ABSTRACT
Graft-vs-host disease (GVHD) of the gastrointestinal (GI) tract is notably a serious complication of allogeneic hematopoietic stem cell transplant (HSCT). However, GI GVHD has rarely been reported in autologous HSCT, and the pathophysiology remains unclear. Diagnosing GVHD after autologous HSCT requires a high level of clinical suspicion, given its nonspecific clinical presentation and endoscopic findings necessitating a histological diagnosis for confirmation. We present a case of autologous GVHD involving the GI tract in a patient with multiple myeloma who responded well to corticosteroids, highlighting the importance of early identification of this rare entity to initiate therapy and improve outcomes.
KEYWORDS: colitis, graft-vs-host disease, autologous, stem cell transplant, gastrointestinal tract
INTRODUCTION
Graft-vs-host disease (GVHD) is a common complication after allogeneic hematopoietic stem cell transplantation (HSCT) with an approximate incidence of 50%.1 On the other hand, GVHD postautologous HSCT has been rarely reported and is referred to as autologous or auto-GVHD.2 Multiple hypotheses have been suggested to explain the pathophysiology of auto-GVHD, but the exact mechanism remains unclear.3 Here, we present a case of gastrointestinal (GI) auto-GVHD in a patient with multiple myeloma (MM) who received auto-HSCT and had a remarkable clinical response after the initiation of corticosteroids. This emphasizes the importance of considering this diagnosis in auto-HSCT recipients presenting with GI symptoms because it is a treatable condition that can be easily overlooked.
CASE REPORT
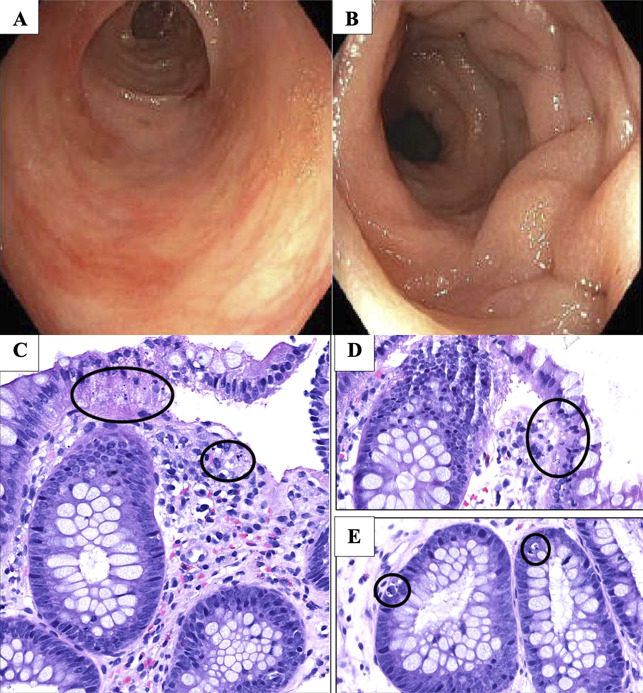
A 63-year-old woman with IgG kappa MM presented to our institution for evaluation of auto-HSCT. She had undergone induction with 6 cycles of lenalidomide, bortezomib, and dexamethasone since diagnosis with minimal to no regimen-related GI toxicity. She underwent consolidation with high-dose melphalan after her admission, followed by auto-HSCT. After transplant, she was taking filgrastim but no other immunosuppressive medications. She subsequently had progressive failure to thrive because of decreased oral intake, nausea, and vomiting requiring initiation of total parenteral nutrition. Despite efforts to maximize her nutritional support, she lost 20% of her total body weight and developed severe hypoalbuminemia to 1.8 g/dL and normocytic anemia with hemoglobin of 8.0 g/dL. Therefore, the decision was made to perform percutaneous endoscopic gastrostomy (PEG) tube placement for feeding. Esophagogastroduodenoscopy showed mild nonspecific gastric erythema in the antrum. Biopsies were not obtained, and PEG tube was successfully placed. She tolerated the procedure well and was transitioned to enteral feeding. Shortly after, she developed severe watery diarrhea with more than 10 stools daily. She had no fevers, abdominal pain, or GI bleeding. She remained hemodynamically stable. Physical examination was pertinent for dry mucous membranes, normal bowel sounds, and mild abdominal distension. Laboratory evaluation showed stable anemia and normal white blood cell count of 7.0 × 103/μL. Comprehensive infectious diarrhea workup with stool studies for Clostridioides difficile, GI pathogen panel, Giardia lamblia and cryptosporidium antigens, ova and parasites, and stool cultures was negative. She was not on any medications that could induce diarrhea, and symptoms did not improve after changing tube feeds to an iso-osmolar formulation and starting scheduled loperamide. Computed tomography of the abdomen showed a fluid-filled colon and rectum without significant abnormalities. Colonoscopy was performed at day 50 post-HSCT revealing diffuse and scattered areas of mild to moderate colitis characterized by erythema, congestion, and granularity in the entire colon most pronounced in the left colon (Figure 1). Targeted and random colonic biopsies showed prominent microscopic bleb formation and associated apoptotic bodies in contiguous crypts consistent with grade 2 of 4 GI auto-GVHD (Figure 1). High-dose intravenous methylprednisolone at 1 mg/kg twice daily was started for 3 days followed by an extended taper. She had complete resolution of the diarrhea and noted improvement in her upper GI symptoms. On her 2-month follow-up, she continued to do well while on low-dose prednisone.
Figure 1.
Colonoscopy findings of the descending and sigmoid colon showing granularity, erythema, congestion, and decreased vascular pattern in (A) and (B). High power magnification reveals colonic mucosa with prominent microscopic bleb formation and associated apoptotic bodies in both the ascending and descending colonic mucosal biopsies (black circles) noted in (C), (D), and (E).
DISCUSSION
Unlike GVHD after allogeneic HSCT, which is mediated through donor T cells and cytokines that lead to end-organ damage, the pathophysiology of auto-GVHD remains unclear.3 Several suggested mechanisms related to decreased self-tolerance, an altered immune system, and the use of newer drugs in MM have been proposed. For example, bortezomib is believed to affect T-cell function to the point of causing GVHD.4,5 Other theories characterize auto-GVHD as one of the manifestations of an engraftment syndrome seen after auto-HSCT. This syndrome is characterized by release of inflammatory cytokines, leading to autoreactive T cells and inhibition of regulatory T cells. This results in immune infiltration and inflammation of the affected tissue.6
Studies have reported that GVHD occurs in 5%-20% of patients after autologous HSCT compared with an incidence of 50% in GVHD after allogeneic HSCT.1,7 Upper GI symptoms of nausea and vomiting tend to be the most common presenting symptoms of auto-GVHD reported in 90% of patients followed by diarrhea.7 Our patient's initial presentation with persistent nausea and vomiting may have been related to upper GI involvement of auto-GVHD; however, biopsies were not obtained at the time of endoscopic PEG tube placement because there was low suspicion for auto-GVHD at that time. Barbash et al reported that nondiarrheal manifestations of GI GVHD are common and may represent an early stage of the disease before progression to involvement of the lower GI tract.8
The differential diagnosis is broad and includes infectious colitis, irritable bowel syndrome, engraftment syndrome, amyloidosis, hormonal disturbances, and medication side effects, among others. Imaging findings are usually nonspecific and can even be normal. Endoscopy can reveal normal to severe colitis.2 The diagnosis is confirmed histologically with apoptotic epithelial cells or crypt cell dropout with lymphocytic infiltrates.8
Several studies have shown that patients with MM are at a higher risk of developing auto-GVHD compared with other patients undergoing auto-HSCT.9 Furthermore, it has been suggested that auto-GVHD in MM patients can follow a more severe course and may not respond to steroids as desired.6,10 This supports the hypothesis that auto-GVHD can be attributed to alterations in the immune system related to the disease itself as well as the immunomodulators used in the conditioning phase.11 Treatment is with high-dose steroids similar to GVHD after allogeneic HSCT. Few studies reported a more severe course for auto-GVHD that was refractory to steroids, either because of superimposed infections or because of progression to an inflammatory bowel disease clinical picture.2,6 Levine et al proposed that anti-TNF may play a role in treating auto-GVHD involving the GI tract in the future in cases refractory to steroids.12
This case highlights the importance of close monitoring of GI symptoms after auto-HSCT, having a high clinical suspicion for auto-GVHD, and considering endoscopic evaluation with tissue sampling. These considerations are particularly important in MM patients receiving immunomodulators because auto-GVHD is a treatable condition and initiating early therapy can improve outcomes.
DISCLOSURES
Author contributions: All authors contributed to the case report. Manuscript preparation of the case and discussion was primarily written by H. Raza and S. Naffouj. G. Guzman and A. Shuja commented on previous versions of the manuscript. All authors read and approved the final manuscript. H. Raza is the article guarantor.
Financial disclosure: None to report.
Previous presentation: This case was previously presented at the American College of Gastroenterology (ACG) Annual Scientific Meeting; October 21–25, Vancouver, BC, Canada.
Informed consent was obtained for this manuscript.
Contributor Information
Sandra Naffouj, Email: snaffo2@uic.edu.
Grace Guzman, Email: graceguz@uic.edu.
Asim Shuja, Email: ashuja@uic.edu.
REFERENCES
- 1.Goddard DS, Ruben BS, Mathes ED, Nixon M, Wolf J, Fox LP. A case of severe cutaneous, GI and liver GVHD in a patient with multiple myeloma, status-post-second auto-SCT. Bone Marrow Transpl. 2010;45(2):409–11. [DOI] [PubMed] [Google Scholar]
- 2.Hammami MB, Talkin R, Al-Taee AM, Schoen MW, Goyal SD, Lai JP. Autologous graft-versus-host disease of the gastrointestinal tract in patients with multiple myeloma and hematopoietic stem cell transplantation. Gastroenterol Res. 2018;11(1):52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazarus HM, Sommers SR, Arfons LM, et al. Spontaneous autologous graft-versus-host disease in plasma cell myeloma autograft recipients: Flow cytometric analysis of hematopoietic progenitor cell grafts. Biol Blood Marrow Transpl. 2011;17(7):970–8. [DOI] [PubMed] [Google Scholar]
- 4.Drobyski WR, Hari P, Keever-Taylor C, Komorowski R, Grossman W.Severe autologous GVHD after hematopoietic progenitor cell transplantation for multiple myeloma. Bone Marrow Transpl. 2009;43(2):169–77. [DOI] [PubMed] [Google Scholar]
- 5.Cogbill CH, Drobyski WR, Komorowski RA. Gastrointestinal pathology of autologous graft-versus-host disease following hematopoietic stem cell transplantation: A clinicopathological study of 17 cases. Mod Pathol. 2011;24(1):117–25. [DOI] [PubMed] [Google Scholar]
- 6.Cornell RF, Hari P, Drobyski WR. Engraftment syndrome after autologous stem cell transplantation: An update unifying the definition and management approach. Biol Blood Marrow Transpl. 2015;21(12):2061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmberg L, Kikuchi K, Gooley TA, et al. Gastrointestinal graft-versus-host disease in recipients of autologous hematopoietic stem cells: Incidence, risk factors, and outcome. Biol Blood Marrow Transpl. 2006;12(2):226–34. [DOI] [PubMed] [Google Scholar]
- 8.Barbash B, Kramer S, Tzimas D, Saitta P. Graft-versus-host disease of the upper gastrointestinal tract after an autologous stem cell transplant. ACG Case Rep J. 2014;2(1):55–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batra A, Cottler-Fox M, Harville T, Rhodes-Clark BS, Makhoul I, Nakagawa M. Autologous graft versus host disease: An emerging complication in patients with multiple myeloma. Bone Marrow Res. 2014;2014:891427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fidler C, Klumpp T, Mangan K, et al. Spontaneous graft versus host disease occurring in a patient with multiple myeloma after autologous stem cell transplant. Am J Hematol. 2012;87(2):219–21. [DOI] [PubMed] [Google Scholar]
- 11.Galustian C, Meyer B, Labarthe MC, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58(7):1033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111(4):2470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]