Highlights
-
•
AiV-A1 replicates in ApoA1 and Ki-67 positive cells of stem cell-derived human small intestinal epithelium model.
-
•
AiV-A1 infection of intestinal cells promotes modulation of the antiviral state.
-
•
AiV-A1 replicates in stem-cell-derived human tracheal/bronchial epithelium model.
-
•
AiV-A1 entry in A549 lung cells is dependent on clathrin, dynamin, and lipid rafts.
Keywords: Aichivirus, Virus entry, Gastrointestinal infection, Lung Infection
Abstract
The role of aichivirus A1 (AiV-A1) in acute gastroenteritis remains controversial and in vitro data illustrating its pathogenesis in suitable human models are scarce. Here, we demonstrate that AiV-A1 isolate A846/88 replicates in ApoA1- (absorptive) and Ki-67-positive (proliferative) enterocytes in stem cell-derived human small intestinal epithelium (HIE) as well as in patient biopsy samples, but not in any of the tested human cell lines. The infection did not result in tissue damage and did not trigger type I and type III interferon (IFN) signalling, whereas the control, human coxsackievirus B3 (strain Nancy), triggered both IFNs. To investigate the tissue tropism, we infected a human tracheal/bronchial epithelium model (HTBE) with AiV-A1 isolates A846/88 and kvgh99012632/2010 and, as a control, with rhinovirus A2 (RV-A2). AiV-A1 isolate kvgh99012632/2010, but not isolate A846/88, replicated in HTBE and induced type III IFN and ISGs signalling. By using various pharmacological inhibitors, we elaborated that cellular entry of AiV-A1 depends on clathrin, dynamin, and lipid rafts and is strongly reliant on endosome acidification. Viral particles co-localised with Rab5a-positive endosomes and promoted leakage of endosomal content. Our data shed light on the early events of AiV-A1 infection and reveal that different isolates exhibit distinct tissue tropism. This supports its clinical importance as a human pathogen with the potential to evolve toward broader tissue specificity.
1. Introduction
According to the Global Burden of Diseases Study from 2019 (Vos et al., 2020) acute gastroenteritis (AGE) was the fifth-leading cause of fatalities for all age groups and the third for children younger than 10 years worldwide. While bacteria and protozoa only account for approximately 10 % of AGE cases in children, viruses are responsible for approximately 70 % (Elliott, 2007). The most prominent viral representatives are rota-, noro-, sapo-, astro-, and adenovirus. Despite more than 20 viruses having been identified as aetiological agents for AGE, it is estimated that in up to 40 % of incidences, the aetiology remains unknown (Holtz et al., 2009). Improved detection methods, such as next-generation sequencing, resulted in the discovery of previously unknown enteric viruses (Oude Munnink and van der Hoek, 2016). However, for aichivirus A1 (AiV-A1), an unequivocal link to AGE still has to be established.
Since its discovery in 1989, during a large-scale outbreak of acute gastroenteritis in Japan involving several elementary schools, AiV-A1, one of the six species of the genus Kobuvirus within the large family of Picornaviridae, has been detected worldwide in human faecal samples (Kitajima and Gerba, 2015). In addition, AiV-A1 was found in environmental specimens, including water from rivers, groundwater, and sewage, suggesting transmission via the faecal-oral route (Reuter et al., 2011). Seroprevalence studies around the world determined that 80–95 % of adults older than 40 years have antibodies against AiV-A1 and to a lesser extent at younger ages (Rivadulla and Romalde, 2020). This suggests a frequent circulation of AiV-A1 in the human population. However, worldwide surveys revealed disease incidence rates of just 0.9 % – 4.1 % fuelling the ambiguity of other epidemiological observations (Jonsson et al., 2012). Additionally, in vitro data are scarce and many infections remain subclinical while symptomatic cases experience diarrhoea, abdominal pain, nausea, vomiting, and fever and are often associated with the consumption of seafood (Yamashita et al., 2001). Compared to European or Asian countries, Tunisia reported a high number of infections and relatively high rates of hospitalisation in children due to AiV-A1, ranking it as the third most frequently detected agent after rotavirus and norovirus (Sdiri-Loulizi et al., 2010). Along these lines, recently developed highly multiplexed antibody measurements on a population scale delineated high-prevalence epidemic waves for Aichivirus A, beside enterovirus D68 (EV-D68), in the summer of 2008 in South Africa (Kelley et al., 2023).
Alongside gastrointestinal ailments, two AiV-A1-positive patients were reported with respiratory symptoms (Reuter et al., 2009; Yamashita et al., 1993). Moreover, AiV-A1 was found to be responsible for severe chronic multi-organ disease and chronic kidney failure in an X-linked agammaglobulinemia patient (Bucciol et al., 2018, 2021). This initial observation led to the discovery of more immune-compromised patients suffering from persistent AiV infection highlighting it as a potential emerging opportunistic pathogen for patients with X-linked agammaglobulinemia and secondary B-cell deficiency (Meyts et al., 2023). Kobuviruses have been detected in a number of hosts from distinct taxon raising the possibility for cross-species transmission (Lu et al., 2018). This is a major concern since many pathogens of relevance for public health are zoonotic as recently seen with the COVID-19 pandemic (Holmes et al., 2021; Woolhouse and Gowtage-Sequeria, 2005). Furthermore, some studies correlated bovine and porcine kobuviruses to diarrhoea in calves and pigs (Hao et al., 2021; Zhai et al., 2017).
The family Picornaviridae includes significant human pathogens (e.g. poliovirus, EV-D68, and EV-71, amongst many others) (King et al., 1999; Yamashita et al., 1998). Currently, one of the 68 picornavirus genera is contributed by Kobuvirus, which encompasses species A, B, C, and D; one of them, Aichivirus A includes ten types (A1–A10) with aichivirus A1 being considered a putative pathogen for humans. So far, two isolates of AiV-A1, A846/88 and kvgh99012632/2010, have been recovered from human samples; types A2–A10 have been detected in various animals (Rivadulla and Romalde, 2020).
The viral particle is non-enveloped with a diameter of approximately 30 nm. It contains a ∼8200 nt long ss(+) RNA genome with one open reading frame encoding a 2433 amino acid polyprotein. This precursor is co-, and post-translationally cleaved into the leader (L) protein, the structural proteins P1 (VP0, VP3, VP1) and the non-structural proteins P2 (2 A, 2 B, 2 C) and P3 (3 A, 3 B, 3 C, 3 D) (Sasaki et al., 2012, 2001). AiV-A1 kvgh99012632/2010 differs in 26 amino acids residues from A846/88 in the capsid proteins (15 in the VP0, 2 in VP3 and 9 in VP1).
The AiV capsid proteins are unusually high in proline and have three poly-l-proline type II (PPII) helices, which are unique in picornaviruses; one in VP0 and two in VP1. The longest PPII helix is at the C-terminus of VP1 pointing outwards from the capsid. It is thus potentially involved in receptor interaction. Incubation of AiV-A1 A846/88 with Vero cells together with a synthetic peptide derived from the long PPII helix reduced the cytopathic effect of the virus by about 40 % at 48 hpi, suggesting competition for the receptor (Zhu et al., 2016).
The mechanism of genome release of AiV-A1 was tentatively explained by comparing the 3D structures of native and empty capsids; heating native virions results in an expanded particle with disordered N-terminus of VP0 (Sabin et al., 2016). This corroborates the hypothesis of ordered egress of the genome observed for other picornaviruses (Bostina et al., 2011; Real-Hohn et al., 2020). AiV-A1 genome release was further investigated by using lipid membrane models and low pH as a trigger, expounding the role of the VP0 N-terminus in the interaction with the cellular membrane and the formation of a pore allowing genome egress (Kelly et al., 2022).
Recently, it was demonstrated that replication of AiV-A1 and cholesterol transport was controlled by interferon-induced transmembrane protein 1, reinforcing its role as an antiviral restriction factor and of AiV-A1’s dependence on cholesterol for the routing to replication centres (Ishikawa-Sasaki et al., 2023). Moreover, the aichivirus 2 A protein was observed to be crucial for RNA (+/-) strand synthesis, diverging from the role of polyprotein proteinase as in other picornaviruses (Sasaki and Taniguchi, 2008).
Although some of the features of AiV-A1 infection have been clarified for isolate kvgh99012632/2010 in different human cell lines and isolated mouse intestinal epithelial cells, a human intestinal cell line supporting productive replication and the cellular tropism in the human intestine remains unknown. Furthermore, an in-depth investigation of cell entry is lacking. Here, we selected the two documented human AiV-A1 isolates for further investigation using intestinal and lung tissue models in combination with human intestinal cell lines to elaborate AiV-A1 cellular and tissue tropism. Additionally, we delineate the early events of AiV-A1 infection, demonstrate infection in a lung tissue model, and highlight the potential for persistent infection.
2. Material and methods
2.1. Cell lines and virus isolates
All cell lines were obtained from ATCC, except from A549, RKO and HCEC-1CT; originally purchased from Sigma, (Sigma-Aldrich, 86,012,804, Germany), these were kindly provided by Dr. Walter Berger (Institute of Cancer Research, Medical University of Vienna, Austria). RKO cells (originally from ATCC, CRL-2577) were a generous gift from Dr. Gijs Versteeg (Max Perutz Labs, Vienna, Austria). HCEC-1CT cells, originally from Jerry W. Shay (University of Texas Southwestern Medical Centre, Dallas, Texas), were a gift from Dr. Thomas Lion (Children's Cancer Research Institute, Vienna, Austria). HeLa, RKO, Vero and Caco-2 cells were cultivated in DMEM, 10 % FBS and 1 % Pen-Strep; for Caco-2 cells the media contained additionally 0.1 mM non-essential amino acids and 1 mM pyruvate. A549 and T84 cells were maintained in Ham's F-12 K Medium instead of DMEM. HCEC-1CT cells were cultured in DMEM and Medium 199 Earle's (4 + 1), 4 mM GlutaMAX™-1 (100x), 2 % Cosmic Calf Serum, 20 ng/ml EGF, 10 μg/ml Insulin, 2 μg/ml apo-transferrin, 5 nM sodium-selenite, 1 μg/ml hydrocortisone and 1 % Pen-Strep. For infection assays, the standard medium was replaced with infection medium (containing just 2 % FBS but otherwise identical) prior to viral inoculation (Table 1).
Table 1.
Immortalized cell lines that were tested for AiV-A1 infection. Cell lines were cultivated in the respective growth medium, as specified by ATCC, and infected in the correspondently adapted infection medium.
| Cell line | Tissue | Growth medium | Infection medium |
|---|---|---|---|
| HeLa | Epithelial, human cervix |
|
|
| RKO | Epithelial, human colon | ||
| Vero | Epithelial, African green monkey kidney | ||
| A549 | Epithelial, human lung |
|
|
| T84 | Epithelial, human colon | ||
| Caco-2 | Epithelial, human colon |
|
|
| HCEC 1CT | Epithelial, human colon |
|
|
Viruses used in this study were (i) AiV-A1 isolate A846/88 (obtained by transfection with RNA transcribed from the plasmid pAV-UCSF (GenBank accession no. JQ281544; a kind donation from Joseph L. DeRisi University of California at San Francisco, USA (Greninger et al., 2012)), (ii) CVB3 isolate Nancy (kind donation from Frank van Kuppeveld, University of Utrecht, Netherlands) and (iii) AiV-A1 kvgh99012632/2010 (generous donation from Dr. Tsung-Hsien Chang, National Defence Medical Centre, Taipei, Taiwan).
2.2. RNA-lipofectamine transfection
AiV-A1 A846/88 RNA was generated by in vitro transcription using the T7 Ribomax Large Scale RNA Production System (Promega, USA) according to the manufacturer's instructions and recovered with TRIzol reagent (TRIzol, Invitrogen, USA); the pAV-UCSF plasmid was linearised by restriction at the 3′ end of the viral genome with HindIII. One, 2, and 4 µl of lipofectamine 2000 (Invitrogen, USA) was mixed with 100 µl of Opti-MEM (Gibco, USA). In parallel, three times 1 µg of viral RNA was mixed with 100 µl of Opti-MEM. Each of the above three (1, 2, and 4 µl) lipofectamine mixes was then combined with one of the diluted RNA mixes and incubated for 10 min at RT, followed by dropwise addition to the medium of the cells and gentle swirling. Three days post transfection the contents of the wells were collected using a cell scraper and progeny virus was released by three freeze-thaw cycles. From the clarified SN infectious virus was determined by endpoint titration expressed as 50 % tissue culture infectious doses (TCID50) per ml.
2.3. Reverse transcription and quantitative PCR (RT-qPCR)
A two-step RT-qPCR was used to measure host and viral gene expression. Total RNA was extracted using the TRIzol reagent according to the manufacturer's instructions. The RNA was reverse transcribed using the M-MLV reverse transcriptase according to the manufacture's (Promega) instruction. In brief, 1 µg RNA was incubated with 0.5 µg of random hexamer primers (Promega). qPCR was then performed using the GoTaq DNA polymerase (Promega) according to the manufacture's instruction with slight modifications; SYTO 82 and primers were added to a final concentration of 5 µM and 0.5 µM, respectively, per reaction. Gene expression was calculated according to the ΔΔCt-method. Viral RNA was normalised either to the human 18S rRNA or to Cyclophillin A (PPIA). Primers used for qPCR analysis are listed in Table S1.
2.4. Susceptibility of different cell lines for AiV-A1 A846/88 and AiV-A1 kvgh99012632/2010
For cold-synchronised infection, each cell line was seeded into three wells, designated mock, T0, and T72 (i.e. hours pi) of a 24-well plate. Medium was replaced with infection medium and the plate was placed at 4 °C for 30 min. Two wells of each cell line received either AiV-A1 A846/88 or AiV-A1 kvgh99012632/2010 (MOI=0.1) while one well was mock-infected. The cells were incubated for 1 h at 4 °C with gentle shaking, allowing maximal virus attachment. The cells were then washed three times with ice-cold PBS, infection media was replenished and the “T0 cells” were collected together with the SN using a cell scraper. The plate was subsequently placed at 37 °C to trigger a synchronized virus internalisation. Three days pi, still attached cells (“T72”) were examined for the development of a CPE via light microscopy and collected as above. For titration and vRNA quantification or for immunofluorescent imaging each immortalized cell line was infected with AiV-A1 kvgh99012632/2010 either with MOI=0.1 or with MOI=1 for 1 h at 37 °C, respectively. After incubation, the cells were washed three times with ice-cold PBS and incubation continued at 37 °C. At the indicated times, the SN were collected and the cells were dissolved in TRIzol reagent. For the immunofluorescent microscopy, the infection was stopped after 8 hpi by paraformaldehyde fixation.
2.5. Human intestinal and tracheal/bronchial epithelium models
The HTBE (EpiAirway AIR-100) and the HIE (EpiIntestinal SMI-100) model tissues were acquired from MatTek (Ashland, USA). Both models were maintained according to the manufacturer's instructions. For infection of the HIE, the inserts were exposed apically and basolaterally either to the AiV-A1 isolate A846/88 or the CVB3 strain Nancy (positive control) with 107 PFU (as determined in Vero cells) in maintenance medium for 1 h at 37 °C. The HIE were then washed once in PBS and further cultivated in maintenance medium (5 ml basolaterally and 200 µl apically). One infection assay comprised five HIE inserts (insert 1 to insert 5). In 24-hour intervals the basolateral and apical media of all tissues were gently collected for titration and replaced with new maintenance medium by gently pipetting along the wall. At the indicated times, the respective membranes together with the attached HIE (insert 1 at T0, insert 2 at T24, etc.) were cut out with a scalpel and combined with TRIzol reagent for RNA extraction and subsequent RT-qPCR.
For infection of the HTBE, the inserts were exposed apically and basolaterally to AiV-A1 kvgh99012632/2010, AiV-A1 A846/88, and rhinovirus A2 (RV-A2, an additional control for productive infection) at 107 PFU in maintenance medium for 1 h at 37 °C. After incubation, the inserts were washed once in PBS and the maintenance medium was replenished at the basolateral side only, (5 ml). For inserts T0 to T48, 200 µl of maintenance medium was added in 24-hour intervals to the apical side for virus collection and subsequent titration as in the HIE, while inserts T72 to T120 only were collected at the indicated times. Additionally, the respective membranes were cut out with a scalpel and combined with TRIzol reagent for RNA extraction and subsequent RT-qPCR. The basolateral media was replenished every 24 h.
2.6. Immunofluorescence microscopy
Media from HIE and HTBE inserts were removed, and the epithelium immediately fixed with 4 % paraformaldehyde in PBS for 30 min at RT. The tissue was then either processed for orthogonal section imaging, which required cryo-sectioning (described further below) or for top-view imaging. For top-view imaging, all steps of the immunofluorescence staining were conducted while the tissue was still on the membrane of the Transwell insert. After fixation, the epithelium was washed three times with cold PBS and the cell membrane was permeabilized with 0.25 % Triton X-100 in PBS for 5 min at RT. The tissue was then washed again three times in PBS for 5 min each and transferred into blocking solution (2 % BSA, 0.1 % Tween 20 in PBS) for 30 min at RT. Subsequently, the tissue was incubated with the primary antibody diluted in blocking solution for 1 h at RT and washed again three times with PBS for 5 min each. This was followed by incubation with the secondary antibody diluted in blocking solution for 1 h at RT. The tissue was then washed once with PBS for 5 min and counterstained with Hoechst 33,258 (final concentration 1 µg/ml in blocking solution) for 10 min at RT. Finally, the epithelium was washed three times with PBS for 5 min each, the membrane carrying the tissue was cut out using a scalpel and mounted with Prolong Glass Antifade Mountant (Invitrogen, P36982, USA). For orthogonal section imaging, a cryosection (described below) was thawed at RT and washed with PBS for 5 min, followed by quenching with 0.1 M glycine in PBS for 10 min at RT. After washing in PBS for 5 min, the section was blocked in 2 % BSA in PBS for 30 min at RT. Then the primary antibody diluted in PBS and 0.1 % Tween 20 was applied for 1 h at RT. After washing with PBS for 5 min, the secondary antibody (in PBS, 0.1 % Tween 20) was applied for 1 h at RT. Working dilutions of antibodies were the same as above. After washing or 5 min with PBS, the nuclei were stained with Hoechst 33,258 as above. Finally, the section was washed once with PBS for 5 min, then with ddH2O and air dried followed by mounting with Prolong Diamond Antifade Mountant (Invitrogen, P36961). The following antibodies were used: anti-dsRNA (J2–1301, English & Scientific Consulting), anti-VP1 AiV-A1 (provided by Dr. Tsung-Hsien Chang, Taiwan described in Chen et al. (2013)), ApoA1 (Invitrogen, PA5–88,109) and Ki-67 (Abcam, ab15580). Images were acquired using a Zeiss Axio Observer Z1 inverted fluorescence microscope and a Zeiss LSM 700 laser scanning confocal microscope. Images were analysed with Fiji software.
2.7. Cryo-sectioning
After fixation, the tissue was washed three times with PBS and incubated in 30 % sucrose in PBS o/n at 4 °C. The tissue was subsequently mounted on cryo-moulds using the Tissue-Tek OCT-compound (Sakura, Netherlands) and shock frozen in isopentane cooled with dry ice. The frozen epithelium was then cut into 5 µm thick orthogonal sections with a Cryostat Microm HM 500 OM (Histocom, Switzerland) and stored at −80 °C until further usage.
2.8. Immunohistochemistry
A cryosection was thawed at RT and washed with ddH2O for 5 min, followed by incubation in Dako REAL Peroxidase-Blocking Solution (DAKO, S2023, USA) for 7 min at RT and washed again three times with TBS (1.4 M NaCl, 26 mM KCl and 247 mM Tris pH 8.0). The section was blocked with 2 % BSA in TBS for 1 h at RT and further incubated with the dsRNA-specific monoclonal antibody J2 (2 µg/ml in TBS and 0.1 % Tween 20). After three washes in TBS, the section was incubated with the secondary antibody conjugated to biotin (DAKO, E0413, 1:200 in TBS and 0.1 % Tween) for 30 min at RT. The section was washed again 3 times for 5 min each with TBS and then incubated with 120 µl of avidin-biotin-complex (ABC) reagent for 30 min at RT. For J2 signal detection, the section was first washed 3 times with PBS for 5 min each; a few drops of DAB+ (Dako, K3468) were then added to the section and incubated for 2 min. Signal development was observed continuously under the microscope. When the signal was strong enough, the reaction was stopped with ddH2O. For the detection of sulphated and carboxylated proteoglycans (mucus, goblet cells, etc.), the section was washed with ddH2O for 5 min, followed by incubation in alcian-blue solution (Merck, 1,016,470,500, Germany) for 5 min. The section was then washed again with running tap water for 3 min and rinsed with ddH2O. Counterstaining was conducted with nuclear fast red-aluminum sulfate solution 0.1 % (Sigma-Aldrich, 1,001,210,500) for 10 min, residual stain was washed off with running tap water for 5 min and the section was rinsed with ddH2O. For storage, the section was dehydrated through immersion into 50 %, 70 % and 100 % EtOH in ddH2O, for 1 min each. It was then incubated in n‑butyl acetate for 5 min, air-dried and mounted with Eukitt mounting medium (Sigma-Aldrich, 25,608–33–7).
2.9. Cytotoxicity assays
The method was adapted from Denani et al. (2021), using the LDH-Glo™ cytotoxicity assay kit (Promega) to measure virus-induced cell death in the HIE. After infection with AiV-A1 A846/88, the apical SN was collected in 24-hour intervals from infected and non-infected (Mock) epithelium and initially diluted 1:5 in LDH Storage Buffer (200 mM Tris–HCl pH 7.3, 10 % Glycerol, 1 % BSA) to reduce enzymatic degradation when stored at –20 °C. As positive control (maximum cell death), epithelia were exposed to Triton X-100 (final concentration 0.25 % in maintenance media) for 30 min at RT. A standard curve, using the LDH positive control included in the kit, was established to determine the linear range of the assay. The maximum cell death samples were serially diluted to fit within the linear range and to infer the necessary dilution factor to be used in the measurement of the LDH activity for the viral infected HIE samples. Based on that outcome, the dilution factor was set to 1:2000 for all samples. The measurements were carried out according to the manufacturer's instructions in 96-well plates in a Synergy H1 plate reader (BioTek, USA).
For the drug screening assay, 5000 A549 cells re-suspended in the growth medium were added to each well of a 384 well-plate and incubated overnight at 37 °C, 5 % CO2. On the next day, the medium was changed to serum-reduced infection medium containing a specific test compound dissolved in 5 % DMSO or only infection medium containing 5 % DMSO as a negative control. The plate was then placed back in the incubator for an additional 24 h at 37 °C, 5 % CO2. After 22 h two wells received Triton-X100 at a final concentration of 1 % serving as a positive control of cytotoxicity. After 24 h, an equal volume of CellTiter-Glo Reagent (Promega, 100 μl/well) was added to all wells, and the luminescence was measured with a VICTOR Nivo plate reader (PerkinElmer, USA) after 5 to 10 min.
2.10. Patient-derived intestinal tissue
A single sample of human intestinal tissue was kindly donated by Univ. Prof. Dr. Martin Klimpfinger from the Klink Favoriten in Vienna, Austria. It consisted of a routine biopsy specimen obtained during a Whipple`s operation from an anonymous patient without any possibility of backtracking. Therefore, according to the managing director Reinhard Undeutsch the approval of an ethics committee of the town of Vienna was not required. Immediately after sample collection, the tissue was washed thoroughly with ice-cold Krebs-Henseleit buffer (KHB) prepared according to de Graaf et al. (2010) (118 mM NaCl, 5 mM KCl, 1.1 mM MgSO4, 1.25 mM CaCl2, 1.2 mM KH2PO4, 25 mM NaHCO3, 25 mM d-glucose, 9.0 mM Hepes, pH 7.4). The layers of serosa, muscularis, and submucosa were removed and the tissue was further cut into smaller pieces (approximately 1 × 1 cm) and maintained in RPMI 1640 medium (Gibco, 11,875,093) containing 10 % FBS (Sigma, F7524) and 1 % Pen-Strep (Sigma, 15,140–122) at 37 °C and 5 % CO2. For infection, the tissue was incubated with 107 PFU of AiV-A1 A846/88 for 1 h at 37 °C, washed with KHB buffer and the incubation continued at 37 °C in RPMI 1640. Twenty-four hours later the tissue was fixed with 4 % paraformaldehyde in PBS at 4 °C o/n and further processed for cryo-sectioning and immunofluorescence staining as outlined above.
2.11. Cell entry and intracellular routing of AiV-A1 isolate KVGH99012632/2010
Cells were grown to 80 % confluency in 6-well plates and treated with inhibitors specific for various entry pathways diluted in infection medium for 30 min at 4 °C prior to AiV-A1 infection. The virus was added at MOI=1 and further incubated at 4 °C for 30 min. The cells were then transferred to 37 °C for additional 8 h. Finally, the medium was discarded, the cells were washed with PBS, TRIzol was added directly to the wells and the lysate was used for RNA extraction und subsequent RT-qPCR.
Chlorpromazine, MβCD and jasplakinolide were found to be toxic for A549 cells after 24 h of incubation. For the RT-qPCR with MβCD and Jasplakinolide, cells were preincubated with each drug for just 15 min at 4 °C, followed by a 1 h incubation in the presence of AiV-A1 at 37 °C. After removing the virus and compound-containing medium, cells were washed with PBS, and fresh infection medium containing 50 mM NH4Cl was added. This weak base efficiently blocks AiV-A1 isolate kvgh99012632/2010 endosomal uncoating as seen for other picornaviruses (Ganjian et al., 2017). The infection was allowed to continue for additional 8 h, the cells were then washed with PBS, lysed with TRIzol and processed as above.
For early endosome labelling, A549 cells were cultured until 50 % confluent and infected for 20 h with a recombinant baculovirus expressing a GFP-tagged Rab5a (green colour). AiV-A1 at (MOI=50) was added without media change and allowed to attach to A549 cells for 30 min at 4 °C. Unattached virus was removed and cells were washed 3 times with cold PBS. New warm infection medium was added, the cells were kept at 37 °C for 30 min, washed as before and fixed in 4% PFA for immunofluorescence analysis.
3. Endosome integrity tracked by live microscopy
Quinacrine (QC) is a fluorophore with a high quantum yield and stable at low pH (Tamura et al., 2006). QC was internalized into the cells simultaneously with AiV-A1 kvgh99012632/2010 or control virus (RV-A2). QC accumulated in intracellular vesicles was used to detect the loss of the fluorescence signal due to endosome leakage, putatively promoted by virus rupturing of the endosomal membrane. A549 cells were grown to 70 % confluency on a glass-bottom dish from MatTek (Ashland, USA). Cells received AiV-A1 (MOI=10) diluted in infection medium containing 1 µM QC and 50 mM NH4Cl or, as a non-disruptive control (Prchla et al., 1995), RV-A2 (MOI=10) also diluted in the same medium. Control cells receiving no virus was evaluated (mock infection). The cells were then incubated at 37 °C in the dark for 30 min and washed three times with PBS containing 50 mM NH4Cl. After the last washing, cells were kept in PBS containing 50 mM NH4Cl and quickly transferred to a light microscope. After selecting the cells to be imaged (field with 5–10 non-overlapping cells), the PBS containing 50 mM NH4Cl was replaced by PBS without NH4Cl and images were directly recorded for 10 min using 15 s intervals. Cells subjected to each condition were treated and imaged individually (n = 3 for each condition). The fluorescence signal intensity of two to three vesicles in the imaged cells was quantified and plotted against time. Only vesicles with diameter about 500 nm were used for quantification. Signal detection and quantification were performed using Fiji 2.9.0 (ImageJ distribution) and plotted using GraphPad Prism 8.
3.1. Statistical analyses
Statistical analyses were performed with GraphPad Prism (Version 8.4.0. (455) for macOS) (GraphPad Software, USA). Data are shown as mean ± standard error of the mean (SEM). Shapiro-Wilk analysis was used to check for normal distribution of data for parametric testing. Two-way ANOVA with Sidak's test for multiple comparisons were used to determine statistical significance.
4. Results
4.1. Identification of human cell lines permissive for AiV-A1 infection
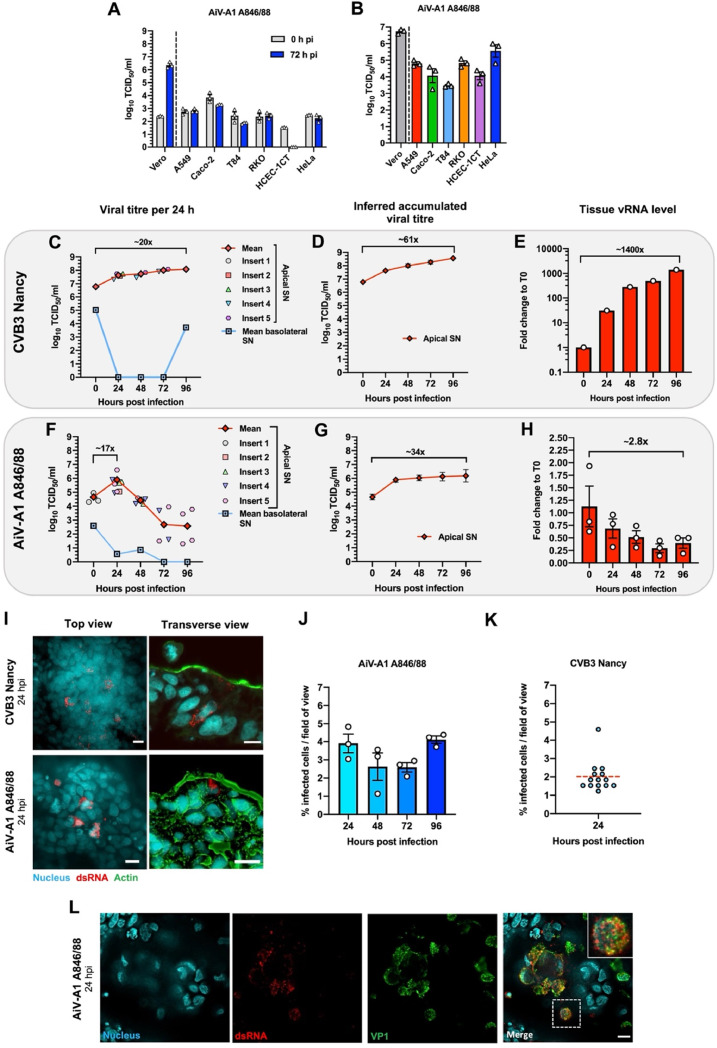
First, we analysed various human intestinal cell lines for permissiveness toward AiV-A1 A846/88 (GenBank accession no. AB040749.1), utilizing Vero cells as positive control (Fig. 1A). We also included the human bronchial cell line A549 since it had been shown to allow AiV-A1 isolate kvgh99012632/2010 replication (Chen et al., 2013). The comparison between the viral titre directly after infection and three days post infection (pi) showed that A846/88 failed to productively infect A549 cells (Fig. 1A). In Vero cells, the titre increased by more than 3 logs under the same conditions. Furthermore, none of the other cell lines were permissive for the A846/88 virus. Except for HCEC-1CT cells, a low titre was observed at 0 h pi, which might indicate residual A846/88 remaining bound to these cells.
Fig. 1.
AiV-A1 A846/88 does not infect prototypic human immortalised cell lines but moderately replicates at the apical side of HIE. (A) AiV-A1 A846/88 was pre-absorbed (MOI=0.1) to the indicated cell lines for 1 h at 4 °C. The cells were washed, medium was added, and the contents of the wells were collected separately at 0 hpi (T0) and 72 hpi (T72). Note that no titre was detected for HCEC-1CT cells at 72 hpi. N = 3. (B) Each cell line was transfected with 1 μg of AiV-A1 A846/88 RNA and different amounts of lipofectamine 2000 (1, 2 and 4 μl); after three days the well contents were collected. Each bar represents the average from the three different lipofectamine amounts. (C–H) HIE-containing wells were simultaneously infected apically and basolaterally with 107 PFU of AiV-A1 A846/88 or CVB3 Nancy for 1 h at 37 °C. After viral challenge, the epithelia were washed once with PBS before continuing the infection at 37 °C. In 24-hour intervals (T0–T96) the basolateral and apical media of the HIE were collected and replaced with new maintenance medium. At the indicated times, the respective membrane was cut out for total RNA extraction. (C and F) Plots of the viral titres in the apical and basolateral SN collected from infected HIE in 24-hour intervals. Note that no progeny virus was detected at some time points in the basolateral SN. Each symbol represents an individual HIE insert. (D and G) Graphs showing the average of the inferred cumulative amount of virus produced per HIE insert at each time point pi. (E and H) vRNA determined by RT-qPCR at the given times. In (F) N = 1 and in (I) N = 3. (I) Top and transverse views of HIE at 24 hpi labelled with mAb J2 antibody (dsRNA - red), Hoechst (nuclei – cyan) and phalloidin (actin filaments - green). Scale bars, 10 μm. (J) Quantification of AiV-A1 A846/88 VP1-positive cells in HIE per 10 fields of view for each time point. N = 3. (K) Quantification of CVB3 dsRNA-positive cells in HIE per 13 fields of view at 24 hpi. N = 1. (L) Immunofluorescence images stained for dsRNA (red) and viral capsid protein VP1 (green) 24 h after AiV-A1 challenge. The box represents the zoomed area shown at the right. Scale bars, 10 μm. Shown is the mean ± SEM.
We thus decided to test the cells' intracellular machinery for its capacity to support virus replication. The virus entry-uncoating steps were bypassed by direct delivery of the A846/88 genome through transfection using 1 µg RNA and different amounts of lipofectamine. Three days post transfection all cell lines shown in Fig. 1B exhibited considerable viral titres, with Vero cells exhibiting a 100-fold increase from day one to day three. This suggests that only Vero cells possess a receptor suitable for AiV-A1 A846/88 entry and uncoating; furthermore, Vero cells are particularly efficient in AiV-A1 replication.
4.2. AiV-A1 A846/88 replicates in a human-derived intestinal epithelium model
Since AiV-A1 A846/88 failed to infect any of the tested cell lines except Vero cells, we turned to a stem cell-derived human small intestinal epithelium (HIE) model, which more closely recapitulates the cellular diversity of the small intestine including conservation of cell polarity. Removable inserts with a porous membrane create a liquid-liquid interface where cell growth and differentiation occur at the membrane surface creating an apical and a basolateral side. Since there was no information on eventually preferred apical or basolateral entry, infection was initiated simultaneously from both sides. Human coxsackievirus B3 strain Nancy (CVB3) was used as a positive control as it had been described to replicate in intestinal enteroids from foetuses (Drummond et al., 2017).
After infection, the membranes with the attached HIE were cut from the inserts and the total cellular and viral RNA were extracted. The control, CVB3, replicated well in the organoids, generating viral titres in the apical and basolateral supernatant (SN) over a period of four days (Fig. 1C). The relatively high titre in the SN collected at the apical side at T0 likely stems from attached input virus, which was not internalized during the first hour of incubation and therefore remained infectious. However, its contribution to the much higher titres measured at later times is negligible. On the basolateral SN, no viral progeny was detected until the fourth day of infection (T96). The titre measured at T0 is the result of the attached and non-internalised virus as stated above. Due to the complete removal of the SN after every other 24 h, the average cumulative titre of apically released CVB3 per HIE insert over hours post-infection (hpi) was calculated. This evaluation demonstrated a viral release that surpassed 108 TCID50/ml at 96 hpi (Fig. 1D). A steady increase of intracellular viral RNA (vRNA) over time was also observed (Fig. 1E).
In contrast to CVB3, the titre of apically released AiV-A1 A846/88 peaked at 24 hpi, reaching 105 TCID50/ml, followed by a steady decrease until 72 hpi eventually levelling off at 96 hpi (Fig. 1F). The appreciable titre at T0 is most likely attributable to non-internalized infectious virions recovered from the SN. In the basolateral SN, apart from the T0 titre arising for the reasons above, a decrease in viral infectious particle shedding was detectable from 24 to 96 hpi. The average of the cumulative titre of the apically released AiV-A1 A846/88 increased from T0 to T24 but then plateaued from 24 hpi to 96 hpi (Fig. 1G). Overall, the apical SN titre for both AiV-A1 A846/88 and CVB3 was dominant when compared to the basolateral side. Interestingly, the amount of AiV-A1 A846/88 vRNA collected from HIE decreased to ∼25 % of the input and then remained stable (Fig. 1H). This differed from the increasing vRNA level as observed for CVB3 (Fig. 1E).
The presence of double-stranded RNA (dsRNA) was assayed with the specific monoclonal antibody (mAb) J2, a gold standard for detecting viral replication (Targett-Adams et al., 2008), as an orthogonal validation of the AiV-A1 A846/88 infection in HIE. Immunofluorescence microscopy at 24 hpi revealed a few infected cells with a typical pattern of distinct foci surrounding the nucleus for both CVB3 and AiV-A1 A846/88 (Fig. 1I) (Harak and Lohmann, 2015). These results indicate that AiV-A1 A846/88 actively replicated in specific cells of the HIE at 24 hpi, the peak of apical virus release. Notably, examination of transverse sections of the infected epithelia located the replication factories of both viruses at the apical side of the tissue.
The number of AiV-A1 A846/88 infected cells remained relatively stable over 4 days of observation with an average of approximately 3 % (Fig. 1J). This is in line with the detection of a small amount of vRNA in the cells and a low virus titre in the apical SN. The lack of a steep increase of infected cells is likely owing to the low overall virus production and the existence of only few susceptible cells as reflected by the modest titres measured in the apical SN at times beyond 24 hpi. The number of CVB3-positive cells was only determined at 24 hpi and corresponded to approximately 2 % infected cells (Fig. 1K).
The detection of VP1 by an antiserum raised against AiV-A1-VP1 (Chen et al., 2013) in combination with mAb J2 showed both signals in close proximity illustrating the existence of viral replication centres (Fig. 1L) further substantiating the productive infection of AiV-A1 in HIE.
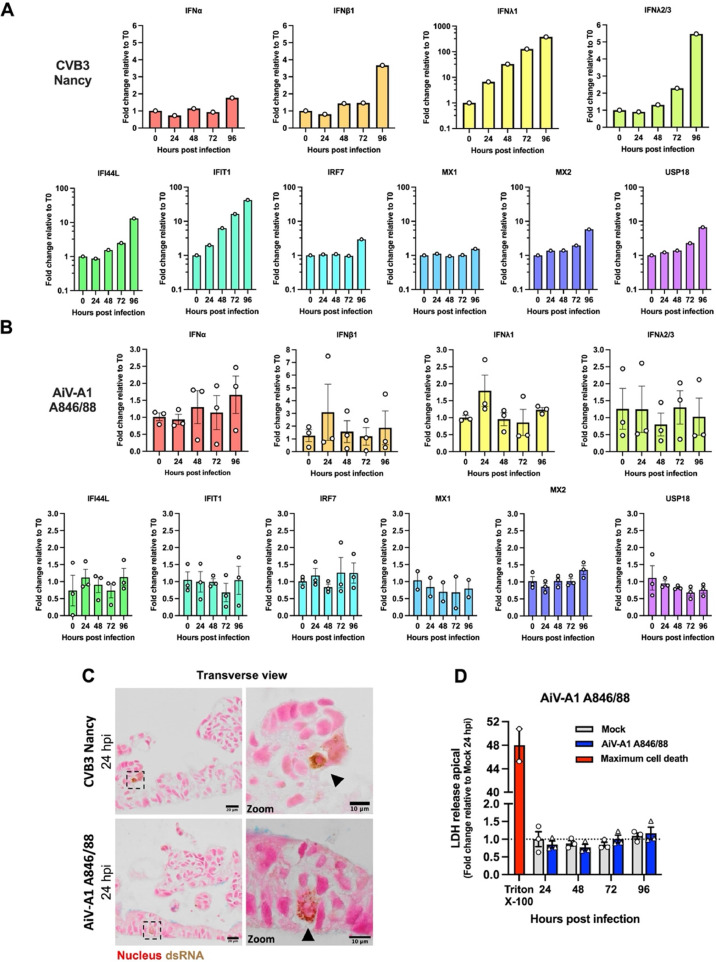
4.3. AiV-A1 A846/88 infection elicits no antiviral response and no damage to the epithelial integrity
As demonstrated above, only a few cells within the HIE became infected by AiV-A1 A846/88, and their number remained practically unchanged until at least 96 hpi. Therefore, a putative strong innate immune response may have curtailed AiV-A1 A846/88 propagation. Therefore, we investigated type I and type III IFN responses, as well as the downstream activation of selected interferon-stimulated genes (ISGs) by RT-qPCR. As a control, HIE was challenged with CVB3, which resulted in a strong induction of type I and type III IFNs, including several ISGs. The strongest increase was detected for IFNλ1 and the antiviral effector protein IFIT1 (Fig. 2A). On the other hand, AiV-A1 A846/88 displayed a strong variability between the three independently produced batches of HIE with overall much lower expression of all selected innate immune genes as compared to CVB3 (Fig. 2B) indicating no noticeable induction.
Fig. 2.
AiV-A1 A846/88 does not induce an antiviral state in HIE and does not damage the epithelial barrier. (A and B) IFN and ISG gene expression determined by RT-qPCR in HIE after AiV-A1 A846/88 and CVB3 infection as indicated (each with 107 PFU). In (A) N = 1 and in (B) N = 3 biological replicates, except MX1 (N = 2). (C) Immunohistochemical micrographs of HIE transverse sections stained with nuclear fast red-aluminum sulfate solution 0.1 % (red) were evaluated for dsRNA (brown, black arrows) via mAb J2 followed by DAB colourisation 24 hpi. Boxes refer to zoomed areas shown at the right. (D) Measurement of cell death by apically released LDH at indicated time points after AiV-A1 A846/88 infection in HIE. N = 3 biological replicates per condition, except maximum cell death (N = 2). Two-way ANOVA with Sidak's test for multiple comparisons was used for statistical analysis. Displayed are the mean ± SEM. ‘ns’, not significant.
Numerous enteric viruses have been reported to cause cell death in the intestinal epithelium resulting in an overall loss of epithelial integrity (e.g. echovirus 11 (E11), SARS-CoV-2, rotavirus) (Drummond et al., 2017; Stanifer and Boulant, 2020; Triana et al., 2021). To test the impact of AiV-A1 A846/88 on HIE viability, released LDH in the apical SN was accessed as a readout for cell damage at different times (24, 48, 72, and 96 hpi). Triton X-100 served as a positive control for the complete destruction of the epithelial barrier. No increase of LDH in the apical SN was detected at any time after AiV-A1 A846/88 infection when compared to uninfected tissues (Fig. 2C).
As orthogonal validation of the LDH assay, the HIE integrity was evaluated by looking for the presence of condensed nuclei in cells replicating AiV-A1 A846/88; transverse sections of infected HIE were labelled with nuclear fast red-aluminium solution and mAb J2 (followed by DAB colourisation). The analysis of the images revealed round and homogeneously coloured nuclei in AiV-A1 A846/88 infected cells with no clear signal for nuclear condensation and the overall epithelial integrity was preserved. A similar pattern was observed in the cells infected with the control virus (CVB3 Nancy) (Fig. 2D). The lack of clear cell damage by both tested viruses is in line with the previous observation of CVB3 RD (Drummond et al., 2017).
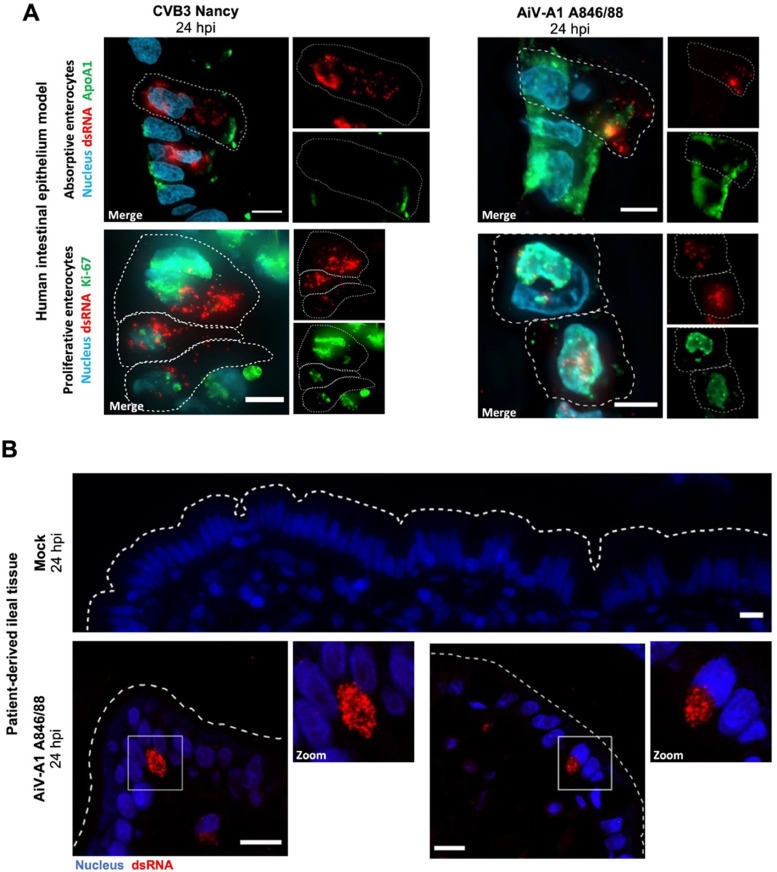
4.4. AiV-A1 A846/88 replicates in absorptive and proliferative enterocytes and infects patient-derived ileum
The above results demonstrated that infection of AiV-A1 846/88 is restricted to a very limited number of cells in HIE, suggesting specialised intestinal cells as targets of AiV-A1 infection. Nevertheless, we decided to test for absorptive and proliferative enterocytes first, as both cell types are relatively abundant and were shown to support SARS-CoV-2 infection (Lamers et al., 2020). To check whether AiV-A1 might have the same cell tropism, HIE was challenged with AiV-A1 A846/88 (CVB3 Nancy was again used as the control virus) and observed by fluorescent microscopy 24 hpi. Infected cells were identified by mAb J2 labelling, and mature absorptive enterocytes and immature proliferative enterocytes were visualized by apolipoprotein A1 (ApoA1) and Ki-67 immunolabelling, respectively. All dsRNA-positive cells were either positive for ApoA1 or Ki-67 (Fig. 3A), pinpointing these cells as putative entry points. In contrast, studies using intestinal crypt-derived organoids revealed that enteroendocrine cells and goblet cells were permissive for echovirus 11 and enterovirus A71, respectively (Drummond et al., 2017) (Good et al., 2019).
Fig. 3.
AiV-A1 A846/88 infects absorptive and proliferative enterocytes and replicates in patient-derived ileum. (A) Immunofluorescence micrographs of HIE after CVB3 and AiV-A1 A846/88 infection (each with 107 PFU) co-stained for dsRNA (red) and for the absorptive or proliferative enterocyte marker ApoA1 and Ki-67 (green), respectively. Dashed lines indicate cell outlines. (B) Patient-derived distal ileal tissue immunostained for dsRNA (red) after AiV-A1 A846/88 infection (107 PFU). Dashed lines indicate luminal interface. White boxes represent zoomed areas shown at the right. Scale bars, 10 μm.
To further corroborate our findings with the intestinal model, we aimed at mimicking a condition most closely representing natural infection. Patient-derived ileal slices were challenged from the apical side with 107 PFU of AiV-A1 A846/88 and kept at 37 °C, 5 % CO2. After 24 hpi we could visualize, by fluorescence microscopy, AiV-A1-positive cells (Fig. 3B); the mAb J2 signal was mainly located at the apical side near the luminal interface.
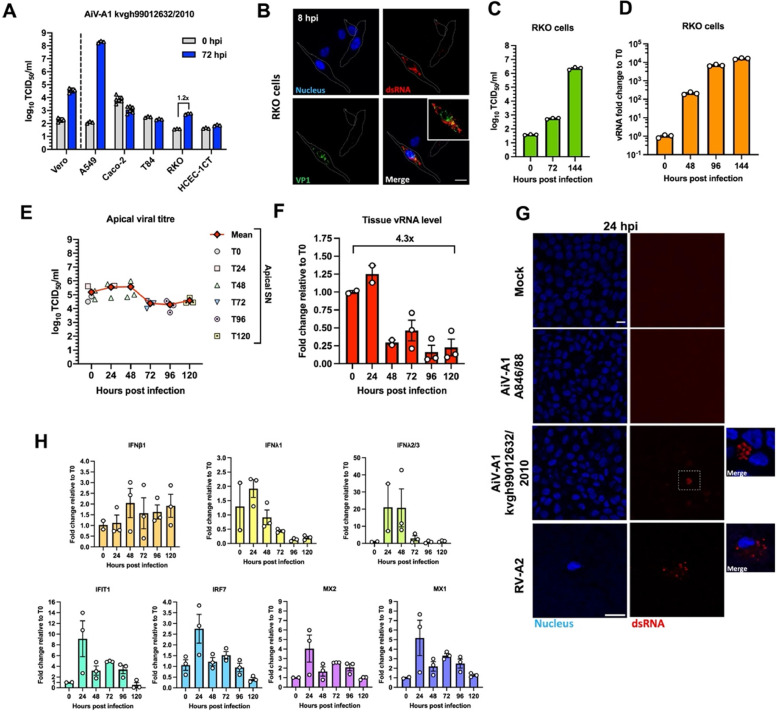
4.5. AiV-A1 kvgh99012632/2010 replicates in RKO cells and human bronchial epithelium
AiV-A1 isolate kvgh99012632/2010, recovered from a diarrhoea patient in Taiwan (Chang et al., 2019), was tested similarly to AiV-A1 A846/88 on various human-derived intestinal cell lines (Fig. 1). The kvgh99012632/2010 isolate replicated well in Vero and A549 cells showing a productive infection with high titres detected at 72 hpi (Fig. 4A). Interestingly, RKO cells were also permissive to the Taiwanese isolate, yielding a more than 10-fold increase in titre. Immunofluorescence confirmed the replication with the detection of dsRNA and VP1 in RKO cells at 8 hpi showing a distinct signal of the replication centres (Fig. 4B). RT-qPCR analysis of RKO cells at different times post-infection (0, 24, 96, 144 h) revealed a continuous and increasing production of AiV-A1 kvgh99012632/2010 (Fig. 4C and D).
Fig. 4.
AiV-A1 kvgh99012632/2010 replicates in RKO cells and triggers type III IFN and ISG expression in HTBE. (A) Virus was pre-absorbed (MOI=0.1) to the indicated cell lines for 1 h at 4 °C. The medium was removed, the cells were washed and the wash was collected (T0). Fresh medium was added, incubation was continued at 37 °C for 72 h (T72) and viral titres in the supernatants were determined N = 3. (B) Immunofluorescence images of AiV-A1 kvgh99012632/2010 infected RKO cells (MOI=1) stained for dsRNA (red) and VP1 (green) at 8 hpi. (C and D) RKO cells were infected with AiV-A1 kvgh99012632/2010 (MOI=0.1) for 1 h at 37 °C. The cells were then washed and at the indicated times SN were collected for titration and cells were dissolved in TRIzol reagent for vRNA quantification. Titration data in (C) from 0 to 72 hpi are taken from cold-synchronisation experiment (Fig. 4A). (E and F) HTBE were simultaneously infected apically and basolaterally with AiV-A1 kvgh99012632/2010 (107 PFU) for 1 h at 37 °C. After viral challenge, the cell layers were washed with PBS before continuing the incubation. While from 0 to 48 hpi the viral titre was determined in 24-hour intervals, between 72 and 120 hpi samples were collected at the indicted times. For RNA extraction, membranes of the respective inserts were cut out at the indicted times. (E) Plot of viral titre from the apical SN. Data from 0 to 48 shows the average inferred cumulative amount of virus produced per HTBE insert at each time point pi. Each individual symbol represents an individual HTBE insert. (F) Replication kinetics of vRNA determined by RT-qPCR. N ≥ 2 biological replicates. (G) Confocal micrographs of AiV-A1 A846/88, AiV-A1 kvgh99012632/2010 and RV-A2 infected HTBE stained for dsRNA (red) and VP1 (green) at 24 hpi. (H) IFN and ISG gene expression analyses in HTBE after AiV-A1 kvgh99012632/2010 infection via RT-qPCR. Shown is the mean ± SEM. Scale bars, 10 µm. Boxes represent zoomed areas shown at the right.
The observed productive infection of AiV-A1 isolate kvgh99012632/2010 of A549 cells (Fig. 4A) together with the report of respiratory symptoms in AiV-infected patients (Reuter et al., 2009) suggest the possibility of AiV-A1 exhibiting an extended tissue tropism beyond the gastrointestinal system. To investigate this, a human tracheal/bronchial epithelium model (HTBE) was infected with AiV-A1 kvgh99012632/2010 similarly as earlier described for HIE infected with AiV-A1 A846/88. The shedding of viral progeny was then measured by titration of the apical SN (collected and exchanged for fresh medium every other 24 h) showing a robust de novo production of AiV-A1 over 5 days of infection (Fig. 4E). Interestingly, the evaluation of vRNA levels over time demonstrated only a slight increase after 24 hpi followed by a reduction to 25 % of the virus input and maintenance of this value until the end of the analysis (Fig. 4F). Besides the increase in vRNA observed at 24 h, the AiV-A1 kvgh99012632/2010 vRNA values match those observed for AiV-A1 A846/88 in HIE (Fig. 1H).
As orthogonal validation, HTBE was infected with AiV-A1 kvgh99012632/2010 for 24 h and immunolabelled for dsRNA as above (Fig. 4G). As a control for productive infection rhinovirus A2 (RV-A2) was employed. Additionally, AiV-A1 A846/88 was included in the test. HTBE was only susceptible to AiV-A1 kvgh99012632/2010 and the control virus RV-A2, highlighting a distinct tropism for the two AiV-A1 isolates.
Aiming at clarifying the above-described cell tropism, we compared the coulombic charge distribution of the two AiV-A1 isolates (Fig. S1). A clear difference in charge distribution is observed in four clusters (*, **, #, §) at the outer face of the capsid's asymmetric unit. These differences might result in distinct specificity/affinity for different receptors in the respective cells used for host invasion. This is in line with the results of the assay that by-passes binding/entry (Fig. 1B).
To analyse whether the transitorily elevated levels of AiV-A1 kvgh99012632/2010 vRNA at 24 hpi could trigger cellular anti-viral defences, IFN and ISGs were measured over 5 days. As a result of the AiV-A1 infection IFNλ2/3 signalling was notably induced at 24 hpi remaining elevated until 48 hpi. The evaluation of the ISGs demonstrated a strong activation of all the evaluated ISGs at 24 hpi (Fig. 4H). This might suggest that the reduced vRNA level after 24 hpi could be resulting from increased activity of interferon signalling.
4.6. AiV-A1 isolate kvgh99012632/2010 exploits clathrin and dynamin and depends on endosomal acidification and cholesterol for entry
Taking the SARS-CoV-2 outbreak as a lesson, understanding the virus entry pathway and compounds interfering with it could aid the selection of a strategy to ameliorate respiratory symptoms. The Taiwanese AiV-A1 isolate replicates in lung-derived cells making it an interesting model to further understand AiV-A1 entry; A549 cells were infected with AiV-A1 kvgh99012632/2010 in the presence of a number of pharmacological inhibitors known to interfere with distinct cellular uptake mechanisms and specific intracellular routing pathways (Table S2).
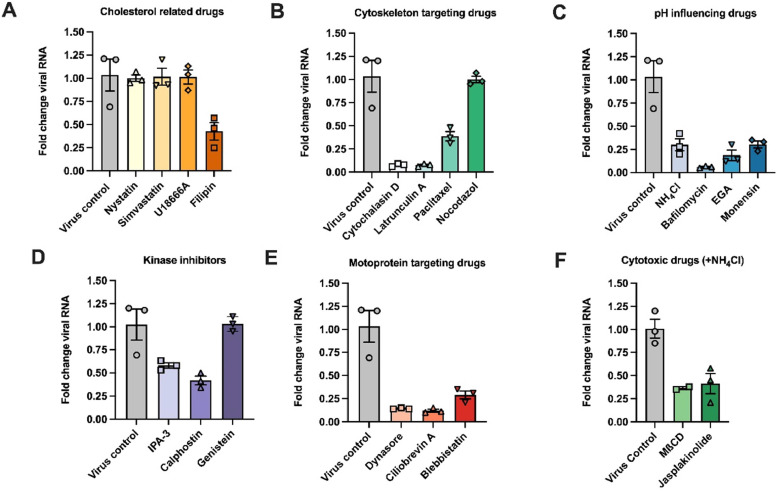
All inhibitors were initially tested for their potential cytotoxicity at the selected concentrations by measuring the ATP levels in the cells after 24 h of incubation with the compounds (Denani et al., 2021). We observed a drastic drop in the cellular ATP levels for chlorpromazine, EIPA, MβCD and jasplakinolide (Fig. S2). The impact of the inhibitors on AiV-A1 kvgh99012632/2010 vRNA production was quantified and normalized to the vRNA recovered from non-treated cells 8 h pi. For simplicity, the analysed compounds are shown as clusters by their functions/characteristics (Fig. 5A-5F).
Fig. 5.
AiV-A1 isolate kvgh99012632/2010 cell entry is dynamin- and cholesterol-mediated and strongly dependent on clathrin and endosomal acidification. (A–E) A549 cells were preincubated with the indicated compounds for 30 min at 4 °C and AiV-A1 kvgh99012632/2010 was added (MOI=1) for additional 30 min at 4 °C. The cells were then transferred to 37 °C for 8 h, lysed and the vRNA was measured by RT-qPCR. (F) A549 were pre-treated for 30 min at 4 °C with the indicated compounds, followed by another incubation for 1 h at 37 °C together with the virus and its subsequent removal by washing with PBS containing NH4Cl. The cells were then incubated for another 8 h at 37 °C, lysed and the vRNA was measured by RT-qPCR.
Cellular cholesterol plays a prominent role in raft/caveolae-mediated endocytosis (Kiss and Botos, 2009) and replication organelles of some picornaviruses including AiV-A1, were observed to be cholesterol-dependent (Ishikawa-Sasaki et al., 2018; van der Schaar et al., 2016). Numerous compounds that differently target the cholesterol metabolism were used in the past. We observed that neither nystatin, which complexes lipid membrane resident cholesterol (Bolard, 1986), nor simvastatin, which inhibits HMG-CoA reductase (Alberts, 1990), nor U18666A, which blocks the cholesterol transporter NPC1 and endogenous cholesterol production (Cenedella, 2009), showed any clear reduction in vRNA of AiV-A1 kvgh99012632/2010 (Fig. 5A). However, filipin that binds free, unesterified cholesterol (Bolard, 1986), caused a drop in AiV-A1 replication by about 50 %.
Cytoskeleton inhibitors such as cytochalasin D, an actin polymerisation blocking agent (Casella et al., 1981), and latrunculin A, which sequester monomeric actin (Yarmola et al., 2000), reduced the vRNA levels to 5 % (Fig. 5B). Paclitaxel, which stabilizes microtubules (Jordan, 2002), resulted in a reduction of viral replication to 40 %. However, nocodazole, which depolymerizes microtubules (Downing, 2000), had practically no effect on the level of replicated vRNA.
After binding to a specific receptor, picornaviruses enter the cell through the endocytic pathway, which is associated with a progressive reduction of the pH in the endosomal lumen. This process is required for other picornaviruses during productive infection (e.g. Foot-and-mouth disease virus (Carrillo et al., 1984) and rhinoviruses (Schober et al., 1998)), as it triggers structural changes and RNA release. Various chemical compounds with distinct mechanism of action were employed (Fig. 5C). NH4Cl, a weak base that can neutralize the intravesicular pH (Ohkuma and Poole, 1978), and monensin, a K+ /H+ exchanger (Mollenhauer et al., 1990), both caused a reduction of 70 % in vRNA production. The V-ATPase inhibitor bafilomycin A1 (Wang et al., 2021) was the most potent, reducing the vRNA levels down to ∼1 %. Additionally, EGA, which inhibits trafficking from early to late endosomes without affecting the endosomal pH (Gillespie et al., 2013), had a major impact on the vRNA production, decreasing it by 85 %. In summary, all lysosomotropic compounds tested that affect endosomal acidification substantially diminished vRNA synthesis.
The kinase inhibitors IPA-3, which block the protein Ser/Thr-kinases Pak1 (Viaud and Peterson, 2009), and calphostin, which inhibits protein kinase C by competing for the binding site of diacylglycerol and phorbol esters (Bruns et al., 1991), decreased the amount of vRNA to 60 % and 40 %, respectively (Fig. 5D). The effect of these inhibitors is tentatively explained due to the importance of Ser/Thr-kinase in macropinocytosis (Dharmawardhane et al., 2000). Genistein, a Tyrosine kinase inhibitor (Spinozzi et al., 1994), did not impact on vRNA production.
Membrane remodelling and intracellular cargo transportation in AiV-A1 entry were accessed through chemical inhibition of molecular motors (Fig. 5E). Dynasore (inhibits dynamin II that is responsible for pinching off vesicles in clathrin-mediated endocytosis (Kirchhausen et al., 2008)) and ciliobrevin A (that affects dynein, which transports vesicles along the microtubules towards the minus-end (Roossien et al., 2015)) led to a reduction in synthesized vRNA by approximately 80 % and 90 %, respectively. Blebbistatin, which blocks myosin II in an actin-detached state (Kovacs et al., 2004), reduced vRNA synthesis by 70 %.
To reduce the adverse effect of the compounds that we found to exhibit substantial cytotoxicity, we shortened the incubation time to 90 min. Treatment with the cholesterol-depleting agent MβCD, which remodels the plasma membrane's mechanics and its interactions with the underlying cytoskeleton (Biswas et al., 2019), and with jasplakinolide, which disrupts actin filaments and induces monomeric actin to assemble into amorphous masses (Bubb et al., 2000), led to a similar decrease in vRNA production by 35 % and 40 %, respectively (Fig. 5F). These results reinforce the importance of membrane remodelling and cargo transportation for AiV-A1 infection. Unfortunately, EIPA (which blocks the activity of the Na+/H+ exchanger via directly interfering with macropinocytosis (Koivusalo et al., 2010)) and chlorpromazine (that inhibits dynamin and clathrin-mediated endocytosis (Daniel et al., 2015)) strongly increased the Ct values of the control housekeeping gene, which disallowed normalization of the measured vRNA levels.
Taken together, experiments with the pharmacological inhibitors suggest that AiV-A1 kvgh99012632/2010 entry depends on dynamin and cholesterol and strongly relies on clathrin-mediated endocytosis and endosomal acidification.
4.7. AiV-A1 kvgh99012632/2010 enters A549 cells via Rab5a-decorated vesicles and promotes their disruption
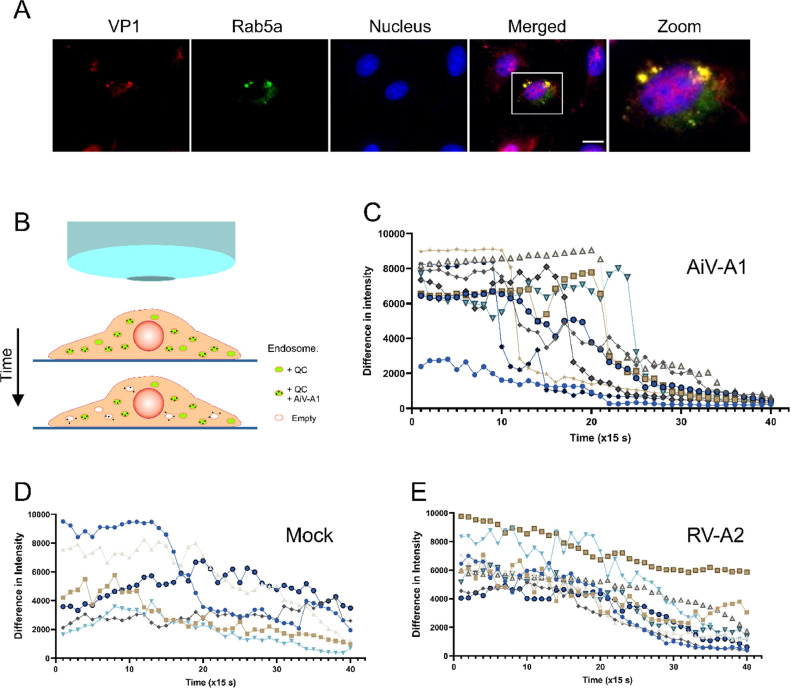
AiV-A1 isolate kvgh99012632/2010 very likely gains access to the cell via clathrin-mediated endocytosis and is shuttled to early endosomes where its productive uncoating depends on endosomal acidification. To further support this finding, A549 cells expressing Rab5a-GFP were challenged with AiV-A1 kvgh99012632/2010 and evaluated by fluorescence microscopy. After pre-absorption for 30 min at 4 °C and subsequent infection for 30 min at 37 °C, Rab5a-positive vesicles containing AiV-A1 particles were observed (Fig. 6A). The lack of an effect of nocodazole on the production of vRNA, as described above, in combination with the finding of AiV-A1 ferrying through early endosomes might suggest that AiV-A1 promotes disruption of the endosomal membrane allowing the viral genome to access the cytosol.
Fig. 6.
AiV-A1 isolate kvgh99012632/2010 enters through early endosomes and promotes membrane permeabilisation. (A) Fluorescence microscopy of A549 cells expressing GFP-tagged Rab5a. Cells were infected with AiV-A1 (MOI=50) and virus attachment was allowed for 30 min at 4 °C. They were then washed 3 times with cold PBS, covered with warm infection medium and further incubated at 37 °C for 30 min, then washed and fixed in 4 % PFA. VP1 (red) was labelled using specific antiserum; the Rab5a signal (green) stems from the fused GFP. Scale bars, 10 μm. (B) Cartoon depicting the live cell microscopy assay illustrating the putative leakage of the early endosomes promoted by AiV-A1. (C-E) A549 cells were infected with AiV-A1, RV-A2 (both at MOI10) or not infected in the presence of NH4Cl (50 mM) and quinacrine (1 µM) in infection medium for 30 min in the dark. After three times washing with PBS containing NH4Cl, PBS without NH4Cl was added and the cells images were recorded immediately over 10 min (40 takes with 15 s interval in-between the takes to reduce photobleaching). Each line represents the signal of one vesicle tracked over time and the values represent the absolute intensity differences calculated for each vesicle individually (2–3 vesicles per cell) in three independent acquisitions per condition and evaluated by using ImageJ, The average was plotted with GraphPad Prism (6–9 vesicles in total). Only vesicles with the same size were evaluated (∼500 nm diameter). Note the abrupt loss of signal around 5 min for AiV-A1.
To test this hypothesis, the integrity of endosomes bearing AiV-A1 kvgh99012632/2010 was evaluated via tracking quinacrine (QC)-containing vesicles over time via live fluorescent microscopy (Fig. 6B). Since AiV-A1 and RV-A2 uncoating is dependent on low pH (see above), infection by both viruses was synchronised via conducting attachment and uptake in PBS containing NH4Cl; uncoating was then initiated by replacing it with PBS without NH4Cl. This strategy also allowed for QC accumulation in the cellular vesicles and adjustment of the conditions for tracking the vesicle signal over 10 min. In the AiV-A1 infected cells, roughly 5 min after removal of the NH4Cl the QC signal fell abruptly (Fig. 6C), suggesting that the QC was released into the cytoplasm via disruption of the endosomal membrane. In contrast, in the mock-infected cells, a progressive reduction in the QC signal was observed (Fig. 6D) as in the cells infected with RV-A2 (Fig. 6E). Lack of detectable endosomal leakage during RV-A2 infection had also been observed by Schober et al. (1998).
5. Discussion
AiV-A1 role in acute gastroenteritis in humans has remained controversial due to ambiguous epidemiological findings paired with scarce in vitro data in relevant human-derived intestinal models. Furthermore, most studies were carried out with monkey-derived Vero cells and human non-intestinal immortalized cell lines (e.g., A549). It was the main goal of this work to employ a model that mimics the architecture and cellular diversity of the human small intestine for its capacity to sustain AiV-A1 replication and to deepen our knowledge on AiV-A1 entry.
By using a stem cell-derived human small intestinal epithelium model (HIE), we detected replication of AiV-A1 A846/88 and the positive control (CVB3 Nancy), at the apical side of proliferative (Ki-67) and absorptive (ApoA1) enterocytes by viral dsRNA co-localisation. Despite infecting the same cell types, AiV-A1 A846/88 and CVB3 replicated with different growth kinetics without damaging the epithelial barrier. While AiV-A1 A846/88 failed to trigger an innate immune response in the HIE, CVB3 robustly induced type III IFN and ISG expression, suggesting a chronic character of AiV-A1 A846/88 infection of intestinal cells due to a modulation of the antiviral state observed by Kung et al. (2020).
Since AiV-A1 kvgh99012632/2010 infected the human lung-derived A549 cell line, we wondered whether a stem cell-derived human tracheal/bronchial epithelium model (HTBE) could also be infected. AiV-A1 isolate kvgh99012632/2010 replicated well in HTBE and selectively induced III IFN and ISGs. This isolate also replicated well in intestinal cells (RKO cells). The same findings could not be reproduced using AiV-A1 isolate A846/88. A summary of all cell lines tested with both AiV-A1 isolates and their different origins is found in Supplementary Fig. S3.
Finally, we found that AiV-A1 isolate kvgh99012632/2010 host cell entry is dependent on clathrin, dynamin, and lipid rafts. Additionally, viral uncoating requires endosomal acidification and AiV-A1 compromises membrane integrity of early-endosomes.
Similar to our observations with AiV-A1, EV-D68 which shares characteristics between enterovirus and rhinovirus also exhibits an expanded tissue tropism for the gastrointestinal tract and the respiratory system (Cassidy et al., 2018). Moreover, AiV-A1 A846/88 replicated in patient-derived ileal tissue at the apical side close to the luminal interface, corroborating our findings in HIE, and demonstrating the utility of this model as an interesting tool for further studies of intestinal viruses.
In summary, our data support the epidemiological observations that AiV-A1 is a causative agent for acute gastroenteritis in humans and, thus, a clinically relevant pathogen. Moreover, AiV-A1 replication is not limited to the gastrointestinal tract but also infects tracheal/bronchial tissue raising awareness for complications in immunocompromised patients, especially in children. More research will be necessary on this mostly understudied virus, including the development of novel therapeutics and strategies to prevent or treat its pathogenesis.
CRediT authorship contribution statement
Martin Jungbauer-Groznica: Methodology, Software, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Konstantin Wiese: Methodology, Software, Formal analysis, Investigation, Data curation, Writing – original draft. Irmgard Fischer: Methodology, Formal analysis, Investigation, Resources. Jan Markus: Methodology, Resources. Tsung-Hsien Chang: Resources. Irene Gösler: Investigation, Resources. Heinrich Kowalski: Conceptualization, Validation, Resources, Writing – original draft, Supervision, Project administration, Funding acquisition. Dieter Blaas: Conceptualization, Validation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition. Antonio Real-Hohn: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Austrian Science Fund (FWF) project P27196 (HK) and P31392 (DB). We thank Univ. Prof. Dr. Martin Klimpfinger from the Klinik Favoriten for providing us with a sample of human small intestinal tissue.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2024.199338.
Contributor Information
Heinrich Kowalski, Email: heinrich.kowalski@meduniwien.ac.at.
Antonio Real-Hohn, Email: antonio.hohn@meduniwien.ac.at.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Alberts A.W. Lovastatin and simvastatin–inhibitors of HMG CoA reductase and cholesterol biosynthesis. Cardiology. 1990;77(4):14–21. doi: 10.1159/000174688. Suppl. [DOI] [PubMed] [Google Scholar]
- Biswas A., Kashyap P., Datta S., Sengupta T., Sinha B. Cholesterol depletion by MbetaCD enhances cell membrane tension and its variations-reducing integrity. Biophys. J. 2019;116(8):1456–1468. doi: 10.1016/j.bpj.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim. Biophys. Acta. 1986;864(3–4):257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- Bostina M., Levy H., Filman D.J., Hogle J.M. Poliovirus RNA is released from the capsid near a twofold symmetry axis. J. Virol. 2011;85(2):776–783. doi: 10.1128/JVI.00531-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R.F., Miller F.D., Merriman R.L., Howbert J.J., Heath W.F., Kobayashi E., Takahashi I., Tamaoki T., Nakano H. Inhibition of protein kinase C by calphostin C is light-dependent. Biochem. Biophys. Res. Commun. 1991;176(1):288–293. doi: 10.1016/0006-291x(91)90922-t. [DOI] [PubMed] [Google Scholar]
- Bubb M.R., Spector I., Beyer B.B., Fosen K.M. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J. Biol. Chem. 2000;275(7):5163–5170. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- Bucciol G., Moens L., Payne K., Wollants E., Mekahli D., Levtchenko E., Vermeulen F., Tousseyn T., Gray P., Ma C.S., Tangye S.G., Van Ranst M., Brown J.R., Breuer J., Meyts I. Chronic aichi virus infection in a patient with X-linked agammaglobulinemia. J. Clin. Immunol. 2018;38(7):748–752. doi: 10.1007/s10875-018-0558-z. [DOI] [PubMed] [Google Scholar]
- Bucciol G., Tousseyn T., Jansen K., Casteels I., Tangye S.G., Breuer J., Brown J.R., Wollants E., Van Ranst M., Moens L., Mekahli D., Meyts I. Hematopoietic stem cell transplantation cures chronic aichi virus infection in a patient with X-linked agammaglobulinemia. J. Clin. Immunol. 2021;41(6):1403–1405. doi: 10.1007/s10875-021-01056-w. [DOI] [PubMed] [Google Scholar]
- Carrillo E.C., Giachetti C., Campos R.H. Effect of lysosomotropic agents on the foot-and-mouth disease virus replication. Virology. 1984;135(2):542–545. doi: 10.1016/0042-6822(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Casella J.F., Flanagan M.D., Lin S. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature. 1981;293(5830):302–305. doi: 10.1038/293302a0. [DOI] [PubMed] [Google Scholar]
- Cassidy H., Poelman R., Knoester M., Van Leer-Buter C.C., Niesters H.G.M. Enterovirus D68 – The New Polio? Front. Microbiol. 2018:9. doi: 10.3389/fmicb.2018.02677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenedella R.J. Cholesterol synthesis inhibitor U18666A and the role of sterol metabolism and trafficking in numerous pathophysiological processes. Lipids. 2009;44(6):477–487. doi: 10.1007/s11745-009-3305-7. [DOI] [PubMed] [Google Scholar]
- Chang Y.T., Kung M.H., Hsu T.H., Hung W.T., Chen Y.S., Yen L.C., Chang T.H. Aichi virus induces antiviral host defense in primary murine intestinal epithelial cells. Viruses. 2019;11(8) doi: 10.3390/v11080763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.S., Chen B.C., Lin Y.S., Chang J.T., Huang T.S., Chen J.J., Chang T.H. Detection of aichi virus with antibody targeting of conserved viral protein 1 epitope. Appl. Microbiol. Biotechnol. 2013;97(19):8529–8536. doi: 10.1007/s00253-012-4644-5. [DOI] [PubMed] [Google Scholar]
- Daniel J.A., Chau N., Abdel-Hamid M.K., Hu L., von Kleist L., Whiting A., Krishnan S., Maamary P., Joseph S.R., Simpson F., Haucke V., McCluskey A., Robinson P.J. Phenothiazine-derived antipsychotic drugs inhibit dynamin and clathrin-mediated endocytosis. Traffic. 2015;16(6):635–654. doi: 10.1111/tra.12272. [DOI] [PubMed] [Google Scholar]
- de Graaf I.A.M., Olinga P., de Jager M.H., Merema M.T., de Kanter R., van de Kerkhof E.G., Groothuis G.M.M. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat. Protoc. 2010;5(9):1540–1551. doi: 10.1038/nprot.2010.111. [DOI] [PubMed] [Google Scholar]
- Denani C.B., Real-Hohn A., de Carvalho C.A.M., Gomes A.M.O., Goncalves R.B. Lactoferrin affects rhinovirus B-14 entry into H1-HeLa cells. Arch. Virol. 2021;166(4):1203–1211. doi: 10.1007/s00705-021-04993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmawardhane S., Schurmann A., Sells M.A., Chernoff J., Schmid S.L., Bokoch G.M. Regulation of macropinocytosis by p21-activated kinase-1. Mol. Biol. Cell. 2000;11(10):3341–3352. doi: 10.1091/mbc.11.10.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing K.H. Structural basis for the interaction of tubulin with proteins and drugs that affect microtubule dynamics. Annu Rev. Cell Dev. Biol. 2000;16:89–111. doi: 10.1146/annurev.cellbio.16.1.89. [DOI] [PubMed] [Google Scholar]
- Drummond C.G., Bolock A.M., Ma C., Luke C.J., Good M., Coyne C.B. Enteroviruses infect human enteroids and induce antiviral signaling in a cell lineage-specific manner. Proc. Natl. Acad. Sci. u S. a. 2017;114(7):1672–1677. doi: 10.1073/pnas.1617363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E.J. Acute gastroenteritis in children. BMJ. 2007;334(7583):35–40. doi: 10.1136/bmj.39036.406169.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganjian H., Zietz C., Mechtcheriakova D., Blaas D., Fuchs R. ICAM-1 binding rhinoviruses enter hela cells via multiple pathways and travel to distinct intracellular compartments for uncoating. Viruses. 2017;9(4) doi: 10.3390/v9040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie E.J., Ho C.L., Balaji K., Clemens D.L., Deng G., Wang Y.E., Elsaesser H.J., Tamilselvam B., Gargi A., Dixon S.D., France B., Chamberlain B.T., Blanke S.R., Cheng G., de la Torre J.C., Brooks D.G., Jung M.E., Colicelli J., Damoiseaux R., Bradley K.A. Selective inhibitor of endosomal trafficking pathways exploited by multiple toxins and viruses. Proc. Natl. Acad. Sci. u S. a. 2013;110(50):E4904–E4912. doi: 10.1073/pnas.1302334110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C., Wells A.I., Coyne C.B. Type III interferon signaling restricts enterovirus 71 infection of goblet cells. Sci. Adv. 2019;5(3):eaau4255. doi: 10.1126/sciadv.aau4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greninger A.L., Knudsen G.M., Betegon M., Burlingame A.L., Derisi J.L. The 3A protein from multiple picornaviruses utilizes the golgi adaptor protein ACBD3 to recruit PI4KIIIbeta. J. Virol. 2012;86(7):3605–3616. doi: 10.1128/JVI.06778-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L., Chen C., Bailey K., Wang L. Bovine kobuvirus-A comprehensive review. Transbound. Emerg. Dis. 2021;68(4):1886–1894. doi: 10.1111/tbed.13909. [DOI] [PubMed] [Google Scholar]
- Harak C., Lohmann V. Ultrastructure of the replication sites of positive-strand RNA viruses. Virology. 2015;479-480:418–433. doi: 10.1016/j.virol.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E.C., Goldstein S.A., Rasmussen A.L., Robertson D.L., Crits-Christoph A., Wertheim J.O., Anthony S.J., Barclay W.S., Boni M.F., Doherty P.C., Farrar J., Geoghegan J.L., Jiang X., Leibowitz J.L., Neil S.J.D., Skern T., Weiss S.R., Worobey M., Andersen K.G., Garry R.F., Rambaut A. The origins of SARS-CoV-2: a critical review. Cell. 2021;184(19):4848–4856. doi: 10.1016/j.cell.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz L.R., Finkbeiner S.R., Zhao G., Kirkwood C.D., Girones R., Pipas J.M., Wang D. Klassevirus 1, a previously undescribed member of the family Picornaviridae, is globally widespread. Virol. J. 2009;6(1):86. doi: 10.1186/1743-422X-6-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa-Sasaki K., Nagashima S., Taniguchi K., Sasaki J. Model of OSBP-mediated cholesterol supply to aichi virus rna replication sites involving protein-protein interactions among Viral proteins, ACBD3, OSBP, VAP-A/B, and SAC1. J. Virol. 2018;92(8) doi: 10.1128/JVI.01952-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa-Sasaki K., Murata T., Sasaki J. IFITM1 enhances nonenveloped viral RNA replication by facilitating cholesterol transport to the Golgi. PLoS. Pathog. 2023;19(5) doi: 10.1371/journal.ppat.1011383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson N., Wahlström K., Svensson L., Serrander L., Lindberg A.M. Aichi virus infection in elderly people in Sweden. Arch. Virol. 2012;157(7):1365–1369. doi: 10.1007/s00705-012-1296-9. [DOI] [PubMed] [Google Scholar]
- Jordan M.A. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem. AntiCancer Agents. 2002;2(1):1–17. doi: 10.2174/1568011023354290. [DOI] [PubMed] [Google Scholar]
- Kelley E.J., Henson S.N., Rahee F., Boyle A.S., Engelbrektson A.L., Nelson G.A., Mead H.L., Anderson N.L., Razavi M., Yip R., Ladner J.T., Scriba T.J., Altin J.A. Virome-wide detection of natural infection events and the associated antibody dynamics using longitudinal highly-multiplexed serology. Nat. Commun. 2023;14(1):1783. doi: 10.1038/s41467-023-37378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J.T., Swanson J., Newman J., Groppelli E., Stonehouse N.J., Tuthill T.J. Membrane interactions and uncoating of aichi virus, a picornavirus that lacks a VP4. J. Virol. 2022 doi: 10.1128/jvi.00082-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1999. Picornaviridae. King, A.M.Q., Brown, F., Christian, P., Hovi, T., Hyypiä, T., Knowles, N.J., Lemon, S.M., Minor, P.D., Palmenberg, A.C., Skern, T., Stanway, G.
- Kirchhausen T., Macia E., Pelish H.E. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol. 2008;438:77–93. doi: 10.1016/S0076-6879(07)38006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A.L., Botos E. Endocytosis via caveolae: alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J. Cell Mol. Med. 2009;13(7):1228–1237. doi: 10.1111/j.1582-4934.2009.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Gerba C.P. Aichi virus 1: environmental occurrence and behavior. Pathogens. 2015;4(2):256–268. doi: 10.3390/pathogens4020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivusalo M., Welch C., Hayashi H., Scott C.C., Kim M., Alexander T., Touret N., Hahn K.M., Grinstein S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 2010;188(4):547–563. doi: 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M., Toth J., Hetenyi C., Malnasi-Csizmadia A., Sellers J.R. Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 2004;279(34):35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- Kung M.H., Lin Y.S., Chang T.H. Aichi virus 3C protease modulates LC3- and SQSTM1/p62-involved antiviral response. Theranostics. 2020;10(20):9200–9213. doi: 10.7150/thno.47077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers M.M., Beumer J., van der Vaart J., Knoops K., Puschhof J., Breugem T.I., Ravelli R.B.G., Paul van Schayck J., Mykytyn A.Z., Duimel H.Q., van Donselaar E., Riesebosch S., Kuijpers H.J.H., Schippers D., van de Wetering W.J., de Graaf M., Koopmans M., Cuppen E., Peters P.J., Haagmans B.L., Clevers H. SARS-CoV-2 productively infects human gut enterocytes. Science (1979) 2020:eabc1669. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Van Dung N., Ivens A., Bogaardt C., O'Toole A., Bryant J.E., Carrique-Mas J., Van Cuong N., Anh P.H., Rabaa M.A., Tue N.T., Thwaites G.E., Baker S., Simmonds P., Woolhouse M.E., Consortium V. Genetic diversity and cross-species transmission of kobuviruses in Vietnam. Virus. Evol. 2018;4(1) doi: 10.1093/ve/vey002. vey002-vey002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyts I., Bucciol G., Jansen K., Wollants E., Breuer J. Aichivirus: an emerging pathogen in patients with primary and secondary B-Cell deficiency. J. Clin. Immunol. 2023;43(3):532–535. doi: 10.1007/s10875-022-01410-6. [DOI] [PubMed] [Google Scholar]
- Mollenhauer H.H., Morre D.J., Rowe L.D. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim. Biophys. Acta. 1990;1031(2):225–246. doi: 10.1016/0304-4157(90)90008-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. u S. a. 1978;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink B.B., van der Hoek L. Viruses causing gastroenteritis: the known, the new and those beyond. Viruses. 2016;8(2) doi: 10.3390/v8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prchla E., Plank C., Wagner E., Blaas D., Fuchs R. Virus-mediated release of endosomal content in vitro: different behavior of adenovirus and rhinovirus serotype 2. J. Cell Biol. 1995;131(1):111–123. doi: 10.1083/jcb.131.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real-Hohn A., Groznica M., Loffler N., Blaas D., Kowalski H. nanoDSF: in vitro label-free method to monitor picornavirus uncoating and test compounds affecting particle stability. Front. Microbiol. 2020;11:1442. doi: 10.3389/fmicb.2020.01442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter G., Boldizsár Á., Papp G., Pankovics P. Detection of Aichi virus shedding in a child with enteric and extraintestinal symptoms in Hungary. Arch. Virol. 2009;154(9):1529–1532. doi: 10.1007/s00705-009-0473-y. [DOI] [PubMed] [Google Scholar]
- Reuter G., Boros A., Pankovics P. Kobuviruses - a comprehensive review. Rev. Med. Virol. 2011;21(1):32–41. doi: 10.1002/rmv.677. [DOI] [PubMed] [Google Scholar]
- Rivadulla E., Romalde J.L. A comprehensive review on human aichi virus. Virol. Sin. 2020 doi: 10.1007/s12250-020-00222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossien D.H., Miller K.E., Gallo G. Ciliobrevins as tools for studying dynein motor function. Front. Cell Neurosci. 2015;9:252. doi: 10.3389/fncel.2015.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin C., Fuzik T., Skubnik K., Palkova L., Lindberg A.M., Plevka P. Structure of Aichi virus 1 and its empty particle: clues towards kobuvirus genome release mechanism. J. Virol. 2016 doi: 10.1128/JVI.01601-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki J., Taniguchi K. Aichi virus 2A protein is involved in viral RNA replication. J. Virol. 2008;82(19):9765–9769. doi: 10.1128/JVI.01051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki J., Kusuhara Y., Maeno Y., Kobayashi N., Yamashita T., Sakae K., Takeda N., Taniguchi K. Construction of an infectious cDNA clone of Aichi virus (a new member of the family Picornaviridae) and mutational analysis of a stem-loop structure at the 5′ end of the genome. J. Virol. 2001;75(17):8021–8030. doi: 10.1128/JVI.75.17.8021-8030.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki J., Ishikawa K., Taniguchi K. 3CD, but not 3C, cleaves the VP1/2A site efficiently during Aichi virus polyprotein processing through interaction with 2A. Virus. Res. 2012;163(2):592–598. doi: 10.1016/j.virusres.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Schober D., Kronenberger P., Prchla E., Blaas D., Fuchs R. Major and minor receptor group human rhinoviruses penetrate from endosomes by different mechanisms. J. Virol. 1998;72(2):1354–1364. doi: 10.1128/jvi.72.2.1354-1364.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sdiri-Loulizi K., Hassine M., Bour J.B., Ambert-Balay K., Mastouri M., Aho L.S., Gharbi-Khelifi H., Aouni Z., Sakly N., Chouchane S., Neji-Guédiche M., Pothier P., Aouni M. Aichi Virus IgG seroprevalence in tunisia parallels genomic detection and clinical presentation in children with gastroenteritis. Clinical Vaccine Immunol. 2010;17(7):1111–1116. doi: 10.1128/CVI.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinozzi F., Pagliacci M.C., Migliorati G., Moraca R., Grignani F., Riccardi C., Nicoletti I. The natural tyrosine kinase inhibitor genistein produces cell cycle arrest and apoptosis in Jurkat T-leukemia cells. Leuk. Res. 1994;18(6):431–439. doi: 10.1016/0145-2126(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Stanifer M., Boulant S. The origin of diarrhea in rotavirus infection. Science (1979) 2020;370(6519):909–910. doi: 10.1126/science.abf1914. [DOI] [PubMed] [Google Scholar]
- Tamura A., Ozawa K., Ohya T., Tsuyama N., Eyring E.M., Masujima T. Nanokinetics of drug molecule transport into a single cell. Nanomedicine (Lond) 2006;1(3):345–350. doi: 10.2217/17435889.1.3.345. [DOI] [PubMed] [Google Scholar]
- Targett-Adams P., Boulant S., McLauchlan J. Visualization of double-stranded RNA in cells supporting hepatitis C virus RNA replication. J. Virol. 2008;82(5):2182–2195. doi: 10.1128/JVI.01565-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triana S., Metz-Zumaran C., Ramirez C., Kee C., Doldan P., Shahraz M., Schraivogel D., Gschwind A.R., Sharma A.K., Steinmetz L.M., Herrmann C., Alexandrov T., Boulant S., Stanifer M.L. Single-cell analyses reveal SARS-CoV-2 interference with intrinsic immune response in the human gut. Mol. Syst. Biol. 2021;17(4):e10232. doi: 10.15252/msb.202110232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaar H.M., Dorobantu C.M., Albulescu L., Strating J., van Kuppeveld F.J.M. Fat(al) attraction: picornaviruses Usurp lipid transfer at membrane contact sites to create replication organelles. Trends. Microbiol. 2016;24(7):535–546. doi: 10.1016/j.tim.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud J., Peterson J.R. An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory domain covalently. Mol. Cancer Ther. 2009;8(9):2559–2565. doi: 10.1158/1535-7163.MCT-09-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T., Lim S.S., Abbafati C., Abbas K.M., Abbasi M., Abbasifard M., Abbasi-Kangevari M., Abbastabar H., Abd-Allah F., Abdelalim A., Etchebest C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Wang J., Hassan A., Lee C.H., Xie X.S., Li X. Molecular basis of V-ATPase inhibition by bafilomycin A1. Nat. Commun. 2021;12(1):1782. doi: 10.1038/s41467-021-22111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse M.E., Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005;11(12):1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Sakae K., Ishihara Y., Isomura S., Utagawa E. Prevalence of newly isolated, cytopathic small round virus (Aichi strain) in Japan. J. Clin. Microbiol. 1993;31(11):2938–2943. doi: 10.1128/jcm.31.11.2938-2943.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Sakae K., Tsuzuki H., Suzuki Y., Ishikawa N., Takeda N., Miyamura T., Yamazaki S. Complete nucleotide sequence and genetic organization of Aichi virus, a distinct member of the Picornaviridae associated with acute gastroenteritis in humans. J. Virol. 1998;72(10):8408–8412. doi: 10.1128/jvi.72.10.8408-8412.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Ito M., Tsuzuki H., Sakae K. Identification of Aichi virus infection by measurement of immunoglobulin responses in an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 2001;39(11):4178–4180. doi: 10.1128/JCM.39.11.4178-4180.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmola E.G., Somasundaram T., Boring T.A., Spector I., Bubb M.R. Actin-latrunculin A structure and function. differential modulation of actin-binding protein function by latrunculin A. J. Biol. Chem. 2000;275(36):28120–28127. doi: 10.1074/jbc.M004253200. [DOI] [PubMed] [Google Scholar]
- Zhai S.L., Zhang H., Lin T., Chen S.N., Zhou X., Chen Q.L., Lv D.H., Wen X.H., Zhou X.R., Jia C.L., Wei W.K. A novel porcine kobuvirus emerged in piglets with severe diarrhoea in China. Transbound. Emerg. Dis. 2017;64(4):1030–1036. doi: 10.1111/tbed.12663. [DOI] [PubMed] [Google Scholar]
- Zhu L., Wang X., Ren J., Kotecha A., Walter T.S., Yuan S., Yamashita T., Tuthill T.J., Fry E.E., Rao Z., Stuart D.I. Structure of human aichi virus and implications for receptor binding. Nat. Microbiol. 2016;1(11):16150. doi: 10.1038/nmicrobiol.2016.150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.