Abstract
We herein report a case of acute neurological symptoms and a fever initially suspected of being encephalitis but later revealed to be dural arteriovenous fistula (dAVF). An 84-year-old woman had a fever and cerebral edema and was initially treated for encephalitis. A review of her magnetic resonance imaging findings revealed abnormal blood flow signals. After cerebral angiography, the patient was finally diagnosed with left transverse-sigmoid sinus dAVF. The present case showed that dAVF can also present with an acute onset and a fever, mimicking acute encephalitis. Because the treatments for encephalitis and dAVF differ greatly, the possibility of dAVF should also be considered when diagnosing encephalitis.
Keywords: acute encephalitis, central fever, dural arteriovenous fistula, MRI, transarterial embolization
Introduction
Most cases of acute encephalitis have an acute onset and present with a fever and neurological symptoms (1). Magnetic resonance imaging (MRI) often shows edematous lesions, with a high signal in images of fluid-attenuated inversion recovery (FLAIR) of the temporal lobe or limbic system (1). When the shunt blood flow of a dural arteriovenous fistula (dAVF) reflows into the superficial or deep cerebral veins, intracranial hemorrhaging, or edema can occur (2). Symptoms vary depending on the shunt site, and most non-bleeding cases develop a chronic-to-subacute course (2).
Acute encephalitis and dAVF often differ in their disease course and can generally be distinguished easily. Nevertheless, we herein report a case of acute neurological symptoms and a fever initially suspected of being encephalitis but eventually diagnosed as dAVF.
Case Report
An 84-year-old woman was admitted to the hospital because of abnormal behavior. She had no known comorbidities or history of significant head trauma, otitis media, tuberculosis, COVID-19, or other infectious diseases. One day prior to admission, she presented with the following symptoms: slower-than-usual reaction, freezing, and immobility. The following day, her symptoms persisted, along with speech and gait difficulties. She was then transported to our hospital.
Upon admission, she had a fever of 38.5°C and a Glasgow Coma Scale rating of E3V2M4. Moderate aphasia, mild left conjugate deviation, and right-dominant limb weakness were also observed. Blood test results showed a white blood cell count of 8,100 /μL and a C-reactive protein level of 0.04 mg/dL; none of the other tests were suggestive of inflammation. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen quantification was performed twice during the disease course and was negative both times. There was no pneumonia or urinary tract infection or any other apparent cause of the fever.
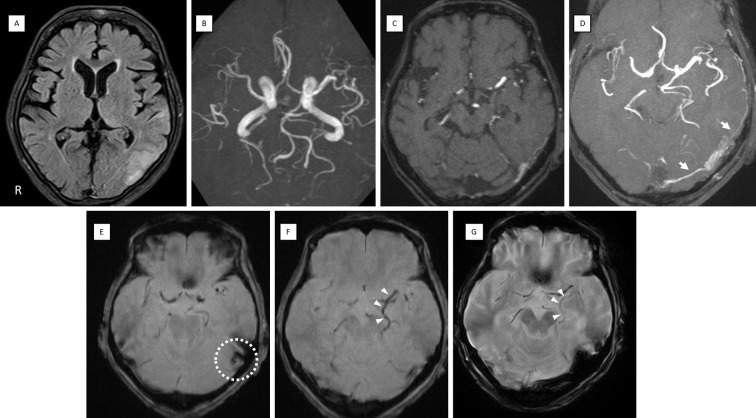
A blood coagulation test showed a mild elevation of D-dimer levels to 3.2 μg/mL. A cerebrospinal fluid (CSF) examination revealed an initial pressure of 12 cmH2O, cell count of 0 /μL, and protein level of 48 mg/dL. Electroencephalography showed occasional generalized slow waves in the theta range but no evident epileptic spikes. Brain MRI showed edematous lesions with a high FLAIR signal in the left temporal to parietal lobes (Fig. 1A). Diffusion-weighted images were normal. Three-dimensional (3D) reconstruction with maximum intensity projection (MIP) of magnetic resonance angiography (MRA) showed no evident abnormalities (Fig. 1B).
Figure 1.
MRI findings on admission. (A) FLAIR showing edema in the temporal to parietal lobes. (B) MRA MIP 3D reconstruction did not reveal any abnormal vessels. (C) MRA source image. (D) Slab MIP (10 mm thickness) of MRA demonstrating abnormal signal propagating into the TS from the MMA and OA (white arrows). T2*-weighted imaging showed a low signal in the left sigmoid sinus suggesting thrombus (E) (dot circle) and dilatation of the left BVR (F) (white arrowheads), which improved on later images after treatment (G) (white arrowheads). BVR: basal vein of Rosenthal, FLAIR: fluid-attenuated inversion recovery, MIP: maximum intensity projection, MMA: middle meningeal artery, MRA: magnetic resonance angiography, OA: occipital artery, TS: transverse sinus
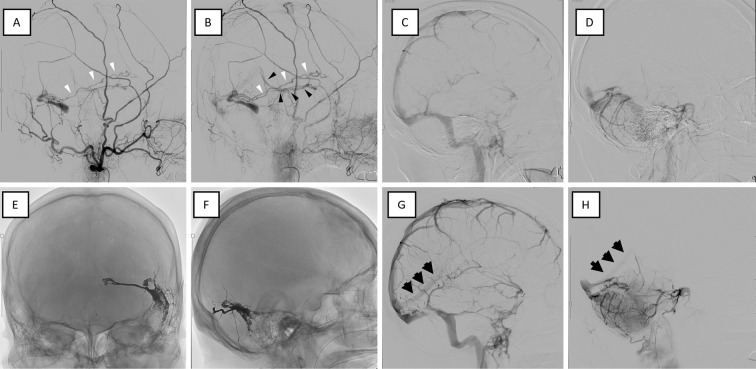
The combination of acute consciousness with fever and abnormal lesions in the temporal lobe was suggestive of acute encephalitis; treatment with acyclovir was initiated. However, a re-review of the MRI source images revealed abnormal blood flow signals. A possible shunt from the ipsilateral occipital artery (OA) and middle meningeal artery (MMA) to the transverse sinus (TS) was observed (Fig. 1C). Slab MIP images delineated the findings more clearly (Fig. 1D). T2*-weighted imaging showed no evident hemorrhaging but disclosed a low signal in the left sigmoid sinus suggesting thrombus (Fig. 1E) and a dilated left basal vein of Rosenthal (BVR) (Fig. 1F). Cerebral angiography showed the left OA (mastoid branch) and left MMA (petrosquamous branch, posterior convexity branch) feeding into the left TS and the left posterior meningeal artery feeding into the left tentorial sinus, along with shunt blood flow from the vein of Labbe into the straight sinus (StS) via the BVR (Fig. 2A, B). Right common carotid and vertebral angiographies showed no shunt blood flow; however, non-perfusion of StS with delayed cerebellar perfusion throughout and the involvement of deep cerebral venous draining insufficiency were observed (Fig. 2C, D).
Figure 2.
Cerebral angiography images before and after treatment. Angiography performed before (A-D) and after (E-H) treatment. (A, B) Lateral view of the left external carotid angiography in the early (A) and late (B) phases. Left external carotid angiography showing TSS dAVF with an isolated sinus and shunt blood flow backward from the vein of Labbe (A, B: white arrowheads) to the BVR (B: black arrowheads). (C, D) Before treatment, the StS was not visible in the venous phase on right internal carotid angiography (C) or vertebrobasilar angiography (D), indicating impaired perfusion of the deep veins. (E, F) The isolated sinus was adequately embolized by TAE with ethylene vinyl alcohol (EVOH) polymer (Onyx®, Medtronic, Irvine, USA). (G, H) After treatment, the StS blood flow normalized (black arrows). BVR: basal vein of Rosenthal, StS: straight sinus, TAE: transarterial embolization, TSS dAVF: transverse-sigmoid sinus dural arteriovenous fistula
The patient was eventually diagnosed with left transverse-sigmoid sinus (TSS) dAVF (isolated sinus, Borden type 3). Transarterial embolization (TAE) with ethylene vinyl alcohol (EVOH) polymer (OnyxⓇ; Medtronic, Irvine, USA) was performed via the posterior convexity branch of MMA. Shunt blood flow completely resolved, and StS perfusion was normalized (Fig. 2E-H). Postoperative T2*-weighted imaging showed that the dilated BVR had been normalized (Fig. 1G).
On postoperative day 2, the patient's symptoms rapidly improved; the patient was able to walk unassisted and was discharged from the hospital. Herpes simplex virus polymerase chain reaction (PCR) test of the CSF was later found to be negative. The patient had a fever of 38.5°C at the time of admission and only then was first noticed to have a fever. Her fever remained in the 37-38°C range after admission, but TAE was performed on the fourth day of admission, and the fever spontaneously resolved on the second day postoperatively. Acyclovir was administered only on the first day of admission, and no other antibiotics or antivirals were used.
Consent for the publication of these findings was obtained from the patient and her family.
Discussion
We reported a case of dAVF that resembled encephalitis. It is considered that three findings contributed to the mimicry: the fever, subacute deterioration of the cognitive function, and edematous changes in the brain on MRI with a lack of MRA abnormalities at first presentation.
Our patient had a fever that resolved after treatment. Patients with dAVF in whom abnormal shunts develop are generally considered not to have pyrexia. Clinical features of dAVF cases associated with a fever have yet to be elucidated; however, there are some reports describing the association between a fever and cerebral vein thrombosis causing deep cerebral venous perfusion defects, such as dAVF. Silburn et al. reported three cases of deep cerebral vein thrombosis presenting as an encephalitic illness accompanied by a fever (3). The mechanism by which a fever develops remains unclear; however, deep cerebral venous perfusion abnormalities involving the draining of hypothalamus can be considered partly responsible. The hypothalamus - center of body temperature regulation - is mainly drained by the premammillary veins, which drain and flow to the BVR (4,5). In the present case, the draining system pertaining to the BVR was disturbed. After the normalization of the disturbed venous flow, the body temperature normalized. There were no evident imaging findings indicating hypothalamic edema or perfusion defects. A “central fever” is still a diagnosis of exclusion. Although there were no abnormalities that could cause a fever, such as infection, according to our examination in the present case, it is still difficult to exclude other possible etiologies that might cause a fever completely. However, temporary dysfunction of the hypothalamus due to a disturbed draining system may have led to the “central fever” in this case, which normalized after treatment.
Our patient presented with a subacute deterioration of the cognitive function. The common clinical course of viral or autoimmune encephalitis is either acute or subacute (1). The clinical presentations of viral or autoimmune encephalitis vary among etiologies; however, the subacute deterioration of the cognitive function is a hallmark of encephalitis (1). In contrast, the clinical presentations of dAVF depend on the shunt pattern, and the common presentation of dAVF has been reported to be rather chronic. To our knowledge, there have been five cases of dAVF other than our own that could be mistaken for inflammatory disease (6-10) (Table). No fever was documented in any of the five cases. Of the six total cases experienced thus far, five showed a rapid clinical course, and five improved with the appropriate diagnosis and treatment. Our patient probably had TSS dAVF originally. However, the sigmoid sinus might have finally occluded and become an isolated sinus, and the blood suddenly flowed from the vein of Labbe into the deep veins, resulting in the sudden worsening of symptoms, mimicking encephalitis. It is important to note that the possibility of dAVF cannot be ruled out, even in the acute course of the disease.
Table.
Cases of DAVF That Were Confused with Encephalitis.
| Reference | Age/ sex |
From onset to hospitalization | Fever | Neurological symptoms | FLAIR high signal | Initial therapy | Final diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| (9) | 51/M | 5 weeks (acute exacerbation 2 days) | Normal | Somnolence, memory impairment, and gait instability | Bilateral thalamus | No mention | Tentorial dAVF | TAE | Improvement |
| (7) | 25/F | 1 month | No mention | Limb weakness, dysphagia, and dyspnea | Pontomedullary junction | Various immunomodulating therapies | Tentorial dAVF | TAE | Mild improvements |
| (8) | 46/M | 2 days | Afebrile | Spatial disorientation and apathy | Bilateral thalamus | Acyclovir,steroid pulse, IVIg | Tentorial dAVF | No mention | Improvement |
| (6) | 80/F | 2 days | No mention | Mild confusion, dysarthria, and left hemiparesis | Pons | High dose steroid | Cavernous sinus dAVF | Attempted TAE/TVE but failed | Severe left paralysis remains |
| (10) | 68/M | 0 day | 37.0°C | Status epilepticus | Left medial temporal lobe | Steroid | Anterior cranial fossa dAVF | Craniotomy | Improvement |
| Our case | 84/F | 2 days | 38.5°C | Freezing, and immobility, and aphasia | Left temporal to parietal lobes | Acyclovir | Transverse-sigmoid sinus dAVF | TAE | Improvement |
dAVF: dural arteriovenous fistula, FLAIR: fluid-attenuated inversion recovery, IVIg: intravenous immunoglobulin, TAE: transarterial embolization, TVE: transvenous embolization
The gold standard for making a dAVF diagnosis remains cerebral angiography (2). While dAVF can be diagnosed using MRA, the diagnostic sensitivity of MRI for detecting intracranial dAVF has been reported to be 58-83%, and the underdiagnosis of dAVF can occur only by MRA (11). MIP is a common reconstruction technique that depicts the maximum intensity on the projection plane along the projection path from any viewing angle of the 3D data. While MIP 3D reconstruction images provide a whole image of the brain vessels at any angle, it is often not sufficient to recognize subtle vascular abnormalities, as in our case. In addition, important findings may be lost in the process of removing structures other than the main artery, which is generally the process involved in 3D reconstruction. Slab MIP, which finely shifts a slab with a certain thickness in parallel, is an effective method for depicting fine blood vessels (12,13). Therefore, it is important to observe the MRA source and slab MIP images along with MIP 3D reconstructed images when dAVF is suspected. SWI and T2*-weighted images are useful for diagnosing intracranial dAVF (14); in the present case, T2*-weighted images showed expansion of the BVR on the lesion side. Although arterial spin labeling (ASL) has been reported to be useful for diagnosing dAVF (10,15), it was not performed in this case. The most important factor to account for in order to avoid missing dAVF is the consideration of dAVF as a differential diagnosis when a cerebral edematous lesion is detected. Subsequently, the aforementioned imaging findings should be reviewed, and cerebral angiography should be performed without hesitation in suspicious cases.
In the present case, there was no CSF pleocytosis. According to a study that analyzed a total of 23 patients with PCR-proven herpes simplex virus, six patients lacked CSF pleocytosis (16). Acute encephalitis cannot be ruled out, even with a normal CSF cell count (1). In the present case, various examinations and the clinical course finally ruled out a fever due to encephalitis or other causes.
Conclusions
We herein report a case of TSS dAVF that required careful distinction from encephalitis. dAVF can have an acute onset and be accompanied by a fever. Because the treatment for encephalitis and dAVF differs, it is important to be aware that dAVF can mimic the clinical features of encephalitis, as observed in this case. Furthermore, MRA resource images and slab MIP images should be carefully observed when edematous lesions are noted. Cerebral angiography should be performed without hesitation.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Ellul M, Solomon T. Acute encephalitis - diagnosis and management. Clin Med (Lond) 18: 155-159, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chaichana KL, Coon AL, Tamargo RJ, Huang J. Dural arteriovenous fistulas: epidemiology and clinical presentation. Neurosurg Clin N Am 23: 7-13, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Silburn PA, Sandstrom PA, Staples C, Mowat P, Boyle RS. Deep cerebral venous thrombosis presenting as an encephalitic illness. Postgrad Med J 72: 355-357, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salamon G, Huang YP, Michotey P, et al. Basal cerebral vein. In: Radiologic Anatomy of the Brain. Salamon G, Huang YP, Eds. Springer, Berlin, 1976: 127-172. [Google Scholar]
- 5. Gutierrez S, Iwanaga J, Dumont AS, Tubbs RS. Direct drainage of the basal vein of Rosenthal into the superior petrosal sinus: a literature review. Anat Cell Biol 53: 379-384, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oka Y, Komatsu K, Abe S, Yoshimoto N, Taki J, Matsumoto S. Acute brainstem dysfunction caused by cavernous sinus dural arteriovenous fistula. Case Rep Neurol Med 2020: 2630959, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen PM, Chen MM, McDonald M, et al. Cranial dural arteriovenous fistula. Stroke 49: e332-e334, 2018. [DOI] [PubMed] [Google Scholar]
- 8. Cheng ZJ, Tu JL, He JL, Li J. Rapidly progressive cognitive impairment caused by intracranial dural arteriovenous fistulas (DAVFs): a case report. Neurol Sci 39: 1293-1296, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Borja MJ, Schaefer PW, Boulter DJ. Case of the season: dural arteriovenous fistula mimicking a bithalamic neoplasm or viral encephalitis. Semin Roentgenol 49: 4-9, 2014. [DOI] [PubMed] [Google Scholar]
- 10. Tamura M, Saito K, Irisa G, et al. [A case of anterior cranial fossa dural arteriovenous fistula involving uncontrolled seizures diagnosed based on characteristic findings on arterial spin-labeling imaging]. No Shinkei Geka 48: 547-552, 2020(in Japanese). [DOI] [PubMed] [Google Scholar]
- 11. Lin YH, Wang YF, Liu HM, Lee CW, Chen YF, Hsieh HJ. Diagnostic accuracy of CTA and MRI/MRA in the evaluation of the cortical venous reflux in the intracranial dural arteriovenous fistula DAVF. Neuroradiology 60: 7-15, 2018. [DOI] [PubMed] [Google Scholar]
- 12. Sasaki M. High-resolution cerebrovascular imaging: current status. Jpn J Neurosurg 26: 476-479, 2017. [Google Scholar]
- 13. Ertl-Wagner BB, Bruening R, Blume J, et al. Relative value of sliding-thin-slab multiplanar reformations and sliding-thin-slab maximum intensity projections as reformatting techniques in multisection CT angiography of the cervicocranial vessels. AJNR Am J Neuroradiol 27: 107-113, 2006. [PMC free article] [PubMed] [Google Scholar]
- 14. Noguchi K, Kuwayama N, Kubo M, et al. Intracranial dural arteriovenous fistula with retrograde cortical venous drainage: use of susceptibility-weighted imaging in combination with dynamic susceptibility contrast imaging. AJNR Am J Neuroradiol 31: 1903-1910, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amukotuwa SA, Marks MP, Zaharchuk G, et al. Arterial spin-labeling improves detection of intracranial dural arteriovenous fistulas with MRI. AJNR Am J Neuroradiol 39: 669-677, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saraya AW, Wacharapluesadee S, Petcharat S, et al. Normocellular CSF in herpes simplex encephalitis. BMC Res Notes 9: 95, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]