Abstract
Objectives
This study examined the effects of the comprehensive medication review of Medicare medication therapy management programs on opioid overuse among Medicare beneficiaries.
Methods
This retrospective study analyzed Medicare data from 2016 to 2017. The intervention group included Medicare beneficiaries who newly received comprehensive medication review in 2017; the control group referred to patients who met the general eligible criteria for the medication therapy management program but did not enroll in 2016 or 2017. Propensity score matching was performed to increase characteristic compatibility between the intervention and control groups. Three measures of opioid overuse were analyzed: use of opioids at a high dosage, use of opioids from multiple providers, and concurrent use of opioids and benzodiazepines. The effects of comprehensive medication review on opioid overuse were analyzed with a multivariate logistic regression with an interaction term between the receipt of comprehensive medication review and the year 2017.
Key Findings
The proportion of concurrent use of opioids and benzodiazepines declined at a greater rate among the recipients (2.21%) than non-recipients (1.55%) of the comprehensive medication review. In the adjusted analysis, the odds ratio of no concurrent use of opioids and benzodiazepines was 5% higher (1.05; 95% confidence interval = 1.02–1.09) among recipients than non-recipients. These significant findings were not found for the other two measures of opioid overuse.
Conclusions
Comprehensive medication review is associated with reduced concurrent use of opioids and benzodiazepines among Medicare beneficiaries. Such service should be incorporated into the current approaches for addressing the opioid epidemic.
Keywords: medication therapy management, comprehensive medication review, disparity, medicare
Introduction
Opioid analgesics have been considered the backbone of pain management for over two decades. Beginning in the mid-1990s with the push to recognize and treat pain as a “fifth vital sign” and the later adoption of pain screenings and rating scales, there have been substantial efforts to improve pain care [1]. To increase patient satisfaction with pain management, opioids became more commonly prescribed [1]. This initiative was reversed in 2016 after research found that treating pain as a fifth vital sign, along with many other factors, contributed to the opioid crisis [1]. Recent research has also begun challenging the classic perception of opioids as the most effective analgesics. For instance, a systematic review of 183 randomized control trials assessing the efficacy of opioids and nonopioid treatments for acute pain found that opioid therapy had similar or reduced effectiveness when compared to non-steroidal anti-inflammatory drugs (NSAIDs) for low back pain, dental surgery pain, and kidney stone pain [2]. These findings are reflected in the 2022 Centers for Disease Control and Prevention (CDC) guideline for opioid prescribing, recommending nonopioid treatments for both acute and chronic pain unless the benefits for the patient are greater than the risks [3].
The increased utilization of opioids has also led to research improving our understanding of the risk of harms associated with their short- and long-term use. Compared to NSAIDs and acetaminophen, the use of opioids for acute pain was found to increase the risk of any short-term adverse effects [2]. In patients with chronic pain, opioid use was associated with a dose-dependent increase in the risk of overdose, opioid dependence diagnosis, myocardial infarction, and all-cause mortality [4]. To identify the number of patients at risk of an opioid overdose, the Pharmacy Quality Alliance developed three high-risk measures: use of opioids at a high dosage, use of opioids from multiple providers, and concurrent use of opioids and benzodiazepines (BZDs) [5]. High doses of opioids can increase the potential for overdose while receiving opioids from multiple providers can lead to an excessive dose if care is not coordinated [6]. Combining opioids and BZDs has been shown to increase the risk of respiratory depression and overdose [7]. To reduce these potential adverse events, the 2016 and 2022 CDC guidelines recommend creating risk mitigation strategies with the patient and avoiding co-prescribing opioids and BZDs unless the benefits outweigh the risks [3].
Medication-related problems have long represented a significant burden to the United States healthcare system. Adverse drug reactions and ineffective or inappropriate medication utilization result in annual healthcare costs exceeding $500 billion in the United States [8, 9]. Due to their increased likelihood of chronic conditions and resultant polypharmacy, older adults are impacted by these medication therapy problems at a much higher rate than the rest of the population [10]. The development of programs to address these difficulties and improve health outcomes began in the 1990s. With the adoption of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, they became officially known as “Medication Therapy Management” (MTM) [11]. This act, enacted in 2006, established Medicare Part D and required MTM to be provided to qualifying beneficiaries [11]. Since then, the eligibility criteria have expanded several times to increase the number of enrollees [12]. To be deemed eligible for the MTM program, beneficiaries must meet the following criteria set by the Centers for Medicare and Medicaid Services (CMS): having multiple chronic diseases, taking multiple Part D drugs, and incurring annual costs for Part D drugs exceeding a set value. However, each plan can set its specific threshold for qualification within the guidelines [12]. Two core services that make up MTM are targeted medication reviews (TMRs) and comprehensive medication review (CMR).13 While TMRs are quarterly assessments of a beneficiary’s medication use, CMR is a more in-depth process that must be offered to enrollees at least once yearly. Although TMRs can be completed using the beneficiary’s records, CMR requires the review to occur either via telehealth or in person with the beneficiary [13]. This allows the pharmacist to collect a direct account from the patient regarding their medications so that they can identify and prioritize any medication-related problems. The provider then works with the patient to create a medication action plan to address these issues and provides the patient with a copy of the review [13].
MTM has been shown to improve health outcomes and adherence for a wide range of chronic disease states. For example, an analysis of 2010 Medicare Part D data found that providing MTM with CMR to beneficiaries with chronic obstructive pulmonary disease, congestive heart failure, and diabetes improved medication adherence [14]. In a pilot study of 706 patients with uncontrolled diabetes and/or hypertension from three Federally Qualified Health Centers who were provided MTM services, 52.84% of patients reached a controlled A1c, 65.21% achieved control of their blood pressure, and 209 interventions were made for medication-related problems concerning safety [15]. Since 2015, CMS has suggested, although not required, Medicare Part D plans to use the MTM program to address opioid overuse issues [16]. However, the effects of MTM programs on opioid-related medication safety outcomes have not been determined. The current paper aims to close this literature gap by investigating the impact of CMR on opioid overdose.
Method
This retrospective study utilized Medicare data from 2016 to 2017, merged with the Area Health Resources Files (AHRF) [17]. Medicare data consisted of Parts A and B claims, Part D Event (PDE) File, the Master Beneficiary Summary File (MBSF), and Part D MTM Data File [18]. Information on individual diagnoses was derived from Parts A and B claims data [18]. The MBSF provided beneficiary demographic and Medicare enrollment information [19]. The PDE File data included beneficiaries’ prescription utilization information, such as medication costs, days of supply, quantity dispensed, and national drug code [20]. The information on whether an individual was enrolled in and received MTM services was obtained from the Part D MTM Data File [21]. To supplement the characteristics of beneficiaries, community-level information on socio-demographics and healthcare capacity within the county of each beneficiary’s residence was collected from AHRF [17].
The sample in this study included only beneficiaries who satisfied the following criteria: (1) aged at least 65 years old and alive at the end of the study period; (2) were continuously covered by Medicare Parts A, B, and D; and (3) met the inclusion/exclusion criteria for an outcome measure of interest: use of opioids at high dosage (OHD), use of opioids from multiple providers (OMP), and the concurrent use of opioids and BZDs (COB) [22]. These outcome measures were developed by PQA, a nonprofit organization devoted to enhancing the quality of medication use and management for the Medicare program, to identify the number of patients at risk of an opioid overdose. Following the PQA measure criteria, beneficiaries were deemed eligible if they filled two or more prescriptions for opioids on separate dates and the total days supply was greater than or equal to 15 days; two opioid measures (OHD and COB), additionally required the first fill of opioid medications to have occurred 90 and 30 days before the end of the measurement year, respectively. Individuals in hospice or with cancer diagnoses were excluded from these three measures [22]. Note that people need to meet the inclusion criteria for one measure to be included in the analysis for that measure.
To evaluate the effects of the CMR service, this paper divided the sample into two groups: an intervention group and a control group. The intervention group in this study included Medicare beneficiaries who were newly enrolled in MTM and received CMR services in 2017. The control group referred to patients who met the general eligible criteria for the MTM program but did not enroll in either 2016 or 2017. The CMS provided sponsors with broad guidance for determining targeted beneficiaries for the MTM program. However, plans had the discretion of selecting specific eligibility thresholds for all criteria within the guidelines [23]. Generally, beneficiaries who met the following three criteria were eligible for the MTM services: (1) had multiple chronic conditions, (2) took multiple medications covered by Part D, and (3) likely to induce annual cost for Part D medications greater than the cost threshold that CMS specified: $3507 for the year 2016 and $3919 for the year 2017. For this study, beneficiaries with three or more chronic conditions, at least eight Part D medications, and annual medication costs above the pre-specified threshold each year were deemed eligible for the MTM program. Three chronic conditions and eight Part D medications were selected because these are the most frequent threshold values that sponsors chose [24, 25]. To determine the number of chronic conditions that a beneficiary had, a list of 25 chronic diseases was used due to their high prevalence and importance in Medicare [26].
Propensity score matching was performed to decrease the heterogeneity in underlying beneficiary characteristics between the intervention and control groups. A logistic regression model was used to obtain each beneficiary’s predicted likelihood of receiving CMRs. All baseline beneficiary- and community-level characteristics were included in the model. The nearest neighbor matching without replacement algorithm was employed for the matching process with a 1:3 ratio. This algorithm generates better-balanced matched pairs of intervention and control groups among other proposed propensity-score matching algorithms [27]. A 1:3 matching ratio between the intervention and control groups was used to increase the sample size. A matching ratio greater than 1:3 was not used for efficiency gain [28]. The propensity score-matched samples for each year were then combined to form the study’s final sample.
Three binary outcome variables were created to identify patients at risk of an opioid overdose: OHD, OMP, and COB. For the OHD measure, an observation was assigned a value of one if a beneficiary took opioids with a daily dosage exceeding 120 morphine milligram equivalents consecutively for 90 or more days. For the OMP measure, a beneficiary had a value of one if they were prescribed opioids from four or more prescribers and received opioids from four or more pharmacies. Finally, for COB, an individual was assigned a value of one if the person filled two or more prescriptions for any BZDs on at least two separate dates and used opioids and BZDs simultaneously for 30 or more days during the treatment period.
This study employed Gelberg-Andersen’s Behavioral Model for Vulnerable Populations as the theoretical framework to determine the model specifications [29]. This model describes health services utilization based on predisposing, enabling, and need factors. In this study, predisposing factors included beneficiary and community-level characteristics that influence a beneficiary’s medication use and the utilization of health services. Beneficiary-level predisposing factors were age, gender, and race/ethnicity. Racial/ethnic groups included non-Hispanic White, Black, Hispanic, Asian/Pacific Islander, and Others (other race/ethnicity, unknown, or American Indian/Alaska Native); American Indian/Alaska Native were grouped with other race/ethnicity and unknowns because of the low validity of the race code in identifying these groups [30]. At the community level, the proportion of married-couple families, the proportion of the population with at least a high school degree, income per capita, and the proportion of people without insurance were included in predisposing factors. Enabling factors represented community-level attributes that influence the accessibility to healthcare services and medications. Metropolitan statistical areas (MSA), Health Professional Shortage Areas (HPSA), and census region were included as enabling factors. Lastly, need factors referred to characteristics that influence a patient’s need for health services and medication use, such as self-perceived or objective health status. As a proxy for a patient’s health status, a risk-adjustment score was used for this study, with a high score signaling poorer health status. Following the CMS’s guideline, the risk score was computed based on beneficiaries’ diagnosis records [31].
Two-stage analyses were carried out to evaluate the effects of CMRs on the utilization of opioid medications among Medicare beneficiaries at risk of an opioid overdose. First, beneficiaries’ characteristics were compared between intervention and control groups before and after the propensity score matching. When testing the hypothesis that characteristics differ between the two groups, a t-test was used for continuous variables, and a Chi-square test was used for categorical variables. This study also explored whether receiving CMRs was associated with a lower risk of experiencing an opioid overdose. Specifically, we compared the proportions of patients at risk of an opioid overdose between the two groups based on the three measures using a Chi-square test. In the second stage, a difference-in-differences method was performed using the multivariate logistic regression analysis with an interaction term between CMR status and the year 2017 as an explanatory variable of interest. The odds ratio (OR) for the interaction term indicated the impact of receiving the CMR service on the opioid. Specifically, the OR greater than one would suggest that compared to non-CMR recipients, beneficiaries who received a CMR were less likely to (1) take a high dosage of opioids, (2) receive opioids from multiple providers, and (3) use opioids and BZDs concurrently. Standard errors were clustered at the county level to account for the potential within-county correlation.
A 5% significance level was employed to determine statistical significance throughout the study. All analyses were performed using SAS Enterprise V.7.1 (Cary, NC) through the CMS Virtual Research Data Center. This study was approved by the Institutional Review Board of the corresponding author’s institution (approval number 17–05326-XM, March 13, 2019).
Results
Differences in baseline characteristics before and after propensity score matching between the intervention and control groups are presented in Tables 1–3 for each measure separately. For the OHD measure (Table 1), the total sample of 50 228 beneficiaries consisted of 12 557 CMR recipients and 37 671 non-CMR recipients. Before matching, individual-level predisposing factors differed between the two groups: for example, CMR recipients were younger (P < .0001) and more likely to be male (P = .0004) than non-CMR recipients. Note that all beneficiaries in the intervention group were matched to beneficiaries in the control group, and characteristics became statistically indistinguishable between CMR- and non-CMR recipients after the matching across all measures. These patterns were similar to the other two measures (Tables 2 and 3).
Table 1.
Propensity score matching—the use of opioids at high dosage.
| Characteristics |
After matching | Before matching | ||||
|---|---|---|---|---|---|---|
| CMR recipients N = 12 557 |
Non-CMR recipients N = 37 671 |
Non-CMR recipients N = 147 201 |
||||
| Number | % | Number | % | Number | % | |
| Predisposing factors | ||||||
| Age, mean (SD)a | 74.47 (6.59) | 74.40 (6.98) | 75.75 (7.57) | |||
| Malea | 3412 | 27.17 | 10 210 | 27.10 | 37 710 | 25.62 |
| Race/ethnicitya | ||||||
| Non-Hispanic White | 10 069 | 80.19 | 30 137 | 80.00 | 117 168 | 79.60 |
| Black | 1403 | 11.17 | 4289 | 11.39 | 15 746 | 10.70 |
| Hispanic | 701 | 5.58 | 2065 | 5.48 | 8550 | 5.81 |
| Asian/Pacific Islander | 165 | 1.31 | 484 | 1.28 | 2908 | 1.98 |
| Others | 219 | 1.74 | 696 | 1.85 | 2829 | 1.92 |
| Proportion of married couples, mean (SD)b |
0.73 (0.06) | 0.73 (0.07) | 0.73 (0.07) | |||
| Proportion of education >= high school, mean (SD)a,b | 0.86 (0.06) | 0.86 (0.06) | 0.87 (0.06) | |||
| Income per capita ($1000), mean (SD)a,b |
44.28 (12.92) | 44.33 (12.67) | 46.28 (14.48) | |||
| Proportion of the uninsured, mean (SD)a,b |
0.10 (0.05) | 0.10 (0.04) | 0.10 (0.05) | |||
| Enabling factors | ||||||
| Metropolitan statistical areaa,b | 8623 | 68.67 | 25 878 | 68.69 | 108 639 | 73.80 |
| Health professional shortage areab | 11 357 | 90.44 | 34 038 | 90.36 | 133 736 | 90.85 |
| Census regionb | ||||||
| Northeast | 1838 | 14.64 | 5590 | 14.84 | 21 796 | 14.81 |
| Midwest | 3040 | 24.21 | 9145 | 24.28 | 33 008 | 22.42 |
| South | 5498 | 43.78 | 16 354 | 43.41 | 66 045 | 44.87 |
| West | 2181 | 17.37 | 6582 | 17.47 | 26 352 | 17.90 |
| Need factor | ||||||
| Risk adjustment summary score, mean (SD)a |
2.29 (1.53) | 2.26 (1.61) | 2.18 (1.51) | |||
Abbreviations: CMR = comprehensive medication review; SD = standard deviation.
aIndicates that characteristics were different between CMR recipients and non-CMR recipients before matching (P < .05).
bCommunity-level factors.
Note that the CMR recipients (intervention group) were 100% matched, and all characteristics were statistically indifferent after the matching.
Table 3.
Propensity score matching—the concurrent use of opioids and benzodiazepines.
| Characteristics |
After matching | Before matching | ||||
|---|---|---|---|---|---|---|
| CMR recipients N = 12 804 |
Non-CMR recipients N = 38 412 |
Non-CMR recipients N = 150 076 |
||||
| Number | % | Number | % | Number | % | |
| Predisposing factors | ||||||
| Age, mean (SD)a | 75.49 (6.61) | 75.41 (6.97) | 76.77 (7.58) | |||
| Malea | 3473 | 27.12 | 10 446 | 27.19 | 38 495 | 25.65 |
| Race/ethnicitya | ||||||
| Non-Hispanic White | 10 272 | 80.22 | 30 719 | 79.97 | 119 461 | 79.60 |
| Black | 1423 | 11.11 | 4330 | 11.27 | 15 976 | 10.65 |
| Hispanic | 715 | 5.58 | 2137 | 5.56 | 8748 | 5.83 |
| Asian/Pacific Islander | 171 | 1.34 | 523 | 1.36 | 3014 | 2.01 |
| Others | 223 | 1.74 | 703 | 1.83 | 2877 | 1.92 |
| Proportion of married couples, mean (SD)b | 0.73 (0.06) | 0.73 (0.07) | 0.73 (0.07) | |||
| Proportion of education >= high school, mean (SD)a,b | 0.86 (0.06) | 0.86 (0.06) | 0.87 (0.06) | |||
| Income per capita (in $1000), mean (SD)a,b | 44.30 (12.92) | 44.36 (12.54) | 46.32 (14.52) | |||
| Proportion of the uninsured, mean (SD)a,b | 0.10 (0.05) | 0.10 (0.04) | 0.10 (0.05) | |||
| Enabling factors | ||||||
| Metropolitan statistical areaa,b | 8806 | 68.78 | 26 412 | 68.76 | 110 841 | 73.86 |
| Health professional shortage areab | 11 578 | 90.42 | 34 769 | 90.52 | 136 356 | 90.86 |
| Census regionb | ||||||
| Northeast | 1879 | 14.68 | 5523 | 14.38 | 22 296 | 14.86 |
| Midwest | 3100 | 24.21 | 9436 | 24.57 | 33 655 | 22.43 |
| South | 5606 | 43.78 | 16 805 | 43.75 | 67 226 | 44.79 |
| West | 2219 | 17.33 | 6648 | 17.31 | 26 899 | 17.92 |
| Need factor | ||||||
| Risk adjustment summary score, mean (SD)a | 2.28 (1.53) | 2.27 (1.62) | 2.17 (1.51) | |||
Abbreviations: CMR = comprehensive medication review; SD = standard deviation.
aIndicates that characteristics were different between CMR recipients and non-CMR recipients before matching (P < .05).
bCommunity-level factors.
Note that the CMR recipients (intervention group) were 100% matched, and all characteristics were statistically indifferent after the matching.
Table 2.
Propensity score matching—the use of opioids from multiple providers.
| Characteristics |
After matching | Before matching | ||||
|---|---|---|---|---|---|---|
| CMR recipients N = 12 792 |
Non-CMR recipients N = 38 376 |
Non-CMR recipients N = 149 693 |
||||
| Number | % | Number | % | Number | % | |
| Predisposing factors | ||||||
| Age, mean (SD)a | 74.49 (6.61) | 74.43 (6.99) | 75.77 (7.58) | |||
| Malea | 3473 | 27.15 | 10 309 | 26.86 | 38 433 | 25.67 |
| Race/ethnicitya | ||||||
| Non-Hispanic White | 10 260 | 80.21 | 30 808 | 80.28 | 119 111 | 79.57 |
| Black | 1423 | 11.12 | 4354 | 11.35 | 15 965 | 10.67 |
| Hispanic | 715 | 5.59 | 2122 | 5.53 | 8735 | 5.84 |
| Asian/Pacific Islander | 171 | 1.34 | 469 | 1.22 | 3006 | 2.01 |
| Others | 223 | 1.74 | 623 | 1.62 | 2876 | 1.92 |
| Proportion of married couples, mean (SD)b |
0.73 (0.06) | 0.73 (0.07) | 0.73 (0.07) | |||
| Proportion of education >= high school, mean (SD)a,b | 0.86 (0.06) | 0.86 (0.06) | 0.87 (0.06) | |||
| Income per capita (in $1000), mean (SD)a,b |
44.30 (12.93) | 44.38 (12.63) | 46.32 (14.52) | |||
| Proportion of the uninsured, mean (SD)a,b |
0.10 (0.05) | 0.10 (0.04) | 0.10 (0.05) | |||
| Enabling factors | ||||||
| Metropolitan statistical areaa,b | 8803 | 68.82 | 26 523 | 69.11 | 110 571 | 73.87 |
| Health professional shortage areab | 11 565 | 90.41 | 34 652 | 90.30 | 136 002 | 90.85 |
| Census regionb | ||||||
| Northeast | 1877 | 14.67 | 5652 | 14.73 | 22 215 | 14.84 |
| Midwest | 3092 | 24.17 | 9380 | 24.44 | 33 565 | 22.42 |
| South | 5605 | 43.82 | 16 773 | 43.71 | 67 081 | 44.81 |
| West | 2218 | 17.34 | 6571 | 17.12 | 26 832 | 17.92 |
| Need factor | ||||||
| Risk adjustment summary score, mean (SD)a | 2.29 (1.53) | 2.27 (1.62) | 2.17 (1.51) | |||
Abbreviations: CMR = comprehensive medication review; SD = standard deviation.
aIndicates that characteristics were different between CMR recipients and non-CMR recipients before matching (P < .05).
bCommunity-level factors.
Note that the CMR recipients (intervention group) were 100% matched, and all characteristics were statistically indifferent after the matching.
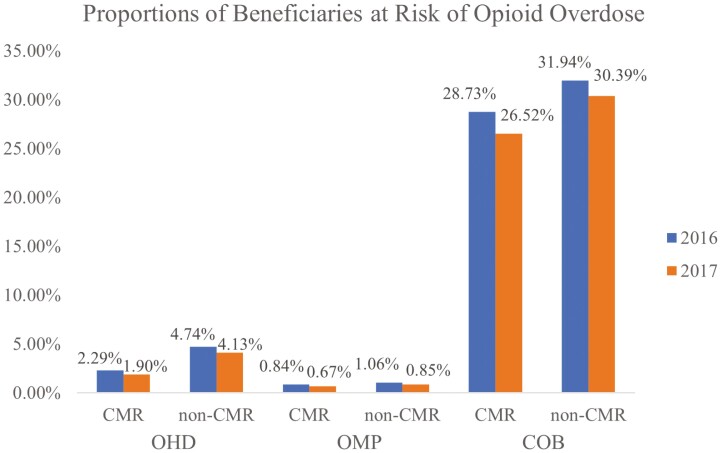
Figure 1 presents the trends in the proportions of Medicare beneficiaries at risk of an opioid overdose across each measure. Regardless of the CMR-receipt status, the proportion of beneficiaries at risk of an opioid overdose declined in 2017 across the board. Based on a Chi-square test, the differences in the proportion of beneficiaries at risk for each measure were statistically significant at the 1% level, except for the OMP measure among the CMR recipients. The proportion of beneficiaries at risk of an opioid overdose declined at a greater rate among CMR recipients than non-CMR recipients for the COB measure; the difference in the proportion of patients who used opioids and BZDs concurrently was 2.21% among CMR recipients (28.73% in 2016 vs. 26.52% in 2017) and 1.55% among non-CMR recipients (31.94% in 2016 vs. 30.39% in 2017).
Figure 1.
Trends in proportions of Medicare beneficiaries at risk of opioid overdose by PQA Opioid Measures. CMR = intervention group; non-CMR = control group. OHD, OMP, and COB stand for the use of opioids at high dosage, the use of opioids from multiple providers, and the concurrent use of opioids and benzodiazepines, respectively. The intervention group showed a decrease in the COB measure at a greater rate than the control group.
Table 4 displays the result from a multivariate logistic regression using a difference-in-differences approach. The signs of estimated coefficients for the key explanatory variable, the interaction terms between the CMR receipt status and the year 2017, were positive across the measures. However, only the OR of the COB measure for the interaction term was statistically significant. Compared to beneficiaries who did not receive the CMR service, the odds ratio of not taking opioids and BZDs concurrently was 5% greater (OR = 1.05; 95% confidence interval (CI) = 1.02–1.09) among CMR recipients.
Table 4.
Effects of comprehensive medication review on the utilization of opioid medications among Medicare beneficiaries.
| OHD | OMP | COB | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% confidence interval | Odds ratio | 95% Confidence interval | Odds ratio | 95% Confidence interval | |
| CMR receipt | 2.05 | 1.81–2.33 | 1.24 | 0.98–1.56 | 1.17 | 1.12–1.22 |
| Year 2017 | 1.09 | 1.04–1.13 | 1.19 | 1.04–1.36 | 1.05 | 1.03–1.07 |
| CMR receipt × year 2017 | 1.05 | 0.94–1.17 | 1.02 | 0.72–1.46 | 1.05 | 1.02–1.09 |
| Predisposing factors | ||||||
| Age | 1.08 | 1.07–1.09 | 1.08 | 1.07–1.10 | 1.029 | 1.026–1.032 |
| Male | 0.68 | 0.63–0.74 | 1.10 | 0.94–1.27 | 1.58 | 1.51–1.65 |
| Race/ethnicity | ||||||
| Black | 1.60 | 1.32–1.94 | 0.90 | 0.74–1.10 | 2.13 | 1.97–2.30 |
| Hispanic | 1.44 | 1.08–1.93 | 1.16 | 0.84–1.60 | 1.41 | 1.25–1.60 |
| Asian/Pacific Islander | 2.29 | 1.40–3.75 | 1.49 | 0.64–3.45 | 2.10 | 1.73–2.55 |
| Others | 1.41 | 1.06–1.87 | 1.11 | 0.66–1.85 | 1.27 | 1.11–1.46 |
| Proportion of married couples | 1.56 | 0.60–4.10 | 4.57 | 1.18–17.68 | 0.92 | 0.59–1.45 |
| Proportion of education >= high schoola | 0.04 | 0.01–0.14 | 0.031 | 0.005–0.196 | 3.56 | 2.02–6.28 |
| Income per capita (in $1000)a | 0.9962 | 0.9927–0.9997 | 0.992 | 0.986–0.998 | 1.001 | 0.998–1.003 |
| Proportion of the uninsureda | 0.04 | 0.01–0.17 | 0.29 | 0.03–2.63 | 2.23 | 1.16–4.31 |
| Enabling factors | ||||||
| Metropolitan statistical areaa | 0.88 | 0.79–0.99 | 0.72 | 0.59–0.87 | 0.96 | 0.90–1.01 |
| Health professional shortage areaa | 1.03 | 0.89–1.20 | 0.95 | 0.72–1.24 | 0.99 | 0.93–1.07 |
| Census regiona | ||||||
| Midwest | 1.45 | 1.23–1.70 | 0.52 | 0.40–0.69 | 1.03 | 0.95–1.12 |
| South | 1.29 | 1.09–1.52 | 0.53 | 0.39–0.72 | 0.81 | 0.74–0.88 |
| West | 0.92 | 0.78–1.08 | 0.42 | 0.32–0.56 | 1.19 | 1.08–1.31 |
| Need factor | ||||||
| Risk adjustment summary score | 0.978 | 0.957–0.999 | 0.86 | 0.83–0.88 | 0.93 | 0.92–0.94 |
Abbreviations: CMR = comprehensive medication review.
Reference groups for categorical variables are as follows: non-CMR recipients, the year 2016, female, non-Hispanic White, non-metropolitan statistical area, non-health professional shortage area, and northeast region.
aCommunity-level factor.
Results also showed that certain beneficiary characteristics were associated with some of the opioid measures (Table 4). Given that the current study found a statistically meaningful effect of CMRs only from the COB measure, this description focused on the associations of covariates with COB. Being older, a greater proportion of the county population with at least a high school degree, a larger proportion of the uninsured, and living in the West were associated with higher likelihoods of not using opioids and BZDs concurrently. Generally, racial/ethnic minority status was also positively related to the COB measure. For instance, Black patients were more likely than non-Hispanic White patients not to take opioids and BZDs concurrently. On the other hand, residing in the South and having a higher risk adjustment score was associated with a lower likelihood of not taking opioids and BZDs concurrently.
Discussion
This retrospective study employed 100% Medicare claims data from 2016 to 2017 to delve into the effect of CMR in addressing the challenges of opioid overdose. This paper found that compared to non-CMR recipients, beneficiaries who received a CMR were more likely not to take opioids and BZDs concurrently. However, this study did not find statistically meaningful evidence to support that receiving a CMR was associated with a lower likelihood of opioid use at high dosages and receiving opioids from multiple providers.
To address the opioid crisis among the elderly, CMS announced the overutilization policy in the 2013 Call Letter to implement a centralized data monitoring program, the Overutilization Monitoring System (OMS) [16]. Its purpose is to identify beneficiaries who meet the OMS criteria for opioid overutilization and limit their opioid utilization [16]. Since the policy’s inception, CMS data has indicated a significant decline in the number of opioid overutilizers [32]. Our findings also reconfirmed the downward trends in all opioid measures of interest irrespective of the CMR status.
MTM programs can be a great tool to fill the gap in the arena of medication management among opioid users. Although the overall impact of MTM services on adverse drug events (ADEs) is yet to be conclusively determined, several papers highlighted the advantages of pharmacist-led intervention in mitigating the risk of opioid-related ADEs [33–37]. Specifically, these interventions were found to be associated with tapering opioid doses and reduced co-prescribing of opioids and BZDs [33–37]. However, opioids are not among the targeted conditions for MTM programs suggested by CMS [16]. Additionally, adequate financial incentives are absent for Part D plans to expand the MTM program. Currently, the costs related to MTM services are included in plans’ annual CMS bids, without plans receiving separate reimbursement for MTM programs from CMS [12]. This may explain CMR’s limited benefits on opioid use.
CMR may have prompted CMR providers to address glaring issues other than the conditions targeted by CMR. COB may be considered a more pressing issue due to the boxed warning about such prescribing patterns from the Food & Drug Administration (FDA) in 2016 [38]. Opioid use issues, in general, may not be as new a concern among CMR providers because opioid use issues had been in the policy conversations in the years leading up to 2016 [39]. This may explain why this study found the effects of CMR only on COB but not other measures.
While statistically significant, the effect size of CMR on the concomitant use of opioids and BZDs was modest. This could potentially be attributed to the widespread awareness of the national opioid use crisis that public education had created. Prescribers may have been more self-policing, and patients may have been more cautious about using these medications. Indeed, a decline in opioid use was observed after the release of the 2016 CDC guidelines on opioid use, and so was the reduction in the practice of co-prescribing opioids and BZDs after the FDA boxed warning on the co-prescribing of opioids and BZDs [38, 40].
The current study offers evidence for potential modifications of CMS policies on the opioid crisis: MTM programs should be incorporated into existing CMS initiatives for opioid monitoring. In 2017, health care costs associated with opioid use disorder reached $31.3 billion in 2017, with Medicare accounting for approximately 10% of the costs [41]. Concurrent use of opioids and BZDs among opioid users was associated with higher odds of utilizing expensive health services and death [3, 7, 42]. MTM providers such as pharmacists are uniquely positioned regarding these issues because of their extensive training in pharmacology and medication management. Policymakers should consider expanding the MTM program within the CMS opioid overutilization policy to mitigate preventable medical expenses related to opioid overdose.
The study findings also suggested that some beneficiary- and county-level factors were associated with opioid overutilization patterns among older adults. These include age, gender, race/ethnicity, insurance, and geographic regions, which is generally consistent with the existing literature [43–48]. For example, age was negatively associated with the risk of any opioid overutilization measures. This was also reported in a CMS report on the national trends in chronic opioid utilization among older adults [43]. One possible explanation for such a negative relationship between age and opioid utilization is that physicians may be reluctant to prescribe opioids to this population because of their higher likelihood of ADEs [48]. Residing in a Southern area was positively associated with opioid overutilization, which also aligns with the extant research [44–47]. The South has experienced the highest opioid overdose rate and the highest rate of prescribing BZDs among primary care physicians among all geographic regions in the US [46, 47]. Further, this study posited that a higher risk adjustment summary score was associated with an increased risk of opioid overutilization. Given that a beneficiary’s diagnosis records are a key determinant of their risk score and the positive association between chronic conditions and opioid use disorder in the literature, such a finding is expected [44, 45].
The results of this paper should be interpreted with the following caveats. First, the study relied on county-level data for socioeconomic and demographic information, except for basic beneficiary-level information, such as age, sex, and race/ethnicity, due to the data limitation. County-level information may not accurately represent the individual beneficiaries’ characteristics. Second, for determining the control group, this study used the mode values for two MTM eligibility criteria: the number of chronic conditions and medications covered by Part D. However, these criteria can vary across Part D plans, so the study results may not be generalizable to beneficiaries enrolled in insurance plans with different eligibility criteria. Finally, opioid overutilization measures in this study were based on recorded prescription fills. However, it is plausible that beneficiaries can experience opioid overdose and associated problems from illicit opioid drugs. Despite the limitations mentioned, this study made a noteworthy contribution to the extant literature by examining the effectiveness of CMRs in addressing opioid overutilization and associated ADEs in the older population.
Conclusion
This paper found a positive association between CMRs and a reduced likelihood of concurrent usage of opioids and BZDs. The study findings highlight the need for incorporating the MTM program as a complementary strategy to the current CMS approaches for addressing the opioid epidemic. By leveraging the unique expertise of pharmacists and other MTM providers through MTM programs, Part D sponsors can better identify at-risk beneficiaries and prevent adverse health outcomes associated with the inappropriate use of opioids. Offering CMRs to all Medicare beneficiaries can also help tackle all medication utilization issues among opioid users, such as those with simultaneous use of opioids and opioid-potentiators other than BZDs. Future research is warranted to examine the effects of CMRs on the use of other opioid-potentiators among beneficiaries who are opioid users.
Acknowledgments
The authors would like to acknowledge the research assistance from Xiangjun Zhang, PhD, Postdoctoral Scholar, and Keuna Truitt, PharmD student, at the University of Tennessee Health Science Center College of Pharmacy.
Contributor Information
Yongbo Sim, Department of Economics, City University of New York, New York, NY, United States; Department of Clinical Pharmacy & Translational Science, University of Tennessee Health Science Center College of Pharmacy, Memphis, TN, United States.
Clayton F Hausberger, University of Tennessee Health Science Center College of Pharmacy, Memphis, TN, United States.
Junling Wang, Department of Clinical Pharmacy & Translational Science, University of Tennessee Health Science Center College of Pharmacy, Memphis, TN, United States.
Conflict of Interest Statement
Yongbo Sim: None. Clayton F. Hausberger: None. Junling Wang: Received funding from AbbVie, Curo, Bristol Myers Squibb, Pfizer, and serves as the Co-Chair of Value Assessment—Heath Outcomes Research Advisory Committee of the Pharmaceutical Research & Manufacturers of America (PhRMA) Foundation.
Funding
Research reported in this publication was supported by the National Institute On Aging of the National Institutes of Health under Award Number R01AG040146. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data Availability
Medicare databases are federal databases sponsored by the Centers for Medicare & Medicaid Services (CMS). These data are available to researchers through the Research Data Assistance Center (ResDAC) at the University of Minnesota, according to a strict protocol for data requests. Users of Medicare databases cannot disclose to, nor share the data with, individuals not listed in the Data Use Agreement.
References
- 1. Scher C, Meador L, Van Cleave JHet al. Moving beyond pain as the Fifth vital sign and patient satisfaction scores to improve pain care in the 21st century. Pain Manag Nurs 2018;19:125–9. 10.1016/j.pmn.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chou R, Wagner J, Ahmed AYet al. Treatments for acute pain: a systematic review. Comparative Effectiveness Review No. 240. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 290- 2015-00009-I.) AHRQ Publication No. 20(21)-EHC006. Rockville, MD: Agency for Healthcare Research and Quality, December 2020. 10.23970/AHRQEPCCER240 [DOI] [Google Scholar]
- 3. Dowell D, Ragan KR, Jones CMet al. CDC Clinical Practice Guideline for prescribing opioids for pain – United States, 2022. MMWR Recomm Rep 2022;71:1–95. 10.15585/mmwr.rr7103a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chou R, Hartung D, Turner Jet al. Opioid treatments for chronic pain. Comparative effectiveness review No. 229. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 290-2015-00009-I.) AHRQ Publication No. 20-EHC011. Rockville, MD: Agency for Healthcare Research and Quality, April 2020. 10.23970/AHRQEPCCER229 [DOI] [PubMed] [Google Scholar]
- 5. Pharmacy Quality Alliance. Measures overview. https://www.pqaalliance.org/measures-overview. (19 August 2023, date last accessed).
- 6. Baumblatt JAG, Wiedeman C, Dunn JRet al. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med 2014;174:796–801. 10.1001/jamainternmed.2013.12711 [DOI] [PubMed] [Google Scholar]
- 7. Sun EC, Dixit A, Humphreys Ket al. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 2017;356:j760. 10.1136/bmj.j760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watanabe JH, McInnis T, Hirsch JD.. Cost of prescription drug-related morbidity and mortality. Ann Pharmacother 2018;52:829–37. 10.1177/1060028018765159 [DOI] [PubMed] [Google Scholar]
- 9. Yoo A, Fennelly JE, Renauer MMet al. Comprehensive medication review service by embedded pharmacists in primary care: innovations and impact. J Am Pharm Assoc (2003) 2022;62:580–7.e1. Epub 2021 Oct 2. 10.1016/j.japh.2021.09.015 [DOI] [PubMed] [Google Scholar]
- 10. Ai AL, Carretta H, Beitsch LMet al. Medication therapy management programs: promises and pitfalls. J Manag Care Spec Pharm 2014;20:1162–82. 10.18553/jmcp.2014.20.12.1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Medicare Prescription Drug, Improvement, and Modernization Act of 2003. Public Law 108-173. December 8, 2003. http://www.gpo.gov/fdsys/pkg/PLAW-108publ173/content-detail.html. (19 August 2023, date last accessed).
- 12. Gray C, Cooke CE, Brandt N.. Evolution of the medicare part D medication therapy management program from inception in 2006 to the present. Am Health Drug Benefits 2019;12:243–51. [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Medicare & Medicaid Services. CY 2022 Medication Therapy Management Program Guidance and Submission Instructions. https://www.cms.gov/files/document/memo-contract-year-2022-medication-therapy-management-mtm-program-submission-v-043021.pdf. Published 2021. (19 August 2023, date last accessed).
- 14. Perlroth D, Marrufo G, Montesinos Aet al. Medication Therapy Management in Chronically Ill Populations: Final Report. https://innovation.cms.gov/files/reports/mtm_final_report.pdf; 2013. (19 August 2023, date last accessed).
- 15. Rodis JL, Sevin A, Awad MHet al. Improving chronic disease outcomes through medication therapy management in federally qualified health centers. J Prim Care Community Health 2017;8:324–31. 10.1177/2150131917701797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Centers for Medicare & Medicaid Services. Announcement of Calendar Year (CY) 2015 Medicare Advantage Capitation Rates and Medicare Advantage and Part D Payment Policies and Final Call Letter. https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Announcements-and-Documents-Items/2015Annoucement. (19 August 2023, date last accessed).
- 17. Health Resources & Services Administration. Area Health Resources Files. https://data.hrsa.gov/topics/health-workforce/ahrf. (20 April 2023, date last accessed). [Google Scholar]
- 18. Research Data Assistance Center. Data file search. https://www.resdac.org/cms-data?tid_1%5B%5D=1. (20 April 2023, date last accessed).
- 19. Research Data Assistance Center. Medicare Master Beneficiary Summary File (MBSF) base. https://resdac.org/cms-data/files/mbsf-base. (20 April 2023, date last accessed). [Google Scholar]
- 20. Research Data Assistance Center. Part D Event (PDE) File. https://resdac.org/cms-data/files/pde. (20 April 2023, date last accessed).
- 21. Research Data Assistance Center. Part D Medication Therapy Management Data file. https://resdac.org/cms-data/files/part-d-mtm. (20 April 2023, date last accessed). [Google Scholar]
- 22. Pharmacy Quality Alliance. Technical Specifications for PQA-Endorsed Measures. Alexandria, VA, USA: PQA. 2018. [Google Scholar]
- 23. Centers for Medicare & Medicaid Services. CY 2016 medication therapy management program guidance and submission instructions. https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Memo-Contract-Year-2016-Medication-Therapy-Management-MTM-Program-Submission-v-040715.pdf. Published 2015. (20 April 2023, date last accessed). [Google Scholar]
- 24. Centers for Medicare & Medicaid Services. 2016 Medicare Part D medication therapy management (MTM) programs. https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/CY2016-MTM-Fact-Sheet.pdf. Published 2016. (20 April 2023, date last accessed). [Google Scholar]
- 25. Centers for Medicare & Medicaid Services. 2017 Medicare Part D medication therapy management (MTM) programs. https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/CY2017-MTM-Fact-sheet.pdf. Published 2017. (20 April 2023, date last accessed). [Google Scholar]
- 26. Daniel GW, Malone DC.. Characteristics of older adults who meet the annual prescription drug expenditure threshold for Medicare medication therapy management programs. J Manag Care Pharm 2007;13:142–54. 10.18553/jmcp.2007.13.2.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med 2014;33:1057–69. 10.1002/sim.6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strom BL. Pharmacoepidemiology. 4th ed. Hoboken (NJ): John Wiley & Sons, 2005. [Google Scholar]
- 29. Gelberg L, Andersen RM, Leak BD.. The behavioral model for vulnerable populations: application to medical care use and outcomes for homeless people. Health Serv Res 2000;34:1273–302. [PMC free article] [PubMed] [Google Scholar]
- 30. Jarrin OF, Nyandege AN, Grafova IBet al. Validity of race and ethnicity codes in Medicare administrative data compared with gold-standard self-reported race collected during routine home health care visits. Med Care 2020;58:e1–8. 10.1097/MLR.0000000000001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Centers for Medicare & Medicaid Services. Medicare managed care manual: Chapter 7—risk adjustment. https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/mc86c07.pdf. Published 2014. (20 April 2023, date last accessed).
- 32. Centers for Medicare & Medicaid Services. Announcement of Calendar Year (CY) 2020 Medicare Advantage Capitation Rates and Medicare Advantage and Part D Payment Policies and Final Call Letter. https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Downloads/Announcement2020.pdf. (19 April 2023, date last accessed).
- 33. Bingham JM, Taylor AM, Boesen KPet al. Preliminary investigation of pharmacist-delivered, direct-to-provider interventions to reduce co-prescribing of opioids and benzodiazepines among a medicare population. Pharmacy (Basel) 2020;8:25. 10.3390/pharmacy8010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Niznik JD, Collins BJ, Armistead LTet al. Pharmacist interventions to deprescribe opioids and benzodiazepines in older adults: a rapid review. Res Social Adm Pharm 2022;18:2913–21. 10.1016/j.sapharm.2021.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farrell J, Miller M, Hennig Ket al. Pharmacist-led multidisciplinary approach to opioid tapering in a private rheumatology practice: patient outcomes. In: Arthritis & Rheumatology. Vol. 73. NJ USA: WILEY, 2021, September, 2216–7. [Google Scholar]
- 36. Tilli T, Hunchuck J, Dewhurst Net al. Opioid stewardship: implementing a proactive, pharmacist-led intervention for patients coprescribed opioids and benzodiazepines at an urban academic primary care centre. BMJ Open Qual 2020;9:e000635. 10.1136/bmjoq-2019-000635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Viswanathan M, Kahwati LC, Golin CEet al. Medication therapy management interventions in outpatient settings [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US), 2014. Nov. Report No.: 14(15)-EHC037-EF. [PubMed] [Google Scholar]
- 38. Bohnert ASB, Guy GP Jr, Losby JL.. Opioid prescribing in the United States before and after the Centers for Disease Control and Prevention’s 2016 Opioid Guideline. Ann Intern Med 2018;169:367–75. Epub 2018 Aug 28. 10.7326/M18-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jones MR, Viswanath O, Peck Jet al. A brief history of the opioid epidemic and strategies for pain medicine. Pain Ther 2018;7:13–21. Epub 2018 Apr 24. 10.1007/s40122-018-0097-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang V, Olfson M, King M.. Opioid and Benzodiazepine Coprescribing in the United States before and after US Food and Drug Administration Boxed Warning. JAMA Psychiatry 2019;76:1208–10. 10.1001/jamapsychiatry.2019.2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Florence C, Luo F, Rice K.. The economic burden of opioid use disorder and fatal opioid overdose in the United States, 2017. Drug Alcohol Depend 2021;218:108350. 10.1016/j.drugalcdep.2020.108350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shehab N, Lovegrove MC, Geller AIet al. US Emergency Department visits for outpatient adverse drug events, 2013-2014. JAMA 2016;316:2115–25. 10.1001/jama.2016.16201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anderson KK, Hendrick F, McClair V.. National trends in high-dose chronic opioid utilization among dually eligible and Medicare-only beneficiaries (2006–2015). Data Analysis Brief: Center for Medicare and Medicaid Services. https://www.cms.gov/medicare-medicaid-coordination/medicare-and-medicaid-coordination/medicare-medicaid-coordination-office/datastatisticalresources/downloads/opioidsdatabrief_2006-2015_10242018.pdf. Published 2018. (19 February 2024, date last accessed). [Google Scholar]
- 44. Wu LT, Zhu H, Ghitza UE.. Multicomorbidity of chronic diseases and substance use disorders and their association with hospitalization: results from electronic health records data. Drug Alcohol Depend 2018;192:316–23. 10.1016/j.drugalcdep.2018.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rajbhandari-Thapa J, Zhang D, Padilla HMet al. Opioid-related hospitalization and its association with chronic diseases: findings from the National Inpatient Sample, 2011-2015. Prev Chronic Dis 2019;16:E157. 10.5888/pcd16.190169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vadiei N, Bhattacharjee S.. Concurrent opioid and benzodiazepine utilization patterns and predictors among community-dwelling adults in the United States. Psychiatr Serv 2020;71:1011–9. 10.1176/appi.ps.201900446 [DOI] [PubMed] [Google Scholar]
- 47. Paulozzi LJ, Mack KA, Hockenberry JM; Division of Unintentional Injury Prevention, National Center for Injury Prevention and Control, CDC. Vital signs: variation among States in prescribing of opioid pain relievers and benzodiazepines – United States, 2012. MMWR Morb Mortal Wkly Rep 2014;63:563–8. [PMC free article] [PubMed] [Google Scholar]
- 48. Spitz A, Moore AA, Papaleontiou Met al. Primary care providers’ perspective on prescribing opioids to older adults with chronic non-cancer pain: a qualitative study. BMC Geriatr 2011;11:35. 10.1186/1471-2318-11-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Medicare databases are federal databases sponsored by the Centers for Medicare & Medicaid Services (CMS). These data are available to researchers through the Research Data Assistance Center (ResDAC) at the University of Minnesota, according to a strict protocol for data requests. Users of Medicare databases cannot disclose to, nor share the data with, individuals not listed in the Data Use Agreement.