Highlights
-
•
This article reviews some of the most clinically relevant data related to metastatic breast cancer presented during 2023.
-
•
Elacestrant (oral SERD) was approved in 2023 for the treatment of patients with pretreated ESR1 mutant HR-positive mBC.
-
•
Capivasertib (AKT inhibitor) was approved in 2023 for patients with pretreated mBC with PIK3CA, PTEN, or AKT1 alterations.
-
•
Sacituzumab govitecan was approved in 2023 for patients with HR-positive tumors previously tereated with chemotherapy.
Keywords: Breast cancer, Metastatic breast cancer, Advanced breast cancer, Capivasertib, Elacestrant, Sacituzumab govitecan, Datopotamab deruxtecan
In recent years, substantial progress has been made in the development of innovative therapies for advanced breast cancer (BC), and 2023 was no exception. This commentary provides an overview of key advances, including research findings published or presented in 2023, focusing on the most impactful studies that have the potential to reshape clinical practice.
1. Hormone receptor (HR) positive breast cancer
Endocrine therapy (ET) remains the cornerstone of the treatment of patients with metastatic HR-positive BC. For several years, cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) in combination with ET have become part of the standard of care for the treatment of patients with HR-positive advanced BC. Three CDK4/6i, palbociclib, ribociclib, and abemaciclib, are approved for the treatment of advanced HR-positive BC and have been used as first-line treatment. The studies that led to the approval of these agents were powered for a primary endpoint of progression-free survival (PFS), which was very similar across the mentioned trials (Table-1) [[1], [2], [3]]. Palbociclib did not meet the secondary endpoint of overall survival (OS) and ribociclib did [[1], [2], [3]]. In 2023, the OS results of the phase III MONARCH 3 study (AI ± abemaciclib) were released and showed a non-statistically significant improvement of 13.1 months in the abemaciclib arm (p = 0.066). The small sample size and the 2:1 randomization could explain this. Moreover, the study was not powered for this secondary endpoint [4]. Ribociclib and abemaciclib are considered by many the preferred CDK4/6i in the metastatic setting, as they met their prespecified endpoint of PFS and showed at least a numerical benefit in the secondary endpoint of OS. Differences in patient population and the characteristics of the drugs could explain the differences in outcomes in the mentioned studies. Patient preference, toxicity profile, and prior use of CDK4/6i in the adjuvant setting should be considered when deciding between these agents.
Table 1.
Selected randomized phase III trials of CDK4/6 inhibitors in the first line setting.
| PALOMA-2 [1,35] | MONALEESA-2 [2] | MONALEESA-7 [36] | MONARCH-3 [3,4] | |
|---|---|---|---|---|
| Regimen | Letrozole ± palbociclib (2:1) | Letrozole ± ribociclib (1:1) | Goserelin + AI or tamoxifen ± ribociclib (1:1) | AI ± abemaciclib (2:1) |
| Eligibility | Postmenopausal women with untreated advanced HR+/HER2- BC | Postmenopausal women with untreated advanced HR+/HER2- BC | Pre/perimenopausal women with untreated advanced HR+/HER2- BC | Postmenopausal women with untreated advanced HR+/HER2- BC |
| Sample size | 666 | 668 | 672 | 493 |
| De novo metastatic disease | 38% | 34% | 41% | 20% |
| Median PFS (CDK vs. placebo) | 27.6 vs. 14.5 months (HR = 0.56, 95%CI 0.46–0.69) | 25.3 vs. 16 months (HR = 0.57; 95%CI 0.45–0.60 | 23.8 vs. 13 months (HR = 0.55, 95%CI 0.44–069) | 28.2 vs. 14.8 (HR-0.53; 95%CI 0.42–0.66) |
| Median OS(CDK vs. placebo) | 53.9 vs. 51.2 months (HR = 0.96; 95%CI 0.77–1.17)a | 63.9 vs. 51.4 months (HR = 0.76 95%CI 0.63–0.93) | 58.7 vs. 48 months (HR 0.76; 95%CI 0.60–0.95) | 53.7 vs. 66.8 months (HR 0.84; 95%CI 0.637–1.01)b |
| Toxicities of interest | Neutropenia, leukopenia, fatigue, nausea, arthralgia, diarrhea | Neutropenia, leukopenia, fatigue, nausea, QTc prolongation, transaminitis | Neutropenia, leukopenia, fatigue, nausea, QTc prolongation, transaminitis | Diarrhea, neutropenia, fatigue, nausea, anemia, abdominal pain |
Abbreviations: AI: aromatase inhibitor; BC: breast cancer; HER2: human epidermal growth factor receptor 2; HR: hazard ratio; HR+: hormone receptor positive; OS: overall survival; PFS: progression free survival.
22% of patients had a disease-free interval off less than 12 months, in the other trials, it ranged from 0 to 7%.
Not statistically significant, p = 0.0664, it was a secondary endpoint.
Among postmenopausal patients, aromatase inhibitors (AI) have been traditionally used in the first line setting, while fulvestrant is used for those with pretreated disease. Questions regarding the optimal endocrine therapy partner to CDK4/6i remain. PARSIFAL was a phase 2 study (n = 486) where patients with untreated metastatic HR-positive disease were randomized to receive palbociclib with either letrozole or fulvestrant [5]. There were no significant differences between the arms. In 2023, the long-term outcomes of these patients of the PARSIFAL-LONG trial were presented (included 80% of the patients in PARSIFAL with a median follow-up of 59.7 months) [6]. No differences between the groups, were observed, and the combined cohorts had excellent long-term outcomes (with a median PFS of 33.2 months and median OS of 65.4 months). Patients with progression within 12 months of initiation of therapy had less favorable outcomes [6]. These findings support the use of AI for untreated patients and fulvestrant for those previously treated. Also support prior studies showing good long-term outcomes in patients treated with CDK4/6i, as discussed below.
Questions about the optimal timing of CDK4/6i have been studied. SONIA, a phase III trial, compared the use of CDK4/6i in the first vs. second-line settings [7]. Over 1000 patients were randomized (1:1) to AI in combination with CDK4/6i followed by fulvestrant at progression or AI followed by fulvestrant in combination with a CDK4/6i at the time of progression. Over 90% of patients received palbociclib. At a median follow-up of 37 months, the groups had no significant difference in PFS2 or OS. Limitations of this study include that most patients were treated with palbociclib and that the current standard of care after progression on CDK4/6i includes combination therapies with targeted agents, discussed below. The findings of the SONIA trial suggest postponing CDK4/6i for the second-line setting could be considered for selected patients. These data could impact low- and middle-income countries with limited resources. However, first-line therapy with ET and CDK4/6i remains the standard of care. Another important question associated with CDK4/6i is the use of these agents beyond progression while changing the ET backbone and continuing CDK4/6i. Three small phase II studies have shown conflicting results (Table-2) and today, continuing CDK4/6i after progression is not considered standard of care [[8], [9], [10]].
Table 2.
Studies assessing the role of CDK4/6 inhibition after progression on CDK4/6 inhibition in metastatic breast cancer.
| MAINTAIN [9] | PACE [8,37] | PALMIRA [10] | |
|---|---|---|---|
| Phase | II | II | II |
| Sample size | 120 | 220 | 198 |
| Design | Fulvestrant or exemestane ± ribociclib in patients with HR+, HER2- BC whose cancer previously progressed on CDK 4/6 inhibitor + ET (1:1) | Arm A (n = 55): fulvestrant Arm B (n = 111): fulvestrant and palbociclib; or Arm C (n = 54): fulvestrant, palbociclib, and avelumab in patients with HR+, HER2- BC whose cancer previously progressed on any CDK 4/6 inhibitor + AI | Fulvestrant or letrozole ± palbociclib in patients with HR+, HER2- BC whose cancer previously progressed on palbociclib inhibitor + ET (2:1) |
| Initial CDK 4/6 inhibitor | Palbociclib (84%), ribociclib (11%) | Palbociclib (90%) | Palbociclib (100%) |
| Continuation CDK 4/6 inhibitor | Ribociclib (100%) | Palbociclib (100%) | Palbociclib (100%) |
| Continuation ET | Fulvestrant (83%), exemestane (17%) | Fulvestrant (100%) | Fulvestrant (90%), letrozole (10%) |
| PFS ET + CDK4/6 inhibitor vs. ET | 5.3 vs. 2.8 months (HR 0.56) | 4.6 vs. 4.8 months (HR 1.11) | 4.9 vs. 3.6 months (HR 0.84) |
Abbreviations: AI: aromatase inhibitor; CDK: cyclin dependent kinase; ER: endocrine therapy; HER2: human epidermal growth factor receptor 2; HR: hazard ratio; HR + hormone receptor positive; PFS: progression free survival * The primary objective was to evaluate PSF between arms A and B.
Novel endocrine therapies have been developed over the past few years. Elacestrant is an oral selective estrogen receptor degrader (SERD) that was approved by the United States Food and Drug Administration (U.S. F.D.A.) in January of 2023 and by the European Medicines Agency (E.M.A) in September of 2023 based on the results of the EMERALD trial [11]. In this phase III study, standard ET (physician's choice) was compared with elacestrant. The latter showed an improvement in median PFS (3.8 vs. 1.9 months) for patients with tumors with ESR1 mutations (48% of the trial population). The benefit was more profound (mPFS of 8.61 months) among patients who had received at least 12 months of therapy with a CDK4/6i, irrespective of concomitant PIK3CA or P53 mutations [12,13]. Common adverse events included nausea, fatigue, vomiting, anorexia, and arthralgia. The approval of elacestrant added a new endocrine therapy to our therapeutic armamentarium in a selected group of patients and other novel therapies are under investigation.
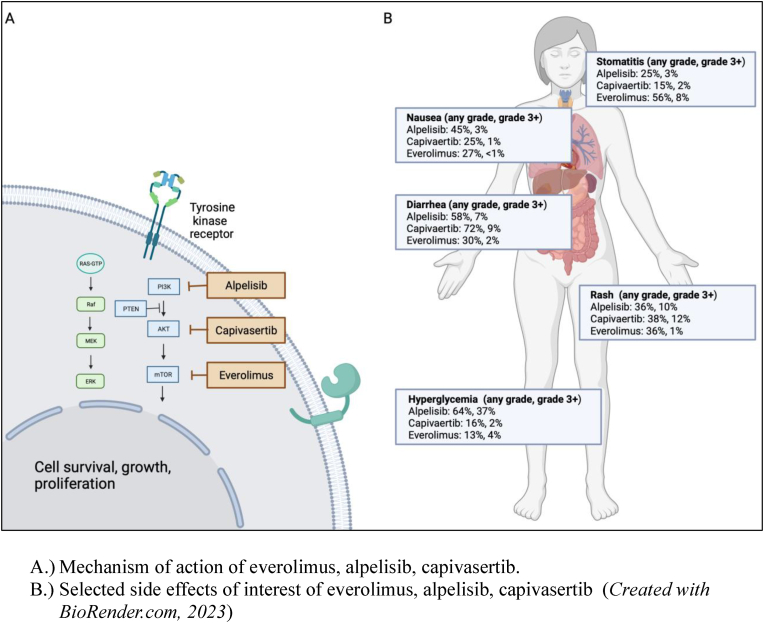
Several drugs targeting the PIK3K/AKT/mTOR pathway have been approved, including everolimus [14], alpelisib [15], and more recently capivasertib [16] (Table-3). In the phase III, CAPItello-291 study, capivasertib (AKT inhibitor) combined with fulvestrant improved PFS compared to fulvestrant alone. The benefit was more profound among patients with alterations in the AKT pathway (7.3 vs. 3.1 months, HR 0.5, 95%CI 0.38–0.65). The most common toxicities were rash (12%) and diarrhea (9.3%) and seem to compare favorably to the side effects of other inhibitors of this pathway, particularly as it relates to hyperglycemia (Figure-1). The U.S. F.D.A. approved this agent in November of 2023 for patients with mBC who have received at least one prior ET and have tumors with PIK3CA, PTEN, or AKT1 alterations. Several other agents targeting this pathway are under investigation, an example is inavolisib, a potent PI3K-alpha inhibitor. In the phase 3 INAVO120 study, 325 patients with metastatic HR-positive BC who had disease recurrence on or within 12 months of adjuvant ET and whose tumors had PIK3CA mutations were randomized 1:1 to receive Palbociclib and fulvestrant ± inavolisib [17]. At a median follow-up of 21 months, the mPFS was 15 vs. 7.2 months (HR 0.43, 95%CI 0.32–0.59, p < 0.0001), favoring the triplet. There was also a numeric improvement in OS; however, longer follow-up is needed since the results are not mature. The ORR was 25 vs. 58%, favoring the triplet. In terms of safety, there was more stomatitis, anemia, hyperglycemia (even though only patients with normal fasting glucose and hemoglobin A1c were eligible), diarrhea, nausea, rash, stomatitis, and dry eye. This is a promising combination with patients with high-risk disease. However, this agent is currently not F.D.A. approved and is, at the time of this commentary, not commercially available.
Table 3.
Agents targeting the PIK3K/AKT/mTOR pathway in metastatic hormone receptor-positive breast cancer.
| Everolimus | Alpelisib | Capivasertib | |
|---|---|---|---|
| Mechanism of action | mTOR inhibitor | PI3Kα-specific inhibitor | AKT inhibitor |
| Trial and phase | BOLERO-2 [14] Phase III |
SOLAR-1 [15] Phase III |
CAPItello-291 [16] Phase III |
| Design | 724 postmenopausal women, randomized 2:1 to everolimus + exemestane vs. placebo + exemestane | 572 patients (341 with PIK3CA alterations) were randomized 1:1 to alpelisib + fulvestrant vs. placebo + fulvestrant | 708 patients (289 with AKT pathway alterations and 489 with prior CDK4/6 inhibitor) randomized 1:1 to capivasertib + fulvestrant vs. placebo + fulvestrant |
| Outcomes | mPFS 10.6 months and 4.1 months, favoring everolimus (HR = 0.36; 95% CI, 0.27–0.47). | PIK3CA WT: mPFS 7.4 vs. 5.6 months. PIK3CA altered: mPFS 11 vs 5.7 months (HR = 0.65; 95%CI, 0.50–0.85). ORR (26.6% vs. 12.8%), clinical benefit (61.5% vs. 45.3%) |
ITT: mPFS 7.2 vs. 3.6 months (HR = 0.60; 95%CI 0.51–0.71) AKT pathway WTa: mPFS 5.2 vs. 3.7 months (HR = 0.79, 95%CI 0.61–1.02) AKT pathway altered: mPFS 7.3 vs. 3.1 months (HR = 0.50; 95%CI, 0.38–0.65) |
| US FDA Approval | 2012 | 2019 for patients with PIK3CA altered advanced HR + breast cancer | 2023 for patients with AKT, PTEN, PIK3CA altered HR + breast cancer |
Abbreviations: CDK4/6: cyclin-dependent kinase 4/6 inhibitor; ITT: intention to treat; mPFS: median progression-free survival; WT: wildtype.
Excluding those with unknown next-generation sequencing results.
Figure-1.
AKT pathway targeted agents approved for the treatment of breast cancer.
A.) Mechanism of action of everolimus, alpelisib, capivasertib.
B.) Selected side effects of interest of everolimus, alpelisib, capivasertib (Created withBioRender.com, 2023).
Antibody-drug conjugates (ADCs) have reshaped the treatment paradigm of patients with metastatic BC. Currently, there are two ADCs approved by the F.D.A. and E.M.A for each BC subtype in the metastatic setting. The pivotal studies leading to the approval of these agents for the treatment of HR-positive BC, are summarized in Table-4. For patients with HR-positive tumors, Sacituzumab Govitecan (SG) was approved by the U.S. F.D.A. and the E.M.A in 2023 for patients who have received at least two prior lines of chemotherapy in the metastatic setting [18]. Trastuzumab Deruxtecan (T-DXd) was approved in 2022 for patients with human epidermal growth factor receptor 2 (HER2) low-expressing metastatic BC who have received at least one prior line of chemotherapy in the metastatic setting [19]. The results of TROPION-Breast01 were recently presented, showing encouraging results for patients with advanced HR-positive BC. In the trial (n = 732) patients were randomized to receive Datopotamab Deruxtecan (Dato-DXd), a humanized anti-TROP2 antibody with a Topo-1 inhibitor payload, or investigator's choice chemotherapy. mPFS was 6.9 moths in the Dato-DXd arm vs 4.5 months (HR = 0.63; 95%CI 0.52–0.76; p < 0.0001) [20]. Dato-DXd was associated with increased dry eye and stomatitis and less neutropenia compared to investigator's choice chemotherapy, and while it is not approved by any regulatory agency, it is likely to become an option in the future.
Table 4.
Selected studies assessing the role of antibody-drug conjugates in the treatment of hormone receptor-positive metastatic breast cancer.
| Datopotumab-DXd TROPION-Breast01 [20] | Sacituzumab govitecan TROPiCS-02 [18] | T-DXd DESTINY Breast-04 [19] | |
|---|---|---|---|
|
ADC Target Payload Linker DAR Bystander effect |
Trop2 Topo1 inhibitor Tumor selective, cleavable 4:1 Yes |
Trop2 Topo1 inhibitor (SN38) Tumor selective, cleavable 8:1 Yes |
HER2 Topo1 inhibitor Tumor selective, cleavable 7–8:1 Yes |
| Study population | Metastatic HR+/HER2- disease, 1–2 prior lines of chemotherapy dato vs TPC | Metastatic HR+/HER2- disease, ET + CDK4/6 → 2–4 prior lines of chemotherapy SG vs TPC | Metastatic HER2-low with 1–2 prior lines of therapy in the metastatic setting |
| Sample size | 732 (1:1 randomization) | 543 (1:1 randomization) | 557 (2:1) ∼90% HR+ |
| PFS | 6.9 vs 4.9 months (HR = 0.63, p < 0.001) | 5.5 vs 4 months (HR = 0.65, <0.001) | 9.6 vs. 4.2 months (HR = 0.37, in HR + group) |
| OS | Not mature | 15.5 vs. 11.2 m. (HR = 0.79, p.013) | 23.9 vs 17.6 m. (.HR = 069, in HR + group) |
| Follow up | 9.7 months | 13 months | 32 months |
| Notes | ORR 36 vs 22%. Stomatitis 50%, alopecia 36%, ILD 3% | Benefit greater in higher Trop 2 expression. Nausea/vomiting and neutropenia | Nausea/vomiting, ILD |
| U.S. FDA approval | No | Yes, February 2023 → at least 2 prior lines of chemotherapy in the metastatic setting | Yes, August 2022 → at least 1 prior line of chemotherapy in the metastatic setting |
Abbreviations: DAR: drug to antibody ratio; DXd: deruxtecan; HER2: human epidermal growth factor receptor 2; HR: hazard ratio, HR+: hormone receptor positive; ILD: interstitial lung disease; ORR: overall response rate; OS: overall survival; PFS: progression free survival; U.S. FDA: United States Food and Drug Administration.
The optimal sequencing strategy for ADCs agents remains unknown, and cross-resistance is a concern as many of the approved agents share antibody targets and/or the mechanism of action of the cytotoxic payload. Several small and retrospective studies have shown that when ADCs are sequenced, the second ADC used is associated with modest response rates and a short duration of response [[21], [22], [23], [24], [25]]. However, how the PFS after a second ADC compares to standard chemotherapy remains unclear. These studies included heterogeneous and often heavily pretreated populations. Further research is needed to answer questions regarding mechanisms of resistance and ADC sequence, and studies are planned.
1.1. Triple negative breast cancer (TNBC)
Until recently, the treatment of patients with TNBC was limited to chemotherapy; however, in recent years, immunotherapy and ADCs have significantly impacted the treatment options for patients with advanced TNBC [19,26,27]. Several studies are assessing the safety and anti-tumor activity of combining immunotherapy and ADCs. In 2023, the results of the BEGONIA trial (arm 7) were presented at the annual ESMO meeting [28]. This phase 1b/2 study assessed the role of Dat-DXd and durvalumab as the first-line treatment for patients with metastatic TNBC. A total of 62 patients were enrolled, and the objective response rate was 79% (95%CI 66.8–88.3), irrespective of PD-L1 expression. The median PFS was 13.8 months, and the median duration of response was 15.5 months. The most common toxicities included nausea, stomatitis, alopecia, constipation, fatigue, and rash. The combination is currently being studied in a phase 3 trials and in a PD-L1 positive patient population.
1.2. ERBB2 overexpressing breast cancer
Several HER2-directed agents have been approved in recent years, significantly improving the outcomes of patients with ERBB2 overexpressing BC; however, those with brain metastases remain to receive limited benefit from our improved therapeutics. T-DXd is approved as a second-line agent for patients with metastatic ERBB2 overexpressing disease, and its role in patients with brain metastases remains unclear. A pooled analysis from the DESTINY-Breast 01, 02 and 03 trials assessed the outcomes of 148 patients with brain metastases (asymptomatic and 70% treated) treated with T-DXd and 83 in the comparator arms in the mentioned studies [29]. Patients with brain metastases treated with T-DXd had higher response rates and longer duration of response relative to the comparator arms, suggesting clinical activity of T-DXd in patients with ERBB2 overexpressing tumors and brain metastases, similar to what has been seen in small studies [30].
Trastuzumab emtansine (T-DM1) was the first ADC approved for the treatment of BC, it has now been studied in combination with other agents. The phase 3 HER2CLIMB-02 study assessed the combination of T-DM1 and the HER2-selective tyrosine kinase inhibitor tucatinib for the treatment of patients with metastatic ERBB2 overexpressing BC, including those with brain metastases (44%, from which had had active and had treated/stable brain metastases) [31]. A total of 460 patients were randomized to T-DM1 with or without tucatinib. At a median follow-up of 24 months, the mPFS was 9.5 vs. 7.4 months (HR = 0.76, 95%CI 0.61–0.95, p = 0.01), favoring the combination. Among patients with brain metastases the mPFS was 7.8 vs. 5.7 months (HR = 0.64, 95%CI 0.46–0.89), favoring the combination. OS data are not mature. There were more grade 3 toxicities (particularly diarrhea and liver function test abnormalities) and toxicities leading to treatment discontinuation in the combination arm (41 vs. 69% and 11 vs. 20%, respectively). Longer follow-up is needed to determine if this combination has a role in clinical practice and to help clinicians determine if a sequential approach of active agents is preferred to a combined approach or vice versa.
In addition to research evaluating new therapies, in 2023, there were also important studies helping us evaluate strategies aimed at decreasing toxicities and improve tolerance. Capecitabine is commonly used for the treatment of patients with all breast cancer subtypes. Two important studies using this agent were presented in ASCO 2023. The standard dose of capecitabine is 1250 mg/m2 twice daily, 14 days out of 21-day cycles. The X-7/7 trial compared the standard dose of the oral chemotherapy to a fixed dose (1500 mg twice daily), 7 days on, 7 days off [32]. A total of 153 patients were enrolled in the study. The 3, 6 and12 months-PFS was similar in both arms, however, patients in the experimental dose, had a significantly milder toxicity profile. D-TORCH was a study assessing the role of 1% topical diclofenac preventing capecitabine associated hand-foot-syndrome [33,34]. A total of 20 patients developed hand-foot syndrome, 6 (7.8%) in the diclofenac arm and 14 (19.7%) in the placebo arm (p = 0.034). X-7/7 and D-TORCH revealed relatively simple and low-cost interventions that can be considered to improve patients’ quality of life.
In conclusion, we reviewed some of the research presented in 2023, which was a very exciting year in the field of breast oncology, with cutting-edge research improving outcomes of patients living with advanced disease. The U.S. F.D.A. and the E.M.A. approved several agents to treat patients with metastatic BC and there is ongoing research to continue to improve patient outcomes of patients living with advanced BC while maintaining a good quality of life.
Declaration of competing interest
Mariana Chavez-MacGregor: Consultant/Advisory Board: Pfizer, Novartis, Astra Zeneca, Lilly, Genentech/Roche, Exact Sciences, Merck, Daiichi-Sankyo. Data Safety Monitoring Committee: Astra Zeneca, Genentech/Roche.
Ilana Schlam: Nothing to disclose.
Funding sources
Mariana Chavez-MacGregor is supported by Susan G. Komen (SAC220221) and the Breast Cancer Research Foundation (BCRF-22-190, BCRF-23-190).
CRediT authorship contribution statement
Ilana Schlam: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Mariana Chavez-MacGregor: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
References
- 1.Finn R.S., Martin M., Rugo H.S., et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 2.Hortobagyi G.N., Stemmer S.M., Burris H.A., et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386(10):942–950. doi: 10.1056/NEJMoa2114663. [DOI] [PubMed] [Google Scholar]
- 3.Johnston S., Martin M., Di Leo A., et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. npj Breast Cancer. 2019;5(1):5. doi: 10.1038/s41523-018-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goetz M.T.M., Houber, Sohn J., Tredan O., et al. SABCS 2023; San Antonio, TX: 2023. Monarch 3: final overall survival results of abemaciclib plus a nonsteroidal aromatase inhibitor as first line therapy for HR+, HER2 advanced breast cancer. [DOI] [PubMed] [Google Scholar]
- 5.Llombart-Cussac A., Pérez-García J.M., Bellet M., et al. Fulvestrant-palbociclib vs letrozole-palbociclib as initial therapy for endocrine-sensitive, hormone receptor–positive, ERBB2-negative advanced breast cancer: a randomized clinical trial. JAMA Oncol. 2021;7(12):1791–1799. doi: 10.1001/jamaoncol.2021.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llombart Cussac, Pr-GaJ A., Bellet M., Dalenc F., Gil-Gil M., et al. PARSIFAL-LONG . SABCS; San Antonio: 2023. Extended follow-up of hormone receptor- positive/HER2-negative advanced breast cancer patients treated with fulvestrant and palbociclib vs letrozole and palbociclib in the PARSIFAL study. Tx2023. [Google Scholar]
- 7.Sonke G.S., Nijhof A.V.O., Wortelboer N., et al. Primary outcome analysis of the phase 3 SONIA trial (BOOG 2017-03) on selecting the optimal position of cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors for patients with hormone receptor-positive (HR+), HER2-negative (HER2-) advanced breast cancer (ABC) J Clin Oncol. 2023;41(17_suppl) LBA1000-LBA1000. [Google Scholar]
- 8.Mayer E.L., Ren Y., Wagle N., et al. Abstract GS3-06: GS3-06 palbociclib after CDK4/6i and endocrine therapy (PACE): a randomized phase II study of fulvestrant, palbociclib, and avelumab for endocrine pre-treated ER+/HER2- metastatic breast cancer. Cancer Res. 2023;83(5_Supplement) GS3-06-GS3-06. [Google Scholar]
- 9.Kalinsky K., Accordino M.K., Chiuzan C., et al. A randomized, phase II trial of fulvestrant or exemestane with or without ribociclib after progression on anti-estrogen therapy plus cyclin-dependent kinase 4/6 inhibition (CDK 4/6i) in patients (pts) with unresectable or hormone receptor–positive (HR+), HER2-negative metastatic breast cancer (MBC): MAINTAIN trial. J Clin Oncol. 2022;40(17_suppl) LBA1004-LBA1004. [Google Scholar]
- 10.Llombart-Cussac A., Harper-Wynne C., Perello A., et al. Second-line endocrine therapy (ET) with or without palbociclib (P) maintenance in patients (pts) with hormone receptor-positive (HR[+])/human epidermal growth factor receptor 2-negative (HER2[-]) advanced breast cancer (ABC): PALMIRA trial. J Clin Oncol. 2023;41(16_suppl) 1001-1001. [Google Scholar]
- 11.Bidard F.C., Kaklamani V.G., Neven P., et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022;40(28):3246–3256. doi: 10.1200/JCO.22.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bardia A., Bidard F.-C., Neven P., et al. Abstract GS3-01: GS3-01 EMERALD phase 3 trial of elacestrant versus standard of care endocrine therapy in patients with ER+/HER2- metastatic breast cancer: updated results by duration of prior CDK4/6i in metastatic setting. Cancer Res. 2023;83(5_Supplement) GS3-01-GS3-01. [Google Scholar]
- 13.Bardia A., O'Shaughnessy J., Francois-Clement B., Neven P., Garcia Saenz J.A., et al., editors. San antonio breast cancer symposium. 2023. Elacestrant vs standard-of-care in ER+/HER2- advanced or metastatic breast cancer (mBC) with ESR1 mutation: key biomarkers and clinical subgroup analyses from the phase 3 EMERALD trial; pp. 5–9. 2023; San Antonio, TX. [Google Scholar]
- 14.Baselga J., Campone M., Piccart M., et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2011;366(6):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.André F., Ciruelos E., Rubovszky G., et al. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 16.Turner N.C., Oliveira M., Howell S.J., et al. Capivasertib in hormone receptor–positive advanced breast cancer. N Engl J Med. 2023;388(22):2058–2070. doi: 10.1056/NEJMoa2214131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jhaveri K.L.I.S., Saura C., Juric D., Loibl S., et al. 2023. Inavolisib or placebo in combination with palbociclib and fulvestrant in patients with PIK3CA-mutated, hormone receptor-positive, HER2-negative locally advanced or metastatic breast cancer: phase III INAVO120 primary analysis SABCS 2023. San Antonio, TX. [Google Scholar]
- 18.Rugo H.S., Bardia A., Marmé F., et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;402(10411):1423–1433. doi: 10.1016/S0140-6736(23)01245-X. [DOI] [PubMed] [Google Scholar]
- 19.Modi S., Jacot W., Yamashita T., et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bardia KJ A., Im S.A., Pernas A., De Laurentiis M., et al. ESMO; Madrid2023: 2023. Datopotamab deruxtecan (Dato-DXd) vs chemotherapy in previously-treated inoperable or metastatic hormone receptor-positive, HER2-negative (HR+/HER2–) breast cancer: primary results from the randomised Phase 3 TROPION-Breast01 trial. [Google Scholar]
- 21.Abelman R.O., Spring L., Fell G.G., et al. Sequential use of antibody-drug conjugate after antibody-drug conjugate for patients with metastatic breast cancer: ADC after ADC (A3) study. J Clin Oncol. 2023;41(16_suppl) 1022-1022. [Google Scholar]
- 22.Abelman R.S.L., Fell G., Davis A.A., Hensing W., et al., editors. Sequencing antibody-drug conjugate after antibody-drug conjugate in metastatic breast cancer (A3 study): multi-institution experience and biomarker analysis. San Antonio Breast Cancer Symposium; San Antonio, Texas: 2023. [Google Scholar]
- 23.Raghavendra A.S.W.Z., Bassett R., Tripathy D., editors. Antibody-drug conjugates (ADCs) in breast cancer: real world analysis of outcomes. San Antonio Breast Cancer Symposium; San Antonio, Tx: 2023. [Google Scholar]
- 24.Huppert L MR, Fisch S, Dempsey N, et al. Multicenter retrospective cohort study of the sequential use of the antibody-drug conjugates (ADCs) trastuzumab deruxtecan (T-DXd) and sacituzumab govitecan (SG) in patients with HER2-low metastatic breast cancer (MBC). San Antonio Breast Cancer Symposium; San Antonio Tx2023..
- 25.Poumeaud F MM, Cabel L, Concalves A, et al. Efficacy of Sacituzumab-Govitecan (SG) post Trastuzumab-deruxtecan (T-DXd) and vice versa for HER2low advanced or metastatic breast cancer (MBC): a French multicentre retrospective study. San Antonio Breast Cancer Symposium; San Antonio, Tx2023..
- 26.Cortes J., Rugo H.S., Cescon D.W., et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022;387(3):217–226. doi: 10.1056/NEJMoa2202809. [DOI] [PubMed] [Google Scholar]
- 27.Bardia A., Hurvitz S.A., Tolaney S.M., et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 28.Schmid PJW P., Ma C.X., Park Y.H., Fernandes R., et al. ESMO; Madrid: 2023. Datopotamab deruxtecan (Dato-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC) [Google Scholar]
- 29.Hurvitz S.M.S., Li W., Hee Park Y., Chung A.P., et al., editors. A pooled analysis of trastuzumab deruxtecan in patients with HER2-positive metastatic breast cancer with brain metastases (BMs) from DESTINY-breast01, -02, and -03. ESMO 2023; Madrid, Spain: 2023. pp. 20–24. October. [Google Scholar]
- 30.Bartsch R., Berghoff A.S., Furtner J., et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med. 2022;28(9):1840–1847. doi: 10.1038/s41591-022-01935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurvitz S LS, O'Shaughnessy J, Okines OFC, Tolaney SM, et al HER2CLIMB-02: primary analysis of a randomized, double-blind phase 3 trial of tucatinib and trastuzumab emtansine for previously treated HER2-positive metastatic breast cancer. San antonio breast cancer symposium; San Antonio, Tx. December 5-9, 20232023..
- 32.Khan Q.J., Bohnenkamp C., Monson T., et al. Randomized trial of fixed dose capecitabine compared to standard dose capecitabine in metastatic breast cancer: the X-7/7 trial. J Clin Oncol. 2023;41(16_suppl) 1007-1007. [Google Scholar]
- 33.Santhosh A., Kumar A., Pramanik R., et al. Randomized double-blind, placebo-controlled study of topical diclofenac in the prevention of hand-foot syndrome in patients receiving capecitabine (the D-TORCH study) Trials. 2022;23(1):420. doi: 10.1186/s13063-022-06353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santhosh A., Sharma A., Batra A., et al. Topical diclofenac in prevention of capecitabine associated HFS in patients with breast cancer: an exploratory subgroup analysis of the D-ToRCH study. JCO Global Oncology. 2023;9(Supplement_1) 18-18. [Google Scholar]
- 35.Finn R.S., Rugo H.S., Dieras V.C., et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): analyses from PALOMA-2. J Clin Oncol. 2022;40(17_suppl) LBA1003-LBA1003. [Google Scholar]
- 36.Lu Y.S., Im S.A., Colleoni M., et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2- advanced breast cancer in MONALEESA-7: a phase III randomized clinical trial. Clin Cancer Res. 2022;28(5):851–859. doi: 10.1158/1078-0432.CCR-21-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer E.L., Wander S.A., Regan M.M., et al. Palbociclib after CDK and endocrine therapy (PACE): a randomized phase II study of fulvestrant, palbociclib, and avelumab for endocrine pre-treated ER+/HER2- metastatic breast cancer. J Clin Oncol. 2018;36(15_suppl) TPS1104-TPS1104. [Google Scholar]