Abstract
Obesity is the fifth leading risk factor for global deaths with numbers continuing to increase worldwide. In the last 20 years, the emergence of pharmacological treatments for obesity based on gastrointestinal hormones has transformed the therapeutic landscape. The successful development of glucagon-like peptide-1 (GLP-1) receptor agonists, followed by the synergistic combined effect of glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 receptor agonists achieved remarkable weight loss and glycemic control in those with the diseases of obesity and type 2 diabetes. The multiple cardiometabolic benefits include improving glycemic control, lipid profiles, blood pressure, inflammation, and hepatic steatosis. The 2023 phase 2 double-blind, randomized controlled trial evaluating a GLP-1/GIP/glucagon receptor triagonist (retatrutide) in patients with the disease of obesity reported 24.2% weight loss at 48 weeks with 12 mg retatrutide. This review evaluates the current available evidence for GLP-1 receptor agonists, dual GLP-1/GIP receptor co-agonists with a focus on GLP-1/GIP/glucagon receptor triagonists and discusses the potential future benefits and research directions.
Keywords: Triple agonists, Triagonists, Glucagon-like peptide 1, Gastric inhibitory polypeptide, Glucagon, Obesity, Weight loss, Retatrutide, Co-agonist
INTRODUCTION
Obesity is a chronic, neurometabolic disease, which has now reached pandemic dimensions with continuing increasing numbers of affected individuals worldwide. The number of people with a body mass index (BMI) ≥30 kg/m² was 603.7 million in 2015 with the figure expected to exceed one billion by 2030 [1]. It is the fifth leading cause of deaths globally with 2.8 million adults dying each year with complications of obesity. Furthermore, obesity accounts for 44% of the diabetes burden, 23% of the ischemic heart disease burden and up to 41% of cancer burden globally [2].
For the last 50 years, bariatric surgery has been the most effective treatment for obesity resulting in approximately 25% weight loss, whilst also improving the control of type 2 diabetes mellitus (T2DM) and reducing cardiovascular and cancer deaths. The mechanisms of bariatric surgery are complex and still not fully understood. During the weight loss phase changes in food selection, reduction in appetite, and changes in gastrointestinal hormone levels are known to play a significant role [3]. Three of the gastrointestinal hormones, which are changed after bariatric surgery: glucagon, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) will be discussed in more detail. The weight loss outcomes are considerably heterogenous after bariatric surgery. Although bariatric surgery is much safer than in the past, many patients do not want surgery because of concerns regarding post-operative morbidity [4]. Therefore, it is unsurprising that the discovery and development of gastrointestinal hormone co-agonists as a pharmacological therapy has transformed the treatment of obesity. This drug class is the most efficacious compared to the limited number of other available obesity medications (for a recent review on these see, Chakhtoura et al. [5]).
The first GLP-1 injectable receptor agonist exenatide was approved by the U.S. Food and Drug Administration (FDA) in 2005 as a glucose-lowering treatment in T2DM [6]. Subsequently, oral formulations and once weekly injections were developed in the 2010s and liraglutide was the first GLP-1 receptor agonist approved for the treatment of obesity in 2014. The Satiety and Clinical Adiposity Liraglutide Evidence (SCALE) Obesity and Prediabetes randomized, double-blind, placebo-controlled trial of 3.0 mg of once daily liraglutide achieved a mean 8.4 kg weight loss in patients with obesity without T2DM [7].
In 2009, the first glucagon and GLP-1 receptor co-agonist was reported, which combined action profiles of two gastrointestinal hormones within a single molecule achieving a synergistic glucose-lowering and weight loss benefit. The technology rapidly progressed with several types of co-agonists forging ahead in clinical trials including GIP/GLP-1 receptor dual agonists with one of the most notable being tirzepatide [8,9]. The Study of Tirzepatide (LY3298176) in Participants With Obesity (SURMOUNT-1) trial found that tirzepatide at 15 mg per week taken over 72 weeks reduced body weight by 20.9% in those with obesity but without diabetes [9]. The remarkable weight loss and glucose-lowering results achieved by the dual peptides paved the way for triple GIP/GLP-1/glucagon receptor triagonists with the hope that the new combinatorial approaches provide further synergistic metabolic effects. This review summarises the most up-to-date developments in triple peptides for the treatment of obesity and diabetes. Due to the breadth of this subject matter, we have limited this review focussing on triagonists, whereby some other promising gut hormone compounds, such as cagrilintide, reviewed elsewhere [10], are not discussed.
GLUCAGON
Glucagon is a peptide hormone processed from a precursor proglucagon by prohormone convertase 2. It is mostly produced in the pancreatic α-cells in response to fasting or hypoglycemia, but some production occurs in the small intestine [11]. Most glucagon receptors are found on hepatocytes, but they are also present in the central nervous system, kidney, gastrointestinal tract, and the pancreas [12]. There are several mechanisms by which glucagon prevents hypoglycemia, such as induction of hepatic gluconeogenesis and glycogenolysis, and the inhibition of hepatic glycogenesis. The sustained action of glucagon causes hyperglycemia and glucose mediated inhibition of glucagon secretion is impaired in patients with T2DM.
Furthermore, glucagon stimulates lipolysis by activating hormone-sensitive lipase in the adipocytes and inhibiting lipogenesis. It also increases ketogenesis in the liver and stimulates the formation of ketone bodies by constantly supplying nonesterified fatty acids to the liver [13]. Hepatic functions such as increasing mitochondrial turnover, improve mitochondrial function and reduce oxidative stress [14,15].
Glucagon promotes satiety and increases energy expenditure in humans. Interestingly, it also stimulates insulin secretion from β-cells, but with much lower potency than GLP-1 with the mechanism potentially due to the weak binding of glucagon at GLP-1 receptor site [11].
Therefore, despite its hyperglycemic effects, glucagon triggers lipid catabolism, energy expenditure and reduces food intake. Fig. 1 summarises these metabolic effects in humans [8,16]. Those characteristics support the use of glucagon in combination with other gastrointestinal hormones that have opposite glucose-lowering effects for the treatment of obesity and T2DM [16].
Fig. 1.
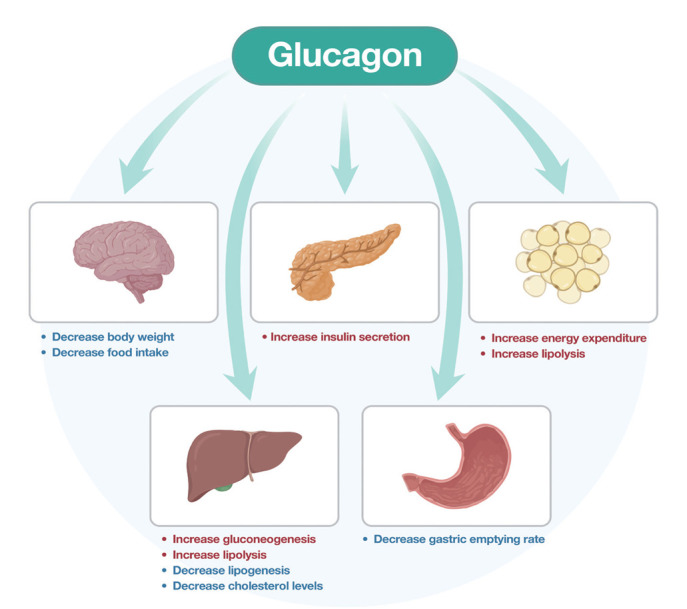
Action of glucagon in humans.
GLUCOSE-DEPENDENT INSULINOTROPIC POLYPEPTIDE
GIP is a 42 amino-acid protein secreted from K cells in the mucosa of the proximal duodenum and jejunum in response to eating and has a marked postprandial increase in secretion [17]. GIP may have a role in α-/β-cell crosstalk. There is evidence that GIP stimulates glycogen secretion even if the glucose is in the hyperglycemic range [18]. It acts on G-protein coupled receptors mostly expressed in pancreatic β-cells, but is also found in adipose tissue, stomach, and bone [19]. Further GIP receptors are in the central nervous system including the hypothalamus, hippocampus, cerebral cortex, and olfactory bulb [20].
In the β-cells, GIP increases insulin release in a glucose-dependent manner. It has protective features on the β-cells survival, and this has a synergistic effect with GLP-1 in healthy humans, but this mechanism is impaired in those with T2DM [21,22]. GIP and GLP-1 have opposite effects on glucagon release with GIP stimulating glucagon release through an increase of intracellular cyclic adenosine monophosphate levels. This enhancing effect on glucagon secretion makes lone GIP agonists unsuitable for the treatment of T2DM [23].
GIP has further effects beyond insulin secretion by stimulating lipogenesis in adipose tissue and reducing lipolysis [24]. The activation of the GIP receptor in the arcuate, dorsomedial and paraventricular nuclei of the hypothalamus decreased food intake in obese mice. The brain GIP receptor signalling pathways play a key role in the regulation of energy balance [25]. Whether GIP antagonism or agonism is the appropriate target for brain receptors is a subject to debate. Despite the above preclinical evidence, there is no significant effect on appetite, energy intake or energy expenditure following a GIP acute infusion over a short period. However, Mathiesen et al. [26] have speculated long-term GIP receptor agonism results in significant GIP receptor downregulation and effectively leads to GIP receptor antagonism with the effect of reduced food intake. Adriaenssens et al. [27] demonstrated that GIP receptor neurons differ in their connectivity, transcriptomic profile, peripheral accessibility, and appetite-controlling mechanisms with vast heterogeneity of the central GIP receptor signalling axis and further studies are needed to clarify the effects of GIP pharmacology on feeding behavior. Fig. 2 illustrates these effects in humans [8,16].
Fig. 2.
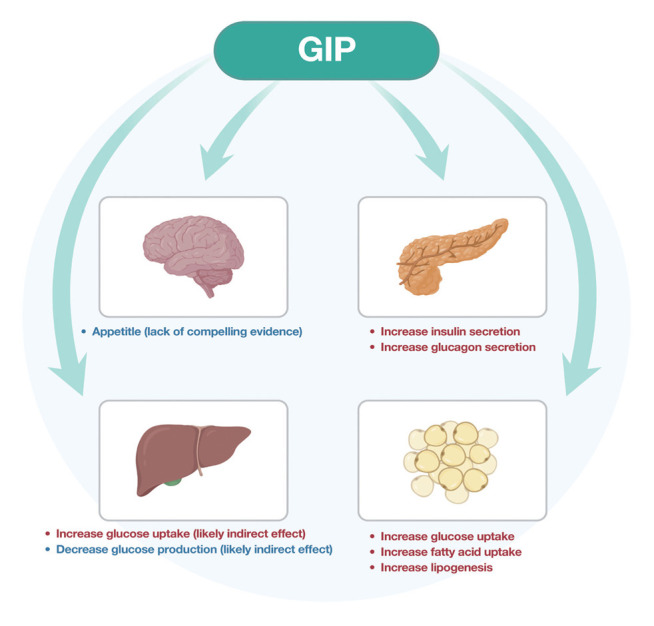
Action of glucose-dependent insulinotropic polypeptide (GIP) in humans.
GLUCAGON-LIKE PEPTIDE-1
GLP-1 is a peptide hormone secreted by intestinal enteroendocrine L-cells, specific neurons of the solitary tract in the brainstem and some α-cells in the pancreas [28]. It is released in response to eating and activates a GLP-1 receptor, which is a class B, G protein-coupled receptor [29]. Its main roles are the stimulation of insulin release [30], suppression of glucagon secretion [31], delay of gastric emptying and reduction in food intake [32]. GLP-1 inhibits food intake and promotes satiety in healthy individuals and those with obesity and diabetes [33]. Its main hormonal actions are illustrated in Fig. 3.
Fig. 3.
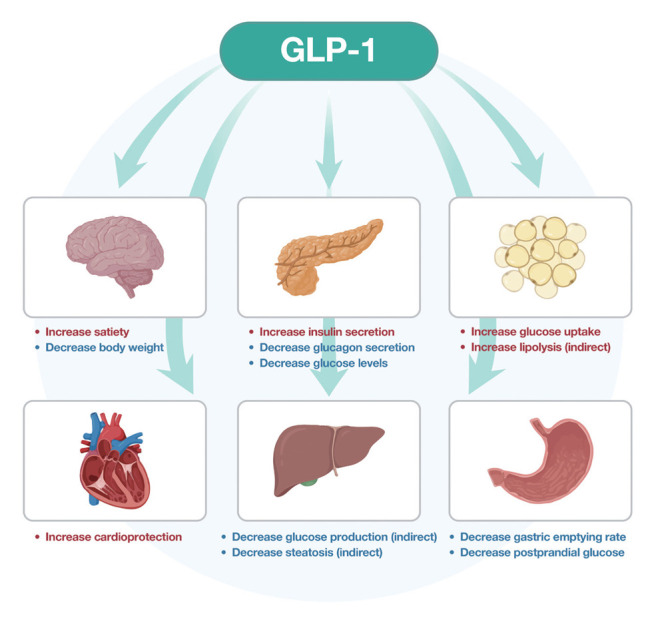
Action of glucagon-like peptide-1 (GLP-1) in humans.
The main challenge during the initial development of GLP-1 receptor agonists was the very short half-life in the circulation of 2 minutes [34]. The enzyme dipeptidyl peptidase-4 (DPP-4) rapidly inactivates GLP-1 by removing two N-terminal amino acids and an intact N-terminal is needed to bind to the GLP-1 receptor [35]. The strategies involved producing formulations of DPP-4-resistant GLP-1 receptor activators.
The first GLP-1 receptor agonist, exenatide was approved by the FDA for the treatment of T2DM in 2005. It is a recombinant form of the peptide exendin-4, which is a GLP-1 receptor agonist with a 53% similarity to the gastrointestinal hormone GLP-1 and it is resistant to DPP-4 inactivation with a 140-minute half-life after subcutaneous administration allowing therapy by a twice daily administration [36]. Liraglutide was the first GLP-1 receptor analogue approved for the management of obesity, thus starting the new era of gastrointestinal peptide agonist pharmacotherapy for the treatment of both diabetes and obesity. Recent comprehensive reviews of the many GLP-1 agonists that have come to the market in the last 20 years have been published elsewhere [8,37]. There have been several head-to-head clinical trials comparing the efficacy of different GLP-1 receptor agonists broadly demonstrating the superiority of semaglutide to comparators [37]. Since then, the Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT) cardiovascular outcomes trial has shown that semaglutide 2.4 mg once weekly was associated with a significant 20% relative risk reduction in major cardiovascular adverse events compared with placebo [38]. In October 2023, A Research Study to See How Semaglutide Works Compared to Placebo in People with Type 2 Diabetes and Chronic Kidney Disease (FLOW) trial assessing the effect of semaglutide versus placebo on the progression of renal impairment in people with T2DM and chronic kidney disease was stopped prematurely due to results of an interim analysis showing significant efficacy of semaglutide with full results due to be released in the first half of 2024 [39]. Overall, GLP-1 agonists are safe with the most common side effects of nausea, vomiting, diarrhoea and constipation being only mild to moderate in severity in most people.
DUAL GLUCAGON/GLUCAGON-LIKE PEPTIDE-1 RECEPTOR CO-AGONISTS
As discussed earlier, glucagon is known to promote hyperglycemia and it seems counterintuitive to use it as treatment in those with diabetes. Despite this effect, it increases satiety, works favourably on energy expenditure and has lipolytic actions. When used in combination with GLP-1, which has glucose-lowering action, it has been shown to be an effective treatment for T2DM and obesity. Oxyntomodulin, produced by the L-cells in the distal small intestine, binds to both glucagon receptor and GLP-1 receptor and is structurally very similar to glucagon. Oxyntomodulin increases energy expenditure and decreases energy intake in animals and humans [40]. The first glucagon/GLP-1 receptor co-agonist was discovered and validated in preclinical models in 2009. The data showed that with weekly administration, it normalized glucose tolerance and led to weight loss by the reduction of food intake and increased energy expenditure in obese mice [41]. Further study confirmed the findings and showed the effects were superior compared to GLP-1 receptor agonist [42].
Following this, there have been two clinical phase 2 trials (SAR425899 and MEDI0382 also known as cotadutide) published evaluating GLP-1/glucagon receptor co-agonists. Their weight loss and antihyperglycemic benefits have been confirmed [43,44]. In a placebo-controlled, double-blind phase I study of ascending doses of cotadutide, there were dose-dependent improvements in glycemic control and increased satiety [45]. There were two further phase IIa studies evaluating cotadutide with the first one showing that it reduced glucose levels in those with obesity and T2DM with the mechanism being greater insulin secretion and delayed gastric emptying [46]. The other phase IIa study also confirmed improved glycemic control in those with obesity and T2DM, but also reported decreased body weight and improvement of blood lipids compared to placebo [44]. Cotadutide is safe with the most common side effects being mild to moderate nausea and vomiting, but with no greater severity or frequency than found in GLP-1 agonists [44].
Cotadutide, but not liraglutide improved hepatic parameters and metabolic dysfunction-associated steatohepatitis (MASH) with similar weight loss with 200 μg cotadutide compared to 1.8 mg liraglutide and greater with 300 μg cotadutide versus 1.8 mg liraglutide [47]. Phase II and III clinical trials are currently underway to evaluate its treatment potential and safety in MASH. Despite initially promising results, SAR425899 has been discontinued after phase 2 trials showed treatment related gastrointestinal disorders (NCT03437720).
Survodutide (BI 456906) is another glucagon/GLP-1 receptor dual agonist with a recent phase II randomized, double-blind study showing reduced glycosylated hemoglobin (HbA1c) levels and bodyweight after 16 weeks’ treatment in participants with T2DM. The mean reduction in HbA1c was similar with low-dose survodutide and semaglutide. There was a survodutide dose-dependent decrease in bodyweight up to –8.7% with the high-dose survodutide producing greater bodyweight reduction compared to semaglutide [48].
Furthermore, the data from a phase II study in participants with obesity and MASH presented at the American Diabetes Association showed those on survodutide achieved 18.7% mean weight loss after 46 weeks compared to the placebo group. There was no plateau in weight loss at 46 weeks with the potential of further weight loss over a longer time period. Forty percentage of the individuals on the two highest doses of survodutide had a mean weight loss of 20%. As with previous dual agonists, the main side effects were gastrointestinal and occurred mostly during the initial dose escalation. Therefore, a more gradual dose escalation will likely decrease the severity and frequency of those specific side effects [49]. There are multiple ongoing phase III studies to investigate survodutide for treatment of obesity, diabetes, cardiovascular disease, and chronic kidney disease.
DUAL GLUCOSE-DEPENDENT INSULINOTROPIC POLYPEPTIDE/GLUCAGON-LIKE PEPTIDE-1 RECEPTOR CO-AGONISTS
As part of the ongoing effort to use synergistic effects of gastrointestinal hormones in the treatment of diabetes and obesity, the first GIP/GLP-1 receptor co-agonist was reported in 2013. The preclinical animal model data suggested that the glucose-lowering, lipid-lowering and insulin secreting effects were greater than those in GLP-1 receptor agonists [50]. As further GIP-GLP-1 receptor co-agonists were developed those findings were confirmed [51]. In 2018, a 39 amino-acid peptide tirzepatide (LY3298176) was developed, which is similar in size to both GLP-1 and GIP with exenatide-derived C-terminal tail and thus has a prolonged half-life. It acts on GIP receptor five times over GLP-1 receptor [50]. A double-blind, randomized phase 2 study comparing various doses (1, 5, 10, 15 mg) of once weekly tirzepatide, dulaglutide (1.5 mg) and placebo, in patients with T2DM, showed tirzepatide was superior on weight loss and glucose-lowering compared to both dulaglutide and placebo [52]. The glucose-lowering efficacy measured by the decrease in HbA1c was dose-dependent in the tirzepatide group and did not plateau [52].
Tirzepatide (LY3298176) in Participants With Type 2 Diabetes Not Controlled With Diet and Exercise Alone (SURPASS) studies have shown that tirzepatide reduced HbA1c and fasting glucose levels more than the titrated insulin degludec, insulin glargine or 1 mg once a week semaglutide independently of the baseline levels of HbA1c. However, about 11% of those with T2DM did not respond even to the highest dose of tirzepatide [53-55]. Furthermore, there has been an indication of tirzepatide reducing liver fat, and improving nephrotic outcomes as evidenced by the lower urine albumin: creatinine ratio and the slower decline of estimated glomerular filtration rate in people with T2DM [56].
SURPASS J-combo study comparing people with T2DM in a Japanese population with a high HbA1c on oral antihyperglycemic monotherapy on 5, 10, or 15 mg of once weekly tirzepatide for 52 weeks showed an improvement of glycemic and lipid control, weight loss and reduction in blood pressure [57]. Following the success and approval of tirzepatide (Mounjaro, Eli Lilly, Indianapolis, IN, USA) in 2022 by FDA for treatment of T2DM, the SURMOUNT programme investigated its use as an anti-obesity medication in those without diabetes. The SURMOUNT-1 phase 3 double-blind, randomized, controlled trial in adults with obesity without diabetes received once weekly tirzepatide (5, 10, or 15 mg) versus placebo for 72 weeks [9]. The results showed high heterogeneity of weight loss from the baseline weight between individuals even at the highest dose of tirzepatide –38.4 to 9.0 kg and mean –22.1 kg. About 2% who were on 15 mg of tirzepatide had no weight loss or even gained weight and were deemed non-responders, which might be related to individual hypothalamic feedback pathway [9]. The side effects of GIP/GLP-1 receptor agonists are most commonly gastrointestinal with nausea, vomiting, diarrhoea, constipation, but similar in severity and frequency to GLP-1 agonists [9]. Tirzepatide has now been approved for chronic weight management by the FDA in 2023 following the clinical trials data.
TRIPLE GLUCAGON-LIKE PEPTIDE-1/GLUCOSE-DEPENDENT INSULINOTROPIC POLYPEPTIDE/GLUCAGON RECEPTOR TRIAGONISTS
In 2015, following the success of dual GIP/GLP-1 receptor co-agonists, the first triple GLP-1/GIP/glucagon receptor triagonist was developed with the rationale that the synergistic effect of the three gastrointestinal hormones will achieve further efficacy compared to the dual gastrointestinal hormone approach [58]. The study confirmed that the newly developed GLP-1/GIP/glucagon receptor triagonist was superior to previous dual receptor co-agonists with greater efficacy in decrease in body weight, reduction in lipid levels and a decrease in insulin secretion [58]. Another study in rodents showed similar results, whilst also highlighted the reversal of steatohepatitis in female mice [59]. Knerr et al. reported greater weight loss in obese mice with the GLP-1/GIP/glucagon receptor triagonist compared to GLP-1 receptor agonists, GIP/GLP-1 receptor co-agonists and GLP-1/glucagon receptor agonists including semaglutide and tirzepatide [60].
The research progressed rapidly with studies performed in primates and healthy humans. In 2022, SAR441255 (GLP-1/GIP/glucagon receptor triagonist) was administered to obese, diabetic primates and healthy lean to overweight humans without diabetes. After 7 weeks, there was 12% reduction compared to baseline body weight in primates without causing hyperglycemia in either group [60]. Following those promising results, a phase 1 study tested ascending doses (3, 9, 20, 40, 80, and 150 μg) of SAR441255 against placebo reported a decrease in both fasting and post prandial blood glucose in the SAR441255 group. The side effects were most commonly gastrointestinal disturbances such as nausea and vomiting and occurred in a similar frequency to those reported with dual receptor co-agonists [61]. Sanofi have since discontinued further work SAR441255 without stating the reason [16].
Another GLP-1/GIP/glucagon receptor triagonist known as retatrutide (previously LY3437943) was developed by Eli Lilly in 2022 leading to weight loss, reduced fat mass, decreased blood glucose and insulin levels in obese mice, which was attributed to increased energy expenditure and lipid oxidation [62]. The same group (Coskun et al. [62]) carried out a phase 1 study with 47 healthy participants without diabetes participants receiving single ascending dose of retatrutide (0.1, 0.3, 1, 3, 4.5, or 6 mg) versus placebo; body weight decreased in a dose-dependent manner and there was a dose-dependent reduction in appetite [62]. The side effects were most commonly gastrointestinal, with 16.7% on the lowest dose up to 100% on the highest dose with no serious adverse events [62].
The next, phase 1b clinical trial in adults with T2DM compared once weekly retatrutide (0.5, 1.5, 3, 3 mg starting dose increased to 6, 3 mg starting dose increased to 12 mg) versus placebo versus dulaglutide 1.5 mg over 12 weeks [63]. As the half-life of retatrutide is 6 days, once weekly administration was sufficient with as expected gastrointestinal disturbance being the most common side effect and occurring in 63% of those on retatrutide, 60% on dulaglutide, and 54% of the placebo cohort [63]. There was a reduction in plasma glucose and HbA1c levels at the three highest doses of retatrutide and a dose-dependent reduction in body weight [63].
Recently, a phase II multicentre, double-blind, randomized controlled trial including 338 adults with obesity were administered retatrutide (1, 2 mg starting dose increased to 4, 2 mg starting dose increased to 8, 2 mg starting dose increased to 12 mg) compared to placebo over 48 weeks [64]. At the highest dose of 12 mg retatrutide, the average weight loss after 48 weeks was 24.2% with reduction up to 30% from the baseline weight in 25% of participants. Furthermore, the trajectory of the weight loss curves suggested that at 48 weeks the plateau of weight was not yet reached and all the participants in the 8 and 12 mg had a weight reduction of 5% or more, which is currently the minimum threshold for efficacy [64]. The weight reductions were accompanied by cardiometabolic profile improvements: decrease in waist circumference, lower systolic and diastolic blood pressure, improved glycemic control including fasting glucose and HbA1c and lower lipid levels. The side effect and safety profile were like those observed with GLP-1 receptor agonists and GIP/GLP-1 receptor co-agonists with mild to moderate gastrointestinal events occurring mostly during dose escalations [64].
To date this is the greatest weight loss reported through a pharmacological agent, but similar results have been observed in some people who underwent bariatric surgery [65]. The mechanism of action is attributed to the GIP/GLP-1 receptor co-agonism being enhanced by glucagon receptor activation and augmentation of energy intake, substrate utilisation and energy expenditure [60]. Although, most participants lost weight, there was heterogenicity, that has been well-documented in obesity studies [66] and those with higher BMI above 35 experienced greater mean percentage weight loss [64]. This may have been because those with lower BMI’s may have had normalisation of their obesity and thus further weight loss was not physiologically possible. This may be similar to patients with diabetes with lower HbA1c having less change than those with higher HbA1c with anti-diabetes medications which don’t cause hypoglycemia.
Finan et al. [58] and Jall et al. [59] showed the excellent efficacy of GLP-1/GIP/glucagon receptor triagonists in reversing fatty liver disease in mice. The effectiveness was superior to both monoagonists and dual agonists. The phase II obesity study of retatrutide also included a MASH substudy to assess its effects on the amount of liver fat. Ninety-eight patients with obesity and MASH underwent liver magnetic resonance imaging and had blood biomarkers of liver injury and fibrosis measured. After 48 weeks treatment with two highest doses of retatrutide, 90% of participants had normalisation of liver fat [67]. This raises the possibility of GLP-1/GIP/glucagon receptor triagonist becoming a new treatment for the reversal of liver fibrosis in MASH.
Another GLP-1/GIP/glucagon receptor triagonist (HM15211) is undergoing preclinical studies for its efficacy in the treatment of T2DM, MASH, and Parkinson’s disease. The current data has shown good safety profile and lowering of hepatosteatosis. In mice models, there was a reduction in hyperglycemia, weight loss and increased energy expenditure compared to liraglutide [68,69].
A GLP-1/glucagon/gastric receptor agonist has been studied in diabetic and obese mice. Gastrin is a neuroendocrine peptide, which acts on the cholecystokinin-2 (CCK2) receptor and is expressed in the pancreas with the rationale being that as it is involved in pancreas development, it might increase β-cell mass and glycemic control [70]. The results of this rodent study showed superior glycemic control effects compared to cotadutide and liraglutide with greater weight loss compared to liraglutide, but similar to the cotadutide [70].
CONCLUSIONS
Undoubtedly, the approval of the first GLP-1 receptor agonist in 2005 has revolutionised the pharmacological treatment of obesity and T2DM. Prior to that, the treatment of obesity has been predominantly limited to lifestyle interventions and bariatric surgery. The further synergistic combination approach with other gastrointestinal hormones and creation of dual GIP/GLP-1 receptor agonists, such as tirzepatide and GLP-1/GIP/glucagon receptor triagonist, retatrutide, has shown unprecedented pharmacologically achieved weight loss with further cardiometabolic benefits in both obesity and T2DM. The results of the phase 3 clinical trial of retatrutide are eagerly awaited, as it is expected that over a longer time period its efficacy in weight loss will be even greater.
Another future direction of utilising GLP-1/GIP/glucagon receptor triagonists is for MASH therapy with the rationale that as GLP-1 receptor agonists halt fibrosis progression, the new therapies might be able to enable fibrosis regression [11]. There is also further ongoing research to understand the mechanisms of GIP agonism versus antagonism to decide on the optimal weight loss and antihyperglycemic approach.
The above clinical studies will have implications on the guideline development including the treatment of T2DM and obesity in young people and balancing the cost with the efficacy of the emerging therapies. Since 2023, there has been a worldwide shortage of GLP-1 receptor agonists, which is expected to continue until late 2024 [71]. Many people with T2DM are left without their essential medications and antidiabetic regimes had to be altered [71]. One of the future challenges is ensuring that the manufactures can keep up with the ongoing demand for the available technology. Predicting therapeutic responses and side effects of these therapies remains a major challenge [72,73].
Footnotes
CONFLICTS OF INTEREST
In the last 5 years Adie Viljoen has received lecture honoraria and/or travel support and/or conducts clinical trials funded by: Amarin, Amgen, Astra Zeneca, Boehringer Ingleheim, Daiichi-Sankyo, Lilly, Menarini, NewAmsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Sanofi, Takeda, and Tosoh.
In the last 5 years Agnieszka Jakubowska conducts trials funded by: Eli Lilly, NewAmsterdam, and Novo Nordisk.
Carel W. le Roux reports grants from the Irish Research Council, Science Foundation Ireland, Anabio, and the Health Research Board. He serves on advisory boards and speakers panels of Novo Nordisk, Herbalife, GI Dynamics, Eli Lilly, Johnson & Johnson, Glia, Irish Life Health, Boehringer Ingelheim, Currax, Zealand Pharma, Keyron, and Rhythm Pharma. Carel W. le Roux is a member of the Irish Society for Nutrition and Metabolism outside the area of work commented on here. He was the chief medical officer and director of the Medical Device Division of Keyron in 2021. Both of these are unremunerated positions. Carel W. le Roux was a previous investor in Keyron, which develops endoscopically implantable medical devices intended to mimic the surgical procedures of sleeve gastrectomy and gastric bypass. No patients have been included in any of Keyron’s studies and they are not listed on the stock market. Carel W. le Roux was gifted stock holdings in September 2021 and divested all stock holdings in Keyron in September, 2021. He continues to provide scientific advice to Keyron for no remuneration. Carel W. le Roux provides obesity clinical care in the Beyond BMI clinic and is a shareholder in the clinic.
REFERENCES
- 1.Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–98. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2015 Obesity Collaborators. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akalestou E, Miras AD, Rutter GA, le Roux CW. Mechanisms of weight loss after obesity surgery. Endocr Rev. 2022;43:19–34. doi: 10.1210/endrev/bnab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim R, Beekley A, Johnson DC, Davis KA. Early and late complications of bariatric operation. Trauma Surg Acute Care Open. 2018;3:e000219. doi: 10.1136/tsaco-2018-000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakhtoura M, Haber R, Ghezzawi M, Rhayem C, Tcheroyan R, Mantzoros CS. Pharmacotherapy of obesity: an update on the available medications and drugs under investigation. EClinicalMedicine. 2023;58:101882. doi: 10.1016/j.eclinm.2023.101882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest. 2017;127:4217–27. doi: 10.1172/JCI97233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 8.Nogueiras R, Nauck MA, Tschop MH. Gut hormone co-agonists for the treatment of obesity: from bench to bedside. Nat Metab. 2023;5:933–44. doi: 10.1038/s42255-023-00812-z. [DOI] [PubMed] [Google Scholar]
- 9.Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–16. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 10.Frias JP, Deenadayalan S, Erichsen L, Knop FK, Lingvay I, Macura S, et al. Efficacy and safety of co-administered once-weekly cagrilintide 2.4 mg with once-weekly semaglutide 2.4 mg in type 2 diabetes: a multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet. 2023;402:720–30. doi: 10.1016/S0140-6736(23)01163-7. [DOI] [PubMed] [Google Scholar]
- 11.Newsome PN, Ambery P. Incretins (GLP-1 receptor agonists and dual/triple agonists) and the liver. J Hepatol. 2023;79:1557–65. doi: 10.1016/j.jhep.2023.07.033. [DOI] [PubMed] [Google Scholar]
- 12.Scheen AJ, Lefebvre PJ. Glucagon, from past to present: a century of intensive research and controversies. Lancet Diabetes Endocrinol. 2023;11:129–38. doi: 10.1016/S2213-8587(22)00349-7. [DOI] [PubMed] [Google Scholar]
- 13.Guzman CB, Zhang XM, Liu R, Regev A, Shankar S, Garhyan P, et al. Treatment with LY2409021, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19:1521–8. doi: 10.1111/dom.12958. [DOI] [PubMed] [Google Scholar]
- 14.Muller TD, Finan B, Clemmensen C, DiMarchi RD, Tschop MH. The new biology and pharmacology of glucagon. Physiol Rev. 2017;97:721–66. doi: 10.1152/physrev.00025.2016. [DOI] [PubMed] [Google Scholar]
- 15.Alberti KG, Johnston DG, Gill A, Barnes AJ, Orskov H. Hormonal regulation of ketone-body metabolism in man. Biochem Soc Symp. 1978;43:163–82. [PubMed] [Google Scholar]
- 16.Tschop M, Nogueiras R, Ahren B. Gut hormone-based pharmacology: novel formulations and future possibilities for metabolic disease therapy. Diabetologia. 2023;66:1796–808. doi: 10.1007/s00125-023-05929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inagaki N, Seino Y, Takeda J, Yano H, Yamada Y, Bell GI, et al. Gastric inhibitory polypeptide: structure and chromosomal localization of the human gene. Mol Endocrinol. 1989;3:1014–21. doi: 10.1210/mend-3-6-1014. [DOI] [PubMed] [Google Scholar]
- 18.El K, Campbell JE. The role of GIP in α-cells and glucagon secretion. Peptides. 2020;125:170213. doi: 10.1016/j.peptides.2019.170213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: similarities and differences. J Diabetes Investig. 2010;1:8–23. doi: 10.1111/j.2040-1124.2010.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology. 1993;133:2861–70. doi: 10.1210/endo.133.6.8243312. [DOI] [PubMed] [Google Scholar]
- 21.Turner DS, Etheridge L, Jones J, Marks V, Meldrum B, Bloom SR, et al. The effect of the intestinal polypeptides, IRP and GIP, on insulin release and glucose tolerance in the baboon. Clin Endocrinol (Oxf) 1974;3:489–93. doi: 10.1111/j.1365-2265.1974.tb02820.x. [DOI] [PubMed] [Google Scholar]
- 22.Calanna S, Christensen M, Holst JJ, Laferrere B, Gluud LL, Vilsboll T, et al. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: systematic review and meta-analyses of clinical studies. Diabetologia. 2013;56:965–72. doi: 10.1007/s00125-013-2841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pederson RA, Brown JC. Interaction of gastric inhibitory polypeptide, glucose, and arginine on insulin and glucagon secretion from the perfused rat pancreas. Endocrinology. 1978;103:610–5. doi: 10.1210/endo-103-2-610. [DOI] [PubMed] [Google Scholar]
- 24.Muller TD, Clemmensen C, Finan B, DiMarchi RD, Tschop MH. Anti-obesity therapy: from rainbow pills to polyagonists. Pharmacol Rev. 2018;70:712–46. doi: 10.1124/pr.117.014803. [DOI] [PubMed] [Google Scholar]
- 25.Adriaenssens AE, Biggs EK, Darwish T, Tadross J, Sukthankar T, Girish M, et al. Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metab. 2019;30:987–96. doi: 10.1016/j.cmet.2019.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathiesen DS, Bagger JI, Bergmann NC, Lund A, Christensen MB, Vilsboll T, et al. The effects of dual GLP-1/GIP receptor agonism on glucagon secretion: a review. Int J Mol Sci. 2019;20:4092. doi: 10.3390/ijms20174092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adriaenssens A, Broichhagen J, de Bray A, Ast J, Hasib A, Jones B, et al. Hypothalamic and brainstem glucose-dependent insulinotropic polypeptide receptor neurons employ distinct mechanisms to affect feeding. JCI Insight. 2023;8:e164921. doi: 10.1172/jci.insight.164921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drucker DJ, Brubaker PL. Proglucagon gene expression is regulated by a cyclic AMP-dependent pathway in rat intestine. Proc Natl Acad Sci U S A. 1989;86:3953–7. doi: 10.1073/pnas.86.11.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graaf Cd, Donnelly D, Wootten D, Lau J, Sexton PM, Miller LJ, et al. Glucagon-like peptide-1 and its class B G protein-coupled receptors: a long march to therapeutic successes. Pharmacol Rev. 2016;68:954–1013. doi: 10.1124/pr.115.011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A. 1987;84:3434–8. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27:740–56. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Lee YS, Shin S, Shigihara T, Hahm E, Liu MJ, Han J, et al. Glucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesis. Diabetes. 2007;56:1671–9. doi: 10.2337/db06-1182. [DOI] [PubMed] [Google Scholar]
- 33.Muller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, et al. Glucagon-like peptide 1 (GLP-1) Mol Metab. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mojsov S. Structural requirements for biological activity of glucagon-like peptide-I. Int J Pept Protein Res. 1992;40:333–43. doi: 10.1111/j.1399-3011.1992.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 35.Goke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, et al. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268:19650–5. [PubMed] [Google Scholar]
- 36.Furman BL. The development of Byetta (exenatide) from the venom of the Gila monster as an anti-diabetic agent. Toxicon. 2012;59:464–71. doi: 10.1016/j.toxicon.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Viljoen A, Bain SC. Glucagon-like peptide 1 therapy: from discovery to type 2 diabetes and beyond. Endocrinol Metab (Seoul) 2023;38:25–33. doi: 10.3803/EnM.2022.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389:2221–32. doi: 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- 39.Gragnano F, De Sio V, Calabro P. FLOW trial stopped early due to evidence of renal protection with semaglutide. Eur Heart J Cardiovasc Pharmacother. 2024;10:7–9. doi: 10.1093/ehjcvp/pvad080. [DOI] [PubMed] [Google Scholar]
- 40.Pocai A. Action and therapeutic potential of oxyntomodulin. Mol Metab. 2013;3:241–51. doi: 10.1016/j.molmet.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pocai A, Carrington PE, Adams JR, Wright M, Eiermann G, Zhu L, et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58:2258–66. doi: 10.2337/db09-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol. 2009;5:749–57. doi: 10.1038/nchembio.209. [DOI] [PubMed] [Google Scholar]
- 43.Corbin KD, Carnero EA, Allerton TD, Tillner J, Bock CP, Luyet PP, et al. Glucagon-like peptide-1/glucagon receptor agonism associates with reduced metabolic adaptation and higher fat oxidation: a randomized trial. Obesity (Silver Spring) 2023;31:350–62. doi: 10.1002/oby.23633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ambery P, Parker VE, Stumvoll M, Posch MG, Heise T, Plum-Moerschel L, et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391:2607–18. doi: 10.1016/S0140-6736(18)30726-8. [DOI] [PubMed] [Google Scholar]
- 45.Ambery PD, Klammt S, Posch MG, Petrone M, Pu W, Rondinone C, et al. MEDI0382, a GLP-1/glucagon receptor dual agonist, meets safety and tolerability endpoints in a single-dose, healthy-subject, randomized, phase 1 study. Br J Clin Pharmacol. 2018;84:2325–35. doi: 10.1111/bcp.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker VE, Robertson D, Wang T, Hornigold DC, Petrone M, Cooper AT, et al. Efficacy, safety, and mechanistic insights of cotadutide, a dual receptor glucagon-like peptide-1 and glucagon agonist. J Clin Endocrinol Metab. 2020;105:dgz047. doi: 10.1210/clinem/dgz047. [DOI] [PubMed] [Google Scholar]
- 47.Nahra R, Wang T, Gadde KM, Oscarsson J, Stumvoll M, Jermutus L, et al. Effects of cotadutide on metabolic and hepatic parameters in adults with overweight or obesity and type 2 diabetes: a 54-week randomized phase 2b study. Diabetes Care. 2021;44:1433–42. doi: 10.2337/dc20-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bluher M, Rosenstock J, Hoefler J, Manuel R, Hennige AM. Dose-response effects on HbA1c and bodyweight reduction of survodutide, a dual glucagon/GLP-1 receptor agonist, compared with placebo and open-label semaglutide in people with type 2 diabetes: a randomised clinical trial. Diabetologia. 2024;67:470–82. doi: 10.1007/s00125-023-06053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Busko M. New York: Medscape; 2023. Survodutide impresses in phase 2 weight-loss trial [Internet] [cited 2024 Jan 31]. Available from: https://www.medscape.com/viewarticle/993928?form=fpf. [Google Scholar]
- 50.Finan B, Ma T, Ottaway N, Muller TD, Habegger KM, Heppner KM, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5:209ra151. doi: 10.1126/scitranslmed.3007218. [DOI] [PubMed] [Google Scholar]
- 51.Gault VA, Kerr BD, Harriott P, Flatt PR. Administration of an acylated GLP-1 and GIP preparation provides added beneficial glucose-lowering and insulinotropic actions over single incretins in mice with type 2 diabetes and obesity. Clin Sci (Lond) 2011;121:107–17. doi: 10.1042/CS20110006. [DOI] [PubMed] [Google Scholar]
- 52.Coskun T, Sloop KW, Loghin C, Alsina-Fernandez J, Urva S, Bokvist KB, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab. 2018;18:3–14. doi: 10.1016/j.molmet.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frias JP, Davies MJ, Rosenstock J, Perez Manghi FC, Fernandez Lando L, Bergman BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503–15. doi: 10.1056/NEJMoa2107519. [DOI] [PubMed] [Google Scholar]
- 54.Ludvik B, Giorgino F, Jodar E, Frias JP, Fernandez Lando L, Brown K, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398:583–98. doi: 10.1016/S0140-6736(21)01443-4. [DOI] [PubMed] [Google Scholar]
- 55.Dahl D, Onishi Y, Norwood P, Huh R, Bray R, Patel H, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA. 2022;327:534–45. doi: 10.1001/jama.2022.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heerspink HJ, Sattar N, Pavo I, Haupt A, Duffin KL, Yang Z, et al. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10:774–85. doi: 10.1016/S2213-8587(22)00243-1. [DOI] [PubMed] [Google Scholar]
- 57.Kadowaki T, Chin R, Ozeki A, Imaoka T, Ogawa Y. Safety and efficacy of tirzepatide as an add-on to single oral antihyperglycaemic medication in patients with type 2 diabetes in Japan (SURPASS J-combo): a multicentre, randomised, open-label, parallel-group, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10:634–44. doi: 10.1016/S2213-8587(22)00187-5. [DOI] [PubMed] [Google Scholar]
- 58.Finan B, Yang B, Ottaway N, Smiley DL, Ma T, Clemmensen C, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21:27–36. doi: 10.1038/nm.3761. [DOI] [PubMed] [Google Scholar]
- 59.Jall S, Sachs S, Clemmensen C, Finan B, Neff F, DiMarchi RD, et al. Monomeric GLP-1/GIP/glucagon triagonism corrects obesity, hepatosteatosis, and dyslipidemia in female mice. Mol Metab. 2017;6:440–6. doi: 10.1016/j.molmet.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knerr PJ, Mowery SA, Douros JD, Premdjee B, Hjollund KR, He Y, et al. Next generation GLP-1/GIP/glucagon triple agonists normalize body weight in obese mice. Mol Metab. 2022;63:101533. doi: 10.1016/j.molmet.2022.101533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bossart M, Wagner M, Elvert R, Evers A, Hubschle T, Kloeckener T, et al. Effects on weight loss and glycemic control with SAR441255, a potent unimolecular peptide GLP-1/GIP/GCG receptor triagonist. Cell Metab. 2022;34:59–74. doi: 10.1016/j.cmet.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Coskun T, Urva S, Roell WC, Qu H, Loghin C, Moyers JS, et al. LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: from discovery to clinical proof of concept. Cell Metab. 2022;34:1234–47. doi: 10.1016/j.cmet.2022.07.013. [DOI] [PubMed] [Google Scholar]
- 63.Urva S, Coskun T, Loh MT, Du Y, Thomas MK, Gurbuz S, et al. LY3437943, a novel triple GIP, GLP-1, and glucagon receptor agonist in people with type 2 diabetes: a phase 1b, multicentre, double-blind, placebo-controlled, randomised, multiple-ascending dose trial. Lancet. 2022;400:1869–81. doi: 10.1016/S0140-6736(22)02033-5. [DOI] [PubMed] [Google Scholar]
- 64.Jastreboff AM, Kaplan LM, Frias JP, Wu Q, Du Y, Gurbuz S, et al. Triple-hormone-receptor agonist retatrutide for obesity: a phase 2 trial. N Engl J Med. 2023;389:514–26. doi: 10.1056/NEJMoa2301972. [DOI] [PubMed] [Google Scholar]
- 65.van Rijswijk AS, van Olst N, Schats W, van der Peet DL, van de Laar AW. What is weight loss after bariatric surgery expressed in percentage total weight loss (%TWL)?: a systematic review. Obes Surg. 2021;31:3833–47. doi: 10.1007/s11695-021-05394-x. [DOI] [PubMed] [Google Scholar]
- 66.Gossmann M, Butsch WS, Jastreboff AM. Treating the chronic disease of obesity. Med Clin North Am. 2021;105:983–1016. doi: 10.1016/j.mcna.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 67.Sanyal AJ. Retatrutide NAFLD: phase 2 trial results in subset of patients with obesity and NAFLD. American Diabetes Association’s 83rd Scientific Sessions; 2023 Jun 23-26; San Diego, CA. [Google Scholar]
- 68.Kim JA, Lee S, Lee SH, Jung SY, Kim YH, Choi IY, et al. Neuroprotective effects of HM15211, a novel long-acting GLP-1/GIP/glucagon triple agonist in the neurodegenerative disease models. Diabetes. 2018;67(Supplement 1):1107–P. [Google Scholar]
- 69.Choi IY, Lee JS, Kim JK, Lee SH, Kim YH, Park YJ, et al. Potent body weight loss, and therapeutic efficacy in a NASH animal model by a novel long-acting GLP-1/Glucagon/GIP tri-agonist (HM15211). American Diabetes Association’s 77th Scientific Sessions; 2017 Jun 9-13; San Diego, CA. [Google Scholar]
- 70.Zhao S, Yan Z, Du Y, Li Z, Tang C, Jing L, et al. A GLP-1/glucagon (GCG)/CCK2 receptors tri-agonist provides new therapy for obesity and diabetes. Br J Pharmacol. 2022;179:4360–77. doi: 10.1111/bph.15860. [DOI] [PubMed] [Google Scholar]
- 71.Iacobucci G. Diabetes: doctors are told not to start new patients on GLP-1 agonists because of shortages. BMJ. 2023;382:p2019. doi: 10.1136/bmj.p2019. [DOI] [PubMed] [Google Scholar]
- 72.Dent R, McPherson R, Harper ME. Factors affecting weight loss variability in obesity. Metabolism. 2020;113:154388. doi: 10.1016/j.metabol.2020.154388. [DOI] [PubMed] [Google Scholar]
- 73.Fruhbeck G, Kiortsis DN, Catalan V. Precision medicine: diagnosis and management of obesity. Lancet Diabetes Endocrinol. 2018;6:164–6. doi: 10.1016/S2213-8587(17)30312-1. [DOI] [PubMed] [Google Scholar]


