Abstract
Metastatic breast cancer could cause various psychological symptoms. Managing Cancer and Living Meaningfully (CALM) is a brief, manualized psychotherapy that has been validated for advanced cancer patients. We conducted a pilot randomized control trial (RCT) to verify the feasibility and preliminary efficacy of CALM therapy in this population. Patients who met the inclusion criteria were randomly assigned into CALM or Wait-list Control (WLC) groups. Patients in the CALM group received CALM therapy and usual care; patients in WLC group first received usual care and then underwent CALM therapy after completing all assessments. All patients were asked to complete three assessments: T0(baseline), T1(3 months), and T2(6 months). The primary outcomes was death anxiety; other outcomes were depression, distress, suicide ideation, attachment security, spiritual well-being and quality of life at the end of life. Analysis of Covariance (ANCOVA) and t-test were used for statistics analysis. Thirty-six patients were randomly assigned to either of the two groups, with 34 patients completing the three assessments. At six months, we found significant between group differences in suicide ideation, distress, and life completion between the CALM and WLC groups. At T2, patients in CALM group reported lower levels of depression (F = 5.016, p = 0.033, partial η2 = 0.143), distress (F = 7.969, p = 0.010, partial η2 = 0.257), attachment avoidance (F = 4.407, p = 0.044, partial η2 = 0.128), and better sense of life completion (F = 5.493, p = 0.026, partial η2 = 0.155) than patients in the WLC group. Compared with results of the T0 assessments, we found significant differences in socres for depression (T2&T0, t = − 2.689, p = 0.011, Cohen’s d = 0.940) and distress (T2&T0, t = − 2.453, p = 0.022, Cohen’s d = 0.965) between the two groups. CALM therapy was well received by the study population, and CALM therapy can reduce depression, distress, attachment avoidance while improving quality of life in Chinese metastatic breast cancer patients. A Phase III RCT was recommended to verify the impact of CALM therapy on psychological burden and survival in this population.
Trial registration: This study is part of the “Preliminary application study for Managing Cancer and Living Meaningfully (CALM) therapy in Chinese advanced cancer patients” clinical trial, with the Trial Registration Number of ChiCTR1900023129 (13/05/2019) in the Chinese Clinical Trial Registry (ChiCTR) website. (https://www.chictr.org.cn/index.html).
Subject terms: Palliative care, Breast cancer, Anxiety, Depression, Cancer, Psychology
Introduction
The latest cancer statistics from the National Cancer Center in China indicate that breast cancer remains the most common type of cancer among women in terms of incidence (45.37‰), while the mortality rate (10.62‰) ranked fifth after lung, stomach, liver, and colorectal cancers1. With increased national medical resources, the 5-year survival rate of cancer in China has improved significantly from 30.9% in 2003 to 40.5% in 2015, and the 5-year survival rate for breast cancer has increased from 73.1 to 82.0%2. Nevertheless, approximately 3%-10% of new breast cancer cases each year still present remote metastases at initial diagnosis. In addtion, 30% of early stages patients eventually progress to advanced stages; and the five-year survival rate for this population is only 20%3. When breast cancer progresses beyond cure, the quality and duration of patient' survival becomes even more challenging; disease invasion and clinical treatment produce more somatic symptoms, such as pain and fatigue, and patients' quality of life diminishes significantly4; In additon, the psychological burden also increases, and there is a preponderance of anxiety and depression, which interact with physical symptoms5. Several studies has demonstrated that 84.1% of patients with advanced breast cancer have clinical anxiety and 25.2% have clinical depression6. The impact of depression is particularly severe in patients with advanced disease. The findings of previous studies have revealed that depression can affect patient survival by decreasing the patient’s ability to care for themselves, thus decreasing patient compliance with antitumor treatment7.
Fear of death is a fundamental aspect of human existence, and this fear is amplified when an individual become truely aware of inevitability of death. Death anxiety is a prevalent psychosocial issue among patients with advanced cancer. Several studies have demonstrated that 32% of advanced cancer patients experience significant levels of death anxiety8 while 43% of advanced non-small cell lung cancer patients experience such level of death anxiety9. A domestic study of Chinese patients reports that 37.6% of young women with advanced breast cancer experience considerable death anxiety10. Factors influencing death anxiety in patients with advanced cancer include depression, demoralization, fear of disease progression, and declining intimacy11. Hence, high-quality psychological interventions for patients with advanced breast cancer should address death anxiety and related factors. Terror Management theory proposes that cultivating a sense of personal worth (a feeling that one's life has meaning and value) is a protective factor for death anxiety and recommends its inclusion in death anxiety interventions12.
Focusing on psychosocial issues in patients with advanced cancer and providing positive and effective psychosocial support has become an important part of the guidelines for quality cancer care in several countries and is strongly recommended by some professional organizations. The Breast Health Global Initiative has developed supportive and palliative care programs for low and middle income countries that include systematic psychosocial and spiritual care13. The National Comprehensive Cancer Network (NCCN) palliative care guidelines recommend the early integration of palliative care into oncology care to improve quality of life for patients with advanced breast cancer14. Notably, Chinese expert consensus on the clinical diagnosis and treatment of advanced breast cancer emphasized multidisciplinary professional care-including psychosocial care15. Kissane et al.16 verified that supportive expressive group therapy (SEGT) can actively improve depression, hopelessness, trauma symptoms and social functions in patients with metastatic breast cancer. However, group therapy challenges, such as high dropout rates, limit its clinical application in patients with advanced cancer. Although individual cognitive therapy has been trialled in patients with metastatic breast cancer, subsequent researches has not verified its efficacy17. Managing Cancer and Living Meaningfully (CALM) is an individual, structured psychological intervention for patients with advanced cancer, with a focus on death anxiety and mood adjustment. Continuous studies have been conducted on CALM therapy in Canada and other countries. The findings of a randomized controlled study with a large sample of advanced cancer patients demonstrate that CALM therapy is significantly effective in improving depression and psychological preparation for the end of life18. Although CALM therapy has been introduced to China for some years and some of its elements has been applied to psychological therapy for advanced cancer patients, online CALM delivery has not been explored prior to this study. Online psychotherapies has demonstrated feasibility for cancer patients19; However, most online psychotherapy focuse on cognitive and behavior therapy and mindfulness approaches with fixed intervention structures20,21. CALM has a relatively flexible structure, and the presentation of the therapeutic domains varies depending on the therapists. No research and clinical application of online CALM therapy has been reported. The COVID-19 pandemic and Community grid management in China limited the feasibility of hospital visits for patients with cancer. Online CALM therapy appeared to be the best option during this period.
Therefore, we conducted this study to explore the following: (1) the feasibility and preliminary efficacy of online CALM therapy for patients with metastatic breast cancer in China; (2) the degree of change that online CALM therapy elicits in this population, as captured by psychometric indicators.
Methods
Patients recruitment
All the patients were recruited from Peking University Cancer Hospital-including outpatients of psycho-oncology department and inpatients at the breast oncology department-between January 2022 to March 2023. This study is part of “Preliminary application study for Managing Cancer and Living Meaningfully (CALM) therapy in Chinese advanced cancer patients (ID: ChiCTR1900023129)” clincal trial, which was registered on the Chinese Clinical Trial Registry (ChiCTR) on 13th May, 2019. This study received ethics approved by Peking University Cancer Hospital Ethics Committee ( No.2019YJZ23). The inclusion criteria were as follows: (1) age ≥ 18 years old; (2) pathologically diagnosed with metastatic breast cancer (UICC TNM stage IV); (3) a ≥15 score on the Death and Dying Distress Scale (DADDS)22; (4) capable of understanding questionnaires; (5) able to provide informed consent. Patients with severe cognitive dysfunction and those undergoing systematic psychosocial interventions were excluded. If the enrolled patients feel uncomfortable with the intervention, they were free to stop the intervention or speak to the therapist without any influence on their clinical cancer treatment.
Study procedure
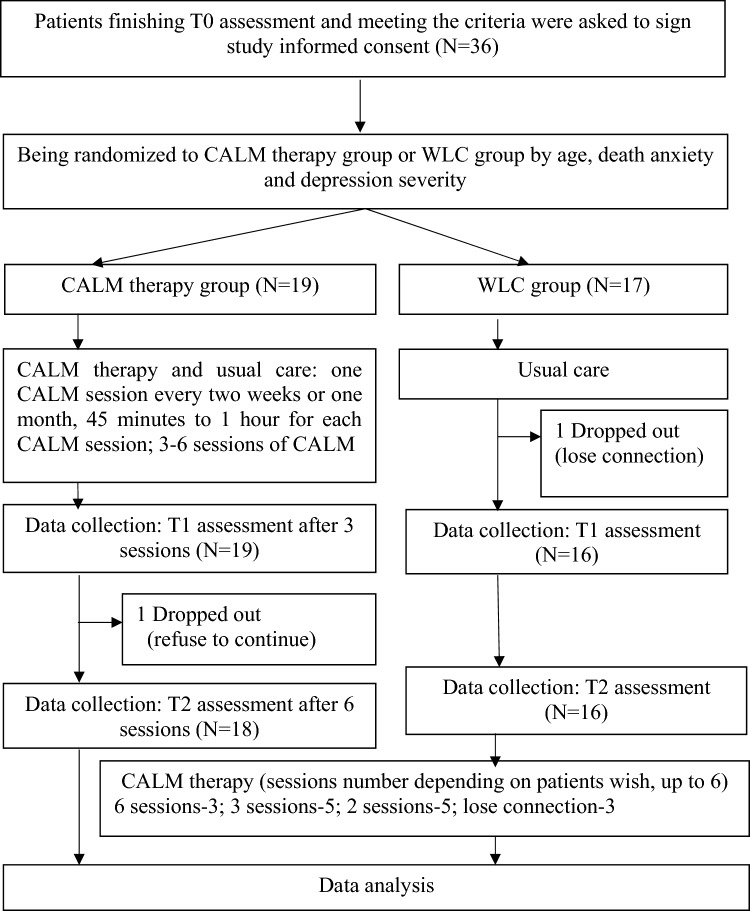
Patients who met the inclusion criteria were asked to register for recruitment: Step 1: Randomised assignment to the CALM group or the wait-list control (WLC) group—the latter involved receiving usual care first and then subsequently receiving CALM therapy or some other complementary psychosocial care after completing several assessments; Step 2: Receiving CALM therapy (CALM group) or first receiving usual care and then waiting to receive psychosocial care later; Step 3: Undergoing T1 and T2 assessments. The detailed study procedure is outlined in Fig. 1.
Figure 1.
Flowchart in this study.
The study participants were assigned to the CALM group or the WLC group in a 1:1 ratio. Dynamic Randomization was computer generated centrally by the Peking University Cancer Hospital Clinical Trials Service Unit: Interactive Web Response System. The randomization was stratified to ensure the study sample was representative of the patients population under study powered by age (≥ 45 or otherwise), PHQ-9 score (≥ 10 or otherwise) and DADDS scores (≥ 45 or otherwise), as these indicators have demonstrated substantial prognostic value and correlation with the outcomes or being the primary outcome itself8,9. This single-centre, prospective, parallel-controlled clinical study was not blinded, as both patients and researchers were aware of the treatment assignment. Patients in CALM group received CALM therapy along with usual care from oncologists and nurses in the breast cancer department as soon as they were recruited to this study. The WLC group initially received only usual care; however, the participants in the WLC group received CALM therapy following their T2 assessment.
As a pilot trial, the key objectives were to examine the feasibility of implementing the online CALM therapy in Chinese patients with metastatic breast cancer, while also allowing pilot test of potential treatment effects. Thus, a smaller sample size was selected similar as a former pilot study23. Mean difference (D) and standard deviation (SD) of efficacy evaluation results were often used as parameters for sample size calculations in randomized controlled studies. A standard deviation (SD) of 2 of the nine-item Patient Health Questionnaire (PHQ-9) was use for sample size calculation in our study. To achieve 80% power for detecting a difference at a 0.05 significance level, sample size calculation using PASS 11.0 software indicated a minimum sample size of 18 for each group. Research coordinators performed the recruitment and group assignment, while a therapist delivered the CALM therapy.
CALM therapy is a brief, structured, individual psychological therapy. CALM therapy focuses on four therapeutic areas: (1) symptom management and communication with medical oncology staff; (2) self-change and relationships with loved ones; (3) sense of meaning and purpose for living; (4) the future and mortality (future, hope, and death). We have added the fifth therapeutic area-(5) fear of cancer progression as the topic was repeated by the patients in the pre-experiment. 3–6 sessions would be carried out in CALM therapy, and 40–60 min for each session. The CALM therapy comprised three to six sessions, with each session lasting 40 to 60 min. Patients in the CALM group received three to six sessions of CALM therapy, along with usual care after T0 assessment, while patients in the WLC group received CALM therapy only after completing theri T1 and T2 assessments. In each CALM session, a brief structure was set out to facilitate the delivery of the therapy by the therapist. The therapy session began with a 10–15 min check-in to assess the patient’s mood and experiences over the past few days. The therapists offered empathetic listening and validation during this segment. For the next 25–40 min, the focus shifted to the core therapeutic areas addressed in CALM therapy. During the last 5 min, therapists worked with patients to summarise the main contents of their discussion during the therapy session and schedule the next session. When this study commenced, COVID-19 restrictions hampered in-person visits to the hospital from which patients were recruited. Therefore, we provided the CALM therapy online via WeChat video calls. The WeChat APP is the most popular online social media platform in China. A previous study has demonstrated the feasibility of WeChat platform-based interventions for patient- education and complementary medical care24. We made some adaptations to enable successful delivery online: (1) before the therapy session began, patients were asked to review and verbally consent to the treatment environment (including the room lighting, privacy protections, and surrounding area) in the therapist’s video chat frame, as seen via the webcam. Only after patients consented would the session begin. (2) The therapist checked whether patients felt comfortable in the remote setting and monitored their engagement in the sessions more frequently. (3) Without assess to body language during online therapy, the therapist focused more intently on empathetic verbal communication, emotional recognition and providing support through thoughtful words and listening responses. The therapist in this study is certified in CAML therapy by the Global Institute of Psychosocial Palliative &End-of-Life Care (GIPPEC). The study participants could stop at any time if they were feeling unwell-with the reasons documented. Data from participants who at least completed the T1 assessment were included in the study analysis.
Originally, we aimed to recruit patients with metastatic triple-negative breast cancer as participants in this study. However, the pre-experiment planning at the single center revealed challenges in recruiting a sufficient number of such patients, as the triple-negative breast cancer cases comprise a small proportion of metastatic breast cancer patients. Therefore, recruitment eligibility was broadened to include all metastatic breast cancer subtypes.
Measurements
Main outcome measurements
Death and Dying Distress Scale (DADDS): The 15-item DADDS assesses distress about death and dying in advanced cancer patients22. Each item on the instrument is scored on a scale of 0 to 5 points. As recommended by Neel et al., a cut-off point of 45 was used to define death anxiety8. The Chinese version of the DADDS has been validated for use with patients with advanced cancer25.
Other outcome measurements
Nine item-patient health questionnaires (PHQ-9): The PHQ-9 is a validated scale for assessing depression symptoms in patients and is based on the criteria for depression disorder outlined in the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV). The Chinese version of the PHQ-9 has demonstrated good validity and reliability among general hospital patients. A ≥10 score was considered indicative of moderate depression26.
Distress Thermometer (DT): The single-item DT is recommended by the distress management group of the NCCN. A score of ≥4 is recommended as the cut-off point for significant distress by both the NCCN and a Chinese validation study27.
Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being (FACIT-sp) This tool was obtained via the FACIT.org website, with a licence to use the simplified Chinese version of the FACIT-Sp in this study. This 12-item scale assesses patients’ spiritual well-being using two subscales: Meaning/Peace (Items 1–8) and Faith (Items 9–12). Each item is scored along a continuum of 0 to 4 (Item 4 and Item 8 are reverse scored), with higher scores indicating better spiritual well-being than lower scores28.
Quality of Life at the End of Life-Cancer (QUAL-EC): The 17-item QUAL-EC assesses quality of life at the end of life in advanced cancer patients, with each item scored on a scale of 1 to 5 points29. The QUAL-EC comprises four subscales: (1) Symptom Burden (Items 1–3), on which higher scores indicate a greater symptom burden than lower scores; (2) Relationship with Healthcare Provider (Items 4–8), on which high scores reflect better outcomes than low scores; (3) Preparation for End of Life (Items 9–12), on which low scores reflect better outcomes than high scores; and (4) Life Completion (Items 13–17), on which high scores reflect better outcomes than low scores.
Modified experiences in close relationship scale (ECR-M16): The 16-item ECR-M16 is a self-reported measure of attachment security with close others. Two of its subscales are calculated separately. Avoidant attachment is assessed by all the odd-numbered items (Items 3, 7, 11 and 15 are reverse scored). Anxiety attachment is assessed by all the even-numbered items. High scores indicate worse outcomes than low scores on both subscales30.
Clinical Evaluation Questionnaire-2 (CEQ-2): The 13-item CEQ-2 elicits patients’ feedback on the feasibility of CALM therapy, covering four CALM domains. Each item is scored on a scale of 0 (not applicable) to 4 (very helpful).
Statistical analysis
The T-test or a non-parametric test was used to compare the results of the measured psychometric indicators for the CALM group and the WLC group at three time points. The T-test was also used to compare the difference between the CALM group and the WLC group in terms of changes in the measured psychometric indicators, with the effect size denoted by the Cohen’s d value. Analysis of covariance (ANCOVA) was used to compare the post-intervention means of the two groups after adjusting for differences at baseline, which can effectively isolate the effect of the CALM intervention on post-intervention scores and report the effect size using the value of partial η2. All statistical analyses were conducted using the IBM Statistical Package for the Social Sciences (SPSS) 26.0.
Ethical approval
The cross-sectional study and this intervention study were approved by the Ethics Committees of Peking University Cancer Hospital (No.2018YJZ24 for cross-sectional study and No.2019YJZ23 for the intervention study). All methods were performed in accordance with the guideline of clinic trials required by the Ethics Committees of Peking University Cancer Hospital.
Consent to participate
We got written informed consent from all participants.
Results
Results of demographic information in the sample
Thirty-six participants consented to participate in this intervention study and were randomly assigned to one of two study groups, with 19 participants assigned to the CALM group and 17 assigned to the WLC group (Fig. 1). Data on the participants who received more than three sessions of CALM therapy and/or at least completed the T0 and T1 assessments were included in the statistical analysis. One participant in the CALM group dropped out after three therapy sessions because of the following notion: ‘thinking about I get well and don’t need psychotherapy’. One participant in the WLC group dropped out after the T0 assessment by refusing to answer the call. The mean age of the participants was 47.26 ± 10.032; most of the participants were unreligious, married, city dwellers and medically insured. We found no between-group differences in any of the demographic characteristics (Table 1).
Table 1.
Differences of demographic information between CALM group and WLC group.
| Variables | Total (N = 36) | WLC Group (n = 17) M ± SD/n (%) | CALM Group (n = 19) M ± SD/n (%) | t /χ2 | p |
|---|---|---|---|---|---|
| Age | 47.26 ± 10.032 | 49.65 ± 10.943 | 45.11 ± 8.888 | 1.373 | 0.179 |
| 18–44 years old | 15 (41.7) | 6 (35.3) | 9 (47.4) | 0.538 | 0.463 |
| ≥ 45 years old | 21 (58.3) | 11 (64.7) | 10 (52.6) | ||
| Religion | 1.092 | 0.296 | |||
| No | 30 (83.3) | 13 (76.5) | 17 (89.5) | ||
| Yes | 6 (16.7) | 4 (23.5) | 2 (33.3) | ||
| Marital status | 2.043 | 0.153 | |||
| Without partner | 7 (19.4) | 5 (29.4) | 2 (10.5) | ||
| Married | 29 (80.6) | 12 (70.6) | 17 (89.5) | ||
| Education status | 1.761 | 0.415 | |||
| ≤ junior middle school | 6 (16.7) | 2 (11.8) | 4 (21.1) | ||
| Senior middle school and junior college | 15 (41.7) | 9 (52.9) | 6 (31.6) | ||
| ≥ college | 15 (41.7) | 6 (35.3) | 9 (47.4) | ||
| Living condition | 0.037 | 0.847 | |||
| In the city | 27 (75.0) | 13 (76.5) | 14 (73.7) | ||
| In rural area | 9 (25.0) | 4 (23.5) | 5 (26.3) | ||
| Average income | 2.386 | 0.303 | |||
| ≤ 3000 RMB/month | 8 (22.2) | 5 (29.4) | 3 (15.8) | ||
| 3000–5000RMB/month | 15 (41.7) | 8 (47.1) | 7 (36.8) | ||
| ≥ 5000RMB/month | 13 (36.1) | 4 (23.5) | 9 (47.4) | ||
| Payment insurance | 0.122 | 0.727 | |||
| Self-pay | 5 (41.7) | 2 (11.8) | 3 (15.8) | ||
| By insurance | 31 (58.3) | 15 (88.2) | 16 (84.2) | ||
***p < 0.001; **p < 0.01; *p < 0.05.
Primary outcomes
(Table 2), Table 3 presents the primary outcome derived using an analysis of covariance (ANCOVA). For DADDS as primary outcome, no significant between-group differences were found at any time point. However, we found disparities in the death anxiety scores, with better outcomes recorded in the CALM group than in the WLC group; the difference was not significant (T1: 26.74 ± 17.672 vs. 32.25 ± 19.814, p = 0.228, partial η2 = 0.045; T2: 23.61 ± 16.256 vs. 29.13 ± 16.974, p = 0.271, partial η2 = 0.039). The changing trend is presented in Fig. 2. In addition, the CALM group recorded a considerably large improvement in the primary DADDS outcome (Table 4). However, the results of t-test indicate this within group change is not statistically significant when compared to that in the WLC group (T0-T1: 10.16 ± 13.263 vs. 2.44 ± 21.420, p = 0.222, Cohen’s d = 0.442; T0-T2: 13.28 ± 14.652 vs. 5.40 ± 24.994, p = 0.268, Cohen’s d = 0.432).
Table 2.
Differences of measurements indicators between CALM group and WLC group in the three times.
| T0 M ± SD/n (%) |
T1 M ± SD/n (%) |
T2 M ± SD/n (%) |
||||
|---|---|---|---|---|---|---|
| PHQ-9 (WLC) | 11.06 ± 6.685 | 9.63 ± 7.145 | 10.33 ± 6.366 | |||
| PHQ-9 (CALM) | 13.42 ± 5.957 | 8.95 ± 6.450 | 6.82 ± 5.235 | |||
| t/χ2 | − 1.120 | 0.295 | 1.711 | |||
| p | 0.271 | 0.770 | 0.097 | |||
| Suicide Ideation (WLC) | Negative 11 (64.7) | Positive 6 (35.3) | Negative 8 (50.0) | Positive 8 (50.0) | Negative 6 (40.0) | Positive 9 (60.0) |
| Suicide Ideation (CALM) | Negative 10 (52.6) | Positive 9 (47.4) | Negative 14 (73.7) | Positive 5 (26.3) | Negative 13 (76.5) | Positive 4 (23.5) |
| t/χ2 | 0.538 | 2.087 | 4.394 | |||
| p | 0.463 | 0.149 | 0.036* | |||
| DADDS (WLC) | 35.18 ± 18.484 | 32.25 ± 19.814 | 29.13 ± 16.974 | |||
| DADDS (CALM) | 36.89 ± 14.282 | 26.74 ± 17.672 | 23.18 ± 16.648 | |||
| t/χ2 | − 0.314 | 0.870 | 1.001 | |||
| p | 0.755 | 0.391 | 0.326 | |||
| DT(WLC) | 3.88 ± 2.421 | 3.00 ± 2.733 | 4.58 ± 2.644 | |||
| DT (CALM) | 4.17 ± 2.203 | 2.42 ± 2.434 | 2.23 ± 2.127 | |||
| t/χ2 | − 0.364 | 0.663 | 2.460 | |||
| p | 0.718 | 0.512 | 0.022* | |||
| FACIT (WLC) | 25.00 ± 10.892 | 23.13 ± 12.371 | 27.00 ± 12.364 | |||
| FACIT (CALM) | 24.84 ± 9.376 | 27.53 ± 8.322 | 32.29 ± 7.819 | |||
| t/χ2 | 0.047 | − 1.252 | − 1.364 | |||
| p | 0.963 | 0.219 | 0.184 | |||
| ECR-Avoidant (WLC) | 27.88 ± 6.051 | 29.88 ± 7.527 | 27.00 ± 12.364 | |||
| ECR-Avoidant (CALM) | 29.00 ± 6.280 | 28.11 ± 5.415 | 32.29 ± 7.819 | |||
| t/χ2 | − 0.542 | 0.807 | 1.469 | |||
| p | 0.591 | 0.425 | 0.152 | |||
| ECR-Anxiety (WLC) | 26.71 ± 10.999 | 27.13 ± 11.407 | 24.80 ± 11.912 | |||
| ECR-Anxiety (CALM) | 31.26 ± 8.366 | 28.42 ± 12.025 | 25.24 ± 11.206 | |||
| t/χ2 | − 1.408 | − 0.325 | − 0.106 | |||
| p | 0.168 | 0.747 | 0.916 | |||
| QUAL-EC-Relationship (WLC) | 17.65 ± 3.605 | 17.44 ± 3.881 | 16.00 ± 4.408 | |||
| QUAL-EC-Relationship (CALM) | 16.63 ± 3.715 | 15.26 ± 4.026 | 17.29 ± 4.883 | |||
| t/χ2 | 0.830 | 1.618 | − 0.783 | |||
| p | 0.412 | 0.115 | 0.440 | |||
| QUAL-EC-Preparation (WLC) | 13.82 ± 3.107 | 14.38 ± 4.515 | 13.13 ± 3.114 | |||
| QUAL-EC- Preparation (CALM) | 14.11 ± 2.885 | 13.16 ± 4.086 | 13.29 ± 3.236 | |||
| t/χ2 | − 0.282 | 0.837 | − 0.143 | |||
| p | 0.780 | 0.409 | 0.887 | |||
| QUAL-EC-Life Completion (WLC) | 18.06 ± 4.630 | 17.31 ± 3.877 | 16.13 ± 3.852 | |||
| QUAL-EC-Life Completion (CALM) | 17.68 ± 3.449 | 17.63 ± 3.022 | 18.71 ± 3.098 | |||
| t/χ2 | 0.277 | − 0.274 | − 2.093 | |||
| p | 0.783 | 0.786 | 0.045* | |||
***p < 0.001; **p < 0.01; *p < 0.05.
Significant values are in bold.
Table 3.
Result from Analysis of Covariance (ANCOVA) in primary and secondary outcomes.
| WLC group | CALM group | F | sig | Effect size from partial Eta Squared (η2) | |
|---|---|---|---|---|---|
| Primary outcome | |||||
| DADDS | |||||
| T0 | 34.69 ± 18.976 | 36.89 ± 14.282 | – | – | – |
| T1 | 32.25 ± 19.814 | 26.74 ± 17.672 | 1.511 | 0.228 | 0.045 |
| T2 | 29.13 ± 16.974 | 23.61 ± 16.256 | 1.206 | 0.271 | 0.039 |
| Secondary outcomes | |||||
| PHQ-9 | |||||
| T0 | 11.00 ± 6.899 | 13.42 ± 5.975 | – | – | – |
| T1 | 9.62 ± 7.145 | 8.95 ± 6.450 | 0.565 | 0.458 | 0.017 |
| T2 | 10.33 ± 6.366 | 6.94 ± 5.104 | 5.016 | 0.033* | 0.143 |
| DT | |||||
| T0 | 3.88 ± 2.421 | 4.17 ± 2.203 | – | – | – |
| T1 | 3.00 ± 2.733 | 2.42 ± 2.434 | 1.328 | 0.258 | 0.041 |
| T2 | 4.58 ± 2.644 | 2.23 ± 2.127 | 7.969 | 0.010* | 0.257 |
| FACIT-sp | |||||
| T0 | 25.00 ± 10.892 | 24.84 ± 9.376 | – | – | – |
| T1 | 23.13 ± 12.371 | 27.53 ± 8.322 | 3.207 | 0.083 | 0.091 |
| T2 | 27.00 ± 12.364 | 32.29 ± 7.819 | 2.683 | 0.113 | 0.090 |
| ECR-Avoidant | |||||
| T0 | 27.88 ± 6.051 | 29.00 ± 6.280 | – | – | – |
| T1 | 29.88 ± 7.527 | 28.11 ± 5.415 | 1.383 | 0.248 | 0.041 |
| T2 | 27.00 ± 12.364 | 32.29 ± 7.819 | 4.407 | 0.044* | 0.128 |
| ECR-Anxiety | |||||
| T0 | 26.71 ± 10.999 | 31.26 ± 8.366 | – | – | – |
| T1 | 27.13 ± 11.407 | 28.42 ± 12.025 | 0.017 | 0.896 | 0.001 |
| T2 | 24.80 ± 11.912 | 25.24 ± 11.206 | 0.038 | 0.847 | 0.001 |
| QUAL-EC-Relationship | |||||
| T0 | 17.65 ± 3.605 | 16.63 ± 3.715 | – | – | – |
| T1 | 17.44 ± 3.881 | 15.26 ± 4.026 | 2.298 | 0.139 | 0.067 |
| T2 | 16.00 ± 4.408 | 17.29 ± 4.883 | 0.695 | 0.411 | 0.023 |
| QUAL-EC- Preparation | |||||
| T0 | 13.82 ± 3.107 | 14.11 ± 2.885 | – | – | – |
| T1 | 14.38 ± 4.515 | 13.16 ± 4.086 | 0.948 | 0.337 | 0.029 |
| T2 | 13.13 ± 3.114 | 13.29 ± 3.236 | 0.116 | 0.736 | 0.004 |
| QUAL-EC- Life Completion | |||||
| T0 | 18.06 ± 4.630 | 17.68 ± 3.449 | – | – | – |
| T1 | 17.31 ± 3.877 | 17.63 ± 3.022 | 0.134 | 0.717 | 0.004 |
| T2 | 16.13 ± 3.852 | 18.71 ± 3.098 | 5.493 | 0.026* | 0.155 |
***p < 0.001; **p < 0.01; *p < 0.05.
Partial η2: 0.01 small effect size, 0.06 moderate effect size, 0.14 large effect size.
Significant values are in bold.
Figure 2.
Changing of depression and death anxiety scores in the three time points.
Table 4.
T-test results of changing range in primary and secondary outcomes.
| WLC Group(n = 17) M ± SD | CALM Group (n = 19) M ± SD | F | sig | t | p | Cohen’s d | 95% confidence interval | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Primary outcomes | |||||||||
| ΔDADDS (T0-T1) | 2.44 ± 21.420 | 10.16 ± 13.263 | 4.565 | 0.040 | − 1.254 | 0.201 | − 0.442 | − 1.113 | − 0.234 |
| ΔDADDS (T0-T2) | 5.40 ± 24.994 | 13.28 ± 14.652 | 2.838 | 0.102 | − 1.127 | 0.268 | − 0.432 | − 1.087 | 0.301 |
| Secondary outcomes | |||||||||
| ΔPHQ-9 (T0-T1) | 1.38 ± 8.049 | 4.47 ± 6.695 | 0.433 | 0.515 | − 1.244 | 0.222 | − 0.422 | − 1.092 | 0.254 |
| ΔPHQ-9 (T0-T2) | 0 ± 5.843 | 6.44 ± 7.587 | 0.387 | 0.539 | − 2.689 | 0.011* | − 0.940 | − 1.657 | − 0.210 |
| ΔDT (T0-T1) | 0.75 ± 3.416 | 2.11 ± 3.197 | 0.017 | 0.896 | − 1.200 | 0.239 | − 0.412 | − 1.067 | − 0.248 |
| ΔDT (T0-T2) | − 0.67 ± 1.723 | 1.79 ± 3.068 | 2.230 | 0.148 | − 2.453 | 0.022* | − 0.965 | − 1.773 | − 0.139 |
| ΔFACIT-sp (T0-T1) | − 2.31 ± 7.863 | 2.68 ± 8.938 | 0.338 | 0.565 | − 1.739 | 0.091 | − 0.590 | − 1.266 | 0.094 |
| ΔFACIT-sp (T0-T2) | 1.87 ± 9.303 | 6.40 ± 8.998 | 0.824 | 0.372 | − 1.357 | 0.186 | − 0.495 | − 1.218 | 0.236 |
| ΔECR-Avoidance (T0-T1) | − 2.06 ± 4.892 | 0.89 ± 7.752 | 3.452 | 0.072 | − 1.319 | 0.196 | − 0.448 | − 1.118 | 0.224 |
| ΔECR-Avoidance (T0-T2) | − 3.67 ± 4.353 | 0.56 ± 6.428 | 3.422 | 0.074 | − 2.162 | 0.038* | − 0.756 | − 1.460 | − 0.040 |
| ΔECR-Anxiety (T0-T1) | − 0.44 ± 13.604 | 2.84 ± 11.644 | 0.285 | 0.597 | − 0.769 | 0.448 | − 0.261 | − 0.927 | 0.409 |
| ΔECR-Anxiety(T0-T2) | 2.27 ± 14.921 | 5.89 ± 9.707 | 0.591 | 0.448 | − 0.840 | 0.407 | − 0.294 | − 0.980 | 0.398 |
| ΔQUAL-Relationship (T0-T1) | 0.19 ± 4.996 | 1.37 ± 5.336 | 0.049 | 0.826 | − 0.671 | 0.507 | − 0.228 | − 0.893 | 0.441 |
| ΔQUAL-Relationship (T0-T2) | 1.20 ± 5.171 | − 0.50 ± 4.902 | 0.002 | 0.964 | 0.968 | 0.341 | 0.338 | − 0.355 | 1.026 |
| ΔQUAL-Preparation (T0-T1) | 0.69 ± 5.375 | − 0.95 ± 3.597 | 1.078 | 0.307 | 1.072 | 0.291 | 0.364 | − 0.310 | 1.032 |
| ΔQUAL-Preparation (T0-T2) | − 0.67 ± 4.100 | − 0.56 ± 3.823 | 0.149 | 0.703 | − 0.080 | 0.936 | − 0.028 | − 0.713 | 0.657 |
| ΔQUAL-Completion (T0-T1) | − 0.63 ± 4.288 | − 0.05 ± 4.156 | 0.058 | 0.811 | − 1.784 | 0.692 | − 0.136 | − 0.801 | 0.531 |
| ΔQUAL-Completion (T0-T2) | − 1.60 ± 3.888 | 1.33 ± 5.280 | 1.767 | 0.193 | − 1.717 | 0.084 | − 0.624 | − 1.321 | 0.083 |
Δ: the changing of measurements indicators in T1 and T2, compared to T0;
***p < 0.001; **p < 0.01; *p < 0.05;
Small effect size: 0 < Cohen’s d ≤ 0.2; Moderate effect size: 0.2 < Cohen’s d ≤ 0.8; large effect size: Cohen’s d > 0.8
Secondary outcomes
A comparison of various psychometric scores for the CALM group and the WLC group is presented in Table 2. No difference was found in T0 and T1 results for the two groups. For the T2-conducted after six sessions, the percentage of participants experiencing suicide ideation in the CALM group in significantly lower than that in the WLC group (23.5% vs. 60.0%, χ2 = 4.394, p = 0.036); Lower distress scores were recorded in the CALM group than that in the WLC group (t = 2.460, p = 0.022); and the scores on Life completion was significantly better in the CALM group than that in the WLC group (t = − 2.093, p = 0.045).
For the secondary outcomes analysed using ANCOVA, the participants in the CALM group had significantly lower levels of depression than those in the WLC group (F = 5.016, p = 0.033) at T2, after adjusting for baseline PHQ-9 scores. Partial η2 = 0.143 indicated a large effect size; in addition, participants in the CALM group reported significantly lower levels of distress (F = 7.969, p = 0.010), as reflected in their DT scores, and attachment avoidance (F = 4.407, p = 0.044), as reflected in their ECR-avoidance scores, than participants in the WLC group. Furthermore, participants in the CALM group reported a significantly higher sense of life completion (F = 5.493, p = 0.026), as reflected in their QUAL-EC-Life Completion scores, than participants in the WLC group, with the effect size ranging from moderate (partial η2 = 0.128 in ECR-avoidance) to large (partial η2 = 0.257 in DT). No significant benefits were found for any of the outcomes in T1 analysed using ANCOVA. We explored the differences between the two groups in the changes in scores on the secondary psychometric indicators before and after the interventions (Table 4). The results showed that the decline in depression and distress score between T0 and T2 was more remarkable in the CALM group than in the WLC group (PHQ-9: T0-T2, t = − 2.689, p = 0.011, Cohen’s d = 0.94; DT: T0-T2, t = − 2.453, p = 0.022. Cohen’s d = 0.965).
Acceptability of CALM therapy in Chinese patients with metastatic breast cancer
The results presented in Table 5 show that study participants felt that the CALM therapy had overall positive effects on helping them to cope with stage IV breast cancer. The percentage of participants scoring ≥ 2 and ≥ 3 on the CEQ-2 increased on all the 13 items from T1 to T2. At T1 (conducted after three CALM therapy sessions), the percentage of participants scoring ≥ 2 on the CEQ-2 ranged from 57.9% on item 7 to 100% on item2 ; at T2, it ranged from 88.9% on item 6 to 100% on item 1, 2, 3, 5, 6, and 12. The participants reported lower levels of benefit from CALM therapy on items related to death and dying (Items 7–9) than on other items; However, at the T2 time point, the scores on these three death/dying-related items were apparently comparatively higher than at T1.
Table 5.
Results from clinical evaluation questionnaire-2.
| Items: to what extent has CALM therapy helped you to: (0 = Not at all; 1 = A little bit; 2 = Somewhat; 3 = Quite a bit; 4 = Very much) | Percentage of scoring ≥ 2 | Percentage of scoring ≥ 3 | ||
|---|---|---|---|---|
| T1 | T2 | T1 | T2 | |
| 1. Freely discuss my concerns about cancer and my treatment options | 18/19 (94.7) | 18/18 (100) | 10/19 (52.6) | 13/18 (72.2) |
| 2. Talk and feel understood about how cancer has affected my life | 19/19 (100) | 18/18 (100) | 13/19 (68.4) | 13/18 (72.2) |
| 3. Deal with changes in my relationships as a result of cancer | 18/19 (94.7) | 18/18 (100) | 9/19 (47.4) | 10/18 (55.6) |
| 4. Explore better ways to communicate with my health care team, my family and others | 15/19 (78.9) | 17/18 (94.4) | 10/19 (52.6) | 16/18 (88.9) |
| 5. Clarify my values and beliefs | 18/19 (94.7) | 18/18 (100) | 11/19 (57.9) | 11/18 (61.1) |
| 6. Talk about my concerns about the future and to be less frightened | 18/19 (94.7) | 18/18 (100) | 12/19 (63.2) | 12/18 (66.7) |
| 7. Plan and prepare for what can happen at the end of life | 11/19 (57.9) | 16/18 (88.9) | 6/19 (31.6) | 9/18 (50.0) |
| 8. Express and manage my fears about dying | 13/19 (68.4) | 17/18 (94.4) | 8/19 (42.1) | 9/18 (50.0) |
| 9. Talk about what will happen to my family | 15/19 (78.9) | 14/18 (77.8) | 8/19 (42.1) | 11/18 (61.1) |
| 10. Say important things to my loved ones | 16/19 (84.2) | 16/18 (88.9) | 9/19 (47.4) | 12/18 (66.7) |
| 11. Make the most of my time | 16/19 (84.2) | 17/18 (94.4) | 11/19 (57.9) | 13/18 (72.2) |
| 12. Enjoy and live in the present | 18/19 (94.7) | 18/18 (100) | 12/19 (63.2) | 17/18 (94.4) |
| 13. Live with an uncertain future | 17/19 (89.5) | 17/18 (94.4) | 10/19 (52.6) | 15/18 (83.3) |
Discussion
This pilot trial examined the feasibility of CALM therapy for patients with metastatic breast cancer and significant death anxiety. The study participants were randomly assigned to one of two groups using computer-implemented dynamic randomization, thus achieving a relatively even distribution of demographic and sociological variables between the two groups. Our hypothesis was that in a sufficiently large study sample size, CALM therapy would significantly alleviate psychological burden and improve quality of life in patients with metastatic breast cancer. Although the differences in death anxiety at matched time points post-intervention did not achieve significance in this small pilot randomized controlled trial, we did observe significant between-group differences in several secondary outcome measures, with the patients benefitting significantly from receiving full six sessions of CALM therapy on the following metrics: depression severity, distress level, attachment avoidance and sense of life completion, with the effect size ranging from moderate to large. The percentage of patients with suicide ideation also decreased more in the CALM group than in the WLC group. Furthermore, the progressive differences in the levels of depression and death anxiety between the two groups shows and apparent and a gradual trend. Further exploration of changes in scores gave us more positive indications of psychological improvement in depression, distress, and attachment avoidance resulting from the CALM therapy, with a large effect size. Based on the results presented in Table 5, it can be seen that the study participants exhibited a high degree of acceptance towards CALM therapy. The largest shifts between T1 and T2 are evident in the psychometric items related to enjoying the present, living with uncertainty, making the most of time, communicating with others and discussing future concerns, which indicates that CALM therapy may have a particular efficacy around psychosocial outcomes related to discovering meaning in life and peace in the face of stage IV breast cancer. A discussion about death and dying was open after three sessions if it fit the patients' preferfences, and these discussions were generally introduced through two opportunities: talking about their future and the uncontrolled aspects of living with advanced cancer life. Significant death anxiety in Chinese advanced cancer patients has been reported by researchers31–33, and talking about death and dying with this population during our research was not as difficult as reported in a previous study34. However, the magnitude of improvements in the domains of ‘planning end-of-life’ and ‘fears of death and dying’ was small, as not all participants were willing to enter into a discussion of death and dying. This indicates that our online CALM therapy content needs to be enhanced in this area in the future researches.
This was the first trial of online CALM therapy for patients with metastatic breast cancer in China. The online delivery model ensured that the intervention and research proceeded at intended pace. Even outside periods of COVID-19 community lockdowns, the patients were still reluctant to visit cancer hospitals-which can be distressful environments that reinforce their cancer patients identity- except when required for anti-cancer treatments and examinations. Limitations of online CALM therapy also existed. Bertuzzi et al. reported common issues, including challenges with technology, low user motivation, and privacy/safety concerns35. We also encountered challenges with technology, as we could not provide non-verbal empathetic response to patients’ strong emotional expressions, such like crying. However, the low motivation and privacy/safety were not remarkable in our study, as most patients agreed to participate, knowing it would be conducted online and hospital visits were not required. The widespread everyday use of WeChat video calls in China may also provide some reassurance about the privacy/safety.
Patients in both groups demonstrated good compliance, with over 94% completing all six CALM sessions and the three assessments. This high compliance rate indicates that CALM therapy was well received by the study population. Compliance in interventional clinical researches can be influenced by inclusion criteria, recruiting procedures and psychotherapy relationship. Published study protocols for randomized clinical trials of CALM therapy have used different inclusion criteria across various study populations. Rodin’s phase III RCT and Miyamoto’s phase 2 trial protocol did not set the threshold scores in the main outcomes36; while Caruso required baseline score ≥ 10 on the Patient Health Questionnaire (PHQ-9) and/or ≥ 20 on the Death and Dying Distress Scale (DADDS)23; Scheffold et al. also limited the inclusion with ≥ 9 on the Patient Health Questionnaire (PHQ-9) and/or ≥ 5 on the Distress Thermometer (DT)37. CALM researchers had not yet agreed on this issue. Our pre-testing revealed that removing threshold score increased recruiting ratio, but decrease the full study compliance in this population in China. In our study, after weighing the intervention impact and population accessibility, we set a DADDS ≥ 15 as inclusion threshold. This was based on the notion that patients with significant death anxiety, who have a considerable need for psychotherapy, may demonstrate positive compliance in clinical trials-in which CALM therapy can serve them well. In addition, engaging breast cancer nurses in the same department from which the patients were recruited facilitated the recruiting process and likely improved the study participants’ compliance.
In the CALM therapy, engaging family members in the intervention process is encouraged. Throughout our pre-experiment and during this pilot trial, we made consistent efforts to request participation from the husbands of patients or their other family members. However, this request was refused in the vast majority of cases. The patients’ husbands believed that only the patients themselves needed psychological therapy, as they did not feel they had any problems that needed addressing. In the context of Chinese culture, men are accustomed to taking on the role of decision-maker rather than caregivers. Even when their wives were suffering from metastatic breast cancer, the husbands were more inclined to participate in deciding on the anti-cancer treatment plans while seldom providing psychological support. Patients also refused our requests to engage their parents or children-whom they were trying to protect. Patients were reluctant to let these family members witness their psychological distress in addition to their physical distress. The reluctance of husbands to participate in therapy sessions and this shielding of vulnerable family members highlight critical cultural barriers and misconceptions about mental health and psychological support. Enhanced education efforts and outreach to destigmatize therapy and elucidate its offerings may enable improved family engagement in the future.
Previous CALM studies in China focused on cancer survivors, examining the effects of CALM therapy on cognitive impairment and immunity markers38. However, the main advantages of CALM therapy are the benefit of psychological, and it improves the overall quality of life for patients with advanced, life-threatening illnesses confronting the finitude of life. All the CALM researches in other countries have examined CALM therapy from this perspective23,39,40. In this study, with a small sample, we verified psychosocial benefits of CALM therapy for Chinese patients with metastatic breast cancer. The encouraging results in this pilot study lay groundwork for an adequately-powered phase III RCT of CALM therapy for Chinese patients with metastatic breast cancer.
Limitations
This first exploration of CALM therapy in Chinese patients with metastatic breast cancer provids encouraging support for a larger phase III RCT to be conducted in this population in China. However, there were several limitations to the study. For instance, because the small sample is small, the results cannot indubitably verify the effects of CALM therapy on depression and death anxiety in patients with metastatic breast cancer. Because we recruited patients from only one tertiary center in Beijing who have generally high levels of education, generalizability is limited, and the results of the study may not reflect outcomes representative of the broader population in China. Although online delivery aided intervention accessibility and pace, there was a challenge with technology: the inability to provide face-to-face empathic support. However, these shortcomings hardly negate the value of our online approach in demonstrating feasibility of CALM therapy and providing support for utilising this approach in the future studies. Indeed, the limitations of this study help to inform such efforts. With only one dropout per arm, compliance was adequate, indicating the acceptability of both study procedures and the CALM therapy. Thus, these positive indicators favor launching a multi-centered phase III study with a large sample size to rigorously evaluate the effects of CALM therapy in the future.
Conclusions
This pilot randomized controlled trial provides preliminary evidence supporting the efficacy of CALM therapy for alleviating depression, distress, death anxiety, suicide ideation in Chinese patients with metastatic breast cancer. Furthermore, CALM therapy has the potential to enhance patients’ sense of life completion at the end of life. A low dropout rate and high intervention compliance indicate that CALM therapy is a welcome and feasible individual psychotherapy for this population in China. These promising findings warrant further rigorous examination in a phase III randomized controlled trial with a large Chinese metastatic breast cancer cohort. The findings also reveal that specially trained therapists can effectively administer online CALM to patients with metastatic breast cancer, which can help ensure intervention accessibility and continuity amidst healthcare disruptions and patients’ reluctance to visit the hospital.
Acknowledgements
We express our sincere thanks to Professor Gary Rodin from Princess Margaret Cancer Centre, the lead investigator of the CALM Global Initiative. We greatly appreciate his invaluable supervision of CALM therapy for the therapist and the conduction of CALM China program. We sincerely appreciate all the patients who were willing to participate in this study and openly share their perspectives on this intervention. Their contributions provided researchers invaluable support beyond the collected data.
Author contributions
Study design: L.T., Y.Z., Y.P., Y.H. Data collection: Y.Z., M.Y. Drafting the manuscript: Y.Z. Critical revision of the manuscript: L.T., Y.P., Y.H. Approval of the final version for publication: All co-authors in this study. All authors agreed with the final draft and provided consent to publish this paper.
Funding
This research had received grant from China Association of Gerontology and Geriatrics.
Data availability
Data supporting the findings of this study and supplementary material are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zheng RS, Zhang SW, Sun KX, Chen R, Wang SM, Li L, Zeng HM, Wei WW, He J. Cancer statistics in China, 2016. Zhonghua Zhong Liu Za Zhi. 2023;45(3):212–220. doi: 10.3760/cma.j.cn112152-20220922-00647. [DOI] [PubMed] [Google Scholar]
- 2.Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003–15: A pooled analysis of 17 population-based cancer registries. Lancet Glob. Health. 2018;6(5):e555–e567. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. In: Yu D, Hung M, editors. Breast Cancer Chemosensitivity. Springer; 2007. pp. 1–22. [DOI] [PubMed] [Google Scholar]
- 4.Hamer J, McDonald R, Zhang L, Verma S, Leahey A, Ecclestone C, Bedard G, Pulenzas N, Bhatia A, Chow R, DeAngelis C, Ellis J, Rakovitch E, Lee J, Chow E. Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Support. Care Cancer. 2017;25(2):409–419. doi: 10.1007/s00520-016-3417-6. [DOI] [PubMed] [Google Scholar]
- 5.Grotmol KS, Lie HC, Loge JH, Aass N, Haugen DF, Stone PC, Kaasa S, Hjermstad MJ. Patients with advanced cancer and depression report a significantly higher symptom burden than non-depressed patients. Palliat. Support. Care. 2019;17(2):143–149. doi: 10.1017/S1478951517001183. [DOI] [PubMed] [Google Scholar]
- 6.Valderrama Rios MC, Sánchez Pedraza R. Anxiety and depression disorders in relation to the quality of life of breast cancer patients with locally advanced or disseminated stage. Rev. Colomb. Psiquiatr. (Engl. Ed.) 2018;47(4):211–220. doi: 10.1016/j.rcp.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Watson M, Haviland JS, Greer S, Davidson J, Bliss JM. Influence of psychological response on survival in breast cancer: a population-based cohort study. Lancet. 1999;354(9187):1331–1336. doi: 10.1016/s0140-6736(98)11392-2. [DOI] [PubMed] [Google Scholar]
- 8.Neel C, Lo C, Rydall A, Hales S, Rodin G. Determinants of death anxiety in patients with advanced cancer. BMJ Support. Palliat. Care. 2015;5:373–380. doi: 10.1136/bmjspcare-2012-000420. [DOI] [PubMed] [Google Scholar]
- 9.Eggen AC, Reyners AKL, Shen G, Bosma I, Jalving M, Leighl NB, et al. Death anxiety in patients with metastatic non-small cell lung cancer with and without brain metastases. J. Pain Symptom Manag. 2020;60(2):422–429.e1. doi: 10.1016/j.jpainsymman.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Zhou YH, Pang Y, Han XK, He SZ, Li JJ, Tang LL. Death anxiety and related factors in young women with advanced breast cancer. Zhong Guo Xin Li Wei Sheng Za Zhi. 2020;34(11):891–897. [Google Scholar]
- 11.Loughan AR, Aslanzadeh FJ, Brechbiel J, Rodin G, Husain M, Braun SE. Death-related distress in adult primary brain tumor patients. Neuro-Oncol. Pract. 2020;7(5):498–506. doi: 10.1093/nop/npaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg J, Vail K, Pyszczynski T. Terror management theory and research: How the desire for death transcendence drives our strivings for meaning and significance. Adv. Motiv. Sci. 2014;1:85–134. doi: 10.1016/bs.adms.2014.08.003. [DOI] [Google Scholar]
- 13.Distelhorst SR, Cleary JF, Ganz PA, Bese N, Camacho-Rodriguez R, Cardoso F, Ddungu H, Gralow JR, Yip CH, Anderson BO, Breast Health Global Initiative Global Summit on Supportive Care and Quality of Life Consensus Panel Members Optimisation of the continuum of supportive and palliative care for patients with breast cancer in low-income and middle-income countries: Executive summary of the breast health global initiative. Lancet Oncol. 2015;16(3):e137–e147. doi: 10.1016/S1470-2045(14)70457-7. [DOI] [PubMed] [Google Scholar]
- 14.Mo L, Urbauer DL, Bruera E, Hui D. Recommendations for palliative and hospice care in NCCN guidelines for treatment of cancer. Oncologist. 2021;26(1):77–83. doi: 10.1002/ONCO.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu B, Hu X, Feng J, Geng C, Jin F, Li H, Li M, Li Q, Liao N, Liu D, Liu J, Liu Q, Lu J, Liu Z, Ma F, Ouyang Q, Pan Y, Shen K, Sun T, Teng Y, Tong Z, Wang B, Wang H, Wang S, Wang S, Wang T, Wang X, Wang X, Wang Y, Wang Z, Wu J, Yan M, Yang J, Yin Y, Yuan P, Zhang J, Zhang P, Zhang Q, Zheng H. Chinese expert consensus on the clinical diagnosis and treatment of advanced breast cancer (2018) Cancer. 2020;15(126 Suppl 16):3867–3882. doi: 10.1002/cncr.32832. [DOI] [PubMed] [Google Scholar]
- 16.Kissane DW, Grabsch B, Clarke DM, Smith GC, Love AW, Bloch S, Snyder RD, Li Y. Supportive-expressive group therapy for women with metastatic breast cancer: Survival and psychosocial outcome from a randomized controlled trial. Psychooncology. 2007;16(4):277–286. doi: 10.1002/pon.1185. [DOI] [PubMed] [Google Scholar]
- 17.Savard J, Simard S, Giguère I, Ivers H, Morin CM, Maunsell E, Gagnon P, Robert J, Marceau D. Randomized clinical trial on cognitive therapy for depression in women with metastatic breast cancer: Psychological and immunological effects. Palliat. Support. Care. 2006;4(3):219–237. doi: 10.1017/s1478951506060305. [DOI] [PubMed] [Google Scholar]
- 18.Rodin G, Lo C, Rydall A, Shnall J, Malfitano C, Chiu A, Panday T, Watt S, An E, Nissim R, Li M, Zimmermann C, Hales S. Managing cancer and living meaningfully (CALM): A randomized controlled trial of a psychological intervention for patients with advanced cancer. J. Clin. Oncol. 2018;36(23):2422–2432. doi: 10.1200/JCO.2017.77.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skrabal Ross X, Gunn KM, Olver I, Willems RA, Lechner L, Mesters I, Bolman CAW. Online psychosocial interventions for posttreatment cancer survivors: An international evidence review and update. Curr. Opin. Support. Palliat. Care. 2020;14(1):40–50. doi: 10.1097/SPC.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 20.Livingston PM, Russell L, Orellana L, Winter N, Jefford M, Girgis A, Austin D, Eric O, Mihalopoulos C, Ugalde A, Chambers R, Phipps-Nelson J, Herath D, Botti M, Rasmussen B, Whitfield K, Ftanou M, Smith AB, Pilatti K, Sara S, Wootten A, Gillan K, Singh M, Campbell D, Pillay B, White V. Efficacy and cost-effectiveness of an online mindfulness program (MindOnLine) to reduce fear of recurrence among people with cancer: Study protocol for a randomised controlled trial. BMJ Open. 2022;12(1):e057212. doi: 10.1136/bmjopen-2021-057212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng L, Yang Y, Chen M, Xu C, Chen Y, Liu R, Cao X, Li M. Effects of an online mindfulness-based intervention on fear of cancer recurrence and quality of life among Chinese breast cancer survivors. Complement. Ther. Clin. Pract. 2022;49:101686. doi: 10.1016/j.ctcp.2022.101686. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro GK, Mah K, Li M, Zimmermann C, Hales S, Rodin G. Validation of the death and dying distress scale in patients with advanced cancer. Psycho-Oncology. 2021;30(5):716–727. doi: 10.1002/pon.5620. [DOI] [PubMed] [Google Scholar]
- 23.Caruso R, Nanni MG, Rodin G, Hales S, Malfitano C, De Padova S, Bertelli T, Murri MB, Bovero A, Miniotti M, Leombruni P, Zerbinati L, Sabato S, Grassi L, CALM-Italian Study Effectiveness of a brief manualized intervention, managing cancer and living meaningfully (CALM), adapted to the Italian cancer care setting: Study protocol for a single-blinded randomized controlled trial. Contemp. Clin. Trials Commun. 2020;20:100661. doi: 10.1016/j.conctc.2020.100661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Meng J, Li H. WeChat-platform-based education and care program as a candidate approach to relieve anxiety, depression, and post-traumatic stress disorder in parents of pediatric and adolescent patients with osteosarcoma. Front. Psychol. 2022;25(13):913940. doi: 10.3389/fpsyg.2022.913940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang L, Zhang Y, Pang Y, Zhou Y, Li J, Song L, He Y, Li Z, Wang Y. Validation of death and dying distress scale-Chinese version and prevalence of death anxiety among patients with advanced cancer. Front. Psychiatry. 2021;20(12):715756. doi: 10.3389/fpsyt.2021.715756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S, Fang Y, Psych HCF, Fan H, Tao J, Conwell MDY. Validation of the nine-item patient health questionnaire to screen for major depression in a Chinese primary care population. Asia Pac. Psychiatry. 2013;5(2):61–68. doi: 10.1111/appy.12063. [DOI] [PubMed] [Google Scholar]
- 27.Tang LL, Zhang YN, Pang Y, Zhang HW, Song LL. Validation and reliability of distress thermometer in Chinese cancer patients. Chin. J. Cancer Res. 2011;23(1):54–58. doi: 10.1007/s11670-011-0054-y.PMID:23467708;PMCID:PMC3587535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu XY, Wei D, Chen YY, Chen QQ, Liang S, Xiang-Hua XU, et al. Reliability and validity of the Chinese version of the functional assessment of chronic illness therapy-spiritual well-being in cancer patients. Chin. J. Nurs. 2016;51(9):1085–1090. [Google Scholar]
- 29.Lo C, Burman D, Swami N, Gagliese L, Rodin G, Zimmerman C. Validation of the QUAL-EC for assessing quality of life in patients with advanced cancer. Eur. J. Cancer. 2011;47(4):554–560. doi: 10.1016/j.ejca.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 30.Lo C, Walsh A, Mikulincer M, Gagliese L, Zimmermann C, Rodin G. Measuring attachment security in patients with advanced cancer: psychometric properties of a modified and brief experiences in close relationships scale. Psychooncology. 2009;18(5):490–499. doi: 10.1002/pon.1417. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Liu X, Liu Z, Wang Y, Feng R, Zheng R, Xie R, Tao H, Wu Y, Li X, Ying W, Wu X. Death anxiety and its relationship with family function and meaning in life in patients with advanced cancer: A cross-sectional survey in China. Asia Pac. J. Oncol. Nurs. 2022;9(10):100134. doi: 10.1016/j.apjon.2022.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong Y, Yuhan L, Youhui G, Zhanying W, Shili Z, Xiaoting H, Wenhua Y. Death anxiety among advanced cancer patients: A cross-sectional survey. Support. Care Cancer. 2022;30(4):3531–3539. doi: 10.1007/s00520-022-06795-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su Z, Zhou Y, Han X, Pang Y, He S, Tang L. Symptom burden in advanced breast cancer patients and its association between death anxiety and psychological distress. Chin. J. Cancer Res. 2022;34(3):298–308. doi: 10.21147/j.issn.1000-9604.2022.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Q, Flaherty JH, Guo JH, Zhou Y, Zhang XM, Hu XY. Attitudes of older Chinese patients toward death and dying. J. Palliat. Med. 2017;20(12):1389–1394. doi: 10.1089/jpm.2017.0014. [DOI] [PubMed] [Google Scholar]
- 35.Bertuzzi V, Semonella M, Castelnuovo G, Andersson G, Pietrabissa G. Synthesizing stakeholders perspectives on online psychological interventions to improve the mental health of the Italian population during the COVID-19 pandemic: An online survey study. Int. J. Environ. Res. Public Health. 2022;19(12):7008. doi: 10.3390/ijerph1912700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamoto S, Yamazaki T, Shimizu K, Matsubara T, Kage H, Watanabe K, Kobo H, Matsuyama Y, Rodin G, Yoshiuchi K. Brief, manualised and semistructured individual psychotherapy programme for patients with advanced cancer in Japan: Study protocol for managing cancer and living meaningfully (CALM) phase 2 trial. BMJ Open. 2022;12(3):e056136. doi: 10.1136/bmjopen-2021-056136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheffold K, Philipp R, Engelmann D, Schulz-Kindermann F, Rosenberger C, Oechsle K, Härter M, Wegscheider K, Lordick F, Lo C, Hales S, Rodin G, Mehnert A. Efficacy of a brief manualized intervention managing cancer and living meaningfully (CALM) adapted to German cancer care settings: Study protocol for a randomized controlled trial. BMC Cancer. 2015;19(15):592. doi: 10.1186/s12885-015-1589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding K, Zhang X, Zhao J, Zuo H, Bi Z, Cheng H. Managing cancer and living meaningfully (CALM) intervention on chemotherapy-related cognitive impairment in breast cancer survivors. Integr. Cancer Ther. 2020;19:1534735420938450. doi: 10.1177/1534735420938450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loughan AR, Willis KD, Braun SE, Rodin G, Lanoye A, Davies AE, Svikis D, Mazzeo S, Malkin M, Thacker L. Managing cancer and living meaningfully (CALM) in adults with malignant glioma: A proof-of-concept phase IIa trial. J. Neurooncol. 2022;157(3):447–456. doi: 10.1007/s11060-022-03988-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehnert A, Koranyi S, Philipp R, Scheffold K, Kriston L, Lehmann-Laue A, Engelmann D, Vehling S, Eisenecker C, Oechsle K, Schulz-Kindermann F, Rodin G, Härter M. Efficacy of the managing cancer and living meaningfully (CALM) individual psychotherapy for patients with advanced cancer: A single-blind randomized controlled trial. Psycho-Oncology. 2020;29(11):1895–1904. doi: 10.1002/pon.5521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this study and supplementary material are available from the corresponding author upon reasonable request.