Key Clinical Message
Patients with an infected charcot spine (ICS) may experience little or no back pain despite severe vertebral destruction. Understanding the pathophysiology underlying ICS and its differential diagnoses is crucial for its accurate diagnosis. Worsening symptoms of chronic charcot spine should raise suspicions of an infection.
Keywords: diagnosis, differential, low Back pain, pain‐free, spondylitis
1. INTRODUCTION
Low back pain is an often encountered complaint among clinical physicians. Although it can be caused by various conditions, there may be diseases that, if overlooked, could lead to serious consequences. Examples include aortic aneurysm, pyogenic spondylitis, metastatic spinal tumors, etc. Charcot spine, which occurs as a result of syphilis or spinal cord injury, exhibits severe deformity despite mild lower back pain. Infected charcot spine (ICS), even if accompanied by fever, may not present lower back pain or only exhibit mild symptoms. As a result, the diagnosis may not be accurately made until the vertebral destruction progress severely and neurological paralysis emerges.
2. CASE
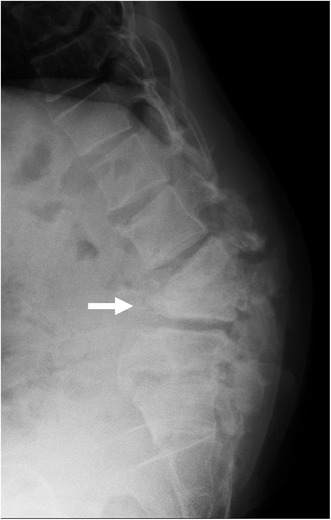
A 44‐year‐old man was admitted with complaints of weakness in the lower extremities. He had no lower back pain and walked unaided. Although he had no history of diabetes or spinal trauma, he had positive syphilis in serology. A physical examination revealed right tibialis anterior muscle weakness, decreased pain sensation in the lateral thigh, decreased vibration sensation in both medial calves, and bladder dysfunction. There was no heat or redness in the lumbar region. Blood tests showed C‐reactive protein (CRP) 30 mg/μL. X‐rays showed severe destruction of the L3 and L4 vertebra (Figure 1, arrow) and angulated kyphosis, and magnetic resonance imaging revealed fluid retention in the destroyed L3 and L4 vertebra (Figure 2, arrow). Under X‐ray fluoroscopy, the L3/4 intervertebral disc puncture revealed a small number of Gram‐negative rods in the fluid sample, and subsequent culture tests revealed Enterobacter cloacae. The severe spinal destruction without back pain was diagnosed as an ICS, and an anterior–posterior lumbar fusion was performed.
FIGURE 1.
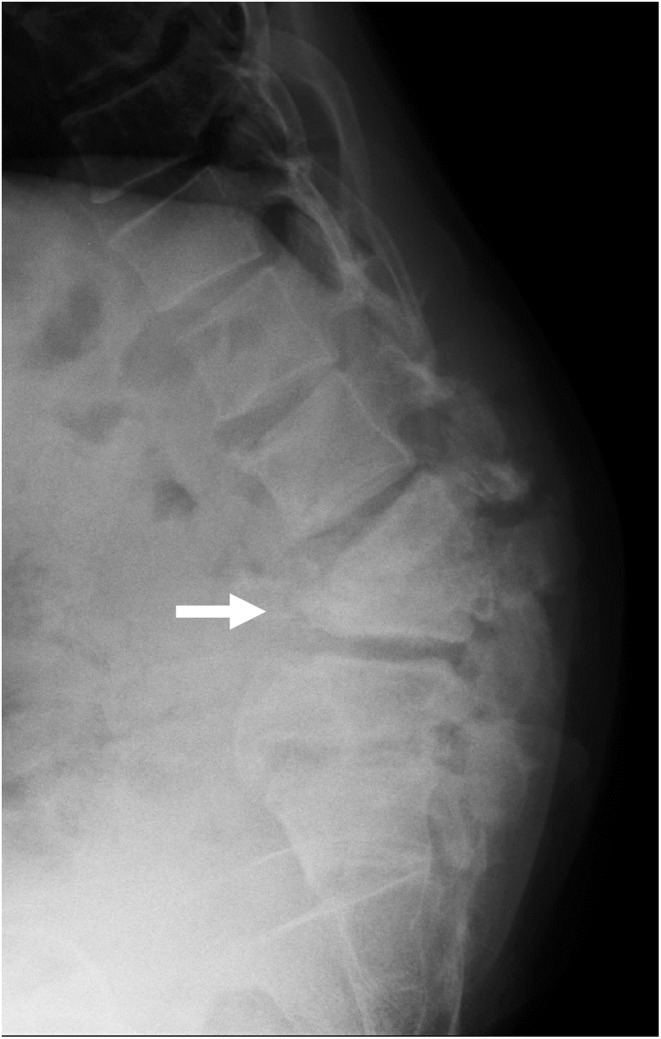
X‐ray of a 44‐year‐old man with infected charcot spine, showing severe destruction of the L4 vertebra(arrow) and angulated kyphosis.
FIGURE 2.
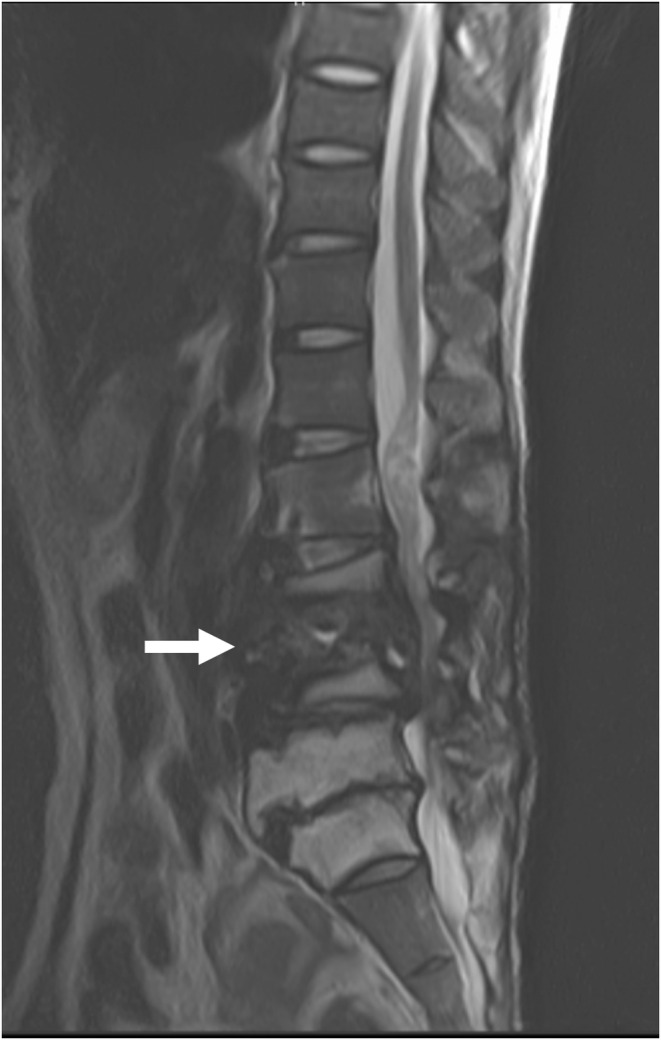
Magnetic resonance imaging showed fluid retention in the destroyed L4 vertebra(arrow).
3. DISCUSSION
Tabes dorsalis occurs 20–30 years after the initial infection of syphilis, causing progressive degeneration of the posterior columns and posterior nerve roots. As a result, proprioception and vibratory sensation are lost, contributing to the development of charcot spine and charcot joints. In this case, the patient had no low back pain, despite a paralyzing severe spinal deformity. The reason for facing difficulty in diagnosing such a condition lies in the pathophysiology of ICS, which results in few complaints of back pain due to diminished sensation, and the rapid progression of vertebral destruction due to concurrent infection. 1 Due to the paucity of symptoms, comprehensive examination by a physician may not be performed, resulting in a delayed diagnosis until there is evidence of progressive vertebral destruction or the onset of nerve palsy. What points should be noted when diagnosing ICS? First, it is crucial to understand the symptoms of charcot spine. Churruca et al. 2 reported that 92% of charcot spine cases exhibit any symptoms. Specifically, these include increased spasticity, reflex disorders, autonomic nervous system symptoms, and pain. Second, having knowledge of differential diagnosis is essential. Differential diagnoses should include pyogenic vertebrates, osteomyelitis, Paget's disease of bone, and destructive tumors. 3 While ICS is rare, it should be included in the differential diagnoses for febrile patients with a history of syphilis or spinal cord injury. Early diagnosis allows prompt initiation of suitable treatments, preventing severe spinal damage and nerve paralysis.
AUTHOR CONTRIBUTIONS
Hiromu Yoshizato: Conceptualization; writing – original draft. Tadatsugu Morimoto: Conceptualization; writing – original draft. Toshihiro Nonaka: Writing – review and editing. Hirohito Hirata: Writing – review and editing. Masaaki Mawatari: Writing – review and editing.
FUNDING INFORMATION
The authors have no current financial arrangement or affiliation with any organization that could directly influence their work.
CONFLICT OF INTEREST STATEMENT
We do not have any conflict of interest.
STATEMENTS RELATING TO ETHICS AND INTEGRITY POLICIES
We declare that we follow the ethical policies of the journal, including patient consent, disclosure of funding information, and data availability.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Yoshizato H, Morimoto T, Nonaka T, Hirata H, Mawatari M. Infected charcot spine. Clin Case Rep. 2024;12:e8490. doi: 10.1002/ccr3.8490
DATA AVAILABILITY STATEMENT
Additional data related to this patient case, beyond what is presented in this publication, are not publicly available to maintain patient consent, confidentiality, and anonymity.
REFERENCES
- 1. Karthik Yelamarthy P, Krishna RT, Mahajan R, et al. Infected charcot spine arthropathy. Spinal Cord Ser Cases. 2018;4:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arco Churruca AD, Vázquez Bravo JC, Álvarez SG, Donat SM, Llona MJ. Charcot arthropathy in the spine. Experience in our centre. About 13 cases. Review of the literature. Rev Esp Cir Ortop Traumatol. 2021;65:461‐468. [DOI] [PubMed] [Google Scholar]
- 3. Suda Y, Saito M, Shioda M, Kato H, Shibasaki K. Infected charcot spine. Spinal Cord. 2005;43:256‐259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional data related to this patient case, beyond what is presented in this publication, are not publicly available to maintain patient consent, confidentiality, and anonymity.


