The evaluation of drug safety and efficacy is an expensive, time-consuming process with a high failure rate. Animal testing was the only non-human testing method to assess drug safety and efficacy before clinical trials for nearly a century. The tragic incidents of mass poisoning that occurred when sulfanilamide was formulated into an elixir spurred the enactment in 1938 of the Federal Food, Drug, and Cosmetic Act that mandated all drugs must be tested for toxicity in animals and truthfully labeled1. In 1961, following the thalidomide disaster in other countries, the FDA enforced its own requirement for clinical trials and the need for preclinical validations of toxicity in animal studies. Since then, drug testing in animals has been the only standard recognized by the FDA before drugs are approved for human subject clinical trials. Following these guidelines, the success rate over the last 25 years of all drugs that entered Phase I clinical trials to reach FDA approval is only 9.6%2. Implicit in this failure rate is the inherent poor correlation between animal efficacy and toxicity data and how that adversely affected the success of drug approval in humans. This high failure rate necessitates the need for alternate methods to be instituted as part of the FDA approval process for clinical trials.
On September 29, 2022, the U.S. Senate unanimously passed Bill S. 5002, which was subsequently approved by the U.S. House on December 23, 2022, giving rise to what is known as the “FDA Modernization Act 2.0”. This landmark act is an attempt to update the 1938 mandate by allowing regulators to consider using new approach methods (NAMs) as a legitimate option to establish drug safety and efficacy instead of using animals.
This bill allows the FDA to consider information other than animal studies, shining a spotlight on significant advancements in biology and technology over the past several decades. Some of these include cell-based approaches, such as human induced pluripotent stem cells (iPSCs), organoids and microphysiological systems3, as well as in silico computer-based modeling, artificial intelligence (AI)) and machine learning4. The ability to pool cell lines from many people has created a powerful method called “clinical trials in a dish” which aims to recreate human variability when monitoring drug safety and efficacy5. The selection of the drug molecule and prediction of its pharmacokinetics and pharmacodynamics (PK/PD) using computational approaches may provide more representative predictions of human studies, with the goal of improving the success rate of the drug approval process.
Paramount to entry into clinical trials is an evaluation of the toxicity of the new drug. One principal metric for new drug failure is cardiotoxicity due to drug-induced arrhythmias, which can be tested using iPSC lines differentiated into cardiomyocytes. While the standard is to use preclinical animal toxicology studies to gauge safety for Phase I clinical trials, it is well known that not all animal toxicology studies necessarily predict toxicology in humans. Some toxic effects may be species-specific, such as biologics or, in many cases, do not occur in humans at all. In the latter case, it could lead to false attrition of drug candidates that would otherwise be highly efficacious at treating human diseases.
Human iPSCs, which are generated by reprogramming adult cells back into pluripotent state, can be differentiated into many different cell types. Relevant cell types derived from patient-specific iPSCs, such as cardiomyocytes, hepatic, or kidney cells, can be used in human-specific models for toxicity testing, generating more accurate results than those produced by animal models. iPSC-derived models can also be used to study the effects of drugs in specific patient populations, such as individuals with genetic mutations or rare diseases5. Using iPSC-derived cardiomyocytes from patients exhibiting LMNA-related dilated cardiomyopathy (DCM) phenotype, we showed improvement in cardiomyocyte function when co-cultured with iPSC-endothelial cells and lovastatin. The study suggests that impaired crosstalk between endothelial cells and cardiomyocytes can contribute to the pathogenesis of LMNA-related DCM, and statin may be an effective therapy for vascular dysfunction in patients with cardiolaminopathy. When these patients were subsequently treated with lovastatin, they showed improvements in endothelial dysfunction. Ethnic variations are also important considerations in drug development as different ethnic groups may respond differently to the same drug due to genetic and physiological differences, sometimes involving adverse effects.
Computational modeling and simulation, applied in structure-based drug design and medical device development, have played pioneering roles in validating the predictive and practical utility of in silico-based methods. The advent of AI has emerged as a transformative force in the field of drug discovery, facilitating the creation of meticulously designed molecules and revolutionizing drug development by preemptively predicting toxicity, thereby reducing the reliance on animal testing. This will lead to more efficient and ethically responsible drug development practices. To that effect, FDA is adopting AI and using “modeling and simulations” for model-informed drug development. FDA’s Center for Drug Evaluation and Research is using quantitative clinical pharmacology, and structure-based approaches to assess the risk of new drugs or drug-impurities that pose risks to the public. Further advancements in in “generative” AI now enable optimization and design of novel drug molecules by accurately predicting their structural features, as well as their potential effectiveness and toxicities. These predictions are based on thorough analysis of chemical structures and their interactions with biological targets. AI can now also optimize clinical trial design and patient selection by analyzing large datasets of patient information, iPSC toxicity, and efficacy studies; this allows the prediction of patients who are most likely to benefit from a particular drug and those who may experience adverse reactions.
Integrating iPSCs, AI, and computational biology into process pipeline is transforming drug discovery and development from what used to be slow and iterative to expedited yet precise process. Advances in “clinical trials in a dish” combining computational biology and human iPSCs may revolutionize drug development and reduce the human risks by providing more relevant human-specific toxicity data. Therefore, embracing these technologies in drug development efforts promise swift identification of effective and safe therapies for patients in need (Figure). The United States Congress has recognized it and FDA regulators are keen to adopt many of these new alternative approaches for drug development.
Figure.
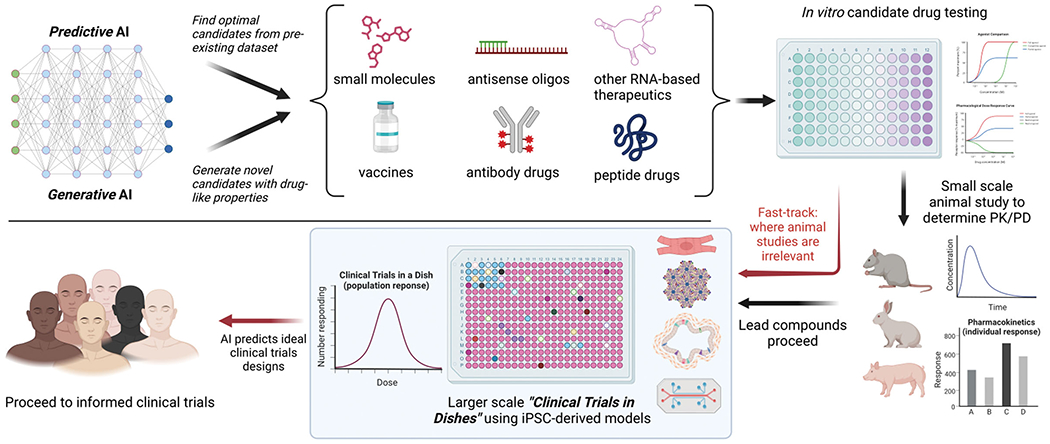
Integration of AI and “Clinical Trials in Dishes” using iPSC-derived models in the drug discovery and development pipeline. The FDA Modernization Act 2.0 facilitates the adoption of innovative approaches for conducting safer and more efficient clinical trials. Created with BioRender.com.
Footnotes
Conflicts of Interest Disclosure: JCW is a co-founder of Greenstone Biosciences. SMA and RVS are paid employees at Greenstone Biosciences, a company that engages in drug discovery using computational modeling and iPSC technology.
References:
- 1.Wax PM. Elixirs, diluents, and the passage of the 1938 federal food, drug and cosmetic act. Ann Intern Med. 1995; 122:456–461. [DOI] [PubMed] [Google Scholar]
- 2.Mullard A. 2022 FDA approvals. Nat Rev Drug Discov. 2023; 22:83–88. [DOI] [PubMed] [Google Scholar]
- 3.Cho S, Discher DE, Leong KW, Vunjak-Novakovic G, Wu JC. Challenges and opportunities for the next generation of cardiovascular tissue engineering. Nat Methods. 2022; 19:1064–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang A, Xing L, Zou J, Wu JC. Shifting machine learning for healthcare from development to deployment and from models to data. Nat Biomed Eng. 2022; 6:1330–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sayed N, Liu C, Ameen M, Himmati F, Zhang JZ, Khanamiri S, Moonen JR, Wnorowski A, Cheng L, Rhee JW, Gaddam S, Wang KC, Sallam K, Boyd JH, Woo YJ, Rabinovitch M, Wu JC. Clinical trial in a dish using iPSCs shows lovastatin improves endothelial dysfunction and cellular cross-talk in LMNA cardiomyopathy. Sci Transl Med. 2020; 12:eaax9276. [DOI] [PMC free article] [PubMed] [Google Scholar]


