braem C., Splinter E., Dekker J., Cathala G., de Laat W., Forne T., Quantitative analysis of chromosome conformation capture assays (3C-qPCR). Nat. Protoc. 2, 1722–1733 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S7
Legends for tables S1 to S4
Tables S1 to S4