Abstract
Background
Since the first case of COVID-19 was diagnosed in Wuhan, China in late 2019, concomitant infections with Herpesviridae were documented that were presented from simple skin manifestations to severe life-threatening conditions that may lead to mortality. In this systematic review, we have included studies conducted in different parts of the world to find out the association of clinical features and outcomes of COVID-19 infection and concomitant Herpesviridae infection.
Methods
A comprehensive search was conducted in electronic databases including Medline through PubMed, Cochrane database, Scopus and Web of science (core collection). Two review authors independently screened the articles and extracted data. The Risk of bias assessment was done by using RoBANS tool.
Results
A total of 919 studies were retrieved and 19 studies were included having data of 539 patients who were infected with both COVID-19 and Herpesviridae. Herpes Simplex-1, Varicella Zoster, Cytomegalovirus, Epstein-Barr virus and Human Herpes Virus-6 were the detected viruses in the included studies. Cytomegalovirus (CMV) reactivation was the most detected concomitant infection. In case of reactivation with more than one Herpes virus mortality among patients were detected along with single viral infection in some studies. Significant association was noted in dosage and usage of steroid and Herpesviridae reactivation in COVID-19 patients. Blood markers such as D-dimer, CRP along with length of stay in the ICU and usage of invasive mechanical ventilation were found to be the significantly associated markers.
Conclusion
Findings from this study will aid clinicians to assess and treat COVID-19 cases with co-infections.
Keywords: COVID-19, Herpesvirus, Reactivation, Co-infection, Systematic review
1. Introduction
Originating from China, the SARS-CoV-2 or COVID-19 virus has wreaked havoc worldwide since December 2019 [1,2] The disease is responsible for mild, moderate to severe type of illness which may lead to severe respiratory distress syndrome that needs urgent hospitalization and sometimes mechanical ventilatory support which may be followed by death. As of 13th September 2023, 770, 563,467 cases were diagnosed as COVID-19 positive and 6,957,216 people died from it [3]. COVID-19 has been the most significant cause of death from the pandemic since the outbreak of the Spanish Flu in the 1900s. The clinical spectrums of COVID-19 infections are of the following: asymptomatic, mild to moderate illness, severe and critical illness [2,4,5] Patients who have severe and critical COVID-19 infection are more likely to have serious complications which may ultimately lead to morbidity and mortality due to suspected triggering of a phase known as ‘cytokine storm’ [2,4,6]. This phase may cause immunosuppression and leucopenia leading to poor outcomes and can be worsened with an underlying superadded viral, bacterial or fungal infection [4,6,7].
Herpesviruses are a group of eight viruses that are known for their ability to cause latent infections and reactivations in immunocompromised patients [8]. They can remain latent in different tissues in the body and can cause various clinical symptoms that can sometimes manifest as opportunistic infections [9]. Among these viruses, Herpes simplex 1 and 2 (HSV-1, HSV-2), Varicella Zoster virus (VZ), Human Herpes Virus 6 (HHV-6), Epstein-Barr virus (EBV) and Cytomegalovirus (CMV) are the common ones causing coinfection or gets reactivated under some circumstances such as decreased immune response, leucopenia, co-morbid conditions, stimuli such as fever, stress, pain etc. [8] Herpes Simplex virus 1, 2, Varicella zoster virus and Human herpes virus 6 mainly cause cutaneous or skin manifestations while Epstein-Barr virus and Cytomegalovirus mainly cause systemic infections. There are some studies that suggest COVID-19 infection can trigger Herpesviridae reactivation [10]. This reactivation is mainly common in critically ill patients or patients who are undergoing invasive mechanical ventilation, prolonged usage of corticosteroids, and getting treated in the intensive care unit even though there is no history of any pre-existing immunodeficient conditions [8,10]. Pre-existing comorbid conditions such as Hypertension, Diabetes Mellitus, Heart disease, Organ transplantation, and Malignancy can trigger the reactivation or can induce the persistence of the virus as a co-infection with COVID-19 [7]. Some published reports confirmed the co-existence of the Herpes virus with laboratory-confirmed COVID-19 cases during this pandemic [6,7,10].
It is suspected that the viral replication and immunomodulatory mechanisms of the COVID-19 virus can trigger the reactivation of Herpesviruses [11,12]. Steroids and some immunomodulatory drugs can also play a role in this suspected mechanism [13].
There are published articles that the viral reactivation or co-infection of viruses in COVID-19 infection can cause significant mortality and morbidity in critically ill patients, though there are no specific clinical guidelines available to treat this viral reactivation and other infections, the lack of early diagnostic tools and appropriate treatment can delay the full recovery of patients from these infections. Adequate information about the prevalence and clinical characteristics of Herpesvirus coinfection or reactivation with COVID-19 can help clinicians to prepare proper guidelines to treat these infections precisely. Regarding this importance, we conducted a systematic review from published articles to find out the relationship between COVID-19 infected patients with concomitant Herpes virus co infections and reactivations and the clinical features and outcomes of these infections in patients.
2. Methods
2.1. Search strategy
This systematic review was conducted following the standard methods and reported as per the PRISMA 2020 statement [14]. The review was registered at the PROSPERO (CRD42022327981). Articles were retrieved from these databases: Medline through Pubmed, Cochrane database, Scopus and Web of Science (core collection) on April 24, 2022. We selected the keywords based on the population, intervention/exposure, comparison, and outcome aspects of the research question. The following key terms were used “Clinical Feature,” “Outcomes,” “Herpes Virus,” “Herpesviridae,” “Co-infection,” “Reactivation,” “Coronavirus disease,” “2019 – nCoV,” “SARS CoV – 2 disease,” “Severe acute respiratory syndrome disease 2,” “WUHAN disease,” “Novel coronavirus disease,” “COVID-19,” “Coronavirus,” “SARS-CoV-2,” “Herpesvirus 1 (alpha) human”, “Herpes Labialis virus” “Human herpesvirus 1” “HHV-1” “HSV-1”, “Herpes simplex rus 1”, “Herpesvirus 2 (alpha) human”, “Herpes Labialis virus” “Human herpesvirus 2” “HHV-2” “HSV-2”, “Herpes simplex virus 2”, “Human Herpesvirus 3”, “Chickenpox virus” “Ocular herpeszoster virus 2” “Varicella-zoster virus” “VZ virus”, “Herpes varicella” “Herpes Zoster virus”, “Cytomegalovirus”, “HCMV”, “Salivary gland virus”, “HHV 5”, “Human Herpes virus 5”, “Herpesvirus (beta) 5 human”, “Roseolovirus”, “HHV-6” “Human betaherpesvirus 6”, “Human Herpesvirus 6”, “HHV 6”, “Human Herpesvirus 6A″, “Human Herpesvirus 6B” “HHV6A” “HHV6B″, “HBLV” “Human B lymphotropic virus”, “HHV-7”, “Human herpesvirus 7”, “Burkitts lymphoma virus”, “E B virus” “Infectious mononucleosis virus”, “Epstein-Barr virus”, “Herpes virus 4 (gamma) human” “HHV-4”, “Human Herpes virus 4”, “EBV”, “HHV-8”, “KSHV”, “Kaposis sarcoma herpesvirus”, “Kaposis sarcoma-Associated herpesvirus”, “Kaposis sarcoma associated herpesvirus”, “Human herpes virus 8” combined with Boolean operators. The Mesh terms (Medical Subject Heading) and text words were used. Additionally, relevant literatures were searched and used to include additional literature. The search strategy for the databases is provided in Supplementary File 1.
2.2. Selection criteria
For this review, we considered any study including cross-sectional studies, case-control studies, cohort studies, quasi-experimental studies, and randomized controlled trials with primary data. Original articles that were published in English and containing primary data were included in this study. Case reports, case series, pre-print papers, editorials were not considered.
Patients who were infected with COVID-19 with no restriction for clinical grading ranging from asymptomatic infection to severe illness with co-infection or reactivation with any virus of Herpesviridae family were included in this study. According to the World Health Organization (WHO) and National Institute of Health (NIH), Asymptomatic or pre-symptomatic infection of COVID-19 is defined as; individuals who tests positive to SARS-CoV-2 with a virologic test. Mild illness can be defined as individuals who have the following symptoms of COVID-19 like fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell but does not have shortness of breath, dyspnea or abnormal chest imaging. Severe illness is individuals who have SPO2<94% on room air at sea level, a respiratory rate of >30breaths per minute, or lung infiltrates >50%. Critical illness is individuals who has respiratory failure, septic shock and/or multiple organ failure [15,16]. Richard J. Whitely denoted Herpes virus infections as; ‘’only 8 of the more than 100 known Herpesviruses routinely only infect humans, they are Herpes Simplex 1 and 2, varicella-zoster virus, cytomegalovirus, Ebstein-Barr virus, Human Herpes virus 6, human herpes virus 7 and Kaposi's sarcoma virus or human herpes virus 8. All of them can establish latent infections in specific tissues, which are different for each virus'’ [17]. Coinfection can be marked as simultaneous or successive infection of a single cell or host by multiple pathogens [18] Traylen et al., illustrated reactivation of virus as the process by which a latent virus switches to a lytic phase of replication [19]. Data from laboratory diagnosis of COVID-19 and Herpes virus were included irrespective of gender, age and geographical distribution.
2.3. Screening of articles
After removal of duplicates, titles and abstracts of all retrieved articles were independently screened by two reviewers using the “Rayyan QCRI software” [20]. Eligible articles that were screened for title-abstract were further subjected to full text screening by other two reviewers independently. Disagreements regarding the inclusion of studies were resolved by review authors after discussing among themselves. Persisting discords were resolved by the opinion of lead author to reach an agreement. The reason for exclusion at full-text screening phase was reported following the “prioritization and sequential exclusion technique” [21].
2.4. Data extraction
The primary outcome of this study was laboratory confirmed COVID-19 cases with coinfection or reactivation of any virus from the Herpesviridae family and the additional outcomes were clinical features, mortality and morbidity associated with these cases. An impromptu data extraction template was developed using an Excel worksheet. Two review authors independently extracted the following data from the included articles; author, year of publication, country, region, study design, sample size, study population (age, race, sex), study duration, co-morbidity, sample type and diagnosis method of COVID-19, clinical feature of COVID-19, concerned Herpes virus name, sample type and diagnosis method of Herpes virus, coinfection or reactivation, type or name of herpes virus lesion, clinical feature of herpes virus infection, received treatment for Herpes virus infection, history of immunosuppression (lymphopenia, LDH level or others), significantly associated blood markers, history of steroid intake, dose and duration of steroid intake, history of tocilizumab intake, dose and duration of tocilizumab intake, history of ICU admission or mechanical ventilation, duration of ICU stay or mechanical ventilation, time length from diagnosis of COVID-19 infection to development of Herpes virus infection, prognosis, statistical analysis (test name, odds ratio, 95% CI, p-value). All disagreements were resolved through discussion among the authors. Data from laboratory diagnosis of COVID-19 with any of the Herpesviridae virus coinfection or reactivation were included. For the laboratory diagnosis of COVID-19 nasopharyngeal swab and throat swab were taken and RT-PCR was the method for diagnosis. In case of Herpes virus diagnosis, blood, serum, plasma, bronchoalveolar lavage fluid, endotracheal aspirates, peripheral blood leukocytes, skin biopsy and tracheal aspirates samples were taken and qPCR, serology for antigen or antibody detection and molecular methods such as PCR were used.
2.5. Risk of bias assessment
The RoBANS tool (Risk of Bias Assessment tool for Non-randomized Studies) which contains six domains (e.g. the selection of participants, confounding variables, the measurement of exposure, the blinding of outcome assessments, incomplete outcome data and selective outcome reporting) was used to assess the risk of bias of this study [22]. RoBANS was considered because it is a validated standard tool specifically designed for assessing the risk of bias in non-randomized studies. The risk of bias was assessed by two review authors independently. Any disputes were resolved by the lead review author.
2.6. Data summary measures and synthesis
A graphical representation of Risk of bias assessment was conducted using stacked bar chart. Percentage and frequency were used for synthesis and presentation of the extracted data. In order to demonstrate the association of clinical features and outcomes of COVID-19 and Herpesviridae co-infection or reactivation a narrative synthesis approach was taken. Due to heterogenicity of the data, meta-analysis was not possible. The demonstration of the findings of the studies regarding clinical features and outcomes of COVID-19 infection with co-infection and reactivation of different viruses of Herpesviridae family were shown in tables.
3. Results
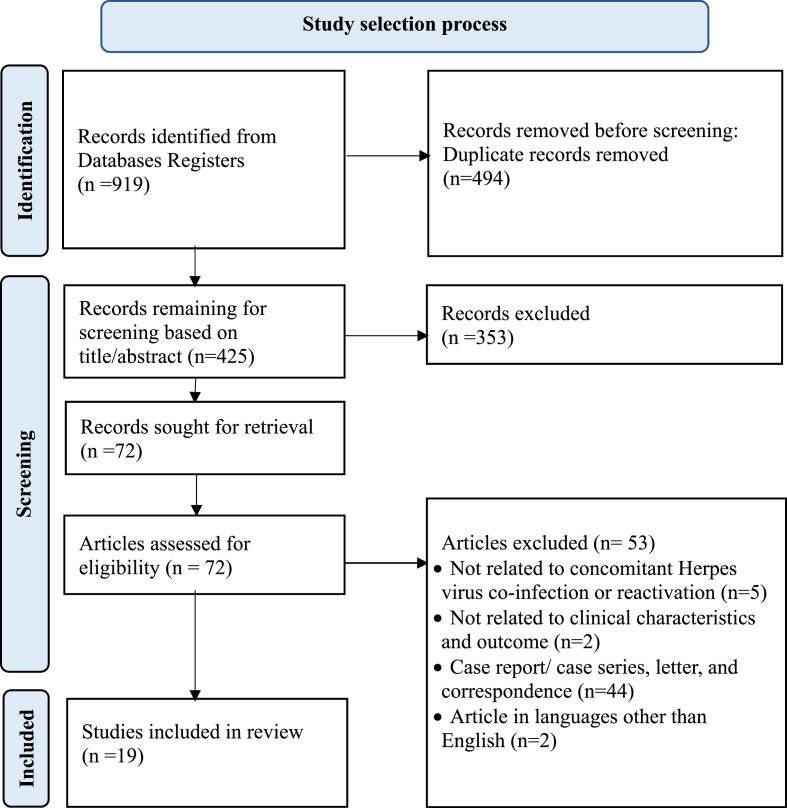
A total of 919 articles were found related to concomitant infection of Herpes virus and COVID-19. After thorough screening and completing all the processes, total 19 articles were included in this study for analysis and data extraction. The detailed flow of systematic review study selection process was shown in Fig. 1.
Fig. 1.
PRISMA flow diagram.
3.1. Study characteristics
Though all of the studies in this review were observational study by nature with heterogenous data, 11 of them are retrospective study and the rest 8 of them are prospective studies [[11], [12], [13],[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38]]. Heterogeneity was observed in terms of population, study design (both prospective and retrospective), exposure reporting, and outcome reporting. All of these studies were conducted between January 2020 to May 2021. A total of 539 participants were included in this study. Studies were conducted in all six WHO regions and the highest 10 studies were from WHO EUR region [11,25,[27], [28], [29], [30], [31], [32],34,36,38]. Though various ethnicities were included, in this review no association was found between ethnicities and Herpesviridae co-infection or reactivation. The included studies identified only five viruses from the Herpesviridae family. The study population had a wide range of age group starting from 4 to 80 years of age including both male and female gender along with various co-morbidities. One study showed the inclusion of Long COVID and short COVID groups as both cases and controls [26]. These study characteristics were briefly included in Table 1.
Table 1.
Characteristics of the included studies.
| Author, year | Country of study and Region | Study design | Sample size | Study duration | Population characteristics |
||
|---|---|---|---|---|---|---|---|
| Age (Mean/median) | Sex | Comorbidity | |||||
| Meshram HS et al., 2022 [24] | India; SEAR |
Retrospective | Total 18; (2 were D+/R-, 6 were D-/R+, 10 were D+/R+, 1 had past CMV infection) | May 2020–Dec 2020 | 45 (53–38) | M:F (14/4) | HTN, DM, Heart Disease, Renal Transplant, Obesity |
| Almutairi N et al., 2022 [23] | Kuwait; EMR |
Prospective | Total 17; 12 cases (another 5 were vaccinated against COVID-19) | March 2020–July 2021 | 53 (37–75) | M:F (14/1) | HTN, DM, COPD |
| Simonnet A et al., 2021 [25] | France; EUR |
Retrospective | Total 34 | March–August 2020 | 58 (26–81) | M:F (25/11) | HTN, DM, Dyslipideamia, Cancer, Immunodeficiency, Myocardiopathy |
| Chen T et al., 2021 [35] | China; WPR |
Retrospective | Total 67 | January–February 2020 | 37 (30–52) | M:F (32/35) | CVD, HTN, DM, CLD, Digestive system disease |
| Lino K et al., 2021 [26] | Brazil; AMR |
Retrospective | Total 60; 13 were HHV-6 positive | April–July 2020 | 52.3 | M:F (8/5) | Cancer, CVD, DM, Therapeutic immunosuppression, obesity |
| Yamamoto Y et al., 2021 [13] | Japan; WPR |
Retrospective | Total 59; 15 cases | April–May 2021 | 67 (59–73) | M:F (13/2) | Obesity, HTN, DM, Dyslipidaemia |
| Giacobbe DR, 2022 [36] | Italy; EUR |
Retrospective | Total 41; 12 cases | April–May 2021 | 65 (60–70) | M; F (33/8) | DM, HTN, COPD, ESRD, CLD, Cancer, HIV infection |
| Fuest KE et al., 2022 [11] | Germany; EUR |
Observational cohort | Total 134; 61 cases | March 2020–January 2021 | 72.5 (60–78) | M:F (98/36 | Malignancy, DM, COPD, HTN |
| Xie Y et al., 2021 [12] | China; WPR |
Retrospective | Total 128; 17 cases | January–March 2020 | 62 (52–68) | M:F (10/7) | Not reported |
| Abadias Grando et al., 2021 [27] | Spain; EUR |
Observational | Total 53; 12 cases | May–October 2020 | 4–50 | M:F (6/6) | Not reported |
| Niitsu T et al., 2021 [37] | Japan; WPR |
Retrospective | Total 26; 6 cases | April 2020–February 2021 | 76.5 (66.25–80) | M:F (3/3) | Not reported |
| Navarro Bielsa A, 2021 [28] | Spain; EUR |
Prospective | Total 63; 12 cases (4 of them are COVID-19 test positive) | May 2020–February 2021 | 34.6 (0.5–74) | M:F (31.8%/61.9%) | Not reported |
| Balc'h P et al., 2020 [29] | France; EUR |
Prospective observational | Total 38; 18 cases (9 HSV, 2 CMV, 7 co reactivation) | March 3, 2020, April 15, 2020 | 59 (54–71) | M:F (12/6) | Not reported |
| Francheschini et al., 2021 [34] | Italy; EUR |
Prospective observational | Total 70; 21 cases | April–May 2020 | 72 (66–76) | DM, HTN, CVD, CKD, Cancer | |
| Reizine F et al., 2021 [30] | France; EUR |
Prospective observational | Total 122; 33 cases | March 2020–April 2021 | 71 (61–73) | M:F (27/6) | Obesity, HTN, DM, previous immunosuppression |
| Saade A et al., 2021 [32] | France; EUR |
Retrospective | Total 100 (38 had immune defect); 63 cases (12 HSV, 58 EBV, 19 CMV) | February–May 2020 | 60 (53–67) | M:F (47/16) | Solid tumor, haematological malignancy, Organ transplantation, HIV infection, Autoimmune or inflammatory disease |
| Meyer A et al., 2021 [38] | France; EUR |
Observational | Total 153; 40 cases (All are HSV-1, none are HSV-2) | February 2020–February 2021 | 61.9 (50.9–70.8) | M:F (115/38) | CVD, Obesity, DM |
| Gold JE et al., 2021 [31] | USA; AMR |
Retrospective | Total 185; 30 cases (Long covid group) 9 (Short term covid group); 20 controls(Long covid group) 9 (Short term covid group | December 2020–February 2021 | 43.8+-13.4 (for long covid) 43.9 + 13.7 (for long covid control) | M:F Long covid (23/7) for control group (14/6) |
Not reported |
| Meng M et al., 2022 [33] | China; WPR |
Retrospective | Total 217; 55 cases | January–March 2020 | 57.19 | M:F (22/31) | Not reported |
3.2. Clinical features and outcome related to COVID-19 and concomitant Herpes virus infection
16 studies showed reactivation of Herpes virus while the rest 3 were associated with co-infection of Herpesviridae and COVID-19 and CMV was the most common virus followed by HSV and EBV. In three studies, the prognosis or clinical outcomes of patients with reactivation of EBV, HHV-6, and Zoster were not mentioned [23,27,31]. Nearly half of the studies omitted the clinical features of COVID-19 infection. Conversely, all but four studies detailed the clinical features of specific Herpes virus infections. One study could not determine specific reactivation or co-infection of Varicella zoster virus [23]. Another one showed (CMV) co-infection after post renal transplantation [24]. Four studies showed both reactivation and co-infection of Human Herpes virus-6 respectively [[25], [26], [27], [28]]. Five studies that investigated the reactivation of more than one Herpesviridae at a time (HSV, EBV, CMV, HHV-6) showed association with mortality [11,25,29,30]. Co-infection or reactivation of Herpes zoster virus was confirmed with pain in the thoracic, lumber, sacral and cranial segments along with secondary bacterial infection in patients [23] In case of reactivation of HHV-6 no association was found with disease severity or mortality but some cutaneous manifestations like maculopapular rash with vesicles, erythema and urticarial lesions were noted. Viruses that are known to be causing systemic infection (CMV, EBV, HSV) were found to have association with mortality and significant association was found with length of stay in the ICU and invasive mechanical ventilation. The summarized clinical features as well as outcomes were shown in Table 2.
Table 2.
Factors related to clinical features and outcomes of COVID-19 and Herpesviridae co-infection or reactivation.
| Author, year | Virus | Reactivation/co-infection | Significantly associated parameters |
Outcome measures | ||||
|---|---|---|---|---|---|---|---|---|
| Clinical feature (%) | Laboratory findings | Percentage of steroid intake with duration and dosage | Outcome | |||||
| Meshram et al., 2022 [24] | CMV | Co-infection after post renal transplantation | Acute Kidney Injury (66), AKI requiring dialysis (11), Oliguria AKI (55), Non oliguric AKI (11) |
Low lymphocyte count, increased D-dimer, CRP, LDH, IL-6, Ferritin | 14 (78%) patients | Recovered: n = 14 (78%), Dead: n = 4 (22%), Days of hospitalization: 12.5 (14-11) |
Not reported | Not reported |
| Almutairi et al., 2022 [23] | Herpes Zoster virus | Concurrent or may be reactivation | Pain (66.67), Thoracic segment (41.67), Cervical (8.33), Cranial (16.67) Lumbar (25), Sacral region (8.33) involvement, secondary bacterial infection (16.67), severe ulceration (8.33) | Leucopenia, lymphopenia, decreased Hemoglobin, raised ESR, CRP, IL-6, AST, ALT | 2 (16%) patients | Hospitalization 2 (16.67%); Oxygen support: 1 (8.67%) |
Not reported | Not reported |
| Simonnet et al., 2021 [25] | EBV, CMV, HHV-6 | reactivation | Features of single, double and triple viremia | Not reported | 30 (88%) patients | ICU discharge: 28 (82%); Death: 6 (18%) (EBV reactivation- 3(50%); EBV + CMV reactivation- 1(17%)); Longer median ICU length of stay: EBV vs No EBV: 15 days vs 8 days |
Longer median ICU length of stay: EBV vs No EBV: <0.05 | Not reported |
| Chen et al., 2021 [35] | EBV | coinfection | fever (61.2), dry cough (52.2), fatigue (46.3), myalgia (26.9), anorexia (23.9) | Raised CRP, LDH level, AST | VCA IgM (+) vs VCA IgM (−): 22 (59.5%) vs 10 (33.3%) | All were discharged | EBV/SARS CoV-2 co-infection vs SARS C0V-2 infection alone: C/F: Fever: 0.03; Inflammatory marker: CRP: 0.02; Corticosteroid use: 0.03 |
EBV/SARS CoV-2 co-infection vs SARS C0V-2 infection alone: C/F: Fever: 3.09 (1.11–8.56) |
| Lino et al., 2021 [26] | HHV-6B | coinfection | fever, cough, throat pain, sneezing, loss of taste, diarrhea, abdominal pain | Not reported | yes | ICU requirement: 9 (69.3); Mortality: 4 (30.7) (Not significant when compared with w/out co-infection group) |
W/out co-infection vs W co-infection: Therapeutic immunosuppresion: 0.01 | Not reported |
| Yamamoto et al., 2021 [13] | CMV | Coinfection/reactivation | Possible CMV DNAemia, gastrointestinal symptoms and pneumonia | D-dimer level | Corticosteroid pulse therapy before ICU admission: Pt with CMV vs w/out CMV: 5/15 (33.3%) vs 8/44 (18.2%); Systemic corticosteroid therapy, days, after ICU admission: Pt with CMV vs W/out CMV: 30 (20–41) vs 13 (11–15) Dexamethasone 6 mg Once daily for 10 days or ICU discharge, If > 10 days, still not extubated, ≤6 mg dexa once dailly until extubation or death. tapering to 1–3 mg once daily |
ICU discharge: 11 (73.4%); Death: 4 (26.6%); |
Characteristics of Pt with CMV and w/out CMV:
|
Risk factor analysis for CMV infection during the ICU stay:
|
| Giacobbe DR., 2022 [36] | HSV-1 | Reactivation | Not reported | Not reported | yes 38 (93%) of total patient population | Crude 30 day mortality: With HSV-1 reactivation vs whole study population: 3/12 (25%) vs 13/41 (32%) |
Not reported | Not reported |
| Fuest et al., 2022 [11] | HSV-1, CMV (No CMV was found in the cohort) | reactivation | Not reported | Not reported | yes 39 (64%) with HSV infection, 49 (67%) with no infection | ICU mortality: HSV-1 (+) vs hSV-1 (−): 35 (57.4%) vs 33 (45.2%) ∗Not significantly associated |
Univariates analysis: HSV vs Non HSV:
Multivariate analysis in HSV positive group as influencing factor for HSV infection:
|
Multivariate analysis in HSV positive group:
|
| Xie et al., 2021 [12] | EBV | reactivation | Tachypnea: 15 (88.2%) Respiratory failure: 13 (76.5) ARDS: 15 (88.2%) | Hyponatraemia: Lymphocyte count, Albumin, D-dimer, Calcium, CRP | Yes Glucocorticoids 1–2 mg/kg for 5–7 days | Mortality: EBV (+)5/17 (29.4%) | EBV vs Non-EBV: C/F: Tachypnea: <0.001; Respiratory failure: 0.001; ARDS: <0.001; Laboratory Ix: Hyponatraemia: <0.001 Lymphocyte count: 0.0002 (Inverse); Albumin: 0.03 (Inverse); D-dimer: <0.0001; Calcium: <0.001; CRP: 0.004 Prognosis: 28-day mortality: 0.0046: 14-day mortality: 0.0046 Multivariate analysis: Better prognosis in non-EBV group: <0.001 |
Multivariate analysis: Better prognosis in non-EBV group: HR: 0.56 (0.116–2.689) |
| Abadias-Grando et al., 2021 [27] | HHV-6 | reactivation | Cutaneous manifestation: maculopapular, ptyriaisis like, perniosis like, vesicular, multiform, seborrhoeic dematitis, urticarial | Not reported | Not reported | Not reported | Not reported | Not reported |
| Niitsu et al., 2021 [37] | CMV | reactivation | CMV antigenaemia; CMV pneumonia |
CMV (+) vs CMV (−): Lymphocyte on ICU admission: 393/μl vs 525/ul |
yes 6 (100%) in CMV group, 20 (100%) in non CMV group | 2 (33.33%) were dead | CMV vs Non-CMV group: Duration of mechanical ventilation, days: 0.010 Bacterial infection: 0.018 Fungal infection: 0.013 Death: 0.046 |
Not reported |
| Navaro Bielsa, 2021 [28] | HHV-6 | Both coinfection and reactivation | Cutaneous manifestation macolopapular eruptions: 24 (38.1); erythema with vesicles or pustules (pseudo-chilblain): 13 (20.6); Vesicular eruption: 8 (12.7); urticarial leisons: 6(9.5); Livedo or necrosis: 5(7.9) | Not reported | Yes Topical and oral corticosteroid |
all were recovered | Not reported | Not reported |
| Balc'h et al., 2020 [29] | HSV, CMV | reactivation | Not reported | Not reported | yes 16 (80%) in no reactivation group and 16 (89%) in reactivation group | 2 (11%) were dead but not statistically relevant when compared with non reactivation group | No-reactivation vs Reactivation: Duration of MV: 0.0001; Ventilator-free days at D28: 0.0008 (−); PaO2: FiO2:(D7): 0.04 (Inverse); PaO2: FiO2:(D14): 0.01 (−); ICU length of stay: 0.0001 |
Not reported |
| Franchesch et al., 2021 [34] | HSV-1 | reactivation | 2 hepatitis (9.5%), 5 herpes labialis (23.8%), 4 pneumonia (19%), 3 gingivostomatitis (14.3%) and 1 encephalitis (4.8%) | Higher level of LDH | yes 16 (76%) in HSV 1 positive, 24 (49%) in negative Methylpredinosolone IV with an initial bolus of 0.5 mg/kg followed by 0.5 mg/kg 4 times daily for 7 days, 0.5 mg/kg 3times daily day 8–10, 0.5 mg/kg 2 times daily day 11–12, 0.5 mg/kg once daily for day 13 and 14. For failed Tocilizumab rx, bouls dose 1g IV for 3 consecutive days |
Invasive mechanical ventilation: 12 (57.1%) Death: 6 (28.6%) |
HSV 1 positive (re-activation) vs negative in COVID pt: C/F: Systolic BP: 0.027 Lab: LDH: 0.022 Intervention: Steroid use: 0.036 Outcome: IMV: 0.005 After logistic regression: Steroids use: Any dose vs No: 0.016 Low dose vs No: 0.027 High dose vs No: 0.043 |
HSV 1 positive (re-activation) vs negative in COVID pt: After logistic regression: Steroids use: Any dose vs No: 5.13 (1.36, 19.32) Low dose vs No: 4.80 (1.20, 19.26) High dose vs No: 6.16 (1.06, 35.74) |
| Reizine et al., 2021 [30] | HSV-1, CMV | reactivation | Not reported | Not reported | yes 85 (95%) in non reactivation, 29 (87%) in reactivation | 1 was dead | No viral reactivation vs Viral reactivation: C/F: Obesity: <0.001; HTN: 0.042; Lab: Duration of lymphopenia (days): 0.001; Clinical course: Duration of (+) resp. SARS CoV-2 RT PCR (days): 0.013; Outcome: Ventilator associated pneumonia: 0.03; Herpesviridae res reactivation: <0.001; Duration of mechanical ventilation (days): 0.018; Length of ICUU stay (days): 0.005 |
Not reported |
| Saade et al., 2021 [32] | HSV, EBV, CMV | reactivation | esophagitis, cutaneous-mucous manifestations | Higher leukocyte count | yes 6 (16%) in non reactivation, 27 (43%) in reactivation group | 23 were dead but statistically proved that it was not realted to viral reactivation. | No Reactivation vs reactivation: Characteristics/Immunosuppressive cond: Valaciclovir prophylaxis: 0.05; Hematopoietic cell transplantation: 0.05; Lab: Leukocytosis on ICU admission: 0.02; Therapeutics: dexamethason: 0.01; Infectious event: 0.04; Bacterial event: 0.02; Pneumonia: 0.05; ICU stay: 0.03 After adjustment using Fine and Gray model: Preexisting hematological malignancy: 0.02; Solid organ transplantation: 0.02 |
After adjustment using Fine and Gray model: Preexisting hematological malignancy: 0.31 (0.11–0.85); Solid organ transplantation: 2.09 (1.13–3.87) |
| Meyer et al., 2021 [38] | HSV-1 | reactivation | fever | Higher level of CRP and LDH | yes 86 (56%) in non reactivation, 22 (55%) in reactivation | Death in ICU: 21 (52.5); Death at day 60: 23 (57.5); HAP/VAP: 33(82.5); ICU-BSI: 18 (45); IMV/ECMO: 35 (87.5) |
Without reactivation vs Reactivation: On ICU admission: C/F: Max body temp: 0.029; Lab: CRP: 0.001; LDH: 0.019; Intervention: MV: 0.009; Initial use of corticosteroid: 0.016; Outcomes: Length of stay in ICU: <0.001; Death in ICU: 0.02; Death at day 60: 0.014; HAP/VAP: <0.001; ICU-BSI: 0.001; IMV/ECMO: <0.001 After adjustment, in multivariable Cox models, increased risk of mortality: 0.01; After adjustment for mortality factors and using acyclovir as a time-dependent covariate, in Cox model, increased risk of mortality at day 60: <0.001; Multivariable specific cause models showed an increased risk of HAP/VAP: 0.037; In blood samples, multivariable cause-specific models, association between HSV-1 reactivation and HAP/VAP: 0.027 |
After adjustment, in multivariable Cox models, increased risk of mortality in all sample: HR 2.05 (1.16–3.62); After adjustment, in multivariable Cox models, increased risk of mortality in blood sample: HR 2.24 (1.23–4.08) After adjustment for mortality factors and using acyclovir as a time-dependent covariate, in Cox model increased risk of mortality at day 60: HR 4.37, 95% CI 2.12–9.02; 0.059). Multivariable specific cause models showed an increased risk of HAP/VAP: csHR 2.38, 95% CI 1.06–5.39; In blood samples, multivariable cause-specific models, association between HSV-1 reactivation and HAP/VAP: HR 2.62; 95% CI 1.12–6.12 |
| Gold et al., 2021 [31] | EBV | reactivation | Fatigue, insomnia, headache, myalgia, confusion, tinitus, hearing loss, skin rashes, covid toes | Not reported | Not reported | Not reported | Long term COVID vs Long term control group; Difference in the fraction of EBV reactivation: <0.001; Short-term COVID vs short-term control group: 0.05; Relationship of EBV EA-D IgG with the number of reported long COVID symptoms: p < 0.001 |
Relationship of EBV EA-D IgG with the number of reported long COVID symptoms: r = 0.34 |
| Meng et al., 2022 [33] | EBV | reactivation | Not reported | Lower Hb level, Higher D-dimer level and bilirubin level. | Not reported | Patients with EBV reactivation have statistically nonsignificant higher mortality rate: 12 [22%] vs. 18 [11%], | EBV serology, Non-survivor vs Survivor: EA-IgG: 0.05; Lab: with reactivation vs w/out reactivation: Hb: 0.007; D-dimer: 0.03; total bilirubin: 0.006; After intervention with Ganciclovir: Lab: Hb level: <0.001; prealbumin level: 0.02; Outcome: Effects on 28 days survival rate: 0.01 (−); Length of stay: 0.006 |
EBV serology, Non-survivor vs Survivor: EA-IgG: −0.00005 (−3.10, 0.00); After intervention with Ganciclovir: Outcome: Effects on 28 days survival rate: 0.98 (0.95, 1.00) |
3.2.1. Significance of steroid use along with immunosuppression, blood markers and other factors
Both topical and systemic steroid usage was noted in 16 studies except in 3 studies where single infection by HHV-6 and EBV was noted [12,26,35] Single reactivation of CMV and HSV-1 and multiple reactivation of HSV, EBV and CMV at a time were noted to have association with dosage and duration of corticosteroid usage [13], [29], [30]. Yamamoto Y et al. [13] and Saade et al. [32] observed that prolonged use of dexamethasone could cause reactivation of CMV [13,32] EBV [32] and HSV [32]. However, only one of these studies identified a statistical significance between prolonged steroid use and the clinical outcomes in patients, though it did not establish viral reactivation as the cause [32]. Chen T et al. [35], and Francheschini et al. [34] reported that significant use of steroids, such as methylprednisolone, can affect outcomes but was not statistically linked to patient mortality. In cases of HHV-6 reactivation or co-infection, both oral and topical steroids were administered, with no reported association between clinical outcomes and steroid use [[26], [27], [28]]. Four studies associated with single reactivation of EBV showed significant relation with blood lymphocyte count level of LDH, sodium, D-dimer level in blood along with length of stay in the ICU in case of critically ill patients [12,31,33,35]. One of these studies showed relation to EBV EA-IgG level with long term COVID symptoms by differentiating patients into long term and short term COVID groups [31]. Clinical features such as fever, tachypnoea, co-morbidities such as obesity and HTN and antiviral treatment such as valacyclovir were associated with outcome in some of the studies. All of these data were shown in Table 2.
3.3. Risk of bias of included studies
The ROBANS tool was used for evaluation of bias in the included studies. The Risk of bias was assessed by considering six domains (see Fig. 2). All the included studies were assessed as low risk of bias for selective outcome reporting (19 studies), and incomplete outcome data (19 studies). No studies had blinding of outcome measurements so it was denoted as unclear risk of bias. A rule of thumb was set for selection bias which was as follows; study population without any additional control group with either positive or negative for COVID-19 were included as low risk bias. If there were inclusion of specific groups (e.g. organ transplantation) related to more susceptibility of COVID-19 infection there were included as high risk of selection bias for study participants (3 studies) [24,25,36]. As for performance bias, in studies if there was any ICU protocol for patients to go for tests of any viruses of Herpesviridae family but the test was not done for patient with COVID-19 in ICU were considered as high risk (8 studies) [12,23,28,31,33,35,37] and studies where COVID-19 patients, who were not in ICU but were tested positive for any of the Herpesviridae family were included as low risk of performance bias (11studies) [11,13,[24], [25], [26],29,30,32,34,36,38].
Fig. 2.
Risk of bias assessment.
4. Discussions
This systematic review contains 19 articles that were selected after thorough search and careful consideration regarding the association between clinical features and outcome of COVID-19 infection with concomitant Herpesviridae infection. CMV was the most commonly detected virus. Among all the included studies, 5/19 studies found association of with mortality in infected patients with single or multiple Herpesviridae reactivation in COVID-19 infection. Though there were history of steroid usage in most of the patients, only 3/16 of them noted to have significant association with its usage. Reactivation of CMV or EBV caused systemic infection and that lead to critical outcome in most of the patients and was significantly associated with length of duration of stay in ICU, Invasive mechanical ventilation, D-dimer level, LDH level and decreased lymphocyte count.
A study found out that almost 95% people infected with COVID-19 can be co-infected with other viral, bacterial or fungal infections [7]. Several herpesviruses are associated with mortality and among them the most important are CMV and EBV [32]. CMV was the most common virus detected in this review. Due to complex innate and adaptive immune system responses, CMV reactivation is found to be related with advancing age along with inflammation and immune activation [37]. Meshram et al. denoted the reactivation of CMV in allogenic kidney transplant recipients with COVID-19 infection and among them 22% were dead though they were given prophylactic ganciclovir and valganciclovir [24]. As the transplant recipient patients were already under immunosuppressive drugs, CMV reactivation occurred which then led to transplant rejection along with clinical disease. The risk of CMV infection in COVID-19 is that it can increase the risk of developing metabolic and cardiovascular complications [[39], [40], [41], [42], [43]]. In case of critically ill COVID-19 patients, reactivation of multiple Herpesviridae at a time can increase the disease severity [39]. Though systemic corticosteroid therapy can improve the condition of patients by modulating the dysregulated immune response caused by COVID-19, it increases the risk of secondary infections. In this review, most of the studies found no statistically significant relationship with the dosage and duration of steroid usage except in two studies conducted in Japan and China that explained the propagation of onset of CMV and EBV reactivation, no mortality was recorded after receiving steroid [12,13]. COVID-19 infection can induce the reactivation of latent CMV by M1 polarization of macrophages, disruption of peripheral blood T-cell differentiation. Then this can decrease the number of naïve T cells which can ultimately lead to cytokine storm specially by increasing the level of IL-6 followed by morbid clinical outcomes in elderly patient [40] Herpes Zoster virus reactivation or co-infection was found to be associated with COVID-19 vaccinations and stem cell transplantation and though it causes mild diseases but rarely causes death [[44], [45], [46], [47], [48], [49], [50]]. In terms of diagnostic values, important blood markers found to be associated with COVID-19 infection and Herpesviridae reactivation are, LDH level, serum ferritin level, hemoglobin level, prealbumin level, blood lymphocyte count (lymphopenia) and D-dimer level in this review and among these Lymphopenia and D-dimer level were the most commonly detected laboratory markers Contradictory finding from study conducted by Saade A et al. showed that viral reactivations were less frequent in patients with hematological malignancy but more frequent in patients with higher leukocyte count [32] Giaconni R et al. also found increased level of blood lymphocytes are associated with CMV reactivation in older patients infected with COVID-19 [41]. Though there were association of immune defects (e.g. lymphopenia) in critically ill COVID-19 patients, no statistically significant evidence was found in ICU patient's outcome with viral reactivation although viral reactivation can be considered as a marker for disease severity [51]. In case of EBV reactivation, older age and female gender were found to be related with reactivation and serum EA-IgG level was statistically significant with mortality and in another study indicated that EBV reactivation can specify the severity of COVID-19 [31,33] These studies have shown that treatment with antiviral such as ganciclovir may have some role in increasing survival rate in EBV reactivated patients. Other factors that are found to be significantly related to Herpesviridae reactivation in this review were critically ill patient with longer stay in the ICU as well as patients who were under mechanical ventilation [25,29,30,37]. Apart from HSV, EBV, CMV, reactivation or co-infection of HHV-6 was found in three studies but no association was found with mortality [[26], [27], [28]]. Only simple skin lesions were noted and those who were infected got recovered. Hepatitis B, Influenza virus, Respiratory syncytial virus can also cause co-infection COVID-19 [[52], [53], [54], [55]]. Patients co-infected with COVID-19 and Influenza are shown to have associated with disease severity and mortality whereas in COVID-19 and Hepatitis B co-infection there is evidence of increased risk of liver injury [54,55]. In Hepatitis B virus co-infection, it is speculated that serum LDH, D-dimer and IL-6 may have some role to this injury [54].
Though there were many studies conducted to assess the association COVID-19 infection and Herpesviridae infection, a systematic review was lacking. In this review, a clear and concise finding regarding primary and latent infection was summarized. In order to find out the specific triggering factors and develop future therapeutic agents in preventing reactivation of latent viral infections this concise knowledge can play its part. Hopefully, the findings from this study can help establish a universal early detection method for latent viruses and thus help predicting the outcomes of COVID-19 infected patients and modify the treatment protocol accordingly.
Among the limitations of this review, first one is that the number of study participants was small. Secondly, the sampling and diagnostic methods for different Herpesvirus infections were not uniform and in most of the cases serological method for diagnosis was preferred over PCR which might hamper with the actual diagnosis. Thirdly, due to lack of homogenous data a meta-analysis could not be conducted. Variations were noted across the studies in aspects such as population characteristics, study designs, methods of exposure reporting, and the manner in which outcomes were reported. Fourthly, the impact of immunomodulatory and antiviral drugs on the clinical outcomes of the COVID-19 and concomitant Herpesviridae infection could not be well defined due to lack of proper data in some studies. And lastly, not enough study was included regarding the association between COVID-19 vaccinations and Herpesviridae reactivation.
5. Conclusion
Early diagnosis of co-infections in COVID-19 caused by virus, bacteria or other organisms should be given priority by estimating the levels of blood markers through proper diagnostic methods. Both the beneficial and harmful effects of treatments given in COVID-19 along with co-infection cases should be evaluated correctly. For better understanding of the pathogenesis of concomitant viral infections, indicative diagnostics markers for early diagnosis along with the role of antiviral or other immunomodulatory drugs in treatment purpose needs to be addressed properly by conducting research on a large scale of participants.
Funding statement
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Research ethics approval
This is a systematic review of published articles. Ethical approval is not applicable.
Data availability statement
The data that support the findings of this study are provided with the manuscript.
Patient consent statement
Not applicable for this study.
Permissions to reproduce materials from other sources
Not applicable.
CRediT authorship contribution statement
Shiny Talukder: Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Paroma Deb: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Monira Parveen Konica: Data curation, Formal analysis, Writing – review & editing. Kaniz E. Zannat: Data curation, Formal analysis, Writing – review & editing. Amirul Huda Bhuiyan: Data curation, Formal analysis, Writing – review & editing. Mahmuda Yeasmin: Data curation, Formal analysis, Writing – review & editing. Md Maruf Ahmed Molla: Data curation, Formal analysis, Writing – review & editing. K.M. Saif-Ur-Rahman: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are grateful to the authors of the included primary studies.
Handling Editor: Patricia Schlagenhauf
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nmni.2024.101233.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Tian S., Hu N., Lou J., et al. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li G., Fan Y., Lai Y., et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO coronavirus (COVID-19) dashboard [Internet]. vol. 2023. Accessed September 20, 2023, 2023. https://covid19.who.int.
- 4.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: emergence, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romagnani P., Gnone G., Guzzi F., et al. The COVID-19 infection: lessons from the Italian experience. J Publ Health Pol. 2020;41:238–244. doi: 10.1057/s41271-020-00229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Y., Wang Y., Shao C., et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aghbash P.S., Eslami N., Shirvaliloo M., Baghi H.B., et al. Viral coinfections in COVID‐19. J Med Virol. 2021;93(9):5310–5322. doi: 10.1002/jmv.27102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ong D.S., Bonten M.J., Spitoni C., et al. Epidemiology of multiple herpes viremia in previously immunocompetent patients with septic shock. Clin Infect Dis. 2017;64(9):1204–1210. doi: 10.1093/cid/cix120. 2017. [DOI] [PubMed] [Google Scholar]
- 9.Getts D.R., Chastain E.M., Terry R.L., Miller S.D. Virus infection, antiviral immunity, and autoimmunity. Immunol Rev. 2013;255(1):197–209. doi: 10.1111/imr.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malekifar P., Pakzad R., Shahbahrami R., et al. Viral coinfection among COVID-19 patient groups: an update systematic review and meta-analysis. BioMed Res Int. 2021 doi: 10.1155/2021/5313832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuest K.E., Erber J., Berg-Johnson W., et al. Risk factors for Herpes simplex virus (HSV) and Cytomegalovirus (CMV) infections in critically-ill COVID-19 patients. Multidisciplinary Respiratory Medicine. 2022;17(1) doi: 10.4081/mrm.2022.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Y., Cao S., Dong H., et al. Clinical characteristics and outcomes of critically ill patients with acute COVID-19 with Epstein-Barr virus reactivation. BMC Infect Dis. 2021;21:1–8. doi: 10.1186/s12879-021-06638-y. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto Y., Shiroyama T., Hirata H., et al. Prolonged corticosteroid therapy and cytomegalovirus infection in patients with severe COVID‐19. J Med Virol. 2022;94(3):1067–1073. doi: 10.1002/jmv.27421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. 2021. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. (2020). Clinical management of COVID-19: interim guidance, 27 May 2020. World Health Organization. Accessed February 22 2023 https://apps.who.int/iris/handle/10665/332196.
- 16.COVID-19 Treatment Guidelines Panel Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/.2021 [PubMed]
- 17.Whitley R.J., Roizman B. Herpes simplex virus infections. Lancet. 2001;357(9267):1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 18.Du Y., Wang C., Zhang Y. Viral coinfections. Viruses. 2022;14(12):2645. doi: 10.3390/v14122645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traylen C.M., Patel H.R., Fondaw W., et al. Virus reactivation: a panoramic view in human infections. Future Virol. 2011;6(4):451–463. doi: 10.2217/fvl.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:1–10. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saif‐Ur‐Rahman K.M., Hasan M., Hossain S., Anwar I., Hirakawa Y., Yatsuya H. Prioritization and sequential exclusion of articles in systematic reviews. Campbell Syst Rev. 2022;18(2):e1229. doi: 10.1002/cl2.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S.Y., Park J.E., Lee Y.J., et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66(4):408–414. doi: 10.1016/j.jclinepi.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Almutairi N., Almutairi A.N., Almazyad M., Alwazzan S. Herpes zoster in the era of COVID 19: a prospective observational study to probe the association of herpes zoster with COVID 19 infection and vaccination. Dermatol Ther. 2022;35(7) doi: 10.1111/dth.15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meshram H.S., Kute V.B., Patel H., Banerjee S., Desai S., Chauhan S. Cytomegalovirus and severe acute respiratory syndrome coronavirus 2 Co-infection in renal transplants: a retrospective study from a single center. Saudi J Kidney Diseas Transpl. 2021;32(4):929–938. doi: 10.4103/1319-2442.338304. [DOI] [PubMed] [Google Scholar]
- 25.Simonnet A., Engelmann I., Moreau A.S., et al. High incidence of Epstein–Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect Dis Now. 2021;51(3):296–299. doi: 10.1016/j.idnow.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lino K., Alves L.S., Raposo J.V., et al. Presence and clinical impact of human herpesvirus‐6 infection in patients with moderate to critical coronavirus disease‐19. J Med Virol. 2022;94(3):1212–1216. doi: 10.1002/jmv.27392. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abadías‐Granado I., Navarro‐Bielsa A., Morales‐Callaghan A.M., et al. COVID‐19‐associated cutaneous manifestations: does human herpesvirus 6 play an aetiological role? Br J Dermatol. 2021;184(6):1187–1190. doi: 10.1111/bjd.19806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarro-Bielsa A., Abadías-Granado I., Morales-Callaghan A.M., et al. Experience with cutaneous manifestations in COVID-19 patients during the pandemic. J Clin Med. 2022;11(3):600. doi: 10.3390/jcm11030600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balc’h L., Pinceaux K., Pronier C., Seguin P., Tadié J.M., Reizine F. Herpes simplex virus and cytomegalovirus reactivations among severe COVID-19 patients. Crit Care. 2020;24(1):1–3. doi: 10.1186/s13054-020-03252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reizine F., Liard C., Pronier C., et al. Herpesviridae systemic reactivation in patients with COVID-19-associated ARDS. J Hosp Infect. 2022;119:189–191. doi: 10.1016/j.jhin.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gold J.E., Okyay R.A., Licht W.E., Hurley D.J. Investigation of long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens. 2021;10(6):763. doi: 10.3390/pathogens10060763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saade A., Moratelli G., Azoulay E., Darmon M. Herpesvirus reactivation during severe COVID-19 and high rate of immune defect. Infect Dis Now. 2021;51(8):676–679. doi: 10.1016/j.idnow.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng M., Zhang S., Dong X., et al. COVID‐19 associated EBV reactivation and effects of ganciclovir treatment. Immun Inflamm Disease. 2022;10(4):e597. doi: 10.1002/iid3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franceschini E., Cozzi-Lepri A., Santoro A., et al. Herpes simplex virus re-activation in patients with SARS-CoV-2 pneumonia: a prospective, observational study. Microorganisms. 2021;9(9):1896. doi: 10.3390/microorganisms9091896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen T., Song J., Liu H., Zheng H., Chen C. Positive Epstein–Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-90351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giacobbe D.R., Di Bella S., Dettori S., et al. Reactivation of herpes simplex virus type 1 (HSV-1) detected on bronchoalveolar lavage fluid (BALF) samples in critically ill COVID-19 patients undergoing invasive mechanical ventilation: preliminary results from two Italian centers. Microorganisms. 2022;10(2):362. doi: 10.3390/microorganisms10020362. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niitsu T., Shiroyama T., Hirata H., et al. Cytomegalovirus infection in critically ill patients with COVID-19. J Infect. 2021;83(4):496–522. doi: 10.1016/j.jinf.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer A., Buetti N., Houhou-Fidouh N., et al. HSV-1 reactivation is associated with an increased risk of mortality and pneumonia in critically ill COVID-19 patients. Crit Care. 2021;25:1–10. doi: 10.1186/s13054-021-03843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Textoris J., Mallet F. Immunosuppression and herpes viral reactivation in intensive care unit patients: one size does not fit all. Crit Care. 2017;21(1):230. doi: 10.1186/s13054-017-1803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Söderberg-Nauclér C. Does reactivation of cytomegalovirus contribute to severe COVID-19 disease? Immun Ageing. 2021;18(1):1–7. doi: 10.1186/s12979-021-00218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giacconi R., Cardelli M., Piacenza F., et al. Effect of cytomegalovirus reactivation on inflammatory status and mortality of older COVID-19 patients. Int J Mol Sci. 2023;24(7):6832. doi: 10.3390/ijms24076832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hraiech S., Bonnardel E., Guervilly C., et al. Herpes simplex virus and Cytomegalovirus reactivation among severe ARDS patients under veno-venous ECMO. Ann Intensive Care. 2019;9(1):1–8. doi: 10.1186/s13613-019-0616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gatto I., Biagioni E., Coloretti I., et al. Cytomegalovirus blood reactivation in COVID-19 critically ill patients: risk factors and impact on mortality. Intensive Care Med. 2022;48(6):706–713. doi: 10.1007/s00134-022-06716-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwanaga J., Fukuoka H., Fukuoka N., Yutori H., Ibaragi S., Tubbs R.S. A narrative review and clinical anatomy of Herpes zoster infection following COVID‐19 vaccination. Clin Anat. 2022;35(1):45–51. doi: 10.1002/ca.23790. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bricout H., Haugh M., Olatunde O., Gil Prieto R. Herpes zoster-associated mortality in Europe: a systematic review. BMC Publ Health. 2015;15:1–14. doi: 10.1186/s12889-015-1753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L.R., Dong L.J., Zhang M.J., Lu D.P. The impact of human herpesvirus 6B reactivation on early complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(10):1031–1037. doi: 10.1016/j.bbmt.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Elsaie M.L., Youssef E.A., Nada H.A. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33(4) doi: 10.1111/dth.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saati A., Al-Husayni F., Malibari A.A., Bogari A.A., Alharbi M. Herpes zoster co-infection in an immunocompetent patient with COVID-19. Cureus. 2020;12(7) doi: 10.7759/cureus.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferreira A.D.F., Romão T.T., Macedo Y.S., et al. COVID‐19 and herpes zoster co‐infection presenting with trigeminal neuropathy. Eur J Neurol. 2020;27(9):1748–1750. doi: 10.1111/ene.14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zerr D.M., Corey L., Kim H.W., Huang M.L., Nguy L., Boeckh M. Clinical outcomes of human herpesvirus 6 reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40(7):932–940. doi: 10.1086/428060. [DOI] [PubMed] [Google Scholar]
- 51.Jaiswal S.R., Arunachalam J., Bhardwaj A., et al. Impact of adaptive natural killer cells, KLRC2 genotype and cytomegalovirus reactivation on late mortality in patients with severe COVID‐19 lung disease. Clinic Translation Immunol. 2022;11(1):e1359. doi: 10.1002/cti2.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X., Jiang Q., Ma Z., et al. Clinical characteristics of hospitalized patients with SARS-CoV-2 and hepatitis B virus co-infection. Virol Sin. 2020;35:842–845. doi: 10.1007/s12250-020-00276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moumbeket Yifomnjou M.H., Monamele G.C., Njankouo‐Ripa M., et al. Viral co‐infection with human respiratory syncytial virus in suspected acute and severe respiratory tract infections during COVID‐19 pandemic in Yaoundé, Cameroon, 2020–2021. Influenza and Other Respiratory Viruses. 2023;17(3) doi: 10.1111/irv.13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin Y., Yuan J., Long Q., et al. Patients with SARS-CoV-2 and HBV co-infection are at risk of greater liver injury. Genes & Diseases. 2021;8(4):484–492. doi: 10.1016/j.gendis.2020.11.005. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alosaimi B., Naeem A., Hamed M.E., et al. Influenza co-infection associated with severity and mortality in COVID-19 patients. Virol J. 2021;18(1):1–9. doi: 10.1186/s12985-021-01594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are provided with the manuscript.