Abstract
The indolent nature and favorable outcomes associated with papillary thyroid microcarcinoma have prompted numerous prospective studies on active surveillance (AS) and its adoption as an alternative to immediate surgery in managing low-risk thyroid cancer. This article reviews the current status of AS, as outlined in various international practice guidelines. AS is typically recommended for tumors that measure 1 cm or less in diameter and do not exhibit aggressive subtypes on cytology, extrathyroidal extension, lymph node metastasis, or distant metastasis. To determine the most appropriate candidates for AS, factors such as tumor size, location, multiplicity, and ultrasound findings are considered, along with patient characteristics like medical condition, age, and family history. Moreover, shared decision-making, which includes patient-reported outcomes such as quality of life and cost-effectiveness, is essential. During AS, patients undergo regular ultrasound examinations to monitor for signs of disease progression, including tumor growth, extrathyroidal extension, or lymph node metastasis. In conclusion, while AS is a feasible and reliable approach for managing low-risk thyroid cancer, it requires careful patient selection, effective communication for shared decision-making, standardized follow-up protocols, and a clear definition of disease progression.
Keywords: Active surveillance, Guideline, Papillary thyroid cancer, Practice guideline, Thyroid neoplasms, Watchful waiting
INTRODUCTION
Thyroid cancer is very common, with its global incidence rate reported as 10.1 per 100,000 in women and 3.1 per 100,000 in men [1]. However, thyroid cancer has a favorable prognosis, with a mortality rate of just 0.5 per 100,000 in women and 0.3 per 100,000 in men [1]. The incidence of thyroid cancer increased dramatically in the 1990s and early 2000s [2]. This dramatic increase has been attributed mainly to the detection of indolent small thyroid cancers, particularly due to the widespread use of medical imaging, especially ultrasonography (US) [3,4]. Autopsy studies have also supported this finding, revealing that occult thyroid cancers were present in up to 35.6% of individuals who died from other causes [5]. Consequently, thyroid cancer has become a focal point in the debates surrounding overdiagnosis and overtreatment [6].
Papillary thyroid microcarcinoma (PTMC), defined as a tumor 1 cm or less in size, has an excellent prognosis [7,8]. The disease-specific mortality of PTMC has been reported to be less than 0.1%, and the recurrence rate is 3% [9]. One reason for these favorable outcomes is the indolent nature of PTMC. When PTMC is monitored without immediate surgery, most cases (>80%) remain stable, with no change in size [10]. Based on PTMC’s indolent nature, a treatment strategy known as active surveillance (AS), which involves watchful waiting rather than immediate surgery, has emerged. Kuma Hospital in Japan pioneered a study in 1993 that observed patients with PTMC without surgery, revealing that only 8% and 3.8% of patients experienced tumor enlargement and lymph node (LN) metastasis, respectively, at a 10-year follow-up [11]. Following the publication of the initial results from Kuma Hospital in 2003 [12], the 2011 Japanese guidelines adopted AS [13]. The Japanese Society of Thyroid Surgeons and the Japanese Association of Endocrine Surgeons (JAES) guidelines (2011) state that PTMC measuring 1 cm or less without clinical LN metastasis, distant metastasis, or significant extrathyroidal extension (ETE) can be considered for AS, although the evidence level is low [13].
Due to concerns about overdiagnosis in thyroid cancer and the favorable outcomes of PTMC as demonstrated in Japanese studies on AS, several prospective studies on AS have been conducted in various countries (Table 1) [10-12,14-43]. These studies have had a significant impact on thyroid cancer management guidelines around the world. The American Thyroid Association (ATA) guidelines published in 2015 recognize AS as an alternative to immediate surgery for PTMC [44]. Currently, various international guidelines offer recommendations on the consideration of AS for thyroid cancer, as detailed in Fig. 1 [13,44-56]. In this review, we discuss the current practice guidelines, with a focus on the indications for AS, the follow-up protocol, and the definition of disease progression.
Table 1.
Prospective Studies on AS
| Institution (trial no.) | Country | Year | No. of participants | Tumor size Included, cm | Primary outcome | Reference (protocol) |
|---|---|---|---|---|---|---|
| Asia | ||||||
| Kuma Hospital | Japan | 1993–2019 (ongoing) | 3,222 AS 2,424 IS | ≤1 | [11,12,14-24] | |
| Cancer Institute Hospital | Japan | 1995–2016 (ongoing) | 421 AS 377 IS | ≤2 | [25] | |
| Seoul National University Hospital, Seoul National University Bundang Hospital, National Cancer Center (MAeSTro) (NCT02938702) | Korea | 2016–2019 | 755 AS 422 IS | ≤1 | Tumor size change LN or distant metastasis | [27-33] (Protocol [26]) |
| Asan Medical Center, Seoul National University Bundang Hospital, Seoul St. Mary’s Hospital, and eight other hospitals (KoMPASS) (KCT0004935) | Korea | 2019–ongoing | Recruiting | ≤1 | Progression-free survival | (Protocol [34]) |
| North America | ||||||
| Memorial Sloan Kettering Cancer Center | US | NA | 291 AS (no IS) | ≤1.5 | Tumor diameter or volume change | [10] |
| Cedars-Sinai Medical Center (NCT02609685) | US | 2014–2021 | 112 AS 110 IS | ≤2.0 | Disease progression (tumor growth, LN, or distant metastasis) | [35] |
| University Health Network (NCT03271892) → Pan-Canadian (Canadian Thyroid Cancer Active Surveillance Study Group) (NCT04624477) | Canada | 2016–ongoing | 155 AS 45 IS→Recruiting | <2.0 | Freqeuncy of patients choosing AS or surgery | [38] (Protocol [36,37]) |
| South America | ||||||
| Head and Neck Cancer Center in Medellin | Colombia | 2015– | 102 AS (no IS) | <1.5 | [39] | |
| Santa Casa de Belo Horizonte | Brazil | 2016–2019 | 77 AS 18 IS | ≤1.2 | [40] | |
| University of Buenos Aires | Argentina | 34 AS (no IS) | ≤1.5 | [41] | ||
| Europe | ||||||
| University Hospital of Pisa (NCT04129281) | Italy | 2014–2020 | 127 AS (no IS) | ≤1.3 | [42,43] |
AS, active surveillance; IS, immediate surgery; MAeSTro, Multicenter Prospective Cohort Study of Active Surveillance on Papillary Thyroid Microcarcinoma; LN, lymph node; KoMPASS, Korean Multicenter Prospective Cohort Study of Active Surveillance or Surgery.
Fig. 1.
Guidelines on active surveillance (AS) for thyroid cancer. The guidelines were written in English, other than the Korean Thyroid Association (KTA) guidelines, which were first written in Korean and then translated into English. MSKCC, Memorial Sloan Kettering Cancer Center; MAeSTro, Multicenter Prospective Cohort Study of Active Surveillance on Papillary Thyroid Microcarcinoma; KoMPASS, Korean Multicenter Prospective Cohort Study of Active Surveillance or Surgery; JATS, Japan Society of Thyroid Surgeons; JAES, Japanese Association of Endocrine Surgeons; ATA, American Thyroid Association; ESMO, European Society for Medical Oncology; ETA, European Thyroid Association; EORTC, European Organization for Research and Treatment of Cancer; SFE, French Society of Endocrinology; AFCE, French Association of Endocrine Surgery; SFNM, French Society of Nuclear Medicine; SBEM, Brazilian Society of Endocrinology and Metabolism; JTA, Japan Thyroid Association; JSTS, Japanese Society of Thyroid Surgeons; NA, not available; PTMC, papillary thyroid microcarcinoma; US, ultrasonography; PTC, papillary thyroid carcinoma; EU-TIRADS, European Union Thyroid Imaging Reporting and Data System; FNA, fine-needle aspiration.
INDICATIONS OR CANDIDATES FOR AS
The initial criteria for AS were derived from studies conducted in Japan. Kuma Hospital in Japan implemented AS for PTMC measuring less than 1 cm, excluding cases where the tumor was located adjacent to the trachea or on the dorsal surface of the thyroid lobe, which could potentially invade the recurrent laryngeal nerve (RLN), or if there was evidence of LN metastasis [15]. With increasing experience and research findings on AS in Japan, Brito et al. [57] proposed a comprehensive framework for categorizing tumors, patients, and medical team characteristics as ideal, appropriate, or inappropriate for AS consideration and decision-making (Table 2). Subsequently, numerous studies and guidelines have adopted this approach. Most guidelines recommend considering AS for tumors that measure 1 cm or less, show no aggressive subtypes on cytology, do not exhibit ETE, and do not have clinical LN or distant metastasis. However, debate continues over the indications for AS. Initially, Japanese groups selected the safest candidates for AS. However, subsequent observations indicated that even with tumor growth or LN metastasis, delayed surgery did not worsen overall survival compared to immediate surgery [20,58]. Consequently, there have been suggestions to expand the criteria for AS. Conversely, some have advocated stricter criteria to exclude tumors with a higher likelihood of progression. These differing viewpoints drive the ongoing debate regarding the selection of candidates for AS. The criteria proposed in guidelines and prospective studies are summarized below.
Table 2.
A Risk-Stratified Approach to Decision-Making in Probable or Proven Papillary Microcarcinoma
| Candidates for observation | Tumor/neck US characteristics | Patient characteristics | Medical team characteristics |
|---|---|---|---|
| Ideal | Solitary thyroid nodule | Older patients (>60 years) | Experienced multidisciplinary management team |
| Well-defined margins | Willing to accept an active surveillance approach | ||
| Surrounded by ≥2 mm normal thyroid parenchyma | Understands that a surgical intervention may be necessary in the future | High-quality neck ultrasonography | |
| No evidence of extrathyroidal extension | Expected to be compliant with follow-up plans | Prospective data collection | |
| Previous US documenting stability | Supportive significant others (including other members of their healthcare team) | Tracking/reminder program to ensure proper follow-up | |
| cN0 | |||
| cM0 | Life-threatening comorbidities | ||
| Appropriate | Multifocal papillary microcarcinomas | Middle-aged patients (18–59 years) | Experienced endocrinologist or thyroid surgeon |
| Subcapsular locations not adjacent to RLN without evidence of extrathyroidal extension | Strong family history of papillary thyroid cancer | ||
| Ill-defined margins | Child bearing potential | Neck ultrasonography routinely available | |
| Background ultrasonographic findings that will make follow-up difficult (thyroiditis, nonspecific lymphadenopathy, multiple other benign-appearing thyroid nodules) | |||
| FDG-avid papillary microcarcinomas | |||
| Inappropriate | Evidence of aggressive cytology on FNA (rare) | Young patients (<18 years) | Reliable neck ultrasonography not available |
| Subcapsular locations adjacent to RLN | Unlikely to be compliant with follow-up plans | ||
| Evidence of extrathyroidal extension | Not willing to accept an observation approach | Little experience with thyroid cancer management | |
| Clinical evidence of invasion of RLN or trachea (rare) | |||
| N1 disease at initial evaluation or identified during follow-up | |||
| M1 disease (rare) | |||
| Documented increase in size of ≥3 mm in a confirmed papillary thyroid cancer tumor |
Adapted from Brito et al. [57], with permission from Mary Ann Libert, Inc.
US, ultrasonography; RLN, recurrent laryngeal nerve; FDG, fluorodeoxyglucose; FNA, fine-needle aspiration.
Tumor characteristics
Tumor size
AS is typically recommended for PTMC measuring 1 cm or less, according to most guidelines. The inclusion criteria of most prospective studies are also aligned with this size limitation. However, some studies have broadened this criterion. For instance, an Italian prospective study proposed that a threshold of 1.3 cm might be a safe alternative to the standard 1.0 cm [42]. This suggestion accounts for the variability in US measurements and the shrinkage that occurs in paraffin-embedded tissue specimens. The precision of US in measuring thyroid nodules can be compromised by factors such as significant inter- and intra-observer variability, heterogeneous background parenchyma due to thyroiditis, and acoustic shadowing caused by calcifications. Moreover, prospective studies that have applied expanded size criteria of 1.5 and 2 cm have reported low rates of tumor progression and an absence of distal metastasis [35,59]. Discussions are ongoing about expanding the size criterion for AS from 1 to 1.5 cm or 2 cm clinical practice, but further research is needed for validation.
Tumor location
There are still many uncertainties regarding the appropriate locations of tumors that are suitable for AS. Fig. 2 depicts the tumor locations recommended for AS in various guidelines. Most guidelines and prospective studies typically do not consider PTMC with ETE to the strap muscles as a candidate for AS. However, the JAES consensus statements (2021) suggest that tumors located on the ventral side of the thyroid, even those with US features indicating invasion into the strap muscles, may not necessarily require immediate surgery. This recommendation is based on the minimal impact of these features on the patient’s quality of life (QOL) and prognosis [50]. ETE exhibits a spectrum, ranging from minor ETE, which is characterized by mere contact or bulging of the thyroid capsule or strap muscles, to gross ETE, which involves significant invasion or replacement of strap muscles (Fig. 2B). The applicability of AS in cases with varying degrees of ETE remains unclear and warrants further research.
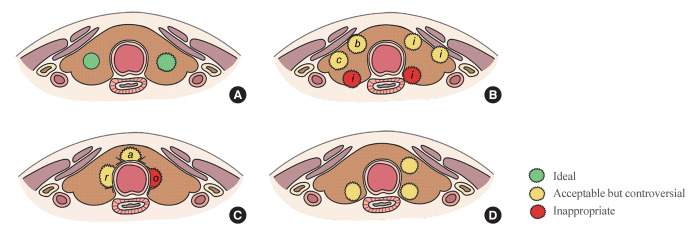
Fig. 2.
Ideal or appropriate tumor location for active surveillance (AS). (A) Ideal tumor location. (B) While tumors on the dorsal side of thyroid are excluded from AS, tumors on the ventral side can be considered for AS even if they exhibit contact with (c) or bulging of (b) the thyroid capsule, or if there is suspected invasion (i) into the strap muscle. (C) While tumors contacting the trachea at an obtuse angle (o) are excluded from AS, tumors contacting the trachea at an acute or right angle (a, r) can be considered for AS. (D) Some guidelines recommend against AS for tumors located close to the trachea or the recurrent laryngeal nerve, and they suggest that AS is appropriate for tumors surrounded by more than 2 mm of normal thyroid parenchyma.
In the observational study conducted at Japan’s Kuma Hospital, PTMCs located adjacent to the trachea or on the dorsal surface of the thyroid were excluded from AS due to their high risk of tracheal or RLN invasion [15]. Following these criteria, some guidelines recommend against selecting such cases for AS, even in the absence of clear evidence of invasion into the trachea or RLN, if the tumor is in close proximity [47,53,60]. However, JAES consensus statements (2021) suggests that tumors smaller than 0.7 cm, those merely touching the trachea, or those located away from the RLN’s course may be eligible for AS [50]. This recommendation is based on evidence that significant tracheal invasion necessitating tracheal cartilage resection only occurred in PTMCs ≥0.7 cm that formed an obtuse angle with the trachea [61]. Similarly, significant invasion requiring dissection of the RLN only occurred in PTMCs ≥0.7 cm without a normal rim between the tumor and the course of the RLN [61]. However, Newman et al. [62] reported that PTMCs >0.9 cm were unsuitable for AS, even if US or computed tomography did not show signs of RLN invasion, as subcapsular tumors located at the paratracheal area and right lateral posterior lobe area may exhibit gross RLN invasion. In summary, the appropriate tumor location for AS is not yet clearly established, indicating the need for additional research on this topic.
Multiplicity
Some guidelines, including those from the European Society for Medical Oncology (ESMO) in 2019, suggest AS only for patients with single or unifocal PTMC, and recommend immediate surgery for those with multifocal or bilateral PTMC [47,56,60]. However, prospective studies that included patients with multiple lesions have reported that the multiplicity is not a risk factor for disease progression [11,15]. Therefore, JAES (2021) and the Brazilian Society of Endocrinology and Metabolism (SBEM) (2022) guidelines state that patients with multiple PTMC can be candidates for AS [50,53].
US characteristics
The Japan Cancer Institute Hospital reported a significant correlation between strong calcification (either macrocalcification or rim calcification) and poor vascularity of the tumor with a nonprogressive tumor status [25]. Based on this observation, the JAES guidelines (2020) note that the presence of strong calcification and poor blood flow observed on US are indicators that tumors are unlikely to grow [48]. However, the role of calcification is debatable, as Oh et al. [63] found that macrocalcification was significantly associated with tumor growth. Recently, Lee et al. [33] reported no association between macrocalcification and tumor progression. The JAES consensus statement (2021) state that the current evidence is insufficient to justify excluding patients with PTMCs from AS based on the degree of calcification or vascularity [50]. Additionally, a Korean prospective study indicated that US features of diffuse thyroid disease and the presence of intratumoral vascularity were associated with tumor growth [33]. Further research is needed to identify the US findings that indicate suitability for AS.
Mutational status
The Italian consensus statement (2018) identifies the BRAFV600E mutation as a potential risk factor for disease progression [46], although the evidence supporting this claim remains limited. Some prospective studies have investigated the prevalence of the BRAFV600E mutation in patients who underwent conversion surgery after AS. At Japan Kuma Hospital, the surgical specimens of 26 patients were analyzed, revealing no significant difference in the frequency of the BRAFV600E mutation among the stable group (64%), the tumor enlargement group (70%), and the LN metastasis group (80%) [64]. Similarly, a Korean prospective study analyzing surgical specimens from 128 patients found no significant difference in the frequency of the BRAFV600E mutation between the stable group (83%) and the disease progression group (80%) [32]. While the BRAFV600E mutation in PTMC has been associated with aggressive clinicopathological characteristics and higher recurrence rates [65,66], its impact is limited unless it coexists with telomerase reverse transcriptase (TERT) mutations, which more significantly affect recurrence [67,68]. However, TERT mutations are infrequent in PTMC, with a reported prevalence of only 0.3% to 0.5% [69,70], suggesting that the clinical impact of the BRAFV600E and TERT mutations might be minor. Further studies are needed to ascertain whether the mutational status, including the BRAFV600E mutation, can predict disease progression in AS. These studies should involve molecular profiling of fine-needle aspiration (FNA) or core needle biopsy specimens and subsequent observational follow-up. Therefore, the JAES consensus statements (2021) state that no reliable molecular markers have been identified to date [50].
Patients’ characteristics
Medical condition
When considering AS for thyroid cancer, a patient’s overall medical condition is a crucial factor. The ATA guidelines (2015) suggest that AS can be considered in patients at high surgical risk because of comorbid conditions, patients with a relatively short expected lifespan (e.g., those with serious cardiopulmonary disease, other malignancies, or very advanced age), and patients with concurrent medical or surgical issues that need to be addressed prior to thyroid surgery [44].
The ESMO guidelines (2019) recommend AS for patients who have not been exposed to radiation in childhood or adolescence [46,47]. However, the evidence supporting this recommendation is limited.
Age
Several prospective studies have consistently shown that younger are more likely to experience disease progression [10,11,21,29,59]. Miyauchi et al. [18] reported that the 10-year disease progression rates during AS were 36% for patients in their 20s, 13%–14% for those in their 30s–40s, and 5%–6% for those in their 50s–60s. Therefore, most guidelines recommend AS for older patients and generally advise against it for patients under the age of 18 to 20 [50,53,60]. While most guidelines do not provide specific age recommendations, the French Society of Endocrinology, French Association of Endocrine Surgery, and French Society of Nuclear Medicine (SFE/AFCE/SFMN) guidelines (2022) suggest that patients aged 45 and older are more suitable for AS [51]. The SBEM (2022) and Korean Thyroid Association (KTA) (2023) guidelines state that AS is ideal for patients aged 60 and above and can be considered appropriate for those between 18 and 59 [53,55].
Family history
Familial differentiated thyroid cancer (DTC) has been shown to have a higher rate of multiplicity, LN metastasis, and recurrence than sporadic DTC [71,72]. However, its disease-specific mortality and overall mortality rates did not significantly differ from those in sporadic DTC [71,73]. The ESMO guidelines (2019) recommend AS for patients without a family history of thyroid cancer [47]. However, Ito et al. [11] found that a family history was not a significant risk factor for disease progression during AS. In line with this, the JAES consensus statements (2021) suggest that patients with a family history of DTC can be considered for AS [50]. The SBEM position statements (2022) indicate that while surgery is typically preferred for familial DTC, AS could be a therapeutic alternative [53].
Concomitant thyroid disorder
The JAES (2021) and SBEM (2022) guidelines state that AS is not contraindicated for patients with Graves’ disease or Hashimoto’s thyroiditis [50,53]. However, in such cases, it is important to note that US evaluation can be challenging due to the heterogeneity of the background thyroid, and high thyroid-stimulating hormone (TSH) levels may potentially stimulate tumor growth.
Childbearing age or pregnancy
Pregnancy and the consequent increase in beta-human chorionic gonadotropin may potentially enlarge thyroid nodules and cancer [74]. However, Kuma Hospital reported that in the majority of cases (92%), AS was possible during pregnancy without any change in tumor size [75]. Therefore, the JAES (2021) and SBEM (2022) guidelines indicate that patients who are planning to conceive, as well as patients who are pregnant, can be candidates for AS [50,53]. Existing guidelines have not addressed the issue of hormone replacement therapy in postmenopausal women because the relationship between hormone replacement therapy and tumor growth of thyroid cancer remains unclear [76].
Sex
Inconsistent results have been reported regarding whether male sex is a prognostic factor in PTMC. Some studies have found higher rates of LN metastasis or recurrence in men, while others have not reported such an association [77,78]. This inconsistency extends to AS studies. A Korean prospective study found a significant correlation between male sex and tumor progression during AS [29,33]. However, other prospective studies on AS have not found any association between them [10,11,35]. As a result, current guidelines do not include sex as a criterion for determining eligibility for AS.
Shared decision-making
Even when tumor characteristics are deemed ideal or appropriate, and the patient’s characteristics align with the criteria outlined above, a collaborative discussion between the patient and the physician is essential for deciding between immediate surgery and AS [46,50,54,55]. This shared decision-making process should consider factors such as QOL, patient-reported outcomes (PROs), and cost-effectiveness. The importance of the patient’s perspective and PROs has gained increasing recognition. The Japan Thyroid Association (JTA) position statements (2021) note that AS is associated with better physical QOL, but also increased anxiety [49]. In a cross-sectional study, Jeon et al. [79] found that the AS group reported better QOL in areas such as neuromuscular, throat/mouth, and scar problems compared to the surgery group. Longitudinal studies have initially shown better physical QOL in AS groups compared to surgery groups, but after 1 to 2 years, the QOL scores between the two groups tend to be comparable [28,80]. Yoshida et al. [81] reported higher anxiety in the AS group than in the surgery group. Furthermore, prospective studies have revealed that 54% to 70% of patients who switched from AS to surgery did so not due to disease progression, but because of personal preference or anxiety [16,32]. The JAES consensus statements (2021) state that there is still a lack of evidence regarding PROs in the management of low-risk PTMC and note that long-term comparative studies are needed on this topic [50].
The JTA position statements (2021) note that the 10-year medical costs of AS were found to be lower than those of immediate surgery in Japan [17,49]. Cost-effectiveness analyses in different countries, such as Hong Kong and Austria, showed that AS was less costly for the first 16 years [82,83], and this trend was also seen in Korea for the first 10 years [30]. In the United States, the cost-effectiveness was found to vary depending on the patient’s disutility [84]. Although cost-effectiveness varies by country, AS may initially be more economical; however, over time, surgery might emerge as the more cost-effective approach. Thus, it is crucial to consider the patient’s financial situation and life expectancy when making the decisions.
FOLLOW-UP PROTOCOL FOR AS
During AS, it is necessary to perform regular US examination by experienced examiners to monitor for any tumor enlargement or LN metastasis. In a prospective study conducted at Kuma Hospital, patients visited the hospital once or twice a year for blood thyroid tests and neck US examinations [21]. In most other prospective studies, patients were scheduled to visit the hospital every 6 months during the first 2 years, followed by annual visits thereafter [10,26,36]. Reflecting these follow-up protocols, various guidelines recommend specific intervals for US examinations, as detailed in Table 3. Currently, there is no definitive evidence regarding when AS can be safely discontinued. Consequently, it is advised to continue AS throughout life [50,51].
Table 3.
Intervals for US Examinations during Active Surveillance
| Society | US evaluation |
|---|---|
| Six Italian Societies (2018) | Every 6 months in the first 2 years and once a year thereafter |
| ESMO (2019) | Every 6–12 months |
| JAES (2020) | Once or twice a year |
| JAES (2021) | Every 6 months in the first 1–2 years and one a year thereafter |
| SFE/AFCE/SFNM (2022) | Every 6 months in the first year and once a year until the end of the 5th year, then at 7 years, then every 2–3 years (level of evidence ++, Grade B) |
| Polish Scientific Societies (2022) | Every 6 months in the first 2 years and once a year thereafter (low quality of evidence, weak recommendation) |
| SBEM (2022) | Every 6 months in the first 2 years and once a year thereafter |
US, ultrasonography; ESMO, European Society for Medical Oncology; JAES, Japanese Association of Endocrine Surgeons; SFE, French Society of Endocrinology; AFCE, French Association of Endocrine Surgery; SFNM, French Society of Nuclear Medicine; SBEM, Brazilian Society of Endocrinology and Metabolism.
The necessity of TSH suppression during AS remains uncertain. Ito et al. [11] suggested that TSH suppression could be beneficial because 50 of 51 patients undergoing TSH suppression during AS showed no disease progression. Korean studies have found a significant association between high TSH levels and tumor progression [33,85]. However, the Japan Cancer Institute Hospital reported no correlation between TSH levels and tumor enlargement during AS [86]. Consequently, the JAES consensus statements (2021) note the lack of evidence for TSH suppression therapy during AS [50]. The SBEM position statements (2022) recommend maintaining TSH levels within the normal range as the safest strategy to prevent nodular growth and the harmful effects of excessive thyroid hormone [53].
The measurement of serum thyroglobulin (Tg) for evaluation of thyroid nodules is not recommended [44]. Although some prospective studies on AS measured Tg levels, no report suggests that serum Tg level is a predictor of tumor progression [15,26,36,42]. This indicates its limited utility in the follow-up of patients on AS.
DEFINITION OF DISEASE PROGRESSION
The definition of disease progression during AS remains a matter of debate. Generally, disease progression is defined based on tumor growth, clinically evident ETE, LN metastasis, or distant metastasis [49]. ETE, LN metastasis, and distant metastasis are universally accepted as indicators of disease progression and indications for surgery in all guidelines and prospective studies. However, the definition of tumor growth or enlargement varies. The most commonly used criterion is an increase in tumor diameter of ≥3 mm, as suggested by Ito et al. [87]. A study at the US Memorial Sloan Kettering Cancer Center defined tumor growth as a tumor size increase of ≥3 mm in the greatest dimension and a tumor volume increase ≥50% compared with baseline [10]. In a Korean multicenter study, tumor growth was defined as a size increase of ≥3 mm in at least one dimension, or ≥2 mm in at least two dimensions [26]. The US Cedars-Sinai Medical Center study defined tumor growth as an increase of ≥ 5 mm in diameter or a volume increase of ≥100% [35]. The definitions of tumor growth also vary across published guidelines, as summarized in Table 4. While some guidelines and studies require meeting the growth criterion only once, the Japan Kuma Hospital and Canadian prospective studies, as well as the SFE/AFCE/SFMN consensus statements (2022), recommend surgery only when the criterion is met twice in consecutive US examinations [20,36]. Two consecutive confirmations are suggested due to the inter- and intra-observer variability in measuring tumor size using US [88], and the possibility that a tumor meeting the growth criterion once may decrease in size subsequently [89]. Moreover, the JAES consensus statements (2021) suggest that a tumor diameter exceeding 1 cm does not always necessitate immediate surgery [50]. The JAES (2021) and SBEM (2022) guidelines suggest that a tumor diameter reaching 13 mm is a surgical indication (Table 4).
Table 4.
Definitions of Tumor Growth and Surgical Indications
| Society | Definition of tumor growth | Surgical indication related to tumor size |
|---|---|---|
| JTA (2021) | Tumor diameter increase ≥3 mm | Samea |
| Tumor volume increase >50% | ||
| JAES (2021) | Tumor diameter increase ≥3 mm | Tumor diameter ≥13 mm |
| Polish Scientific Societies (2022) | Tumor diameter increase ≥3 mm | Samea |
| SBEM (2022) | Tumor diameter increase >3 mm | Tumor diameter increase >3 mm |
| Tumor diameter ≥13 mm |
JTA, Japan Thyroid Association; JAES, Japanese Association of Endocrine Surgeons; SBEM, Brazilian Society of Endocrinology and Metabolism.
The surgical indication related to tumor size is the same as the definition of tumor growth.
During follow-up, new thyroid nodules may be detected and confirmed as PTMC through FNA. Such newly detected cases of PTMC can be considered either intrathyroidal metastases or new, separate cancers. Currently, no consensus exists in guidelines or prospective studies on whether newly developed PTMC within the thyroid gland indicates disease progression or warrants surgery. Only one ongoing Korean prospective study has categorized the cytopathological diagnosis of a new thyroid cancer lesion as disease progression, but its results are yet to be published [34].
AS WITHOUT FINE-NEEDLE ASPIRATION
With the increasing adoption of AS for pathologically proven low-risk PTMC, there has been growing debate about the necessity of performing FNA on sonographically suspicious subcentimeter thyroid nodules that do not exhibit ETE or LN metastasis. The ATA (2015) and European Thyroid Association (ETA) (2017) guidelines recommend FNA only for thyroid nodules that are 1 cm or larger even if thyroid nodules exhibit a high suspicion US pattern [44,90]. The ETA guidelines (2017) also state that subcentimeter nodules exhibiting highly suspicious US features can be managed either through AS or FNA [90]. Therefore, in countries that strictly follow thyroid nodule evaluation guidelines, the number of pathologically proven low-risk PTMCs may be low. The role of AS in these settings may therefore be less relevant [91]. The majority of guidelines lean toward suggesting AS without FNA for subcentimeter thyroid nodules [92]. More recently, prospective studies are being conducted on AS for highly suspicious subcentimeter thyroid nodules, even without prior FNA confirmation [93]. However, the JTA position statements (2021) continue to recommend FNA for nodules measuring 0.5 to 1 cm that are strongly suspected of being malignant based on US findings [49].
AS IN KOREA
The KTA published guidelines on AS in 2016 and updated them in 2023. The 2016 KTA guidelines stated that AS could be considered for low-risk PTMC patients with a tumor size of 1 cm or less, no aggressive subtype on cytology, no ETE, and no clinical LN or distant metastasis [45]. In contrast, the 2023 KTA guidelines generally recommend AS for the same patient group, indicating a more assertive approach towards AS [55]. This shift was influenced by the results of multiple AS studies conducted in Korea [26,29,30,32,33]. The 2023 guidelines advise considering AS primarily for patients over the age of 60 but do not provide additional criteria. Furthermore, they do not address the follow-up protocol for AS or the definition of disease progression, underscoring the necessity for more comprehensive guidelines on AS.
CONCLUSIONS
Over the past decade, there has been a significant paradigm shift with the introduction of AS as a new treatment option for low-risk thyroid cancer. This article explores the current landscape of AS, drawing on insights from various guidelines and prospective studies. While AS is a feasible and reliable option for managing low-risk thyroid cancer, it carries the risk of cancer progression. Consequently, careful patient selection and proper implementation are crucial. Central to this approach is the identification of ideal or appropriate candidates for AS, considering tumor characteristics such as size, location, number, and US findings, as well as patient factors like medical condition, age, and family history. Additionally, PROs, such as QOL, and cost-effectiveness should be taken into account. It is critical to discuss these factors with patients to facilitate informed, shared decision-making. For patients who opt for AS, the establishment of standardized follow-up protocols and a precise definition of disease progression are essential for effective monitoring. Moreover, ongoing research to discover markers that can predict disease progression is crucial for improving the efficacy and safety of AS in the management of low-risk thyroid cancer.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Patient-Doctor Shared Decision Marking Research Center (PDSDM), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HV23C1828). This research was supported by the BK21 FOUR Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (5120200513755).
Footnotes
CONFLICTS OF INTEREST
Young Joo Park is an editor-in-chief of the journal. But she was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022;10:264–72. doi: 10.1016/S2213-8587(22)00035-3. [DOI] [PubMed] [Google Scholar]
- 2.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317:1338–48. doi: 10.1001/jama.2017.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic?: the increasing impact of overdiagnosis. N Engl J Med. 2016;375:614–7. doi: 10.1056/NEJMp1604412. [DOI] [PubMed] [Google Scholar]
- 4.Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”: screening and overdiagnosis. N Engl J Med. 2014;371:1765–7. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 5.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid: a “normal” finding in Finland: a systematic autopsy study. Cancer. 1985;56:531–8. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Ullmann TM, Papaleontiou M, Sosa JA. Current controversies in low-risk differentiated thyroid cancer: reducing overtreatment in an era of overdiagnosis. J Clin Endocrinol Metab. 2023;108:271–80. doi: 10.1210/clinem/dgac646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzaferri EL. Management of low-risk differentiated thyroid cancer. Endocr Pract. 2007;13:498–512. doi: 10.4158/EP.13.5.498. [DOI] [PubMed] [Google Scholar]
- 8.Hay ID. Management of patients with low-risk papillary thyroid carcinoma. Endocr Pract. 2007;13:521–33. doi: 10.4158/EP.13.5.521. [DOI] [PubMed] [Google Scholar]
- 9.Mehanna H, Al-Maqbili T, Carter B, Martin E, Campain N, Watkinson J, et al. Differences in the recurrence and mortality outcomes rates of incidental and nonincidental papillary thyroid microcarcinoma: a systematic review and meta-analysis of 21 329 person-years of follow-up. J Clin Endocrinol Metab. 2014;99:2834–43. doi: 10.1210/jc.2013-2118. [DOI] [PubMed] [Google Scholar]
- 10.Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg. 2017;143:1015–20. doi: 10.1001/jamaoto.2017.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24:27–34. doi: 10.1089/thy.2013.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito Y, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. 2003;13:381–7. doi: 10.1089/105072503321669875. [DOI] [PubMed] [Google Scholar]
- 13.Takami H, Ito Y, Okamoto T, Yoshida A. Therapeutic strategy for differentiated thyroid carcinoma in Japan based on a newly established guideline managed by Japanese Society of Thyroid Surgeons and Japanese Association of Endocrine Surgeons. World J Surg. 2011;35:111–21. doi: 10.1007/s00268-010-0832-6. [DOI] [PubMed] [Google Scholar]
- 14.Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Papillary microcarcinoma of the thyroid: how should it be treated? World J Surg. 2004;28:1115–21. doi: 10.1007/s00268-004-7644-5. [DOI] [PubMed] [Google Scholar]
- 15.Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010;34:28–35. doi: 10.1007/s00268-009-0303-0. [DOI] [PubMed] [Google Scholar]
- 16.Oda H, Miyauchi A, Ito Y, Yoshioka K, Nakayama A, Sasai H, et al. Incidences of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid. 2016;26:150–5. doi: 10.1089/thy.2015.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oda H, Miyauchi A, Ito Y, Sasai H, Masuoka H, Yabuta T, et al. Comparison of the costs of active surveillance and immediate surgery in the management of low-risk papillary microcarcinoma of the thyroid. Endocr J. 2017;64:59–64. doi: 10.1507/endocrj.EJ16-0381. [DOI] [PubMed] [Google Scholar]
- 18.Miyauchi A, Kudo T, Ito Y, Oda H, Sasai H, Higashiyama T, et al. Estimation of the lifetime probability of disease progression of papillary microcarcinoma of the thyroid during active surveillance. Surgery. 2018;163:48–52. doi: 10.1016/j.surg.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Miyauchi A, Kudo T, Ito Y, Oda H, Yamamoto M, Sasai H, et al. Natural history of papillary thyroid microcarcinoma: kinetic analyses on tumor volume during active surveillance and before presentation. Surgery. 2019;165:25–30. doi: 10.1016/j.surg.2018.07.045. [DOI] [PubMed] [Google Scholar]
- 20.Miyauchi A, Ito Y, Fujishima M, Miya A, Onoda N, Kihara M, et al. Long-term outcomes of active surveillance and immediate surgery for adult patients with low-risk papillary thyroid microcarcinoma: 30-year experience. Thyroid. 2023;33:817–25. doi: 10.1089/thy.2023.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito Y, Miyauchi A, Fujishima M, Noda T, Sano T, Sasaki T, et al. Thyroid-stimulating hormone, age, and tumor size are risk factors for progression during active surveillance of low-risk papillary thyroid microcarcinoma in adults. World J Surg. 2023;47:392–401. doi: 10.1007/s00268-022-06770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasaki T, Miyauchi A, Fujishima M, Ito Y, Kudo T, Noda T, et al. Comparison of postoperative unfavorable events in patients with low-risk papillary thyroid carcinoma: immediate surgery versus conversion surgery following active surveillance. Thyroid. 2023;33:186–91. doi: 10.1089/thy.2022.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto M, Miyauchi A, Ito Y, Fujishima M, Sasaki T, Kudo T. Active surveillance outcomes of patients with low-risk papillary thyroid microcarcinoma according to levothyroxine treatment status. Thyroid. 2023;33:1182–9. doi: 10.1089/thy.2023.0046. [DOI] [PubMed] [Google Scholar]
- 24.Fujishima M, Miyauchi A, Ito Y, Kudo T, Noda T, Sano T, et al. Active surveillance is an excellent management technique for identifying patients with progressive low-risk papillary thyroid microcarcinoma requiring surgical treatment. Endocr J. 2023;70:411–8. doi: 10.1507/endocrj.EJ22-0559. [DOI] [PubMed] [Google Scholar]
- 25.Fukuoka O, Sugitani I, Ebina A, Toda K, Kawabata K, Yamada K. Natural history of asymptomatic papillary thyroid microcarcinoma: time-dependent changes in calcification and vascularity during active surveillance. World J Surg. 2016;40:529–37. doi: 10.1007/s00268-015-3349-1. [DOI] [PubMed] [Google Scholar]
- 26.Moon JH, Kim JH, Lee EK, Lee KE, Kong SH, Kim YK, et al. Study protocol of multicenter prospective cohort study of active surveillance on papillary thyroid microcarcinoma (MAeSTro) Endocrinol Metab (Seoul) 2018;33:278–86. doi: 10.3803/EnM.2018.33.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong SH, Ryu J, Kim MJ, Cho SW, Song YS, Yi KH, et al. Longitudinal assessment of quality of life according to treatment options in low-risk papillary thyroid microcarcinoma patients: active surveillance or immediate surgery (interim analysis of MAeSTro) Thyroid. 2019;29:1089–96. doi: 10.1089/thy.2018.0624. [DOI] [PubMed] [Google Scholar]
- 28.Moon JH, Ryu CH, Cho SW, Choi JY, Chung EJ, Hah JH, et al. Effect of initial treatment choice on 2-year quality of life in patients with low-risk papillary thyroid microcarcinoma. J Clin Endocrinol Metab. 2021;106:724–35. doi: 10.1210/clinem/dgaa889. [DOI] [PubMed] [Google Scholar]
- 29.Lee EK, Moon JH, Hwangbo Y, Ryu CH, Cho SW, Choi JY, et al. Progression of low-risk papillary thyroid microcarcinoma during active surveillance: interim analysis of a multicenter prospective cohort study of active surveillance on papillary thyroid microcarcinoma in Korea. Thyroid. 2022;32:1328–36. doi: 10.1089/thy.2021.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim K, Choi JY, Kim SJ, Lee EK, Lee YK, Ryu JS, et al. Active surveillance versus immediate surgery for low-risk papillary thyroid microcarcinoma patients in South Korea: a cost-minimization analysis from the MAeSTro Study. Thyroid. 2022;32:648–56. doi: 10.1089/thy.2021.0679. [DOI] [PubMed] [Google Scholar]
- 31.Hwangbo Y, Choi JY, Lee EK, Ryu CH, Cho SW, Chung EJ, et al. A cross-sectional survey of patient treatment choice in a multicenter prospective cohort study on active surveillance of papillary thyroid microcarcinoma (MAeSTro) Thyroid. 2022;32:772–80. doi: 10.1089/thy.2021.0619. [DOI] [PubMed] [Google Scholar]
- 32.Hwang H, Choi JY, Yu HW, Moon JH, Kim JH, Lee EK, et al. Surgical outcomes in patients with low-risk papillary thyroid microcarcinoma from MAeSTro Study: immediate operation versus delayed operation after active surveillance: a multicenter prospective cohort study. Ann Surg. 2023;278:e1087–95. doi: 10.1097/SLA.0000000000005841. [DOI] [PubMed] [Google Scholar]
- 33.Lee JY, Kim JH, Kim YK, Lee CY, Lee EK, Moon JH, et al. US predictors of papillary thyroid microcarcinoma progression at active surveillance. Radiology. 2023;309:e230006. doi: 10.1148/radiol.230006. [DOI] [PubMed] [Google Scholar]
- 34.Jeon MJ, Kang YE, Moon JH, Lim DJ, Lee CY, Lee YS, et al. Protocol for a Korean multicenter prospective cohort study of active surveillance or surgery (KoMPASS) in papillary thyroid microcarcinoma. Endocrinol Metab (Seoul) 2021;36:359–64. doi: 10.3803/EnM.2020.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho AS, Kim S, Zalt C, Melany ML, Chen IE, Vasquez J, et al. Expanded parameters in active surveillance for low-risk papillary thyroid carcinoma: a nonrandomized controlled trial. JAMA Oncol. 2022;8:1588–96. doi: 10.1001/jamaoncol.2022.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawka AM, Ghai S, Tomlinson G, Rotstein L, Gilbert R, Gullane P, et al. A protocol for a Canadian prospective observational study of decision-making on active surveillance or surgery for low-risk papillary thyroid cancer. BMJ Open. 2018;8:e020298. doi: 10.1136/bmjopen-2017-020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawka AM, Ghai S, Tomlinson G, Baxter NN, Corsten M, Imran SA, et al. A protocol for a Pan-Canadian prospective observational study on active surveillance or surgery for very low risk papillary thyroid cancer. Front Endocrinol (Lausanne) 2021;12:686996. doi: 10.3389/fendo.2021.686996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawka AM, Ghai S, Rotstein L, Irish JC, Pasternak JD, Gullane PJ, et al. A quantitative analysis examining patients’ choice of active surveillance or surgery for managing low-risk papillary thyroid cancer. Thyroid. 2022;32:255–62. doi: 10.1089/thy.2021.0485. [DOI] [PubMed] [Google Scholar]
- 39.Sanabria A. Experience with active surveillance of thyroid low-risk carcinoma in a developing country. Thyroid. 2020;30:985–91. doi: 10.1089/thy.2019.0522. [DOI] [PubMed] [Google Scholar]
- 40.Rosario PW, Mourao GF, Calsolari MR. Active surveillance in adults with low-risk papillary thyroid microcarcinomas: a prospective study. Horm Metab Res. 2019;51:703–8. doi: 10.1055/a-1015-6684. [DOI] [PubMed] [Google Scholar]
- 41.Smulever A, Pitoia F. Active surveillance in papillary thyroid carcinoma: not easily accepted but possible in Latin America. Arch Endocrinol Metab. 2019;63:462–9. doi: 10.20945/2359-3997000000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molinaro E, Campopiano MC, Pieruzzi L, Matrone A, Agate L, Bottici V, et al. Active surveillance in papillary thyroid microcarcinomas is feasible and safe: experience at a single Italian center. J Clin Endocrinol Metab. 2020;105:e172–80. doi: 10.1210/clinem/dgz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campopiano MC, Matrone A, Rago T, Scutari M, Prete A, Agate L, et al. Assessing mPTC progression during active surveillance: volume or diameter increase? J Clin Med. 2021;10:4068. doi: 10.3390/jcm10184068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yi KH, Lee EK, Kang HC, Koh Y, Kim SW, Kim IJ, et al. 2016 Revised Korean Thyroid Association management guidelines for patients with thyroid nodules and thyroid cancer. Int J Thyroidol. 2016;9:59–126. [Google Scholar]
- 46.Pacini F, Basolo F, Bellantone R, Boni G, Cannizzaro MA, De Palma M, et al. Italian consensus on diagnosis and treatment of differentiated thyroid cancer: joint statements of six Italian societies. J Endocrinol Invest. 2018;41:849–76. doi: 10.1007/s40618-018-0884-2. [DOI] [PubMed] [Google Scholar]
- 47.Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1856–83. doi: 10.1093/annonc/mdz400. [DOI] [PubMed] [Google Scholar]
- 48.Ito Y, Onoda N, Okamoto T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan Associations of Endocrine Surgeons: core questions and recommendations for treatments of thyroid cancer. Endocr J. 2020;67:669–717. doi: 10.1507/endocrj.EJ20-0025. [DOI] [PubMed] [Google Scholar]
- 49.Horiguchi K, Yoshida Y, Iwaku K, Emoto N, Kasahara T, Sato J, et al. Position paper from the Japan Thyroid Association task force on the management of low-risk papillary thyroid microcarcinoma (T1aN0M0) in adults. Endocr J. 2021;68:763–80. doi: 10.1507/endocrj.EJ20-0692. [DOI] [PubMed] [Google Scholar]
- 50.Sugitani I, Ito Y, Takeuchi D, Nakayama H, Masaki C, Shindo H, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: consensus statements from the Japan Association of Endocrine Surgery task force on management for papillary thyroid microcarcinoma. Thyroid. 2021;31:183–92. doi: 10.1089/thy.2020.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leboulleux S, Lamartina L, Lecornet Sokol E, Menegaux F, Leenhardt L, Russ G. SFE-AFCE-SFMN 2022 Consensus on the management of thyroid nodules: follow-up: how and how long? Ann Endocrinol (Paris) 2022;83:407–14. doi: 10.1016/j.ando.2022.10.010. [DOI] [PubMed] [Google Scholar]
- 52.Jarzab B, Dedecjus M, Slowinska-Klencka D, Lewinski A, Adamczewski Z, Anielski R, et al. Guidelines of Polish National Societies Diagnostics and Treatment of Thyroid Carcinoma: 2018 update. Endokrynol Pol. 2018;69:34–74. doi: 10.5603/EP.2018.0014. [DOI] [PubMed] [Google Scholar]
- 53.Ward LS, Scheffel RS, Hoff AO, Ferraz C, Vaisman F. Treatment strategies for low-risk papillary thyroid carcinoma: a position statement from the Thyroid Department of the Brazilian Society of Endocrinology and Metabolism (SBEM) Arch Endocrinol Metab. 2022;66:522–32. doi: 10.20945/2359-3997000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koot A, Soares P, Robenshtok E, Locati LD, de la Fouchardiere C, Luster M, et al. Position paper from the endocrine task force of the European Organisation for Research and Treatment of Cancer (EORTC) on the management and shared decision making in patients with low-risk micro papillary thyroid carcinoma. Eur J Cancer. 2023;179:98–112. doi: 10.1016/j.ejca.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 55.Park YJ, Lee EK, Song YS, Kang SH, Koo BS, Kim SW, et al. 2023 Korean Thyroid Association management guidelines for patients with thyroid nodules. Int J Thyroidol. 2023;16:1–31. [Google Scholar]
- 56.Durante C, Hegedus L, Czarniecka A, Paschke R, Russ G, Schmitt F, et al. 2023 European Thyroid Association clinical practice guidelines for thyroid nodule management. Eur Thyroid J. 2023;12:e230067. doi: 10.1530/ETJ-23-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brito JP, Ito Y, Miyauchi A, Tuttle RM. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid. 2016;26:144–9. doi: 10.1089/thy.2015.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chou R, Dana T, Haymart M, Leung AM, Tufano RP, Sosa JA, et al. Active surveillance versus thyroid surgery for differentiated thyroid cancer: a systematic review. Thyroid. 2022;32:351–67. doi: 10.1089/thy.2021.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakai T, Sugitani I, Ebina A, Fukuoka O, Toda K, Mitani H, et al. Active surveillance for T1bN0M0 papillary thyroid carcinoma. Thyroid. 2019;29:59–63. doi: 10.1089/thy.2018.0462. [DOI] [PubMed] [Google Scholar]
- 60.Jarzab B, Dedecjus M, Lewinski A, Adamczewski Z, Bakula-Zalewska E, Baldys-Waligorska A, et al. Diagnosis and treatment of thyroid cancer in adult patients: recommendations of Polish Scientific Societies and the National Oncological Strategy. 2022 Update. Endokrynol Pol. 2022;73:173–300. doi: 10.5603/EP.a2022.0028. [DOI] [PubMed] [Google Scholar]
- 61.Ito Y, Miyauchi A, Oda H, Kobayashi K, Kihara M, Miya A. Revisiting low-risk thyroid papillary microcarcinomas resected without observation: was immediate surgery necessary? World J Surg. 2016;40:523–8. doi: 10.1007/s00268-015-3184-4. [DOI] [PubMed] [Google Scholar]
- 62.Newman SK, Harries V, Wang L, McGill M, Ganly I, Girshman J, et al. Invasion of a recurrent laryngeal nerve from small well-differentiated papillary thyroid cancers: patient selection implications for active surveillance. Thyroid. 2022;32:164–9. doi: 10.1089/thy.2021.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oh HS, Kwon H, Song E, Jeon MJ, Kim TY, Lee JH, et al. Tumor volume doubling time in active surveillance of papillary thyroid carcinoma. Thyroid. 2019;29:642–9. doi: 10.1089/thy.2018.0609. [DOI] [PubMed] [Google Scholar]
- 64.Yabuta T, Matsuse M, Hirokawa M, Yamashita S, Mitsutake N, Miyauchi A. TERT promoter mutations were not found in papillary thyroid microcarcinomas that showed disease progression on active surveillance. Thyroid. 2017;27:1206–7. doi: 10.1089/thy.2016.0645. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y, Sadow PM, Suh H, Lee KE, Choi JY, Suh YJ, et al. BRAF(V600E) is correlated with recurrence of papillary thyroid microcarcinoma: a systematic review, multi-institutional primary data analysis, and meta-analysis. Thyroid. 2016;26:248–55. doi: 10.1089/thy.2015.0391. [DOI] [PubMed] [Google Scholar]
- 66.Li F, Chen G, Sheng C, Gusdon AM, Huang Y, Lv Z, et al. BRAFV600E mutation in papillary thyroid microcarcinoma: a meta-analysis. Endocr Relat Cancer. 2015;22:159–68. doi: 10.1530/ERC-14-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32:2718–26. doi: 10.1200/JCO.2014.55.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moon S, Song YS, Kim YA, Lim JA, Cho SW, Moon JH, et al. Effects of coexistent BRAFV600E and TERT promoter mutations on poor clinical outcomes in papillary thyroid cancer: a meta-analysis. Thyroid. 2017;27:651–60. doi: 10.1089/thy.2016.0350. [DOI] [PubMed] [Google Scholar]
- 69.Kim MJ, Kim JK, Kim GJ, Kang SW, Lee J, Jeong JJ, et al. TERT promoter and BRAF V600E mutations in papillary thyroid cancer: a single-institution experience in Korea. Cancers (Basel) 2022;14:4928. doi: 10.3390/cancers14194928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang H, Park H, Ryu HJ, Heo J, Kim JS, Oh YL, et al. Frequency of TERT promoter mutations in real-world analysis of 2,092 thyroid carcinoma patients. Endocrinol Metab (Seoul) 2022;37:652–63. doi: 10.3803/EnM.2022.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uchino S, Noguchi S, Kawamoto H, Yamashita H, Watanabe S, Yamashita H, et al. Familial nonmedullary thyroid carcinoma characterized by multifocality and a high recurrence rate in a large study population. World J Surg. 2002;26:897–902. doi: 10.1007/s00268-002-6615-y. [DOI] [PubMed] [Google Scholar]
- 72.Capezzone M, Secchi C, Fralassi N, Cantara S, Brilli L, Ciuoli C, et al. Should familial disease be considered as a negative prognostic factor in micropapillary thyroid carcinoma? J Endocrinol Invest. 2019;42:1205–13. doi: 10.1007/s40618-019-01039-0. [DOI] [PubMed] [Google Scholar]
- 73.Ito Y, Kakudo K, Hirokawa M, Fukushima M, Yabuta T, Tomoda C, et al. Biological behavior and prognosis of familial papillary thyroid carcinoma. Surgery. 2009;145:100–5. doi: 10.1016/j.surg.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Kung AW, Chau MT, Lao TT, Tam SC, Low LC. The effect of pregnancy on thyroid nodule formation. J Clin Endocrinol Metab. 2002;87:1010–4. doi: 10.1210/jcem.87.3.8285. [DOI] [PubMed] [Google Scholar]
- 75.Ito Y, Miyauchi A, Kudo T, Ota H, Yoshioka K, Oda H, et al. Effects of pregnancy on papillary microcarcinomas of the thyroid re-evaluated in the entire patient series at Kuma Hospital. Thyroid. 2016;26:156–60. doi: 10.1089/thy.2015.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cao Y, Wang Z, Gu J, Hu F, Qi Y, Yin Q, et al. Reproductive factors but not hormonal factors associated with thyroid cancer risk: a systematic review and meta-analysis. Biomed Res Int. 2015;2015:103515. doi: 10.1155/2015/103515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oh HS, Park S, Kim M, Kwon H, Song E, Sung TY, et al. Young age and male sex are predictors of large-volume central neck lymph node metastasis in clinical N0 papillary thyroid microcarcinomas. Thyroid. 2017;27:1285–90. doi: 10.1089/thy.2017.0250. [DOI] [PubMed] [Google Scholar]
- 78.Siddiqui S, White MG, Antic T, Grogan RH, Angelos P, Kaplan EL, et al. Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma. Thyroid. 2016;26:807–15. doi: 10.1089/thy.2015.0429. [DOI] [PubMed] [Google Scholar]
- 79.Jeon MJ, Lee YM, Sung TY, Han M, Shin YW, Kim WG, et al. Quality of life in patients with papillary thyroid microcarcinoma managed by active surveillance or lobectomy: a cross-sectional study. Thyroid. 2019;29:956–62. doi: 10.1089/thy.2018.0711. [DOI] [PubMed] [Google Scholar]
- 80.Liu C, Zhao H, Xia Y, Cao Y, Zhang L, Zhao Y, et al. Active surveillance versus immediate surgery: a comparison of clinical and quality of life outcomes among patients with highly suspicious thyroid nodules 1 cm or smaller in China. Eur J Surg Oncol. 2023;49:106917. doi: 10.1016/j.ejso.2023.04.016. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida Y, Horiuchi K, Okamoto T. Patients’ view on the management of papillary thyroid microcarcinoma: active surveillance or surgery. Thyroid. 2020;30:681–7. doi: 10.1089/thy.2019.0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lang BH, Wong CK. A cost-effectiveness comparison between early surgery and non-surgical approach for incidental papillary thyroid microcarcinoma. Eur J Endocrinol. 2015;173:367–75. doi: 10.1530/EJE-15-0454. [DOI] [PubMed] [Google Scholar]
- 83.Lin JF, Jonker PK, Cunich M, Sidhu SB, Delbridge LW, Glover AR, et al. Surgery alone for papillary thyroid microcarcinoma is less costly and more effective than long term active surveillance. Surgery. 2020;167:110–6. doi: 10.1016/j.surg.2019.05.078. [DOI] [PubMed] [Google Scholar]
- 84.Venkatesh S, Pasternak JD, Beninato T, Drake FT, Kluijfhout WP, Liu C, et al. Cost-effectiveness of active surveillance versus hemithyroidectomy for micropapillary thyroid cancer. Surgery. 2017;161:116–26. doi: 10.1016/j.surg.2016.06.076. [DOI] [PubMed] [Google Scholar]
- 85.Kim HI, Jang HW, Ahn HS, Ahn S, Park SY, Oh YL, et al. High serum TSH level is associated with progression of papillary thyroid microcarcinoma during active surveillance. J Clin Endocrinol Metab. 2018;103:446–51. doi: 10.1210/jc.2017-01775. [DOI] [PubMed] [Google Scholar]
- 86.Sugitani I, Fujimoto Y, Yamada K. Association between serum thyrotropin concentration and growth of asymptomatic papillary thyroid microcarcinoma. World J Surg. 2014;38:673–8. doi: 10.1007/s00268-013-2335-8. [DOI] [PubMed] [Google Scholar]
- 87.Ito Y, Miyauchi A. A therapeutic strategy for incidentally detected papillary microcarcinoma of the thyroid. Nat Clin Pract Endocrinol Metab. 2007;3:240–8. doi: 10.1038/ncpendmet0428. [DOI] [PubMed] [Google Scholar]
- 88.Park CS, Kim SH, Jung SL, Kang BJ, Kim JY, Choi JJ, et al. Observer variability in the sonographic evaluation of thyroid nodules. J Clin Ultrasound. 2010;38:287–93. doi: 10.1002/jcu.20689. [DOI] [PubMed] [Google Scholar]
- 89.Ito Y, Miyauchi A, Kudo T, Higashiyama T, Masuoka H, Kihara M, et al. Kinetic analysis of growth activity in enlarging papillary thyroid microcarcinomas. Thyroid. 2019;29:1765–73. doi: 10.1089/thy.2019.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. 2017;6:225–37. doi: 10.1159/000478927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Loncar I, van Dijk SP, Metman MJ, Lin JF, Kruijff S, Peeters RP, et al. Active surveillance for papillary thyroid microcarcinoma in a population with restrictive diagnostic workup strategies. Thyroid. 2021;31:1219–25. doi: 10.1089/thy.2020.0845. [DOI] [PubMed] [Google Scholar]
- 92.Do Cao C, Haissaguerre M, Lussey-Lepoutre C, Donatini G, Raverot V, Russ G. SFE-AFCE-SFMN 2022 Consensus on the management of thyroid nodules: initial work-up for thyroid nodules. Ann Endocrinol (Paris) 2022;83:380–8. doi: 10.1016/j.ando.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 93.Zhuge L, Huang Z, Cai H, Wang S, Niu L, Li Z. The optimal age threshold for stratifying the risks of disease progression in patients with highly suspicious sub-centimeter thyroid nodules. Ann Surg Oncol. 2023;30:5463–9. doi: 10.1245/s10434-023-13497-1. [DOI] [PubMed] [Google Scholar]