Abstract
Background and study aims Besides increasing adequacy, rapid on-site evaluation (ROSE) during endoscopic ultrasound (EUS) or endoscopic retrograde cholangiopancreatography (ERCP) may impact choices and timing of subsequent therapeutic procedures, yet has been unexplored.
Patients and methods This was a retrospective evaluation of a prospectively maintained database of a tertiary, academic centre with availability of ROSE and hybrid EUS-ERCP suites. All consecutive patients referred for pathological confirmation of suspected malignancy and jaundice or gastric outlet obstruction (GOO) between Jan-2020 and Sep-2022 were included.
Results Of 541 patients with underlying malignancy, 323 (59.7%) required same-session pathological diagnosis (male: 54.8%; age 70 [interquartile range 63–78]; pancreatic cancer: 76.8%, biliary tract adenocarcinoma 16.1%). ROSE adequacy was 96.6%, higher for EUS versus ERCP. Among 302 patients with jaundice, ERCP-guided stenting was successful in 83.1%, but final drainage was completed in 97.4% thanks to 43 EUS-guided biliary drainage procedures. Twenty-one patients with GOO were treated with 15 EUS-gastroenterostomies and six duodenal stents. All 58 therapeutic EUS procedures occurred after adequate ROSE. With ERCP-guided placement of stents, the use of plastic stents was significantly higher among patients with inadequate ROSE (10/11; 90.9%) versus adequate sampling (14/240; 5.8%) P <0.0001; OR 161; 95%CI 19–1352). Median hospital stay for diagnosis and palliation was 3 days (range, 2–7) and median time to chemotherapy was 33 days (range, 24–47).
Conclusions Nearly two-thirds of oncological candidates for endoscopic palliation require contemporary pathological diagnosis. ROSE adequacy allows, since the index procedure, state-of-the-art therapeutics standardly restricted to pathologically confirmed malignancies (e.g. uncovered SEMS or therapeutic EUS), potentially reducing hospitalization and time to oncological treatments.
Keywords: Endoscopic ultrasonography, Pancreatobiliary (ERCP/PTCD), Tissue diagnosis, Fine-needle aspiration/biopsy, Intervention EUS
Introduction
Pancreatobiliary malignancies represent an increasing burden, especially in terms of cancer-related mortality 1 2 . A prompt and accurate diagnosis is crucial for timely access to curative surgery or chemotherapy and might impact on disease-specific survival 3 4 5 .
Endoscopic ultrasound (EUS) is a fundamental investigation in this setting, being the gold standard for pathological diagnosis of most pancreatobiliary diseases 6 , and it is increasingly acquiring therapeutic relevance for symptom palliation 7 . Rapid on-site evaluation (ROSE) by cytopathologists has been extensively evaluated as an additional tool to reduce false-negative results of EUS-guided fine-needle aspiration (FNA) sampling in pancreatobiliary malignancies 8 , despite the need for specific expertise and additional management costs.
Although EUS-FNA + ROSE may be unnecessary when endoscopists use needles specifically designed for obtaining histological cores, the so-called fine-needle biopsy (FNB) 9 , false-negative samples may be encountered even after EUS-FNB, and it is necessary to wait for the histopathological report to eventually assess the adequacy of the material.
Notably, most evidence belongs to the setting of pancreatic solid lesions, whereas sampling of non-mass-forming biliary strictures remains more challenging 10 , and ROSE has been suggested to increase adequacy of sampling obtained through endoscopic retrograde cholangiopancreatography (ERCP) 11 .
Moreover, besides adequate pathological confirmation of a disease, a fraction of patients with pancreatobiliary disease require prompt treatment of symptoms such as jaundice or gastric outlet obstruction (GOO) that are frequent at disease onset. Because their treatment strictly depends on their etiology, the absence of a confirmed malignancy might preclude some therapeutic maneuvers, such as the placement of uncovered self-expandable metal stents (SEMS) or the performance of some EUS-guided interventions, due to difficult 12 or impossible removal. Indeed, EUS-guided choledochoduodenostomy [EUS-CDS], hepaticogastrostomy [EUS-HGS] or gastroenterostomy (EUS-GE) are restricted to malignant diseases 13 .
For all these reasons, the absence of a malignancy confirmation may result in a delay of symptom palliation, while a ROSE-confirmed malignancy conversely may lead to same-session diagnosis and definitive palliation of cancer-related symptoms potentially resulting in fewer interventions and a shorter hospital stay and time to anticancer treatment. Notwithstanding, no study to date has evaluated the role of ROSE in impacting such therapeutic decisions.
The aim of this study, therefore, was to evaluate all consecutive patients with suspected malignancy requiring both cyto-histological characterization of a suspected pancreatobiliary neoplasia and additional therapeutic maneuvers for jaundice or GOO, with the aim to analyze: 1) the prevalence of this scenario; 2) the diagnostic impact of ROSE in terms of adequacy; and 3) the clinical impact of ROSE-assessed adequacy in subsequent therapeutic management.
Patients and methods
This was a retrospective evaluation of a prospectively maintained endoscopic database at San Raffaele Hospital (Milan, Italy), a tertiary, academic, referral center with availability of ROSE and hybrid endoscopic suites allowing same-session diagnostic EUS, ERCP, and therapeutic EUS.
All consecutive patients referred to the endoscopy unit for treatment of jaundice and/or GOO between January 2020 and September 2022 were queried. Patients with suspected malignancy were screened to evaluate how malignancy confirmation was obtained. Patients with same-session EUS/ERCP with ROSE were finally included.
Endpoints
The aims of this study were to analyze: 1) the rate (proportion) of therapeutic procedures requiring contemporary EUS-/ERCP-guided sampling of a suspected malignancy; 2) the rate (proportion) of ROSE-assessed adequacy of first endoscopic sampling; 3) the rate (proportion) of technical success of jaundice or GOO endoscopic palliation, with focus on the need to adopt procedures usually restricted to confirmed malignancies; 4) total length of hospital stay for diagnosis and palliation; and 5) time to chemotherapy initiation/resumption.
Patients
Inclusion criteria were as follows: 1) for final confirmation of malignancy, the gold standard for malignancy was a cyto-histological positive sample obtained through any technique (EUS, ERCP, liver biopsy of a metastasis, forceps biopsy during luminal endoscopy, surgical specimen) or by a clear clinico-radiological neoplastic evolution of the disease; 2) symptom palliation in either jaundice (bilirubin ≥2 mg/dL) or GOO (GOO Scoring System [GOOSS]) <2 14 , no intake or liquids only) in the presence of a radiologically or endoscopically confirmed biliary or upper gastrointestinal stenosis; and 3) first-time referral for an endoscopic therapeutic procedure; 4) clinical follow-up of at least 30 days
Exclusion criteria were as follows: 1) benign disease, either with a clear benign indication for the procedures (e.g., choledocholithiasis; treatment of postsurgical biliary fistula) or by exclusion of malignancy in indeterminate stenoses (either by histological confirmation of resected patients or clear clinico-radiological exclusion of malignancy after at least 12 months FU); 2) need for symptom palliation in patients with malignancies characterized in a previous diagnostic procedure; 3) patients who already received treatment for the same symptom (e.g. ERCP performed in another hospital); and 4) follow-up <30 days.
Definitions
Same-session diagnostic and symptom palliation was defined as a diagnostic EUS performed before ERCP, enteral stenting, or therapeutic EUS, in the same room, under the same sedation.
ROSE adequacy was defined as confirmation of the presence of enough material to confirm the clinico-radiological suspicion of malignancy. Technical success was defined as the completion of the intended procedure. In case therapeutic EUS was used as a rescue of failed ERCP, separate technical success was reported for ERCP alone and for overall biliary drainage, independent of the adopted procedure.
Hospital stay and time to chemotherapy were calculated from the day of the procedure to the day of hospital discharge and initiation of oncological treatment, respectively.
The complete list of collected variables is reported in Supplementary Statement 1
Endoscopic procedures
All procedures were performed under deep sedation or general anesthesia, in a fluoroscopy-equipped room.
EUS was performed using linear echoendoscopes (EG38-J10U, Pentax Medical). In our center, EUS-FNA is usually performed starting with a 25G Menghini-type FNA needle; however, the use of larger-caliber needles or FNB design is adopted at the discretion of the endoscopists.
ERCPs were performed using duodenoscopes (ED3470TK, ED34i10 T, Pentax Medical) by expert endoscopists who performed >200 procedures per year. Cannulation is usually performed with a sphincterotome over the wire, followed by contrast injection, double-guidewire technique, and pre-cut or transpancreatic sphincterotomy at the discretion of the endoscopists. ERCP-guided sampling is usually started with over-the-wire brushing catheters, with secondary use of biopsy forceps or cholangioscopy at the discretion of the endoscopists. Retrograde biliary stenting is usually performed through SEMS, with plastic stents restricted to resectable hilar malignancies or inadequate sampling. For distal malignant stenoses, a partially-covered SEMS (PC-SEMS) is usually preferred 15 , whereas uncovered SEMS are usually preferred in unresectable hilar malignancies.
In case of ERCP failure (either biliary access, or stenting of a desired biliary segment), EUS-guided biliary drainage is usually performed in the same session (typically EUS-CDS for distal stenoses and EUS-HGS for proximal stenoses). EUS-CDS is performed through free-hand placement of an 8×8 mm or a 6×8 mm LAMS (Hot Axios, Boston Scientific) between the common bile duct and the duodenum 16 . EUS-HGS is performed by EUS-guided access of a left intrahepatic duct through a 19G needle, followed by contrast injection, guidewire cannulation, tract creation through a 6F cystotome (Endo-flex GmbH), and placement of a partially-covered stent (Giobor, Taewoong) 17 .
As for GOO, enteral stenting was performed by through-the-scope placement of an uncovered 22-mm-wide SEMS across the stenosis 18 . EUS-GE was performed using the Wireless Simplified EUS-GE Technique (WEST) 19 , involving an oro-jejunal tube for jejunal distension and free-hand placement of an electrocautery-enhanced 20-mm LAMS (Hot Axios, Boston Scientific) 18 .
ROSE technique
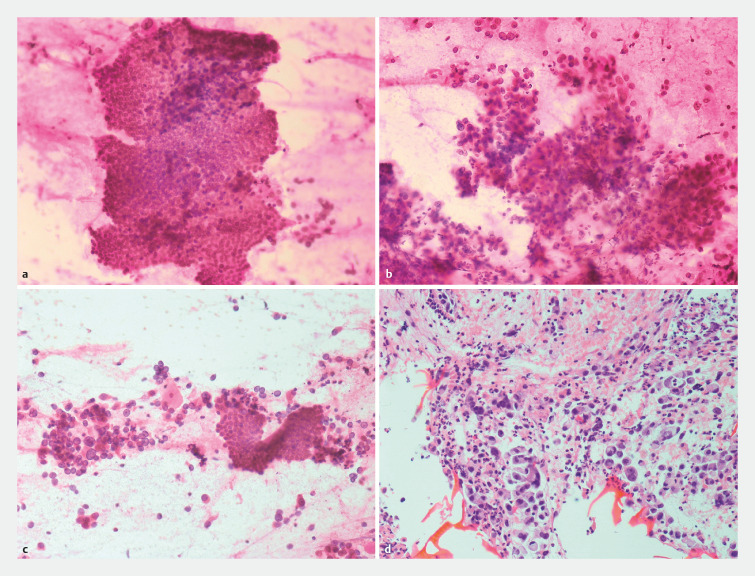
EUS-FNA or ERCP-guided samples were given to the on-site cytologist for ROSE and the endoscopist waited for the response regarding the adequacy to either perform additional passes or move on to additional diagnostic modalities or therapeutic procedures. The smears were prepared immediately after obtaining the specimen. Smears were fixed in absolute alcohol and stained with a rapid 2-minute hematoxylin-eosin stain ( Fig. 1 ). Once the slides were prepared, they were examined by an on-site cytologist and real-time evaluation of the sample adequacy was performed. A sample was considered adequate based on whether there was enough material representative of the site of sampling and compatible with the clinical suspicion of malignancy. The diagnosis was based on classic cytologic criteria, i.e nuclear shape and dimension, such as nuclei enlargement with irregularities and grooves, high nuclear-cytoplasmic ratio, pleomorphism, eventual necrotic background, and the architectural crowding with formation of 3D structures. The on-site cytologist was not blinded to patient clinical and radiological history.
Fig. 1.
Pathological smears. a EUS-FNA sampling; haematoxylin-eosin staining (20x): normal ductal epithelium. b EUS-FNA sampling; haematoxylin-eosin staining (20x): biliary adenocarcinoma. c ERCP-guided brushing; haematoxylin-eosin staining (20x): normal ductal epithelium close to a fragment of adenocarcinoma. d EUS-FNA sampling; hematoxylin-eosin staining (40x); cell block from EUS-FNA showing abundant material representing adenocarcinoma.
Ethics
This study was conducted in compliance with the Declaration of Helsinki and Good Clinical Practice. This retrospective study was approved by the Ethics Committee (Id: 178/INT/2020).
Statistics
Descriptive statistics are reported as frequencies (proportions) and medians (interquartile ranges). Comparisons were performed through the Chi-squared or Fisher’s test for qualitative data. P <0.05 was considered significant. All analyses were performed using Medcalc (Ostende, Belgium).
Results
Between January 2020 and September 2022, 541 patients with underlying malignancy were referred to San Raffaele Pancreatobiliary Endoscopy Unit for jaundice or GOO palliation. Of those, 218 had already received a pathological diagnosis of their malignancy, while 323 (59.7%) required same-session pathological confirmation before a therapeutic procedure, and represent the cohort under analysis.
Characteristics of included patients are reported in Table 1 . Of the patients, 54.8% were male, the median age was 70 years [range, 63–78], the primary disease was pancreatic cancer in 76.8% and cholangiocarcinoma in 16.1%. The neoplasm was resectable/borderline resectable in 33.1% of cases and locally advanced in 47.7%, while a higher rate of metastasis was seen among patients treated for GOO versus jaundice (38.1% versus 17.9%, P =0.02).
Table 1 Characteristics of included patients, separated according to presenting symptom.
| Variable | Jaundice (N=302) | GOO (N=21) |
| GOO, gastric outlet obstruction. | ||
| Age, median [IR] | 70 [68–71] | 70 [62–76] |
| Male, n (%) | 165 (54.6%) | 11 (52.4%) |
| Primary disease, n (%) | ||
| Pancreatic cancer | 231 (76.5%) | 17 (80.9%) |
| Cholangiocarcinoma | 52 (17.2%) | 1 (4.8%) |
| Ampullary/duodenal cancer | 8 (2.6%) | 2 (9.5%) |
| Metastatic lesion | 5 (1.8%) | / |
| Other malignancies | 6 (1.9%) | 1 (4.8%) |
| Oncological staging, n (%) | ||
| Resectable/borderline resectable | 229 (75.8%) | 1 (4.8%) |
| Locally advanced | 17 (5.6%) | 12 (57.2%) |
| Metastatic | 55 (18.2%) | 8 (38.1%) |
Diagnostic adequacy
EUS was chosen as the upfront modality for obtaining pathological diagnosis in 318 cases (98.5%) ( Table 2 ). ERCP-guided sampling was used in 16 cases (4.9%), but only in five cases (1.5%) it was used without any prior EUS attempt, whereas in 11 cases, it followed inadequate EUS sampling.
Table 2 Characteristics of sampling procedures.
| Variable | N=323 |
| ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; FU, follow up. * Treated with over-the-scope clip closure. | |
| Upfront procedure | |
| EUS, n (%) | 318 (98.5) |
| ERCP, n (%) | 5 (1.5) |
| ERCP after inadequate EUS, n (%) | 11 (3.4) |
| First-session adequacy, n (%) | 312 (96.6) |
| EUS adequacy, n (%) | 304/318 (95.6) |
| ERCP adequacy, n (%) | 8/16 (50) |
| Inadequate samples, n (%) | 11 (3.4) |
| Final diagnosis obtained by | |
| Subsequent EUS | 1 |
| Subsequent ERCP | 4 |
| Surgical specimen | 2 |
| Clinico-radiological FU | 4 |
| Adverse events during sampling | 1/316 (0.3) |
| Duodenal perforation * | 1 |
Total adequacy of first round of sampling ( Table 2 ) was 96.6%. This rate was significantly higher for EUS versus ERCP (95.6% versus 50%, P <0.0001). Among the 11 patients (3.4%) with inadequate sampling, in five cases, pathological confirmation was obtained at subsequent EUS or ERCP (in 1 case by cholangioscopy-guided biopsies), while it was obtained through surgical resection and clinical and radiological follow-up in two and four cases, respectively.
Sampling adequacy was significantly higher in distal versus proximal biliary stenosis ( Supplementary Table 1 ), both overall (97.1% versus 90%, P =0.05) and when attempted via EUS (96.3% versus 85%, P =0.01).
Sampling adequacy was significantly higher in pancreatic cancer versus biliary tract cancer ( Supplementary Table 2 ), both overall (98.4% versus 90.4%, P =0.002), and when attempted via EUS (97.9% versus 87.2%, P <0.001).
Symptom palliation
Jaundice
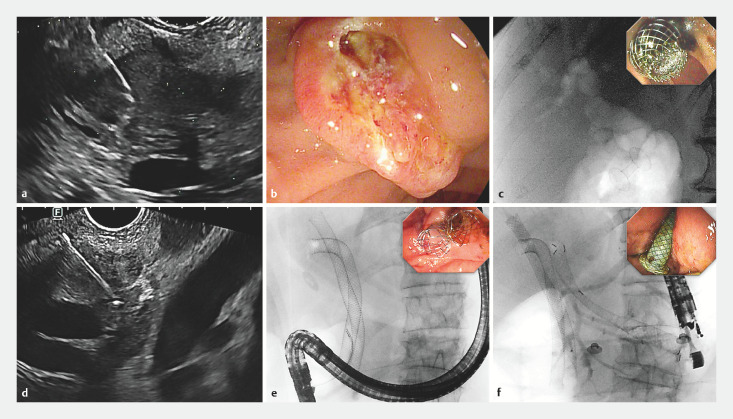
Among 302 patients with jaundice ( Table 3 ), ERCP-guided stenting was successful in 83.1%, but final endoscopic drainage was completed in 97.4%, through 37 EUS-choledochoduodenostomies ( Fig. 2 a–c ), five EUS-hepaticogastrostomies ( Fig. 2 d–f ) and one EUS-gallbladder drainage. Only seven patients (2.3%) required percutaneous transhepatic biliary drainage, whereas one patient underwent surgical bypass.
Table 3 Characteristics of therapeutic procedures.
| Variable | Jaundice N=302 | Variable | GOO N=21 |
| AE, adverse event; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; CDS, choledochoduodenostomy; GOO, gastric outlet obstruction; GBD, gallbladder drainage; GE, gastro-enterostomy; HGS, hepaticogastrostomy; IQR, interquartile range; LAMS, lumen apposing metal stent; PTBD, percutaneous transhepatic biliary drainage. * Sum of times for the diagnostic part, patient repositioning and instrument exchange, and the therapeutic part. | |||
| Endoscopic technical success, n (%) | 294 (97.4) | 20 (95.2) | |
| ERCP | 251 (83.1) | EUS-GE | 15 (71.4) |
| EUS-CDS | 37 (12.3) | Enteral Stenting | 6 (28.6) |
| EUS-HGS | 5 (1.7) | ||
| EUS-GBD | 1 (0.3) | ||
| Rescue of endoscopic failure, n (%) | |||
| PTBD | 7 (2.3) | ||
| Surgery | 1 (0.3) | Surgery | 1 (4.8) |
| AEs n (%) | 38 (12.6) | 3 (14.3) | |
| Post-ERCP acute pancreatitis | 17 (5.6) | ||
| Cholecystitis | 11 (3.6) | ||
| Cholangitis | 4 (1.3) | ||
| Bleeding | 5 (1.7) | Bleeding | 1 (4.8) |
| LAMS misdeployment | 1 (0.3) | LAMS misdeployment | 1 (4.8) |
| Vomiting | 1 (4.8) | ||
| Procedure time * [IQR], minutes | 90 [69.3–109.5] | 101 [84.3–107.5] | |
| Hospital stay [IQR], days | 3 [2–7] | 6.5 [4.5–11] | |
| Time to CHT [IQR], days | 34 [25–49] | 26.5 [20–28] | |
Fig. 2.
Management of Jaundice. a–c Patient with pancreatic adenocarcinoma. a EUS-FNA sampling of a pancreatic head lesion, adequate for malignancy. b Failed ERCP nowithstanding pre-cut fistulotomy. c Biliary drainage achieved through EUS-guided choledochoduodenostomy, as seen by the fluoroscopic visualization of aerobilia through the LAMS (inlet: endoscopic visualization of the LAMS at the end of the procedure). d–f Patient with Klatskin tumor and jaundice. a EUS-FNA of an unresectable hilar lesion with infiltration of the biliary carrefour; FNA was adequate for malignancy. b ERCP was performed with retrograde stenting of the right lobe (two uncovered SEMS in the right dorsal and right ventral ducts), whereas access to the left lobe was impossible. c same-session EUS-guided hepaticogastrostomy was performed to achieve complete biliary drainage.
The incidence of adverse events (AEs) was 12.6%, including a 5.6% rate of post-ERCP pancreatitis. Median hospital stay for diagnosis and symptom palliation was 3 days (range, 2–7) and median time to chemotherapy was 34 days (range, 25–49).
For ERCP-guided stenting ( Supplementary Table 2 and Supplementary Table 3 ), the use of plastic stenting and uncovered stenting was significantly higher in proximal stenoses and among cholangiocarcinomas, whereas most distal stenoses and pancreatic cancer-related strictures were treated with PC-SEMS.
The rate of plastic stenting was significantly higher among patients with inadequate ROSE (10/11 [90.9%]) than among those with adequate sampling (14/240 [5.8%], P <0.001, OR 161, 95% confidence interval 19–1352), whereas most remaining plastic stenting was due to a resectable hilar malignancy.
GOO
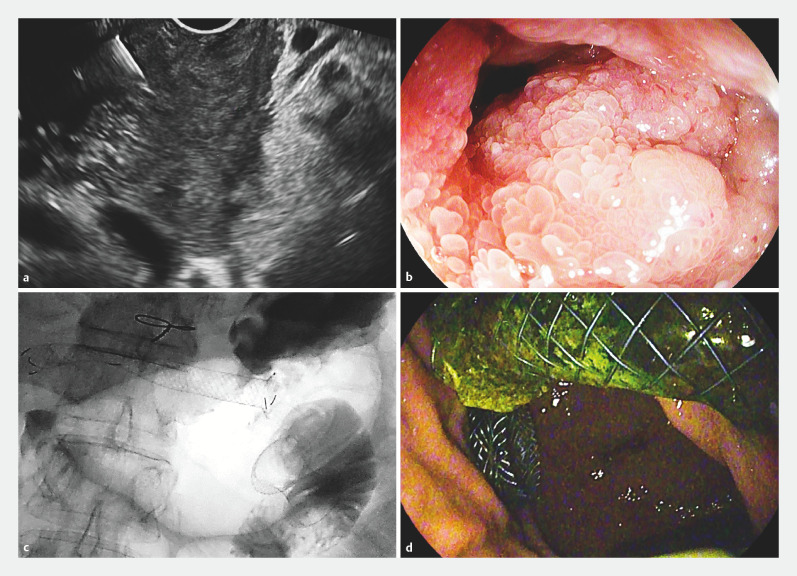
Among the 21 patients requiring GOO palliation ( Table 3 ), EUS-gastroenterostomy was performed in 15 ( Fig. 3 ) and endoscopic placement of uncovered duodenal SEMS in six, with a technical success rate of 95.2% at first procedure and an AE rate of 14.3%.
Fig. 3.
Management of a double obstruction. Pancreatic with adenocarcinoma and double biliary and gastric outlet obstruction. a EUS revealed a pancreatic head leasion determining biliary duct and duodenal infiltration; FNA was adequate for malignancy. b A symptomatic duodenal neoplastic obstruction impeded access to the papillary region. c,d EUS-guided gastroenterostomy and hepaticogastrostomy were performed in the same session ( c fluoroscopy; d endoscopy).
Median hospital stay for diagnosis and symptoms’ palliation was 6.5 days [range, 4.5–11] and median time to chemotherapy was 26.5 days [range, 22–30].
All 58 therapeutic EUS procedures occurred after adequate ROSE.
Discussion
ROSE has been extensively evaluated as an add-on to increase diagnostic accuracy of EUS-guided FNA sampling. Despite conflicting results of studies and meta-analysis, ROSE seems to be associated with an increased diagnostic yield and decreased need for repeated sampling 8 29 30 . However, the need for specific cytopathological expertise, additional costs and procedure time, have restricted the use of ROSE to a limited number of centers 8 . Furthermore, the advantage of ROSE is increasingly debated to be trivial in light of the introduction of needles with “core” design (EUS-FNB) 20 . However, most evidence is associated with pancreatic solid lesions and it does not account for some additional theoretical advantages of ROSE in clinical practice, which have been poorly investigated in the available literature. Specifically, no paper has analyzed the potential impact on timing and choices of subsequent therapeutic procedures.
In our series, almost 60% of patients needing palliation of jaundice or GOO required same-session pathological confirmation of the suspected malignancy, thus suggesting that a large majority of patients referred for endoscopic palliation would benefit from same-session diagnostics and therapeutics, where available.
Second, as expected, ROSE availability has resulted in an extremely high (97%) rate of sampling adequacy in this series. Moreover, our data provide some additional insights about variables affecting sampling adequacy, as this was higher in EUS versus ERCP samples (95.6% versus 50%, P <0.0001), in proximal versus distal biliary stenoses (97.1% versus 90%, P =0.05), and in pancreatic cancer versus cholangiocarcinoma (98.4% versus 90.4%, P =0.002). The relatively low yield of ERCP-guided sampling in this series is likely due to the selection of patients, being mostly used after inadequate EUS-guided sampling, which was a relatively rare event in this series. These data support the previously reported evidence that EUS-FNA has higher accuracy than ERCP-guided brushing in biliary stenoses, especially those that were extrahepatic, extrinsic and mass-forming 6 21 . This might support EUS as the primary sampling modality independently on the level of the stenosis, especially because the most commonly reported drawback is needle-tract seeding. Nonetheless, pancreatic cancer needle-tract seeding is extremely rare 22 , reported mostly as seeding nodules arising in the gastric wall that can be easily removed surgically 23 , and it has been proved that EUS-FNA does not increase the rate of peritoneal spread 24 and does not impact overall and recurrence-free survival 25 26 27 . As for proximal cholangiocarcinoma, needle-tract seeding was initially suggested by anecdotal cases (N=5) in a small series of transperitoneal FNA sampling, the majority of which were performed via a percutaneous rather than EUS-guided route 28 , while subsequent larger experiences demonstrated no influence of preoperative sampling on overall and progression-free survival 24 31 . Furthermore, optimization of intraductal (ERCP-guided) sampling would require the more expensive use of cholangioscopy 6 , which we usually restrict to cases with inadequate first-round sampling.
More important, in our series, an adequate ROSE allowed same-session diagnostics and state-of-the-art therapeutics typically restricted to pathologically confirmed malignancies, leading to an overall median hospital stay of 3 days (range, 2–7) and a median time to chemotherapy of 33 days (range, 24–47). Despite the absence of a control group, these results inherently suggest that the availability of ROSE and hybrid suites allowing EUS, ERCP, and therapeutic EUS might contribute to reducing the time to obtain pathological confirmation of a neoplasia and long-lasting symptoms palliation. This time minimization does not intrinsically depend on ROSE, but on the reduced rate of false-negative sampling and the reduced need for reintervention deriving from using state-of-the-art therapeutics. Conversely, in facilities where diagnostic EUS and operative procedures are performed in different rooms, two procedures are required, and they are usually not planned on the same day. In the same setting, in case of failed ERCP, EUS-guided rescue drainage might require rescheduling the procedure in a different session or room. Moreover, the availability of pathological confirmation of malignancy is considered mandatory for some specific therapeutic modalities. Indeed, to date, EUS-guided biliary drainage is restricted to pathologically confirmed malignancies, as also suggested by the only available guidelines on this topic 13 . In our experience, the possibility of performing same-session EUS-CDS or EUS-HGS has increased the technical success rate for biliary drainage from 83% (retrograde stenting) to 97% (combined retrograde and EUS-guided drainage); this also means that the need for percutaneous transhepatic biliary drainage (PTBD), with its known morbidity burden 32 33 , might be significantly contained where adequate endoscopic expertise is available, and that definitive biliary drainage might be obtained during the first endoscopic procedure in almost all cases, provided an adequate ROSE is available.
Pathological diagnosis might also impact choices regarding ERCP stenting: plastic stenting or fully-covered SEMS (FC-SEMS) are usually preferred by centers not performing ROSE, because an uncovered design might significantly complicate removability of stents in case of an eventual benign etiology or when additional sampling is required 34 . However, plastic stenting has demonstrated a significantly higher rate of jaundice recurrence, even in the neoadjuvant setting, and this might result in unplanned readmission or chemotherapy interruptions in these patients 12 . Although FC-SEMS might be a good compromise in distal stenoses, PC-SEMS seems associated with longer patency and might be preferrable in case of confirmed malignancies 15 . Moreover, FC-SEMS are not recommended for hilar strictures, due to the risk of obstructing side biliary branches 34 ; therefore, an unconfirmed malignancy would preclude the placement of better-performing UC-SEMS. Consistently, the use of plastic stenting in our series was significantly higher in case of inadequate sampling.
The potential advantages of ROSE-assessed adequacy are even more apparent when dealing with management of GOO. In this scenario, management of benign versus malignant GOO involves completely different procedures, ranging from medical treatment, balloon dilation or surgical bypass in the former to uncovered SEMS or, more recently, EUS-guided gastroenterostomy in the latter. Therefore, in the absence of ROSE, the definitive treatment of GOO must be deferred, usually by temporary placement of a nasogastric decompression tube, while awaiting pathological confirmation of malignancy.
For all these reasons, we believe that availability of ROSE and hybrid EUS, ERCP, and therapeutic EUS suites can play a major role in the management of patients with pancreatobiliary malignancies.
This study has several limitations. First, despite an accurate and extensive search, the retrospective nature might have led to exclusion of some patients/events of interest. Second, these results were obtained in a tertiary, academic, multidisciplinary referral center with cytopathological expertise and high-volume experience in pancreatic pathology, pancreatobiliary endoscopy, and therapeutic EUS. The generalizability of the findings outside this setting cannot be assured. In addition, the absence of a control group necessitates caution in the interpretation of the data, which should still be considered speculative with need of confirmation. Nevertheless, the authors attempted unsuccessfully to procure a control group of patients treated for similar indications without the use of ROSE from other centers. Finally, while it is tempting to speculate that shorter hospitalization and time to active treatment may result in lower costs, better quality of life, and longer disease-specific survival, these data were not collected in our study.
Conclusions
To the best of our knowledge, this is the only available report describing how availability of ROSE might impact timing and efficacy of symptom palliation in patients with pancreatobiliary malignancies. While awaiting prospective, controlled comparisons on this topic, the present findings suggest that availability of ROSE and hybrid EUS/ERCP/therapeutic EUS expertise could contribute to reducing hospitalization and access time for oncological treatments for patients with newly diagnosed pancreatobiliary malignancies, especially those with more challenging scenarios, such as proximal/intrinsic biliary strictures, GOO, or double obstruction.
Acknowledgement
The Authors would like to thank the commitment of the cytologists Teresa Carbone, Miriam Cosciotti, Maria Grazia La Monaca, Franca Toffolo and Roberta Valagussa.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Supplementary Material
References
- 1.Gordon-Dseagu VL, Devesa SS, Goggins M et al. Pancreatic cancer incidence trends: evidence from the Surveillance, Epidemiology and End Results (SEER) population-based data. Int J Epidemiol. 2018;47:427–439. doi: 10.1093/ije/dyx232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Swords DS, Mone MC, Zhang C et al. Initial misdiagnosis of proximal pancreatic adenocarcinoma is associated with delay in diagnosis and advanced references stage at presentation. J Gastrointest Surg. 2015;19:1813–1821. doi: 10.1007/s11605-015-2923-z. [DOI] [PubMed] [Google Scholar]
- 4.Pitter JG, Lukács G, Csanádi M et al. Clinical impact of treatment delay in pancreatic cancer patients revisited. Int J Cancer. 2018;142:2621–2622. doi: 10.1002/ijc.31263. [DOI] [PubMed] [Google Scholar]
- 5.Sanjeevi S, Ivanics T, Lundell L et al. Impact of delay between imaging and treatment in patients with potentially curable pancreatic cancer. Br J Surg. 2016;103:267–275. doi: 10.1002/bjs.10046. [DOI] [PubMed] [Google Scholar]
- 6.Pouw RE, Barret M, Biermann K et al. Endoscopic tissue sampling - Part 1: Upper gastrointestinal and hepatopancreatobiliary tracts. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:1174–1188. doi: 10.1055/a-1611-5091. [DOI] [PubMed] [Google Scholar]
- 7.Vanella G, Bronswijk M, Arcidiacono PG et al. Current landscape of therapeutic EUS: Changing paradigms in gastroenterology practice. Endosc Ultrasound. 2023;12:16–28. doi: 10.4103/EUS-D-21-00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polkowski M, Jenssen C, Kaye P et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline - March 2017. Endoscopy. 2017;49:989–1006. doi: 10.1055/s-0043-119219. [DOI] [PubMed] [Google Scholar]
- 9.Crinò SF, Di Mitri R, Nguyen NQ et al. Endoscopic ultrasound-guided fine-needle biopsy with or without rapid on-site evaluation for diagnosis of solid pancreatic lesions: a randomized controlled non-inferiority trial. Gastroenterology. 2021;161:899–9.09E7. doi: 10.1053/j.gastro.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Fujii-Lau LL, Thosani NC, Al-Haddad M et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in the diagnosis of malignancy in biliary strictures of undetermined etiology: Summary and Recommendations. Gastrointest Endosc. 2023;98:685–693. doi: 10.1016/j.gie.2023.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Archibugi L, Mariani A, Ciambriello B et al. High sensitivity of ROSE-supported ERCP-guided brushing for biliary strictures. Endosc Int Open. 2021;9:E363–E370. doi: 10.1055/A-1322-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawas T, Al Halabi S, Parsi MA et al. Self-expandable metal stents versus plastic stents for malignant biliary obstruction: a meta-analysis. Gastrointest Endosc. 2015;82:256–2.67E9. doi: 10.1016/j.gie.2015.03.1980. [DOI] [PubMed] [Google Scholar]
- 13.Van Der Merwe SW, Van Wanrooij RLJ, Bronswijk M et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:185–205. doi: 10.1055/a-1717-1391. [DOI] [PubMed] [Google Scholar]
- 14.Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol. 2002;97:72–78. doi: 10.1111/j.1572-0241.2002.05423.x. [DOI] [PubMed] [Google Scholar]
- 15.Vanella G, Coluccio C, Cucchetti A et al. Fully versus partially-covered self-expandable metal stents for palliation of distal malignant biliary obstruction: a meta-analysis. Gastrointest Endosc. 2023 doi: 10.1016/j.gie.2023.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Vanella G, Bronswijk M, Dell’Anna G et al. Classification, risk factors, and management of lumen apposing metal stent dysfunction during follow-up of endoscopic ultrasound-guided choledochoduodenostomy: Multicenter evaluation from the Leuven-Amsterdam-Milan Study Group. Dig Endosc. 2023;35:377–388. doi: 10.1111/den.14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanella G, Bronswijk M, Maleux G et al. EUS-guided intrahepatic biliary drainage: a large retrospective series and subgroup comparison between percutaneous drainage in hilar stenoses or postsurgical anatomy. Endosc Int Open. 2020;08:E1782–E1794. doi: 10.1055/a-1264-7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanella G, Dell’Anna G, Capurso G et al. EUS-guided gastroenterostomy for management of malignant gastric outlet obstruction: a prospective cohort study with matched comparison with enteral stenting. Gastrointest Endosc. 2023;98:337–3.47E7. doi: 10.1016/j.gie.2023.04.2072. [DOI] [PubMed] [Google Scholar]
- 19.Bronswijk M, Vanella G, Petrone MC et al. EUS-guided gastroenterostomy: Less is more! The wireless EUS-guided gastroenterostomy simplified technique. VideoGIE. 2020;5:442. doi: 10.1016/j.vgie.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Facciorusso A, Gkolfakis P, Tziatzios G et al. Comparison between EUS-guided fine-needle biopsy with or without rapid on-site evaluation for tissue sampling of solid pancreatic lesions: A systematic review and meta-analysis. Endosc Ultrasound. 2022;11:458. doi: 10.4103/EUS-D-22-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diogo Turiani Hourneaux Moura A, Guidamarães Hourneaux de Moura E, Eiji Matuguma S et al. EUS-FNA versus ERCP for tissue diagnosis of suspect malignant biliary strictures: a prospective comparative study. Endosc Int Open. 2018;06:E769–E777. doi: 10.1055/s-0043-123186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenssen C, Alvarez-Sánchez MV, Napoléon B et al. Diagnostic endoscopic ultrasonography: assessment of safety and prevention of complications. World J Gastroenterol. 2012;18:4659–4676. doi: 10.3748/wjg.v18.i34.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Archibugi L, Ponz de Leon Pisani R, Petrone MC et al. Needle-tract seeding of pancreatic cancer after EUS-FNA: a systematic review of case reports and discussion of management. Cancers (Basel) 2022;14:6130. doi: 10.3390/cancers14246130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Facciorusso A, Crinò SF, Gkolfakis P et al. Needle tract seeding after endoscopic ultrasound tissue acquisition of pancreatic lesions: a systematic review and meta-analysis. Diagnostics (Basel) 2022;12:2113. doi: 10.3390/diagnostics12092113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ngamruengphong S, Xu C, Woodward TA et al. Risk of gastric or peritoneal recurrence, and long-term outcomes, following pancreatic cancer resection with preoperative endosonographically guided fine needle aspiration. Endoscopy. 2013;45:619–626. doi: 10.1055/s-0033-1344216. [DOI] [PubMed] [Google Scholar]
- 26.Ngamruengphong S, Swanson KM, Shah ND et al. Preoperative endoscopic ultrasound-guided fine needle aspiration does not impair survival of patients with resected pancreatic cancer. Gut. 2015;64:1105–1110. doi: 10.1136/gutjnl-2014-307475. [DOI] [PubMed] [Google Scholar]
- 27.Kojima H, Kitago M, Iwasaki E et al. Peritoneal dissemination of pancreatic cancer caused by endoscopic ultrasound-guided fine needle aspiration: A case report and literature review. World J Gastroenterol. 2021;27:294–304. doi: 10.3748/wjg.v27.i3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heimbach JK, Sanchez W, Rosen CB et al. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford) 2011;13:356–360. doi: 10.1111/j.1477-2574.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iglesias-Garcia J, Lariño-Noia J, Abdulkader I et al. Rapid on-site evaluation of endoscopic-ultrasound-guided fine-needle aspiration diagnosis of pancreatic masses. World J Gastroenterol. 2014;20:9451–9457. doi: 10.3748/wjg.v20.i28.9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matynia AP, Schmidt RL, Barraza G et al. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:697–705. doi: 10.1111/jgh.12431. [DOI] [PubMed] [Google Scholar]
- 31.Chafic AH El, Dewitt J, LeBlanc JK et al. Impact of preoperative endoscopic ultrasound-guided fine needle aspiration on postoperative recurrence and survival in cholangiocarcinoma patients. Endoscopy. 2013;45:883–889. doi: 10.1055/s-0033-1344760. [DOI] [PubMed] [Google Scholar]
- 32.Pedersoli F, Schröder A, Zimmermann M et al. Percutaneous transhepatic biliary drainage (PTBD) in patients with dilated vs. nondilated bile ducts: technical considerations and complications. Eur Radiol. 2020;31:3035–3041. doi: 10.1007/s00330-020-07368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behera RK, Srivastava DN, Kumar P et al. Right-sided versus left-sided percutaneous transhepatic biliary drainage in the management of malignant biliary obstruction: a randomized controlled study. Abdominal Radiology. 2021;46:768–775. doi: 10.1007/s00261-020-02651-y. [DOI] [PubMed] [Google Scholar]
- 34.Dumonceau JM, Tringali A, Papanikolaou IS et al. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910–930. doi: 10.1055/a-0659-9864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.