Abstract
Syphilis is caused by treponema pallidum. If untreated, or inadequately treated, during pregnancy, it can result in congenital syphilis (CS), which is classified as early and late. Early CS displays before 2 years of age. We herein describe 2 cases of early CS, whose clinical onset included liver failure, edema, organomegaly, and respiratory distress. We focus on liver, intestinal, and brain ultrasound (US) and other peculiar radiological findings. To date, there are no scientific data on intestinal and brain US findings in patients with early CS whereas data on abdominal US are scarce. Increasing knowledge about US findings in early CS could be useful to improve the diagnostic and therapeutic approach to these patients.
Keywords: Syphilis, Congenital syphilis, Neonatal ultrasound, Ultrasound, Intestinal ultrasound, Brain ultrasound
Introduction
Syphilis is caused by treponema pallidum. If untreated, or inadequately treated, during pregnancy, it can result in congenital syphilis (CS). CS is a major public health problem worldwide and is a major cause of fetal and neonatal mortality [1,2]. CS can lead to severe sequelae, including cerebral palsy, hydrocephalus, sensorineural hearing loss, and musculoskeletal deformity [3,4]. CS is classified as early and late CS. Early CS displays before 2 years of age [5], [6], [7]. Herein we describe 2 cases of early CS with a particular focus on abdominal, intestinal, and brain US findings.
Case 1 presentation
A 40-day-old Caucasian newborn was admitted to the pediatric resuscitation unit for liver failure. At first evaluation, physical examination showed hepato-splenomegaly and jaundice. Blood tests showed leukocytosis (white blood cells: 29,300/uL), anemia (hemoglobin: 7.1 g/dL), thrombocytopenia (platelets: 38,000/uL), and coagulopathy (PT 39 %, aPTT 41.9 seconds, fibrinogen 112 mg/dL, INR 1,9, AT III 8 %). Hepatic and renal functional markers were as follows: Aspartate transaminase (AST) 545 U/L, Alanine transaminase (ALT) 132 U/L, Gamma-glutamyl transferase (GGT) 220 U/L, bilirubin 13.1 mg/dL, creatinine 0.29 mg/dL, azotemia 25 mg/dL. C-reactive protein (CRP) was increased with a value of 48.5 mg/L (normal values: 0-5 mg/L) and Procalcitonin (PCT) was 6.79 ng/mL (normal values: 0-0.5 ng/mL). Further abnormal examinations were as follows: lactate dehydrogenase (LDH) 879 U/L, albumin 2.1 g/dL (normal values > 3.4), glycemia 21 mg/dL, Na 110 mEq/L, K 3.3 mEq/L. The cardiological examination showed rhythmic tones and absence of heart murmurs. Percutaneous oxygen saturation was 98% and the arterial blood pressure was 90/60 mmHg. Electrocardiogram showed cardiac sinus rhythm, with a rate of 120 beats per minute (bpm), atrioventricular conduction within limits, normo-oriented electrical axis, normal ventricular recovery, and cQT. Color Doppler echocardiography showed no significant pathological findings.
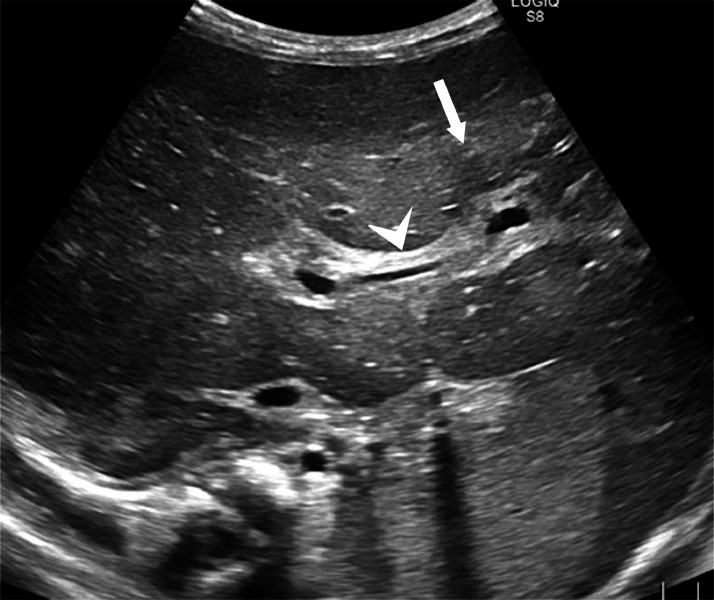
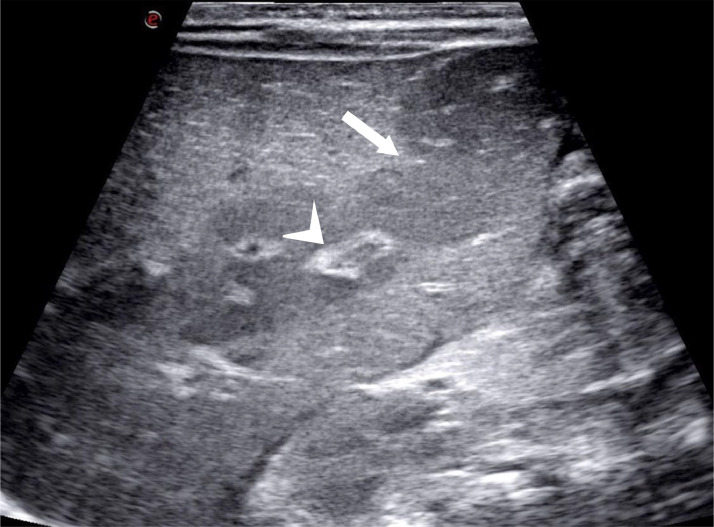
Abdominal ultrasound depicted a liver of increased volume, with the longitudinal diameter of the right lobe measuring 90 mm. The liver had wavy margins, a diffusely and modestly heterogeneous echo-structure with areas of tenuous and nuanced hypo-echogenicity distributed on a “geographical map” without nodular organization. The latter was more pronounced at the level of the hepatic hilum and the periportal level. Gallbladder was scarcely distended, with thickened walls with “unlaminated” appearance. There was no appreciable dilation of the biliary tract but there was marked thickening of the periportal-biliary spaces, especially at the level of the central branches (Fig. 1). The Portal vein was not ectatic. The evaluable portions of the pancreas (body and head) were normal. The spleen was markedly enlarged, with the longitudinal diameter measuring 93 mm. The spleen appeared slightly hyperechoic, with a homogeneous echo-structure. Kidneys were markedly hyperechoic, as well (Fig. 2).
Fig. 1.
Abdominal ultrasound showing an enlarged liver, with a diffusely and modestly heterogeneous echo structure as a “geographical map” but without nodular organization (arrow); a marked thickening of the periportal-biliary spaces (arrowhead) more represented at the level of the central branches but also well detectable in the peripheral structures.
Fig. 2.
Abdominal ultrasound showing an enlarged and hyperechoic spleen (*), with a homogeneous echo-structure; a markedly hyperechogenic kidney (arrow) and peri-splenic free fluid (arrowheads).
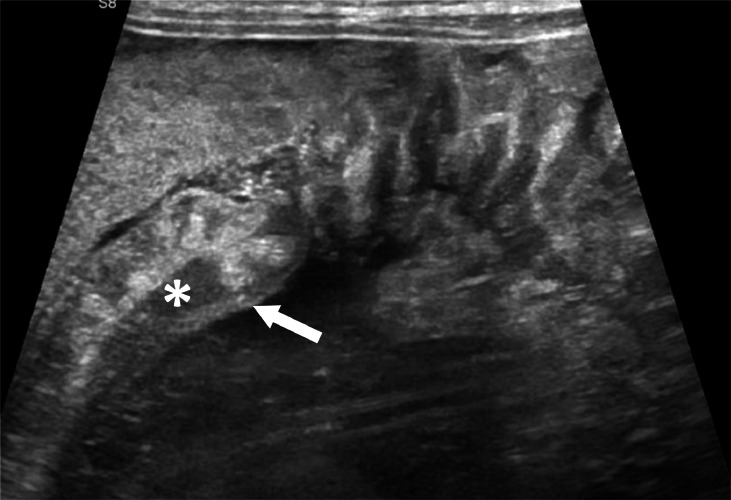
Intestinal US showed a moderately thick-walled colon, especially in its transverse and descending portions (Fig. 3). The thickening was “non-stratified” with complete loss of stratification of bowel wall and increased vascularity on color doppler. A moderate amount of free fluid in the abdomen, mainly localized at peri-hepatic and peri-splenic levels, was reported.
Fig. 3.
Intestinal US showing a moderately thick-walled colon (arrow). The thickening is ``non-stratified'' with complete loss of stratification of bowel wall (*).
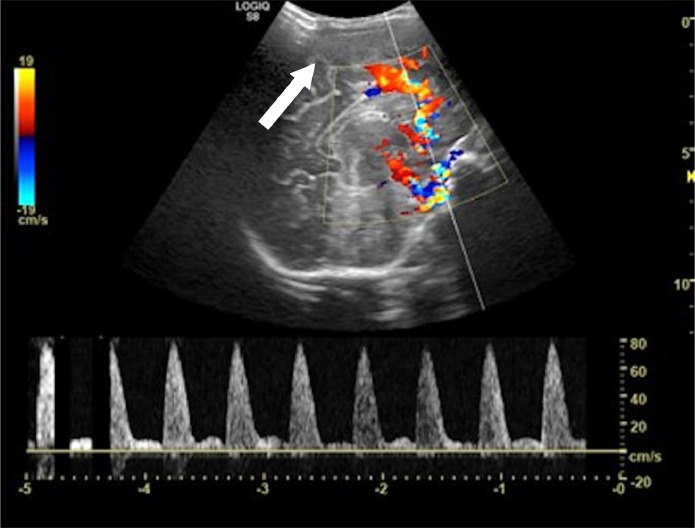
Brain US showed moderate flattening of the cerebral convolutions and thickening of the subcortical parenchyma that appeared faintly and diffusely hypoechoic. Resistance indexes (RI) increased at the level of the anterior cerebral artery (ACA) with a sampled value of 0.91 (Fig. 4).
Fig. 4.
Brain US showing a moderate flattening of the cerebral convolutions and thickening of the subcortical parenchyma that appeared faintly and diffusely hypoechoic (arrow). Resistance indexes (RI) increased at the level of the anterior cerebral artery (ACA) with a sampled value of 0.91.
The radiograph of the abdomen showed meteoric over-distension of some intestinal loops. Brain CT examination showed no significant pathological findings. Radiograph of the forearm and the arm bilaterally showed widespread periosteal apposition of the diaphysis of the upper limb. A markedly irregular periosteal reaction was appreciated at the level of the right radius with interruption of the same in the mid-distal diaphyseal area. The radiograph of the lower limbs showed the diffuse periosteal reaction of the diaphysis of the femur, tibia, and fibula bilaterally. Chest X-ray showed no significant pathological findings. The peripheral blood smear showed anisocytosis with the presence of schistocytes and dacryocytes, leukocytosis with 50% neutrophils, and the presence of activated lymphocytes; lymphocyte subpopulations showed increased NK cells and B lymphocytes. A bone marrow aspiration of the right tibia was performed, and its culture was positive for syphilis. The specific treatment for early CS was therefore promptly initiated along with supportive therapies (transfusion of blood products, plasma, and ATIII).
Case 2 presentation
A 4-month-old infant was admitted to the emergency room for respiratory distress. Physical examination showed rhinorrhea, eyelid edema, edema of the lower limbs, and organomegaly. The patient did not present sensory alterations, fever, or alterations of the bowel function. The patient was born at full term from physiological pregnancy and had presented at birth with noisy breathing and episodes of apnea. The infant had presented an episode of bronchiolitis 3 weeks before the admission. The ear, nose, and throat examination revealed anterior stenosis of the nasal pits. Laboratory tests showed neutrophilic leukocytosis (white blood cells: 17960/uL) normochromic normocytic anemia (hemoglobin: 6.1 g/dL, red blood cells: 2480000/uL), hyponatremia, hypoalbuminemia with a value of 2.4 g/dL (normal values: > 3.4) and increased inflammation indices (CRP: 80.2 mg/L). Hepatic and renal functional makers were as follows: AST 43 U/L, ALT 42 U/L, bilirubin 0.29 mg/dL, creatinine 0.19 mg/dL, azotemia 26 mg/dL.
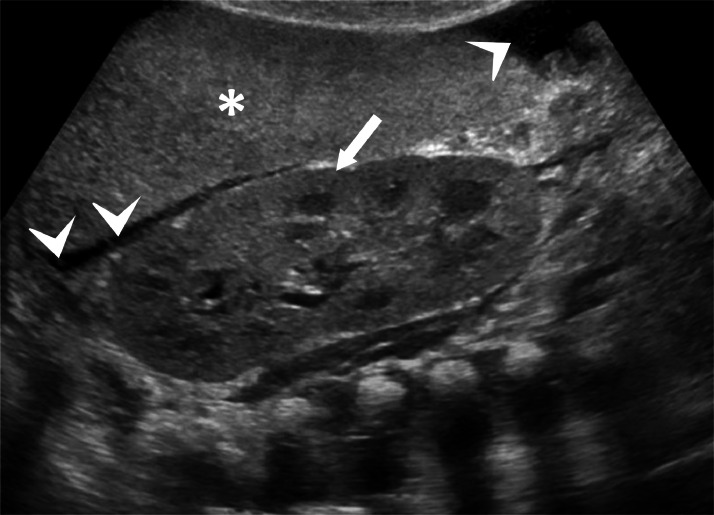
Abdominal ultrasound showed a liver of increased volume, with a diffusely uneven echo-structure like a “geographical map” with the presence of large, mildly hypoechoic areas without nodular organization (Fig. 5). There was a marked thickening of the biliary tract walls and the vascular structure walls. Kidneys showed no cortico-medullary differentiation and fluid effusion was reported into the pelvic cavity.
Fig. 5.
Abdominal ultrasound showing an enlarged liver, with a diffusely uneven echo-structure like a “geographical map” with the presence of hypoechoic and hyperechoic areas without nodular organization (arrow) and marked thickening of the biliary tract walls and the vascular structure walls (arrowhead).
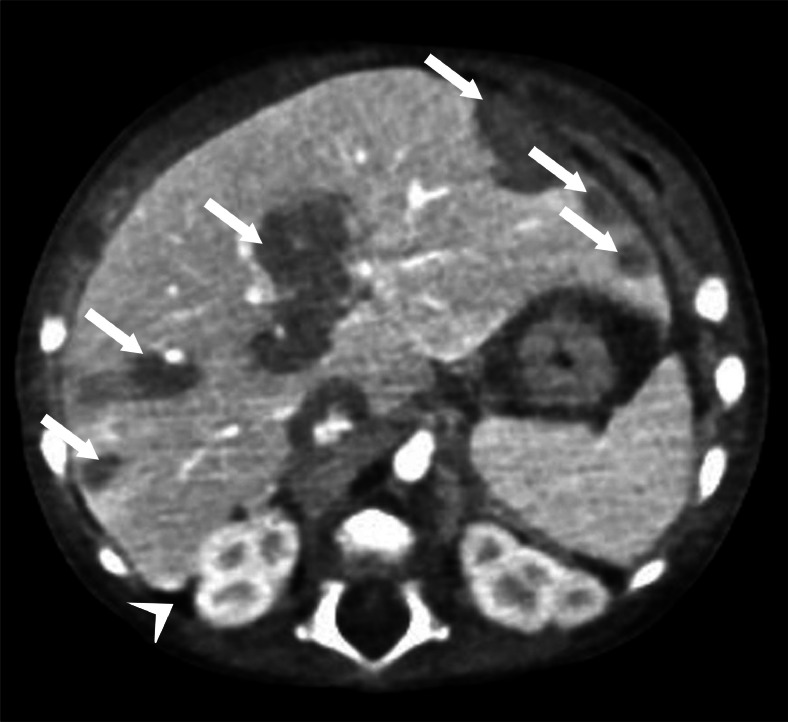
The CT examination showed an enlarged liver with diffusely inhomogeneous densitometry (Fig. 6) with multiple coarse, tenuously hypodense areas of infiltrating character, scattered throughout the parenchyma, some with subcapsular extension, others arranged along the periportal spaces and perihepatic intra-abdominal fluid effusion, peri splenic, between the intestinal loops and in the pelvic cavity. The CT study also showed some bilateral pulmonary thickenings grossly nodule and tending to confluence, mainly sub-mantle, predominantly posterior, and more evident in the lower lobes. An area of consolidation was also evident at the level of the upper and lower right lobes. Bilateral pleural effusion was also reported at the posterior mid-basal level. Moreover, a widespread periosteal reaction affected the diaphysis of long bones. The chest X-ray showed para-mediastinal opacification and inhomogeneous right inter-cleidoylar hypo-diaphania. The vaso-bronchial texture was diffusely accentuated, especially in the left para-mediastinal area. Early CS was suspected, and blood and liquor samples were promptly performed showing the following results: quantitative Rapid Plasma Reagine (RPR) of 1:256, quantitative TPHA over 1:1280, and positive RPR on liquor sample.
Fig. 6.
CT examination showing an enlarged liver with diffusely inhomogeneous densitometry with multiple coarse, tenuously hypodense areas of infiltrating character, scattered throughout the parenchyma, some with subcapsular extension, others arranged along the periportal spaces (arrows); intra-abdominal fluid effusion (arrowhead).
Discussion
Newborns with CS are in most cases asymptomatic. Nevertheless, early CS may display the following clinical manifestations: skin rash, snuffle, jaundice, hepatomegaly, splenomegaly, fever, generalized lymphadenopathy, and failure to thrive [8]. Early CS can cause Coombs-negative hemolytic anemia, thrombocytopenia, neurosyphilis, pneumonia, hepatitis, and skeletal abnormalities [8]. The abdominal US performed in our patients showed liver and spleen volume enlargement. Hepatomegaly - as well as spleen enlargement - is a common finding of fetal syphilis [9,10]. To the best of our knowledge, a similar US finding in early CS has never been reported (thickening of the periportal-biliary spaces, hepatic parenchymal and margin alterations, gallbladder's wall thickening, with an “unlaminated” appearance). Pathophysiology of hepatomegaly may be secondary to acute syphilitic hepatitis, increased extra-medullary hematopoiesis and/or hepatic congestion due to heart failure, either from myocarditis or from acute fetal anemia [11]. In our 2 cases, US findings could be due to one or more of these concomitant conditions. Of note, a retrospective cohort study of 235 women showed that US findings of fetal syphilis are strongly associated with a diagnosis of CS at delivery, but a normal US does not necessarily eliminate the possibility of CS at delivery [12]. The abdominal US of clinical cases above-described, showed hyperechogenic spleen and hyperechogenic kidneys, suggesting local suffering. Intestinal obstruction and bleeding are uncommon complications of CS and syphilitic ileitis has already been described in literature [13]. Nevertheless, we found no mention in literature of intestinal US findings of CS. Case 1 intestinal US showed a thick-walled colon, with complete loss of stratification of bowel wall and increased vascularity on color Doppler. These intestinal ultrasound findings could be due to syphilitic involvement of the colon. It is reported [14] that the periosteal reaction is the most common abnormality in early CS. Our experience confirms this data by showing widespread periosteal reaction affecting the diaphyses of long bones on CT scans of Case 2 and by showing widespread periosteal apposition of the diaphysis of the upper limb and periosteal reaction of the diaphysis of the femur, tibia, and fibula bilaterally on radiographs of Case 1. A study [11] conducted on 202 infants with early CS showed that radiologic changes in the long bones were seen in > 95 % of the babies and the most frequent were the following: wide band of decreased radiodensity in the metaphysis, saw-tooth appearance, “pared fingernail,” Wimburger's sign, fracture and single-layered periosteal reaction. It is known that signs of CS in an affected child can include meningitis and hydrocephalus. Intracranial involvement results in meningovascular and parenchymal syphilis, we could therefore observe an enhancement of the leptomeninges, that may extend along the perivascular space into the brain parenchyma and appears as an enhancing parenchymal mass [15] while inflammatory vasculitis can result in arterial infarctions [16]. Interestingly, case 1 showed no detectable changes on brain CT while trans-fontanellar brain ultrasound showed changes, such as flattening of the cerebral convolutions, thickening of the subcortical parenchyma that appeared faintly and diffusely hypoechoic and an increase in the resistance index (IR) of the anterior cerebral artery (ACA) of 0.91. These findings, which we have not found mention in the literature, could suggest an initial involvement of the central nervous system by Syphilis. Chest X-ray of Case 1 showed no significant pathological findings while chest X-ray of Case 2 showed an Opacification, a hypodiaphania and the vaso-bronchial texture was diffusely accentuated. The diagnosis of CS pneumonitis can be suspected on chest X-ray and should be included in the differential diagnosis of any baby who presents with an interstitial pattern on chest radiography [17]. The application of US to the study of newborns has seen increasing progresses over the last years but there is a lack of scientific data about intestine and brain US findings in patients with early CS. Furthermore, information on abdominal US findings in such patients are incomplete. Hence the need to increase the knowledge about US findings in early CS to improve the diagnostic and therapeutic workup of these patients.
Conclusions
In patients with suspected neonatal pathology, performing US examination of the abdomen, intestine, and brain, may - in association with a correct clinical and laboratory assessment - direct toward the correct diagnosis. To the best of our knowledge, data on intestinal, abdominal, and brain US findings in patients with early CS are lacking or inconclusive. That presents us with a new challenge: to increase the knowledge about US findings in early CS to improve the diagnostic work-up and therapeutic approach to these patients.
Patient consent
The authors declare that written, informed consent for the publication was obtained.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Cooper JM, Sánchez PJ. Congenital syphilis. Semin Perinatol. 2018;42(3):176–184. doi: 10.1053/j.semperi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Medoro AK, Sánchez PJ. Syphilis in neonates and infants. Clin Perinatol. 2021;48(2):293–309. doi: 10.1016/j.clp.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Rowe CR, Newberry DM, Jnah AJ. Congenital syphilis: a discussion of epidemiology, diagnosis, management, and nurses’ role in early identification and treatment. Adv Neonatal Care. 2018;18(6):438–445. doi: 10.1097/ANC.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 4.Arnold SR, Ford-Jones EL. Congenital syphilis: a guide to diagnosis and management. Paediatr Child Health. 2000;5(8):463–469. doi: 10.1093/pch/5.8.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bembry W, Anderson M, Nelson S. Congenital syphilis: the great pretender strikes back. A case report. Clin Pediatr (Phila) 2018;57:992–996. doi: 10.1177/0009922817738343. [DOI] [PubMed] [Google Scholar]
- 6.Dobson SR. UpToDate, Waltham; MA, USA: 2018. Congenital syphilis: clinical features and diagnosis. [Google Scholar]
- 7.Singhal P, Patel P, Marfatia YS. A case of congenital syphilis with Hutchinson’s triad. Indian J Sexually Trans Dis AIDS. 2011;32(1):34–36. doi: 10.4103/2589-0557.81252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung AKC, Leong KF, Lam JM. A case of congenital syphilis presenting with unusual skin eruptions. Case Rep Pediatr. 2018;2018:4. doi: 10.1155/2018/1761454. . 1761454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan L, Twickler MT, Sánchez PJ, Wendel GD., Jr. Fetal syphilis: correlation of sonographic findings and rabbit infectivity testing of amniotic fluid. J Ultrasound Med. 1993;2:97–101. doi: 10.7863/jum.1993.12.2.97. [DOI] [PubMed] [Google Scholar]
- 10.Hollier LM, Harstad TWSanchez PJ, Twickler DM, Wendel GD., Jr. Fetal syphilis: clinical and laboratory characteristics. Obstet Gynecol. 2001;97(6):947–953. doi: 10.1016/s0029-7844(01)01367-9. [DOI] [PubMed] [Google Scholar]
- 11.Hira SK, Bhat GJPatel JB, Din SN, Attili RV, Patel MI, et al. Early congenital syphilis: clinico-radiologic features in 202 patients. Sex Trans Dis. 1985;12(4):177–183. doi: 10.1097/00007435-198510000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Rac MWF., Bryant SN, McIntire DD, Cantey JB, Twickler DM, Wendel GD, Jr, et al. Progression of ultrasound findings of fetal syphilis after maternal treatment. Am J Obstet Gynecol. 2014;211(4):426.e1–426.e6. doi: 10.1016/j.ajog.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 13.Ajayi NA, Marven SKaschula RO, Millar A, Rode H. Intestinal ulceration, obstruction, and haemorrhage in congenital syphilis. Pediatr Surg Int. 1999;15(5–6):391–393. doi: 10.1007/s003830050608. [DOI] [PubMed] [Google Scholar]
- 14.Cremin BJ, Fisher RM. The lesions of congenital syphilis. Br J Radiol. 1970;43(509):333–341. doi: 10.1259/0007-1285-43-509-333. [DOI] [PubMed] [Google Scholar]
- 15.Barkovich AJ. 4th ed. Lippincott Williams & Wilkins; Philadelphia: 2005. Pediatric neuroradiology; pp. 801–868. [Google Scholar]
- 16.Ressler JA, Nelson M. Central nervous system infections in the pediatric population. Neuroimaging Clin North Am. 2000;10:427–443. [PubMed] [Google Scholar]
- 17.Pieper CH, van Gelderen WF, Smith J, Kirsten GF, Möhrcken S, Gie RP. Chest radiographs of neonates with respiratory failure caused by congenital syphilis. Pediatr Radiol. 1995;25(3):198–200. doi: 10.1007/BF02021534. [DOI] [PubMed] [Google Scholar]