Abstract
Background. Lipomas are common superficial soft tissue tumors of mature adipocytes. In contrast, well-differentiated/dedifferentiated liposarcoma typically presents in the retroperitoneum as large masses. We provide clinicopathologic and follow-up details of 9 retroperitoneal/intra-abdominal benign lipomatous tumors (BLT) and discuss the utility of ancillary fluorescence in situ hybridization (FISH) in distinguishing from their malignant counterparts. Design. Clinicopathologic details and histology of 9 intra-abdominal and retroperitoneal lipomas were studied along with ancillary CD10 immunohistochemistry (IHC) and FISH for MDM2 and CDK4 amplification. Results. There were 6 females and 3 males. Median age at diagnosis was 52 years (range 36-81 years). Seven were identified incidentally and 2 presented with primary complaints. On imaging, 7 were considered suspicious for liposarcoma. Grossly, the tumors ranged from 3.4 to 41.2 cm (median 16.5 cm). Histologically, all cases showed well-differentiated BLT, further classified as lipoma (n = 7; 1 with metaplastic ossification, 2 with prominent vessels, and 4 ordinary lipomas) and lipoma-like hibernoma (n = 2)—the latter 2 showed intramuscular lesions with interspersed brown fat. CD10 IHC showed strong staining in the 2 hibernomas, whereas the staining was weak in the remaining. MDM2 and CDK4 amplification were negative by FISH in all. Follow-up (median 18 months) did not show recurrence on clinical or imaging evaluation. Conclusion. Retroperitoneal/intra-abdominal BLT are extremely rare and are indistinguishable clinically and radiographically from liposarcoma. This necessitates molecular confirmation even when the histology is convincingly benign, for a confident diagnosis. Our cohort shows that conservative excision without removal of abutted organs is sufficient in most cases.
Keywords: lipoma, liposarcoma, retroperitoneum, MDM2, CDK4
Introduction
Lipomas are common benign tumors of fully mature adipocytes that tend to present in the upper back, proximal extremities, and abdominal region as a superficial soft tissue mass. Rarely, these may present in the deeper subcutaneous tissue or in viscera. 1 In 2009, Macarenco et al presented the largest series of 19 well-differentiated adipocytic tumors 2 prior to which, and because of the overwhelming predilection of well-/de-differentiated liposarcoma (WD-/DD-LPS) at this site, large fatty tumor of the retroperitoneum would be presumed to be liposarcoma. Using cytogenetic and molecular genetic characteristics, they confirmed that not all lipomatous tumors in the retroperitoneum are liposarcomas and a small subset represents benign lipomas. These tumors are, however, still uncommon and pose diagnostic challenges clinically and histologically. In this retrospective study, we describe the clinicopathologic features and provide follow-up information on 9 abdominal and retroperitoneal lipomas that we diagnosed since 2009 with the help of fluorescence in situ hybridization (FISH) assay for MDM2 amplification. We further discuss differential diagnoses and utilization of FISH techniques for a confident diagnosis.
Materials and Methods
The study was approved by the institutional review board. Specimens were retrieved from the electronic database of the hospital laboratory using the terms “retroperitoneal,” “intra-abdominal,” and “lipoma” with the exclusion of “liposarcoma,” diagnosed after 2009 up to 2022. Concomitantly, a search was performed to obtain the number of lipomas diagnosed in our lab during the same timeframe. An extended search for tumors prior to 2009 using a variety of terminology did not show additional cases in the archives.
Clinicopathologic and follow-up information were obtained from the medical records and from the referring physicians. One section per centimeter of the tissue was submitted, according to the grossing protocol in our laboratory. The diagnosis was confirmed based on morphologic, immunohistochemical, and molecular (FISH) features. Hematoxylin and eosin-stained sections of formalin-fixed paraffin-embedded (FFPE) blocks were reviewed.
As CD10 has recently shown to be selectively highly expressed in hibernomas, 3 we retrospectively performed CD10 on all tumors to illustrate any difference in expression between tumors with and without brown fat. For these immunohistochemical studies, 4-μm-thick sections were cut from FFPE tissue blocks and mounted on positively charged slides. Sections were processed on a Bond-III automated staining system (Leica Biosystems), deparaffinized, and then subjected to heat-induced epitope retrieval. Sections were then stained with CD10 (RTU mouse monoclonal antibody; 56C6; catalog # PA0131; Leica Biosystems) on all. In 2 lesions, to exclude a spindle cell lipoma presenting at an unusual site or an atypical spindle cell lipomatous tumor, 4 immunostaining for Retinoblastoma-1 antigen (RB1, clone 1F-8; 1:50; Thermo Fisher Scientific) and CD34 (RTU mouse monoclonal antibody; QBEND10; Leica Biosystems) were performed at review.
Prospectively, at the time of diagnosis, FISH for mouse double minute 2 (MDM2) was performed on all, and for cyclin-dependent kinase 4 (CDK4) on 5 tumors. Retrospectively, CDK4) FISH was performed on remaining 4 tumors at the time of this review. FISH for MDM2 and CDK4 gene amplification was performed using 4-μm FFPE tissue sections of the tumors using commercially available dual DNA probes. For MDM2, dual DNA probes targeting MDM2 (12q15) and a centromeric region of chromosome 12 (SE12) (Kreatech Repeat-Free™ Poseidon™, Leica Biosystems) was used. The MDM2 (12q15) gene region probe is directly labeled with PlatinumBright™550 (red). The centromeric12 control probe is directly labeled with PlatinumBright495 (green). To detect CDK4 amplification, commercially available dual DNA probes for CDK4 (12q13) and a Satellite Enumeration 12 (SE12) to centromeric region of chromosome 12 (Kreatech Repeat-Free™) were used. The CDK4 (12q13) gene region probe is direct labeled with PlatinumBright™550 (red). The SE12 control probe is directly labeled with PlatinumBright™495 (green). In addition to the ratio of MDM2 or CDK4 copy signal against CEP12 or SE12 signal of more than 2.0, the morphologic feature of the amplified signals in cluster more than 6/cell was considered specific for WD-/DD-LPS. Immunohistochemical stain for MDM2 was not performed since its specificity and sensitivity is inferior to FISH. On the 2 cases with a question of spindle cell lipoma or atypical spindle cell lipomatous tumor, FISH for RB1 (RB1/13q14; Vysis LSI 13, Abbott Laboratories) was performed.
Results
Clinical and Imaging Findings
Clinical, imaging, and follow-up information are summarized in Table 1. From a total of 1039 lipomas diagnosed at our institution, 9 (0.77%) were located in the abdominal cavity or retroperitoneum. Among these patients, 6 were females and 3 were males. The median age at identification of the masses was 52 years (range 36-81 years). Only 2 patients presented with a related complaint while the tumors were detected incidentally in 7 patients. Among the 2 with clinical symptoms, one patient noticed asymmetry of the abdomen and the other complained of progressive abdominal bloating and back pain. In the remaining 7 patients, the tumor was detected in 5 by imaging for unrelated reasons; in one patient, the tumor was noticed perioperatively during surgery for a urinary bladder cystocele repair, while in the other, the primary physician noticed a lump on abdomen palpation in an annual visit and was subsequently imaged. The comorbidities that prompted imaging in those 5 patients included: primary biliary cirrhosis, lumbar radiculopathy, surveillance after surgery for sigmoid colon adenocarcinoma with lymph node metastasis, flank pain due to renal stones, and follow-up for recently diagnosed endometrial carcinoma. In the patient who had colorectal surgery 4 years’ prior, no soft tissue masses were identified perioperatively or in the interim.
Table 1.
Clinicopathologic Characteristics of Patients.
| Patient | Age (years)/Sex | Clinical history | Imaging findings and impression | Operative procedure | Gross size (largest dimension) |
MDM2 FISH |
CDK4 FISH |
Follow-up (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 81/Female | Recurrent UTI; cystocele and diverticulum of urinary bladder | Not visualized on imaging, incidentally found perioperatively | Complete excision | 3.4 cm | Neg | Neg | DOC |
| 2 | 64/Female | Cirrhosis; PBC; Liver and kidney transplant; mass detected during imaging for other diseases | 15.8 cm circumscribed, fat attenuation mass with internal septation in the right retroperitoneum with mild mass effect on the inferior pole of right kidney; liposarcoma | Complete excision after separation from Gerota's fascia | 26 cm | Neg | Neg | ANER (129); followed by imaging for 92 months (for transplant), then clinically |
| 3 | 36/Male | Self-visualized asymmetry of right lower abdominal wall | 10 cm encapsulated and lobulated fat-containing mass filling the right lower quadrant extending to below the right kidney and reaching up to the anterior abdominal wall and iliopsoas musculature; lipoma | Complete excision of mass after separation from iliopsoas musculature | 13.1 cm | Neg | Neg | ANER (20); followed by imaging |
| 4 | 59/Male | Lumbar radiculopathy with incidental detection of “fatty” mass in abdomen | 16 cm, minimally complex fat-containing lesion in the anteromedial aspect of left psoas muscle with lobulated, partially calcified focus along with the upper aspect; “atypical lipoma” versus WDLS | Complete excision after separation from musculature | 16.5 cm | Neg | Neg | ANER (21); followed by imaging for 6 months, then clinically |
| 5 | 44/Female | Abdominal bloating and back pain | 20 cm mass in the left retroperitoneum, predominantly fatty with multiple septations and encasing the left kidney with mass effect; suspicious for well-differentiated liposarcoma | Conglomerate of tumors completely removed in piecemeal | 41.2 cm; 2 additional resected portions—14 cm and 14.2 cm | Neg | Neg | ANER (20); followed clinically |
| 6 | 52/Male | Sigmoid colon adenocarcinoma metastatic to lymph nodes, mass detected on surveillance imaging 4 years post-colectomy | 12 cm, slowly growing retroperitoneal mass with variably thick encapsulation of up to 3 mm and suspicious solid component, displacing the left kidney anteriorly; lipoma with low suspicion for liposarcoma | Several separate tumors in the abdomen visualized perioperatively, incomplete removal for debulking | 13.2 cm; separate pieces 10.8 cm in aggregate | Neg | Neg | ANER (16); followed by imaging |
| 7 | 40/Female | Flank pain due to renal stones; mass discovered incidentally on imaging for renal stones, previous 2 c-sections, cholecystectomy (interval unknown) | 16.7 cm mass in right lower pelvis with displacement of uterus and bladder to the left and superior displacement of the right ovary, suspicious for WDLS versus “atypical” lipoma | Complete excision | 19.2 cm; smaller excised peritoneal nodule showed a benign multicystic mesothelioma | Neg | Neg | Recent surgery (6-month follow-up); ANER by imaging |
| 8 | 70/Female | Recently diagnosed serous endometrial carcinoma, imaging revealed a large retroperitoneal mass. | 14.6 cm fat-containing mass in the right pararenal space with enhancing solid component, suspicious for retroperitoneal liposarcoma | Partial resection | 8.9 cm | Neg | Neg | Recent surgery (3 months follow-up) |
| 9 | 68/Female | Right lower quadrant mass detected incidentally by PCP on physical examination; occasional nausea | 15.8 cm mass within the lower abdomen and pelvis with several soft tissue septations; impinging on adjacent bowel; favored to be a low-grade liposarcoma | Partial resection | In multiple pieces, 15 cm in aggregate | Neg | Neg | ANER; presented after 15 months with ventral abdominal incisional hernia due to protrusion of residual lipoma; FISH for MDM2 amplification was negative in the reexcision. Imaging prior to reexcision did not show growth of residual tumor |
Abbreviations: FISH, fluorescence in situ hybridization; UTI, urinary tract infection; DOC, died of other cause(s); PBC, primary biliary cirrhosis; ANER, alive and no evidence of recurrence; WDLS, well-differentiated liposarcoma; PCP, primary care physician.
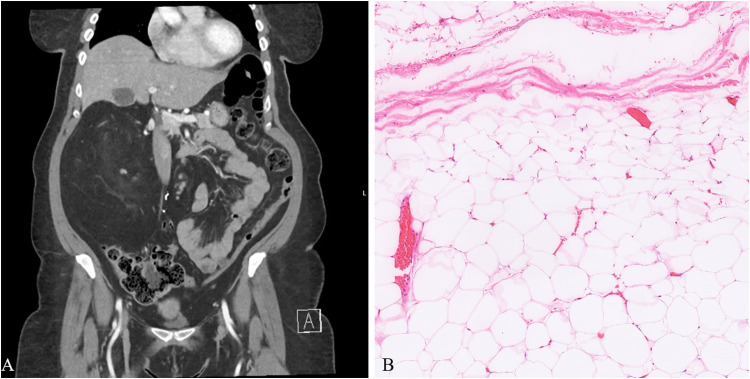
On computed tomography or magnetic resonance imaging, the masses were described as encapsulated of variable thickness, lobulated, complex, and with internal septations. The masses displaced the ipsilateral kidney or bowel in 7 examples. A suspicion of liposarcoma was raised in 7. In one tumor (patient 4), a lobulated, calcified focus was identified, which correlated histologically (see below). Two lesions showed encasement of iliopsoas muscle. Radiographic appearance of one example (patient 8) is shown in Figure 1A.
Figure 1.
A, Computed tomography scan shows a large, 14.6 cm fat-containing mass in the right retroperitoneal, pararenal space with slight enhancement of a small solid component. (B) “Giant” lipoma arising in the retroperitoneum showing typical features of mature adipocytes with thin fibrous strands traversing through fat lobules (patient 5).
Follow-up was available for all patients. The median follow-up period was 18 months (range 3-129 months); follow-up for more than 12 months was available in 6 patients. There was no evidence of recurrence in any patient. Patient 9 presented to the surgeon 15 months after initial resection for ventral incisional hernia due to residual pelvic intra-abdominal lipoma; no clinical or radiographic recurrence was suspected.
Gross Features
The median size of the tumors was 16.5 cm (range 3.4-41.2 cm). In 2 samples, there were additional fragmented pieces of the tumor with similar gross appearance. A thin, translucent capsule was present in all tumors. The cut surfaces of all tumors showed yellow, homogenous, lobulated appearances. There were no visible areas of hemorrhage or necrosis. Fibrous tissue was noted grossly in 3 tumors (patients 3, 5, and 6); however, this was not replacing the lobulated cut surface of the specimens. In patient 5, firm areas of calcification were also present. If needed, additional sections were submitted from any concerning areas. The small 3.4 cm lipoma (patient 1) perioperatively and grossly appeared distinct from lobulated peritoneal fat.
Microscopic, Immunohistochemical, and FISH Features
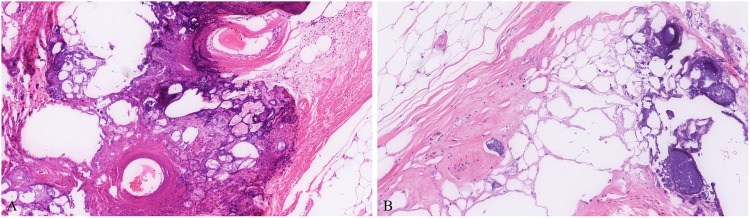
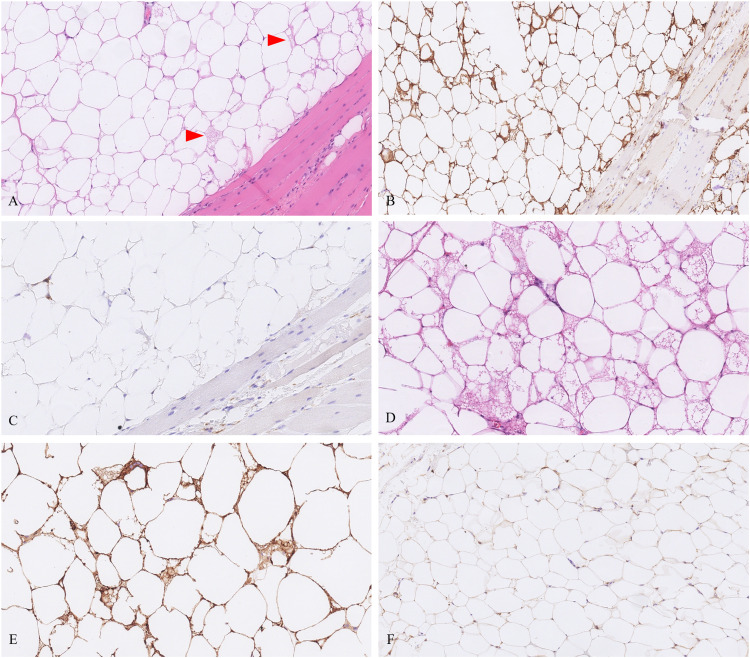
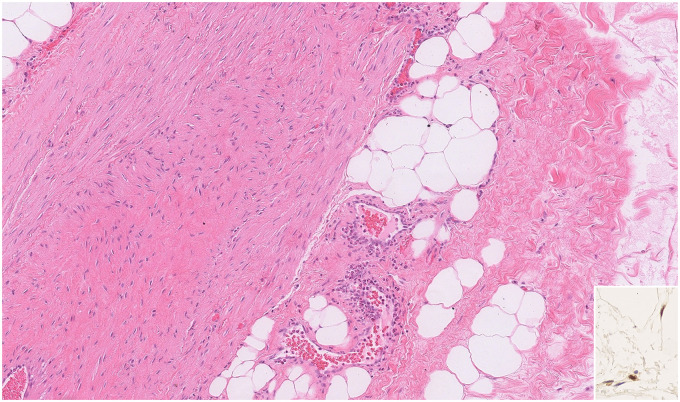
Microscopic sections of all 9 tumors showed nearly identical morphology with only slight variability. All tumors were thinly encapsulated. All neoplasms were composed of mature adipocytes with distinct cell boundaries and peripherally placed thin nuclei (Figure 1B). One tumor showed prominent osseous metaplasia with focal myxoid change (Figure 2). In 2 tumors, a prominent admixture of brown fat cells was noted (patients 3 and 6; Figure 3). Both these lesions also showed entrapped skeletal muscle, compatible with intramuscular lipoma-like hibernoma and were reclassified as such. Four tumors showed multiple thick-walled blood vessels and traversing fibrous bands. In 2 of those 4 tumors (patients 2 and 5), the extent of vessel wall thickness (Figure 4) prompted the reviewing pathologists to perform ancillary testing at the time of diagnosis to rule out differential diagnoses such as angiomyolipoma or lipoleiomyoma but were not supported by additional testing and hence were ultimately classified as lipomas with prominent vessels at the time of diagnosis. No atypical hyperchromatic stromal cells were identified in any specimen.
Figure 2.
Rare tumors can show metaplastic ossification (A and B) and can be worrisome for dedifferentiation. In this example (patient 4), calcification and ossification were present near areas of fat necrosis (B).
Figure 3.
A, Tumor from patient 3 showed an intramuscular retroperitoneal lipoma-like hibernomas with scattered rare brown fat cells (arrowheads). (B) CD10 showed strong staining in this example. (C) CD68 is shown in the same areas, which helps to discriminate from histiocytes. (D) The tumor from patient 6 showed prominent brown fat cell population with strong CD10 staining (E) as compared to negative or faint positivity in ordinary retroperitoneal lipoma without brown fat (F).
Figure 4.
Lipoma with prominent vasculature. A thick vessel wall with intervening adipocytes may prompt additional workup to exclude other differential diagnoses. In such examples, spindle cell lipoma or atypical spindle cell lipomatous tumor can be considered in the differential which was excluded based on morphology and retained RB1 staining (inset). In addition, CD34 was negative (not shown).
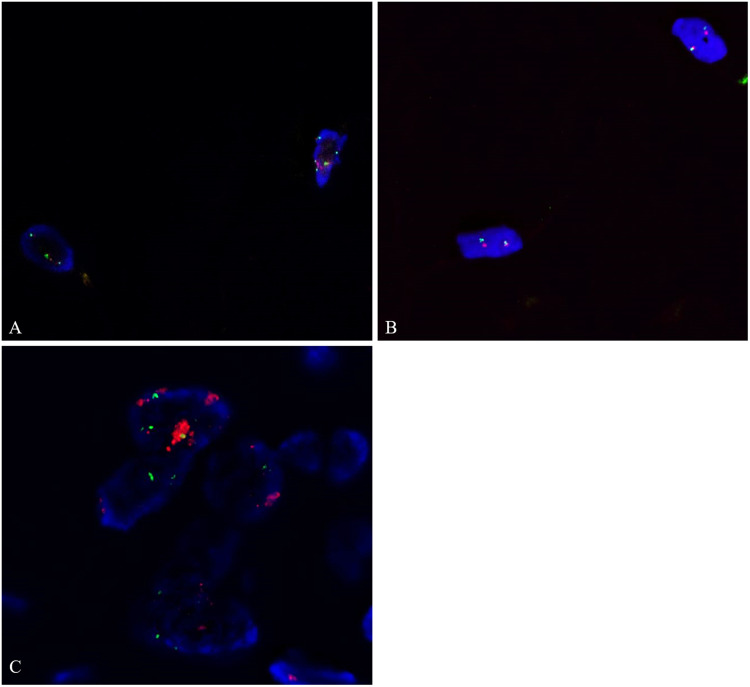
CD10 immunohistochemistry (IHC) was performed to detect any variation of staining between the lipomatous tumors with and without the hibernomatous components (Figure 3B, E, and F). CD10 showed diffuse and robust positivity in tumors from patients 3 and 6. It was negative to very faintly positive in 3 tumors and showed mild to moderate reactivity in 4 tumors. The staining was located predominantly in the cell membranes with very rare cells positive in the stroma of the fibrous bands. For the 2 tumors with thick-walled blood vessels where spindle cell lipoma was considered in the histologic differential at the time of this review (patients 2 and 5), CD34 was negative and RB1 was retained in the adipocyte and stromal nuclei (Figure 4 inset). RB1 FISH on 1 of 2 tumors showed retained probe signals in more than 80% of the cells. In the other tumor, there was poor probe hybridization in the adipocytes as well as native endothelium, likely due to older archival tissue and hence was inconclusive. Even in the absence of the confirmatory FISH assay, the lack of supportive morphologic and immunohistochemical features was sufficient to exclude a spindle cell lipoma in both instances. FISH studies in all 9 tumors did not show amplification of MDM2 or CDK4 (Figure 5).
Figure 5.
Fluorescence in situ hybridization for MDM2 (A) and CDK4 (B) shows a lack of amplification. (C) An example of dedifferentiated liposarcoma with MDM2 amplification is shown for comparison (red signal in A and C, MDM2 probe; red signal in B, CDK4 probe; green signal in A-C, CEP12 probe).
Discussion
Lipoma is a common, benign soft tissue tumor of mature adipocytes that present in the superficial subcutis of the extremities and body. It typically presents as a painless mass with up to 5% of patients having multiple lesions and a small number having an association with PTEN hamartoma syndrome. Although it is considered mainly a tumor of soft tissue and skeletal muscle, lipomas are also reported in unusual sites such as the airways, gastrointestinal tract, and head and neck region, including intracranial sites and salivary glands.1,5-8 Lipomas are usually less than 5 cm in size but rarely can grow to large sizes. In their review, Sanchez et al suggested the prefix of “giant” for any lipoma that exceeds the 10-cm cutoff. 9 Their review, however, did not include any intrabdominal or retroperitoneal lipomas.
When arising in the subcutaneous soft tissue in small size, the diagnosis is straightforward. Ruling out other possibilities becomes necessary when lipomas present in unusual sites or if they are remarkably large. Lipomas have been recognized to occur in the retroperitoneum but prior to, and since, Macarenco et al's large series, 2 it is only described in case reports of unusual presentations,10-19 such that additional experience in clinical–pathological findings and differential diagnostic considerations are difficult to find. In this study, we describe the clinicopathologic features of 9 additional abdominal cavity lipomas that were histologically and molecularly distinguished from WD-LPS.
Nearly all patients presented within the fourth to sixth decades. In contrast to previous larger descriptions, 2 the masses in most patients in our cohort were detected incidentally on imaging to monitor co-morbidities. When symptomatic, patients presented with visible abdominal asymmetry and bloating similar to that of WD-/DD-LPS. Given the large potential space of the retroperitoneum, the asymptomatic presentation is conceivable. As expected, symptoms result from the massive size and impingement on adjacent structures, including urinary urgency and frequency, bloating, nausea, and constipation. 17 Rarely, these tumors present in pregnancy.13,16,20 On imaging, the possibility of liposarcoma was raised in 7 out of 9 patients because of the alarmingly large size and complexity imparted by the fibrous septae. In one patient, the multilobulated mass encased the kidney, further convincing for a liposarcoma. Origin from the iliopsoas muscle was detected radiographically in 2 patients (histologically both were intramuscular lipomas).
Microscopically, typical features of lipoma were appreciated in each instance. These included thin lobules of mature adipocytes, traversed by thin fibrous bands, with an outer fibrous capsule. Two tumors in our cohort showed a smaller number of brown fat cells, signifying that these were in fact lipoma-like hibernomas. Hibernomas are slow-growing benign tumors and are usually present in the subcutis. We hypothesize that the empty retroperitoneal space likely allowed these hibernomas to grow and mature, ultimately demonstrating lipoma-like morphology. Recently, Gjorgova-Gjeorgjievski et al showed that CD10 is consistently expressed in hibernomas. 3 This was replicated in both retroperitoneal lipoma-like hibernomas where CD10 showed robust and diffuse expression within adipocyte membranes. Our experience with CD10 was slightly different than the abovementioned series 3 in the sense that our comparison group of intra-abdominal lipomas (rather than ordinary extremity lipomas) did show some CD10 positivity, albeit never expressing the same intensity as the 2 hibernomas.
The differential diagnoses of lipomas in the retroperitoneum include WD-/DD-LPS, renal, or extrarenal angiomyolipoma (PEComa), and lipoleiomyoma—WD-/DD-LPS holding the most diagnostic significance and challenge. WD-LPS is a malignant adipocytic lesion with a predilection for the retroperitoneum. The lipoma-like (adipocytic) variant of WD-LPS closely mimics its benign counterpart. Although the different subtypes (inflammatory, sclerosing) often coexist, the presence of just the adipocytic component in a biopsy can be challenging. Features that aid in distinction include substantial variation in cell size with the presence of nuclear atypia in fat or stromal spindle cells. 21 Progression of WD-LPS results in dedifferentiation, characterized by an abrupt transition to nonlipogenic sarcoma, in most instances to a high-grade type.22-24 Both WD-LPS and DD-LPS are cytogenetically characterized by the formation of supernumerary ring chromosomes, forming via an unknown mechanism, that contains amplified sequences in chromosome regions 12q14-q15. 25 This results in amplification of MDM2 gene that drives oncogenesis in liposarcoma.25,26 Several other genes are co-amplified in the amplicon that includes CDK4 (most common), TSPAN31, HMGA2, YEATS4, CPM, and FRS2. 27 MDM2 protein is an inhibitor of p53 transcriptional activity; gene amplification results in loss of p53 tumor suppressor function, leading to oncogenesis. 28 This genetic alteration can be detected by FISH as well as IHC targeting the nuclear-localized protein.29,30 Recently, multiplex ligation-dependent probe amplification technique was also described by Creytens et al to detect MDM2/CDK4 amplifications on FFPE with the high concordance with FISH. 31 Given the relatively insensitive nature of immunohistochemical studies in differentiated lipomatous tumors, FISH is considered the more reliable and cost-effective option compared to other molecular tests.29,30 Sirvent et al also note that IHC alone is often insufficient to solve diagnostic problems. 29 Further, MDM2 immunostain can show dim staining in histiocytes in traumatized lipomas, an important but underrecognized pitfall. 32 In our laboratory, we rely solely on FISH detection for MDM2 amplification. Apart from confirming the morphologic impression, this test is warranted for any “problematic” fatty tumors meeting any criteria described before. 33 In all our patients, the clinical and radiographic suspicion of WD-LPS was high due to the location and unusually large size. Microscopically, however, the lack of cell size variation and absence of atypical stromal cells supported the benign nature. MDM2 and CDK4 amplification was absent by FISH in all tumors, thus underscoring its utility for a confident diagnosis.
While discussing WD-/DD-LPS, 2 other important, albeit rare, situations warrant attention—first, hibernomas that morphologically mimic atypical lipomatous tumor/WD-LPS 34 and second, WD-/DD-LPS with hibernoma-like histology. 35 In both instances, careful microscopic search for atypical stromal cells or nuclei and areas of dedifferentiation is critical. In addition, ancillary tests for MDM2 overexpression or MDM2 amplification aid in distinction.34,35 Uncoupling protein 1 (UCP-1) is a recently described protein transporter and is considered a sensitive and specific marker of brown fat differentiation when compared to white/abdominal fat. 36 Further, Kojima et al tested UCP-1 on their cohort of liposarcomas with brown fat differentiation and found that UCP-1 is expressed in these foci, 35 which could be very useful to confirm brown fat differentiation when needed.
In contrast to WD-/DD-LPS, the genetic basis of some lipomas is attributed to translocations of HMGA2 (High Mobility Group AT-Hook 2) gene to various partners (LPP, CXCR7, EBF, LHFP, and NFIB).37-39 HMGA2 acts as a transcriptional regulating factor and rearrangements result in deregulated activity. Although a commercial HMGA2 FISH break apart probe is widely available, its utility in diagnosing ordinary lipoma is quite limited. In fact, it is more desirable to detect the absence of MDM2 amplification 40 to exclude liposarcoma. HMGA2 translocations were reported by Macarenco et al in 42% of their retroperitoneal lipomas. 2 In our experience, the absence of MDM2 amplification was sufficient to reach a confident diagnosis.
Few other tumors with lipomatous elements in the area warrant mention for differential diagnosis, particularly when only lipomatous elements present in small core biopsy material. In addition to histology, radiologic/imaging correlation is also very important in the diagnosis of retroperitoneal lipoma against these tumors with lipomatous elements in small biopsy material. Angiomyolipoma is a benign mesenchymal tumor belonging to a family of lesions characterized by the proliferation of perivascular epithelioid cells, termed PEComa. These tumors have a wide anatomic distribution and can present in renal or extrarenal sites.41-43 These tumors are composed of a varying quantity of adipose tissue, spindled and epithelioid smooth muscle cells, and thick-walled blood vessels. A unique feature of this tumor category is the coexpression of smooth muscle and melanocytic markers, including HMB-45, Melan-A, MITF, SMA, desmin, and caldesmon.42,44 “Fat-rich” angiomyolipomas have been described, defined by more than 75% of tumor composed of mature fat. 45 In a subset of our cases, we found thick-walled blood vessels with associated fibrous bands in the lobules of adipocytes. In some foci, these spindle cells appeared to emanate from the vessel wall, reminiscent of angiomyolipoma. However, both markers of smooth muscle and melanocytic differentiation were absent which aided in ruling out angiomyolipomas. A subset of PEComas is driven by TFE3 fusions with corresponding nuclear TFE3 expression. In females, a large retroperitoneal fatty mass would also generate the differential of a uterine lipoleiomyoma. These are uncommon mesenchymal tumors that are primarily present in the uterus, however, rare extrauterine examples also exist.46,47 In the largest reported series by Wang et al, the tumors were mostly small but did occasionally reach up to 35.5 cm. 46 Eight out of 50 tumors in their series showed adipocytes comprising 76% to 100% of the lesion. “Fat-rich” lipoleiomyoma was in the differential with angiomyolipoma in the same 2 specimens in our series. As mentioned earlier, negative muscle markers were supportive of a pure adipocytic tumor. PNL2 is a novel antibody that has shown high sensitivity and specificity for angiomyolipoma and PEComa, 48 although its utility in pure adipocytic tumors has yet to be established. In any case, the differential diagnosis is usually not difficult in excision specimens when other nonlipomatous elements are also present in these tumors.
Spindle cell lipoma arising in an unusual location or an atypical spindle cell lipomatous tumor4,49,50 can be considered if the morphological features are supportive, especially when these features are dominant in a needle core biopsy. In such situations, a combination of CD34 with RB1 IHC is useful for confirmation or exclusion; RB1 FISH is a complementary or alternative tool. These RB1-altered tumors are considered to lie on a spectrum 50 and so far have shown benign behavior with a small risk of recurrence in the latter. 4
Although common in subcutaneous lipomas, fat necrosis was only seen in one retroperitoneal lipoma because of the concealed nature and the extremely low probability of mechanical trauma. Nevertheless, histiocytes can simulate lipoblasts (pseudolipoblasts) and can create confusion. As alluded to earlier, MDM2 immunostain can show spurious positivity and should not be overcalled. 32 One tumor in our cohort showed marked chondrous and osseous metaplasia. These changes, although expected in long-standing lipomas, are rarely reported in giant lipomas. 51 In fact, the presence of osseous and chondroid metaplasia in a retroperitoneal fatty tumor is more concerning for DD-LPS, than a lipoma. In that scenario, however, the osteocartilaginous elements would be expected to show clear-cut malignant features.52,53
In summary, our series provides another cohort confirming the presence of lipomas in the retroperitoneum, which were diagnosed prospectively with the help of MDM2 FISH. Even though these are extremely rare (<1%), retroperitoneal and intra-abdominal lipomas could present with an ominous radiographic presentation, often escaping attention for a lengthy period. In our experience, they tend to achieve “giant” sizes as the empty retroperitoneum allows for growth without detection. The histologic features are typical of lipoma, except that rarely they may show the presence of brown fat or metaplastic changes. Molecular confirmation by FISH for lack of MDM2 amplification is highly recommended to render a reliable diagnosis of retroperitoneal lipoma. Our findings support that adequate sampling (1 section per centimeter), careful microscopic evaluation, and absence of MDM2 gene amplification (by FISH) are sufficient for a confident diagnosis, without the need for additional ancillary or molecular testing. Pathologists and clinicians should be aware that not all huge fatty tumors in the retroperitoneum are well-differentiated liposarcomas and the use of FISH assay on routine paraffin section for MDM2 amplification could effectively and reliably confirm the diagnosis of lipoma in the retroperitoneum.
Footnotes
Author Contributions: Wrote the manuscript: FM and PJZ; acquisition, analysis, or interpretation of data: all authors; final approval of manuscript: all authors.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
ORCID iD: Faizan Malik https://orcid.org/0000-0001-8597-5074
References
- 1.WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours. 5th ed. IARC; 2020. [Google Scholar]
- 2.Macarenco RS, Erickson-Johnson M, Wang X, et al. Retroperitoneal lipomatous tumors without cytologic atypia: are they lipomas? A clinicopathologic and molecular study of 19 cases. Am J Surg Pathol. 2009;33(10):1470-1476. doi: 10.1097/PAS.0b013e3181b278bf [DOI] [PubMed] [Google Scholar]
- 3.Gjorgova-Gjeorgjievski S, Fritchie K, Folpe AL. CD10 (neprilysin) expression: a potential adjunct in the distinction of hibernoma from morphologic mimics. Hum Pathol. 2021;110:12-19. doi: 10.1016/j.humpath.2020.12.012 [DOI] [PubMed] [Google Scholar]
- 4.Marino-Enriquez A, Nascimento AF, Ligon AH, Liang C, Fletcher CD. Atypical spindle cell lipomatous tumor: clinicopathologic characterization of 232 cases demonstrating a morphologic spectrum. Am J Surg Pathol. 2017;41(2):234-244. doi: 10.1097/PAS.0000000000000770 [DOI] [PubMed] [Google Scholar]
- 5.Eghwrudjakpor PO, Kurisaka M, Fukuoka M, Mori K. Intracranial lipomas. Acta Neurochir (Wien). 1991;110(3-4):124-128. doi: 10.1007/BF01400679 [DOI] [PubMed] [Google Scholar]
- 6.Agaimy A, Ihrler S, Markl B, et al. Lipomatous salivary gland tumors: a series of 31 cases spanning their morphologic spectrum with emphasis on sialolipoma and oncocytic lipoadenoma. Am J Surg Pathol. 2013;37(1):128-137. doi: 10.1097/PAS.0b013e31826731e0 [DOI] [PubMed] [Google Scholar]
- 7.Yehia, L, Keel, E, Eng, C. The clinical spectrum of PTEN mutations. Annu Rev Med. 2020;71:103-116. [DOI] [PubMed] [Google Scholar]
- 8.Boland JM, Fritchie KJ, Erickson-Johnson MR, Oliveira AM, Colby TV, Folpe AL. Endobronchial lipomatous tumors: clinicopathologic analysis of 12 cases with molecular cytogenetic evidence supporting classification as “lipoma”. Am J Surg Pathol. 2013;37(11):1715-1721. doi: 10.1097/PAS.0b013e3182a115c9 [DOI] [PubMed] [Google Scholar]
- 9.Sanchez MR, Golomb FM, Moy JA, Potozkin JR. Giant lipoma: case report and review of the literature. J Am Acad Dermatol. 1993;28(2 Pt 1):266-268. doi: 10.1016/s0190-9622(08)81151-6 [DOI] [PubMed] [Google Scholar]
- 10.Katebi Kashi P, Dengler KL, Hamilton CA. Lipoma or liposarcoma? Robotic resection of a retroperitoneal mass. J Obstet Gynaecol Can. 2021;44(10):1095-1096.e1. doi: 10.1016/j.jogc.2021.06.004 [DOI] [PubMed] [Google Scholar]
- 11.Laurens JR, Frankel AJ, Smithers BM, Strutton G. A rare case of a giant retroperitoneal lipoma with multiple limb and trunk lipomata without familial multiple lipomatosis. J Surg Case Rep. 2022;2022(3):rjac121. doi: 10.1093/jscr/rjac121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen ZY, Chen XL, Yu Q, Fan QB. Giant retroperitoneal lipoma presenting with abdominal distention: a case report and review of the literature. World J Clin Cases. 2022;10(5):1675-1683. doi: 10.12998/wjcc.v10.i5.1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassan B, Fatteh M, Abiad M, Khalifeh M, Chamsy D. Retroperitoneal lipoma in pregnancy: between surgical intervention and postpartum deferral. Int J Gynaecol Obstet. 2022;158(3):762-763. doi: 10.1002/ijgo.14287 [DOI] [PubMed] [Google Scholar]
- 14.Arab M, Noei Teymoordash S, Talayeh M, et al. Retroperitoneal lipoma, a rare cause of pelvic mass in women. Caspian J Intern Med. 2021;12(Suppl 2):S495-S499. doi: 10.22088/cjim.12.0.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nardi WS, Diaz Saubidet H, Porto EA, Quildrian SD. Resection of a giant retroperitoneal lipoma herniating through the inguinal canal. BMJ Case Rep. 2021;14(1). doi: 10.1136/bcr-2020-239301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell K, Fuller K, Thomay A, Shapiro R. Diagnosis and surgical management of a retroperitoneal lipoma in pregnancy. Case Rep Obstet Gynecol. 2020;2020:6309417. doi: 10.1155/2020/6309417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weniger M, D'Haese JG, Kunz W, et al. En-bloc resection of a giant retroperitoneal lipoma: a case report and review of the literature. BMC Res Notes. 2015;8:75. doi: 10.1186/s13104-015-1038-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammad AM, Yakubu AA. Giant retroperitoneal lipoma in an infant. J Surg Tech Case Rep. 2010;2(1):33-34. doi: 10.4103/2006-8808.63722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singaporewalla RM, Thamboo TP, Rauff A, Cheah WK, Mukherjee JJ. Acute abdominal pain secondary to retroperitoneal bleeding from a giant adrenal lipoma with review of literature. Asian J Surg. 2009;32(3):172-176. doi: 10.1016/S1015-9584(09)60390-0 [DOI] [PubMed] [Google Scholar]
- 20.Wei D, Shen L, Yang K, Fang F. Giant retroperitoneal lipoma in a pregnant patient. J Obstet Gynaecol. 2013;33(5):522. doi: 10.3109/01443615.2013.788621 [DOI] [PubMed] [Google Scholar]
- 21.Evans HL. Atypical lipomatous tumor, its variants, and its combined forms: a study of 61 cases, with a minimum follow-up of 10 years. Am J Surg Pathol. 2007;31(1):1-14. doi: 10.1097/01.pas.0000213406.95440.7a [DOI] [PubMed] [Google Scholar]
- 22.McCormick D, Mentzel T, Beham A, Fletcher CD. Dedifferentiated liposarcoma. Clinicopathologic analysis of 32 cases suggesting a better prognostic subgroup among pleomorphic sarcomas. Am J Surg Pathol. 1994;18(12):1213-1223. doi: 10.1097/00000478-199412000-00004 [DOI] [PubMed] [Google Scholar]
- 23.Weiss SW, Rao VK. Well-differentiated liposarcoma (atypical lipoma) of deep soft tissue of the extremities, retroperitoneum, and miscellaneous sites. A follow-up study of 92 cases with analysis of the incidence of “dedifferentiation”. Am J Surg Pathol. 1992;16(11):1051-1058. doi: 10.1097/00000478-199211000-00003 [DOI] [PubMed] [Google Scholar]
- 24.Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3(6):507-523. doi: 10.1097/00000478-197912000-00004 [DOI] [PubMed] [Google Scholar]
- 25.Halvorson LM, Kaiser UB, Chin WW. The protein kinase C system acts through the early growth response protein 1 to increase LHbeta gene expression in synergy with steroidogenic factor-1. Mol Endocrinol. 1999;13(1):106-116. doi: 10.1210/mend.13.1.0216 [DOI] [PubMed] [Google Scholar]
- 26.Dei Tos AP, Doglioni C, Piccinin S, et al. Coordinated expression and amplification of the MDM2, CDK4, and HMGI-C genes in atypical lipomatous tumours. J Pathol. 2000;190(5):531-536. doi: [DOI] [PubMed] [Google Scholar]
- 27.Creytens D, Van Gorp J, Speel EJ, Ferdinande L. Characterization of the 12q amplicons in lipomatous soft tissue tumors by multiplex ligation-dependent probe amplification-based copy number analysis. Anticancer Res. 2015;35(4):1835-1842. [PubMed] [Google Scholar]
- 28.Haines DS. The mdm2 proto-oncogene. Leuk Lymphoma. 1997;26(3-4):227-238. doi: 10.3109/10428199709051772 [DOI] [PubMed] [Google Scholar]
- 29.Sirvent N, Coindre JM, Maire G, et al. Detection of MDM2-CDK4 amplification by fluorescence in situ hybridization in 200 paraffin-embedded tumor samples: utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real-time PCR. Am J Surg Pathol. 2007;31(10):1476-1489. doi: 10.1097/PAS.0b013e3180581fff [DOI] [PubMed] [Google Scholar]
- 30.Binh MB, Sastre-Garau X, Guillou L, et al. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. Am J Surg Pathol. 2005;29(10):1340-1347. doi: 10.1097/01.pas.0000170343.09562.39 [DOI] [PubMed] [Google Scholar]
- 31.Creytens D, van Gorp J, Ferdinande L, Speel EJ, Libbrecht L. Detection of MDM2/CDK4 amplification in lipomatous soft tissue tumors from formalin-fixed, paraffin-embedded tissue: comparison of multiplex ligation-dependent probe amplification (MLPA) and fluorescence in situ hybridization (FISH). Appl Immunohistochem Mol Morphol. 2015;23(2):126-133. doi: 10.1097/PDM.0000000000000041 [DOI] [PubMed] [Google Scholar]
- 32.Stojanov IJ, Marino-Enriquez A, Bahri N, Jo VY, Woo SB. Lipomas of the oral cavity: utility of MDM2 and CDK4 in avoiding overdiagnosis as atypical lipomatous tumor. Head Neck Pathol. 2019;13(2):169-176. doi: 10.1007/s12105-018-0928-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clay MR, Martinez AP, Weiss SW, Edgar MA. MDM2 Amplification in problematic lipomatous tumors: analysis of FISH testing criteria. Am J Surg Pathol. 2015;39(10):1433-1439. doi: 10.1097/PAS.0000000000000468 [DOI] [PubMed] [Google Scholar]
- 34.Al Hmada Y, Schaefer IM, Fletcher CDM. Hibernoma mimicking atypical lipomatous tumor: 64 cases of a morphologically distinct subset. Am J Surg Pathol. 2018;42(7):951-957. doi: 10.1097/PAS.0000000000001061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kojima N, Komiyama M, Shinoda Y, et al. Liposarcoma with hibernoma-like histology: a clinicopathologic study of 16 cases. Am J Surg Pathol. 2022;46(10):1319-1328. doi: 10.1097/PAS.0000000000001911 [DOI] [PubMed] [Google Scholar]
- 36.Malzahn J, Kastrenopoulou A, Papadimitriou-Olivgeri I, et al. Immunophenotypic expression of UCP1 in hibernoma and other adipose/non adipose soft tissue tumours. Clin Sarcoma Res. 2019;9:8. doi: 10.1186/s13569-019-0118-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacaria M, El Demellawy D, McGowan-Jordan J. A rare case of pediatric lipoma with t(9;12)(p22;q14) and evidence of HMGA2-NFIB gene fusion. Cancer Genet. 2017;216-217:100-104. doi: 10.1016/j.cancergen.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 38.Panagopoulos I, Gorunova L, Bjerkehagen B, Lobmaier I, Heim S. The recurrent chromosomal translocation t(12;18)(q14∼15;q12∼21) causes the fusion gene HMGA2-SETBP1 and HMGA2 expression in lipoma and osteochondrolipoma. Int J Oncol. 2015;47(3):884-890. doi: 10.3892/ijo.2015.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bianchini L, Birtwisle L, Saada E, et al. Identification of PPAP2B as a novel recurrent translocation partner gene of HMGA2 in lipomas. Genes Chromosomes Cancer. 2013;52(6):580-590. doi: 10.1002/gcc.22055 [DOI] [PubMed] [Google Scholar]
- 40.Antonescu CR, Organization WH. Soft Tissue and Bone Tumours. International Agency for Research on Cancer; 2020. [Google Scholar]
- 41.Bennett JA, Braga AC, Pinto A, et al. Uterine PEComas: a morphologic, immunohistochemical, and molecular analysis of 32 tumors. Am J Surg Pathol. 2018;42(10):1370-1383. doi: 10.1097/PAS.0000000000001119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010;41(1):1-15. doi: 10.1016/j.humpath.2009.05.011 [DOI] [PubMed] [Google Scholar]
- 43.Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29(12):1558-1575. doi: 10.1097/01.pas.0000173232.22117.37 [DOI] [PubMed] [Google Scholar]
- 44.Makhlouf HR, Ishak KG, Shekar R, Sesterhenn IA, Young DY, Fanburg-Smith JC. Melanoma markers in angiomyolipoma of the liver and kidney: a comparative study. Arch Pathol Lab Med. 2002;126(1):49-55. doi: 10.5858/2002-126-0049-MMIAOT [DOI] [PubMed] [Google Scholar]
- 45.Mehta V, Venkataraman G, Antic T, Rubinas TC, Le Poole IC, Picken MM. Renal angiomyolipoma, fat-poor variant–a clinicopathologic mimicker of malignancy. Virchows Arch. 2013;463(1):41-46. doi: 10.1007/s00428-013-1432-2 [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Kumar D, Seidman JD. Uterine lipoleiomyomas: a clinicopathologic study of 50 cases. Int J Gynecol Pathol. 2006;25(3):239-242. doi: 10.1097/01.pgp.0000192273.66931.29 [DOI] [PubMed] [Google Scholar]
- 47.Schaefer SL, Strong AL, Bahroloomi S, et al. Large intraperitoneal lipoleiomyoma in a pre-menopausal woman: a case report. World J Surg Oncol. 2021;19(1):144. doi: 10.1186/s12957-021-02256-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gulavita P, Fletcher CDM, Hirsch MS. PNL2: An adjunctive biomarker for renal angiomyolipomas and perivascular epithelioid cell tumours. Histopathology. 2018;72(3):441-448. doi: 10.1111/his.13369 [DOI] [PubMed] [Google Scholar]
- 49.Creytens D, Mentzel T, Ferdinanden L, et al. “Atypical” pleomorphic lipomatous tumor: a clinicopathologic, immunohistochemical and molecular study of 21 cases, emphasizing its relationship to atypical spindle cell lipomatous tumor and suggesting a morphologic spectrum (atypical spindle cell/pleomorphic lipomatous tumor). Am J Surg Pathol. 2017;41(11):1443-1455. doi: 10.1097/PAS.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 50.Creytens D, van Gorp J, Savola S, Ferdinande L, Mentzel T, Libbrecht L. Atypical spindle cell lipoma: a clinicopathologic, immunohistochemical, and molecular study emphasizing its relationship to classical spindle cell lipoma. Virchows Arch. 2014;465(1):97-108. doi: 10.1007/s00428-014-1568-8 [DOI] [PubMed] [Google Scholar]
- 51.Simsek T, Sonmez A, Aydogdu IO, Eroglu L, Karagoz F. Giant fibrolipoma with osseous metaplasia on the thigh. J Plast Reconstr Aesthet Surg. 2011;64(5):e125-e127. doi: 10.1016/j.bjps.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 52.Yoon RS, Benevenia J, Beebe KS, Hameed M. Dedifferentiated liposarcoma of thigh with chondrosarcomatous dedifferentiated component. Am J Orthop (Belle Mead NJ). 2010;39(11):E114-E118. [PubMed] [Google Scholar]
- 53.Yoshida A, Ushiku T, Motoi T, Shibata T, Fukayama M, Tsuda H. Well-differentiated liposarcoma with low-grade osteosarcomatous component: an underrecognized variant. Am J Surg Pathol. 2010;34(9):1361-1366. doi: 10.1097/PAS.0b013e3181ebcc45 [DOI] [PubMed] [Google Scholar]