Abstract
The role of NRF2 in kidney biology has received considerable interest over the past decade. NRF2 transcriptionally controls genes responsible for cellular protection against oxidative and electrophilic stress and has anti‐inflammatory functions. NRF2 is expressed throughout the kidney and plays a role in salt and water handling. In disease, animal studies show that NRF2 protects against tubulointerstitial damage and reduces interstitial fibrosis and tubular atrophy, and may slow progression of polycystic kidney disease. However, the role of NRF2 in proteinuric glomerular diseases is controversial. Although the NRF2 inducer, bardoxolone methyl (CDDO‐Me), increases glomerular filtration rate in humans, it has not been shown to slow disease progression in diabetic kidney disease and Alport syndrome. Furthermore, bardoxolone methyl was associated with negative effects on fluid retention, proteinuria, and blood pressure. Several animal studies replicate findings of worsened proteinuria and a more rapid progression of kidney disease, although considerable controversy exists. It is clear that further study is needed to better understand the effects of NRF2 in the kidney. This review summarizes the available data to clarify the promise and risks associated with targeting NRF2 activity in the kidney.
Keywords: bardoxolone methyl, chronic kidney disease, electrophiles, oxidative stress, proteinuria
1. INTRODUCTION
The nuclear factor erythroid 2‐related factor 2 (NRF2) system is an important mechanism for mitigating cellular stress. NRF2 upregulates genes that alleviate oxidative stress and detoxify electrophilic compounds that threaten cellular homeostasis (Yamamoto et al., 2018). NRF2 also plays an important role in reducing inflammation. Not surprisingly, NRF2 has been implicated in a variety of human diseases, including heart, lung, liver, and kidney diseases as well as in cancer biology (Yamamoto et al., 2018).
NRF2 is a member of the NF‐E2 family of Cap'n'Collar basic leucine zipper DNA‐binding transcription factors (Moi et al., 1994). NRF2 is restrained by its endogenous inhibitor Kelch‐like ECH‐associated protein 1 (KEAP1). Under normal conditions, KEAP1 ubiquitinates NRF2 and directs it for proteasomal degradation (Cullinan et al., 2004; Kobayashi et al., 2004; Nguyen et al., 2003; Zhang et al., 2004). These two proteins work in concert, with KEAP1 serving as a sensor for reactive oxygen species and electrophiles (Nezu, Suzuki, & Yamamoto, 2017). These modify thiol side chains of key cysteine residues on KEAP1, disrupting its interaction with NRF2 or its ability to degrade it (Yamamoto et al., 2018). NRF2 accumulates, translocates to the nucleus, and forms a heterodimer with small musculo‐aponeurotic fibrosarcoma (sMAF) transcription factors (Itoh et al., 1997). The sMAF‐NRF2 heterodimer binds to antioxidant and electrophile response elements to upregulate target genes including NAD(P)H quinone dehydrogenase 1 (NQO1), glutathione S‐transferases (GSTs), catalase (CAT), and heme oxygenase 1 (HMOX) (Figure 1). These and other upregulated genes have roles in mitigating oxidative stress, participating in detoxification pathways, and increasing glutathione synthesis, among other effects (Nezu, Suzuki, & Yamamoto, 2017). In short, the activation of NRF2 by oxidative or electrophilic stress leads to the upregulation of cellular defenses against these insults.
FIGURE 1.
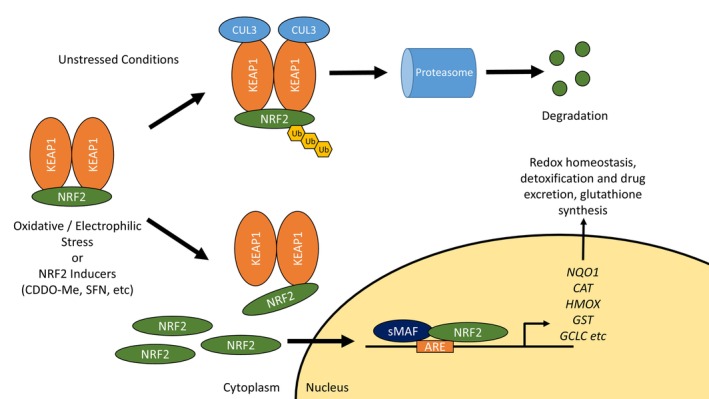
The KEAP1/NRF2 Pathway. KEAP1 is the endogenous inhibitor of NRF2, directing it for ubiquitination and subsequent degradation via the proteasome. However, in the presence of oxidative stress, electrophiles, or NRF2‐inducing agents, KEAP1 is modified and NRF2 is allowed to accumulate. Free NRF2 translocates to the nucleus and partners with sMAF transcription factors to upregulate gene expression. Many of these genes have antioxidant, detoxifying, or other cytoprotective properties. KEAP1, Kelch‐like associated protein 1; NRF2, nuclear factor 2 erythroid 2; sMAF, small musculo‐aponeurotic fibrosarcoma; ARE, antioxidant response element; NQO1, NAD(P)H quinone dehydrogenase 1; CAT, catalase; HMOX, heme oxygenase 1; GST, glutathione S‐transferases; GCLC, glutamate‐cysteine ligase catalytic subunit.
NRF2 has anti‐inflammatory effects, including negative effects on NF‐κB and gene transcription of IL‐1β and IL‐6 (van der Horst et al., 2022). NRF2 also impacts metabolism, obesity, and insulin resistance (Chartoumpekis & Kensler, 2013). In aggregate, these functions indicate a critical role for NRF2 in health and disease.
A number of chemicals have been isolated or developed to induce NRF2. These include sulforaphane, dimethyl fumarate, and the triterpenoids bardoxolone methyl (CDDO‐Me) and bardoxolone imidazole (CDDO‐Im). These act by disrupting KEAP1‐mediated NRF2 degradation (Yamamoto et al., 2018). In addition, NRF2 is regulated by glycogen synthase kinase‐3β (GSK‐3β) and β‐TrCP, the phosphatidylinositol‐3‐kinase/AKT (PI3K/AKT) pathway, extracellular signal‐regulated kinase (ERK), C‐Jun N‐terminal kinase (JNK), and PKR‐like endoplasm reticulum kinase (PERK) (Guerrero‐Hue et al., 2020).
2. NRF2 EXPRESSION IN THE KIDNEY
According to the Human Protein Atlas, NRF2 has higher expression in renal tubules compared to glomeruli under normal conditions (Uhlen et al., 2015). However, our group and others have observed glomerular upregulation during disease (Jiang et al., 2010; Jiang et al., 2014; Rush et al., 2021). Although its protein abundance is informative, NRF2 activity may be more accurately reflected in the expression of target genes such as NQO1. In the Human Protein Atlas, NQO1 is found in both glomeruli and distal tubules (Uhlen et al., 2015).
In mice, NRF2 protein is present in proximal (Cuevas et al., 2015) and distal (Liu et al., 2019) tubules with some glomerular expression (Henique et al., 2016). Interestingly, NQO1 is constitutively expressed in the proximal tubule, but can be induced in the distal tubules via administration of NRF2 inducers or by genetic knockdown of KEAP1 (Jobbagy et al., 2020). Glomerular expression of NQO1 is enhanced during disease (Moon et al., 2020). Interestingly, severe injuries can maladaptively decrease NRF2 activity and Nqo1 expression in renal tubules (Bondi et al., 2022).
3. THE ROLE OF NRF2 IN KIDNEY PHYSIOLOGY
Using a transcriptomic and proteomic approach, Shelton and colleagues determined that kidneys from Nrf2 knockout mice are deficient in genes and proteins that regulate glutathione synthesis and conjugation, redox balance, metabolism and removal of xenobiotics, and generation of NAD(P)H (Shelton et al., 2015). Ingenuity Pathway Analysis similarly revealed roles in “xenobiotic metabolism signaling,” “aryl hydrocarbon receptor signaling,” “pregnane X receptor (PXR)/retinoid X receptor (RXR) activation,” and “glutathione‐mediated detoxification.” These findings confirm the known roles for NRF2 in antioxidant and detoxifying functions and also suggest an important role in regulating general kidney homeostasis (Shelton et al., 2015).
NRF2 activity appears to have a reversible pharmacodynamic effect on glomerular filtration rate (GFR). The NRF2 inducer, bardoxolone methyl, lowers serum creatinine in humans (Hong et al., 2012), an effect that ultimately led to its investigation for the treatment of chronic kidney disease (CKD). Multiple human trials have confirmed this effect, as well as reversal after drug washout (de Zeeuw et al., 2013; Pergola et al., 2011; Warady et al., 2022). This is a true effect on glomerular filtration, since bardoxolone methyl increases inulin clearance (Nangaku et al., 2020). The mechanism in humans is unknown, but in rodents NRF2 activity increases expression of the sodium‐glucose cotransporter 2 (SGLT2) (Zhao et al., 2021). Higher SGLT2 expression could increase GFR through effects on tubulo‐glomerular feedback (Vallon & Verma, 2021). Other experimental studies showed that NRF2 enhancement could increase glomerular surface area (and GFR) via effects on mesangial (Ding et al., 2013) or podocyte contractility (Kidokoro et al., 2023).
NRF2 affects renal salt and water handling. In elegant mouse studies, high NRF2 activity induced by genetic Keap1 deletion reduced aquaporin‐2 expression, leading to nephrogenic diabetes insipidus (DI) and hydronephrosis (Noel et al., 2016; Suzuki et al., 2017). Meanwhile, mice with Keap1 hypomorphism (reduced expression instead of a complete deletion) exhibited only mild hyposthenuria and normal urinary concentrating ability. The hypomorphic mice also had reduced expression of the Na+‐Cl− cotransporter (NCC) and were surprisingly protected against lithium‐induced DI (Jobbagy et al., 2020). The puzzling difference in phenotype could be related to the graded increase in NRF2 activity caused by Keap1 hypomorphism (high) compared to complete knockout (very high). This would be an example of hormesis (Bhakta‐Guha & Efferth, 2015), with a deleterious outcome being determined by the greater NRF2 activity in the knockout.
The effect of NRF2 on blood pressure appears to be complex and context‐dependent. Many studies demonstrate that NRF2 activity increases blood pressure. In humans, bardoxolone methyl increased or tended to increase blood pressure (de Zeeuw et al., 2013; Pergola et al., 2011). However, at least one study in mice showed that bardoxolone methyl can reduce blood pressure (Hisamichi et al., 2018). Our work using radiotelemetry found that Keap1 hypomorphic mice exhibit higher blood pressure, specifically during the inactive daytime portion of their diurnal cycle (mice are nocturnal). Unlike wild‐type mice, these hypomorphic mice also had defects in blood pressure dipping during sleep when challenged with chronic angiotensin II infusion (Rush et al., 2021). In agreement with this, genetic Nrf2 deletion or pharmacologic inhibition lowered systolic blood pressure in mice (Zhao et al., 2018).
In another study using chronic angiotensin II infusion, the NRF2 inducer, tert‐butylhydroquinone, amplified early (Day 0–3) angiotensin II‐induced increases in blood pressure while decreasing late (Day 8–12) blood pressures, in an NRF2‐dependent manner (Wang et al., 2018). Another inducer, cinnamaldehyde, reduced blood pressure in diabetic mice and improved vasodilation in explanted arteries (Wang et al., 2020). Similarly, the NRF2 inducer, resveratrol, reduced blood pressure in spontaneously hypertensive rats (Javkhedkar et al., 2015), and in an oxidant‐induced hypertension model, NRF2 inhibition increased blood pressure and markers of oxidative stress (Farooqui et al., 2021). Currently, it is not clear why NRF2 increases blood pressure in some studies and reduces it in others. Further study to understand how and by what mechanisms NRF2 affects blood pressure in normal and disease states is required.
4. ROLES IN KIDNEY DISEASE
4.1. Acute kidney injury (AKI)
Abundant evidence demonstrates that NRF2 activity is protective in renal ischemia–reperfusion injury (IRI). Nrf2 knockout mice exhibited increased injury (serum creatinine and histologic tubular injury) and mortality compared to wild‐type animals (Liu et al., 2009). Conversely, mice having genetic Keap1 modifications increasing NRF2 activity (Keap1 hypomorphic mice and tubule‐specific Keap1 deletion) were protected from IRI‐AKI (Nezu, Souma, et al., 2017). Lymphocyte NRF2 activity is also important, as upregulation of NRF2 specifically in CD4+ T cells protected against IRI (Kurzhagen et al., 2023; Noel et al., 2015), while activation in myeloid cells (macrophages and neutrophils) did not (Nezu, Souma, et al., 2017).
Pharmacologic NRF2 enhancement is also effective when administered within a therapeutic window. Sulforaphane, RTA‐408 (omaveloxolone), CDDO‐Me, and CDDO‐Im all reduced injury when administered at least 24 h prior to IRI (Han et al., 2017; Liu et al., 2014; Wu et al., 2011; Yoon et al., 2008). However, protection was not observed if CDDO‐Im treatment was delayed (starting 3 h prior to IRI or later) (Liu et al., 2014). Septic AKI is reduced by the induction of NRF2 activity prior to injury (Chen et al., 2022), and NRF2 protects against heme‐induced renal damage after intravascular hemolysis (Rubio‐Navarro et al., 2019). Interestingly, preconditioning with remote ischemia protects against AKI, and this is associated with increased NRF2 (Liu & Gong, 2015). Furthermore, prolonged intestinal ischemia causes AKI, but postconditioning with additional but brief cycles of intestinal ischemia and reperfusion similarly increased kidney NRF2 and decreased renal injury (Chen et al., 2020).
NRF2 protects against a wide variety of nephrotoxin‐induced AKI, consistent with its detoxifying and antioxidant functions. NRF2 activation reduces kidney injury caused by exposure to arsenic, cadmium, cisplatin, contrast, and methotrexate (Guerrero‐Beltran et al., 2010; Mapuskar et al., 2023; Ran et al., 2022; Salama et al., 2021; Xu et al., 2021; Younis et al., 2021), while Nrf2 knockout mice are more susceptible to cisplatin‐induced nephrotoxic injury (Liu et al., 2009). At least some of this protection may be due to direct toxin elimination, but NRF2 may also protect against post‐exposure evolution of disease. Future studies can differentiate these effects with carefully timed activation of NRF2.
4.2. AKI‐to‐CKD progression
Patients with AKI have a higher risk of developing renal fibrosis and CKD (Basile et al., 2016). Studies from our laboratory and others show that higher NRF2 activity (via constitutive genetic modifications in mice) reduced fibrosis at late timepoints after IRI‐AKI (Nezu, Souma, et al., 2017; Tan et al., 2016). Nezu and colleagues further demonstrated that CDDO‐Im started 1 day after injury mitigated the development of fibrosis, but delaying administration until day 7 did not (Nezu, Souma, et al., 2017). In aged rats, basal expression of NRF2 is decreased and is associated with worse renal fibrosis after IRI, which is rescued with CDDO‐Me starting as late as 3 days after injury (Jo et al., 2023). These data reveal a post‐injury therapeutic window in which NRF2 enhancement is protective against the development of fibrosis and CKD. This is clinically relevant since most human AKI is recognized after injury has already occurred.
Since NRF2 activity reduces kidney injury, we sought to examine how its endogenous activity levels are affected by AKI. While some investigators found an increase in NRF2 activity after AKI (Leonard et al., 2006), in our hands there was prolonged suppression, which would predispose the kidney to further injury (Tan et al., 2016). In both mild and severe IRI (induced by short and long ischemia times), we found that kidney NQO1 expression was immediately reduced 1 day after injury (Bondi et al., 2022). Over the next 10 days, mice with mild injury restored normal NRF2 activity and NQO1 protein levels, recovered kidney function, and avoided renal fibrosis. Mice with severe injury exhibited permanent NRF2 suppression, nonrecovery of kidney function, and progression of renal fibrosis and CKD. Our data suggest that restoration of NRF2 activity leads to renal recovery while prolonged suppression promotes CKD. The latter may occur through the activity of hypoxia‐inducible factor‐1α (HIF‐1α), which inhibited NRF2 activity in severe injury (Bondi et al., 2022).
In one study, a regulatory role was found for GSK‐3β, a protein that facilitates NRF2 nuclear exclusion and degradation (Lu et al., 2019). In mice exhibiting recovery after folic‐acid induced AKI, GSK‐3β expression was low and NRF2 activity was high. In mice with progression to CKD, high GSK‐3β expression inhibited NRF2 activity, albeit to a level that was still higher than baseline. Inhibition of GSK‐3β with lithium chloride prevented AKI‐to‐CKD progression, even if delayed until 7 days after the injury (Lu et al., 2019).
In summary, lower NRF2 levels are associated with severe AKI. Meanwhile, early interventions to increase NRF2 activity are critical for protection and can affect the long‐term development of fibrosis and CKD. Additional studies are needed to determine how different types of AKI are affected by NRF2, and whether NRF2 activity can prevent human AKI and AKI‐to‐CKD progression.
4.3. Nonproteinuric CKD
NRF2 activity preserves normal kidney architecture and prevents progression of CKD. In unilateral ureteral obstruction (UUO), Nrf2 knockout mice had increased fibrosis while Keap1 hypomorphism reduced fibrosis (Kong et al., 2017; Tan et al., 2016). Pretreatment with CDDO‐Me protects against CKD induced by aristolochic acid (Wu et al., 2014), which is converted into electrophilic compounds that adduct to DNA (Han et al., 2019). Kidney injury in hyperuricemic nephropathy was worsened in Nrf2 knockout mice and improved with sulforaphane (Qiao et al., 2023).
Autosomal dominant polycystic kidney disease (ADPKD) is an inherited disorder leading to cyst formation in both kidneys. ADPKD patients exhibit oxidative stress and lower NRF2 activity, while in mice, cyst growth was accelerated in Nrf2 knockouts and reduced in animals treated with sulforaphane (Lu, Sun, et al., 2020). Tolvaptan, a vasopressin antagonist and known treatment for ADPKD, activates the NRF2 pathway in cells of the outer medulla (Fujiki et al., 2019). The FALCON trial was designed to test whether bardoxolone methyl slows ADPKD but was terminated in 2023 (https://clinicaltrials.gov/study/NCT03918447) when results of other clinical trials led the sponsor to end all of its CKD trials (see below).
4.4. Proteinuric CKD
Glomerular diseases such as diabetic nephropathy, IgA nephropathy, and focal segmental glomerulosclerosis (FSGS) remain important causes of kidney failure. Several clinical trials tested whether bardoxolone methyl (CDDO‐Me) is an effective treatment for these diseases. Unfortunately, there is no convincing evidence that NRF2 enhancement slows the progression of proteinuric CKD in humans.
Cancer patients treated with bardoxolone methyl exhibit increased eGFR (Hong et al., 2012), a finding that spawned several clinical trials targeting diabetic kidney disease (BEAM, BEACON, TSUBAKI, AYAME). All confirmed an early increase in eGFR (Akizawa et al., 2023; de Zeeuw et al., 2013; Nangaku et al., 2020; Pergola et al., 2011). However, the BEACON trial was terminated early due to serious adverse events including heart failure exacerbations and death, suggesting an effect on fluid retention. Bardoxolone methyl also increased albuminuria and blood pressure and caused hypomagnesemia and weight loss (de Zeeuw et al., 2013). The final trial (AYAME) examined extended treatment with bardoxolone methyl (at least 3 years) in a diabetic kidney disease population with exclusions for heart failure (Nangaku et al., 2023). In results presented recently, treatment with bardoxolone methyl did not change time to onset of ESRD (https://www.kyowakirin.com/media_center/news_releases/2023/pdf/e20230510_01.pdf; Akizawa et al., 2023).
Bardoxolone methyl was also tested in Alport syndrome patients in the CARDINAL trial. The phase 3 portion of this trial examined the effects of 2 years of exposure to bardoxolone methyl, with a 4‐week drug washout each year to address concerns about drug‐induced reversible changes in GFR. The investigators found mean improvement in eGFR after the 1‐ and 2‐year washout periods (Warady et al., 2022). However, the Food and Drug Administration (FDA) declined the new drug application, citing concerns about an insufficient washout period, increases in albuminuria and blood pressure, and weight loss in a pediatric population. Further, the FDA stated that increases in GFR appeared to be a “one‐time benefit that does not grow over time,” calling into question its overall utility in disease treatment (https://www.fda.gov/media/154630/download). In the aftermath of CARDINAL and AYAME, Reata Pharmaceuticals and its partner Kyowa Kirin terminated all of their clinical trials of bardoxolone methyl in kidney disease in 2023 (https://www.kyowakirin.com/media_center/news_releases/2023/pdf/e20230510_01.pdf).
The mechanisms for the albuminuria and GFR effects are still under study. Nonhuman primate research suggested that megalin downregulation (and lower tubular protein reabsorption) led to the increased albuminuria with bardoxolone methyl exposure (Reisman et al., 2012). An alternative explanation is that bardoxolone methyl increases intraglomerular pressure, which would hasten CKD progression, explain the increased GFR, and is the opposite effect of proven treatments such as renin angiotensin system (RAS) inhibitors (Baigent & Lennon, 2018).
Several animal studies confirm deleterious effects of NRF2 activity in proteinuric CKD. A bardoxolone methyl analog, RTA 405, worsened proteinuria and kidney injury in diabetic rats, even when combined with RAS inhibition. Concerns about purity of the drug led to studies with another analog, RTA dh404, which also worsened disease and caused kidney pseudotumors (Zoja et al., 2013). Reata Pharmaceuticals repeated this study and did not see these adverse effects, but their data still reported a large numerical increase in proteinuria in animals treated with either analog (Chin et al., 2013).
Since NRF2 inducers could have off‐target effects, we examined Keap1 hypomorphic mice with global NRF2 hyperactivation (Rush et al., 2021). These mice had no increase in proteinuria at baseline but did exhibit increased proteinuria compared to wild‐type mice in three different glomerular injury models (Adriamycin, angiotensin II, and albumin overload). The proteinuria was accompanied by more severe glomerular injury and renal fibrosis. While Keap1 hypomorphic mice exhibited higher blood pressures, the measured difference (~7 mmHg in mean arterial pressure) was not likely to explain the very large difference in proteinuria. Conversely, Nrf2 knockout mice were protected from proteinuria. CDDO‐Im administration increased proteinuria in wild‐type but not the Nrf2 knockout mice, showing dependence on Nrf2 for the effect. (Rush et al., 2021).
In elegant studies, Zhao and colleagues showed that NRF2 can worsen proteinuria through SGLT2 (Zhao et al., 2021). While combined Akita diabetic Nrf2 knockout mice were protected from proteinuria and renal fibrosis, the transgenic reintroduction of Nrf2 in just the renal tubules was sufficient to restore kidney disease. The mutant mice expressed higher levels of SGLT2, and NRF2 directly promoted its gene expression in vitro (Zhao et al., 2021). Similar effects were found in the db/db mouse (Su et al., 2023). These findings point strongly to an SGLT2‐dependent mechanism for the increases in proteinuria and GFR since it is well‐known that SGLT2 inhibitors reduce GFR and proteinuria (Vallon & Verma, 2021).
However, it is acknowledged that many animal studies show that NRF2 is protective in glomerular disease. Nrf2 knockout mice exhibited greater oxidative stress, glomerular injury and proteinuria compared to wild‐type mice in streptozotocin‐induced diabetic kidney disease (Jiang et al., 2010). Sulforaphane ameliorated diabetic nephropathy and obesity‐related glomerular disease (Cui et al., 2012; Lu, Zhang, et al., 2020; Zheng et al., 2011). Long‐term administration of RTA dh404 in 5/6 nephrectomy in rats restored NRF2 protein expression, attenuated oxidative stress, proinflammatory, and profibrotic pathways, and reduced inflammation, glomerulosclerosis, and interstitial fibrosis (Aminzadeh et al., 2014). A recent study in Akita Nrf2 knockout mice demonstrated that lack of Nrf2 led to worse glomerular and tubular injury, inflammation, and renal fibrosis (Liu et al., 2022).
The reasons for the divergent results from animal studies are not immediately clear, but differences in strength of NRF2 activity may play a role. In the 5/6 nephrectomy model in rats, a lower dose of RTA dh404 protected against glomerulosclerosis, interstitial fibrosis, and inflammation, while a higher dose worsened proteinuria, interstitial fibrosis, oxidative and inflammatory pathways, and paradoxically reduced NRF2 activity (Vaziri et al., 2015). Some studies, including our own unpublished observations, show that high doses of NRF2 inducers are cytotoxic or fatal (Hisamichi et al., 2018). Indeed, our studies using Keap1 hypomorphs or CDDO‐Im did lead to strong upregulation of Nqo1 levels, but the fact that Nrf2 knockout mice were protected from proteinuria suggests even low NRF2 activity can be harmful (Rush et al., 2021).
NRF2 is not the only pathway with dual effects in biology. HIF‐1α and HIF‐2α, although both regulated by prolyl hydroxylases, have opposing effects on fibrosis and inflammation (Packer, 2021). The Wnt/β‐catenin pathway is another example, in which early and transient activation of the pathway is associated with renal recovery after injury, while prolonged and sustained activation leads to AKI‐to‐CKD progression (Xiao et al., 2016). Experimental design should carefully address the titratable level of NRF2 induction, method by which it is induced (pharmacologic versus genetic), and the exact timing and the cell types in which induction is achieved.
In our opinion, the current evidence does not support NRF2 enhancement in the treatment of proteinuric kidney diseases. The “benefit” of higher GFR in humans treated with bardoxolone methyl is controversial and has not been proven to provide long‐term benefit in slowing progression to ESRD. Deleterious increases in proteinuria and blood pressure have been observed in humans and recapitulated in animals when NRF2 is induced. NRF2 also appears to have poorly understood dose‐dependent effects. Future studies are needed to clarify the exact mechanisms of NRF2 effects in proteinuric diseases.
5. CONCLUSION
In the kidney, NRF2 plays a role in mitigating oxidative and electrophilic stress and inflammation and in regulation of physiological processes including salt, water, and glucose handling and blood pressure. To date, the positive results from experimental models of AKI and nonproteinuric CKD demonstrate potential therapeutic value in pharmacologically enhancing NRF2 activity. Unfortunately, the use of NRF2 to treat proteinuric CKD with glomerular injury may harm patients and worsen kidney disease (Figure 2). Although the clinical trials in proteinuric CKD were appropriately terminated, study of NRF2 should not be completely abandoned. Future studies must elucidate the pros and cons of NRF2 in different disease states, at acute and chronic stages, and at different levels of activation. Potential side effects, including effects on albuminuria, magnesium, blood pressure, weight loss, and fluid retention, also require study and mitigation. This research should remain a priority, not only for the purposes of treating disease, but to better understand the impact of NRF2 activity on kidney physiology and pathology.
FIGURE 2.
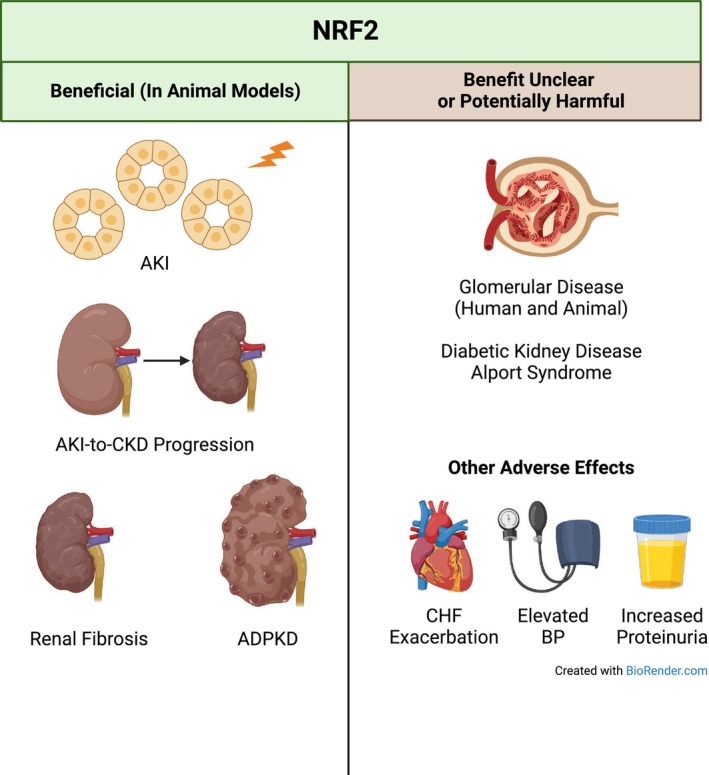
Overview of the effects of NRF2. Animal studies have shown that NRF2 activation, when properly timed, can mitigate AKI, AKI‐to‐CKD progression, renal fibrosis, and cystogenesis in autosomal dominant polycystic kidney disease (ADPKD). Conversely, in glomerular diseases such as human diabetic kidney disease and Alport syndrome, NRF2 has not been shown to have a clear effect to slow disease progression. Many animal studies also show deleterious effects in glomerular disease models. Furthermore, NRF2 appears to have negative effects on congestive heart failure (CHF) and fluid accumulation, blood pressure (BP), and even kidney disease and proteinuria.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
R.J.T. was supported by a Department of Veterans Affairs Merit Award I01BX005680, Department of Defense W81XWH‐22‐1‐0845, NIH R01 DK131991, DK064005 and the Pittsburgh Center for Kidney Research (P30 DK079307 and U54 DK137329). C.D.B. was supported by National Institutes of Health T32DK061296, American Heart Association 20POST35200358, and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases K01DK124357.
Bondi, C. D. , Hartman, H. L. , & Tan, R. J. (2024). NRF2 in kidney physiology and disease. Physiological Reports, 12, e15961. 10.14814/phy2.15961
DATA AVAILABILITY STATEMENT
Data sharing not applicable—no new data generated.
REFERENCES
- Akizawa, T. , Yamawaki, K. , Ichikawa, T. , Mukai, K. , & Nangaku, M. (2023). AYAME study: Randomized, double‐blind, placebo‐controlled phase 3 study of bardoxolone methyl in diabetic kidney disease (DKD) patients. Journal of American Society of Nephrology, 34, B1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminzadeh, M. A. , Reisman, S. A. , Vaziri, N. D. , Khazaeli, M. , Yuan, J. , & Meyer, C. J. (2014). The synthetic triterpenoid RTA dh404 (CDDO‐dhTFEA) restores Nrf2 activity and attenuates oxidative stress, inflammation, and fibrosis in rats with chronic kidney disease. Xenobiotica, 44, 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baigent, C. , & Lennon, R. (2018). Should we increase GFR with bardoxolone in Alport syndrome? Journal of American Society of Nephrology, 29, 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile, D. P. , Bonventre, J. V. , Mehta, R. , Nangaku, M. , Unwin, R. , Rosner, M. H. , Kellum, J. A. , Ronco, C. , & Group AXW . (2016). Progression after AKI: Understanding maladaptive repair processes to predict and identify therapeutic treatments. Journal of American Society of Nephrology, 27, 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakta‐Guha, D. , & Efferth, T. (2015). Hormesis: Decoding two sides of the same coin. Pharmaceuticals (Basel), 8, 865–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi, C. D. , Rush, B. M. , Hartman, H. L. , Wang, J. , Al‐Bataineh, M. M. , Hughey, R. P. , & Tan, R. J. (2022). Suppression of NRF2 activity by HIF‐1alpha promotes fibrosis after ischemic acute kidney injury. Antioxidants, 11, 1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoumpekis, D. V. , & Kensler, T. W. (2013). New player on an old field; the keap1/Nrf2 pathway as a target for treatment of type 2 diabetes and metabolic syndrome. Current Diabetes Reviews, 9, 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R. , Zeng, Z. , Zhang, Y. Y. , Cao, C. , Liu, H. M. , Li, W. , Wu, Y. , Xia, Z. Y. , Ma, D. , & Meng, Q. T. (2020). Ischemic postconditioning attenuates acute kidney injury following intestinal ischemia‐reperfusion through Nrf2‐regulated autophagy, anti‐oxidation, and anti‐inflammation in mice. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 34, 8887–8901. [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Wang, H. , Hu, B. , Chen, X. , Zheng, M. , Liang, L. , Lyu, J. , & Zeng, Q. (2022). Transcription factor nuclear factor erythroid 2 p45‐related factor 2 (NRF2) ameliorates sepsis‐associated acute kidney injury by maintaining mitochondrial homeostasis and improving the mitochondrial function. European Journal of Histochemistry, 66, 3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin, M. , Lee, C. Y. , Chuang, J. C. , Bumeister, R. , Wigley, W. C. , Sonis, S. T. , Ward, K. W. , & Meyer, C. (2013). Bardoxolone methyl analogs RTA 405 and dh404 are well tolerated and exhibit efficacy in rodent models of type 2 diabetes and obesity. American Journal of Physiology Renal Physiology, 304, F1438–F1446. [DOI] [PubMed] [Google Scholar]
- Cuevas, S. , Yang, Y. , Konkalmatt, P. , Asico, L. D. , Feranil, J. , Jones, J. , Villar, V. A. , Armando, I. , & Jose, P. A. (2015). Role of nuclear factor erythroid 2‐related factor 2 in the oxidative stress‐dependent hypertension associated with the depletion of DJ‐1. Hypertension, 65, 1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, W. , Bai, Y. , Miao, X. , Luo, P. , Chen, Q. , Tan, Y. , Rane, M. J. , Miao, L. , & Cai, L. (2012). Prevention of diabetic nephropathy by sulforaphane: Possible role of Nrf2 upregulation and activation. Oxidative Medicine and Cellular Longevity, 2012, 821936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan, S. B. , Gordan, J. D. , Jin, J. , Harper, J. W. , & Diehl, J. A. (2004). The Keap1‐BTB protein is an adaptor that bridges Nrf2 to a Cul3‐based E3 ligase: Oxidative stress sensing by a Cul3‐Keap1 ligase. Molecular and Cellular Biology, 24, 8477–8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zeeuw, D. , Akizawa, T. , Audhya, P. , Bakris, G. L. , Chin, M. , Christ‐Schmidt, H. , Goldsberry, A. , Houser, M. , Krauth, M. , Lambers Heerspink, H. J. , McMurray, J. J. , Meyer, C. J. , Parving, H. H. , Remuzzi, G. , Toto, R. D. , Vaziri, N. D. , Wanner, C. , Wittes, J. , Wrolstad, D. , … Investigators, B. T. (2013). Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. The New England Journal of Medicine, 369, 2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, Y. , Stidham, R. D. , Bumeister, R. , Trevino, I. , Winters, A. , Sprouse, M. , Ding, M. , Ferguson, D. A. , Meyer, C. J. , Wigley, W. C. , & Ma, R. (2013). The synthetic triterpenoid, RTA 405, increases the glomerular filtration rate and reduces angiotensin II‐induced contraction of glomerular mesangial cells. Kidney International, 83, 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui, Z. , Mohammad, R. S. , Lokhandwala, M. F. , & Banday, A. A. (2021). Nrf2 inhibition induces oxidative stress, renal inflammation and hypertension in mice. Clinical and Experimental Hypertension, 43, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki, T. , Ando, F. , Murakami, K. , Isobe, K. , Mori, T. , Susa, K. , Nomura, N. , Sohara, E. , Rai, T. , & Uchida, S. (2019). Tolvaptan activates the Nrf2/HO‐1 antioxidant pathway through PERK phosphorylation. Scientific Reports, 9, 9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero‐Beltran, C. E. , Calderon‐Oliver, M. , Tapia, E. , Medina‐Campos, O. N. , Sanchez‐Gonzalez, D. J. , Martinez‐Martinez, C. M. , Ortiz‐Vega, K. M. , Franco, M. , & Pedraza‐Chaverri, J. (2010). Sulforaphane protects against cisplatin‐induced nephrotoxicity. Toxicology Letters, 192, 278–285. [DOI] [PubMed] [Google Scholar]
- Guerrero‐Hue, M. , Rayego‐Mateos, S. , Vazquez‐Carballo, C. , Palomino‐Antolin, A. , Garcia‐Caballero, C. , Opazo‐Rios, L. , Morgado‐Pascual, J. L. , Herencia, C. , Mas, S. , Ortiz, A. , Rubio‐Navarro, A. , Egea, J. , Villalba, J. M. , Egido, J. , & Moreno, J. A. (2020). Protective role of Nrf2 in renal disease. Antioxidants, 10, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J. , Xian, Z. , Zhang, Y. , Liu, J. , & Liang, A. (2019). Systematic overview of aristolochic acids: Nephrotoxicity, carcinogenicity, and underlying mechanisms. Frontiers in Pharmacology, 10, 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, P. , Qin, Z. , Tang, J. , Xu, Z. , Li, R. , Jiang, X. , Yang, C. , Xing, Q. , Qi, X. , Tang, M. , Zhang, J. , Shen, B. , Wang, W. , Qin, C. , & Zhang, W. (2017). RTA‐408 protects kidney from ischemia‐reperfusion injury in mice via activating Nrf2 and downstream GSH biosynthesis gene. Oxidative Medicine and Cellular Longevity, 2017, 7612182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henique, C. , Bollee, G. , Lenoir, O. , Dhaun, N. , Camus, M. , Chipont, A. , Flosseau, K. , Mandet, C. , Yamamoto, M. , Karras, A. , Thervet, E. , Bruneval, P. , Nochy, D. , Mesnard, L. , & Tharaux, P. L. (2016). Nuclear factor erythroid 2‐related factor 2 drives podocyte‐specific expression of peroxisome proliferator‐activated receptor gamma essential for resistance to crescentic GN. J Am Soc Nephrol, 27, 172–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamichi, M. , Kamijo‐Ikemori, A. , Sugaya, T. , Hoshino, S. , Kimura, K. , & Shibagaki, Y. (2018). Role of bardoxolone methyl, a nuclear factor erythroid 2‐related factor 2 activator, in aldosterone‐ and salt‐induced renal injury. Hypertension Research, 41, 8–17. [DOI] [PubMed] [Google Scholar]
- Hong, D. S. , Kurzrock, R. , Supko, J. G. , He, X. , Naing, A. , Wheler, J. , Lawrence, D. , Eder, J. P. , Meyer, C. J. , Ferguson, D. A. , Mier, J. , Konopleva, M. , Konoplev, S. , Andreeff, M. , Kufe, D. , Lazarus, H. , Shapiro, G. I. , & Dezube, B. J. (2012). A phase I first‐in‐human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clinical Cancer Research, 18, 3396–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, K. , Chiba, T. , Takahashi, S. , Ishii, T. , Igarashi, K. , Katoh, Y. , Oyake, T. , Hayashi, N. , Satoh, K. , Hatayama, I. , Yamamoto, M. , & Nabeshima, Y. (1997). An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochemical and Biophysical Research Communications, 236, 313–322. [DOI] [PubMed] [Google Scholar]
- Javkhedkar, A. A. , Quiroz, Y. , Rodriguez‐Iturbe, B. , Vaziri, N. D. , Lokhandwala, M. F. , & Banday, A. A. (2015). Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 308, R840–R846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, T. , Huang, Z. , Lin, Y. , Zhang, Z. , Fang, D. , & Zhang, D. D. (2010). The protective role of Nrf2 in streptozotocin‐induced diabetic nephropathy. Diabetes, 59, 850–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, T. , Tian, F. , Zheng, H. , Whitman, S. A. , Lin, Y. , Zhang, Z. , Zhang, N. , & Zhang, D. D. (2014). Nrf2 suppresses lupus nephritis through inhibition of oxidative injury and the NF‐kappaB‐mediated inflammatory response. Kidney International, 85, 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, M. J. , Kim, J. E. , Bae, S. Y. , Cho, E. , Ahn, S. Y. , Kwon, Y. J. , & Ko, G. J. (2023). Impaired NRF2 inhibits recovery from ischemic reperfusion injury in the aging kidney. Antioxidants, 12, 1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobbagy, S. , Vitturi, D. A. , Salvatore, S. R. , Pires, M. F. , Rowart, P. , Emlet, D. R. , Ross, M. , Hahn, S. , St Croix, C. , Wendell, S. G. , Subramanya, A. R. , Straub, A. C. , Tan, R. J. , & Schopfer, F. J. (2020). Nrf2 activation protects against lithium‐induced nephrogenic diabetes insipidus. JCI Insight, 5, e128578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro, K. , Kadoya, H. , Cherney, D. Z. I. , Kondo, M. , Wada, Y. , Umeno, R. , Kishi, S. , Nagasu, H. , Nagai, K. , Suzuki, T. , Sasaki, T. , Yamamoto, M. , Kanwar, Y. S. , & Kashihara, N. (2023). Insights into the regulation of glomerular filtration rate by the Keap1‐Nrf2 pathway. Kidney360, 4, 1454–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, A. , Kang, M. I. , Okawa, H. , Ohtsuji, M. , Zenke, Y. , Chiba, T. , Igarashi, K. , & Yamamoto, M. (2004). Oxidative stress sensor Keap1 functions as an adaptor for Cul3‐based E3 ligase to regulate proteasomal degradation of Nrf2. Molecular and Cellular Biology, 24, 7130–7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, W. , Fu, J. , Liu, N. , Jiao, C. , Guo, G. , Luan, J. , Wang, H. , Yao, L. , Wang, L. , Yamamoto, M. , Pi, J. , & Zhou, H. (2017). Nrf2 deficiency promotes the progression from acute tubular damage to chronic renal fibrosis following unilateral ureteral obstruction. Nephrology, Dialysis, Transplantation, 33, 771‐783. [DOI] [PubMed] [Google Scholar]
- Kurzhagen, J. T. , Noel, S. , Lee, K. , Sadasivam, M. , Gharaie, S. , Ankireddy, A. , Lee, S. A. , Newman‐Rivera, A. , Gong, J. , Arend, L. J. , Hamad, A. R. A. , Reddy, S. P. , & Rabb, H. (2023). T cell Nrf2/Keap1 gene editing using CRISPR/Cas9 and experimental kidney ischemia‐reperfusion injury. Antioxidants & Redox Signaling, 38, 959–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard, M. O. , Kieran, N. E. , Howell, K. , Burne, M. J. , Varadarajan, R. , Dhakshinamoorthy, S. , Porter, A. G. , O'Farrelly, C. , Rabb, H. , & Taylor, C. T. (2006). Reoxygenation‐specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia‐reperfusion injury. The FASEB Journal, 20, 2624–2626. [DOI] [PubMed] [Google Scholar]
- Liu, M. , Deng, M. , Luo, Q. , Dou, X. , & Jia, Z. (2019). High‐salt loading downregulates Nrf2 expression in a sodium‐dependent manner in renal collecting duct cells. Frontiers in Physiology, 10, 1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M. , Grigoryev, D. N. , Crow, M. T. , Haas, M. , Yamamoto, M. , Reddy, S. P. , & Rabb, H. (2009). Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney International, 76, 277–285. [DOI] [PubMed] [Google Scholar]
- Liu, M. , Reddy, N. M. , Higbee, E. M. , Potteti, H. R. , Noel, S. , Racusen, L. , Kensler, T. W. , Sporn, M. B. , Reddy, S. P. , & Rabb, H. (2014). The Nrf2 triterpenoid activator, CDDO‐imidazolide, protects kidneys from ischemia‐reperfusion injury in mice. Kidney International, 85, 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Uruno, A. , Saito, R. , Matsukawa, N. , Hishinuma, E. , Saigusa, D. , Liu, H. , & Yamamoto, M. (2022). Nrf2 deficiency deteriorates diabetic kidney disease in Akita model mice. Redox Biology, 58, 102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , & Gong, R. (2015). Remote ischemic preconditioning for kidney protection: GSK3beta‐centric insights into the mechanism of action. American Journal of Kidney Diseases, 66, 846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, M. , Wang, P. , Qiao, Y. , Jiang, C. , Ge, Y. , Flickinger, B. , Malhotra, D. K. , Dworkin, L. D. , Liu, Z. , & Gong, R. (2019). GSK3beta‐mediated Keap1‐independent regulation of Nrf2 antioxidant response: A molecular rheostat of acute kidney injury to chronic kidney disease transition. Redox Biology, 26, 101275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. , Sun, Y. , Liu, Z. , Lu, Y. , Zhu, X. , Lan, B. , Mi, Z. , Dang, L. , Li, N. , Zhan, W. , Tan, L. , Pi, J. , Xiong, H. , Zhang, L. , & Chen, Y. (2020). Activation of NRF2 ameliorates oxidative stress and cystogenesis in autosomal dominant polycystic kidney disease. Science Translational Medicine, 12, eaba3613. [DOI] [PubMed] [Google Scholar]
- Lu, Y. , Zhang, Y. , Lou, Y. , Cui, W. , & Miao, L. (2020). Sulforaphane suppresses obesity‐related glomerulopathy‐induced damage by enhancing autophagy via Nrf2. Life Sciences, 258, 118153. [DOI] [PubMed] [Google Scholar]
- Mapuskar, K. A. , Pulliam, C. F. , Zepeda‐Orozco, D. , Griffin, B. R. , Furqan, M. , Spitz, D. R. , & Allen, B. G. (2023). Redox regulation of Nrf2 in cisplatin‐induced kidney injury. Antioxidants, 12, 1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moi, P. , Chan, K. , Asunis, I. , Cao, A. , & Kan, Y. W. (1994). Isolation of NF‐E2‐related factor 2 (Nrf2), a NF‐E2‐like basic leucine zipper transcriptional activator that binds to the tandem NF‐E2/AP1 repeat of the beta‐globin locus control region. Proceedings of the National Academy of Sciences of the United States of America, 91, 9926–9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, S. J. , Jeong, J. Y. , Kim, J. H. , Choi, D. H. , Choi, H. , Chang, Y. K. , Na, K. R. , Lee, K. W. , Lee, C. H. , Choi, D. E. , & Hwang, J. H. (2020). The potential roles of NAD(P)H:Quinone oxidoreductase 1 in the development of diabetic nephropathy and Actin polymerization. Scientific Reports, 10, 17735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangaku, M. , Kanda, H. , Takama, H. , Ichikawa, T. , Hase, H. , & Akizawa, T. (2020). Randomized clinical trial on the effect of bardoxolone methyl on GFR in diabetic kidney disease patients (TSUBAKI study). Kidney Int Rep, 5, 879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangaku, M. , Takama, H. , Ichikawa, T. , Mukai, K. , Kojima, M. , Suzuki, Y. , Watada, H. , Wada, T. , Ueki, K. , Narita, I. , Kashihara, N. , Kadowaki, T. , Hase, H. , & Akizawa, T. (2023). Randomized, double‐blind, placebo‐controlled phase 3 study of bardoxolone methyl in patients with diabetic kidney disease: Design and baseline characteristics of the AYAME study. Nephrology, Dialysis, Transplantation, 38, 1204–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezu, M. , Souma, T. , Yu, L. , Suzuki, T. , Saigusa, D. , Ito, S. , Suzuki, N. , & Yamamoto, M. (2017). Transcription factor Nrf2 hyperactivation in early‐phase renal ischemia‐reperfusion injury prevents tubular damage progression. Kidney International, 91, 387–401. [DOI] [PubMed] [Google Scholar]
- Nezu, M. , Suzuki, N. , & Yamamoto, M. (2017). Targeting the KEAP1‐NRF2 system to prevent kidney disease progression. American Journal of Nephrology, 45, 473–483. [DOI] [PubMed] [Google Scholar]
- Nguyen, T. , Sherratt, P. J. , Huang, H. C. , Yang, C. S. , & Pickett, C. B. (2003). Increased protein stability as a mechanism that enhances Nrf2‐mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. The Journal of Biological Chemistry, 278, 4536–4541. [DOI] [PubMed] [Google Scholar]
- Noel, S. , Arend, L. J. , Bandapalle, S. , Reddy, S. P. , & Rabb, H. (2016). Kidney epithelium specific deletion of kelch‐like ECH‐associated protein 1 (Keap1) causes hydronephrosis in mice. BMC Nephrology, 17, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel, S. , Martina, M. N. , Bandapalle, S. , Racusen, L. C. , Potteti, H. R. , Hamad, A. R. , Reddy, S. P. , & Rabb, H. (2015). T lymphocyte‐specific activation of Nrf2 protects from AKI. J Am Soc Nephrol, 26, 2989–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer, M. (2021). Mechanisms leading to differential hypoxia‐inducible factor signaling in the diabetic kidney: Modulation by SGLT2 inhibitors and hypoxia mimetics. American Journal of Kidney Diseases, 77, 280–286. [DOI] [PubMed] [Google Scholar]
- Pergola, P. E. , Raskin, P. , Toto, R. D. , Meyer, C. J. , Huff, J. W. , Grossman, E. B. , Krauth, M. , Ruiz, S. , Audhya, P. , Christ‐Schmidt, H. , Wittes, J. , Warnock, D. G. , & Investigators, B. S. (2011). Bardoxolone methyl and kidney function in CKD with type 2 diabetes. The New England Journal of Medicine, 365, 327–336. [DOI] [PubMed] [Google Scholar]
- Qiao, P. , Sun, Y. , Wang, Y. , Lin, S. , An, Y. , Wang, L. , Liu, J. , Huang, Y. , Yang, B. , & Zhou, H. (2023). Activation of NRF2 signaling pathway delays the progression of Hyperuricemic nephropathy by reducing oxidative stress. Antioxidants, 12, 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran, F. , Yang, Y. , Yang, L. , Chen, S. , He, P. , Liu, Q. , Zou, Q. , Wang, D. , Hou, J. , & Wang, P. (2022). Capsaicin prevents contrast‐associated acute kidney injury through activation of Nrf2 in mice. Oxidative Medicine and Cellular Longevity, 2022, 1763922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman, S. A. , Chertow, G. M. , Hebbar, S. , Vaziri, N. D. , Ward, K. W. , & Meyer, C. J. (2012). Bardoxolone methyl decreases megalin and activates nrf2 in the kidney. J Am Soc Nephrol, 23, 1663–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio‐Navarro, A. , Vazquez‐Carballo, C. , Guerrero‐Hue, M. , Garcia‐Caballero, C. , Herencia, C. , Gutierrez, E. , Yuste, C. , Sevillano, A. , Praga, M. , Egea, J. , Cannata, P. , Cortegano, I. , de Andres, B. , Gaspar, M. L. , Cadenas, S. , Michalska, P. , Leon, R. , Ortiz, A. , Egido, J. , & Moreno, J. A. (2019). Nrf2 plays a protective role against intravascular hemolysis‐mediated acute kidney injury. Frontiers in Pharmacology, 10, 740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush, B. M. , Bondi, C. D. , Stocker, S. D. , Barry, K. M. , Small, S. A. , Ong, J. , Jobbagy, S. , Stolz, D. B. , Bastacky, S. I. , Chartoumpekis, D. V. , Kensler, T. W. , & Tan, R. J. (2021). Genetic or pharmacologic Nrf2 activation increases proteinuria in chronic kidney disease in mice. Kidney International, 99, 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama, S. A. , Mohamadin, A. M. , & Abdel‐Bakky, M. S. (2021). Arctigenin alleviates cadmium‐induced nephrotoxicity: Targeting endoplasmic reticulum stress, Nrf2 signaling, and the associated inflammatory response. Life Sciences, 287, 120121. [DOI] [PubMed] [Google Scholar]
- Shelton, L. M. , Lister, A. , Walsh, J. , Jenkins, R. E. , Wong, M. H. , Rowe, C. , Ricci, E. , Ressel, L. , Fang, Y. , Demougin, P. , Vukojevic, V. , O'Neill, P. M. , Goldring, C. E. , Kitteringham, N. R. , Park, B. K. , Odermatt, A. , & Copple, I. M. (2015). Integrated transcriptomic and proteomic analyses uncover regulatory roles of Nrf2 in the kidney. Kidney International, 88, 1261–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, K. , Zhao, S. L. , Yang, W. X. , Lo, C. S. , Chenier, I. , Liao, M. C. , Pang, Y. C. , Peng, J. Z. , Miyata, K. N. , Cailhier, J. F. , Ethier, J. , Lattouf, J. B. , Filep, J. G. , Ingelfinger, J. R. , Zhang, S. L. , & Chan, J. S. D. (2023). NRF2 deficiency attenuates diabetic kidney disease in Db/Db mice via Down‐regulation of angiotensinogen, SGLT2, CD36, and FABP4 expression and lipid accumulation in renal proximal tubular cells. Antioxidants, 12, 1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, T. , Seki, S. , Hiramoto, K. , Naganuma, E. , Kobayashi, E. H. , Yamaoka, A. , Baird, L. , Takahashi, N. , Sato, H. , & Yamamoto, M. (2017). Hyperactivation of Nrf2 in early tubular development induces nephrogenic diabetes insipidus. Nature Communications, 8, 14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, R. J. , Chartoumpekis, D. V. , Rush, B. M. , Zhou, D. , Fu, H. , Kensler, T. W. , & Liu, Y. (2016). Keap1 hypomorphism protects against ischemic and obstructive kidney disease. Scientific Reports, 6, 36185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen, M. , Fagerberg, L. , Hallstrom, B. M. , Lindskog, C. , Oksvold, P. , Mardinoglu, A. , Sivertsson, A. , Kampf, C. , Sjostedt, E. , Asplund, A. , Olsson, I. , Edlund, K. , Lundberg, E. , Navani, S. , Szigyarto, C. A. , Odeberg, J. , Djureinovic, D. , Takanen, J. O. , Hober, S. , … Ponten, F. (2015). Proteomics. Tissue‐based map of the human proteome. Science, 347, 1260419. [DOI] [PubMed] [Google Scholar]
- Vallon, V. , & Verma, S. (2021). Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annual Review of Physiology, 83, 503–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst, D. , Carter‐Timofte, M. E. , van Grevenynghe, J. , Laguette, N. , Dinkova‐Kostova, A. T. , & Olagnier, D. (2022). Regulation of innate immunity by Nrf2. Current Opinion in Immunology, 78, 102247. [DOI] [PubMed] [Google Scholar]
- Vaziri, N. D. , Liu, S. , Farzaneh, S. H. , Nazertehrani, S. , Khazaeli, M. , & Zhao, Y. Y. (2015). Dose‐dependent deleterious and salutary actions of the Nrf2 inducer dh404 in chronic kidney disease. Free Radical Biology & Medicine, 86, 374–381. [DOI] [PubMed] [Google Scholar]
- Wang, C. , Luo, Z. , Carter, G. , Wellstein, A. , Jose, P. A. , Tomlinson, J. , Leiper, J. , Welch, W. J. , Wilcox, C. S. , & Wang, D. (2018). NRF2 prevents hypertension, increased ADMA, microvascular oxidative stress, and dysfunction in mice with two weeks of ANG II infusion. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 314, R399–R406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P. , Yang, Y. , Wang, D. , Yang, Q. , Wan, J. , Liu, S. , Zhou, P. , & Yang, Y. (2020). Cinnamaldehyde ameliorates vascular dysfunction in diabetic mice by activating Nrf2. American Journal of Hypertension, 33, 610–619. [DOI] [PubMed] [Google Scholar]
- Warady, B. A. , Pergola, P. E. , Agarwal, R. , Andreoli, S. , Appel, G. B. , Bangalore, S. , Block, G. A. , Chapman, A. B. , Chin, M. P. , Gibson, K. L. , Goldsberry, A. , Iijima, K. , Inker, L. A. , Kashtan, C. E. , Knebelmann, B. , Mariani, L. H. , Meyer, C. J. , Nozu, K. , O'Grady, M. , … Chertow, G. M. (2022). Effects of bardoxolone methyl in Alport syndrome. Clinical Journal of the American Society of Nephrology, 17, 1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. , Liu, X. , Fan, J. , Chen, W. , Wang, J. , Zeng, Y. , Feng, X. , Yu, X. , & Yang, X. (2014). Bardoxolone methyl (BARD) ameliorates aristolochic acid (AA)‐induced acute kidney injury through Nrf2 pathway. Toxicology, 318, 22–31. [DOI] [PubMed] [Google Scholar]
- Wu, Q. Q. , Wang, Y. , Senitko, M. , Meyer, C. , Wigley, W. C. , Ferguson, D. A. , Grossman, E. , Chen, J. , Zhou, X. J. , Hartono, J. , Winterberg, P. , Chen, B. , Agarwal, A. , & Lu, C. Y. (2011). Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARgamma, and HO‐1. American Journal of Physiology. Renal Physiology, 300, F1180–F1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, L. , Zhou, D. , Tan, R. J. , Fu, H. , Zhou, L. , Hou, F. F. , & Liu, Y. (2016). Sustained activation of Wnt/beta‐catenin signaling drives AKI to CKD progression. J Am Soc Nephrol, 27, 1727–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, G. , Gu, Y. , Yan, N. , Li, Y. , Sun, L. , & Li, B. (2021). Curcumin functions as an anti‐inflammatory and antioxidant agent on arsenic‐induced hepatic and kidney injury by inhibiting MAPKs/NF‐kappaB and activating Nrf2 pathways. Environmental Toxicology, 36, 2161–2173. [DOI] [PubMed] [Google Scholar]
- Yamamoto, M. , Kensler, T. W. , & Motohashi, H. (2018). The KEAP1‐NRF2 system: A thiol‐based sensor‐effector apparatus for maintaining redox homeostasis. Physiological Reviews, 98, 1169–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, H. Y. , Kang, N. I. , Lee, H. K. , Jang, K. Y. , Park, J. W. , & Park, B. H. (2008). Sulforaphane protects kidneys against ischemia‐reperfusion injury through induction of the Nrf2‐dependent phase 2 enzyme. Biochemical Pharmacology, 75, 2214–2223. [DOI] [PubMed] [Google Scholar]
- Younis, N. S. , Elsewedy, H. S. , Shehata, T. M. , & Mohamed, M. E. (2021). Geraniol averts methotrexate‐induced acute kidney injury via Keap1/Nrf2/HO‐1 and MAPK/NF‐kappaB pathways. Current Issues in Molecular Biology, 43, 1741–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. D. , Lo, S. C. , Cross, J. V. , Templeton, D. J. , & Hannink, M. (2004). Keap1 is a redox‐regulated substrate adaptor protein for a Cul3‐dependent ubiquitin ligase complex. Molecular and Cellular Biology, 24, 10941–10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, S. , Ghosh, A. , Lo, C. S. , Chenier, I. , Scholey, J. W. , Filep, J. G. , Ingelfinger, J. R. , Zhang, S. L. , & Chan, J. S. D. (2018). Nrf2 deficiency upregulates intrarenal angiotensin‐converting Enzyme‐2 and angiotensin 1‐7 receptor expression and attenuates hypertension and nephropathy in diabetic mice. Endocrinology, 159, 836–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, S. , Lo, C. S. , Miyata, K. , Ghosh, A. , Zhao, X. , Chenier, I. , Cailhier, J. F. , Ethier, J. , Lattouf, J. B. , Filep, J. G. , Ingelfinger, J. R. , Zhang, S. L. , & Chan, J. S. D. (2021). Overexpression of Nrf2 in renal proximal tubular cells stimulates sodium‐glucose Co‐transporter 2 expression and exacerbates Dysglycemia and kidney injury in diabetic mice. Diabetes, 70, 1388–1403. [DOI] [PubMed] [Google Scholar]
- Zheng, H. , Whitman, S. A. , Wu, W. , Wondrak, G. T. , Wong, P. K. , Fang, D. , & Zhang, D. D. (2011). Therapeutic potential of Nrf2 activators in streptozotocin‐induced diabetic nephropathy. Diabetes, 60, 3055–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoja, C. , Corna, D. , Nava, V. , Locatelli, M. , Abbate, M. , Gaspari, F. , Carrara, F. , Sangalli, F. , Remuzzi, G. , & Benigni, A. (2013). Analogs of bardoxolone methyl worsen diabetic nephropathy in rats with additional adverse effects. American Journal of Physiology Renal Physiology, 304, F808–F819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable—no new data generated.


