Abstract
Introduction
Patients with sarcoidosis have a lower survival rate than the general population, in part due to cardiovascular disease, infections and neoplasms. Our objective was to evaluate the impact of haematological neoplasms (HN) and lymphomas on sarcoidosis patient mortality in a nation-wide analysis conducted in Spain, a country with a population of 47 million.
Methods
Retrospective and observational comparison of the HN related deaths in sarcoidosis patients and the general Spanish population reported in the Spanish Hospital Discharge Database. To determine the impact of sarcoidosis on the risk of dying from each HN lineage, a binary logistic regression considering age, female sex, tobacco and alcohol consumption, was performed.
Results
In the period 2016 and 2019, 139,531 in-hospital deaths from neoplasms were certified in Spain (77 in patients with sarcoidosis). Patients with sarcoidosis died at younger age than the general Spanish population (72.9 vs 77.6, p<0.001). Sarcoidosis patients presented a higher mortality risk from HN (20.8% vs 8.9%, p=0.001, OR=2.64, 95% CI 1.52-4.59), attributable to the higher proportion of deaths from non-Hodgkin lymphoma (NHL), (9.2% vs 2.9%, p=0.006, OR= 3.33, 95% CI 1.53-7.25) from both B cell (6.6% vs 2.5%, p=0.044, OR= 2.62, 95% 1.06-6.5) and T/NK cell lineages (2.6% vs 0.3%, p=0.024, OR= 7.88, 95% CI 1.92-32.29) as well as HN with uncertain behavior and myeloproliferative disorders (2.6% vs 0.3%, p=0.018, OR= 11.88, 95% CI 2.88-49.02). The mean age of sarcoidosis patients who died from HN (63.6 vs 71.9, p=0.032) and non-Hodgkin lymphoma (56.9 vs 71, p=0.009) was lower than that of the general population
Conclusion
Patients with sarcoidosis present a higher risk of premature death from HN, including NHL from B, T/NK cell lineage and myeloproliferative disorders in comparison with the general Spanish population. In addition to developing strategies that might help to attenuate their occurrence and impact, such as decreasing the immunosuppressive burden, specific early-detection programs for these conditions should be investigated and considered carefully.
Keywords: Sarcoidosis, Haematological neoplasm, Lymphoma, Mortality, Sarcoidosis-lymphoma syndrome
Highlights
-
•
Mortality from haematological neoplasm (HN) is higher in patients with sarcoidosis.
-
•
The early-age mortality from HN supports its strong impact on their premature death.
-
•
This risk was attributable to non-Hodgkin lymphoma from B, T/NK cell lineage and myeloproliferative disorders.
Abbreviations
- AS
Ankylosing spondylitis
- CT
Computed tomography
- DM/PM
Dermato-polymyositis
- FDG
Fluorodeoxyglucose
- HN
Haematological neoplasm.
- IBD
Inflammatory bowel disease
- ICD
International Classification of Diseases
- MG
Myasthenia gravis
- NHL:
Non-Hodgkin lymphoma
- PET
positron emission therapy
- RA
Rheumatoid arthritis
- SjS
Sjögren's syndrome
- SLE
Systemic lupus erythematosus
- SNHDD
Spanish Hospital Discharge Database
- SON
Solid organ neoplasm.
- SSc
Systemic sclerosis
1. Introduction
Sarcoidosis is a systemic chronic autoimmune disease characterized by the formation of non-caseating granulomas [[1], [2], [3]]. The cause of sarcoidosis, and the exaggerated immune response in which activated macrophages and dendritic cells shift the T-cell pathways towards Th1 differentiation, leading to subsequent granuloma formation, is still unknown. However, some reports have suggested that sarcoidosis might be a dysregulated antigenic response to certain environmental exposures, for example to microorganisms [2,4,5]. Therefore, the pathogen-associated molecular patterns of phagocytosed and partly degraded microbial antigens, such as mycobacteria and propionibacteria, can trigger or amplify inflammation, leading to granuloma formation and the heterogeneous clinical phenotype of sarcoidosis in lungs, lymphatic system, eyes and skin, among others tissues [1].
Patients with sarcoidosis have a lower survival rate than the general population, mostly due to advanced pulmonary fibrosis, respiratory failure, pulmonary hypertension and, less commonly, from cardiac, central nervous system and hepatic involvement [2,[6], [7], [8]]. Moreover, a higher risk of cardiovascular disease, infections and neoplasms, has been recently highlighted as an important cause of premature death in this population [[1], [2], [3],6,7].
Previous reports have identified an association between sarcoidosis and lymphoma, which led Bricker, to propose the term ‘sarcoidosis-lymphoma syndrome’ [[9], [10], [11]]. However, solid information on this association is lacking, since most reports are based on small-size, monocentric and retrospective studies from previous decades [9,[12], [13], [14]]. Hence, other reports have failed to confirm this hypothesis [[15], [16], [17]]. Furthermore, to date there are a lot of unanswered questions regarding the haematological neoplasms (HN) and lymphoma risk on sarcoidosis patients, including if both entities truly coexist or if sarcoidosis could be indeed a long-term risk factor for HN and lymphoma, for example [5,18]. Finally, the impact of HN and lymphomas on the mortality of sarcoidosis patients has not been defined.
In light of the above, our objective was to evaluate the impact of HN and lymphomas on sarcoidosis patient mortality in a nation-wide analysis conducted in Spain, a country with a population of 47 million.
2. Materials and methods
We performed an analysis on data extracted from the Spanish Hospital Discharge Database (SNHDD), a public access registry belonging to the Spanish Government. The SNHDD includes demographic and epidemiological data and up to 20 discharge diagnoses carried out during admission and defined from January 1st, 2016 by the International Classification of Diseases (ICD-10). We compared the proportion and lineage of the HN related deaths of sarcoidosis patients and the general Spanish population.
2.1. Study population
We selected hospital admissions, from 2016 to 2019 for patients with a diagnosis within the ICD-10-CM code D86 (sarcoidosis) at any position in the diagnostic list. Organ involvement (lung, nodal, skin, central nervous system, eye, kidney, heart, joint and myositis) were also retrieved according to the D86 code subsections. Cases with limited cutaneous sarcoidosis (D86.3), without affecting any other organ or presenting systemic features, were not included. Furthermore, patients who presented any other autoimmune disease which might impact the neoplasm related risk in the sarcoidosis population were also excluded [[19], [20], [21], [22]]. These conditions were also identified according to the respective ICD-10-CM diagnostic codes, including systemic lupus erythematosus (SLE) (M32), Sjögren's syndrome (SjS) (M35), systemic sclerosis (SSc) (M34), dermato-polymyositis (DM/PM) (M33), rheumatoid arthritis (RA) (M05 and M06), ankylosing spondylitis (AS) (M45), psoriasis (L40), myasthenia gravis (MG) (G70.0) or inflammatory bowel disease (IBD) (K50 and K51). All in-hospital deaths notified in Spain during 2016–2019 were retrieved from the SNHDD, and were used as the control group to compare with the sarcoidosis patients.
2.2. Variables and neoplasm-related deaths
According to the database structure and design, the main diagnosis was the defining reason for admission, and the cause of death if that occurred. Therefore, all main diagnoses of the deceased patients were decodified, analyzed and clustered. Only those admissions and deaths attributable to neoplasms (from ICD-10-CM code C.00 to D.49), other than sarcoidosis itself (D86), were analyzed.
Following the ICD-10-CM coding criteria, neoplasm related deaths were classified as malign neoplasms, which include solid organ malign neoplasms (C00–C80), haematological malign neoplasms (C81–C96), in situ neoplasms (D00-D09), benign solid organ neoplasms (D10-D36 and D3A), and unknown or non-specified behavior neoplasms (D37-D49), in turn also including solid organ and HN. Myelodysplastic syndromes (MDS), based on their worse prognosis and mortality rate, were considered malign haematological neoplasms. Finally, the main HN lineages, and subsequent subclassifications, such as lymphoma, leukemia or MDS, among others, were considered and analyzed separately.
2.3. Statistical analysis
The epidemiological and demographic data, as well as the proportion of deaths attributable to the distinct neoplasm types and lineages, with the main focus on HN, were compared between sarcoidosis patients and the general Spanish population. Categorical variables were reported as frequencies and percentages while continuous variables were presented as mean and standard deviation. The significance of differences between the two groups was determined by the Chi-square or Student's t-test, as appropriate. In addition, we performed a binary logistic regression analysis for each HN group and lineage to determine the impact of sarcoidosis on the risk of dying from each HN group and lineage. Age, female sex, tobacco and alcohol consumption (according to ICD-10 CM codes F17 or Z72.0 and F10, K70 or I42.6, respectively) were adjusted for. For all the analyses, a significance level of 0.05 was set. Statistical analysis was performed using SPSS version 26.0 (IBM, Spain).
2.4. Ethics
The study complies with the Declaration of Helsinki and was approved by the local research ethics committee (Expedient number PI 237-23). The data were provided after all potential patient identifiers had been deleted and data were given anonymously. Due to the design of the study, and according to Spanish law, informed consent was not required.
3. Results
3.1. Sarcoidosis patient characteristics
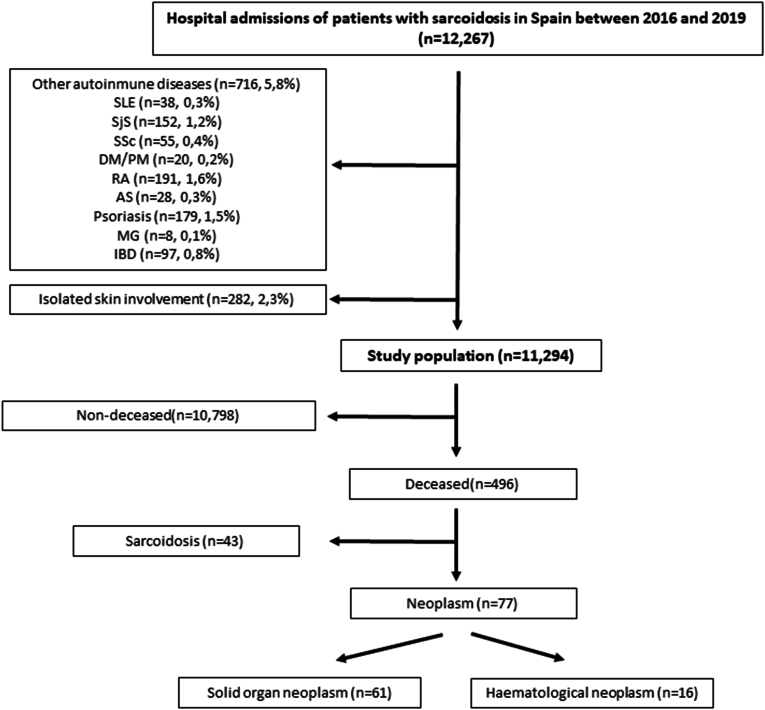
Between 2016 and 2019, 12,267 hospital admissions of patients with sarcoidosis were identified in the Spanish National Registry (Fig. 1). The admissions of patients with other autoimmune diseases related to a potential higher malignancy risk (716, 5.8%), as well as those with isolated sarcoidosis skin involvement (282, 2.3%) were excluded as discussed above.
Fig. 1.
Flow chart of the study population.
Out of 11,294 admissions in patients with sarcoidosis, 52.9 % were female, with a mean age of 62.8 years (Table 1). Sarcoidosis organ involvement was as follows: lung in 4921 (43.6%), nodal in 1397 (12.4%), skin in 238 (2.1%), central nervous system in 60 (0.5%), eye in 53 (0.5%), kidney in 93 (0.8%), heart in 151 (1.3%), articular in 106 (0.9%), myositis in 29 (0.3%) and others, including hepatitis or Heerfordt syndrome, in 1609 (9.5%)
Table 1.
Patient characteristics.
| Admissions of patients with Sarcoidosis (N = 11,294) | |
|---|---|
| Patients/admissions characteristics | |
| Female, N (%) | 5975 (52.9) |
| Age (years) (Mean, SD) | 62.8 (15.8) |
| Ethnicity, N (%) | |
| Arabic | 263 (2.3) |
| Asian | 22 (0.2) |
| Black | 152 (1.3) |
| Caucasian | 8568 (75.9) |
| Hindu | 12 (0.1) |
| Latin/Hispanic | 115 (1) |
| Sarcoidosis activity-related admission, N (%) | 2001 (17.7) |
| Age (years) (Mean, SD) | 53.9 (15.8) |
| Neoplasm-related admission, N (%) | 759 (6.7) |
| Age (years) (Mean, SD) | 63.8 (13.4) |
| Sarcoidosis organ involvement, N (%) | |
| Lung | 4921 (43.6) |
| Nodal | 1397 (12.4) |
| Skin | 238 (2.1) |
| Central nervous system | 60 (0.5) |
| Eye | 53 (0.5) |
| Kidney | 93 (0.8) |
| Heart | 151 (1.3) |
| Articular | 106 (0.9) |
| Myositis | 29 (0.3) |
| Others | 1609 (9.5) |
| Outcomes | |
| Deaths, N (%) | 496 (4.4) |
| Age (years) (Mean, SD) | 72.9 (12.2) |
| From sarcoidosis activity | 43 (8.7) |
| Age (years) (Mean, SD) | 70 (11.3) |
| From neoplasms | 77 (15.5) |
| Age (years) (Mean, SD) | 67.7 (13.3) |
| Admission-length (days), (Mean, SD) | 8.4 (10) |
| ICU admission, N (%) | 643 (5.7) |
| ICU admission length (days), (Mean, SD) | 5.7 (9.3) |
SD: Standard deviation, ICU: Intensive care unit.
Regarding outcomes, 643 (5.7%) patients were admitted to the Intensive Care Unit (ICU) and 496 (4.4 %) died (Table 1, Fig. 1). The 2001 patients (17.7%) admitted due to sarcoidosis activity were younger than the 759 patients (6.7%) admitted due to neoplasms (53.9 vs 63.8, p < 0.001). On the other hand, only 43 patients (8.7%) died as a result of sarcoidosis activity but 77 (15.5%) from neoplasms (p = 0.001).
3.2. Differences in neoplasm related deaths in sarcoidosis patients and the general Spanish population
The mean Spanish population between 2016 and 2019 was 46,704,229 inhabitants. In this period, 705,557 in-hospital deaths were identified. These patients died at older age than the sarcoidosis patients who died during in-hospital admission (77.6 vs 72.9, p < 0.001).
Overall, 139,531 (19.8%) of all deaths identified in Spain in this period were attributable to neoplasms. Table 2 identifies several differences when the proportion of neoplasm related deaths in sarcoidosis patients was compared with the general Spanish population. In sarcoidosis patients, 78.9% of neoplasm related deaths were attributable to solid organ neoplasm (SON) (vs 91.1% in the general Spanish population, p = 0.001) and 20.8% to HN (vs 8.9% in the Spanish population, p = 0.001). In addition, the mean age of sarcoidosis patients who died from neoplasm (67.4 vs 70.7, p = 0.034), and from HN (63.6 vs 71.9, p = 0.032) was lower than that of the general population. No age differences were found between the two groups for SON (68.8 vs 70.6, p = 0.296).
Table 2.
Differences in neoplasm related deaths for Sarcoidosis patients and the general Spanish population for the period 2016–2019.
| Neoplasm-related deaths N (%) |
Mean age (years), (SD) |
|||||
|---|---|---|---|---|---|---|
| Non-sarcoidosis | Sarcoidosis | p | Non-sarcoidosis | Sarcoidosis | p | |
| Total | 139,453 | 77 | – | 70.7 (13.5) | 67.4 (13.3) | 0.034 |
| SON | 127,093 (91.1) | 60 (78.9) | 0.001 | 70.6 (13.3) | 68.8 (13.5) | 0.296 |
| HN | 12,360 (8.9) | 16 (20.7) | 0.001 | 71.9 (15.5) | 63.6 (12.5) | 0.032 |
| Malign neoplasm | 136,807 (98.1) | 73 (96.1) | 0.176 | 70.6 (13.5) | 67.6 (13.6) | 0.064 |
| Malign SON | 124,843 (89.5) | 59 (77.6) | 0.002 | 70.5 (13.3) | 68.9 (13.6) | 0.375 |
| Malign HN | 11,989 (8.6) | 14 (17.9) | 0.003 | 71.7 (15.6) | 62.2 (12.8) | 0.023 |
| Benign SON | 727 (0.5) | 0 | 1 | 72.3 (13.5) | – | – |
| UB neoplasm | 1695 (1.2) | 3 (3.9) | 0.066 | 78.7 (13.9) | 68.3 (8.1) | 0.196 |
| UB SON | 1326 (1) | 1 (1.3) | 0.516 | 78.6 (14.7) | 59 | 0.182 |
| UB HN | 369 (0,3) | 2 (2.6) | 0.018 | 79 (10.6) | 74 (2) | 0.419 |
| In situ carcinoma | 187 (0.1) | 0 | 1 | 73.4 (12.4) | – | – |
SD: Standard deviation, SON: Solid organ neoplasm, HN: Haematological neoplasm, UB: Uncertain behaviour.
Values in bold highlight statistically significant difference.
3.3. Haematological neoplasms in sarcoidosis patients
As the rate of HN deaths in patients with sarcoidosis and the general Spanish population differed, a more detailed comparison, considering the different HN lineages, was performed (Table 3). In order to determine the impact of sarcoidosis on the risk of death from each HN, a binary logistic regression analysis, taking into account age, sex, alcohol and tobacco consumption, was carried out for each neoplasm lineage.
Table 3.
Differences in Haematological neoplasm related deaths for Sarcoidosis patients and the general Spanish population by lineage.
| Neoplasm-related deaths N (%) |
Mean age (years), (SD) |
||||||
|---|---|---|---|---|---|---|---|
| Non-sarcoidosis | Sarcoidosis | p | Non-sarcoidosis | Sarcoidosis | p | OR (95% CI)a | |
| Haematological | 12,362 (8.9) | 16 (20.5) | 0.001 | 71.9 (15.5) | 63.6 (12.5) | 0.032 | 2.64 (1.52–4.59) |
| Malign haematological | 11,993 (8.6) | 14 (18.4) | 0.006 | 71.7 (15.6) | 62.32 (12.8) | 0.023 | 2.30 (1.28–4.11) |
| Lymphoma | 4241 (3) | 7 (9.2) | 0.008 | 70.7 (14.7) | 56.9 (14) | 0.013 | 3.09 (1.42–6.73) |
| Hodgkin Lymphoma | 261 (0.2) | 0 | 1 | 65.5 (18.4) | – | – | – |
| Non-Hodgkin Lymphoma | 3980 (2.9) | 7 (9.2) | 0.006 | 71 (14.4) | 56.9 (14) | 0.009 | 3.33 (1.53–7.25) |
| B cell lineage | 3541 (2.5) | 5 (6.6) | 0.044 | 71.7 (14.2) | 59 (10.1) | 0.046 | 2.62 (1.06–6.50) |
| T/NK cell lineage | 439 (0.3) | 2 (2.6) | 0.024 | 65.6 (14.6) | 51.5 (26.1) | 0.174 | 7.88 (1.92–32.29) |
| Leukemia | 4627 (3.3) | 4 (5.3) | 0.322 | 70.2 (18.1) | 70.5 (10.8) | 0.972 | 1.50 (0.55–4.10) |
| Myeloid lineage | 3193 (2.3) | 2 (2.6) | 0.694 | 71.2 (15.5) | 61.5 (0.71) | 0.377 | 1.09 (0.27–4.45) |
| Lymphoid lineage | 1005 (0.7) | 1 (1.3) | 0.423 | 64.7 (24.3) | 83 | 0.452 | 1.56 (0.22–11.30) |
| Multiple myeloma | 2163 (1.6) | 2 (2.6) | 0.342 | 74 (11) | 68 (5.7) | 0.445 | 1.70 (0.42–6.93) |
| Myelodisplastic syndrome | 885 (0.6) | 1 (1.3) | 0.384 | 78.7 (12) | 55 | 0.049 | 2.37 (0.33–17.16) |
| Others | 77 (0.1) | 0 | 1 | 71 (17.1) | – | – | – |
| Unknown behaviour | 369 (0.3) | 2 (2.6) | 0.018 | 79 (10.5) | 73 | 0.419 | 11.88 (2.88–49.02) |
| Myeloproliferative disorders | 242 (0.2) | 1 (1.3) | 0.124 | 78.4 (10.1) | 75 | 0.592 | |
| Others | 127 (0.1) | 1 (1.3) | 0.067 | 80.2 (11.3) | 73 | 0.528 | - |
SD: Standard deviation, OR: Odds ratio, CI: Confidence interval.
Values in bold highlight statistically significant differences.
After adjustment for age, sex, alcohol and tobacco consumption.
The adjusted multivariate analysis confirmed that sarcoidosis patients presented a higher mortality rate from HN (20.5% vs 8.9%, p = 0.001, OR = 2.64, 95% CI 1.52–4.59) than the general Spanish population. This difference was attributable to the higher proportion of deaths from non-Hodgkin lymphoma (NHL), (9.2% vs 2.9%, p = 0.009, OR = 3.33, 95% CI 1.53–7.25) from both B cell (6.6% vs 2.5%, p = 0.044, OR = 2.62, 95% 1.06–6.50) and T/NK cell lineages (2.6% vs 0.3%, p = 0.024, OR = 7.88, 95% CI 1.92–32.29) as well as HN with uncertain behavior (2.6% vs 0.3%, p = 0.018, OR = 11.88, 95% CI 2.88–49.02). Finally, it is noteworthy that the sarcoidosis patients who died from NHL were younger (56.9 vs 71, p = 0.009) than those in the general Spanish population.
4. Discussion
This large nationwide epidemiological study analyses the impact of HN on patients with sarcoidosis and reveals that mortality due to HN is up to three times higher than in the general population. In addition, the early-age mortality from HN and lymphomas in our study supports that both have a strong impact on the premature death of sarcoidosis patients.
In 1986, Brincker reported an analysis of 17 patients with sarcoidosis who presented lymphoproliferative disorders years after sarcoidosis onset and diagnosis [10]. Given this association, the author proposed the term ‘sarcoidosis-lymphoma syndrome’. Since them, several studies have tried to assess this issue and this concept has been nuanced [1,6,7,9,12]. Thus, the higher lymphoproliferative disease risk in patients with sarcoidosis seen in later studies has been related to chronic active disease, extra-thoracic involvement, the development of new organ involvement, recurrent disease or even to the persistence of high levels of angiotensin-converting enzyme. However, other authors have shown conflicting results [[15], [16], [17]]. Romer et al. did not find any association between lymphoma and sarcoidosis after a more rigorous pathologic confirmation of sarcoidosis. Similarly, a very recent study from Ungprasert et al. neither showed a higher risk of haematological or lymphoproliferative malignancy in patients with sarcoidosis compared to non-sarcoidosis subjects [23]. Finally, a population-based study from Korea published in 2021, despite confirming a higher risk from lymphoma in patients with sarcoidosis, showed that lymphoma diagnosis practically coincided with sarcoidosis onset and diagnosis, unlike lymphoma in patients with rheumatoid arthritis or Sjögren's syndrome [20]. The above differences probably reflect the complex relationship between the two entities and the ‘sarcoidosis-lymphoma syndrome’ concept ambiguity. We believe our study helps to clarify this.
Our nation-wide population study showed an up to 3-fold mortality risk from HN in patients with sarcoidosis, mostly related to NHL from B cell and T/NK cell lineages, as well as to other HN such as myeloproliferative disorders. To our knowledge, only the Swedish group pointed out that patients who were previously admitted owing to sarcoidosis presented a higher mortality rate from NHL than the patients not admitted because of sarcoidosis [14,24]. Therefore, our results not only confirm the association of sarcoidosis and lymphoma but also determine its significance in terms of mortality. Other than B and T/NK cell lineage lymphomas, myeloproliferative disorders were responsible for the higher death rate due to HN.
Another important point arising from our study is the early age at which patients with sarcoidosis died from HN and lymphomas. While the mean age of the patients admitted because of sarcoidosis activity and diagnosis was 53.9 years, similar to previously described [1,2], the sarcoidosis patients who died from HN and lymphomas were even 9 and 15 years younger, respectively, than the general population who died from the same cause. Therefore, and obviously taking into account the limitations of our study design and the lack of certain data, these results suggest that sarcoidosis is indeed a risk factor for both HN and lymphoma development and mortality. Hence, HN and lymphomas should be contemplated as long-term complications of sarcoidosis that have to be considered seriously in the management and surveillance of this population [5,7,9,12]. By contrast, no age differences were found regarding SON and therefore our results do not support that SON lead to premature death of patients with sarcoidosis. Unfortunately, and despite these sound figures, we were not able to clarify important questions about the ‘sarcoid-lymphoma syndrome’ and the sarcoidosis-like diseases, as well as the diagnostic complexity and frequent misclassification of both [5,6,12]. Accordingly, further prospective studies, evaluating histological features, disease course, immunosuppression, chemotherapy and check-point inhibitors drugs will have to confirm our findings.
Several limitations of this study have to be considered. As mentioned, important information regarding disease course (early vs late disease), clinical features and histopathologic data, as well as immunosuppression drugs, could have allowed more solid conclusions. Secondly, this analysis was restricted to hospital admissions, with the resultant limitation in power and the potential selection bias. However, we mainly evaluated categorical variables such as deaths from HN, which are difficult to misclassify. Third, the prevalence of sarcoidosis could not be properly assessed in the databases. Therefore, the rate, risk or incidence of each neoplasm could not be calculated and only deaths could be compared. Hence, deaths from neoplasm type or lineage in sarcoidosis patients and the general Spanish population were compared in terms of deaths from neoplasm, giving an estimated proportion and death risk and not an absolute risk ratio. This type of analysis might produce slight variations in the death risk from each neoplasm type in sarcoidosis patients. Thus, and despite the previous reservations, we believe that our study from a nationwide analysis of a large sample size and for a long study period, yields consistent results which confirm those seen in smaller studies and evaluates a robust outcome such as mortality and not incidence.
In conclusion, patients with sarcoidosis present a higher risk of premature death from HN, including NHL from B, T/NK cell lineage and myeloproliferative disorders in comparison with the general Spanish population. In addition to developing strategies that might help to attenuate their occurrence and impact, such as decreasing the immunosuppressive burden, specific early-detection programs for these conditions should be investigated and considered carefully.
For this purpose, other authors have described that there are certain clinical, analytical or radiological features that can suggest lymphoma development or onset within sarcoidosis [1,9,12,18]. Accordingly, chronic active, recurrent or refractory disease, extra-thoracic or atypical manifestations, new organ involvement after remission, rising or sustained angiotensin-converting enzyme, lactate dehydrogenase, beta-2 macroglobulin levels and serum cryoglobulins, are red flags suggesting a more exhaustive assessment. Otherwise, Fluorodeoxyglucose (FDG) positron emission therapy (PET)/computed tomography (CT) is a very useful tool used for both sarcoidosis diagnosis and monitoring [9]. However, both sarcoid and lymphoma are FDG avid and the distinction between benign and malignant disorders on the basis of a PET scan is difficult. Current reports have shown that this imaging technique can correctly characterize sarcoidosis and lymphoma lesions with very good performance, sometimes supported by machine learning combined with radiomics [25]. Despite the above, histopathology remains to date the sarcoidosis diagnosis cornerstone, given that correct classification based on imaging can create a diagnostic challenge and misclassification it is still not infrequent and has important consequences [1].
Funding
None.
Ethics
The study complies with the Declaration of Helsinki and was approved by the local research ethics committee (Expedient number PI 237-23). The data were provided after all potential patient identifiers had been deleted and data were given anonymously. Due to the design of the study, and according to Spanish law, informed consent was not required.
CRediT authorship contribution statement
Víctor Moreno-Torres: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. María Martínez-Urbistondo: Writing – review & editing, Validation, Investigation, Conceptualization. Pedro Durán-del Campo: Writing – review & editing, Validation, Investigation, Conceptualization. Pablo Tutor: Writing – review & editing, Validation, Investigation, Conceptualization. Begoña Rodríguez: Writing – review & editing, Validation, Investigation, Conceptualization. Raquel Castejón: Writing – review & editing, Validation, Investigation, Conceptualization. Susana Mellor-Pita: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- 1.Brito-Zerón P., Pérez-Álvarez R., Ramos-Casals M. Sarcoidosis, Med Clin (Barc). 2022;159:195–204. doi: 10.1016/j.medcli.2022.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Valeyre D., Prasse A., Nunes H., Uzunhan Y., Brillet P.-Y., Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383:1155–1167. doi: 10.1016/S0140-6736(13)60680-7. [DOI] [PubMed] [Google Scholar]
- 3.Drent M., Crouser E.D., Grunewald J. Challenges of sarcoidosis and its management. N. Engl. J. Med. 2021;385:1018–1032. doi: 10.1056/NEJMra2101555. [DOI] [PubMed] [Google Scholar]
- 4.Chen E.S., Moller D.R. Etiology of sarcoidosis. Clin. Chest Med. 2008;29:365–377. doi: 10.1016/j.ccm.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Tana C., Giamberardino M.A., Di Gioacchino M., Mezzetti A., Schiavone C. Immunopathogenesis of sarcoidosis and risk of malignancy: a Lost Truth? Int. J. Immunopathol. Pharmacol. 2013;26:305–313. doi: 10.1177/039463201302600204. [DOI] [PubMed] [Google Scholar]
- 6.Tana C., Drent M., Nunes H., Kouranos V., Cinetto F., Jessurun N.T., Spagnolo P. Comorbidities of sarcoidosis. Ann. Med. 2022;54:1014–1035. doi: 10.1080/07853890.2022.2063375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brito-Zerón P., Acar-Denizli N., Sisó-Almirall A., Bosch X., Hernández F., Vilanova S., Villalta M., Kostov B., Paradela M., Sanchez M., Ramírez J., Muxí A., Berruezo A., Galceran-Chaves C., Xaubet A., Agustí C., Sellarés J., Ramos-Casals M. The burden of comorbidity and complexity in sarcoidosis: impact of associated chronic diseases. Lung. 2018;196:239–248. doi: 10.1007/s00408-017-0076-4. [DOI] [PubMed] [Google Scholar]
- 8.Spagnolo P., Rossi G., Trisolini R., Sverzellati N., Baughman R.P., Wells A.U. Pulmonary sarcoidosis. Lancet Respir. Med. 2018;6:389–402. doi: 10.1016/S2213-2600(18)30064-X. [DOI] [PubMed] [Google Scholar]
- 9.Goswami T., Siddique S., Cohen P., Cheson B.D. The sarcoid-lymphoma syndrome. Clin. Lymphoma, Myeloma & Leukemia. 2010;10:241–247. doi: 10.3816/CLML.2010.n.052. [DOI] [PubMed] [Google Scholar]
- 10.Brincker H. The sarcoidosis-lymphoma syndrome. Br. J. Cancer. 1986;54:467–473. doi: 10.1038/bjc.1986.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oskuei A., Hicks L., Ghaffar H., Hoffstein V. Sarcoidosis–lymphoma syndrome: a diagnostic dilemma. BMJ Case Rep. 2017 doi: 10.1136/bcr-2017-220065. bcr-2017-220065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerri S., Fontana M., Balduzzi S., Potenza L., Faverio P., Luppi M., D'Amico R., Spagnolo P., Clini E., Luppi F. Clinical differences in sarcoidosis patients with and without lymphoma: a single-centre retrospective cohort analysis. Eur. Respir. J. 2019;54 doi: 10.1183/13993003.02470-2018. [DOI] [PubMed] [Google Scholar]
- 13.Arkema E.V., Rossides M., Cozier Y.C. Sarcoidosis and its relation to other immune-mediated diseases: epidemiological insights. J. Autoimmun. 2023 doi: 10.1016/j.jaut.2023.103127. [DOI] [PubMed] [Google Scholar]
- 14.Ji J., Shu X., Li X., Sundquist K., Sundquist J., Hemminki K. Cancer risk in hospitalized sarcoidosis patients: a follow-up study in Sweden. Ann. Oncol. 2009;20:1121–1126. doi: 10.1093/annonc/mdn767. [DOI] [PubMed] [Google Scholar]
- 15.Seersholm N., Vestbo J., Viskum K. Risk of malignant neoplasms in patients with pulmonary sarcoidosis. Thorax. 1997;52:892–894. doi: 10.1136/thx.52.10.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekström Smedby K., Vajdic C.M., Falster M., Engels E.A., Martínez-Maza O., Turner J., Hjalgrim H., Vineis P., Seniori Costantini A., Bracci P.M., Holly E.A., Willett E., Spinelli J.J., La Vecchia C., Zheng T., Becker N., De Sanjosé S., Chiu B.C.-H., Dal Maso L., Cocco P., Maynadié M., Foretova L., Staines A., Brennan P., Davis S., Severson R., Cerhan J.R., Breen E.C., Birmann B., Grulich A.E., Cozen W. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029–4038. doi: 10.1182/blood-2007-10-119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romer F., Hommelgaard P., Schou G. Sarcoidosis and cancer revisited: a long-term follow-up study of 555 Danish sarcoidosis patients. Eur. Respir. J. 1998;12:906–912. doi: 10.1183/09031936.98.12040906. [DOI] [PubMed] [Google Scholar]
- 18.El Jammal T., Pavic M., Gerfaud-Valentin M., Jamilloux Y., Sève P. Sarcoidosis and cancer: a complex relationship. Front. Med. 2020;7 doi: 10.3389/fmed.2020.594118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Z., Liu H., Yang Y., Zhou J., Zhao L., Chen H., Fei Y., Zhang W., Li M., Zhao Y., Zeng X., Zhang F., Yang H., Zhang X. The five major autoimmune diseases increase the risk of cancer: epidemiological data from a large‐scale cohort study in China. Cancer Commun. 2022;42:435–446. doi: 10.1002/cac2.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J.S., Kim I.H., Byun J.M., Chang J.H. Population-based study on the association between autoimmune disease and lymphoma: national health insurance service-national sample cohort 2002–2015 in Korea. J. Autoimmun. 2021;121 doi: 10.1016/j.jaut.2021.102647. [DOI] [PubMed] [Google Scholar]
- 21.Moreno-Torres V., Tarín C., Ruiz-Irastorza G., Castejón R., Gutiérrez-Rojas Á., Royuela A., Durán-del Campo P., Mellor-Pita S., Tutor P., Rosado S., Sánchez E., Martínez-Urbistondo M., de Mendoza C., Yebra M., Vargas J.-A. Trends in hospital admissions and death causes in patients with systemic lupus erythematosus: Spanish national registry. J. Clin. Med. 2021;10:5749. doi: 10.3390/jcm10245749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terwiel M., Grutters J.C., van Moorsel C.H.M. Clustering of immune-mediated diseases in sarcoidosis. Curr. Opin. Pulm. Med. 2019;25:539–553. doi: 10.1097/MCP.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 23.Ungprasert P., Crowson C.S., Matteson E.L. Risk of malignancy among patients with sarcoidosis: a population‐based cohort study. Arthritis Care Res. 2017;69:46–50. doi: 10.1002/acr.22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shu X., Ji J., Sundquist K., Sundquist J., Hemminki K. Survival in cancer patients with previous hospitalization for sarcoidosis: a Swedish population-based cohort study during 1964–2006. Ann. Oncol. 2011;22:1427–1434. doi: 10.1093/annonc/mdq614. [DOI] [PubMed] [Google Scholar]
- 25.Lovinfosse P., Ferreira M., Withofs N., Jadoul A., Derwael C., Frix A.-N., Guiot J., Bernard C., Diep A.N., Donneau A.-F., Lejeune M., Bonnet C., Vos W., Meyer P.E., Hustinx R. Distinction of lymphoma from sarcoidosis on 18 F-FDG PET/CT: evaluation of radiomics-feature–guided machine learning versus human reader performance. J. Nucl. Med. 2022;63:1933–1940. doi: 10.2967/jnumed.121.263598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.