Abstract
Background
Being considered a life-threatening condition, cesarean scar pregnancy (CSP) leads to loss of fertility, severe bleeding, and even maternal mortality. We intended to assess the effect of double-balloon cervical ripening catheter insertion on CSP termination before nine weeks of gestation.
Method
All participants were diagnosed CSP by abdominal and transvaginal ultrasound. The cases were treated with a sterile, double-balloon cervical ripening catheter inserted with real-time transabdominal ultrasound guidance and removed three days later. The control group consisted of patients treated with systemic methotrexate with or without fetal reduction.
Results
Thirty-five patients were eligible for double-balloon ripening and 32 for MTX therapy; the treatment in cases failed in five of the patients. Success rate difference between two methods was insignificant (Pearson Chi-square: 0.383, p-value: 0.536). There were significant differences regarding the time to normal menstruation (OR: 1.303) and the thickness of the myometrium after surgery (OR: 4.721), but there was no significant difference in the time resolve of either β-HCG or residue of pregnancy.
Conclusion
Double-balloon cervical ripening insertion yields acceptable results for terminating CSP. This strategy does not cause bleeding and even prevents it with its tamponade properties. Additionally, this treatment is minimally invasive simple with low morbidity.
Keywords: Cesarean section, Cesarean scar pregnancy, Double-balloon insertion, Fertility
Introduction
Being considered as a major life-threatening condition, cesarean scar pregnancy (CSP) refers to the implantation and growth of the gestational sac in the fibrous scar tissue of the prior cesarean section (CS) (1). The CS delivery rate is increasing worldwide, especially among the developing countries, with an annual incidence of 18.5 million cases (2). Hence, it is not surprising to detect the rising CSP rate since its first description four decades ago (3). Although the accurate incidence of CSP is unclear, the estimated incidence of CSP is reported to range from 1 in 1800 to 1 in 2216 pregnancies due to its underdiagnosis, with the rate of 6.1% of all ectopic pregnancies among women with prior history of CS in the USA (4).
The increasing prevalence of CS and its associated complications, most significantly CSP and placenta accreta, necessitates obstetricians to be more familiar with this condition, to have early diagnosis, and to recognize the best treatment with the least complications. The diagnosis is based on ultrasound findings, including empty uterine and cervical cavity, and gestational sac implantation in a cesarean scar (1). Expectant management is not globally accepted as it causes high maternal morbidity and mortality. There is a wide variety of treatment strategies for pregnancy termination including medical, radiological and surgical interventions, alone or in combination. However, in case of unwise treatment, many of the known treatments lead to loss of fertility, severe bleeding, and even, maternal mortality. A treatment strategy relies on various determinants such as gestational age, pregnancy viability, family planning, and the surgeon’s skill. In treatment, the primary goal is to maintain maternal health and the secondary goal is to maintain fertility (5).
Several studies have highlighted the application of a double-balloon catheter to control postpartum hemorrhage (6). The application of such a catheter has also been considered for CSP termination. This method is minimally invasive, is well tolerated by patients, prevents hemorrhagic complications, maintains fertility, and does not require additional invasive interventions in most cases. It is noteworthy that the application of a double-balloon catheter is well-recognized among obstetricians as a conventional method for cervical ripening in labor induction. To the best of our knowledge, since the introduction of the method by Timor‐Tritsch and his coworkers, few studies have assessed its efficacy for CSP termination (7–9). The current study began concurrent with other studies after the publication by Timor‐Tritsch secondary to the significance of his results, added to the literature regarding the feasibility and effectiveness of double-balloon cervical ripening catheter insertion in terminating CSPs before 9 weeks of gestation, and presented associated complications and challenges that may be encountered by obstetricians. Furthermore, we also compared the results with the conventional method of systemic methotrexate therapy which might precipitate the chances of adverse drug reactions.
Methods
Study Design
This prospective case–control study evaluated the efficacy of double-balloon catheter placement for CSP termination. The cases and the control groups were taken consecutively, and the patients were not randomly allocated to either group. Participants were women with history of a prior CS, increased β-HCG level, and menstrual retardation. CSP was diagnosed by abdominal and transvaginal ultrasound (TVS) in all the participants using the following criteria: retained gestational sac/placenta in the previous cesarean scar, the empty uterine cavity or cervical canal, the gestational sac in the anterior lower uterine segment with or without a fetal pole or cardiac activity, and a vascular pattern in the previous cesarean scar (4). The inclusion criteria were the established CSP by TVS, 5–9 weeks of gestation, clear declaration for CSP termination following adequate explanations, desire for future fertility, and a stable hemodynamic state. The treatment failure was considered as the increased β-HCG level in need of further intervention, including methotrexate (MTX) administration or dilatation and curettage (D&C), and severe hemorrhage in need of additional measures such as hysterectomy. In this study we tried to evaluate as to whether this procedure could lead to better outcomes. Therefore, we compared the results with a control group including 32 subjects who received medical treatment with systemic MTX with or without fetal reduction in regard to the myometrial thickness after procedure, time to residual resolve, and time to the resolve of β-HCG (10, 11).
Procedure
The participants were admitted to a hospital except three of the patients residing in the hospital city. Prophylactic antibiotics (IV cefazolin 2 gr 30 min before procedure) and conscious sedation with mask-assisted ventilation were used for all the patients. Moreover, a Hegar dilator was used in some of the patients to dilate the internal OS. A sterile, gel-lubricated, double-balloon cervical ripening catheter (Cook® Cervical Ripening Balloon, Cook Ob/GYN, Spencer, IN) (see Fig. 1) was inserted under the real-time transabdominal ultrasound guidance. However, Doppler ultrasonography was not used due to practical issues. First, the upper anchor balloon was placed adjacently to the gestational sac site and inflated with sterile normal saline up to a level compressing the upper portion of the gestational sac. Then, the lower balloon was inflated with sterile normal saline to compress the gestational sac in the lower uterine segment. The inflation of the balloons was continued until the gestational sac was completely compressed. The stoppage of fetal cardiac activity was confirmed according to ultrasound evaluations. The antibiotic administration was continued employing Cefazolin 2 gr QID and Gentamicin 80 mg TDS until the catheter removal. The pain was managed by using a suppository nonsteroidal anti-inflammatory agent (50 mg diclofenac TDS) for all the cases and by decreasing the lower balloon volume for one. Ultrasound studies were performed the day after treatment to evaluate fetal cardiac activity, the gestational sac, and the vascularization pattern. The catheter was removed 72 h after its placement, and the admitted patients were discharged in case of a stable hemodynamic state. (7, 12, 13).
Fig. 1.

Cook® Cervical Ripening Balloon with Stylet*. * figure provided by Cook® Group Incorporated
The control group consisted of hemodynamically stable CSP patients who had no contraindications to medical treatment (e.g., rupture, vaginal bleeding, etc.). After ultrasonographic evaluation, if the fetal heart activity was present, the patient underwent fetal reduction and systemic MTX therapy and if not present only systemic MTX was administered. The dosage of MTX was decided based on β-HCG levels, and the patient was followed by serial measurement of β-HCG levels until negative. The patient was followed up to resolve of remnants and normal menstruation. The failure in treatment included the need for hysterectomy or suction and curettage or any surgical intervention.(Table 1) (10).
Table 1.
Medical treatment protocol for ectopic pregnancy. Any case that faces rupture or has any kind of surgical management indication is considered as a failure of medical treatment
| Protocol | Evaluation |
|---|---|
|
Single Dose (Methotrexate 50 mg/m2 IM) (β-hCG < 4999 IU/L) |
A simple dose of MTX on day 1 |
| Measurement of β-hCG on days 1, 4, and 7 then β-hCG is measured weekly until undetectable | |
| And if the difference is below 15%, an extra dose is given; then, β-hCG is measured weekly until undetectable | |
|
Double Dose (Methotrexate 50 mg/m2 IM) (5000 IU/L ≤ β-hCG < 9999 IU/L) |
MTX is given on days 1 and 4 |
| Measurement of β-hCG on days 1, 7 | |
| And if the difference between days 1 and 7 is below 15%, third dose is given | |
|
Multiple Dose (1 mg/kg IM) (10,000 IU/L ≤ β-hCG) |
MTX is given on days 1, 3, 5, and 7 |
| Folinic acid is also administered on days 2, 4, 6, 8 1mg/kg IM | |
| MTX is given until β-hCG level decreases more than 15% in 48 h or four doses of methotrexate given; then, β-hCG is measured weekly until undetectable |
Follow-Up and Outcome Evaluation
In cases, the β-HCG level was measured before and 24 and 72 h after the procedure, and then, weekly until the level became negative. The β-HCG level was considered negative if it was less than 5 mIU/ml in two consecutive measurements. If β-HCG did not decrease, the treatment was considered failed and a second treatment option was planned. In control group, β-HCG levels were measured weekly until negative. In all patients TVS was performed 2 months after the established negative β-HCG level to evaluate CSP remnants. The participants were instructed to have contraception for at least 6 months after the treatment. Moreover, the participants were followed to examine their menstruation status following the treatment as well as their potential future pregnancy. Follow-up ultrasound assessment was used to evaluate the uterus in respect of myometrial and endometrial status.
Statistical Analysis
The data were analyzed using SPSS version 22 and expressed by mean (± standard deviation) or number (percent). A repeated measurement test was applied to investigate the intervention effect on the β-HCG level. P-value below 0.05 was considered statistically significant (Figs. 2 and 3).
Fig. 2.
The provided figure illustrates the application of double-balloon cervical ripening catheter for termination of cesarean scar pregnancy in 7th week. A: ultrasonographic imaging shows gestational sac and fetus, B: ultrasonographic imaging shows the fetus being compressed between the inflated balloons, C: Doppler ultrasonographic imaging showing fetal cardiac activity, D: assessing the gestational age using crown-rump length
Fig. 3.
The ß-HCG level declines during the study period for patients responding successfully to the treatment
Results
Sixty-seven patients were eligible for the study, 35 for cases and 32 for control group. In the case group, two of the patients had severe hemorrhage, one of them underwent hysterectomy (7 days after balloon deflation), and the other underwent D&C followed by laparotomy for wedge resection (7 days after balloon deflation) (Table 2). The upper anchor balloon was inflated at 35.44 ± 8.56 cc, and the lower balloon was inflated at 21.28 ± 4.9 cc. The β-HCG level increased in three of the patients, although they had a decreased β-HCG level following the administration of a single dose of MTX (50 mg/m2). Accordingly, the treatment was successful for 30 (85.7%) of the patients with treatment failure in only five of them (14.3%; Table 4). The average maternal age and gestational age were 33.17 ± 4.98 years and 7.12 ± 0.85 weeks, respectively. Previous history of abortion and curettage was positive for 22 (62.8%) and 15 (42.8%) of the patients, respectively. Of the patients, one had previous history of ectopic pregnancy and one had previous history of CSP. Fetal cardiac activity was detectable during the initial ultrasonographic studies in 21 (60%) of the patients. The average myometrium thickness was 2.66 + 0.38 (1.7–3.3) mm and 4.06 + 0.39 (3.4–5) mm between the gestational sac and the bladder before and 2 months after the intervention, respectively, which had a significant increment (P < 0.0001). The β-HCG level became negative in 63.67 ± 28.46 days (Table 3, Fig. 4). The β-HCG level before treatment (p = 0.545), previous history of infertility (p = 1.000), history of abortion (p = 0.630), number of previous CSs (p = 0.380), gestational age (p = 0.147), and myometrium thickness before intervention (p = 0.792) were not associated with the treatment failure. Normal menstruation occurred after 10.27 ± 2.82 weeks, and pregnancy remnants disappeared in an average of 3.3 ± 0.99 months. Pregnancy occurred in three of the patients, of whom two had full-term normal labor and one desired abortion.
Table 2.
The characteristics of the failed patients
| Patient number | Age (Year) | Gestational age (weeks + day) | FHR (bpm) | Myometrium diameter (mm) | Gestation number | Labor number | Abortion number | ß-HCG levels after balloon insertion and before intervention | Intervention | ß-HCG levels before intervention |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39 | 7W | Neg | 2.8 | 2 | 1 | 0 | 19026→14500→11800→10500→23320→38100 | MTX | 5469→187→13.8→ negative |
| 2 | 35 | 8W | Neg | 2.7 | 3 | 2 | 0 | 122000→118700→106789→19160→43657 | MTX | 1832→201→73→Neg. |
| 3 | 41 | 6W + 3d | Pos | 4 | 3 | 2 | 0 | 32759→30490→28450 | Hysterectomy | Neg. |
| 4 | 30 | 6W | Pos | 2.7 | 3 | 3 | 0 | 36540→24500→136200 | D&C and then laparotomy for wedge resection | Neg. |
| 5 | 35 | 8W + 2d | Pos | 3 | 4 | 2 | 1 | 27810→22300→19700→21700 | MTX | 15110→9730→4820→2300→780→230→42→Neg |
Table 4.
Pearson Chi-square: 0.383 P-value: 0.536 exact P-value: 0.711
| EP site | Success (percent) | Failure (percent) |
|---|---|---|
| Double balloon | 30 (85.7%) | 5 (14.3%) |
| MTX | 29 (90.6%) | 3 (9.4%) |
Table 3.
The β-HCG level before and 24 and 72 h after the intervention
| β-HCG level | Mean | Standard deviation | Minimum | Maximum | 95% Confidence interval | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| Before treatment | 64,905 | 57,362.40 | 1440 | 219,836 | 41,893.126 | 83,031.141 |
| 24 h after treatment | 38,802 | 39,673.56 | 670 | 161,636 | 23,988.481 | 53,617.186 |
| 72 h after treatment | 30,423 | 32,149.47 | 331 | 103,787 | 18,419.125 | 42,428.742 |
| The last follow-up | < 5 | – | – | – | – | – |
| p-value | < 0.0001 | |||||
Fig. 4.
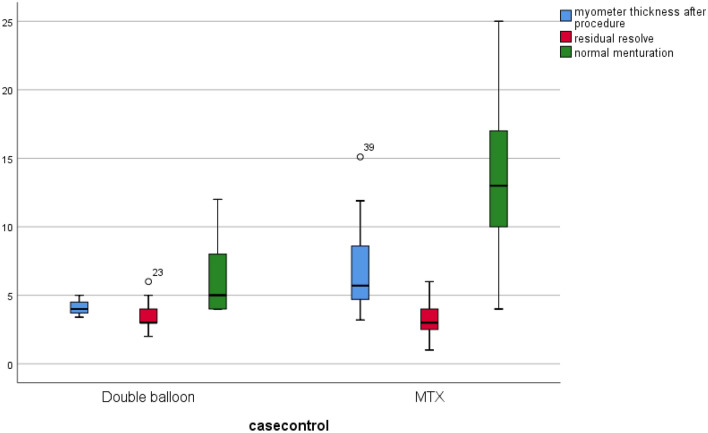
The box-and-whisker plot shows the averages and quartiles of myometrial thickness (in millimeters), time to resolve of pregnancy residuals (in weeks), and the weeks to normal menstruation
In order to assess the success rate of this procedure we compared the success rate among the patients who were treated with methotrexate and double-balloon ripening. Our results actively demonstrate that there is no difference between the two methods (Pearson Chi-square: 0.383, exact p-value: 0.536) (Table 4).
We also compared the results of two methods in regard to the weeks to normal menstruation, myometrial thickness after procedure, time to resolve of residue of pregnancy, and the time to a negative β-HCG levels. The results show that while there were significant differences regarding the time to normal menstruation, and the thickness of myometrium after surgery, there was no significant difference in the time to negative β-HCG level and time to resolve of residue of pregnancy. The analysis was furthered using regression models and showed that the values for myometrial thickness and weeks to normal menstruation are statistically significant after regression. The data also show that while the odds ratio myometrial thickness after the procedure is markedly high (OR: 4.721), the ratio is not as significantly high for weeks to normal menstruation (OR: 1.303) (Table 5).
Table 5.
Data show mean and standard deviations and P-values
| Characteristics | Double balloon | MTX | P-value |
|---|---|---|---|
| Age | 33.17 (4.985) | 33.78 (5.160) | 0.624 |
| Initial β-HCG | 57,362.404 (9696.01) | 37,724.543 (6668.820) | 0.004 |
| Gestational age | 6.94 (0.90) | 7.00 (1.39) | 0.841 |
| Time to residual resolve | 6.3 (0.98) | 6.9 (2.03) | 0.159 |
| Weeks to normal menstruation | 10.26 (2.81) | 16.36 (8.71) | 0.001 |
| Days to β-HCG resolve | 63.67 (28.4) | 61.60 (27.2) | 0.775 |
| Myometrial thickness after procedure | 4.05 (0.39) | 7.00 (3.32) | < 0.000 |
| Myometrial thickness before procedure | 2.66 (0.38) | 2.83 (0.52) | 0.129 |
| Regression models | |||
| Characteristics | Wald test | Odds Ratio | P-value |
| Weeks to normal menstruation | 9.050 | 1.303 | 0.003 |
| Myometer thickness after procedure | 6.741 | 4.721 | 0.009 |
Discussion
The current study investigated the effect of the application of a double-balloon cervical ripening catheter on CSP termination without prior MTX administration in the largest sample reported to date, and the results were compared with patients who were treated with MTX (± fetal reduction), without any other procedures such as dilation and curettage. According to the results, 85.7% of the patients were treated successfully with double balloon and the remaining percentage of them required further intervention. The MTX therapy was successful with 90.6%. This difference was not statistically significant. The failed cases did not have remarkable characteristics to let us predict the possible failure of the method. The average β-HCG level showed 43% and 56% decrements before compared to 24 and 72 h after catheter placement, respectively (10, 14). The time to resolution of β-HCG and the time to resolve of pregnancy showed no difference between the groups CSP is considered as one of the serious causes of maternal morbidity and mortality. In cases with CSP, obstetricians mostly suggest pregnancy termination. So far, many treatment protocols have been suggested with various complications; the best protocol is the one with minimal invasiveness which preserves fertility. The insertion of a Foley catheter is a known treatment for cervical ripening in labor induction, and its application has been extended recently as a treatment strategy for termination of cervical and CSPs. Vo et al. (2019) reported the use of Foley balloon catheter placement followed by dilatation and curettage in 311 women diagnosed with CSP. They showed that 90.7% of the participants were treated with this protocol successfully (8). Foley catheter insertion is technically challenging and limited to lower gestational ages (15). Insertion of a double-balloon catheter for terminating cervical and cesarean scar pregnancy was first described by Timor-Tritsch in 2016 (7). The method was successfully used for seven individuals with CSPs and with the average gestational age of 6–7 weeks. They concluded that the method was minimally invasive and efficient for terminating CSPs (7, 14). The findings of the present study indicated that about 86% of the patients with an average gestation age of 7 weeks were treated successfully. The results of this study show that the myometrial thickness after procedure and time to normal menstruation is significantly higher in double-balloon group. However, as we did not record myometrial thickness in the control group before treatment, we could not evaluate differences in this regard and the cases might have had relatively thicker myometrium. And this result might have been affected by the fact that the cases and controls consisted of 2 consecutive groups rather than random allocation. The treatment failed in five of the cases needing additional interventions, including MTX, D&C, wedge resection, and hysterectomy, while 3 cases in controls failed and 2 were treated with hysteroscopic resection of remnant and 1 was treated with hysterotomy. This shows the necessity of patient follow-up to apply further appropriate treatment in case of failure. Two main goals are achieved with double-balloon method: preventing hemorrhage and ceasing embryonic cardiac activity. This method could also be used in more developed and better formed gestational sacs that are more likely to progress, while malformed sacs are more likely to be treated with MTX. The higher β-HCG levels in cases are an indicator for this difference. Monteagudo et al. (2018) reported the results of Cook balloon placement and adjuvant systemic MTX (9). Thirty-seven patients were treated successfully without any further intervention (9), fairly comparable with the result of the current study without neoadjuvant administration. In 2020, Spazzini et al. reported that this technique was efficient for CSP termination during the first trimester for a 36-year-old woman with two prior CSs (16). The patient was discharged 5 days later, and the ß-HCG level was negative on day 21 (16).
During the management of CSPs, related complications including bleeding as the most significant one frequently occur, and 44% of individuals with CSP face such complications (17). Even the most noninvasive benign intervention is associated with the occurrence of such complications. Two of the cases (5.7%) had severe bleeding in this study; one of them underwent hysterectomy and the other underwent D&C followed by laparotomy and wedge resection. Dark vaginal bleeding or pain occurred in 36 patients in the study of Monteagudo et al., and one patient underwent total abdominal hysterectomy 27 days after the treatment due to severe life-threatening hemorrhage (9). Moreover, blood loss more than 200 ml occurred in two patients (0.6%) in the study of Vo et al. (8), but other complications such as infection and perforation did not occur. We found that spotting and dark vaginal bleeding occurred in 30 of the cases, which were resolved 8 days after balloon insertion. Pain is one of the common complications of this technique, probably due to uterus distension caused by the inflation of balloons. Timor-Tritsch et al. in a letter to an editor suggested that the administration of 600–800 mg ibuprofen two hours before the procedure and 400–600 mg every six to eight hours with or without acetaminophen after the procedure could remarkably relieve the pain (12). We employed another NSAID, diclofenac 50 mg TDS in suppository form, which resulted in good pain control in all except one of the patients needing balloon deflation to decrease the lower balloon volume. Meanwhile, no patient suffered from pain in MTX group. Adkins et al. (2019) reported the use of Cook balloon for CSP termination. They reported the rupture of hysterotomy scar and intraperitoneal gestational sac repulsion, known as the floating fetus; we encountered no such complications in our cases (18).
The best-known method for follow-up of ectopic pregnancy and efficiency assessment of the applied treatment is to chase the β-HCG level. The intervention yields successful results in case the β-HCG level reaches 5 mUI/mL and the gestational sac and vascularization are resolved (4). Some studies have indicated that the β-HCG level may increase before beginning to decrease (4), and that vascularization gradually disappears by dropping down the β-HCG level. Vo et al. demonstrated that several factors, including gestational age before 6 weeks, β-HCG level below 11,000 mUI/ml, and gestational sac volume less than 5 cm3 after 2 weeks of treatment, were associated with the protocol success (8). All the gestational ages in this study were above 5 weeks. The median time for the β-HCG level decline was reported 49 days (28–97) in Timor-Tritsch’s study (12). In the study of Vo et al., the average β-HCG level prior to the treatment was 80,017.5 mIU/mL, the average time to become negative was 3.84 weeks (8), and the time for the gestational sac volume and the resolution of vascularization was 5.08 and 4.07 weeks, respectively. Their findings also showed that the β-HCG level declined rapidly until 4th week of gestation and, then, declined steadily and reached a normal range at 12th week (8). In the study of Monteagudo et al., the median time for the β-HCG level to become negative was similar to that between gestational ages (50, 41, 50, 49, and 49 days for pregnancies with 5, 6, 7, 8, and 10 weeks of gestation, respectively) (9). In the present study, the average β-HCG level before the treatment was 57,362.4and it took an average of 63 days to become negative. The TVS performed 2 months after the negative result of the β-HCG level in all the patients showed resolved vascularization, and in two CSP remnant was resected hysteroscopically in control group. Normal pregnancy occurred in three of the cases at least 6 months after the treatment was completed, with two normal full-term labors and one abortion in the first trimester due to patient’s desire. In 3 controls we had full-term pregnancies (Fig. 5).
Fig. 5.
The box-and-whisker plot shows the averages and quartiles show the days to resolve of beta HCG
Limitations
The small sample size was the main limitation in the present study since the study reported one of the biggest CSP population treated with Cook catheter insertion without neoadjuvant therapy. In this study we lacked myometrial thickness measuring in control groups which prevented assessment. On the other hand, the cases and controls were not randomly allocated and included two consecutive groups. A prospective multi-center study with a larger sample size, including both types of CSP, is required in this regard.
Conclusion
Double-balloon cervical ripening insertion leads to acceptable results for terminating CSP. This strategy has a relatively close success rate with conventional MTX therapy, and it does not cause bleeding, and even, prevents it with its tamponade properties. Additionally, the treatment is minimally invasive, simple with low morbidity, and familiar to obstetricians and gynecologists. Most importantly, the fertility will be preserved the youngest patients’ desire. As a result, the treatment can be considered as an acceptable candidate for the first-line treatment.
Authors contributions
FG and AT contributed in developing the research idea and composing and revising the manuscript. EI, SB, AG contributed in composing and revising the manuscript. AH contributed in study design, composing and revising manuscript, and statistical analysis.
Funding
Not applicable.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical consideration
This study was approved by the Research Deputy and the Ethics Committee of the Tehran University of Medical Sciences (Reference number: IR. TUMS.IKHC.REC.1397.312) and conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and all subsequent revisions.
Informed consent
A written informed consent form was obtained from all the participants.
Footnotes
Azadeh Tarafdari: Associate professor and consultant of Obstetrics and Gynaecology at Tehran University of Medical Sciences; Alireza Hadizadeh: post-doctoral research fellow at Tehran University of Medical Sciences; Elnaz Irandoost: Obstetrician and Gynaecologist at Tehran University of Medical Sciences; Sedigheh Borna: professor and consultant of Obstetrics and Gynaecology at Tehran University of Medical Sciences; Azin Ghamari: post-doctoral research fellow at Tehran University of Medical Sciences; Fahimeh Ghotbizadeh-vahdani: Associate professor and consultant of Obstetrics and Gynaecology at Tehran University of Medical Sciences.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maymon R, Halperin R, Se M, et al. Ectopic pregnancies in a Caesarean scar: review of the medical approach to an iatrogenic complication. Hum Reprod Update. 2004;10(6):515–523. doi: 10.1093/humupd/dmh042. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons L, Belizan JM, Lauer JA, et al. Inequities in the use of cesarean section deliveries in the world. American J Obstet Gynecol. 2012;206(4):331–e1. doi: 10.1016/j.ajog.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Larsen J, Solomon M. Pregnancy in a uterine scar sacculus-an unusual cause of postabortal haeinorrhage. S Afr med J. 1978;53:142. [PubMed] [Google Scholar]
- 4.Timor-Tritsch IE, Monteagudo A, Santos R, et al. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. American J Obstet Gynecol. 2012;207(1):44e1–e13. doi: 10.1016/j.ajog.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Miller R, Timor-Tritsch IE, Gyamfi-Bannerman C. Society for maternal-fetal medicine (SMFM) consult series# 49: cesarean scar pregnancy. American J Obstet Gynecol. 2020;222(5):2–14. doi: 10.1016/j.ajog.2020.01.030. [DOI] [PubMed] [Google Scholar]
- 6.Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. American J Obstet Gynecol. 2020;222(4):293–e1. doi: 10.1016/j.ajog.2019.11.1287. [DOI] [PubMed] [Google Scholar]
- 7.Timor-Tritsch IE, Monteagudo A, Bennett T-A, et al. A new minimally invasive treatment for cesarean scar pregnancy and cervical pregnancy. American J Obstet Gynecol. 2016;215(3):3511–8. doi: 10.1016/j.ajog.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Vo TM, Van T, Nguyen L, Tran Q. Management of cesarean scar pregnancy among vietnamese women. Gynecol Minimal Invasive Ther. 2019;8(1):12. doi: 10.4103/GMIT.GMIT_8_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monteagudo A, Calì G, Rebarber A, Cordoba M, Fox NS, Bornstein E, et al. Minimally invasive treatment of cesarean scar and cervical pregnancies using a cervical ripening double balloon catheter: expanding the clinical series. J Ultrasound Med. 2019;38(3):785–793. doi: 10.1002/jum.14736. [DOI] [PubMed] [Google Scholar]
- 10.Tarafdari A, Bandarian M, Hantoushzadeh S, et al. Assessing the risk factors and management outcomes of ectopic pregnancy: a retrospective case-control study. Int J Reproduct Biomed. 2023;21(5):403–98. doi: 10.18502/ijrm.v21i5.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarafdari A, Malek M, PahlevanFalahy E, Hadizadeh A. IUD perforation and embedment within omentum: a rare and perplexing incidence. Clin Case Rep. 2022;10(4):e05732. doi: 10.1002/ccr3.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timor-Tritsch IE, Monteagudo A, Agten AK. Recap-Minimally invasive treatment for cesarean scar pregnancy using a double-balloon catheter: additional suggestions to the technique. Am J Obstet Gynecol. 2017;217(4):496–497. doi: 10.1016/j.ajog.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Tsui K-H, Lin L-T, Yu K-J, Chen S-F, Chang W-H, Yu S, et al. Double-balloon cervical ripening catheter works well as an intrauterine balloon tamponade in post-abortion massive hemorrhage. Taiwan J Obstet Gynecol. 2012;51(3):426–429. doi: 10.1016/j.tjog.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Fatehnejad M, Hadizadeh A, Tayebi A, et al. (2023) Assessment of the clinical outcomes and complications of hysteroscopic and laparoscopic approaches in the treatment of symptomatic isthmocele: an observational study. Int J Gynecol Obstet. [DOI] [PubMed]
- 15.Fang Q, Sun L, Tang Y, Qian C, Yao X. Quantitative risk assessment to guide the treatment of cesarean scar pregnancy. Int J Gynecol Obstet. 2017;139(1):78–83. doi: 10.1002/ijgo.12240. [DOI] [PubMed] [Google Scholar]
- 16.Spazzini MD, Villa A, Maffioletti C, Mariuzzo F, Calì G. First-trimester treatment of cesarean scar pregnancy using a cervical ripening—double-balloon catheter: a case report. J Clin Ultrasound. 2020;48(5):298–300. doi: 10.1002/jcu.22838. [DOI] [PubMed] [Google Scholar]
- 17.Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson C. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol: Off J Int Soc Ultrasound Obstet Gynecol. 2003;21(3):220–227. doi: 10.1002/uog.56. [DOI] [PubMed] [Google Scholar]
- 18.Adkins JM, Thampy R, Thupili CR. Floating fetus: a rare complication of balloon tamponade treatment of caesarean scar ectopic pregnancy. BMJ Case Reports CP. 2019;12(1):e228500. doi: 10.1136/bcr-2018-228500. [DOI] [PMC free article] [PubMed] [Google Scholar]