Abstract
Erdheim-Chester disease (ECD) is a rare histiocytic disease that affects multiple systems in the body. While it typically targets long bones, cardiovascular structures, the retroperitoneum, and the central nervous system, reports of tendon and skeletal muscle involvement are scarce. This review presents 2 cases: a case of ECD involving the left Achilles tendon and left abductor hallucis, as well as an unusual manifestation of ECD in the thigh musculature. In Case 1, studies involved a 39-year-old man who initially presented with bone and pituitary involvement. An order for 18F-FDG PET/CT imaging was placed by marked swelling in the patient's left ankle and observed soft tissue fullness on foot radiographs, which revealed a soft tissue mass involving the left Achilles tendon, which arose along the tendon-muscle junction and involved the left abductor hallucis muscle. In Case 2, studies involved a 41-year-old man who initially presented with involvement of the cardiovascular system and retroperitoneum. 18F-FDG PET/CT scan showed an infiltrative right atrial mass and hypermetabolic lesion in the left external obturator muscle, extending to the left pectineus and right quadratus femoris muscle. Involvement of the Achilles tendon and skeletal muscle involvement, including left abductor hallucis muscle and medial thigh muscles, is one of the rare manifestations of ECD. Diagnostic delays were frequent due to the condition's rarity and nonspecific multisystemic symptoms. This should be considered in patients who present with myositis, tendinopathy, and bone pain and have other unexplained multisystemic problems.
Keywords: Erdheim-Chester disease, Non-Langerhans cell histiocytosis, Achilles tendon, [18F] FDG PET scan, BRAF proto-oncogene, Muscle involvement
Introduction
Erdheim-Chester disease (ECD) is a rare disorder that includes a group of conditions called histiocytosis, characterized by the abnormal accumulation of histiocytes in various tissues and organs of the body [1]. The typical age of ECD diagnosis ranges from 45 to 60 years, with a slight predominance in males [2]. In ECD, CD68 and CD163 are expressed by histiocytic cells, but not S100 protein or CD1a, as opposed to Langerhans cell histiocytosis (LCH), in which histiocytes express S100 and CD1a [3]. Additionally, an activating mutation of the proto-oncogene BRAF, specifically the V600E mutation, is a common genetic alteration found in more than one-half of ECD patients [4]. The clinical spectrum of ECD is broad and typically involves the endocrine, cardiovascular, and central nervous systems. ECD patients also show retroperitoneum, renal, and respiratory involvements [5]. While laboratory results generally include an elevated erythrocyte sedimentation rate and elevated C-reactive protein levels, which are nonspecific and nondiagnostic, imaging is typically helpful for diagnosis. Common CT abnormalities include “coated aorta,” as well as, “hairy kidneys,” the latter corresponds to histiocytic infiltration into the perirenal fascia [6,7]. Clinical outcome is influenced by the extent and location of the disease; it has been shown that cardiac and central nervous system involvement are associated with poorer prognosis [8]. A literature search revealed no more than one case report on tendon involvement [9]. Moreover, only a few reports are available on skeletal muscle involvement in patients with ECD [10], [11], [12]–13].
We present an ECD patient with left Achilles tendon and left abductor hallucis involvement. We also review one ECD case with multiorgan ECD involvement with thigh musculature involvement.
Case presentations
Case 1
A 39-year-old male with no significant medical history presented with severe bilateral lower extremities pain. At the same time, he was suffering from flu like symptoms, excessive thirst, and increase urine volume. Evaluations revealed pituitary involvement which caused endocrine dysfunction. Imaging studies, lab studies, and biopsies of his distal femur were performed and there was evidence of osteosclerosis of the bilateral femurs, tibiae, and radii on radiographs.
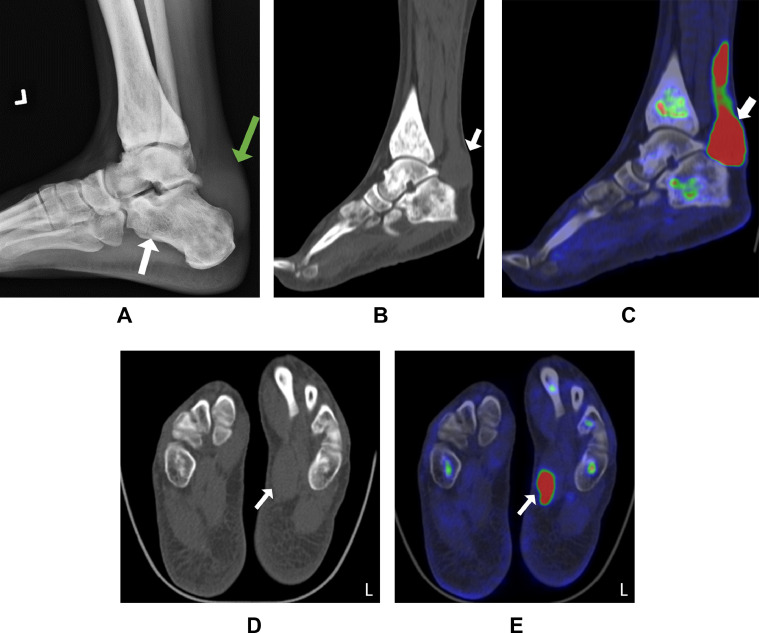
Due to his left ankle's significant swelling and evidence of soft tissue fullness on the radiographs of his foot, a PET scan was ordered (Fig. 1). His 18F-FDG PET/CT scan, after 6 hours of fasting, revealed soft tissue mass surrounding the left calcaneus and posterior ankle and increased tracer uptake along the Achilles tendon (Fig. 1). The left abductor hallucis muscle showed uptake on an 18F-FDG PET/CT scan (Fig. 1).
Fig. 1.
Representative radiograph and 18F-FDG PET/CT scan of left ankle and foot soft tissue involvement in a 39-year-old male. (A) Lateral plain radiograph showing soft tissue fullness above the calcaneus (green arrow) and osteosclerosis in the hindfoot (white arrow). (B, C) 18F-FDG PET/CT scan showing an avid soft tissue mass superior to the left calcaneus and along the Achilles tendon (white arrow). (D, E) 18F-FDG PET/CT scan showing increased uptake in the left abductor hallucis muscle (white arrow).
The presence of many foamy histiocytes was found in the microscopic analysis of the distal femur biopsy. Immunohistochemistry revealed that these histiocytes were positive for CD68 and negative for S-100 protein. The diagnosis of ECD was confirmed after reviewing the pathology of non-LCH and clinical features such as bone and pituitary involvement. Following the diagnosis, the patient was begun on treatment with 3 million units of interferon 3 times a week.
Case 2
A 41-year-old male with controlled myasthenia gravis presented with bilateral ptosis, extraocular muscular weakness, and diplopia, which worsened after starting prednisone. Periorbital masses were noted, and sinus pathology revealed histiocytes, but this was not further characterized.
After suffering from these conditions for 15 years, he was admitted to the hospital due to fractures in his left knee and elbow following a ground-level fall. A radiograph revealed inhomogeneous intramedullary osteosclerosis of his bones, indicating he was suffering from bone disease at the time. There was no definite diagnosis at the time.
Ten years later, he arrived in an emergency room with chest pain, shortness of breath, dizziness, and headache. After fasting for 6 hours, an 18F-FDG PET/CT scan showed an infiltrative right atrial mass with mild to moderate FDG uptake spreading to the subcarinal area and lymph nodes in the right paratracheal, paraesophageal, and right and left hilar regions.
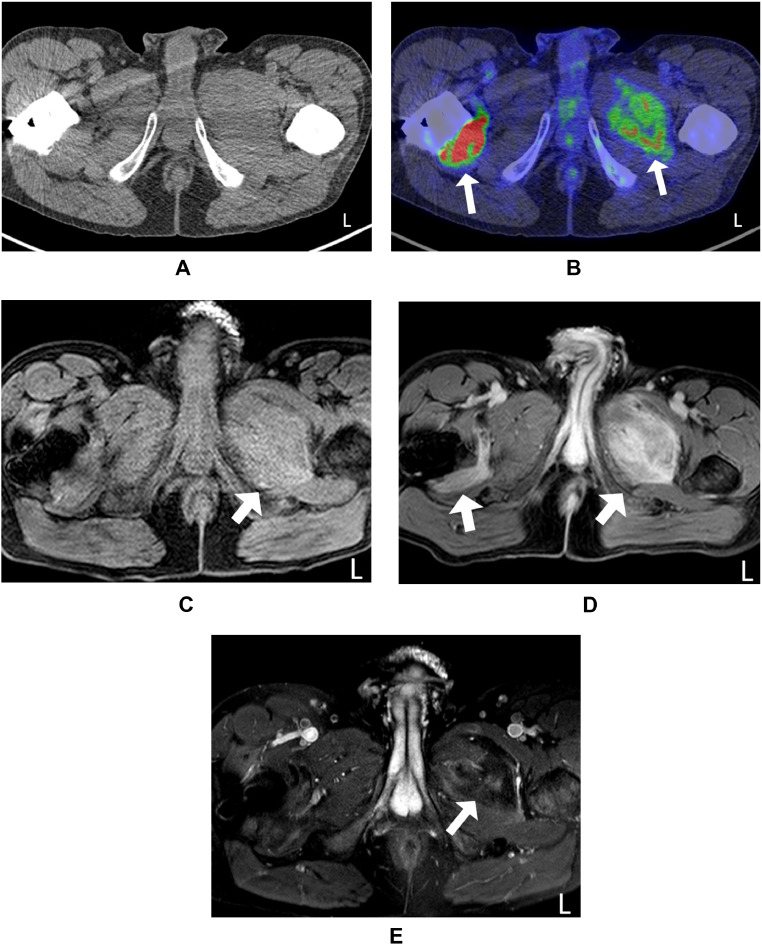
18F-FDG PET/CT scan of the lower extremities revealed a hypermetabolic destructive lesion in the left external obturator muscle with extension to the left pectineus muscle and right quadratus femoris muscle (Fig. 2).
Fig. 2.
(A, B) Representative 18F-FDG PET/CT scan and MRI of the medial thigh muscle involvement in a 41-year-old male. 18F-FDG PET/CT scan showing increased uptake in the left external obturator muscle with extension to the left pectineus muscle and right quadratus femoris muscle (arrow). (E) Axial subtraction contrast-enhanced T1-W image showing low enhancement (arrow), (C) Pre-contrast T1-W image showing increased signal intensity (arrow), (D) Post contrast T1-W image showing high signal intensity (arrow) in bilateral medial thigh muscles.
The proximal left femur's bone samples exhibited discrete areas of histiocytic infiltration in the bone marrow. Bone marrow biopsy facilitated the diagnosis of ECD. He was started on 3 million units of alpha-interferon after the ECD diagnosis.
Discussion
ECD is the “L” category histiocytosis neoplasm that, like Langerhans, features activating MAP kinase mutations. ECD commonly affects the long bones and soft tissues. However, this multisystemic disease can also involve the muscles and tendons in rare cases.
To our knowledge, only one previous case of ECD with tendon involvement has been reported [9]. In a previous report by Nadjiri et al., a 36-year-old man had significant swelling at the posterior aspect of both ankles, with histological examination revealing foamy histiocytes and Touton-type giant cells in the biopsy of his Achilles' tendons [9]. In comparison, the patient in Case 1 presented with bone pain in the areas around the knees and ankles, along with redness and swelling of the left ankle. Our case study is the first report to show the abductor hallucis in addition to Achilles tendon involvement. Additionally, our case study is the first to show tendon involvement on 18F-FDG PET/CT scan in ECD, while the reported case by Nadjiri et al. only included the MRI and bone scintigraphy.
On the other hand, the patient reported in the above-mentioned study, involvement was verified through biopsy of the Achilles tendon, whereas our case lacks a pathology report [9]. A few prior studies showed muscular involvement determined by the pathology report and imaging findings, including MRI and PET, scans [10,11]. Our study demonstrated, for the first time, evidence of muscle involvement in ECD using 18F-FDG PET/CT, revealing the presence of destructive hypermetabolic lesions in a bilateral pattern. Tan et al. presented the first case report of muscle involvement in Erdheim-Chester, accompanied by extensive visceral disease. The diagnosis of infiltrative ECD was confirmed by biopsy of the rectus abdominis [11]. In Case 2, the patient presented with involvement of the right quadriceps femoris muscle, characterized by a lymphogranuloma, and showed obvious enhancement on gadolinium-enhanced T1-weighted image [10].
18F-FDG PET/CT scan is a popular technique in oncologic imaging. Furthermore, FDG accumulates in inflammatory lesions, including inflammatory cells that utilize glucose [14]. 18F-FDG PET scan detects systemic inflammatory disorders such as rheumatoid arthritis, vasculitis, and polymyalgia rheumatica [15], [16]–17]. 18F-FDG PET has been shown to detect inflammatory muscle lesions in myositis. A quick and accurate method to evaluate myositis is preferred for the successful clinical application of 18F-FDG PET/CT. Therefore, after prolonged fasting for at least 5 hours, 18F-FDG PET/CT was useful for ECD restaging at cardiac and soft tissue levels [18]; both our patients were fast for 6 hours before their 18F-FDG PET/CT scan. Also, previous reports showed that 18F-FDG PET/CT scan has proven helpful in differentiating various conditions, including ECD, from diseases like Langerhans histiocytosis, Rosai–Dorfman Disease, IgG4-related disease, idiopathic retroperitoneal fibrosis, and sarcoidosis [19]. Furthermore, there are a few reports that compared functional imaging versus anatomical imaging [20], [21]–22]. For instance, in a study by Arnaud et al., sensitivity and specificity analyses compared 18F-FDG PET/CT scan to CT or MRI, revealing nearly 100% specificity but variable sensitivity across organ systems. Moreover, 18F-FDG PET/CT scan helps choose the best site for biopsy [23]. Additionally, previous observations suggested that 18F-FDG PET/CT scan avidity may precede radiographic evidence of sclerosis in ECD, warranting exploration of whether avidity precedes changes in other imaging modalities in future studies [24,25].
Conclusion
ECD can present with rare manifestations, including Achilles tendon and thigh muscle involvement. Due to its rarity and nonspecific multisystemic manifestations, there is often a diagnostic delay. Musculotendinous involvement should be considered in people with suspected or known ECD who show signs and symptoms of muscle disease or tendinopathies. 18F-FDG PET/CT scan results may aid in detecting the diagnosis of rare manifestations, including Achilles tendon and thigh muscle involvement, which may be a sign of ECD disease, even though evaluating the effectiveness of functional imaging may be an important place to begin further research into the pathophysiology of disease in muscle and tendon.
Approvals and Disclosures
Our institutional review board approved study was compliant with Health Insurance Portability and Accountability Act (HIPPA) and all patients enrolled in the study signed the written informed consent. We have no financial disclosures pertaining to this article.
Patient consent
Written informed consent was obtained for publication from the patient(s) or their legal representative(s)/guardian(s).
Footnotes
Acknowledgments: This work was supported by the Intramural Research programs of the National Human Genome Research Institute, the National Heart, Lung and Blood Institute, the Center for Cancer Research-National Cancer Institute and the National Institutes of Health Clinical Center, Bethesda, Maryland, USA. The authors thank Yolanda L. Jones, National Institutes of Health Library Editing Services, for editing assistance.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Kevin O'Brien, Email: obrienke@mail.nih.gov.
Ashkan A. Malayeri, Email: ashkan.malayeri@nih.gov.
References
- 1.Estrada-Veras JI, O'Brien KJ, Boyd LC, Dave RH, Durham B, Xi L. The clinical spectrum of Erdheim-Chester disease: an observational cohort study. Blood Adv. 2017;1(6):357–366. doi: 10.1182/bloodadvances.2016001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa I, Costa FAS, Bittar CS, Rizk SI, Abdo ANR, Siqueira SAC, et al. Cardiac tamponade as the first manifestation of Erdheim-Chester disease. JACC CardioOncology. 2020;2(2):324–328. doi: 10.1016/j.jaccao.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal G, Heaney ML, Collin M, Cohen-Aubart F, Vaglio A, Durham BH, et al. Erdheim-Chester disease: consensus recommendations for evaluation, diagnosis, and treatment in the molecular era. Blood. 2020;135(22):1929–1945. doi: 10.1182/blood.2019003507. [DOI] [PubMed] [Google Scholar]
- 4.Haroche J, Charlotte F, Arnaud L, von Deimling A, Hélias-Rodzewicz Z, Hervier B, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120(13):2700–2703. doi: 10.1182/blood-2012-05-430140. [DOI] [PubMed] [Google Scholar]
- 5.Goyal G, Young JR, Koster MJ, Tobin WO, Vassallo R, Ryu JH, et al. The Mayo Clinic Histiocytosis Working Group consensus statement for the diagnosis and evaluation of adult patients with histiocytic neoplasms: Erdheim-Chester disease, langerhans cell histiocytosis, and rosai-dorfman disease. Mayo Clin Proc. 2019;94(10):2054–2071. doi: 10.1016/j.mayocp.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Mirmomen SM, Sirajuddin A, Nikpanah M, Symons R, Paschall AK, Papageorgiou I, et al. Thoracic involvement in Erdheim-Chester disease: computed tomography imaging findings and their association with the BRAF(V600E) mutation. Eur Radiol. 2018;28(11):4635–4642. doi: 10.1007/s00330-018-5421-3. [DOI] [PubMed] [Google Scholar]
- 7.Nikpanah M, Kim L, Mirmomen SM, Symons R, Papageorgiou I, Gahl WA, et al. Abdominal involvement in Erdheim-Chester disease (ECD): MRI and CT imaging findings and their association with BRAF(V600E) mutation. Eur Radiol. 2018;28(9):3751–3759. doi: 10.1007/s00330-018-5326-1. [DOI] [PubMed] [Google Scholar]
- 8.Cohen Aubart F, Idbaih A, Galanaud D, Law-Ye B, Emile JF, Charlotte F, et al. Central nervous system involvement in Erdheim-Chester disease: an observational cohort study. Neurology. 2020;95(20):e2746–e2e54. doi: 10.1212/WNL.0000000000010748. [DOI] [PubMed] [Google Scholar]
- 9.Nadjiri J, Woertler K, Specht K, Harrasser N, Toepfer A. Erdheim-Chester disease with bilateral Achilles tendon involvement. Skeletal Radiol. 2016;45(10):1437–1442. doi: 10.1007/s00256-016-2447-y. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto T, Mizuno K. Erdheim-Chester disease with intramuscular lipogranuloma. Skeletal Radiol. 2000;29(4):227–230. doi: 10.1007/s002560050598. [DOI] [PubMed] [Google Scholar]
- 11.Tan AP, Tan LK, Choo IH. Erdheim-Chester disease involving breast and muscle: imaging findings. Am J Roentgenol. 1995;164(5):1115–1117. doi: 10.2214/ajr.164.5.7717216. [DOI] [PubMed] [Google Scholar]
- 12.Ambrosini V, Savelli F, Merli E, Zompatori M, Nanni C, Allegri V, et al. 18F-FDG PET/CT detects muscle involvement in Erdheim-Chester disease. Clin Nucl Med. 2012;37(2):196–197. doi: 10.1097/RLU.0b013e31823e9d54. [DOI] [PubMed] [Google Scholar]
- 13.Tan AHS, Dhanda S, Jagmohan P, Singh P, Hallinan J, Quek ST. Erdheim-Chester disease: Imaging spectrum of multisystemic manifestations. Ann Acad Med, Singapore. 2022;51(11):742–744. [PubMed] [Google Scholar]
- 14.Gotthardt M, Bleeker-Rovers CP, Boerman OC, Oyen WJ. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med Technol. 2013;41(3):157–169. doi: 10.2967/jnumed.110.076232. [DOI] [PubMed] [Google Scholar]
- 15.Kubota K, Ito K, Morooka M, Minamimoto R, Miyata Y, Yamashita H, et al. FDG PET/CT for rheumatoid arthritis: basic considerations and whole-body PET/CT. Ann N Y Acad Sci. 2011;1228(1):29–38. doi: 10.1111/j.1749-6632.2011.06031.x. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs M, Briel M, Daikeler T, Walker UA, Rasch H, Berg S, et al. The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis. Eur J Nucl Med Mol Imaging. 2012;39(2):344–353. doi: 10.1007/s00259-011-1967-x. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita H, Kubota K, Takahashi Y, Minaminoto R, Morooka M, Ito K, et al. Whole-body fluorodeoxyglucose positron emission tomography/computed tomography in patients with active polymyalgia rheumatica: evidence for distinctive bursitis and large-vessel vasculitis. Mod Rheumatol. 2012;22(5):705–711. doi: 10.1007/s10165-011-0581-x. [DOI] [PubMed] [Google Scholar]
- 18.Owada T, Maezawa R, Kurasawa K, Okada H, Arai S, Fukuda T. Detection of inflammatory lesions by 18F-FDG PET in patients with polymyositis and dermatomyositis. J Rheumatol. 2012;39(8):1659–1665. doi: 10.3899/jrheum.111597. [DOI] [PubMed] [Google Scholar]
- 19.Kumar P, Singh A, Gamanagatti S, Kumar S, Chandrashekhara SH. Imaging findings in Erdheim-Chester disease: what every radiologist needs to know. Pol J Radiol. 2018;83:e54–e62. doi: 10.5114/pjr.2018.73290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirchner J, Hatzoglou V, Buthorn JB, Bossert D, Sigler AM, Reiner AS, et al. 18F-FDG PET/CT versus anatomic imaging for evaluating disease extent and clinical trial eligibility in Erdheim-Chester disease: results from 50 patients in a registry study. Eur J Nucl Med Mol Imaging. 2021;48(4):1154–1165. doi: 10.1007/s00259-020-05047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnaud L, Malek Z, Archambaud F, Kas A, Toledano D, Drier A, et al. 18F-fluorodeoxyglucose-positron emission tomography scanning is more useful in followup than in the initial assessment of patients with Erdheim-Chester disease. Arthritis Rheum. 2009;60(10):3128–3138. doi: 10.1002/art.24848. [DOI] [PubMed] [Google Scholar]
- 22.Nikpanah M, Dehghani Firouzabadi F, Farhadi F, Mirmomen SM, Ahlman MA, Huda F. Skeletal involvement in Erdheim-Chester disease: multimodality imaging features and association with the BRAF(V600E) mutation. Clin Imaging. 2024;106 doi: 10.1016/j.clinimag.2023.110067. [DOI] [PubMed] [Google Scholar]
- 23.Jois B, Ananthasivan R, Rawat P, Rakshit S. Role of (18) F-FDG PET/CT in Erdheim-Chester disease in the era of multimodality imaging. Indian J Radiol Imaging. 2021;31(3):729–734. doi: 10.1055/s-0041-1736164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White TV, Silvester NC, Otero HJ. Non-sclerotic bone involvement in Erdheim-Chester: PET/CT and MRI findings in a 15-year-old boy. Pediatr Radiol. 2016;46(9):1345–1349. doi: 10.1007/s00247-016-3594-y. [DOI] [PubMed] [Google Scholar]
- 25.Young JR, Johnson GB, Murphy RC, Go RS, Broski SM. 18)F-FDG PET/CT in Erdheim-Chester disease: imaging findings and potential BRAF mutation biomarker. J Nucl Med. 2018;59(5):774–779. doi: 10.2967/jnumed.117.200741. [DOI] [PubMed] [Google Scholar]