Abstract
Autophagy, a conserved degradation and reuse process, plays a crucial role in plant cellular homeostasis during abiotic stress. Although numerous autophagy–related genes (ATGs) that regulate abiotic stress have been identified, few functional studies have shown how they confer tolerance to copper (Cu) stress. Here, we cloned a novel Vitis vinifera ATG6 gene (VvATG6) which was induced by 0.5 and 10 mM Cu stress based on transcriptomic data, and transgenic Arabidopsis thaliana, tobacco (Nicotiana tabacum), and grape calli were successfully obtained through Agrobacterium-mediated genetic transformation. The overexpression of VvATG6 enhanced the tolerance of transgenic lines to Cu. After Cu treatment, the lines that overexpressed VvATG6 grew better and increased their production of biomass compared with the wild-type. These changes were accompanied by higher activities of antioxidant enzymes and a lower accumulation of deleterious malondialdehyde and hydrogen peroxide in the transgenic plants. The activities of superoxide dismutase, peroxidase, and catalase were enhanced owing to the elevation of corresponding antioxidant gene expression in the VvATG6 overexpression plants under Cu stress, thereby promoting the clearance of reactive oxygen species (ROS). Simultaneously, there was a decrease in the levels of expression of RbohB and RbohC that are involved in ROS synthesis in transgenic plants under Cu stress. Thus, the accelerated removal of ROS and the inhibition of its synthesis led to a balanced ROS homeostasis environment, which alleviated the damage from Cu. This could benefit from the upregulation of other ATGs that are necessary for the production of autophagosomes under Cu stress. To our knowledge, this study is the first to demonstrate the protective role of VvATG6 in the Cu tolerance of plants.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-024-01415-y.
Keywords: Autophagy, Cu stress, VvATG6, Transgenic grape calli, ROS synthesis and scavenging
Introduction
As an important micronutrient for plants, copper (Cu) is one of the essential cofactors for various biological activities, including photosynthesis, transpiration, and stomatal movement, but excessive amounts are highly toxic to plants and thereby impact crop yield, fruit quality, and food safety (Huang et al. 2021; Wu et al. 2021). To control fruit and leaf fungal diseases, the long-term and inappropriate application of Cu-based fungicides causes toxicity in vineyards, and the soil Cu concentrate was found to reach up to 487.62 mg kg−1, which dramatically exceeds the national secondary standard value (150 mg kg−1) (Wang et al. 2015a). Furthermore, the production of excess Cu induces the accumulation of reactive oxygen species (ROS) and causes oxidative stress, thus resulting in membrane lipid peroxidation, a reduction in the selective permeability of the plasma membrane, and the exosmosis of cellular contents, which ultimately affects the normal operation of plant physiological and metabolic processes (Li et al. 2018). To prevent this oxidative stress, plant cells must have the ability to quickly remove excess ROS to maintain a normal physiological and metabolic balance. Enzymatic and non-enzymatic systems are effective antioxidant systems to remove excess ROS in plants (Ahmad et al. 2010). These systems include the antioxidant enzymes superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR) and others, as well as glutathione (GSH), ascorbic acid (AsA), proline, and other non-enzymatic antioxidants (Geng et al. 2018). Both systems are essential to prevent the damage caused by ROS and thus promote tolerance to stress.
Previous studies have identified that autophagy plays an important role in plant growth, immune response, aging, and abiotic stress, and the rapid activation of its related genes ATGs is crucial in mediating and regulating plant physiological and biochemical reactions. For example, the overexpression of MdATG18a, MdATG9, and MdATG10 was shown to confer tolerance to alkalinity, low nitrogen, and salt stress in apple (Malus domestica) plants (Li et al. 2020; Huo et al. 2020b, 2020a). In contrast, the knockdown of ATG10 and ATG18f significantly reduced the tolerance to drought and the formation of autophagosomes in tomato (Solanum lycopersicum) (Wang et al. 2015b). Currently, the induction of autophagy caused by Cu stress was reported in grape and sweet orange (Citrus x. aurantium f. aurantium) (Shangguan et al. 2018; Fu et al. 2020). Xia et al. (2023) found that Cu-tolerant grapevine significantly induced the expression of autophagy-related genes. However, there is no direct evidence that the overexpression or knockout of ATGs can change the tolerance of grapevine to Cu.
Previous studies have shown that ATG6 can be activated by multiple abiotic stresses, such as treatment with hydrogen peroxide (H2O2), nitrogen deficiency, high salinity, temperature stress, and aluminum toxicity, which indicates a potential role of ATG6 in the response to various environmental stressors in plants (Zeng et al. 2017). Cao et al. (2022) reported that the overexpression of ATG6 enhanced tolerance to low nitrogen stress in tomato by improving the activities of nitrate reductase and nitrite reductase, carbon fixation and photosynthetic capacity. However, atg6 mutants have shown the opposite trend in the plants. In rice (Oryza sativa), OsATG6a and OsATG6b were upregulated during drought but downregulated under high and low temperature stress, respectively, while OsATG6c was upregulated under drought and high and low temperatures (Rana et al. 2012). In barley (Hordeum vulgare), the expression of HvATG6 was also up-regulated by H2O2 treatment, high salinity, drought, and low temperatures (Zeng et al. 2017). These results suggest that the stress-specific responses of ATG6 help plants to more effectively adapt to different stress conditions, but the biological mechanisms and roles of these genes under abiotic stress remain unclear. Interestingly, our previous study found that the number of autophagosomes increased at 8 h and slightly decreased at 64 h but was still more than that at 0 h in grapevine under Low-ECS (0.5 mM copper sulfate [CuSO4]), with an increase in the expression of VvATG6 (Chen et al. 2021). In addition, a quantitative analysis of the data showed that the level of expression of VvATG6 increased rapidly for 36 h in grapevine after treatment with Cu (Shangguan et al. 2018). Therefore, we hypothesized that VvATG6 plays a key role in maintaining the homeostasis of grape leaf metabolites under Cu stress. In this study, we will explore whether the overexpression of VvATG6 contributes to the tolerance to Cu in plants through molecular biological means and what pathway is used to achieve this process to provide ideas to screen plants resistant to this heavy metal.
Materials and methods
Plant materials, growth conditions, and stress treatments
The biannual potted cultivar ‘Shine Muscat’ (‘SM’, Vitis vinifera × Vitis labruscana) grapevine was planted at temperatures of 25 °C day/15 °C night. The cultivation substrate was mixed with organic matter: vermiculite: perlite (2:1:1). Mature leaves with similar sizes and growth status were treated with water (control), 0.5 and 10 mM CuSO4 for 0, 8, and 64 h until the leaves dripped water. All the leaves were immediately frozen in liquid nitrogen and stored at –80 °C for further assays of the spatiotemporal pattern of expression of the VvATG6 gene.
A. thaliana, N. tabacum, and ‘Gamay’ (V. vinifera) calli were used for the genetic transformations and assays of Cu tolerance. They were cultured in a growth chamber at 25 °C and 70% humidity under a light/dark (16:8 h) photoperiod. For the seed germination and root development analyses of Arabidopsis, approximately 80 T3 seeds from each wild type (WT) and transgenic line were surface-sterilized and evenly sown on one-half MS agar media that contained 0, 50, and 75 μM CuSO4. The seeds were vernalized at 4 °C for 48 h before growth at 25 °C under a 16 h photoperiod. Seedlings with fully emerged radicle tips were scored for seed germination, and the rate of germination was calculated every 12 h for 120 h. The root length and levels of gene expression were measured after 7 and 10 d, respectively. Simultaneously, the seedlings in one-half MS media were transplanted to the cultivation substrate for further growth. After 21 days, the leaves of the seedlings were treated with 75 μM CuSO4 for 3,3’-diaminobenzidine (DAB) staining. To select the optimal concentration for the subsequent Cu stress experiment, 6 to 8-week-old WT and overexpressed N. tabacum seedlings were sprayed with 0, 200, 400, and 500 μM CuSO4 to observe their status under stress. Finally, 500 μM Cu was determined as the appropriate concentration to analyze the resistance of transgenic tobacco. After 500 μM Cu treatment, the WT and transgenic tobacco leaves were stained with DAB at 0 and 24 h. The content of malondialdehyde (MDA), antioxidant enzyme activities and their related levels of gene expression were measured at 0, 8, and 64 h. In addition, 8-week-old N. benthamiana seedlings were prepared for subcellular localization and bimolecular fluorescence complementary (BiFC) analyses. The 3- to 4-week-old WT and overexpressed grape calli were treated with 0, 25, 50, 75, 100, and 125 μM CuSO4 to observe the optimal concentration for the subsequent Cu stress experiment, and calli of the same size were inoculated on RG solid media (Gamborg B-53.21 g L−1 + casein acid hydrolysate 0.25 g L−1 + sucrose 20 g⋅L−1 + myo-inositol 0.1 g L−1 + kinetin (KT) 100 uL L−1 + naphthalene acetic acid (NAA) 100 uL L−1 + NaOH 200 uL L−1 + Phytagel 2.5 g L−1) that contained 100 μM CuSO4. The contents of MDA and H2O2 and the levels of related gene expression were measured at 0 and 12 d.
Sequence alignments and phylogenetic and ProtParam analyses
We used the NCBI database (https://www.ncbi.nlm.nih.gov/) to download 13 ATG6 amino acid sequences of grapevine ATG6 representatives. A phylogenetic tree was constructed using the neighbor-joining (NJ) method (Saitou and Nei 1987) together with the amino acid sequences of ATG6 proteins from various species. These parameters were used in the NJ method: bootstrap (1000 replicates), complete deletion, and amino: p-distance. Next, the Evolview online website (https://www.evolgenius.info/) was performed to visualize the phylogenetic tree. Multiple sequence alignments were performed on full-length ATG6 amino acid sequences using MEGA v. 6 with the default parameters, and GeneDoc software was then used to visualize them. In addition, we used the CD-Search tool (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) to analyze the VvATG6 protein conservative structure domain and the ProtParam tool (https://web.expasy.org/protparam/) to predict its physical and chemical properties.
Subcellular localization assays
CDS without the stop codon of VvATG6 were inserted into the pCAMBIA1305-GFP vector to generate the pCAMBIA1305-VvATG6-GFP fusion vector. The primers are listed in Table S1. The fusion vectors were then introduced into Agrobacterium tumefaciens strain EHA105 and then infiltrated into the N. benthamiana leaves. After 48–72 h, the infected tissues were observed for fluorescence signals using an LSM 780 confocal microscope (Zeiss, Oberkochen, Germany).
Yeast two-hybrid (Y2H) assays
Yeast (Saccharomyces cerevisiae) Y2H assays were performed according to the manufacturer’s instructions and those of their cDNA plasmid library (Shanghai OE Biotech Co., Ltd., Shanghai, China). The full-length CDS of VvATG6 were inserted into the Y2H bait vector pGBKT7, and then the plasmids were sequenced to ensure the successful insertion of the sequences. The cDNA plasmid library vector (containing pGADT7) and pGBKT7-VvATG6 were co-transformed into Y2H Gold yeast cells and grown on SD/-Leu/-Trp-deficient medium. The grown colonies were then transferred to SD/-Ade/-His/-Leu/-Trp-deficient media. The positive colonies obtained were detected by PCR, and sequence alignment via the NCBI database was screened for proteins that could interact with ATG6.
Subsequently, the full-length CDS of the candidate genes that were screened above were recombined into the Y2H prey vector pGADT7. Different combinations of these plasmids and pGBKT7-VvATG6 were co-transformed into Y2H Gold yeast cells. The cells were plated on yeast synthetic dropout media that lacked Trp and Leu and cultured at 28 °C. To screen for interactions, those colonies were transferred to yeast synthetic dropout media that was supplemented with X-α-gal but lacked Trp, Leu, His, and Ade and used the empty pGADT7 prey vector and pGBKT7 bait vector as the negative controls. The primers used above are listed in Table S1.
Bimolecular fluorescence complementary assays
The entire CDS of VvATG6 and candidate genes that may interact with VvATG6 were inserted into the pUC-SPYCE and the pUC-SPYNE vector, respectively, and then were sequenced to ensure the sequence correctness. Recombinant plasmid DNA was co-transformed into A. tumefaciens EHA105 and then injected into the N. benthamiana leaves with a needleless syringe, while using the empty vectors as the control. The leaves were cultured at 25 °C in weak light for 48–72 h. The distribution of yellow fluorescent protein was observed with a confocal fluorescence microscope (Zeiss). The primers are listed in Table S1.
Cloning of VvATG6 and relative gene expression analysis
Total RNA was extracted from the grapevines, A. thaliana, N. tabacum leaves, and grape calli using a CTAB-based method, and the cDNA was synthesized with a Hifair® II 1st Strand cDNA Synthesis SuperMix for qPCR (gDNA digester plus) kit from Shanghai Yisheng Biotechnology Co., Ltd. (Shanghai, China). For cloning VvATG6, the coding sequence (CDS) was obtained via semi-quantitative PCR (RT-PCR) from cDNA derived from the leaves of ‘SM’ grapevine using the specific primers listed in Table S1. For the real-time quantitative reverse transcription PCR (qRT-PCR), reverse transcription was performed with 100–500 ng of total RNA from each sample, followed by the PCR amplification of 1 μL of the product. Reaction mixtures of 20 μL that contained 10 μL of Hifair II SuperMix Plus were then conducted in neutralization reactions according to the manufacturer’s instructions. The concentration of cDNA was diluted to 100 ng μL−1, and 1 μL aliquots were used for qRT-PCR using Hieff™ qPCR SYBR® Green Master Mix with Low Rox Plus. The relative levels of expression of the genes were calculated and analyzed by the 2−ΔΔCT method (Livak and Schmittgen 2001). In addition, the VvActin, AtActin, and NtActin genes were used as the internal reference genes for normalization (Table S2).
Vector construction and plant transformation
The full-length CDS of VvATG6 was inserted into pCAMBIA1305 to construct the overexpression vectors. The recombinant plasmids were introduced into the WT plants using the Agrobacterium tumefaciens EHA105-mediated floral dip method in A. thaliana (Clough and Bent 1998) and the leaf disk method in tobacco (Gallois and Marinho 1995). Seeds of the transgenic plants were harvested individually and screened with 25 μg mL−1 hygromycin (Hyg). The T3 homozygous transgenic lines were used for further phenotypic analysis.
The grape calli were transformed by introducing the VvATG6-pCAMBIA1302 recombinant plasmid into the ‘Gamay’ grape calli using the Agrobacterium tumefaciens EHA105-mediated method described by Dai et al. (2015). The grape calli with VvATG6 overexpression were obtained as described for improved methods by Li et al. (2022). Briefly, the embryogenic callus was placed in a triangular flask that contained Agrobacterium culture for 15 min. The supernatant was then discarded, and 40 mL of RG liquid media was poured in to dilute the Agrobacterium. The process was repeated three times. A co-culture was subsequently performed in the G media (RG + acetosyringone [AS] 100 mg L−1 + agar 8.5 g L−1) for 48 h. Afterward, the calli were inoculated into the screening 1 medium (RG + cephalosporin 125 mg L−1 + Phytagel 2.5 g L−1) for 7 d and then transferred to the screening 2 medium (RG + cephalosporin 125 mg L−1 + paromomycin 60 mg L−1 + agar 8.5 g L−1), and subcultured every 1–2 weeks. The resistant calli that grew stably were used for further assays. The primers described above are listed in Table S1.
Hydrogen peroxide tissue staining and measurements of the physiological indices
Herein, the level of the generation of H2O2 was detected using a histochemical staining protocol, and the content of H2O2 was measured using an H2O2 assay kit (Solarbio, Beijing, China). In addition, the content of malondialdehyde (MDA) and the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) were determined as described by Chen et al. (2021).
Statistical analysis
All the experiments were repeated independently three times. A one-way analysis of variance (ANOVA) and Duncan’s tests were used to compare the results using GraphPad Prism v. 8.0.2 (GraphPad, La Jolla, CA, USA). In addition, the differences between the treatments were considered statistically significant at P < 0.05 (Sun et al. 2018b).
Accession numbers
The information on genes and accession is provided in Tables S1 and S2.
Results
VvATG6 is involved in regulating the response of grapevine to Cu stress and its bioinformatic analysis
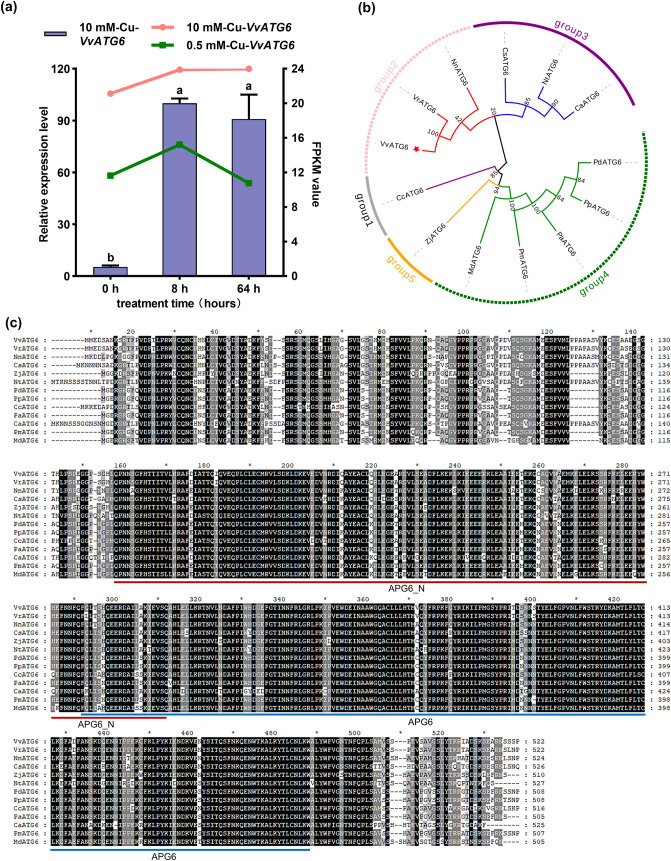
The VvATG6 transcript was induced by Cu in the leaves of ‘SM’ grapevine plants, with respective upregulation being 18.87-fold at 8 h and 17.16-fold at 64 h (Fig. 1a and Table S3). This was consistent with that detected in the transcriptome data where the levels of transcription increased or increased first and then decreased under Cu stress (Chen et al. 2021, Fig. 1a and Table S3). It was hypothesized that VvATG6 was involved in the regulation of the grapevine response to Cu stress.
Fig. 1.
VvATG6 is involved in the regulation of grapevine response to Cu stress and its bioinformatic analysis. a Changes in the level of expression of VvATG6 in the leaves of grapevine plants after treatment with or without different concentrations of Cu stress. The data are shown as the means of three replicates ± SE. Different letters indicate significant differences between the treatments based on a one-way ANOVA and Duncan’s multiple range test (P < 0.05). b Phylogenetic relationships of VvATG6 and other ATG6 proteins. VvATG6 is marked with an asterisk. The accession numbers for other ATG6 proteins listed are as follows: VvATG6, XP_002277370.1; VrATG6, XP_034709134.1; NnATG6, XP_010261575.1; CsATG6, XP_028065346.1; ZjATG6, XP_015888692.1; NtATG6, NP_001313137.1; PdATG6, XP_034205678.1; PpATG6, XP_007219547.1; CcATG6, XP_006444857.1; PaATG6, XP_021815270.1; CaATG6, XP_027115412.1; PmATG6, XP_008233183.1; and MdATG6, NP_001281034.1. c Analysis of the homology of VvATG6 and the other ATG6 amino acid sequences. The red/blue underline represents the APG6_N/APG6 conservative domain. Cu — copper, SE — standard error
The full-length CDS of VvATG6 was cloned from the ‘SM’ grapevine leaves by RT-PCR, which resulted in a length of 1569 bp. The full-length DNA sequence of the gene (GenBank accession number: VIT_00027092001) was obtained by searching the NCBI database with the cDNA sequence as a probe. To understand the sequence conservation of ATG6 genes among plants, the NCBI online website was used to search for ATG6 protein sequences from riverbank grape (V. riparia), N. tabacum, M. domestica, peach (Prunus persica), Chinese plum (P. mume), jujube (Ziziphus jujuba), sweet cherry (P. avium), almond (P. dulcis), coffee (Coffea arabica), lotus (Nelumbo nucifera), tea (Camellia sinensis), and clementine (Citrus clementina). A phylogenetic analysis showed that the VvATG6 protein was closely related to the ATG6 proteins of V. riparia and N. nucifera, which suggested that the function of the ATG6 gene in these species could be conserved (Fig. 1b).
Next, CD-Search was used to analyze the conserved domain of the ATG6 protein. The results showed that the ATG6 protein of these species shared two identically conserved domains, APG6_N and APG6 (Fig. 1c). Moreover, a ProtParam analysis showed that the VvATG6 gene, with a predicted molecular weight of 59.30 kDa, an isoelectric point of 5.68, instability index (II) of 44.21, and grand average of hydropathicity (GRAVY) of − 0.53, encoded 522 amino acids (Table S4).
In this study, we also analyzed the localization of a pCAMBIA1305-VvATG6-GFP fusion protein in the cells by agroinfiltration into the leaves of N. benthamiana. The pCAMBIA1305-GFP fusion protein was utilized as a control. The confocal microscopy results showed that green and red fluorescence signals from the expression of pCAMBIA1305-GFP eventually spread throughout the cells (Fig. 2a). In contrast, the green fluorescent signal from the expression of pCAMBIA1305-VvATG6-GFP could only be found at the cell margins and in the nucleus (Fig. 2b). Thus, these results indicated that the VvATG6 protein was localized to the nucleus and cell membrane.
Fig. 2.
Subcellular localization of VvATG6. Subcellular localization of the pCAMBIA1305-GFP fusion protein a and the pCAMBIA1305-VvATG6-GFP fusion protein b. All the fusions were expressed in Nicotiana benthamiana plants. At the far left of the images are cells with the red mCherry signal. GFP is the green GFP signal. The light is bright-field images of the same cells, and images at the end are overlays of the bright-field and fluorescence images. In (b) we labeled four cells, and the four red triangles represent the nuclei of four cells. An irregular polygon surrounded by a green curve is the cell membrane (a, b). GFP—green fluorescent protein
Screening and validation of the interaction of VvATG6 with candidate proteins
To identify proteins that may interact with VvATG6, we performed a split ubiquitin-yeast Y2H analysis. The results of toxicity and self-activation of the VvATG6 protein showed that PGBKT7-VvATG6 had no toxic effects on the growth of yeast cells and had no self-activation activity (Fig. S1a–e). Next, we screened 20 proteins that may participate in the process of plant responses to stress (Table S5). The 20 candidate genes described above were cloned as full-length coding sequences (primers are shown in Table S1), and fusion vectors were then constructed with pGADT7, respectively. These fusion vectors were transformed into Y2HGold yeast cells with the PGBKT7-VvATG6 plasmid, respectively. As shown in Figs. 3a and S2, all the co-transformed fusion proteins grew normally on SD/-Trp/-Leu media, while only six positive clones grew normally on different dilutions of the SD/-Ade/-Trp/-Leu/-His media with different dilutions, including VvBADH, VvGSO1, VvRBSC-1A, VvEPXH2, VvMORF2, and VvGPDHC1, indicating that these proteins had a strong interaction with VvATG6.
Fig. 3.
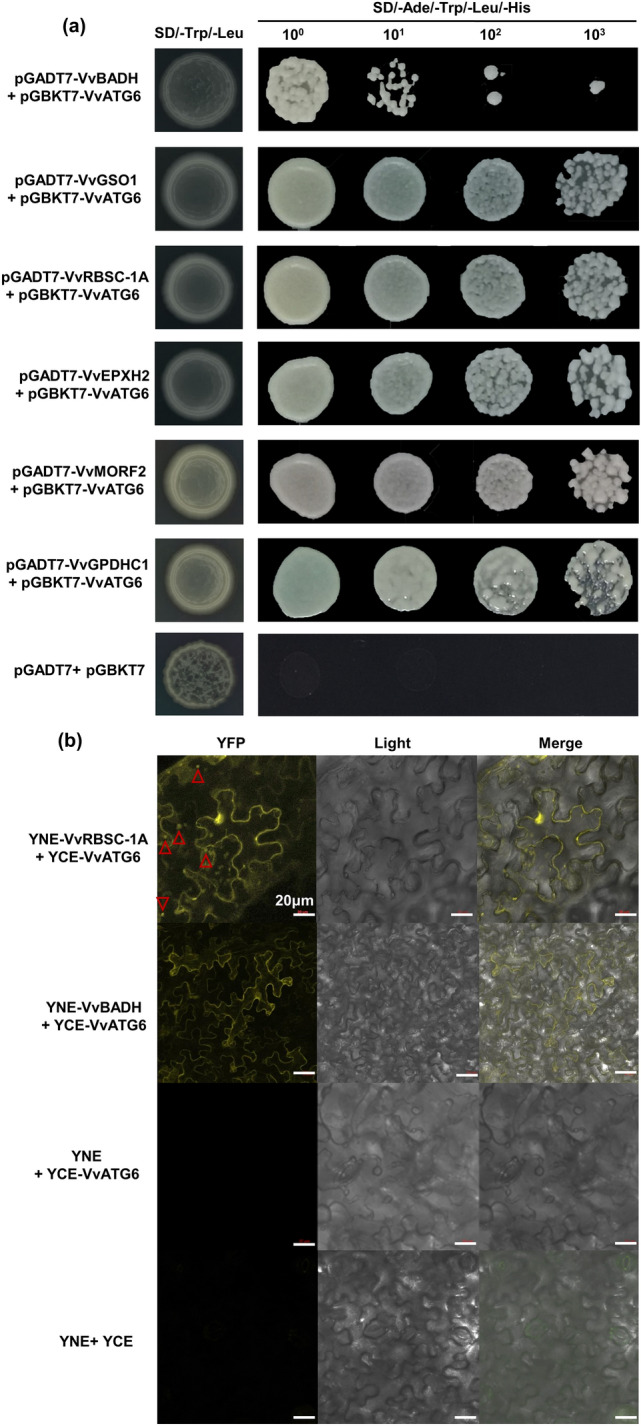
Screening and validation of the VvATG6 interaction candidate proteins. a The Y2H validation of the proteins that interacted with VvATG6. SD/-Trp/-Leu, SD media without tryptophan and leucine; SD/-Ade/-Trp/-Leu/-His, SD media without adenine, histidine, tryptophan and leucine; 10n, dilution of the yeast; pGADT7 + pGBKT7, negative control. b BiFC assay of the VvATG6 interaction candidate proteins in (a). At the far left of images are cells with a yellow YFP signal. Light is the bright-field images of the same cells, and the images at the end are overlays of the bright-field and fluorescence images. We labeled five cells, and the five red triangles represent the nuclei of these cells. An irregular polygon that is surrounded by a yellow curve is the cell membrane. BIFC—bimolecular fluorescent complementation, Y2H—yeast 2-hybrid assay, YFP–yellow fluorescent protein
Furthermore, a BiFC assay was performed to verify the interactive relationship between VvATG6 and the six candidate proteins described above. The results indicated that the control groups YCE + YNE and YNE + YCE-VvATG6 had no yellow fluorescence, while YNE-VvBADH + YCE-VvATG6 only had yellow fluorescence in the cell membrane; YNE-VvRBSC-1A + YCE-VvATG6 had strong fluorescence in the nucleus and cell membrane (Fig. 3b). It was hypothesized that VvATG6 interacted with VvBADH and VvRBSC-1A in the nucleus or cell membrane. The results of the other four proteins were similar to those of the control groups (Fig. S3a).
We took into consideration that our transcriptome data showed that the levels of gene transcripts (VvBADH4, GrapeSMv01_17g0867; and VvRBSC-1A, GrapeSMv01_17g0415) that encode these two interacting proteins were affected under Cu stress. Our results showed that the expression of VvBADH4 increased under the Cu treatment, while VvRBSC-1A was downregulated first and then upregulated, indicating they might be involved in the process of plant response to Cu stress (Fig. S3b and Table S16).
Overexpression of VvATG6 can promote seed germination and root growth in transgenic Arabidopsis thaliana under Cu stress
To investigate whether VvATG6 contributes to Cu tolerance in plants, we obtained three independent transgenic overexpression A. thaliana lines, including line1 (L1), line2 (L2), and line3 (L3). The phenotypic observations, semi-quantitative PCR, and quantitative real-time PCR analysis indicated that the VvATG6 was successfully integrated into the genome of A. thaliana genome (Fig. S4a–c and Table S7).
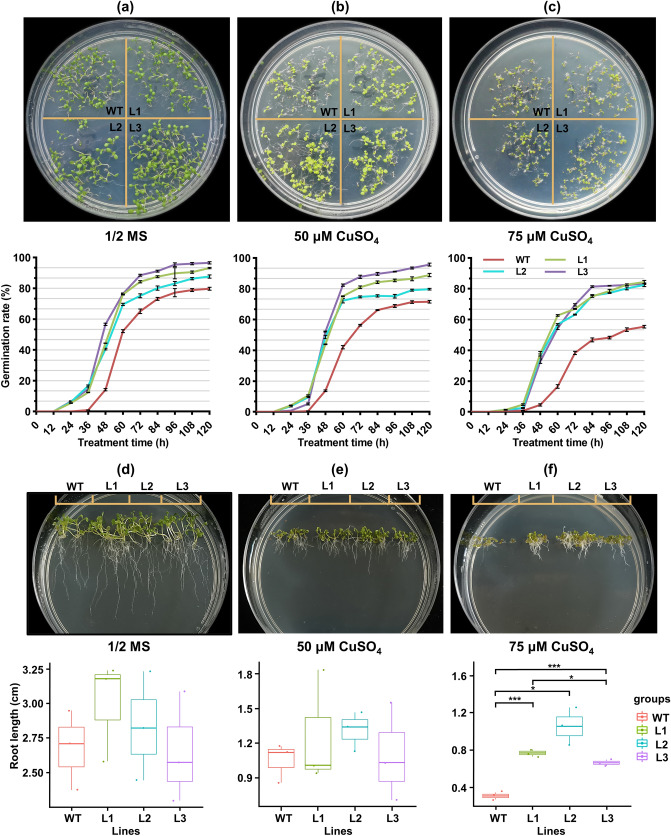
Then the germination rate of the seeds in the A. thaliana lines was counted every 12 h for 120 h, and the phenotype was observed after 10 d by exposing the WT and overexpression plants to 0, 50, and 75 μM CuSO4. The results showed that the time of germination of the overexpressed lines generally appeared after 12 h, while that of the WT appeared after 24 h, indicating that the overexpressed lines germinated earlier than those of the WT, and the rate of germination was also higher than that of the WT (Fig. 4a–c and Table S8). Specifically, in one-half MS media, the rate of seed germination of the overexpressed plants increased by 10.26–21.24% compared with the WT. Under one-half MS media that contained 50 μM Cu, the rate of germination of the overexpressed plants increased by 10.08–33.10% compared with the WT. Under one-half MS media that contained 75 μM Cu, the rate of germination of the overexpressed plants increased by 51.43%–54.28% compared with the WT. Clearly, compared with the WT, the rate of seed germination of the overexpressed plants increased by a larger factor under Cu stress than that in the untreated media. This indicated that the overexpressed plants were more tolerant to Cu stress than the WT plants, which resulted in a higher rate of germination.
Fig. 4.
Overexpression of VvATG6 can promote seed germination and root growth in transgenic Arabidopsis thaliana under Cu stress. (a–c) Germination status and germination rate statistics of the WT and overexpressed VvATG6 lines on one-half MS media that contained 0, 50, and 75 μM CuSO4. (d–f) The root development status and root length statistics of the WT and overexpressed VvATG6 lines on one-half MS media that contained 0, 50, and 75 μM CuSO4. The data are shown as three replicates. *P < 0.05 as shown by a one-way ANOVA and Duncan’s multiple range test. ANOVA—analysis of variance, CuSO4—copper sulfate, L1—line 1, L2—line 2, L3—line 3, WT—wild type
Next, we observed that there was no significant difference in the growth of roots between the WT and overexpressed plants under one-half MS media and one-half MS media that contained 50 μM Cu. In contrast, the growth of the roots of overexpressed plants increased by 148.39%–241.94% compared with the WT in one-half MS media that contained 75 μM Cu. This indicated that the overexpression of VvATG6 effectively alleviated the negative effects of Cu stress on the A. thaliana seedling root growth (Fig. 4d–f and Table S9). Taken together, these results suggested that VvATG6 contributed to Cu tolerance in A. thaliana by promoting seed germination and root growth under Cu stress.
Overexpression of VvATG6 positively regulated the expression of antioxidant-related genes in transgenic Arabidopsis thaliana under Cu stress
When stained with DAB in A. thaliana, which is used to examine the accumulation of H2O2, no dyeing was observed in the leaves of WT and overexpressed plants without Cu stress, while the WT leaves showed more intensely colored patches compared with the transgenic lines in the presence of Cu stress, thus, indicating that the overexpression lines accumulated less H2O2 and were subjected to less oxidative stress (Fig. 5a). Thus, we hypothesized that VvATG6 could potentially regulate the antioxidant system to mitigate the negative effects of Cu toxicity on A. thaliana.
Fig. 5.
Overexpression of VvATG6 enhanced the antioxidant ability of transgenic Arabidopsis thaliana under Cu stress. a In situ accumulation of H2O2 in the leaves treated with (right panels) and without (left panels) Cu stress revealed by DAB staining. b Changes in the levels of expression of the antioxidant-related genes in WT and transgenic A. thaliana with (64 h) or without (0 h) Cu treatment. Data are shown as the means of three replicates ± SE. Different letters indicate significant differences between the treatments according to a one-way ANOVA and Duncan’s multiple range test (P < 0.05). Cu—copper, DAB—3,3’-diaminobenzidine, H2O2—hydrogen peroxide, SE—standard error, WT—wild type
To test this possibility, we first used quantitative PCR (qPCR) to investigate the antioxidant-related genes in A. thaliana seedlings and found that the levels of transcription of AtGPX6, AtPOD, AtAPX3, AtMDHAR3, and AtCAT2 were significantly upregulated in the overexpression lines compared with WT under Cu stress, suggesting that antioxidant system participated in the Cu stress (Fig. 5b and Table S10). Interestingly, compared with the WT, the level of expression of AtMDHAR3 did not change significantly under the control conditions, while it increased by 6.29- to 44.20-fold under Cu stress. This indicated that AtMDHAR3 played crucial roles in the resistance of plants to Cu. In addition, VvATG6 was stably expressed in all the lines, and the level of expression of the transgenic lines was significantly higher than that of the WT under Cu stress (Fig. 5b and Table S10). Additionally, through the overexpression of VvATG6 in tobacco (Fig. S5a–c and Table S11), we also found that the transgenic plants did reduce the levels of H2O2 and MDA compared with the WT plants under Cu stress to some extent and increased the activities of SOD, POD and CAT, as well as the levels of expression of the related antioxidant genes like NtCAT1, NtAPX3, and NtGPX6 (Fig. S5d–j and Tables S12–13). Briefly, the overexpression of VvATG6 could confer tolerance to copper by affecting the plant’s antioxidant system.
Overexpression of VvATG6 might activate the antioxidant system by intensifying autophagic activity to enhance the tolerance of grape calli to Cu
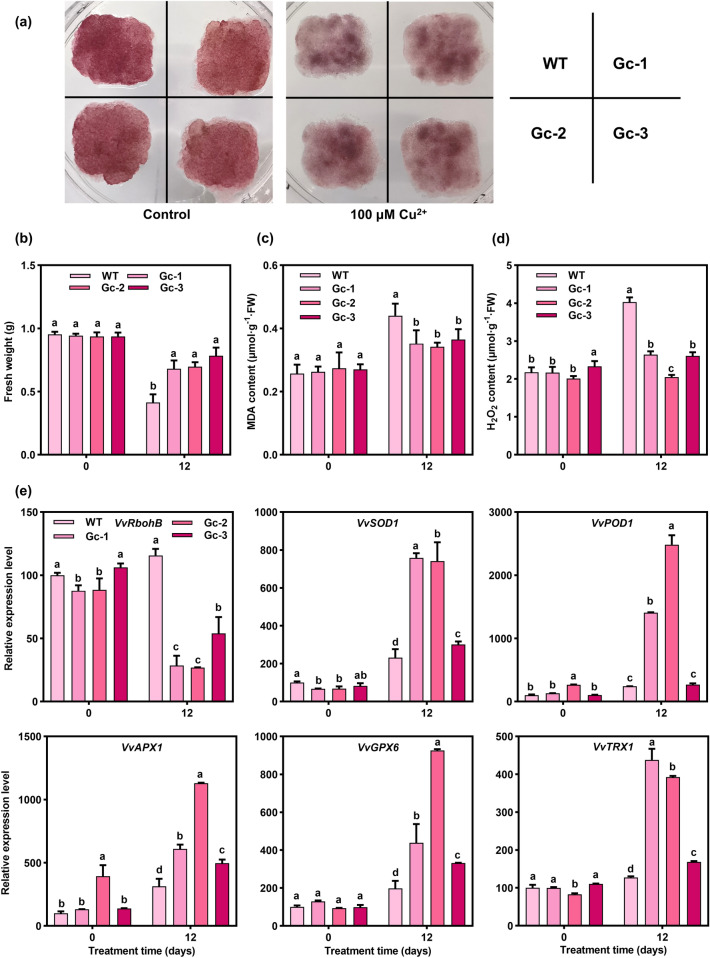
To determine how VvATG6 affects the plant antioxidant system, we obtained grape calli that overexpressed VvATG6, including Gc-1, Gc-2, and Gc-3 (Fig. S6a–c and Table S14). As shown in Fig. 6a, no significant changes were observed in the WT and transgenic calli under control conditions, while after 12 d of Cu treatment, all the lines were darkened, and their growth was inhibited. However, the transgenic lines were inhibited less strongly than those of the WT. More specifically, compared with the control conditions, after 12 d of Cu treatment, the fresh weight of the grape calli decreased by 130.75% in the WT, 38.68% in Gc-1, 38.68% in Gc-2, and 19.50% in Gc-3 (Fig. 6b and Table S15). Measurements of MDA and H2O2 showed that the transgenic lines had much less substantial damage and accumulated less ROS than the WT under Cu treatment, respectively (Fig. 6c–d and Table S15). Moreover, after the Cu treatment, the levels of expression of the genes involved in ROS synthesis and scavenging were decreased and increased in the transgenic lines compared with the WT lines, respectively (Fig. 6e and Table S16). These results suggested that the overexpression of VvATG6 improved the ability of grape calli to tolerate Cu by activating the antioxidant system.
Fig. 6.
Overexpression of VvATG6 might activate the ROS homeostasis system to enhance the tolerance of grape calli to Cu. a Phenotypic observation of the WT and overexpressed grape callus treated with 100 µM CuSO4 for 0 and 12d. b The fresh weight in the WT and transgenic grape calli with (12 d) or without (0 d) Cu treatment. Content of c malondialdehyde (MDA), d hydrogen peroxide (H2O2) in the WT and transgenic grape calli with (12 d) or without (0 d) Cu treatment. e Changes in the levels of expression of antioxidant-related genes in the WT and transgenic grape calli with (12 d) or without (0 d) Cu treatment. Data are shown as the means of three replicates ± SE. Different letters indicate significant differences between the treatments according to a one-way ANOVA and Duncan’s multiple range test (P < 0.05). ANOVA—analysis of variance, Cu—copper, CuSO4—copper sulfate, SE—standard error, WT—wild type
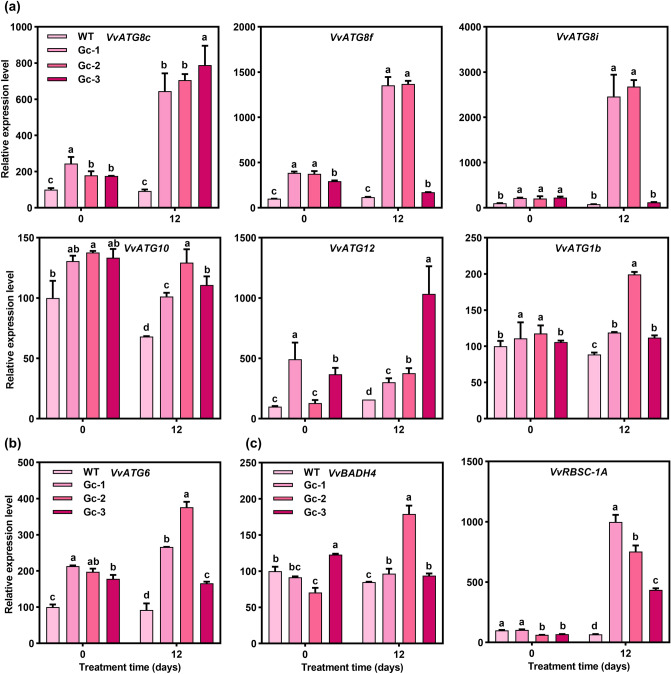
To analyze whether the autophagic activity changed in grape calli under Cu stress, we examined the levels of expression of other important VvATGs after 12 d of Cu treatment (Fig. 7a and Table S16). The levels of expression of these tested VvATGs showed a slight increase in its levels in the transgenic grape calli under the control conditions. In response to the Cu stress, except for VvATG10 and VvATG1b; VvATG8c, VvATG8f, VvATG8i, and VvATG12 changed indistinctively in the WT plants, while they were dramatically induced among the transgenic lines. Moreover, the level of expression of VvATG8c, which directly mediates the amplification of autophagosomes (Xia et al. 2012), was not significantly induced by Cu treatment in the WT plants, but it was induced to 6.98-, 7.64-, and 8.53-fold in the Gc-1, 2, and 3 plants by Cu stress, respectively. Moreover, the pattern of expression of VvATG6 was similar to that of the VvATGs (Fig. 7b and Table S16). Additionally, the levels of expression of VvBADH4 and VvRBSC-1A in the transgenic lines were much higher than those in the WT lines (Fig. 7c and Table S16). Taken together, these results demonstrate that the overexpression of VvATG6 promotes the occurrence of autophagy to affect the antioxidant system in grape calli under Cu stress.
Fig. 7.
Overexpression of VvATG6 promoted the occurrence of autophagy in grape calli under Cu stress. a Changes in the expression of autophagy-related genes in the WT and transgenic grape calli with (12 d) or without (0 d) Cu treatment. b Changes in the level of expression of VvATG6 were detected in grape calli under Cu stress. c The levels of VvBADH4 and VvRBSC-1A transcripts associated with these two interacting proteins were measured in grape calli under Cu stress. Data are shown as the means of three replicates ± SE. Different letters indicate significant differences between treatments according to a one-way ANOVA and Duncan’s multiple range test (P < 0.05). ANOVA—analysis of variance, Cu—copper, SE—standard error, WT—wild type
Discussion
Autophagy is a conserved cellular process that functions in the maintenance of physiological and metabolic balance (Michaeli et al. 2016). In this study, we characterized the role of VvATG6, a grapevine gene associated with autophagy, in the response to Cu stress. The Cu treatment resulted in a limited rate of germination and root growth in A. thaliana plants, brown tobacco leaves, and slow growth in grape calli. However, this limitation was alleviated to some extent in the lines that overexpressed VvATG6. This difference was manifested by better growth and a higher production of biomass in the transgenic plants under Cu stress (Figs. 4a–f and 6a–b). Therefore, we concluded that the overexpression of VvATG6 can improve the Cu tolerance of plants. This was consistent with previous studies that showed that the upregulation of ATG6 contributes to the plant response to abiotic stress (Rana et al. 2012; Zeng et al. 2017; Cao et al. 2022).
In this study, we showed that the overexpression of VvATG6 contributed to the plant tolerance to Cu owing to the activation of its antioxidant system. Similar phenomena have been observed in previous studies. For example, the overexpression of MdATG18a in apple improved resistance to Marssonia blotch disease caused by Diplocarpon mali owing to its enhanced antioxidant activity (Sun et al. 2018a). Plants are prone to generate and accumulate harmful ROS under excessive Cu stress, which leads to lipid peroxidation and electrolyte leakage among other negative effects in the cells and ultimately affects crop growth (Chen et al. 2021; Ren et al. 2022). Autophagy may be necessary to clear the ROS and oxidation products (Qi et al. 2021). In this study, we found that the plants that overexpressed VvATG6 accumulated lower levels of ROS compared with the WT plants under Cu stress (Figs. 5a, S5d, and 6d). Conversely, the level of accumulation of ROS in the roots of atg mutant was higher than that of the WT after waterlogging (Guan et al. 2019). MDA is a proxy that is commonly used to evaluate abiotic stress tolerance based on the degree of membrane damage (Tang et al. 2013). In this study, the MDA content of the VvATG6 transgenic plants was significantly lower than that of the WT under Cu stress (Fig. S5e, and 6c). These results showed that the transgenic plants suffered less oxidative damage than the WT plants.
Autophagy can also improve the antioxidant system by regulating the activity of antioxidant enzymes and the level of expression of the antioxidant-related genes (Jia et al. 2021). Enzyme defense systems that remove ROS include SOD, CAT, POD, and APX (Mansoor et al. 2022). The SOD first converts oxide (O2−) into O2 and H2O2 by a disproportionation reaction that reduces the formation of hydroxide (OH−) (Lu et al. 2017). Subsequently, CAT, POD and APX rapidly degrade H2O2 into H2O and O2 (Mhamdi et al. 2010). Our results showed that the transgenic plants had higher activities of SOD, POD, and CAT than the WT after Cu treatment (Fig. S5f–h). Notably, we also found that with the extension of stress time, the activities of POD and SOD decreased, which could be because the enzyme structures were irreversibly damaged by long-term treatment (Xia et al. 2023). Furthermore, the overexpression of the corresponding genes of the antioxidant enzymes resulted in an increase in the potential tolerance of the plants to metal stress conditions (Lee et al. 2007). For example, the overexpression of SaCu/Zn SOD in Sedum alfredii enhanced the plant’s antioxidative defense capacity and has been implicated as the reason why the plants were tolerant to cadmium (Cd) (Li et al. 2017). Our findings supported the hypothesis that the levels of expression of SOD, POD, APX, and CAT in the OE-VvATG6 lines under Cu stress were much higher than those in the WT (Figs. 5b, S5i, and 6e). As an antioxidant system, the ascorbic acid-glutathione (AsA-GSH) cycle plays an important role in removing the H2O2 produced by plants under stress (Sun et al. 2018b). We also observed stronger increases in the levels of APX, MDHAR, GPX, and TRX in the transgenic plants compared with the WT under Cu stress (Figs. 5b, S5i, and 6e). These results were consistent with the significant upregulation of these genes in Cu-tolerant grapevine (Xia et al. 2023). The synthesis of ROS is regulated by the Rboh gene family (Chapman et al. 2019). In this study, we found that the levels of expression of RbohB and RbohC decreased in the transgenic lines under Cu treatment (Figs. S5i and 6e), indicating that the overexpression of VvATG6 inhibited the synthesis of ROS. These results suggest that overexpressing VvATG6 improves the antioxidant system and thereby, promotes the degradation of ROS in response to Cu stress.
ATG6 has been reported to be responsible for the nucleation of autophagosomes (Baskaran et al. 2014), and the overexpression of this gene could also promote expression of other ATGs. Indeed, in this study, we found that the exogenous overexpression of VvATG6 in grape calli stressed by Cu triggered the upregulation of the endogenous ATGs (Fig. 7a). ATG1 mediates the initiation step (Noda and Fujioka 2015), and we found that the overexpression of VvATG6 significantly increased the levels of expression of VvATG1b in the transgenic lines compared with the WT under Cu stress (Fig. 7a). ATG8 is a key member of the ubiquitin–like conjugation systems that are responsible for the maturation of autophagosomes (Nakatogawa 2013). Additionally, the VvATG8s were more highly expressed in the transgenic lines than in the WT under Cu stress (Fig. 7a). Overall, these results demonstrate that VvATG6 promotes autophagy by upregulating the other key ATGs necessary for autophagosome production, which could also enhance the antioxidant system. In our previous studies, we also found that Cu stress can induce the autophagosome in grapevine leaves (Chen et al. 2021), but whether it is the increase of ATG6 that causes the increase of autophagosomes under Cu stress remains unclear. Thus, this will also be the direction of our future research. This study not only proved the function of ATGs, which is a key factor in the tolerance of grape to copper, but also provided a theoretical basis for the further discovery and characterization of the genes for the tolerance of copper in grape.
Conclusion
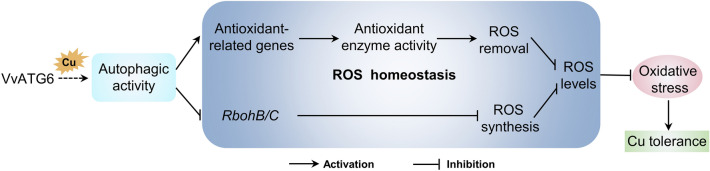
Our results demonstrated that the overexpression of VvATG6 enhanced the tolerance of plants to Cu by improving their antioxidative levels (Fig. 8). First, the levels of expression of the antioxidant-related genes were higher in the transgenic plants, which would promote the activity of antioxidant enzymes, such as SOD, POD, and CAT, thereby accelerating the removal of ROS under Cu stress. Moreover, in response to Cu treatment, lower levels of RbohB and RbohC in the plants that overexpressed VvATG6 led to a suppression of ROS synthesis. These might be a cause of the accumulation of less ROS in transgenic plants under Cu stress, thereby alleviating the oxidative stress on the physiological processes in plants. Additionally, we found that VvATG6 regulates autophagy by influencing the other genes related to this process, thereby enhancing the tolerance of Cu to plants. However, the molecular mechanisms involved in this process merit further investigation. This study increases our understanding of the role that autophagy plays in promoting tolerance to Cu in plants.
Fig. 8.
A hypothetical model for VvATG6 contributing to copper stress tolerance by enhancing the antioxidant ability
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This project was funded by Jiangsu Agriculture Science and Technology Innovation Fund (CX (23) 3063), Jiangsu Province key research and development plan (modern agriculture) project (BE2022381), Ningxia Hui Autonomous Region key R&D Plan (2023BCF01001), Jiangsu Modern Agricultural Industrial Technology System Construction Project (JATS [2022] 457), Shandong Province Key R&D Plan (Agricultural Improved Breed Project, 2022LZGCQY1018), and Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (RAPD). We also thanks for the support of Bioinformatics Center of Nanjing Agricultural University.
Author Contributions
JX conducted the experiments, managed and formalized the data, and edited the manuscript. ZW conducted the experiments and analyzed data. SL, XF, and AH conducted the experiments. JF and LS was responsible for project administration and funding acquisition. All authors read and approved the manuscript.
Data availability
The analyzed transcriptome data are available in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database under the accession number PRJNA694453, PRJNA770426, and PRJNA865402.
Declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiaxin Xia and Zicheng Wang have contributed equally.
References
- Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol. 2010;30(3):161–175. doi: 10.3109/07388550903524243. [DOI] [PubMed] [Google Scholar]
- Baskaran S, Carlson L-A, Stjepanovic G, Young LN, Kim DJ, Grob P, Stanley RE, Nogales E, Hurley JH. Architecture and dynamics of the autophagic phosphatidylinositol 3-kinase complex. Elife. 2014;3:e05115. doi: 10.7554/eLife.05115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Zheng X, Xie D, Zhou H, Shao S, Zhou J. Autophagic pathway contributes to low-nitrogen tolerance by optimizing nitrogen uptake and utilization in tomato. Horticulture Res. 2022;9:ac068. doi: 10.1093/hr/uhac068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JM, Muhlemann JK, Gayomba SR, Muday GK. RBOH-dependent ROS synthesis and ROS scavenging by plant specialized metabolites to modulate plant development and stress responses. Chem Res Toxicol. 2019;32(3):370–396. doi: 10.1021/acs.chemrestox.9b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Fang X, Wang Z, Shangguan L, Liu T, Chen C, Liu Z, Ge M, Zhang C, Zheng T. Multi-omics analyses on the response mechanisms of ‘Shine Muscat’grapevine to low degree of excess copper stress (Low-ECS) Environ Pollut. 2021;286:117278. doi: 10.1016/j.envpol.2021.117278. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Dai L, Zhou Q, Li R, Yangjian D, He J, Wang D, Cheng S, Zhang J, Wang Y. Establishment of a picloram-induced somatic embryogenesis system in Vitis vinifera cv. chardonnay and genetic transformation of a stilbene synthase gene from wild-growing Vitis species. Plant Cell, Tissue Organ Culture (PCTOC) 2015;121(2):397–412. doi: 10.1007/s11240-015-0711-9. [DOI] [Google Scholar]
- Fu XZ, Zhou X, Xu YY, Hui QL, Chun CP, Ling LL, Peng LZ. Comprehensive analysis of autophagy-related genes in sweet orange (Citrus sinensis) highlights their roles in response to abiotic stresses. Int J Mol Sci. 2020;21(8):2699. doi: 10.3390/ijms21082699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallois P, Marinho P. Leaf disk transformation using Agrobacterium tumefaciens-expression of heterologous genes in tobacco. Plant Gene Transfer Expression Protocols. 1995;1995:39–48. doi: 10.1385/0-89603-321-X:39. [DOI] [PubMed] [Google Scholar]
- Geng A, Wang X, Wu L, Wang F, Wu Z, Yang H, Chen Y, Wen D, Liu X. Silicon improves growth and alleviates oxidative stress in rice seedlings (Oryza sativa L.) by strengthening antioxidant defense and enhancing protein metabolism under arsanilic acid exposure. Ecotoxicol Environ Saf. 2018;158:266–273. doi: 10.1016/j.ecoenv.2018.03.050. [DOI] [PubMed] [Google Scholar]
- Guan B, Lin Z, Liu D, Li C, Zhou Z, Mei F, Li J, Deng X. Effect of waterlogging-induced autophagy on programmed cell death in Arabidopsis roots. Front Plant Sci. 2019;10:468. doi: 10.3389/fpls.2019.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HY, Ren QQ, Lai YH, Peng MY, Zhang J, Yang LT, Huang ZR, Chen LS. Metabolomics combined with physiology and transcriptomics reveals how Citrus grandis leaves cope with copper-toxicity. Ecotoxicol Environ Saf. 2021;223:112579. doi: 10.1016/j.ecoenv.2021.112579. [DOI] [PubMed] [Google Scholar]
- Huo L, Guo Z, Jia X, Sun X, Wang P, Gong X, Ma F. Increased autophagic activity in roots caused by overexpression of the autophagy-related gene MdATG10 in apple enhances salt tolerance. Plant Sci. 2020;294:110444. doi: 10.1016/j.plantsci.2020.110444. [DOI] [PubMed] [Google Scholar]
- Huo L, Guo Z, Zhang Z, Jia X, Sun Y, Sun X, Wang P, Gong X, Ma F. The apple autophagy-related gene MdATG9 confers tolerance to low nitrogen in transgenic apple callus. Front Plant Sci. 2020;11:423. doi: 10.3389/fpls.2020.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Jia X, Li T, Wang Y, Sun X, Huo L, Wang P, Che R, Gong X, Ma F. MdATG5a induces drought tolerance by improving the antioxidant defenses and promoting starch degradation in apple. Plant Sci. 2021;312:111052. doi: 10.1016/j.plantsci.2021.111052. [DOI] [PubMed] [Google Scholar]
- Lee S-H, Ahsan N, Lee K-W, Kim D-H, Lee D-G, Kwak S-S, Kwon S-Y, Kim T-H, Lee B-H. Simultaneous overexpression of both CuZn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. J Plant Physiol. 2007;164(12):1626–1638. doi: 10.1016/j.jplph.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Li Z, Han X, Song X, Zhang Y, Jiang J, Han Q, Liu M, Qiao G, Zhuo R. Overexpressing the Sedum alfredii Cu/Zn superoxide dismutase increased resistance to oxidative stress in transgenic Arabidopsis. Front Plant Sci. 2017;8:1010. doi: 10.3389/fpls.2017.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhang K, Gill RA, Islam F, Farooq MA, Wang J, Zhou W. Ecotoxicological and interactive effects of copper and chromium on physiochemical, ultrastructural, and molecular profiling in Brassica napus L. Biomed Res Int. 2018 doi: 10.1155/2018/9248123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu C, Sun X, Liu B, Zhang X, Liang W, Huo L, Wang P, Ma F, Li C. Overexpression of MdATG18a enhances alkaline tolerance and GABA shunt in apple through increased autophagy under alkaline conditions. Tree Physiol. 2020;40(11):1509–1519. doi: 10.1093/treephys/tpaa075. [DOI] [PubMed] [Google Scholar]
- Li P, Yu D, Gu B, Zhang H, Liu Q, Zhang J. Overexpression of the VaERD15 gene increases cold tolerance in transgenic grapevine. Sci Hortic. 2022;293:110728. doi: 10.1016/j.scienta.2021.110728. [DOI] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu T, Meng Z, Zhang G, Qi M, Sun Z, Liu Y, Li T. Sub-high temperature and high light intensity induced irreversible inhibition on photosynthesis system of tomato plant (Solanum lycopersicum L.) Front Plant Sci. 2017;8:365. doi: 10.3389/fpls.2017.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor S, Ali Wani O, Lone JK, Manhas S, Kour N, Alam P, Ahmad A, Ahmad P. Reactive oxygen species in plants: from source to sink. Antioxidants. 2022;11(2):225. doi: 10.3390/antiox11020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G. Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot. 2010;61(15):4197–4220. doi: 10.1093/jxb/erq282. [DOI] [PubMed] [Google Scholar]
- Michaeli S, Galili G, Genschik P, Fernie AR, Avin-Wittenberg T. Autophagy in plants–what's new on the menu? Trends Plant Sci. 2016;21(2):134–144. doi: 10.1016/j.tplants.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Nakatogawa H. Two ubiquitin-like conjugation systems that mediate membrane formation during autophagy. Essays Biochem. 2013;55:39–50. doi: 10.1042/bse0550039. [DOI] [PubMed] [Google Scholar]
- Noda NN, Fujioka Y. Atg1 family kinases in autophagy initiation. Cell Mol Life Sci. 2015;72:3083–3096. doi: 10.1007/s00018-015-1917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Xia FN, Xiao S. Autophagy in plants: Physiological roles and post-translational regulation. J Integr Plant Biol. 2021;63(1):161–179. doi: 10.1111/jipb.12941. [DOI] [PubMed] [Google Scholar]
- Rana R, Dong S, Ali Z, Huang J, Zhang H. Regulation of ATG6/Beclin-1 homologs by abiotic stresses and hormones in rice (Oryza sativa L.) Genet Molecular Res. 2012;11(4):3676–3687. doi: 10.4238/2012.August.17.3. [DOI] [PubMed] [Google Scholar]
- Ren QQ, Huang ZR, Huang WL, Huang WT, Chen HH, Yang LT, Ye X, Chen LS. Physiological and molecular adaptations of Citrus grandis roots to long-term copper excess revealed by physiology, metabolome and transcriptome. Environ Exp Bot. 2022;203:105049. doi: 10.1016/j.envexpbot.2022.105049. [DOI] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Shangguan L, Fang X, Chen L, Cui L, Fang J. Genome-wide analysis of autophagy-related genes (ARGs) in grapevine and plant tolerance to copper stress. Planta. 2018;247(6):1449–1463. doi: 10.1007/s00425-018-2864-3. [DOI] [PubMed] [Google Scholar]
- Sun X, Huo L, Jia X, Che R, Gong X, Wang P, Ma F. Overexpression of MdATG18a in apple improves resistance to Diplocarpon mali infection by enhancing antioxidant activity and salicylic acid levels. Horticulture Res. 2018;5:57. doi: 10.1038/s41438-018-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Wang P, Jia X, Huo L, Che R, Ma F. Improvement of drought tolerance by overexpressing MdATG18a is mediated by modified antioxidant system and activated autophagy in transgenic apple. Plant Biotechnol J. 2018;16(2):545–557. doi: 10.1111/pbi.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Cai H, Ji W, Luo X, Wang Z, Wu J, Wang X, Cui L, Wang Y, Zhu Y. Overexpression of GsZFP1 enhances salt and drought tolerance in transgenic alfalfa (Medicago sativa L.) Plant Physiol Biochem. 2013;71:22–30. doi: 10.1016/j.plaphy.2013.06.024. [DOI] [PubMed] [Google Scholar]
- Wang QY, Liu JS, Wang Y, Yu HW. Accumulations of copper in apple orchard soils: distribution and availability in soil aggregate fractions. J Soils Sediments. 2015;15:1075–1082. doi: 10.1007/s11368-015-1065-y. [DOI] [Google Scholar]
- Wang Y, Cai S, Yin L, Shi K, Xia X, Zhou Y, Yu J, Zhou J. Tomato HsfA1a plays a critical role in plant drought tolerance by activating ATG genes and inducing autophagy. Autophagy. 2015;11(11):2033–2047. doi: 10.1080/15548627.2015.1098798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Huang H, Peng M, Lai Y, Ren Q, Zhang J, Huang Z, Yang L, Rensing C, Chen L. Adaptive Responses of Citrus grandis leaves to copper toxicity revealed by RNA-Seq and physiology. Int J Mol Sci. 2021;22(21):12023. doi: 10.3390/ijms222112023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Xiao D, Liu D, Chai W, Gong Q, Wang NN. Heterologous expression of ATG8c from soybean confers tolerance to nitrogen deficiency and increases yield in Arabidopsis. PLoS ONE. 2012;7(5):e37217. doi: 10.1371/journal.pone.0037217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Chen C, Liu T, Liu C, Liu S, Fang J, Shangguan L. Germplasm resource evaluation and the underlying regulatory mechanisms of the differential copper stress tolerance among Vitis species. Environ Exp Bot. 2023;206:105198. doi: 10.1016/j.envexpbot.2022.105198. [DOI] [Google Scholar]
- Zeng X, Zeng Z, Liu C, Yuan W, Hou N, Bian H, Zhu M, Han N. A barley homolog of yeast ATG6 is involved in multiple abiotic stress responses and stress resistance regulation. Plant Physiol Biochem. 2017;115:97–106. doi: 10.1016/j.plaphy.2017.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The analyzed transcriptome data are available in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database under the accession number PRJNA694453, PRJNA770426, and PRJNA865402.