Abstract
Human pluripotent stem cells (hPSCs), in particular embryonic stem cells and induced pluripotent stem cells, have received enormous attention in cardiovascular regenerative medicine, owing to their ability to expand and differentiate into functional cardiomyocytes and other cardiovascular cell types. Despite the potential applications of hPSCs for tissue regeneration in patients suffering from cardiovascular disease, whether hPSC-based therapies can be safe and efficacious remains inconclusive, with strong evidence from clinical trials lacking. Critical factors limiting therapeutic efficacy are the degree of maturity and purity of the hPSC-derived differentiated progeny, and the tumourigenic risk associated with residual undifferentiated cells. In this Review, we discuss recent advances in cardiac-cell differentiation from hPSCs and in the direct reprogramming of non-myocyte cells for cardiovascular regenerative applications. We also discuss approaches for the delivery of cells to diseased tissue, and how such advances are contributing to progress in cardiac tissue engineering for tackling heart disease.
Pluripotency is defined as the capacity of cells to differentiate into multiple mature lineages, such as cardiomyocytes, hepatocytes, neurons or blood cells. When considering their sources, hPSCs ― which have the ability to form all adult cell types ― can be divided in two groups: human embryonic stem cells (hESCs), derived from the embryo’s inner cell mass at the blastocyst stage after in vitro fertilization or somatic nuclear transfer; and human induced pluripotent stem cells (hiPSCs), generated via the ectopic expression of defined transcription factors1,2. Both hESCs and hiPSCs have unlimited self-renewal potential and therefore represent potentially unrestricted cell sources for the production of tissues for regenerative medicine3. However, their high capacity for self-renewal comes with safety concerns, owing to the possible formation of teratomas and to the instability of their genetic material4. A major challenge of stem cell therapy is to eliminate these risks in preclinical studies before the initiation of clinical trials.
The successful application of hPSCs as clinical therapy requires control over the maintenance of their pluripotent phenotype and their bona fide differentiation into terminal lineages. hPSCs can retain their pluripotency and self-renewal capacity when cultured in maintenance media containing self-renewal factors. Withdrawal of these factors then triggers the spontaneous differentiation of hPSCs into a mixture of cells representing all three germ layers. Directed differentiation can thus be evoked when pluripotency maintenance media are replaced with differentiating media supplemented with factors that activate or inhibit particular intracellular signalling pathways. During this process, cells lose their pluripotency and self-renewal capacity, and acquire cell-type-specific functions and markers. Different factors can be added into differentiation media to mimic the embryonic developmental signalling to obtain specific cell types5.
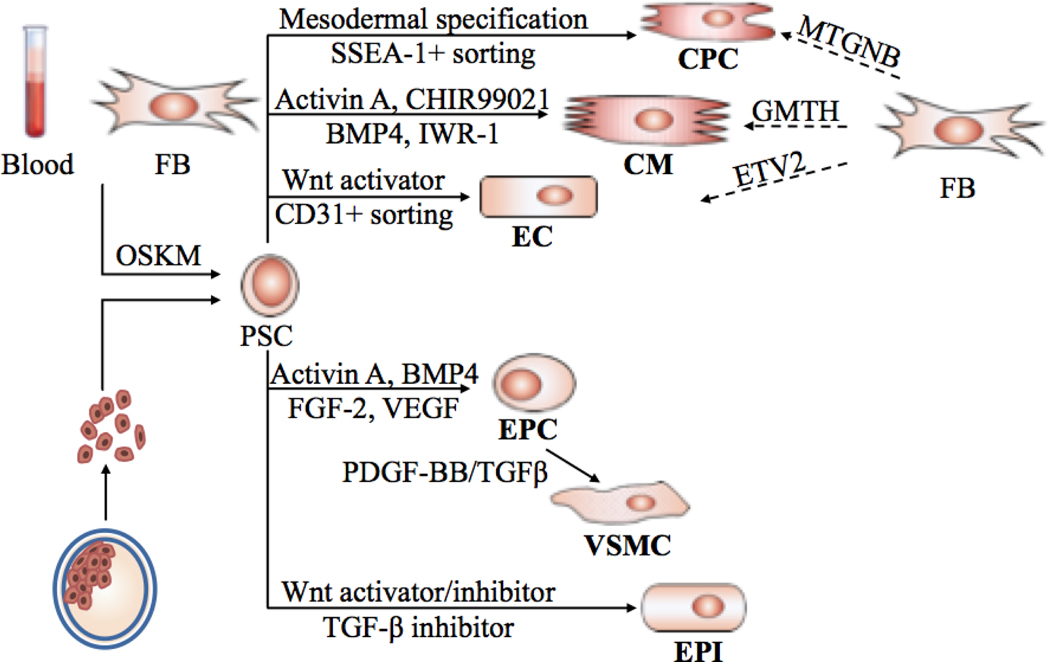
The mammalian heart consists of many cell types, including atrial and ventricular cardiomyocytes, cardiac fibroblasts, endothelial cells, smooth muscle cells, and cells forming the conduction system such as pacemaker cells and Purkinje fibres. Given that cell death is a major aspect in tissue damage during injury and heart failure, cellular replacement using hPSC-derived cardiac cells is a promising strategy for stem-cell-based regenerative therapy. In this Review, we discuss the use of hPSCs and their progeny for the repair of the cardiovascular system, and summarize the in vitro cardiac-lineage differentiation protocols developed to date. We also discuss the direct reprogramming of non-myocytes into cardiomyocytes or cardiac progenitors (Fig. 1).
Fig. 1. Pluripotent stem cell therapy for cardiovascular regeneration.
Patient-specific hiPSCs reprogrammed from somatic cells or hESCs derived from fertilized embryos are differentiated into cells with different cardiac lineages: cardiomyocytes (CMs), endothelial cells (ECs), cardiac progenitor cells (CPCs), endothelial progenitor cells (EPCs), epicardial cells (EPIs) and vascular smooth muscle cells (VSMCs). Fibroblasts (FBs) are reprogrammed directly into CMs, CPCs or ECs via transfection of transcription factors. OSKM: Oct3/4, Sox2, Klf4, and c-Myc. MTGNB: Mesp1, Tbx5, Gata4, Nkx2.5, and Baf60c. GMTH: Gata4, Mef2c, Tbx5, and Hand2. ETV2, ETS variant 2.
hPSC-derived cardiomyocytes for cardiac repair
One of the most studied cell types are hPSC-derived cardiomyocytes (hPSC-CMs), which have shown promising results in multiple studies for improving cardiac function in animal models6–10. Several key steps are involved in the preparation of hPSC-CMs for transplantation, including cardiomyocyte differentiation, purification and maturation. The efficiency of cardiomyocyte differentiation has improved dramatically over the past decade, rising from 1–10% efficiency using the embryoid-body formation protocol11 to >90% using the monolayer protocol combined with metabolic purification to remove undifferentiated cells12,13. Most cardiomyocyte-differentiation protocols involve the modulation of signalling pathways involving Wnt, Activin A or bone morphogenetic protein-4 (BMP-4) in order to recall early cardiac development5. Although the differentiation and purification of hPSC-CMs has been progressively optimized, successful and efficient maturation has thus far eluded the field. hPSC-CMs show immature phenotypes, as measured by low sarcomere alignment, small action potentials in electrophysiology assessments, and fetal-like transcriptome profiles. Importantly, hPSC-CMs can exhibit heterogeneous electrophysiological phenotypes and spontaneous automaticity, carrying a risk of life-threatening arrhythmias when transplanted8,14.
The challenges of controlling hPSC-CM maturation have been tackled with a variety of approaches, including targeting internal pathways, recreating the optimal environment, and providing proper physical stimulation (Box 1). To investigate differences between mature and immature hPSC-CMs, hESC-CMs have been cultured for over a year and have been found to have let-7 as the most highly upregulated microRNA. Consequently, overexpression of let-7 family members in hESC-CMs enhanced cell size, sarcomere length, the force of contraction, and respiratory capacity15. Additionally, for maturation of hPSC-CMs, metabolic approaches have been used to increase the use of fatty acids as the energy source. On the basis of metabolic differences between hPSC-CMs and primary cardiomyocytes, insulin, dexamethasone and 3-isobutyl-1-methylxanthine were found to drive metabolic maturation via the increase of mitochondrial oxidation, lipogenesis and lipolysis16. Nevertheless, these approaches only target single phenotypic features and cannot generate cells that are as mature as adult human cardiomyocytes.
Box 1. Four main strategies used to replicate the environment of cardiomyocytes to promote maturation of cardiomyocytes derived from pluripotent stem cells.
| Cell Biology Approaches • Extended Culture • Co-Culture ○ Endothelial cells ○ Fibroblasts • Cell Fusion ○ To form larger and multinucleated cells |
Genetic Approaches • Overexpression of cardiac genes ○ Ion channels or calcium handling proteins (e.g., Kir 2.1, calsequestrin) • Gene expression network perturbation ○ MicroRNA (e.g., let 7, miR-1) |
| Biophysical approaches • Tissue engineering • Electric pacing • Mechanical loading • Conductive substrate • Substrate stiffness • Micropatterning |
(Bio)chemical approaches • Growth Factors • Hormones (e.g., thyroid/glucocorticoid hormones) • Adrenergic agonists • ROS removal |
hPSC-CM differentiation has also been achieved via specific environmental cues. For example, to replicate the environment of the naive myocardium, hPSC-CM maturity has been boosted by injecting cells into fetal animal hearts so as to provide the microenvironment for cardiomyocyte maturation17,18. Although increased sarcomere alignment was observed, the low throughput of the approach and the use of model animals as a cell source limit the future application of this approach.
Approaches relying on a scalable biopolymer-based microenvironment to enhance maturation have shown some potential. The ideal matrix stiffness for promoting cardiomyocyte maturation has been studied extensively, and is thought to be ~10 kPa. The matrix can incorporate patterns or elongated folds for optimal cell differentiation. For instance, hPSC-CMs have been cultured on 10 kPa polyacrylamide substrates patterned with Matrigel in 2,000 μm2 rectangles, with aspect ratios between 5:1 and 7:1, using mechanical output and myofibril alignment as the measurement of maturity19; in this study, the mechanical output was highest in 7:1 hPSC-CMs. On the other hand, another study in which action potentials were used to evaluate cardiomyocyte maturity revealed that cardiomyocytes had the longest action potential when cultured on 9 kPa hydrogel20.
In the quest for developing an optimal microenvironment for hPSC-CM differentiation, an on-going challenge is to determine the optimal permutation of parameters, such as type of material, its stiffness, matrix patterns and dimensions, and its co-culture with other cell types. To that end, physical cues have been used to stimulate maturation, for example with the use of gold nanowires to promote cardiomyocyte maturation, which increased electrical signal propagation across the cells and improved electrical synchronicity21. Others have found that transversal, shearing, or stretch stimuli can induce the proper maturation cues22.
Despite these advances, the in vivo therapeutic use of hPSC-CMs requires a degree of maturity that is different from those encountered in in vitro disease modelling and drug screening. To date, the immaturity of hPSC-CMs is mainly defined by the comparison between adult cardiomyocytes and hPSC-CMs with respect to sarcomere organization, sarcoplasmic reticulum function, metabolism, energy handling, ion-channel density and conduction, calcium kinetics, and gene expression (reviewed in ref. 23). Whereas immature hPSC-CMs have been transplanted and engrafted in animal hearts, the survival of primary adult cardiomyocytes after transplantation is poor24. Mature hPSC-CMs may therefore be more sensitive to ischaemic stress, and have little or no capacity for cell regeneration, leading to poor survival. Additional studies are required to find the optimal balance between cell maturity and therapeutic efficacy (Fig. 2).
Fig. 2. The trajectory from pluripotent stem cells to fully differentiated cells through progenitor cells.
Both progenitors and fully differentiated cells can be candidates for transplantation. Pluripotent stem cells are however not suitable because of their high self-renewal capacity and potential tumorigenicity.
Cardiac repair with hPSC-derived non-myocyte cells
The survival of cardiomyocytes transplanted into heart tissue depends on other cell types that provide the necessary local support, including the supply of oxygen and extracellular matrix. In addition to hPSC-CMs, non-myocyte cells also have the therapeutic potential to repair a damaged heart.
Endothelial cells
Endothelial cells make up the interior surface of blood vessels, and are required for angiogenesis and vasculogenesis. As with cardiomyocyte differentiation, endothelial cells can be differentiated from hPSCs (hPSC-ECs) through the modulation of Activin/Nodal or Wnt pathways. The first hESC-EC generated from embryoid bodies involved spontaneous differentiation of hESCs in the presence of serum, followed by cell sorting with platelet endothelial cell-adhesion molecule-1 antibodies25. Improved protocols in the presence of different growth factors and small molecules26–28 have been inspired by this approach. More recently, monolayer protocols in which hPSCs are grown and differentiated on surfaces coated with Matrigel followed by antibody purification with one or more endothelial markers (such as CD31 and CD144) have been reported29–33.
The transplantation of hPSC-ECs can revascularize tissues and promote vascular regeneration34,35. In this context, hESC-ECs have been used to treat myocardial infarction in a rodent model, where the transplanted endothelial cells formed functional vessels and improved cardiac function36. Also, by incorporating hiPSC-ECs and hiPSC-CMs into engineered cardiac tissue sheets neovascularization and engraftment survival four weeks after transplantation could be increased37. Furthermore, in a porcine model of acute myocardial infarction, the engraftment of tri-lineage cardiac patches with hiPSC-CMs, hiPSC-ECs, and hiPSC-derived smooth muscle cells in combination with IGF-1–fibrin led to improvements in cardiac function without evidence of arrhythmias38. Similar to hPSC-CMs, hPSC-ECs purified by selecting for endothelial cell markers exhibited functional heterogeneity and displayed arterial, venous and lymphatic subtype markers39. Further refinements in protocols for subtype-specific hPSC-EC differentiation are expected.
Vascular smooth muscle cells
Vascular smooth muscle cells (VSMCs) are another major component of blood vessels, with both contractile and factor-release functions. The transplantation of smooth muscle cells into scar tissue can improve heart function40. To differentiate VSMCs, hPSCs are directed towards the mesoderm lineage by modulation of the Activin/Nodal pathway, followed by cell sorting to purify the cells of interest. The co-transplantation of hPSC-derived vascular smooth muscle cells with other cells can enhance the formation of blood vessels41,42.
Cardiac progenitor cells
Cardiac progenitor cells (CPCs) are a group of resident cells in the heart that can proliferate and differentiate into mature cardiomyocytes following cardiac injury. CPCs are not yet well understood, and surface markers are often used to define them. Although terminally differentiated cell types such as cardiomyocytes and endothelial cells have been tested for their efficacy at heart repair in preclinical models, it is debatable whether progenitor cells that are tissue-committed yet still multipotent would be more suitable for regeneration, owing to their potential to expand and differentiate into multiple cardiac cell types after transplantation. The differentiation protocol for generating hPSC-derived cardiac progenitor cells relies on embryoid-body formation and fluorescence-activated cell sorting (FACS)-based selection of CPC surface markers such as Flk-1 (KDR in humans)43,44, CD13/ROR2 (ref. 45) and SSEA-1 (ref. 46). Although hPSC-CPCs possess the ability to generate vascularized myocardium grafts after transplantation, their tumorigenic risk must be evaluated thoroughly, owing to the possibility of hPSC contamination and cell dedifferentiation.
Endothelial progenitor cells
Endothelial progenitor cells (EPCs) are circulating cells that participate in the formation of new blood vessels by adhering to endothelial cells at sites of hypoxic or ischaemic injury. As with CPCs, how EPCs should be defined remains unresolved. EPCs have been defined on the basis of the cell-surface markers CD31, CD34 and KDR (refs. 47,48), whereas others have defined EPCs on the basis of their colony-forming capacity47,49. Protocols generating EPCs from hPSCs rely on embryoid-body formation50,51 or on a combination of factors that include Activin A, BMP-4, FGF-2 and VEGF (ref. 52).
Epicardial cells
The epicardium, the outer layer of the heart, has the ability to form epicardial-derived cells such as cardiac fibroblasts, cardiac muscle cells, coronary smooth muscle cells and vascular endothelial cells. Generation of hPSC-derived epicardial cells (EPIs) involves the temporal modulation of the BMP and WNT signalling pathways53–55. Treatment with TGF-β inhibitors enables the long-term culture of hPSC-EPIs without the spontaneous epithelial–mesenchymal transition53 occurring. Epicardial cells have the ability to differentiate into smooth muscle cells, fibroblasts and endothelial cells56, and can protect the heart following myocardial injury57,58. Interestingly, epicardial Hippo signalling plays a key role in the immunosuppressive response following myocardial infarction59‘; yet it remains unclear whether hPSC-EPIs retain these properties in a therapeutic setting.
Benefits and barriers of combinatorial stem cell therapy
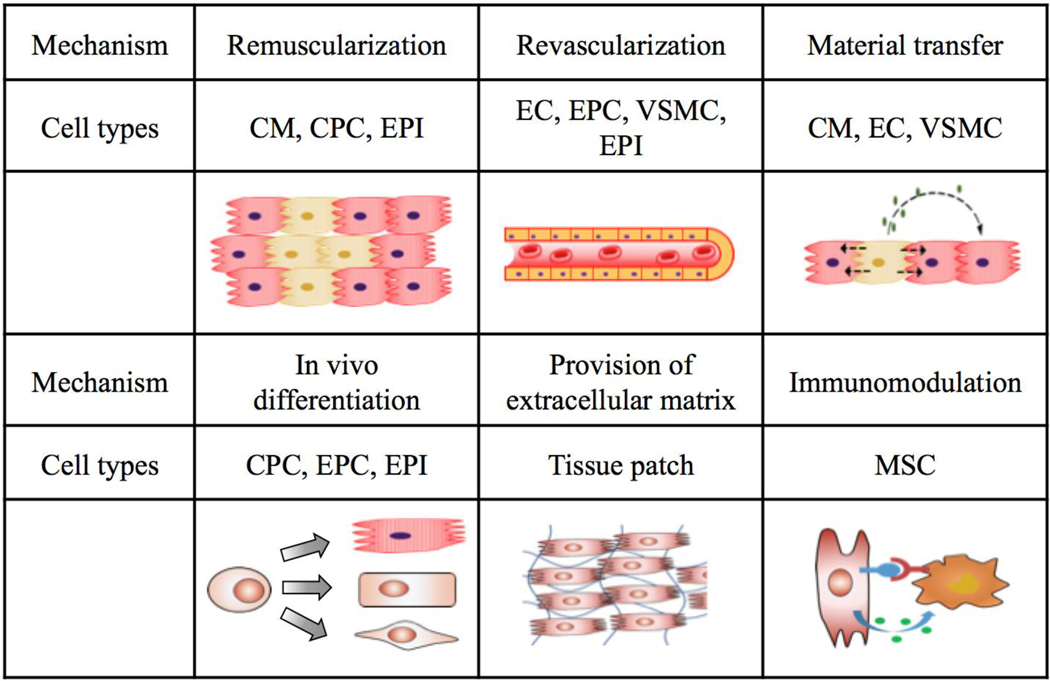
The idea that cardiac stem cell therapy may benefit from using multiple cell types is based on the following hypotheses60: (i) no single cell type can regenerate all cardiac cells in vivo; (ii) both cardiomyocytes and non-cardiomyocyte cells are required to repair the damaged heart; and (iii) synergistic effects from the interaction of multiple cell types are known to improve implant survival (Fig. 3). These hypotheses, especially the third one, need to be tested in preclinical studies.
Fig. 3. Mechanisms of cell-based therapy for cardiovascular regeneration.
Depending on cell types and delivery routes, one or more mechanisms may be used. When a combination of cell types is introduced, synergistic effects may occur to promote therapeutic efficacy. Progenitor cells including CPCs and EPCs, as well as epicardial cells, can further differentiate into other functional cells and may regenerate multiple cell types after transplantation. Extracellular matrix may be supplied through tissue patches to support stem cell survival and reverse heart remodelling.
In particular, a side-by-side comparison is needed to determine the optimal combination of cell types. The comparison of different cell types for cardiac repair is difficult because of the different models and procedures required for their generation. A comparison among hPSC-CMs, hPSC-CPCs and bone marrow mononuclear cells61 showed that injecting a total of 10 × 106 hPSC-CMs and hPSC-CPCs into rat hearts improved cardiac contractility, whereas bone-marrow-derived mononuclear cells enhanced host vascularization without improving contractility. Additional experiments will thus be needed to test whether the delivery of cell types with complementary roles may lead to improved effects.
Moreover, the optimal stoichiometry and delivery approach of implanted cells still need experimental validation. When using three cell types (hPSC-CMs, hPSC-ECs and hPSC-SMCs) in a 1:1:1 ratio for transplantation in a fibrin patch loaded with insulin growth factors38, 27.1% ± 5% of surviving transplanted cells were hiPSC-CMs, 34.2% ± 10% were hiPSC-ECs, and 40.5% ± 1% were hiPSC-SMCs at 4 weeks after injury, resulting in a ratio of 0.67:0.84:1. When the same three types of cells were used in a ratio of 2:1:1 (ref. 62), the ratio became 0.74:0.52:1 (CMs:ECs:SMCs) at week 1 post transplantation, and 0.78:1.16:1 at week 4. This ratiometric change is likely to be due to different cell-replication rates and to tolerance to ischaemic stress; in both cases, hPSC-CMs suffered the most from cell loss. More work is needed to evaluate the effect of cell ratio on therapy efficacy.
Cardiac repair with direct reprogramming
hPSC-derived cells may have a high risk of tumorigenicity, owing to possible contamination of the transplanted suspension with residual undifferentiated cells. Therefore, the possibility of directly reprogramming non-myocyte cells to differentiate into cardiomyocytes is being explored. Unlike cardiac differentiation from hPSCs, reprogramming of non-myocyte cells into cardiomyocytes or CPCs could regenerate injured heart tissue by either in vitro reprogramming followed by transplantation, or by directly injecting the reprogramming factors into infarcted sites for re-muscularization. This has been reviewed elsewhere63,64.
Induced cardiomyocytes
Fibroblasts can be directly reprogrammed into cardiomyocytes via transfection of cardiac transcription factors65,66. Inspired by the pioneering reprogramming of somatic cells into hPSCs by ectopic expression of Yamanaka factors, fibroblasts can be induced to undergo transdifferentiation into cardiomyocytes via overexpression of cardiomyocyte master transcription factors. Known as GMT factors, the overexpression of Gata4, Mef2c, and Tbx5 using retrovirus vectors67 or Sendai virus68 in mouse fibroblasts resulted in cardiac lineage transdifferentiation. Unlike hPSC-based cell therapy, induced cardiomyocytes can be generated in vivo through gene therapy to reprogram cardiac fibroblasts into functional cardiomyocytes69.
Despite these achievements in cardiomyocyte transdifferentiation, clinical trials of in vivo direct reprogramming should be approached with caution. First, the efficiency of direct reprogramming to induced cardiomyocytes is low70. Because of the lack of consensus on cardiomyocyte markers (such as Troponin T, αMHC, or Nkx2.5) and phenotypes (such as spontaneous beating), the efficiency of transdifferentiation can range from 0% (ref. 70) to 10% (ref. 68). Second, results from rodent models cannot be reliably translated into human trials, owing to molecular and physiological differences between the two species. For example, GMT alone is insufficient in generating induced cardiomyocytes from human fibroblasts, and additional factors (such as MESP1, HAND2, or specific microRNAs) are needed to enhance reprogramming65,66. Similarly to hPSC-CMs, induced cardiomyocytes have an immature phenotype, as indicated by myofilament disarray, electrophysiology, and global gene expression. Additional head-to-head comparisons between hPSC-CMs and induced cardiomyocytes are necessary in order to evaluate the therapeutic potential of these two cell types.
Induced CPCs (iCPCs)
Two studies71,72 have reported different strategies for the direct reprogramming of adult mouse fibroblasts into cardiovascular progenitor cells. Whereas one study71 started with an extensive search for CPC-inducing transcription factors and revealed five (MTGNB: Mesp1, Tbx5, Gata4, Nkx2.5, and Baf60c) factors that were sufficient to induce CPCs, the other study72 transiently overexpressed the four Yamanaka factors in combination with a JAK inhibitor to induce mesodermal specification, followed by a treatment of various combinations of modulators to induce expandable CPCs. Unlike what was shown previously for induced cardiomyocytes, both these studies also showed that iCPCs are expandable, with the ability to propagate for >18 passages in vitro. They also showed that iCPCs can generate different cardiac lineages in vivo, and improve cardiac function and survival following transplantation into a myocardial infarction animal model.
Reprogrammed endothelial cells (rECs)
Direct lineage conversion with cell-type-specific transcription factors has been attempted to generate rECs directly from somatic cells. The ETS (E26 transformation-specific or E-twenty-six) family of transcription factors, especially ETV2 (ETS variant 2; also known as ER71), drive endothelial-cell development, and their overexpression leads to conversion of somatic cells into rECs (refs. 73). In lieu of the overexpression of endothelial-cell transcription factors, a cocktail of growth factors combined with an immunostimulant was also sufficient to induce rEC transdifferentiation74. Although not tested in a cardiac injury model, rEC transplantation has been shown to increase neovascularization and to enhance blood perfusion in a rodent limb ischaemia model73,74.
Delivery and transplantation strategies
A central determinant in the survival and efficacy of transplanted hPSC-CMs is the delivery method. Thus far, two primary delivery methods have been explored: direct injection, and transplantation via a gel patch. Each method has its own advantages and disadvantages, with injection being the most used method of delivery in preclinical trials to date. However, progress in the development of gel patches shows them as an increasingly attractive alternative, and has enabled their integration into cardiac tissue to stabilize transplanted hPSC-CMs, as evidenced by progressive vascularization of the patches and by their stable engraftment into the host after transplantation22,75–77.
The transplantation of hPSC-CMs via gel patches offers a host of advantages. The engineered tissue offers contractile, paracrine and passive mechanical support. In designing a patch for cell transplantation, two key factors are the selection of patch material and the design of the patch architecture. It is essential to select a biopolymer that is scalable and capable of integrating into the cardiac tissue while faithfully relaying the signals and contractions of the seeded hPSC-CMs. Additional functionalities, such as an advantageous degradation rate, are also important aspects when selecting polymers for cell delivery. Although many biopolymers have been proposed, thus far collagen, fibrin, alginate and Matrigel have been the most applied78. For example, collagen type-I hydrogels have been used extensively, owing to their integral role in the native heart’s extracellular matrix, ability to advance the maturation of hPSC-CMs, and well-characterized properties for widespread use in multiple clinical scenarios22,79. Alginate, an algae-derived polysaccharide, is also an attractive material, owing to its low cost, biocompatibility and highly tunable properties. Alginate has been investigated for patients with myocardial infarction in an attempt to reduce the demands on the injured heart, and as a patch for the transplantation of hPSC-CMs. Cardiac-cell-seeded microporous alginate scaffolds have been successfully implanted into infarcted rat hearts80,81.
Although also popular, fibrin has been less extensively studied than the aforementioned materials, perhaps because as a standalone material it cannot be optimized for cardiomyocyte support78; however, it has been studied for its resemblance to the main protein constituent of engineered heart tissue. Using hESC-CMs, a 2D cell monolayer and a 3D fibrin-based cardiac patch were found to increase cardiac maturity with higher conduction velocities, longer sarcomeres, and increased expression of contractility genes. Not only could cardiomyocytes be seeded onto fibrin patches; the constructs also promoted maturity82. In a fibrin patch containing hESC-derived cardiac progenitors, the left ventricular ejection fraction (LVEF) was found to improve after 2 months in rats with the cardiac patch, without evidence of arising teratomas, thus validating the efficacy and safety of a fibrin-based patch46. Subsequently, a clinical trial of hESC-derived CPCs in patients with severe ischemic left ventricular dysfunction83 reported no tumour formation or arrhythmias in all patients during a 1-year follow-up. Interestingly, although this trial was not powered to assess efficacy, a slight improvement in wall motion and an overall increase in ejection fraction was observed.
Whereas the major consideration in evaluating a cell-delivery approach is to maximize viable cell retention in the target tissue, other variables such as cell differentiation and maturation can also affect the choice of delivery strategies. To support the survival of fully differentiated cardiomyocytes, the appropriate extracellular matrix, as well as growth factors, can be co-injected to provide a favourable microenvironment84. When endothelial cells or vascular progenitor cells are delivered, pro-angiogenic factors (such as VEGF) can be encapsulated in the hydrogels or in other biomaterials to support blood-vessel formation. Recent developments in 3D bioprinting have created additional opportunities to generate multicellular engineered-tissue constructs with pre-defined biomaterials and microstructure85. By using a 3D bioprinter, cardiac-muscle patches can be created with multiple cell types embedded for transplantation62,86. Even for hPSCs, which are not suitable for direct transplantation owing to the risk of teratomas formation, acellular approaches, such as those involving extracellular vesicles isolated from culture supernatants, can induce cardiac repair87.
Outlook
The heart has a very limited endogenous regenerative capacity; yet, lessons learned from developmental biology have brought us closer to the goal of generating appropriate types of cells to repair damaged hearts. Despite notable progress in the development of cell-differentiation protocols, hurdles in the way of clinical translation, such as achieving appropriate cell maturity, cell purity, and the ability to generate enough cells for therapeutic efficacy, remain mostly unsurpassed. Preclinical studies are necessary to identify the best combinations of cell types for myocardial regeneration.
Other applications of hPSCs in cardiovascular medicine include disease modelling and drug discovery. Using primary patient-derived cells, hiPSCs have been used for studying cardiovascular disorders caused by genetic mutations, and the emergence of genome-editing tools has further expanded the application of hiPSCs in precision medicine. Advances in hiPSC technology also facilitate the screening for personalized pharmacological drugs (thoroughly reviewed elsewhere88).
There are further obstacles to the translation of cardiac stem cell therapy. Primarily, a lack of knowledge of the mechanisms of regeneration89; in this context, incorporating molecular imaging in animal experiments to track cell fate after transplantation may help elucidate the mechanisms90. And great caution is advised when evaluating differences between species before translating animal results into human trials91. Naturally, economic, regulatory and ethical issues persist4. Because cardiac stem cell therapy requires large investments in preclinical research and in clinical trials, closer collaboration between government agencies, non-profit foundations, private and strategic investors, universities, and research institutes is necessary in order for cell therapies to hopefully revolutionize the understanding of heart regeneration and ameliorate heart disease.
Acknowledgements
This work was supported in part by research grants from the National Institute of Health R01 HL126527, R01 HL133272, R24 HL117756, American Heart Association 17MERIT33610009 (to JCW), and iHeart Research Dorothy Dee & Marjorie Helene Boring Trust Award (to AZ).
Footnotes
Competing interests
The authors declare no competing financial or non-financial interests.
References
- 1.Takahashi K. & Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676, doi: 10.1016/j.cell.2006.07.024 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Yu J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920, doi: 10.1126/science.1151526 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Zhao MT et al. Molecular and functional resemblance of differentiated cells derived from isogenic human iPSCs and SCNT-derived ESCs. Proceedings of the National Academy of Sciences of the United States of America 114, E11111-E11120, doi: 10.1073/pnas.1708991114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neofytou E, O’Brien CG, Couture LA & Wu JC Hurdles to clinical translation of human induced pluripotent stem cells. J Clin Invest 125, 2551–2557, doi: 10.1172/JCI80575 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burridge PW, Sharma A. & Wu JC Genetic and epigenetic regulation of human cardiac reprogramming and differentiation in regenerative medicine. Annu Rev Genet 49, 461–484, doi: 10.1146/annurev-genet-112414-054911 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawamura M. et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 126, S29–37, doi: 10.1161/circulationaha.111.084343 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Shiba Y. et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489, 322–325, doi: 10.1038/nature11317 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong JJ et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277, doi: 10.1038/nature13233 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong SG et al. Microfluidic single-cell analysis of transplanted human induced pluripotent stem cell-derived cardiomyocytes after acute myocardial infarction. Circulation 132, 762–771, doi: 10.1161/circulationaha.114.015231 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiba Y. et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538, 388–391, doi: 10.1038/nature19815 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Zwi L. et al. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation 120, 1513–1523, doi: 10.1161/CIRCULATIONAHA.109.868885 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Burridge PW et al. Chemically defined generation of human cardiomyocytes. Nat Methods 11, 855–860, doi: 10.1038/nmeth.2999 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burridge PW et al. Chemically defined culture and cardiomyocytes differentiation of human pluripotent stem cells. Current Protocols In Human Genetics. 15, 21–23, doi: 10.1002/0471142905.hg2103s87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gepstein L. et al. In vivo assessment of the electrophysiological integration and arrhythmogenic risk of myocardial cell transplantation strategies. Stem Cells (Dayton, Ohio) 28, 2151–2161, doi: 10.1002/stem.545 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Kuppusamy KT et al. Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proceedings of the National Academy of Sciences of the United States of America 112, E2785–2794, doi: 10.1073/pnas.1424042112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen JY et al. Maturation-based model of arrhythmogenic right ventricular dysplasia using patient-specific induced pluripotent stem cells. Circulation Journal : Official Journal of the Japanese Circulation Society 79, 1402–1408, doi: 10.1253/circj.CJ-15-0363 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Kadota S, Pabon L, Reinecke H. & Murry CE In vivo maturation of human induced pluripotent stem cell-derived cardiomyocytes in neonatal and adult rat hearts. Stem Cell Reports 8, 278–289, doi: 10.1016/j.stemcr.2016.10.009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho GS, Tampakakis E, Andersen P. & Kwon C. Use of a neonatal rat system as a bioincubator to generate adult-like mature cardiomyocytes from human and mouse pluripotent stem cells. Nat Protoc 12, 2097–2109, doi: 10.1038/nprot.2017.089 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro AJ et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proceedings of the National Academy of Sciences of the United States of America 112, 12705–12710, doi: 10.1073/pnas.1508073112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boothe SD et al. The Effect of substrate stiffness on cardiomyocyte action potentials. Cell Biochem Biophys 74, 527–535, doi: 10.1007/s12013-016-0758-1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dvir T. et al. Nanowired three-dimensional cardiac patches. Nat Nanotechnol 6, 720–725, doi: 10.1038/nnano.2011.160 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiburcy M. et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation 135, 1832–1847, doi: 10.1161/circulationaha.116.024145 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayed N, Liu C, Wu JC. Translation of human induced pluripotent stem cells: from clinicla trial in a dish to precision medicine. Journal of the American College of Cardiology 67, 2161–76. doi: 10.1016/j.jacc.2016.01.083 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riegler J, Gillich A, Shen Q, Gold JD & Wu JC Cardiac tissue slice transplantation as a model to assess tissue-engineered graft thickness, survival, and function. Circulation 130, S77–86, doi: 10.1161/CIRCULATIONAHA.113.007920 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J. & Langer R. Endothelial cells derived from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America 99, 4391–4396, doi: 10.1073/pnas.032074999 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James D. et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nature Biotechnology 28, 161–166, doi: 10.1038/nbt.1605 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nourse MB et al. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arterioscler Thromb Vasc Biol 30, 80–89, doi: 10.1161/ATVBAHA.109.194233 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu SJ et al. Robust generation of hemangioblastic progenitors from human embryonic stem cells. Regen Med 3, 693–704, doi: 10.2217/17460751.3.5.693 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Samuel R. et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America 110, 12774–12779, doi: 10.1073/pnas.1310675110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusuma S. et al. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proceedings of the National Academy of Sciences of the United States of America 110, 12601–12606, doi: 10.1073/pnas.1306562110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song W, Kaufman DS & Shen W. Efficient generation of endothelial cells from human pluripotent stem cells and characterization of their functional properties. J Biomed Mater Res A 104, 678–687, doi: 10.1002/jbm.a.35607 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Kane NM et al. Derivation of endothelial cells from human embryonic stem cells by directed differentiation: analysis of microRNA and angiogenesis in vitro and in vivo. Arterioscler Thromb Vasc Biol 30, 1389–1397, doi: 10.1161/ATVBAHA.110.204800 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Lian X. et al. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Reports 3, 804–816, doi: 10.1016/j.stemcr.2014.09.005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J. et al. Functional characterization of human pluripotent stem cell-derived arterial endothelial cells. Proceedings of the National Academy of Sciences of the United States of America 114, E6072-E6078, doi: 10.1073/pnas.1702295114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rufaihah AJ et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol 31, e72–79, doi: 10.1161/ATVBAHA.111.230938 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z. et al. Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PLoS ONE 4, e8443, doi: 10.1371/journal.pone.0008443 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masumoto H. et al. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci Rep 4, 6716, doi: 10.1038/srep06716 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye L. et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 15, 750–761, doi: 10.1016/j.stem.2014.11.009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rufaihah AJ et al. Human induced pluripotent stem cell-derived endothelial cells exhibit functional heterogeneity. Am J Transl Res 5, 21–35 (2013). [PMC free article] [PubMed] [Google Scholar]
- 40.Li RK, Jia ZQ, Weisel RD, Merante F. & Mickle DA Smooth muscle cell transplantation into myocardial scar tissue improves heart function. Journal of Molecular and Cellular Cardiology 31, 513–522, doi: 10.1006/jmcc.1998.0882 (1999). [DOI] [PubMed] [Google Scholar]
- 41.Cheung C. & Sinha S. Human embryonic stem cell-derived vascular smooth muscle cells in therapeutic neovascularisation. Journal of Molecular and Cellular Cardiology 51, 651–664, doi: 10.1016/j.yjmcc.2011.07.014 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Ayoubi S, Sheikh SP & Eskildsen TV Human induced pluripotent stem cell-derived vascular smooth muscle cells: differentiation and therapeutic potential. Cardiovasc Res 113, 1282–1293, doi: 10.1093/cvr/cvx125 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Mauritz C. et al. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. European Heart Journal 32, 2634–2641, doi: 10.1093/eurheartj/ehr166 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Yang L. et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453, 524–528, doi: 10.1038/nature06894 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Skelton RJ et al. CD13 and ROR2 Permit isolation of highly enriched cardiac mesoderm from differentiating human embryonic stem cells. Stem Cell Reports 6, 95–108, doi: 10.1016/j.stemcr.2015.11.006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menasche P. et al. Towards a clinical use of human embryonic stem cell-derived cardiac progenitors: a translational experience. European Heart Journal 36, 743–750, doi: 10.1093/eurheartj/ehu192 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Asahara T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 (1997). [DOI] [PubMed] [Google Scholar]
- 48.Friedrich EB, Walenta K, Scharlau J, Nickenig G. & Werner N. CD34-/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circulation Research 98, e20–25, doi: 10.1161/01.RES.0000205765.28940.93 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Patel J. et al. Functional definition of progenitors versus mature endothelial cells reveals key soxF-dependent differentiation process. Circulation 135, 786–805, doi: 10.1161/CIRCULATIONAHA.116.024754 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Feng Q. et al. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells 28, 704–712, doi: 10.1002/stem.321 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Cimato T. et al. Neuropilin-1 identifies endothelial precursors in human and murine embryonic stem cells before CD34 expression. Circulation 119, 2170–2178, doi: 10.1161/CIRCULATIONAHA.109.849596 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prasain N. et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nature Biotechnology 32, 1151–1157, doi: 10.1038/nbt.3048 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bao X. et al. Long-term self-renewing human epicardial cells generated from pluripotent stem cells under defined xeno-free conditions. Nat Biomed Eng 1, doi: 10.1038/s41551-016-0003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bao X. et al. Directed differentiation and long-term maintenance of epicardial cells derived from human pluripotent stem cells under fully defined conditions. Nat Protoc 12, 1890–1900, doi: 10.1038/nprot.2017.080 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao J. et al. Efficient Differentiation of TBX18+/WT1+ Epicardial-Like Cells from Human Pluripotent Stem Cells Using Small Molecular Compounds. Stem Cells Dev 26, 528–540, doi: 10.1089/scd.2016.0208 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cano E. et al. Extracardiac septum transversum/proepicardial endothelial cells pattern embryonic coronary arterio-venous connections. Proceedings of the National Academy of Sciences of the United States of America 113, 656–661, doi: 10.1073/pnas.1509834113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei K. et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 525, 479–485, doi: 10.1038/nature15372 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang GN et al. C/EBP transcription factors mediate epicardial activation during heart development and injury. Science 338, 1599–1603, doi: 10.1126/science.1229765 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramjee V. et al. Epicardial YAP/TAZ orchestrate an immunosuppressive response following myocardial infarction. J Clin Invest 127, 899–911, doi: 10.1172/JCI88759 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hatzistergos KE & Vedenko A. Cardiac cell therapy 3.0: The beginning of the end or the end of the beginning? Circulation Research 121, 95–97, doi: 10.1161/CIRCRESAHA.117.311293 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernandes S. et al. Comparison of human embryonic stem cell-derived cardiomyocytes, cardiovascular progenitors, and bone marrow mononuclear cells for cardiac repair. Stem Cell Reports 5, 753–762, doi: 10.1016/j.stemcr.2015.09.011 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao L. et al. Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-Dimensionally printed scaffold. Circulation Research 120, 1318–1325, doi: 10.1161/circresaha.116.310277 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ebert AD, Diecke S, Reprogramming and transdifferentiation for cardiovascular development and regenerative medicine: where do we stand?. EMBO molecular medicine 7, 1090–103. doi: 10.15252/emmm.201504395.(2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ebrahimi B. In vivo reprogramming for heart regeneration: A glance at efficiency, environmental impacts, challenges and future directions. Journal of Molecular and Cellular Cardiology 108, 61–72, doi: 10.1016/j.yjmcc.2017.05.005 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Nam YJ et al. Reprogramming of human fibroblasts toward a cardiac fate. Proceedings of the National Academy of Sciences of the United States of America 110, 5588–5593, doi: 10.1073/pnas.1301019110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao N. et al. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science 352, 1216–1220, doi: 10.1126/science.aaf1502 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Ieda M. et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386, doi: 10.1016/j.cell.2010.07.002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyamoto K. et al. Direct in vivo reprogramming with sendai virus vectors improves cardiac function after myocardial infarction. Cell Stem Cell 22, 91–103 e105, doi: 10.1016/j.stem.2017.11.010 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Qian L. et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598, doi: 10.1038/nature11044 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen JX et al. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circulation Research 111, 50–55, doi: 10.1161/CIRCRESAHA.112.270264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lalit PA et al. Lineage reprogramming of fibroblasts into proliferative induced cardiac progenitor cells by defined factors. Cell Stem Cell 18, 354–367, doi: 10.1016/j.stem.2015.12.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y. et al. Expandable cardiovascular progenitor cells reprogrammed from fibroblasts. Cell Stem Cell 18, 368–381, doi: 10.1016/j.stem.2016.02.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee S. et al. Direct reprogramming of human dermal fibroblasts Into endothelial cells using ER71/ETV2. Circulation Research 120, 848–861, doi: 10.1161/CIRCRESAHA.116.309833 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sayed N. et al. Transdifferentiation of human fibroblasts to endothelial cells: role of innate immunity. Circulation 131, 300–309, doi: 10.1161/CIRCULATIONAHA.113.007394 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shadrin IY et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nature Communications 8, 1825, doi: 10.1038/s41467-017-01946-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jackman CP et al. Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation. Biomaterials 159, 48–58, doi: 10.1016/j.biomaterials.2018.01.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weinberger F. et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Science Translational Medicine 8, 363ra148, doi: 10.1126/scitranslmed.aaf8781 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Abilez OJ, Wu JC Stem cell reprogramming: A 3D boost. Nature Materials 15, 259–61, doi: 10.1038/nmat4583 (2016). [DOI] [PubMed] [Google Scholar]
- 79.Abilez OJ, et al. Passive stretch induces structural and functional maturation of engineered heart muscle as predicted by computational modeling. Stem Cells 36, 265–277, doi: 10.1002/stem.2732 (2017).. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruvinov E. & Cohen S. Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook: From ocean algae to patient bedside. Adv Drug Deliv Rev 96, 54–76, doi: 10.1016/j.addr.2015.04.021 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Orr S. et al. TGF-beta affinity-bound to a macroporous alginate scaffold generates local and peripheral immunotolerant responses and improves allocell transplantation. Acta Biomater 45, 196–209, doi: 10.1016/j.actbio.2016.08.015 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Zhang D. et al. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 34, 5813–5820, doi: 10.1016/j.biomaterials.2013.04.026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Menasche P. et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. Journal of the American College of Cardiology 71, 429–438, doi: 10.1016/j.jacc.2017.11.047 (2018). [DOI] [PubMed] [Google Scholar]
- 84.Johnson TD & Christman KL Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin Drug Deliv 10, 59–72, doi: 10.1517/17425247.2013.739156 (2013). [DOI] [PubMed] [Google Scholar]
- 85.Liu C, et al. Modeling human disease with induced pluripotent stem cells: from 2D to 3D and beyond Development 145, 156–166, doi: 10.1242/dev.156166 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ong CS et al. Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes. Sci Rep 7, 4566, doi: 10.1038/s41598-017-05018-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adamiak M. et al. Induced pluripotent stem cell (iPSC)-derived extracellular vesicles are safer and more effective for cardiac repair Than iPSCs. Circulation Research 122, 296–309, doi: 10.1161/CIRCRESAHA.117.311769 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi Y, Inoue H, Wu JC & Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov 16, 115–130, doi: 10.1038/nrd.2016.245 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nguyen PK, Neofytou E, Rhee JW & Wu JC Potential strategies to address the major clinical barriers facing stem cell regenerative therapy for cardiovascular disease: a review. JAMA Cardiol 1, 953–962, doi: 10.1001/jamacardio.2016.2750 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qin X. et al. Photoacoustic imaging of embryonic stem cell-derived cardiomyocytes in living hearts with ultrasensitive semiconducting polymer nanoparticles. Advanced Functional Materials, 1704939, doi: 10.1002/adfm.201704939 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao X. et al. Comparison of non-human primate versus human induced pluripotent stem cell-derived cardiomyocytes for treatment of myocardial infarction. Stem Cell Reports, doi: 10.1016/j.stemcr.2018.01.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]