Highlights
-
•
Larger left atrial volume index more likely to have a recurrence of AF after direct current cardioversion.
-
•
Increase in left atrial volume index by 1 ml/m2 increases the risk of overall recurrence of AF post DCCV by 6 %.
-
•
The utility of indexed LA volume measurements exceeds absolute LA volume.
-
•
Age and duration of atrial fibrillation predict recurrence of AF after direct current cardioversion.
Keywords: Atrial fibrillation, Cardioversion, DCCV, Recurrence, Left Atrium
Abstract
This systematic review and meta-analysis was conducted to determine the clinical relevance of echocardiographically measured left atrial (LA) size to predict the recurrence of atrial fibrillation (AF) after direct current cardioversion (DCCV). A search was performed on Medline (Ovid), Embase (Elsevier), Cochrane Central Register of Controlled Trials (CENTRAL) in Cochrane Library, Wiley and Web of Science (Clarivate) to identify relevant studies. Amongst the initial 4066 citations identified, 31 fulfilled the criteria for inclusion in the data analysis incorporating 2725 patients with a mean follow-up period of 6.5 months. The weighted mean left atrial volume index (LAVI) was 40.56 ml/m2 (95 %CI:37.24–43.88) in the sinus rhythm (SR) maintenance group versus 48.69 ml/m2 (95 % CI: 44.42–52.97) in the AF recurrence group with P value of < 0.001, left atrial diameter (LAD) was 42.06 mm (95 %CI: 41.08–43.05) in the SR maintenance group versus 45.13 mm (95 %CI: 44.09–46.16) in the AF recurrence group, P value < 0.001. Effect size analysis of LAVI showed that each unit increase in LAVI resulted in an increase in the risk of AF recurrence by 6 % (95 % CI: 3 %–10 %). Age and AF duration were also statistically significant between the two groups however comorbidities, use of beta blockers or amiodarone were not significantly different. This meta-analysis shows that AF duration, LAVI, LAD and age predict the risk of recurrence of atrial fibrillation post electrical cardioversion with LAVI being the most clinically relevant echocardiographic feature.
1. Introduction
The worldwide prevalence of AF is estimated at 4,977 cases per million inhabitants and has increased substantially over the last 20 years) [1]. It is expected that, due to the ageing population, the number of patients living with AF will continue to increase, in turn contributing to rising healthcare costs [1].
The recommended management of AF is the Atrial Fibrillation Better Care (ABC) holistic pathway which involves A’ Anticoagulation/Avoid stroke; ‘B’ Better symptom management; ‘C’ Cardiovascular and Comorbidity optimisation [2]. One aspect of ‘B’ Better symptom management is rhythm control which attempts to restore and maintain sinus rhythm (SR) by engaging one or a combination of treatment approaches including synchronised direct current cardioversion (DCCV), anti-arrhythmic medications, and/or catheter ablation [2]. Current evidence dictates that the primary indication for rhythm control is to reduce AF-related symptoms and improve quality of life (QoL) [2]. AF ablation should be considered in those with HFrEF who have been selected for rhythm control to reduce heart failure (HF) hospitalization and, potentially, mortality [2]. Performing DCCV is the recommended management of AF in hemodynamically compromised AF patients, it is more effective than chemical cardioversion and results in immediate restoration of SR [2]. Pharmacological cardioversion or DCCV are both effective management options in stable patients [2].
The advantages of DCCV include a high initial success rate of 68–98 %, however, sustaining SR long-term is not reliably achieved [3]. A multi-centre double-blind, placebo-controlled study that assessed the efficacy and safety of two anti-arrhythmic medications in preventing the recurrence of AF after successful DCCV in 848 patients showed that 83 % of patients receiving placebo had recurrence within 1 year [4]. Relapse of AF following DCCV is associated with increased mortality. [3] It is therefore imperative to identify appropriate patient cohorts for DCCV and manage reversible factors related to recurrence [2], [3], [4].
Several factors in individual studies have been associated with an increased risk of AF recurrence after elective cardioversion including older age, female gender, obesity, previous cardioversion, chronic obstructive pulmonary disease (COPD), renal impairment, structural heart disease, left atrium (LA) dimensions, and heart failure (HF) [3].
Evidence from several studies supports an association between LA size before or immediately after DCCV and the risk of AF recurrence [5], [6], [7], [8], [9]; however, other studies have failed to observe a relationship [10], [11]. These original studies were all performed in single centres, had small numbers and used different modalities of determining LA size, limiting the conclusions that can be drawn. A systematic review and meta-analysis of these studies would provide insights into whether SR maintenance after successful elective DCCV may be influenced by LA size measured by transthoracic echocardiogram. The purpose of this review was to determine the clinical relevance of echocardiographically measured left atrial size to predict the recurrence of atrial fibrillation after DCCV.
2. Protocol registration
A protocol registered with PROSPERO International Prospective Register of Systematic Reviews outlines the methods for this systematic review (CRD42022308169) [12]. The Preferred Reporting Items for Systematic Reviews (PRISMA) and Synthesis guidelines were followed throughout the review and reporting of the findings [13].
3. Search strategy and screening
Searches were conducted on the 14th December 2021 in Medline (Ovid), Embase (Elsevier), Cochrane Central Register of Controlled Trials (CENTRAL) in Cochrane Library, Wiley (Issue 11, 2021) and Web of Science (Clarivate). We applied no limits for study type, or year of publication. Three investigators (MG, DR, LH) worked alongside ST, an experienced medical librarian, to develop the search strategy. The search used a combination of Medical Subject Headings (MeSH) and key words for the terms “atrial fibrillation”, “electric cardioversion” and “recurrence”. The full search strategies for each database are included in Appendix A.
After removing all duplicates, title and abstract screening was conducted by MG, DR, ST and LH to select studies which met eligibility criteria through the web-based software platform Covidence (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia, available at https://www.covidence.org). All articles were screened by two investigators with criteria set for either MG or DR to screen each article.
Due to the large volume of studies included from the initial title and abstract screening, the project was divided into parts to focus on specific variables. Consequently, the abstracts of studies that reported on echocardiographic factors were screened. Studies were included that reported on echocardiographic factors which were clinically relevant and widely described.
Reference checks of related systematic reviews, metanalysis and conference articles was done, the resulting studies were included for full text review. Full-text screening was conducted by MG, DR, LH and ST to identify eligible articles which were evaluated before inclusion in the analysis. Disagreements were resolved by consensus.
4. Inclusion and exclusion criteria
Studies were eligible for inclusion if they were primary research articles, published in English language from January 1980 onwards, RCTs, non-RCTs and cohort studies and incorporated:
-
–
adult patients > 18 years,
-
–
patients with both AF and external electrical cardioversion,
-
–
outcomes at least 30 days post external electrical cardioversion
Exclusion criteria were 1) case studies, cross-sectional studies, 2) follow up less than 30 days 3) animal studies, 4) non-English studies, 5) only report pharmacological cardioversion, 6) abstracts with insufficient data e.g., conference proceedings, and 7) trial protocols.
5. Data extraction and synthesis
Two authors (MG, DR) performed data extraction with a purpose-built data extraction template, which was verified by LH and ST. Data extracted included article characteristics (e.g., title, year, author, country, journal and type of study), study characteristics (e.g., number of participants, number of lost to follow up, mean age, demographic factors, AF duration, comorbidities and medications), and primary and secondary outcomes of interest from the article.
6. Statistical analysis
Quantitative data related to outcomes for studies investigating AF following DCCV was analysed, and a t-test was conducted to compare patients who maintained sinus rhythm (SR) and those who reverted to AF for demographic details, co-morbidities and medications used to manage AF before DCCV. Efficacy outcomes related to age, body mass index, AF duration and echocardiographic findings were meta-analysed. Forest plots were presented alongside the I2 statistic to evaluate heterogeneity for LAVI and LAD. A p-value of < 0.05 was considered to represent statistical significance. All analyses were performed using Stata 17 (Stat Corp., College Station, Tx, USA).
7. Quality assessment
Two unblinded reviewers independently evaluated the quality of each of the included studies using the JBI Critical Appraisal Tool. Discrepancies between reviewers were solved by consensus. The percentage for each feature was independently evaluated among the studies.
Regression-based Egger’s test and the non-parametric trim-and-fill method were used to assess for publication bias.
8. Study selection and patient characteristics
At initial screening, 4066 studies were identified. After the exclusion of nonrelevant studies, case reports, and reviews by title and abstracts, 74 studies were retrieved for full-text screening of which 12 were obtained from reference checks (Fig. 1). Thirty-one studies were included in the final analysis after meeting the eligibility criteria (Table 1). Out of the 31 studies included, 3 studies were retrospective cohort studies and 28 were prospective cohort studies. The total number of patients from the 31 studies was 2830; 105 patients were lost to follow-up resulting in 2725 patients being analysed. The mean period of follow-up was 6.5 months, and 12 studies had a mean follow-up duration of < 3 months.
Fig. 1.
Flow chart of the systematic review and reasons for exclusion of studies.
Table 1.
Summary of the characteristics of the included studies.
| Study | Country | Study Design | Patients (N) | Duration of follow-up (Mean, months) | Lost to follow up (N) |
|---|---|---|---|---|---|
| Akdemir et al. 2013 [5] | Turkey | Prospective Cohort | 50 | 1 | 0 |
| Altun et al. 2015 [23] | Turkey | Prospective Cohort | 32 | 6 | 0 |
| Ari et al. 2008 [24] | Turkey | Prospective Cohort | 58 | 6 | 0 |
| Aribas et al. 2013 [25] | Turkey | Prospective Cohort | 156 | 6 | 7 |
| Bernard- Brunet et al. 2014 [26] | France | Prospective Cohort | 29 | 1 | 9 |
| Besli et al. 2015 [27] | Turkey | Prospective Cohort | 75 | 1 | 0 |
| Budeus et al. 2006 [28] | Germany | Prospective Cohort | 151 | 9 | 33 |
| Cho et al. 2015 [29] | Korea | Prospective Cohort | 163 | 12 | 0 |
| Caputo et al. 2011 [30] | Italy | Prospective Cohort | 51 | 12 | 0 |
| Degiovanni et al. 2018 [31] | Italy | Prospective Cohort | 96 | 1 | 0 |
| Gurses et al. 2019 [32] | Turkey | Prospective Cohort | 90 | 3 | 0 |
| Kim et al. 2014 [33] | Korea | Retrospective Cohort | 171 | 5 | 34 |
| Lin et al. 2002 [10] | Taiwan | Prospective Cohort | 36 | 7 | 0 |
| Luong et al. 2015 [6] | Canada | Retrospective Cohort | 95 | 6 | 13 |
| Luong et al. 2016 [7] | Canada | Retrospective Cohort | 95 | 6 | 0 |
| Meurling et al. 2006 [34] | Sweden | Prospective Cohort | 32 | 2 | 0 |
| Mukherjee et al. 2013 [35] | USA | Prospective Cohort | 82 | 3 | 0 |
| Naji et al. 2011 [36] | Slovenia | Prospective Cohort | 85 | 24 | 6 |
| Park et al. 2010 [11] | Korea | Prospective Cohort | 53 | 6 | 0 |
| Roijer et al. 2001 [37] | Sweden | Prospective Cohort | 62 | 1 | 0 |
| Rondano et al. 2010 [38] | Italy | Prospective Cohort | 130 | 12 | 0 |
| Toufan et al. 2017 [8] | Iran | Prospective Cohort | 51 | 3 | 0 |
| Verhorst et al. 1997 [39] | Netherlands | Prospective Cohort | 50 | 12 | 0 |
| Walek et al. 2020 [9] | Poland | Prospective Cohort | 117 | 12 | 0 |
| Watanabe et al. 2006 [40] | Japan | Prospective Cohort | 84 | 12 | 0 |
| Eren et al. 2020 [41] | Turkey | Prospective Cohort | 306 | 7 | 0 |
| Fujimoto et al. 2018 [42] | Japan | Prospective Cohort | 141 | 1 | 0 |
| Maffe et al. 2015 [43] | Italy | Prospective Cohort | 104 | 14 | 0 |
| Moreno-Ruiz et al 2019 [44] | Mexico | Prospective Cohort | 81 | 6 | 0 |
| Muller et al. 2014 [45] | Germany | Prospective Cohort | 54 | 3 | 3 |
| Weijs et al. 2018 [46] | Netherlands | Prospective Cohort | 50 | 1 | 0 |
9. Baseline characteristics of patients
The total population consisted of 1447 patients in the SR-maintaining group and 1278 patients in the AF-recurrence group. The baseline clinical and echocardiographic characteristics of these patients are summarised in Table 2. Overall, 61.7 % of patients were male in the SR- maintenance group and 59.7 % in the AF-recurrence group. There was no statistically significant differences between other factors including demographics, comorbidities, or use of beta blockers or amiodarone.
Table 2.
Summary of baseline characteristics of patients who maintained SR compared to patients with AF recurrence.
| Maintaining SR N = 1447 |
Recurrent AF N: 1278 |
P value | No of studies | |
|---|---|---|---|---|
| Demographics | ||||
| Males/Females | 721/447 | 667/450 | 25 | |
| Co-morbidities Mean (SD) | ||||
| Patients with CAD | 10.78 (13.48) | 8.94 (10.03) | 0.193 | 18 |
| Patients with heart failure/ cardiomyopathy | 8.36 (8.24) | 10.54 (12.36) | 0.282 | 11 |
| Patients with hypertension | 27.25 (20.43) | 26.91 (20.56) | 0.918 | 24 |
| Patients with diabetes | 7.46 (4.98) | 7.7 (6.63) | 0.844 | 20 |
| Medications Mean (SD) | ||||
| Patients on beta blockers | 25.38 (19.26) | 22.2 (16.85) | 0.230 | 15 |
| Patients on amiodarone | 21.28 (11.88) | 18.2 (12.46) | 0.301 | 10 |
AF: atrial fibrillation; CAD: coronary artery disease; SR: sinus rhythm.
SD and p-values at 95% confidence interval.
10. Association of patient characteristics with recurrence of AF after DCCV
Table 3 provides a summary of meta-analysis data. Patients with recurrence of AF were older compared to patients who maintained SR (weighted mean 64.94 [95 % CI: 63.22–66.66] versus 63.20 years [95 % CI: 61.45–64.94], p = 0.016, respectively). AF duration was significantly different with mean duration of 197.82 days (95 %CI: 153.88–241.75) in the SR group versus 223.1 days (95 %CI: 176.73–269.46) in the AF recurrence group, p = 0.019.
Table 3.
Metanalysis of demographic and echocardiogram variables of patients who maintained SR compared to patients with AF recurrence.
| Maintaining SR N = 1447 |
Recurrent AF N = 1278 |
P value | No of studies | |
|---|---|---|---|---|
|
Demographic factors Mean (95 % confidence interval) | ||||
| Age years | 63.20 (61.45–64.94) | 64.94 (63.22–66.66) | 0.016 | 28 |
| BMI kg/m2 | 27.17 (26.08–28.25) | 27.73 (26.49–28.96) | 0.239 | 12 |
| AF duration days | 197.82 (153.88–241.75) | 223.1 (176.73–269.46) | 0.019 | 20 |
|
Echocardiographic findings Mean (95 % confidence interval) | ||||
| LA area cm2 | 17.55 (3.98–31.11) | 20.74 (5.19–36.28) | 0.034 | 4 |
| LA volume ml | 70.23 (55.66–84.81) | 77.70 (65.60–89.82) | 0.268 | 4 |
| LA volume indexed mL/m2 | 40.56 (37.24–43.88) | 48.69 (44.42–52.97) | <0.001 | 18 |
| LA diameter mm | 42.06 (41.08–43.05) | 45.13 (44.09–46.16) | <0.001 | 23 |
| LVEF % | 57.11 (55.17–59.05) | 56.49 (54.64–58.33) | 0.194 | 28 |
Data presented as weighted means and SD at 95% confidence interval in parenthesis.
BMI; body mass index, AF; atrial fibrillation, LA; left atrium, LVEF; left ventricular ejection fraction, SR; sinus rhythm.
There was no statistically significant difference between other factors including demographics, comorbidities, or use of beta blockers or amiodarone.
11. Association of echocardiographic LA measurements with recurrence of AF after DCCV
A summary of differences in LA size amongst the patients maintaining SR and patients with AF recurrence is presented in Table 3. The weighted mean LA diameter was 42.06 mm (95 %CI: 41.08–43.05) in the SR maintenance group versus 45.13 mm (95 %CI: 44.09–46.16) in the AF recurrence group, p < 0.001.
Patients with recurrence of AF had larger LA area compared to those who maintained SR (weighted mean 20.74 cm2; 95 %CI: 5.19–36.28 versus 17.55 cm2; 95 % CI: 3.98–31.11), respectively; p = 0.034. The weighted mean LAVI was 40.56 ml/m2 (95 %CI:37.24–43.88) in the SR maintenance group versus 48.69 ml/m2 (95 % CI: 44.42–52.97) in the AF recurrence group with p < 0.001. There were no significant differences in LV ejection fraction and non-indexed LA volume between patients with and without AF recurrence.
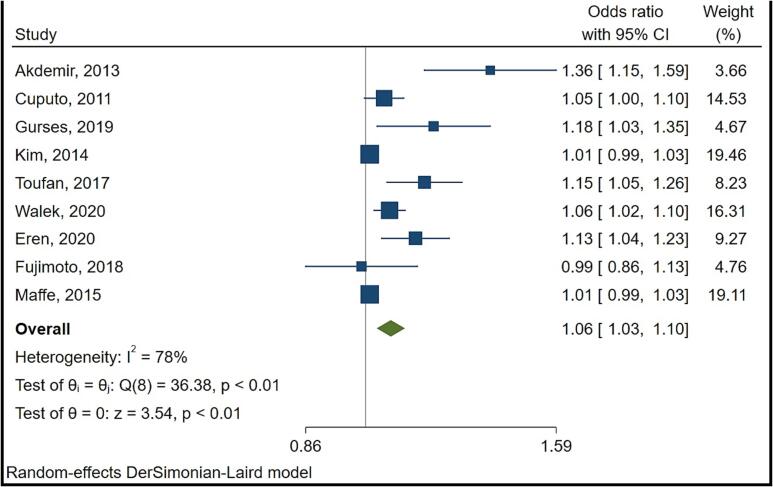
There were 18 studies that reported on LAVI of which 9 studies reported data in a format appropriate for meta-analysis. Fig. 2 provides a summary result for LAVI. Random effects meta-analysis showed a high level of heterogeneity (I2 = 78 %) with findings suggesting that for each 1 unit increase in LAVI, the odds of AF relapse is higher by 6 % (95 % confidence interval 3 % to 10 %).
Fig. 2.
Forest plot of the effect metanalysis of left atrial volume indexed (LAVI) on risk of atrial fibrillation recurrence post electrical cardioversion.
12. Quality assessment
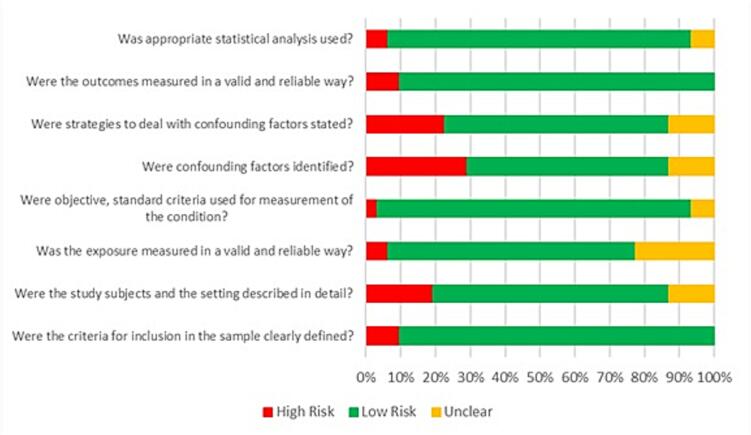
Results of the quality assessment of included studies are summarised in Fig. 3. The details of quality assessment for individual studies can be found in the supplementary material [Supplementary Table 1]. Almost 30 % of the studies did not report on confounding factors. Amongst the studies which identified confounding factors, less than 25 % reported strategies to mitigate the risk of bias. More than 90 % of the studies included details of the inclusion criteria.
Fig. 3.
Assessment of the methodological quality of the studies using the JBI Critical Appraisal Tool.
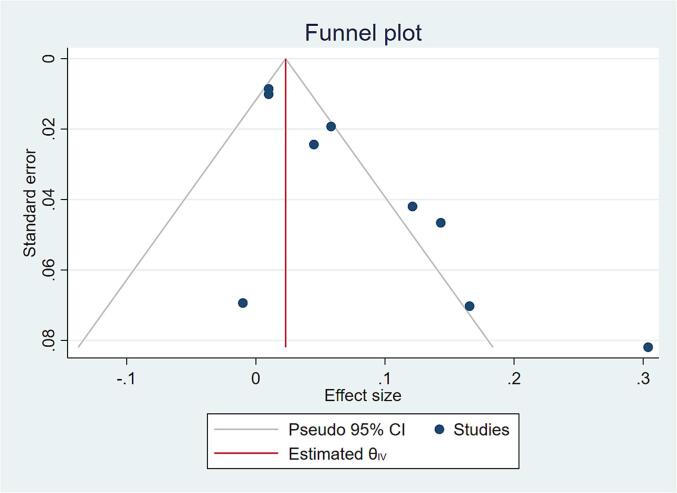
Funnel plot and Egger’s test show strong evidence of a small studies effect, possibly publication bias. When the trim-and-fill method is used to account for the bias the overall summary effect is reduced from 0.06 (95 % CI 0.03 to 1.0) to 0.05 (95 % CI 0.01 to 0.08) (Fig. 4).
Fig. 4.
Funnel plot on study results to assess risk of bias.
This systematic review and meta-analysis was conducted to determine the association between echocardiographic measurement of LA size and the recurrence of AF post successful elective DCCV. To our knowledge, there has not been a similar study published that assessed the correlation between LA size and recurrence of AF after successful DCCV.
The most important finding of our systematic review and meta-analysis is that patients with larger LAVI, LA area and LAD were more likely to have a recurrence of AF after successful DCCV. For example, an increase in LAVI by 1 ml/m2 increases the risk of overall recurrence of AF post DCCV by 6 %. Another vital finding is the importance of indexation of LA volume measurements as LA volume alone was not predictive, supporting contemporary society recommendations for reporting LAVI on echocardiogram [14].
Additionally, LAD and LA area measurements were significant for predicting AF recurrence without being indexed for body surface area. However, the narrow difference in the weighted mean of LA diameter (42.06 mm vs 45.13 mm) and LA Area (17.55 cm2 vs 20.72 cm2) between the two groups, raises questions as to whether these measurements would be clinically relevant. Interestingly, the mean values for LA area in both the groups were within normal reference limit (≤20 cm2) [14].
In patients with AF, the LA usually enlarges in the superior-inferior or medial–lateral axis since it is constrained posteriorly by the tracheal bifurcation and anteriorly by the aortic root and right ventricular outflow tract [15]. Therefore, measurement of the antero-posterior (AP) diameter may often neglect the changes in other LA dimensions which are better captured with LA volume [16].
There was no significant difference in LVEF between the two groups, which may have been expected given most of the included studies excluded patients with moderate to severely reduced ejection fraction. Moreover, medication usage was a not a factor associated with in maintenance of SR, although only beta blockers and amiodarone were reported frequently enough to be analysed. This lack of association may also be due to heterogeneity in dosage, timing of initiation and cessation of these medications.
Our analysis also shows that more advanced age is a significant predictor of recurrence of AF. Age has also been associated with recurrence of AF post cardioversion in multiple studies with variable methodologies and definitions of age groups [17], [18], [19], [20]; additionally, in clinical practice, older patients are less likely to be offered DCCV. [47].
It is well known from previous studies that prolonged AF duration is associated with lower rates of successful cardioversion and higher recurrence post successful cardioversion [17], [18], [21]. Our analysis also reiterates that longer AF duration is associated with higher likelihood of AF recurrence post successful DCCV. Chronic AF promotes electrical and structural remodelling so that patients with chronic AF appear to have larger atria and are more resistant to DCCV [22].
Based on the findings we suggest AF patients with normal (16‐34 ml/m2) or mildly enlarged LAVI (35‐41 ml/m2) proceed to DCCV if rhythm control is decided upon. In those with moderate (42–48 ml/m2) to severely (>48 ml/m2) enlarged LA, given recurrence is more likely, we suggest they should undergo appropriate risk and benefit discussion with shared decision making prior to DCCV.
Future studies areas of study may include more complex measurements of LA function such as strain and reservoir function and their impact on maintenance of SR. The identified echocardiographic variables could be incorporated into selecting cohorts in future randomised trials of rhythm control strategies.
13. Limitations
A few considerations must be taken into account in this systematic review and metanalysis. The studies did not segregate patients with short duration AF, for example < 7 days, which may have different patterns of AF recurrence. Independent predictors of recurrence could not be determined by further statistical analysis such as meta-regression since individual patient data and supplementary data could not be accessed for the included studies. The studies included had a high degree of heterogeneity with variable duration of follow-up. Incomplete information regarding some variables such as smoking habits, alcohol intake, and use of medications such as angiotensin converting enzyme inhibitor (ACEI), angiotensin II receptor blockers (ARB) and statins could have a role in recurrence of AF. Moreover, patient variables used in adjustment of confounders were not uniform or clearly mentioned in the studies included. Furthermore, three of the studies included were retrospective thus bias related to incomplete data reporting cannot be excluded. Additionally, patients may have undetected recurrences of AF however continuous patient monitoring for long durations may not be practical. We did not adjust the analysis for contributing factors such as age and AF duration as we did not have access to the original data from the various studies.
14. Conclusion
In this systematic and meta-analysis of patients undergoing elective DCCV for AF, significant predictors of AF recurrence include age, AF duration, LA area, diameter and indexed volume. In particular LA volume, when indexed for BSA, appears to be a strong and clinically relevant predictor of AF recurrence after successful cardioversion. These findings suggest LAVI should be factored into decision making around utility of DCCV in patients with AF.
CRediT authorship contribution statement
Dipesh Raniga: Writing – review & editing, Writing – original draft, Project administration, Methodology, Formal analysis, Data curation, Conceptualization. Mina Goda: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Laetitia Hattingh: Writing – review & editing, Supervision, Software, Project administration, Methodology, Formal analysis, Data curation, Conceptualization. Sarah Thorning: Writing – review & editing, Formal analysis, Data curation. Matthew Rowe: Writing – review & editing, Supervision, Resources. Laurie Howes: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101364.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Lippi G., Sanchis-Gomar F., Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke. 2021;16(2):217–221. doi: 10.1177/1747493019897870. [DOI] [PubMed] [Google Scholar]
- 2.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 3.Ecker V., Knoery C., Rushworth G., Rudd I., Ortner A., Begley D., et al. A review of factors associated with maintenance of sinus rhythm after elective electrical cardioversion for atrial fibrillation. Clin. Cardiol. 2018;41(6):862–870. doi: 10.1002/clc.22931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fetsch T., Bauer P., Engberding R., Koch H.P., Lukl J., Meinertz T., et al. Prevention of atrial fibrillation after cardioversion: results of the PAFAC trial. Eur. Heart J. 2004;25(16):1385–1394. doi: 10.1016/j.ehj.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Akdemir R., Gunduz H., Erbilen E., Ozer I., Albayrak S., Unlu H., et al. Atrial fibrillation after electrical shock: a case report and review. Emerg. Med. J.: EMJ. 2004;21(6):744–746. doi: 10.1136/emj.2003.005496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luong C., Thompson D.J.S., Bennett M., Gin K., Jue J., Barnes M.E., et al. Right atrial volume is superior to left atrial volume for prediction of atrial fibrillation recurrence after direct current cardioversion. Can. J. Cardiol. 2015;31(1):29–35. doi: 10.1016/j.cjca.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Luong C.L., Thompson D.J.S., Gin K.G., Jue J., Nair P., Lee P.-K., et al. Usefulness of the Atrial Emptying Fraction to Predict Maintenance of Sinus Rhythm After Direct Current Cardioversion for Atrial Fibrillation. Am. J. Cardiol. 2016;118(9):1345–1349. doi: 10.1016/j.amjcard.2016.07.066. [DOI] [PubMed] [Google Scholar]
- 8.Toufan M., Kazemi B., Molazadeh N. The significance of the left atrial volume index in prediction of atrial fibrillation recurrence after electrical cardioversion. J. Cardiovasc. Thoracic Res. 2017;9(1):54–59. doi: 10.15171/jcvtr.2017.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walek P., Sielski J., Starzyk K., Gorczyca I., Roskal-Walek J., Wozakowska-Kaplon B. Echocardiographic assessment of left atrial morphology and function to predict maintenance of sinus rhythm after electrical cardioversion in patients with non-valvular persistent atrial fibrillation and normal function or mild dysfunction of left ventricle. Cardiol. J. 2020;27(3):246–253. doi: 10.5603/CJ.a2019.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin J.-M., Lin J.-L., Lai L.-P., Tseng Y.-Z., Stephen Huang S.K. Predictors of clinical recurrence after successful electrical cardioversion of chronic persistent atrial fibrillation: clinical and electrophysiological observations. Cardiology. 2002;97(3):133–137. doi: 10.1159/000063329. [DOI] [PubMed] [Google Scholar]
- 11.Park S.-M., Kim Y.-H., Choi J.-I., Pak H.-N., Kim Y.-H., Shim W.-J. Left atrial electromechanical conduction time can predict six-month maintenance of sinus rhythm after electrical cardioversion in persistent atrial fibrillation by Doppler tissue echocardiography. J. Am. Soc. Echocardiogr.: Off. Publ. Am. Soc. Echocardiogr. 2010;23(3):309–314. doi: 10.1016/j.echo.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 12.M.G. Goda, L. Hattingh, D. Raniga, S. Thorning, Factors that predict long term maintenance of sinus rhythm or recurrence of atrial fibrillation after elective external electrical cardioversion, PROSPERO CRD42022308169, 2022.
- 13.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Lemire F., Tajik A.J., Hagler D.J. Asymmetric left atrial enlargement; an echocardiographic observation. Chest. 1976;69(6):779–781. doi: 10.1378/chest.69.6.779. [DOI] [PubMed] [Google Scholar]
- 16.Lester S.J., Ryan E.W., Schiller N.B., Foster E. Best method in clinical practice and in research studies to determine left atrial size. Am. J. Cardiol. 1999;84(7):829–832. doi: 10.1016/s0002-9149(99)00446-4. [DOI] [PubMed] [Google Scholar]
- 17.Pisters R., Nieuwlaat R., Prins M.H., Le Heuzey J.Y., Maggioni A.P., Camm A.J., et al. Clinical correlates of immediate success and outcome at 1-year follow-up of real-world cardioversion of atrial fibrillation: the Euro Heart Survey. Europace. 2012;14(5):666–674. doi: 10.1093/europace/eur406. [DOI] [PubMed] [Google Scholar]
- 18.Marchese P., Bursi F., Delle Donne G., Malavasi V., Casali E., Barbieri A., et al. Indexed left atrial volume predicts the recurrence of non-valvular atrial fibrillation after successful cardioversion. Eur. J. Echocardiogr. 2011;12(3):214–221. doi: 10.1093/ejechocard/jeq176. [DOI] [PubMed] [Google Scholar]
- 19.Boriani G., Diemberger I., Biffi M., Domenichini G., Martignani C., Valzania C., et al. Electrical cardioversion for persistent atrial fibrillation or atrial flutter in clinical practice: predictors of long-term outcome. Int. J. Clin. Pract. 2007;61(5):748–756. doi: 10.1111/j.1742-1241.2007.01298.x. [DOI] [PubMed] [Google Scholar]
- 20.Jaakkola S., Lip G.Y., Biancari F., Nuotio I., Hartikainen J.E., Ylitalo A., et al. Predicting Unsuccessful Electrical Cardioversion for Acute Atrial Fibrillation (from the AF-CVS Score) Am. J. Cardiol. 2017;119(5):749–752. doi: 10.1016/j.amjcard.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Toso E., Blandino A., Sardi D., Battaglia A., Garberoglio L., Miceli S., et al. Electrical cardioversion of persistent atrial fibrillation: acute and long-term results stratified according to arrhythmia duration. Pacing Clin. Electrophysiol. 2012;35(9):1126–1134. doi: 10.1111/j.1540-8159.2012.03453.x. [DOI] [PubMed] [Google Scholar]
- 22.Frick M., Frykman V., Jensen-Urstad M., Ostergren J., Rosenqvist M. Factors predicting success rate and recurrence of atrial fibrillation after first electrical cardioversion in patients with persistent atrial fibrillation. Clin. Cardiol. 2001;24(3):238–244. doi: 10.1002/clc.4960240313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altun I., Pamukcu B., Yildiz C.E., Arkaya S.C., Guz G., Yilmaz A., et al. Cardiotrophin-1: A new predictor of atrial fibrillation relapses after successful cardioversion. Bosn. J. Basic Med. Sci. 2015;15(3):68–73. doi: 10.17305/bjbms.2015.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ari H., Binici S., Ari S., Akkaya M., Koca V., Bozat T., et al. The predictive value of plasma brain natriuretic peptide for the recurrence of atrial fibrillation six months after external cardioversion. Turk Kardiyoloji Dernegi Arsivi : Turk Kardiyoloji Derneginin Yayin Organidir. 2008;36(7):456–460. [PubMed] [Google Scholar]
- 25.Arıbaş A., Akıllı H., Gül E.E., Kayrak M., Demir K., Duman C., et al. Can neutrophil/lymphocyte ratio predict recurrence of non-valvular atrial fibrillation after cardioversion? Anadolu Kardiyol Derg. 2013;13(2):123–130. doi: 10.5152/akd.2013.036. [DOI] [PubMed] [Google Scholar]
- 26.Bernard-Brunet A., Saint Etienne C., Piver E., Zannad N., Pages J.-C., Fauchier L., et al. Incomplete recovery of mechanical and endocrine left atrial functions one month after electrical cardioversion for persistent atrial fibrillation: a pilot study. J. Transl. Med. 2014;12:51. doi: 10.1186/1479-5876-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Besli F., Basar C., Kecebas M., Turker Y. Improvement of the myocardial performance index in atrial fibrilation patients treated with amiodarone after cardioversion. J. Interventional Cardiac Electrophysiol.: Int. J. Arrhythmias Pacing. 2015;42(2):107–115. doi: 10.1007/s10840-014-9965-0. [DOI] [PubMed] [Google Scholar]
- 28.Budeus M., Wieneke H., Sack S., Erbel R., Perings C. Long-term outcome after cardioversion of atrial fibrillation: prediction of recurrence with P wave signal averaged ECG and chemoreflexsensitivity. Int. J. Cardiol. 2006;112(3):308–315. doi: 10.1016/j.ijcard.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Cho K.-I., Kim B.-J., Cha T.-J., Heo J.-H., Kim H.-S., Lee J.-W. Impact of duration and dosage of statin treatment and epicardial fat thickness on the recurrence of atrial fibrillation after electrical cardioversion. Heart Vessels. 2015;30(4):490–497. doi: 10.1007/s00380-014-0505-8. [DOI] [PubMed] [Google Scholar]
- 30.Caputo M., Urselli R., Capati E., Navarri R., Sinesi L., Furiozzi F., et al. Usefulness of left ventricular diastolic dysfunction assessed by pulsed tissue Doppler imaging as a predictor of atrial fibrillation recurrence after successful electrical cardioversion. Am. J. Cardiol. 2011;108(5):698–704. doi: 10.1016/j.amjcard.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 31.Degiovanni A., Boggio E., Prenna E., Sartori C., De Vecchi F., Marino P.N., et al. Association between left atrial phasic conduit function and early atrial fibrillation recurrence in patients undergoing electrical cardioversion. Clin. Res. Cardiol.: Off. J. German Cardiac Soc. 2018;107(4):329–337. doi: 10.1007/s00392-017-1188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurses K.M., Yalcin M.U., Kocyigit D., Canpinar H., Ates A.H., Canpolat U., et al. Serum Galectin-3 Level Predicts Early Recurrence following Successful Direct-Current Cardioversion in Persistent Atrial Fibrillation Patients. Persistan Atriyal Fibrilasyonlu Hastalarda Serum Galektin-3 Seviyeleri Basarili Elektriksel Kardiyoversiyondan Sonra Erken Nuksu Ongordurur. 2019;47(7):564–571. doi: 10.5543/tkda.2019.58399. [DOI] [PubMed] [Google Scholar]
- 33.Kim H., Lee J.-P., Yoon H.-J., Park H.-S., Cho Y.-K., Nam C.-W., et al. Association between Doppler flow of atrial fibrillatory contraction and recurrence of atrial fibrillation after electrical cardioversion. J. Am. Soc. Echocardiogr.: Off. Publ. Am. Soc. Echocardiogr. 2014;27(10):1107–1112. doi: 10.1016/j.echo.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Meurling C.J., Roijer A., Waktare J.E.P., Holmqvist F., Lindholm C.J., Ingemansson M.P., et al. Prediction of sinus rhythm maintenance following DC-cardioversion of persistent atrial fibrillation - the role of atrial cycle length. BMC Cardiovasc. Disord. 2006;6:11. doi: 10.1186/1471-2261-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukherjee R., Akar J.G., Wharton J.M., Adams D.K., McClure C.D., Stroud R.E., et al. Plasma profiles of matrix metalloproteinases and tissue inhibitors of the metalloproteinases predict recurrence of atrial fibrillation following cardioversion. J. Cardiovasc. Transl. Res. 2013;6(4):528–535. doi: 10.1007/s12265-013-9471-2. [DOI] [PubMed] [Google Scholar]
- 36.Naji F., Sabovic M. Lipoprotein(a) and inflammation in patients with atrial fibrillation after electrical cardioversion. J. Negat. Results Biomed. 2011;10:15. doi: 10.1186/1477-5751-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roijer A., Meurling C.J., Eskilsson J., Olsson B. Left atrial appendage outflow velocity index is superior to conventional criteria for prediction of maintenance of sinus rhythm after cardioversion. An echocardiographic study in patients with atrial fibrillation of a few months' duration. Scandinavian Cardiovasc. J. : SCJ. 2001;35(2):119–124. doi: 10.1080/140174301750164817. [DOI] [PubMed] [Google Scholar]
- 38.Rondano E., Dell'Era G., De Luca G., Piccinino C., Bellomo G., Marino P.N. Left atrial asynchrony is a major predictor of 1-year recurrence of atrial fibrillation after electrical cardioversion. J. Cardiovasc. Med. (hagerstown, Md). 2010;11(7):499–506. doi: 10.2459/JCM.0b013e32833757b5. [DOI] [PubMed] [Google Scholar]
- 39.Verhorst P.M., Kamp O., Welling R.C., Van Eenige M.J., Visser C.A. Transesophageal echocardiographic predictors for maintenance of sinus rhythm after electrical cardioversion of atrial fibrillation. Am. J. Cardiol. 1997;79(10):1355–1359. doi: 10.1016/s0002-9149(97)00139-2. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe E., Arakawa T., Uchiyama T., Kodama I., Hishida H. High-sensitivity C-reactive protein is predictive of successful cardioversion for atrial fibrillation and maintenance of sinus rhythm after conversion. Int. J. Cardiol. 2006;108(3):346–353. doi: 10.1016/j.ijcard.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 41.H. Eren, U. Kaya, L. Ocal, A. Senbas, M. Kalcik, The presence of fragmented QRS may predict the recurrence of nonvalvular atrial fibrillation after successful electrical cardioversion, Ann. Noninvasive Electrocardiol.: Off. J. Int. Soc. Holter Noninvasive Electrocardiol., Inc. 2020;25(1):e12700. [DOI] [PMC free article] [PubMed]
- 42.Fujimoto Y., Yodogawa K., Maru Y.-J., Oka E., Hayashi H., Yamamoto T., et al. Advanced interatrial block is an electrocardiographic marker for recurrence of atrial fibrillation after electrical cardioversion. Int. J. Cardiol. 2018;272:113–117. doi: 10.1016/j.ijcard.2018.07.135. [DOI] [PubMed] [Google Scholar]
- 43.Maffe S., Paffoni P., Dellavesa P., Cucchi L., Zenone F., Bergamasco L., et al. Prognostic value of total atrial conduction time measured with tissue Doppler imaging to predict the maintenance of sinus rhythm after external electrical cardioversion of persistent atrial fibrillation. Echocardiography (mount Kisco, NY). 2015;32(3):420–427. doi: 10.1111/echo.12702. [DOI] [PubMed] [Google Scholar]
- 44.Moreno-Ruiz L.A., Madrid-Miller A., Martinez-Flores J.E., Gonzalez-Hermosillo J.A., Arenas-Fonseca J., Zamorano-Velazquez N., et al. Left atrial longitudinal strain by speckle tracking as independent predictor of recurrence after electrical cardioversion in persistent and long standing persistent non-valvular atrial fibrillation. Int. J. Cardiovasc. Imaging. 2019;35(9):1587–1596. doi: 10.1007/s10554-019-01597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muller P., Schiedat F., Dietrich J.-W., Shin D.-I., Kara K., Mugge A., et al. Reverse atrial remodeling in patients who maintain sinus rhythm after electrical cardioversion: evidence derived from the measurement of total atrial conduction time assessed by PA-TDI interval. J. Echocardiogr. 2014;12(4):142–150. doi: 10.1007/s12574-014-0227-z. [DOI] [PubMed] [Google Scholar]
- 46.Weijs B., Limantoro I., Delhaas T., de Vos C.B., Blaauw Y., Houben R.P.M., et al. Cardioversion of persistent atrial fibrillation is associated with a 24-hour relapse gap: Observations from prolonged postcardioversion rhythm monitoring. Clin. Cardiol. 2018;41(3):366–371. doi: 10.1002/clc.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.NHFA CSANZ Atrial Fibrillation Guideline Working Group, Brieger D, Amerena J, et al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian Clinical Guidelines for the Diagnosis and Management of Atrial Fibrillation 2018. Heart Lung Circ. 2018;27(10):1209-1266. doi:10.1016/j.hlc.2018.06.1043. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.