Abstract
Disruption of the mouse Atm gene, whose human counterpart is consistently mutated in ataxia-telangiectasia (A-T) patients, creates an A-T mouse model exhibiting most of the A-T-related systematic and cellular defects. While ATM plays a major role in signaling the p53 response to DNA strand break damage, Atm−/− p53−/− mice develop lymphomas earlier than Atm−/− or p53−/− mice, indicating that mutations in these two genes lead to synergy in tumorigenesis. The cell cycle G1/S checkpoint is abolished in Atm−/− p53−/− mouse embryonic fibroblasts (MEFs) following γ-irradiation, suggesting that the partial G1 cell cycle arrest in Atm−/− cells following γ-irradiation is due to the residual p53 response in these cells. In addition, the Atm−/− p21−/− MEFs are more severely defective in their cell cycle G1 arrest following γ-irradiation than Atm−/− and p21−/− MEFs. The Atm−/− MEFs exhibit multiple cellular proliferative defects in culture, and an increased constitutive level of p21 in these cells might account for these cellular proliferation defects. Consistent with this notion, Atm−/− p21−/− MEFs proliferate similarly to wild-type MEFs and exhibit no premature senescence. These cellular proliferative defects are also rescued in Atm−/− p53−/− MEFs and little p21 can be detected in these cells, indicating that the abnormal p21 protein level in Atm−/− cells is also p53 dependent and leads to the cellular proliferative defects in these cells. However, the p21 mRNA level in Atm−/− MEFs is lower than that in Atm+/+ MEFs, suggesting that the higher level of constitutive p21 protein in Atm−/− MEFs is likely due to increased stability of the p21 protein.
Ataxia-telangiectasia (A-T) is an autosomally recessive human genetic disease characterized by pleiotropic defects in multiple systems. Affected patients suffer from growth retardation, neuronal degeneration in the cerebellum leading to ataxia, dilated blood vessels in the eye and facial area, gonadal defects, immunodeficiency, a high incidence of cancer, and hypersensitivity to ionizing radiation (20). Cells derived from A-T patients are defective in their checkpoint responses to ionizing radiation and are hypersensitive to ionizing radiation (22, 27). Following the induction of strand break damage induced by ionizing radiation, normal cells arrest their cell cycle at three cell cycle checkpoints: at the G1/S border, at S phase, and at the G2/M border (12). However, all three cell cycle checkpoints in A-T cells are defective in response to ionizing radiation. The cell cycle checkpoint defects of A-T cells have been suggested to account for the cellular hypersensitivity of these cells to ionizing radiation (22, 27).
A gene consistently mutated in A-T patients, denoted ATM, has been identified through linkage mapping and positional cloning (9, 26). The ATM gene encodes a large kinase which is similar to a family of kinases involved in DNA metabolism and cell cycle checkpoint control in response to DNA damage (26, 34). While the ATM kinase family members contain a kinase domain similar to that of phosphatidylinositol 3-kinase (PI-3 kinase), none of them have been shown to have any lipid kinase activity (13). Instead, a number of ATM family members, including FRAP, DNA-PK, and ATM, display protein kinase activity (3, 4, 11, 16). In addition, immunofluorescence studies using an anti-ATM antibody have shown that ATM is ubiquitously expressed in all murine tissues and is mainly localized in the nucleus, consistent with the notion that ATM may be involved in the detection of DNA strand break damage and in the activation of cell cycle checkpoints following DNA strand break damage (6).
To clarify the function of ATM and create a mouse model to study the basis of the pleiotropic defects in A-T patients, we disrupted the Atm gene in mice through homologous recombinations (33). Mice homozygous for this mutation express most of the A-T phenotypes, including neural degeneration, growth retardation, abolished germ cell development, immune defects, and a high incidence of thymic lymphomas (19, 31). Furthermore, primary cells derived from the Atm−/− mice displayed cellular defects characteristic of A-T, including hypersensitivity to γ-irradiation and defective cell cycle G1/S and S-phase checkpoint control following γ-irradiation (3, 33). In addition, Atm−/− mouse embryonic fibroblasts (MEFs) exhibit defective cellular proliferation, inefficient G1- to S-phase cell cycle progression and premature senescence in culture (33). Similar systematic and cellular defects have been reported in two independently generated Atm−/− mouse strains (1, 8). Therefore, Atm plays important roles in both cellular responses to strand break damage and normal cellular growth.
p53 is required for the cell cycle G1 arrest following γ-irradiation (18). The impaired p53 response to γ-irradiation in Atm−/− cells could account for the defective cell cycle G1 arrest in these cells (15, 17, 33). In addition, the increased constitutive level of p21CIP1/WAF1 observed in Atm−/− MEFs might account for the cellular proliferative defects observed in these mutant cells because p21 is involved in the inhibition of G1- to S-phase cell cycle progression (5, 7, 30). To test the roles of the defective p53 response and the abnormal p21 protein level in the cellular and developmental defects observed in Atm−/− mice, Atm−/− p21−/− and Atm−/− p53−/− mice were generated.
The majority of Atm−/− p53−/− mice die embryonically. The born double mutant mice are runted and develop lymphomas sometimes of both B and T origin by 2 months of age, indicating a synergy of the Atm and p53 mutations in tumorigenesis since Atm−/− mice develop thymic lymphomas by 4 months of age. Similar to p53−/− cells, Atm−/− p53−/− cells are completely defective in their cell cycle G1 arrest following γ-irradiation, indicating that the impaired but not abolished p53 response in Atm−/− cells contributes to the partial cell cycle G1 arrest following γ-irradiation in these cells (33). However, this synergy in tumorigenesis is not observed in Atm−/− p21−/− mice. The development of Atm−/− p21−/− mice is grossly similar to that of Atm−/− mice. However, when compared with wild-type and p21−/− MEFs, the Atm−/− p21−/− MEFs proliferate normally at both low and high passages, consistent with the notion that the increased constitutive level of p21 in Atm−/− MEFs causes the cellular proliferation defects observed in these cells. In addition, because little p21 is observed in Atm−/− p53−/− MEFs, the increased p21 protein level in Atm−/− MEFs is p53 dependent and likely due to a more stable p21 protein, because the p21 mRNA level in Atm−/− MEFs is lower instead of higher than that in Atm+/+ MEFs.
MATERIALS AND METHODS
Generation of Atm−/− p21−/− and Atm−/− p53−/− mice.
The p21−/− and p53−/− mice were described previously (5, 14). Because Atm−/− mice are sterile (31), the p21−/− mice were bred with Atm+/− mice to generate Atm+/− p21+/− mice, which were intercrossed to generate Atm+/− p21−/− mice. The Atm+/− p21−/− mice were then intercrossed to generate Atm−/− p21−/− mice. A similar breeding scheme was attempted to generate Atm−/− p53−/− mice but attempts to intercross Atm+/− p53−/− mice have failed. So Atm+/− p53+/− mice were intercrossed instead to generate Atm−/− p53−/− mice.
Flow cytometric analysis of thymocytes.
Thymi were surgically removed from mice and single-cell suspensions were prepared as previously described (31). For two-color flow cytometric analysis, one-half million cells were silmutaneously stained with phycoerythrin-conjugated anti-CD4 antibody and fluorescein isothiocyanate (FITC)-conjugated anti-CD8 antibody. After being washed with staining buffer (3% fetal bovine serum [FBS] in phosphate-buffered saline [PBS]), stained cells were analyzed using a FACScan (Becton-Dickinson) and CellQuest software. All antibodies were obtained from Pharmingen.
Generation and culture of MEFs.
MEFs were derived from day 14 or day 16 embryos as previously described (33). The Atm−/− p21−/− or Atm−/− p53−/− MEFs were derived from embryos obtained through the intercrosses of Atm+/− p21−/− mice or Atm+/− p53+/− mice, respectively. MEFs were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, 5 mM glutamine, 50 μM β-mecaptoethanol, 50 U of penicillin per ml, and 50 U of streptomycin per ml at 37°C with 5% CO2.
γ-Irradiation treatment of MEFs and cell cycle analysis.
MEFs were synchronized at the G0 phase of the cell cycle by culturing in medium supplemented with 0.1% FBS for 96 h as previously described (7). The G0-synchronized cells were trypsinized and irradiated in suspension with a 137Cs γ-ray source. Subsequently, the irradiated and untreated MEFs were plated in 10-cm-diameter plates at a density of 0.8 × 106 to 1 × 106 cells/plate in normal growth medium supplemented with 10 μM bromodeoxyuridine (BrdU). After 24 h of BrdU labeling, cells were harvested, fixed in 70% ethanol, and stored at −20°C until analysis.
The analyses of DNA content and synthesis were performed as previously described (33). DNA content was revealed by staining with propidium iodide, and DNA synthesis was revealed by staining with FITC-conjugated anti-BrdU antibody (Southern Biotech., Inc.). The stained cells were analyzed using a FACScan and the CellQuest program as previously described (33).
Northern blot analysis.
Total RNA was prepared from harvested MEFs with Trizol reagent (Sigma) according to the manufacturer’s protocol. Total RNA (15 μg) was electrophoresed on a 17.5% formaldehyde-1% agarose gel and was transferred to a nylon membrane (Amersham) as previously described (32). The full-length mouse p21 cDNA was used as a probe to hybridize to the membrane as previously described (21). To standardize the amount of RNA loaded into each lane, the same filter was stripped and hybridized with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe as previously described (32). The hybridized filter was sequentially exposed to both X-ray films and PhosphorImager screens. The amount of p21 and GAPDH mRNA in each sample was quantitated with an ImageQuant program (Molecular Dynamics).
Assays for in vitro cellular proliferation and G1/S cell cycle progression.
Cellular proliferation assays were performed as previously described (7, 33). Briefly, 105 MEFs were plated onto each 35-mm-diameter plate, and each day after plating, MEFs from three plates of each genotype were trypsinized and counted with a hematocytometer. To synchronize MEFs at G0, a subconfluent culture was washed with PBS and placed in DMEM containing 0.1% FBS for 96 h (7). The synchronized MEFs were harvested and released into DMEM supplemented with 10% FBS and 10 μM BrdU for 24 h. Cells in S phase were analyzed using flow cytometry as described above.
Western blot analysis.
Western blot analysis of p21 protein levels in MEFs was performed as previously described (33). Protein extracts derived from 3 × 105 cells were loaded into each lane, separated by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes (Schleicher and Schuell). The filter was probed with a polyclonal rabbit anti-p21 antibody obtained from Santa Cruz Biochemicals, and then was incubated with horseradish peroxidase-conjugated secondary antibody and developed with enhanced chemiluminescence (Amersham). To verify that an equivalent amount of protein was loaded into each lane, the same filter was subsequently probed with a polyclonal rabbit anti-tubulin antibody (ICN Biomedicals, Inc.) and developed as described above.
RESULTS
Developmental phenotypes in Atm−/− p53−/− and Atm−/− p21−/− mice.
Because p53−/− Atm+/− mice are not healthy and usually develop testicular sarcomas, attempts to use these mice to generate Atm−/− p53−/− mice have failed so far. (Testicular sarcomas are also a common malignancy found in the strain of p53−/− mice that we used [14].) Therefore, Atm+/− p53+/− mice were intercrossed to generate Atm−/− p53−/− mice. While the predicted frequency of Atm−/− p53−/− mice in the offspring of this intercross should be 1/16, only about 2% of over 350 offspring genotyped were Atm−/− p53−/− mice, suggesting that about 60% of these double mutant mice die prenatally. In addition, the born Atm−/− p53−/− mice are apparently runted at the weaning age and thus the prevalence of growth retardation phenotypes is hard to judge.
To generate Atm−/− p21−/− mice, Atm+/− p21−/− mice were generated and intercrossed. Consistent with the predicted frequency of 1/4, about 25% of the offspring from the Atm+/− p21−/− crosses were double mutant animals, indicating that there is no prenatal death during the early development of the double mutant mice (data not shown).
Atm−/− mice exhibit several developmental defects, including growth retardation and defective T-cell development (31). While the Atm−/− p53−/− mice are runted and appear unhealthy by the weaning age, the Atm−/− p21−/− mice are healthy at this stage of development and were studied for this spectrum of developmental defects. Similar to Atm−/− mice (31), the 1- to 2-month-old Atm−/− p21−/− mice are also growth retarded with a body weight about 79% ± 7% of that of the p21−/− control mice (data derived from six sets of Atm−/− p21−/− and Atm+/+ p21−/− mice).
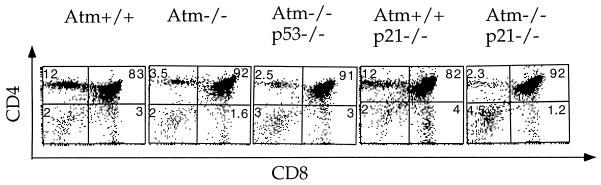
T-cell developments in Atm−/− p21−/− mice were analyzed using flow cytometry and cell counting. While the total number of thymocytes in Atm−/− mice is on average 40% ± 10% of that of Atm+/+ control mice (33), the total number of thymocytes in the Atm−/− p53−/− and Atm−/− p21−/− mice in this study averaged 50% ± 5% and 52% ± 12%, respectively, of those of p53−/− and p21−/− control mice (data derived from four sets of Atm−/− p53−/− and p53−/− control mice and seven sets of 1- to 2-month-old Atm−/− p21−/− and Atm+/+ p21−/− control mice). In addition, when determined by flow cytometry for the expression of CD4 and CD8 surface markers, CD4+ and CD8+ single-positive mature thymocytes were still significantly reduced in the thymi of Atm−/− p21−/− and Atm−/− p53−/− mice compared to those of Atm+/+ p21−/− and Atm+/+ p53−/− control mice, respectively (Fig. 1). Therefore, the thymocyte differentiation from the CD4+ CD8+ stage to the CD4+ or CD8+ stage is consistently defective in Atm−/−, Atm−/− p21−/−, and Atm−/− p53−/− mice.
FIG. 1.
Flow cytometric analysis of T-cell development in 1- to 2-month-old Atm+/+, Atm−/−, Atm−/− p53−/−, Atm+/+ p21−/−, and Atm−/− p21−/− mice. Cells residing in the lymphoid gate were analyzed and the percentages of total cells in a particular gate are indicated. Consistent data were obtained from two Atm−/− p53−/− mice and seven sets of Atm+/+ p21−/− and Atm−/− p21−/− mice.
Lymphomas in Atm−/− p53−/− and Atm−/− p21−/− mice.
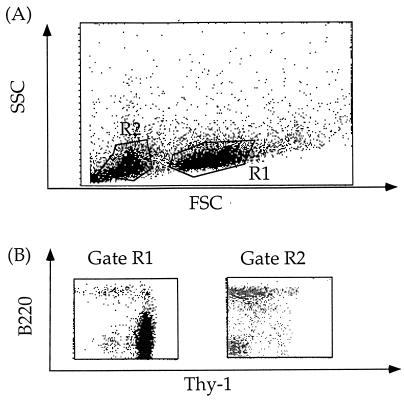
While Atm−/− mice invariably develop thymic lymphomas by 4 months of age, seven Atm−/− p53−/− mice developed thymic and/or peripheral lymphomas by 2 months of age. When analyzed with flow cytometry, three of the seven lymphomas derived from Atm−/− p53−/− mice appear to have been composed of two populations of tumor cells of different sizes (Fig. 2A). When the analysis was gated on these two populations of tumor cells, the smaller tumor cells appeared to be mainly composed of B220+ cells, which are also Thy-1− CD4−, indicating that they are phenotypically of B-cell origin (Fig. 2B and data not shown). The larger tumor cells expressed some T-cell markers, including CD4 and Thy-1, but were B220−, suggesting that they were of T-cell origin (Fig. 2B and data not shown). Therefore, the Atm−/− p53−/− mice can develop lymphomas of both B- and T-cell origin.
FIG. 2.
FACScan profile of lymphomas in Atm−/− p53−/− mice. Peripheral lymphomas derived from a 6-week-old Atm−/− p53−/− mouse were analyzed for cell size (A) and surface expression of pan-T-cell marker Thy-1 and pan-B-cell marker B220 (B). The plot of the forward scatter (FSC) versus the sizing scatter (SSC) indicates two populations of uniform lymphoma cells in the tumor, as indicated by R1 and R2 gates.
Similar to Atm−/− mice, Atm−/− p21−/− mice invariably develop thymic lymphomas by 5 months of age, and no other malignancies have been detected in these animals at an elevated frequency. In addition, similar to the thymic lymphoma cells derived from Atm−/− mice, the thymic lymphoma cells observed in Atm−/− p21−/− mice are mostly of CD4+ CD8+ immature T-cell origin (data not shown) (31).
Cell cycle G1 arrest following γ-irradiation in Atm−/− p53−/− and Atm−/− p21−/− MEFs.
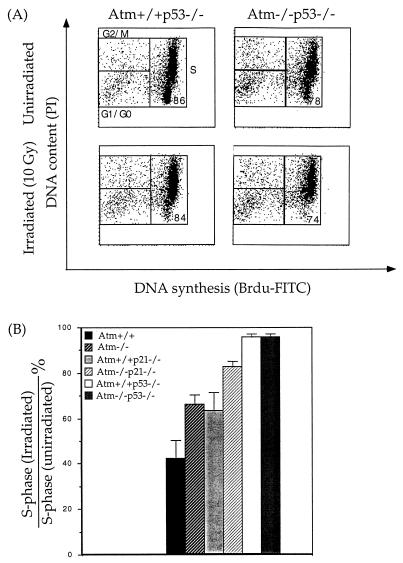
In response to γ-irradiation, Atm−/− MEFs are partially defective in their cell cycle G1 arrest, presumably due to a greatly reduced and delayed p53 upregulation in response to γ-irradiation (33). In addition, the constitutively higher basal protein level of p21 in Atm−/− MEFs might also affect the cell cycle G1 arrest of these cells in response to γ-irradiation (33). To test these possibilities, the cell cycle G1 arrest following γ-irradiation in Atm−/− p53−/− and Atm−/− p21−/− MEFs was evaluated as previously described (7). Briefly, MEFs synchronized at G0 by serum starvation were treated with 0 or 10 Gy of γ irradiation and released into medium supplemented with 10% FBS and 10 μM BrdU. After 24 h, the percentages of S-phase cells in the irradiated and untreated MEFs were assayed with flow cytometry as previously described (31) (Fig. 3A). Following γ-irradiation, there is essentially no cell cycle G1 arrest in p53−/− and p53−/− Atm−/− MEFs while there is more than 50% reduction of S-phase cells in wild-type MEFs (Fig. 3B). In addition, the Atm−/− p21−/− MEFs are more severely defective in their cell cycle G1 arrest following γ-irradiation than p21−/− and Atm−/− MEFs (Fig. 3B).
FIG. 3.
Cell cycle G1 arrest following γ-irradiation in MEFs of various genotypes. (A) Representative FACScan profile of untreated and irradiated Atm+/+ p53−/− and Atm−/− p53−/− MEFs. The 2N and 4N DNA contents were revealed by propidium iodide (PI) staining, and DNA synthesis was revealed by staining with FITC-conjugated anti-BrdU antibody. Cells residing in G0/G1, S, and G2/M phases are indicated by boxes. The percentages of S-phase cells are also indicated. (B) Quantitative analysis of the reduction of S-phase cells following γ-irradiation in Atm+/+, Atm+/+ p21−/−, Atm−/− p21−/−, Atm+/+ p53−/−, and Atm−/− p53−/− MEFs. Two independent experiments were performed and in each experiment, three irradiated samples of MEFs of each genotype (except in the case of Atm−/− MEFs, for which one set of samples is involved) and untreated controls were compared. Mean values with standard derivations (indicated by error bars) are presented.
Growth properties of Atm−/− p21−/− and Atm−/− p53−/− MEFs.
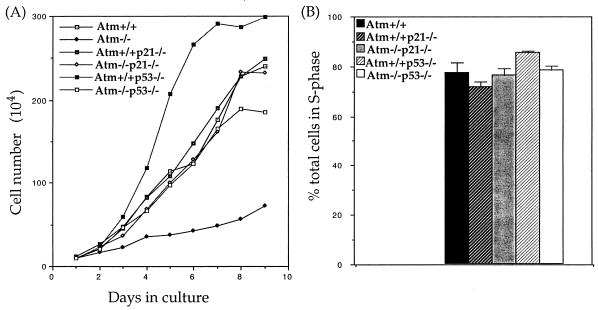
The Atm−/− MEFs exhibit defects in cellular proliferation, including slower proliferation rate, lower saturation density, inefficient G1- to S-phase cell cycle progression, and premature senescence, possibly due to the increased basal level of p21 in these mutant cells (33). Therefore, the cellular proliferation of Atm−/− p21−/− and Atm−/− p53−/− MEFs were examined as previously described (33). At earlier passages, Atm−/− p21−/−, Atm−/− p53−/−, p21−/−, and wild-type MEFs proliferate similarly and reach similar saturation densities, while as expected, Atm−/− MEFs proliferate more slowly (Fig. 4A). In addition, Atm−/− p21−/− and Atm−/− p53−/− MEFs are capable of proliferating at high passages (passage 6), while Atm−/− MEFs are senescent (data not shown) (26, 33).
FIG. 4.
In vitro cellular proliferation and G1/S-phase cell cycle progression following serum stimulation of MEFs of various genotypes. (A) Proliferation curves and saturation densities of Atm+/+, Atm−/−, Atm+/+ p21−/−, Atm−/− p21−/−, Atm+/+ p53−/−, and Atm−/− p53−/− MEFs at passage 3. The cell numbers represent the averages of three plates counted at each time point. (B) Quantitative analysis of the percentage of S-phase cells after serum stimulation for 24 h of G0-synchronized Atm+/+, Atm+/+ p21−/−, Atm−/− p21−/−, Atm+/+ p53−/−, and Atm−/− p53−/− MEFs. The BrdU-labeled MEFs were analyzed with flow cytometry, and the S-phase cells were revealed by staining with anti-BrdU antibody. Three independent experiments were performed and the mean values with standard derivations (indicated by error bars) are presented.
The cell cycle G1- to S-phase progression was examined as previously described (33). Atm+/+, Atm+/+ p21−/−, Atm−/− p21−/−, Atm+/+ p53−/−, and Atm−/− p53−/− MEFs synchronized at G0 through serum starvation were serum stimulated for 24 h, and the percentages of cells in S phase were determined with flow cytometry. Similar percentages of S-phase cells were detected in all the MEF samples tested (Fig. 4B). Therefore, all the cellular proliferation defects tested in Atm−/− MEFs are rescued in Atm−/− p53−/− and Atm−/− p21−/− MEFs.
p21 protein and mRNA levels in various MEFs.
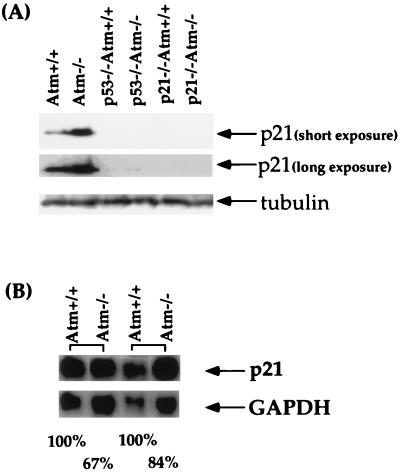
To test whether the increased basal protein level of p21 in Atm−/− mice is dependent on p53, the p21 protein level in Atm−/− p53−/− MEFs was analyzed by Western blotting. As described previously, a much higher level of p21 was detected in Atm−/− MEFs than Atm+/+ MEFs (Fig. 5A) (31). However, little p21 protein was detected in both p53−/− and Atm−/− p53−/− MEFs (Fig. 5A). As expected, no p21 could be detected in Atm−/− p21−/− and Atm+/+ p21−/− MEFs (Fig. 5A).
FIG. 5.
p21 protein and mRNA levels in MEFs of various genotypes. (A) p21 protein levels in Atm+/+, Atm−/−, Atm+/+ p53−/−, Atm−/− p53−/−, Atm+/+ p21−/−, and Atm−/− p21−/− MEFs. Both the short exposure and the long exposure of the same Western blot are presented. The genotypes are shown at the top. p21 and the β-tubulin are indicated by arrows. (B) p21 mRNA levels in Atm−/− and Atm+/+ control MEFs. The p21 mRNA and loading control GAPDH are indicated by arrows. The genotypes are indicated at the top. The levels of p21 and GAPDH mRNA in each sample were quantitated using an ImageQuant program with a PhosphorImager (Molecular Dynamics). After the p21 mRNA level of each sample was standardized with its GAPDH mRNA level, the percentile ratio of the p21 mRNA level in Atm−/− MEFs versus that in Atm+/+ MEF controls was determined and is indicated at the bottom.
To test whether the increased level of p21 protein in Atm−/− MEFs is due to a higher level of p21 mRNA in these cells, total RNA was prepared from passage 3 Atm−/− and Atm+/+ control MEFs and analyzed by Northern blotting using the full-length mouse p21 cDNA as a probe (21). While the p21 mRNA could be easily identified in both Atm+/+ and Atm−/− MEF samples, a lower p21 mRNA level was detected in the Atm−/− MEF samples compared to that of Atm+/+ MEF controls derived from the same pregnant female, indicating that the increased level of constitutive p21 protein in Atm−/− MEFs is not due to the higher p21 mRNA level in these cells (Fig. 5B). Consistent data were obtained from an additional two sets of Atm+/+ and Atm−/− MEFs (data not shown).
DISCUSSION
Atm−/− cells show an impaired p53 upregulation in response to γ-irradiation and an increased level of p21, either or both of which could be responsible for their cellular defects and increased tumorigenesis. To study this issue, we generated Atm−/− p53−/− and Atm−/− p21−/− mice and derived MEFs from them. The cellular proliferative defects observed in Atm−/− MEFs are absent in Atm−/− p21−/− and Atm−/− p53−/− MEFs. In addition, the cell cycle G1 checkpoint response to γ-irradiation is more severely defective in Atm−/− p53−/− and Atm−/− p21−/− MEFs than in Atm−/− MEFs. While the developmental defects and tumorigenesis in the Atm−/− p21−/− mice are similar to those in Atm−/− mice, the majority of Atm−/− p53−/− mice die prenatally and the surviving Atm−/− p53−/− mice develop tumors earlier than the Atm−/− and p53−/− mice, suggesting a cooperation of Atm and p53 in mouse embryonic development as well as in tumor suppression.
An increased p21 protein level can inhibit cell cycle G1/S transition and is also correlated with cellular senescence (10, 23–25, 30). Therefore, an increased constitutive p21 protein level in Atm−/− MEFs could account for the cellular proliferative defects, including slower proliferation, inefficient cell cycle G1/S progression, and premature senescence (33). Consistent with this notion, none of the cellular proliferative defects observed in Atm−/− MEFs were evident in Atm−/− p21−/− MEFs, indicating that the increased constitutive p21 level is indeed responsible for the cellular proliferative defects observed in Atm−/− cells. In addition, the proliferative defects observed in Atm−/− MEFs are also rescued in Atm−/− p53−/− MEFs, probably due to the fact that a minimum level of p21 is expressed in any p53−/− MEFs. Thus, the p21 expression in cycling Atm−/− MEFs is apparently p53 dependent, just as it is in wild-type cells. Since in normal-cycling MEFs, p53 is thought to transcriptionally activate p21 mRNA expression through binding to the two p53 binding sites in the p21 promoter region, the regulation of p21 expression by p53 in Atm−/− MEFs is most likely at the transcriptional level (21).
The increased p21 level in the Atm−/− MEFs might be due to either a higher basal activity of the p53-p21 pathway leading to an increased level of p21 mRNA or a more stable p21 protein in the Atm−/− MEFs. However, a lower level of p21 mRNA was detected in the Atm−/− MEFs than in the Atm+/+ control MEFs, indicating that the higher level of constitutive p21 protein in the Atm−/− MEFs is not due to a higher p21 mRNA level in these cells but is likely due to the increased stability of p21 protein in these cells. This finding is also consistent with the notion that ATM might be involved in the maintenance of the p53 protein level not only after excessive DNA damage but also during normal cellular proliferation.
While the cellular proliferative defects in Atm−/− cells are rescued in Atm−/− p21−/− cells, the growth retardation observed in Atm−/− mice is not rescued in the Atm−/− p21−/− mice because these double mutant mice are still smaller than their p21−/− control littermates. Therefore, the growth retardation in Atm−/− mice cannot be due solely to the cellular proliferative defects in Atm−/− cells. Instead, other potential defects, such as the abnormal production of growth factors due to the neural defects in Atm−/− mice, might account for the growth retardation in Atm−/− mice (19). In addition, there is a significant reduction of the total number of thymocytes in Atm−/− mice, possibly due to defective thymocyte proliferation or impaired V(D)J recombination or both (31). However, in contrast to the findings obtained for the Atm−/− p21−/− and Atm−/− p53−/− MEFs, there is no apparent rescue of thymus cellularity in the Atm−/− p21−/− and Atm−/− p53−/− mice, indicating that p53 and p21 are not involved in the thymus hypoplasia in Atm−/− mice. However, this does not rule out the possibility that defective thymocyte proliferation contributes to the thymus hypoplasia in Atm−/− mice.
The cell cycle G1 arrest following γ-irradiation is abolished in p53−/− MEFs but is partially defective in Atm−/− MEFs (15, 17, 18, 33). Consistent with the notion that the cell cycle G1 arrest in response to γ-irradiation is p53 dependent (18), the p53 upregulation in response to γ-irradiation in Atm−/− cells is greatly impaired and delayed (15, 17, 33). Therefore, our findings that Atm−/− p53−/− MEFs are completely deficient in their cell cycle G1 arrest in response to γ-irradiation indicate that the residual p53 response to γ-irradiation in Atm−/− cells contributes to the partial G1 cell cycle arrest in Atm−/− MEFs. Thus, in response to DNA strand break damage, ATM plays a major role in signaling the p53 upregulation but there exist ATM-independent signaling pathways that can partially compensate for this ATM activity.
While ATM plays a major role in signaling the p53 response to γ-irradiation, the Atm−/− p53−/− mice develop lymphomas earlier than Atm−/− mice, indicating that mutations in these two genes can cooperate in tumorigenesis. Two observations could account for this synergy in tumorigenesis. First, the cell cycle checkpoint defects occurring in response to DNA damage induced by γ-irradiation are more severe in Atm−/− p53−/− cells than in Atm−/− cells. Secondly, the p53-dependent apoptosis of thymocytes in response to γ-irradiation is only partially defective in Atm−/− thymocytes but is completely abolished in Atm−/− p53−/− cells (29, 33). In addition, ATM signals the upregulation of p53 only in response to DNA strand break damage but is not involved in such signaling in the cellular responses to DNA damage induced by UV or base mismatch (33, 33a). Therefore, while only the cellular response to DNA strand break damage is defective in Atm−/− cells, cellular responses to multiple forms of DNA damage and other cellular stresses are defective in Atm−/− p53−/− cells, thus providing the potential basis for the synergy of tumorigenesis in Atm−/− p53−/− mice. The Atm−/− p53−/− mice also develop lymphomas earlier than p53−/− mice (14). Therefore, other defective but p53-independent cellular responses to strand break damage in Atm−/− cells, such as the defective S-phase cell cycle checkpoint which occurs in response to strand break damage, can account for the earlier onset of tumors in Atm−/− p53−/− mice (3).
An independently generated Atm−/− p53−/− mouse line was reported while this paper was in preparation (29). The findings for our Atm−/− p53−/− mice are in general agreement with those for the reported mouse line. While this paper was being reviewed, two reports described the meiosis, tumorigenesis, and apoptosis responses in two independently generated Atm−/− p21−/− mouse lines (2, 28).
ACKNOWLEDGMENTS
This project was partially supported by a National Institute of Health grant to D.B. and grants from AT Childrens Projects to D.B. and Y.X. Y.X. was partly supported by the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation. D.B. is an American Cancer Society Research Professor.
REFERENCES
- 1.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley J N, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 2.Barlow C, Liyanage M, Moens P B, Deng C X, Ried T, Wynshaw-Boris A. Partial rescue of the prophase I defects of Atm-deficient mice by p53 and p21 null alleles. Nat Genet. 1997;17:462–466. doi: 10.1038/ng1297-462. [DOI] [PubMed] [Google Scholar]
- 3.Baskaran R, Wood L D, Whitaker L L, Connon C E, Morgan S E, Xu Y, Barlow C, Baltimore D, Wynshaw-Boris A, Kastan M B, Wang J Y. Ataxia telangiectasia mutant protein activates c-Abl tyrosine kinase in response to ionizing radiation. Nature. 1997;387:516–519. doi: 10.1038/387516a0. [DOI] [PubMed] [Google Scholar]
- 4.Brown E J, Beal P A, Keith C T, Chen J, Shin T B, Schreiber S L. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature. 1995;377:441–446. doi: 10.1038/377441a0. [DOI] [PubMed] [Google Scholar]
- 5.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Lee E. The product of the ATM gene is a 370-kDa nuclear phosphoprotein. J Biol Chem. 1996;271:33693–33697. doi: 10.1074/jbc.271.52.33693. [DOI] [PubMed] [Google Scholar]
- 7.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 8.Elson A, Wang Y, Daugherty C J, Morton C C, Zhou F, Campos-Torres J, Leder P. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc Natl Acad Sci USA. 1996;93:13084–13089. doi: 10.1073/pnas.93.23.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatti R A, et al. Localization of an ataxia-telangiectasia gene to chromosome 11q22-23. Nature. 1988;336:577–580. doi: 10.1038/336577a0. [DOI] [PubMed] [Google Scholar]
- 10.Halevy O, Novitch B G, Spicer D B, Skapek S X, Rhee J, Hannon G J, Beach D, Lassar A B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 11.Hartley K O, Gell D, Smith G C, Zhang H, Divecha N, Connelly M A, Admon A, Lees-Miller S P, Anderson C W, Jackson S P. DNA-dependent protein kinase catalytic subunit: a relative of phosphatidylinositol 3-kinase and the ataxia telangiectasia gene product. Cell. 1995;82:849–856. doi: 10.1016/0092-8674(95)90482-4. [DOI] [PubMed] [Google Scholar]
- 12.Hartwell L H, Kastan M B. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 13.Hunter T. When is a lipid kinase not a lipid kinase? When it is a protein kinase. Cell. 1995;83:1–4. doi: 10.1016/0092-8674(95)90225-2. [DOI] [PubMed] [Google Scholar]
- 14.Jacks T, Remington L, Williams B O, Schmitt E M, Halachmi S, Bronson R T, Weinberg R A. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 15.Kastan M B, Zhan Q, el-Deiry W S, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fornace A J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 16.Keegan K S, Holtzman D A, Plug A W, Christenson E R, Brainerd E E, Flaggs G, Bentley N J, Taylor E M, Meyn M S, Moss S B, Carr A M, Ashley T, Hoekstra M F. The Atr and Atm protein kinases associate with different sites along meiotically pairing chromosomes. Genes Dev. 1996;10:2423–2437. doi: 10.1101/gad.10.19.2423. [DOI] [PubMed] [Google Scholar]
- 17.Khanna K K, Lavin M F. Ionizing radiation and UV induction of p53 protein by different pathways in ataxia-telangiectasia cells. Oncogene. 1993;8:3307–3312. [PubMed] [Google Scholar]
- 18.Kuerbitz S J, Plunkett B S, Walsh W V, Kastan M B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci USA. 1992;89:7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuljis R O, Xu Y, Aguila M C, Baltimore D. Degeneration of neurons, synapses, and neuropil and glial activation in a murine Atm knockout model of ataxia-telangiectasia. Proc Natl Acad Sci USA. 1997;94:12688–12693. doi: 10.1073/pnas.94.23.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmann A R. Pages 83–102. In: Bridges B A, Harnden D G, editors. Ataxia-telangiectasia: a cellular and molecular link between cancer, neuropathology and immune deficiency. Chichester, United Kingdom: Wiley Interscience; 1982. [Google Scholar]
- 21.Macleod K F, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995;9:935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 22.Meyn M S. Ataxia-telangiectasia and cellular responses to DNA damage. Cancer Res. 1995;55:5991–6001. [PubMed] [Google Scholar]
- 23.Nakanishi M, Adami G R, Robetorye R S, Noda A, Venable S F, Dimitrov D, Pereira-Smith O M, Smith J R. Exit from G0 and entry into the cell cycle of cells expressing p21Sdi1 antisense RNA. Proc Natl Acad Sci USA. 1995;92:4352–4356. doi: 10.1073/pnas.92.10.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noda A, Ning Y, Venable S F, Pereira-Smith O M, Smith J R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 25.Parker S B, Eichele G, Zhang P, Rawls A, Sands A T, Bradley A, Olson E N, Harper J W, Elledge S J. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995;267:1024–1027. doi: 10.1126/science.7863329. . (Comments.) [DOI] [PubMed] [Google Scholar]
- 26.Savitsky T, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 27.Shiloh Y. Ataxia-telangiectasia: closer to unraveling the mystery. Eur J Hum Genet. 1995;3:116–138. doi: 10.1159/000472285. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y A, Elson A, Leder P. Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc Natl Acad Sci USA. 1997;94:14590–14515. doi: 10.1073/pnas.94.26.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westphal C H, Rowan S, Schmaltz C, Elson A, Fisher D E, Leder P. atm and p53 cooperate in apoptosis and suppression of tumorigenesis, but not in resistance to acute radiation toxicity. Nat Genet. 1997;16:397–401. doi: 10.1038/ng0897-397. [DOI] [PubMed] [Google Scholar]
- 30.Xiong Y, Hannon G J, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Ashley T, Brainerd E E, Bronson R T, Meyn M S, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Baldassare M, Fisher P, Rathbun G, Oltz E M, Yancopoulos G D, Jessell T M, Alt F W. LH-2: a LIM/homeodomain gene expressed in developing lymphocytes and neural cells. Proc Natl Acad Sci USA. 1993;90:227–231. doi: 10.1073/pnas.90.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Baltimore D. Dual roles of ATM in the cellular response to radiation and in cell growth control. Genes Dev. 1996;10:2401–2410. doi: 10.1101/gad.10.19.2401. [DOI] [PubMed] [Google Scholar]
- 33a.Xu, Y. Unpublished data.
- 34.Zakian V A. ATM-related genes: what do they tell us about functions of the human gene? Cell. 1995;82:685–687. doi: 10.1016/0092-8674(95)90463-8. [DOI] [PubMed] [Google Scholar]