Abstract
Micro- and nano-plastics (MNPs) pollution has become a pressing global environmental issue, with growing concerns regarding its impact on human health. However, evidence on the effects of MNPs on human health remains limited. This paper reviews the three routes of human exposure to MNPs, which include ingestion, inhalation, and dermal contact. It further discusses the potential routes of translocation of MNPs in human lungs, intestines, and skin, analyses the potential impact of MNPs on the homeostasis of human organ systems, and provides an outlook on future research priorities for MNPs in human health. There is growing evidence that MNPs are present in human tissues or fluids. Lab studies, including in vivo animal models and in vitro human-derived cell cultures, revealed that MNPs exposure could negatively affect human health. MNPs exposure could cause oxidative stress, cytotoxicity, disruption of internal barriers like the intestinal, the air–blood and the placental barrier, tissue damage, as well as immune homeostasis imbalance, endocrine disruption, and reproductive and developmental toxicity. Limitedly available epidemiological studies suggest that disorders like lung nodules, asthma, and blood thrombus might be caused or exacerbated by MNPs exposure. However, direct evidence for the effects of MNPs on human health is still scarce, and future research in this area is needed to provide quantitative support for assessing the risk of MNPs to human health.
Keywords: Microplastics, Nanoplastics, Environmental exposure, Human system homeostasis, Health effects, Risk assessment
Graphical abstract
Highlights
-
•
Human exposure to micro- and nano-plastics (MNPs) via inhalation, ingestion, and dermal contact are summarized.
-
•
MNPs have an intrinsic capability to escape and translocate to the circulatory system.
-
•
MNPs have the potential to disrupt homeostasis, leading to oxidative stress, cytotoxicity, tissue damage, and systemic dysfunction.
1. Introduction
Microplastics (MPs) are plastic fragments with a particle size of ≤5 mm, while nanoplastics (NPs) are typically considered to have a particle size of ≤1 μm [1]. Despite consistent efforts to reduce plastic manufacturing and enhance plastic recycling, an estimated 250 million metric tons (Mt) of plastic waste will enter the aquatic system, and 460 million Mt will enter the soil system from 2016 to 2040 [2]. Plastic waste that enters the environment includes primary micro- and nano-plastics (MNPs), such as those added to personal care and cosmetic products [3], as well as secondary MNPs produced by the fragmentation of larger plastics through physical, chemical, and biological processes [4]. MNPs are ubiquitous in the global biosphere and can be found in oceans [5], lakes [6], rivers [7], indoor air [8], outdoor air [9], and soil [10], as well as in seafood [11], drinking water [12], beverages [13], and salt [14].
Humans are inevitably and continuously exposed to MNPs, raising concern about their potential risk to human health [[15], [16], [17], [18], [19]]. However, it is unclear whether MNPs directly affect human health due to limitations in human tissue sampling, lack of epidemiological investigations and in situ detection methods. Current studies have shown that MNPs not only exhibit particulate toxicity to organisms but also induce chemical toxicity [20,21]. The toxicity of particles with similar sizes to MNPs, such as PM10 (aerodynamic equivalent diameter less than 10 μm), PM2.5 (aerodynamic equivalent diameter less than 2.5 μm), and engineered nanoparticles, has extensively been studied [22]. Epidemiological studies have shown a significant correlation between PM2.5 and human respiratory morbidity and mortality [22]. In addition, long-term exposure to engineered nanoparticles can cause lung damage and cardiovascular disease [23]. To date, there is a paucity of epidemiological studies examining the potential health effects of MNPs in humans. However, available evidence from in vitro studies using human cells and in vivo studies using animal models, such as mice and rats, indicates that exposure to MNPs may induce inflammation, oxidative stress, cytotoxicity, and respiratory disease [24,25]. Moreover, it is important to note that MNPs not only contain a range of plastic additives, including dyes, plasticizers, and antioxidants but also serve as carriers of persistent organic chemicals, heavy metals, and pathogenic microorganisms, all of which can be toxic and have potential carcinogenic and mutagenic effects on human health [20,26]. There is thus an urgent need to understand the potential impact of MNPs on human health.
We collected and analyzed the available literature mostly published before June 2023 based on the database of Web of Science, ScienceDirect and Google Scholar using the keyword “microplastics” OR “nanoplastic”, and the articles were grouped with different categories including “atmosphere or air”, “seafood, drinking water, salt, sugar, or honey”, “translocation or accumulation”, “system, lung, intestinal, or placenta”, and “toxicity, human, cell, or rat” to summarize the new progresses. We found that MNPs have been detected not only in human feces [27], urine [28], and sputum [29] but also in the lungs [30] and intestines [31]. Moreover, they have been found to enter the blood [32], thrombus [33], closed body fluids [34], liver [35], and even the placenta [36]. Meanwhile, the number of studies on the impact of MNPs on the health of model animals or human cells has exponentially increased, yielding fresh insights into our understanding of the effects of MNPs on human health. This review aims to discuss the exposure pathways of MNPs, the potential uptake, transport, and accumulation mechanisms of MNPs in the human body, and the potential toxicity to human organ systems. By summarizing current knowledge, this review hope to provide insights for further research to better understand the impact of MNPs on human health.
2. Pathways of exposure of MNPs to humans
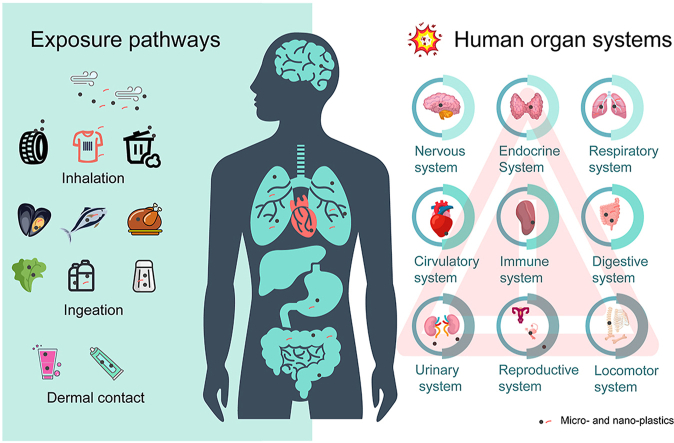
In order to evaluate the impact of MNPs on human health, it is crucial to elucidate the pathways and levels of human exposure. The three primary routes of human exposure to MNPs are inhalation, ingestion, and dermal contact. Therefore, it is imperative to thoroughly investigate these pathways and their associated exposure levels to accurately assess the potential risks and hazards of MNPs to human health.
2.1. Inhalation
2.1.1. Indoor air
It is estimated that individuals spend approximately 89% of their daily time indoors, highlighting the significance of MNPs concentration in indoor air to human health [8]. The different functions of indoor spaces affect the abundance of MNPs. In Paris, the abundance of MPs ranged 1.1–18.2 fibers/m3 in apartment air and 4.0–59.4 fibers/m3 in offices [9]. In nail salons, the abundance of MPs in the environment is 46 particles/m3 [37]. The deposition rate of MPs also varies in different indoor environments, with the highest in the home [up to 1.96 × 104 particles/(m2·day)] and lowest in the classroom [6.20 × 103 particles/(m2·day)] [38]. Zhang et al. [8] detected 5.5 times as many MPs in the dormitory air [9.9 × 103 particles/(m2·day)] as in the office [1.8 × 103 particles/(m2·day)]. The abundance or deposition rate of MPs in indoor air can vary significantly from room to room. This variation is primarily influenced by factors such as the room’s function, the flow of people, and the concentration of MPs in the outdoor air [8]. The variability can be attributed to differences in the detection methods employed by researchers [8,9]. Additionally, Zhan et al. [39] detected an abundance of MPs in the indoor air of electronic waste dismantling facilities, ranging from 2.6 to 11 particles/m3. These MNPs pose a greater risk to human health, as they may contain flame retardants, heavy metals, or poly-brominated diphenyl ethers (PBDEs). Currently, researchers mainly used two methods of collection, active, and passive sampling, but the data from both methods cannot be compared because passive sampling can only respond to the amount of MNPs that can be deposited in the air [40]. At present, the majority of MPs in indoor air are fiber with sizes >20 μm [41]. Future research needs to focus on MNPs with particle sizes <2.5 μm, as suspended MNPs with smaller particle sizes are more easily inhaled by people.
Infants or children tend to spend more time indoors than adults [42]. However, the abundance of MPs in indoor air below 1 m is very poorly documented. In addition, infants and children are more likely to inhale or ingest indoor dust, which also contains high levels of MPs. Concentrations of polyethylene terephthalate (PET) and polycarbonate (PC) in indoor dust in 12 countries ranged from 38 to 1.2 × 105 μg/g and <0.11 to 1,700 μg/g, respectively [43]. In Shiraz, the abundance of MPs in school dust was 195 particles/g [44]. Therefore, different living situations and ages need to be considered when assessing the health risks of MNPs in indoor air to humans.
2.1.2. Outdoor air
The outdoor environment is more extensive, and the air is more mobile than indoors. The concentration of MNPs is generally lower in the outdoor environment than indoors. In Wenzhou, China, the outdoor abundance of MPs (189 ± 85 particles/m3) was one order of magnitude lower than indoors (1,583 ± 1,180 particles/m3) [45]. Similarly, in Paris, France, the abundance of MPs in indoor and outdoor air ranges 1–60 particles/m3 and 0.3–1.5 particles/m3, respectively [9]. The abundance of MPs in outdoor air exhibited regional differences, with MPs being more abundant in urban air than in rural air, and in northern Chinese cities than in southern cities [45,46]. For example, Liu et al. [47] estimated that Shanghai residents inhaled approximately 21 particles/day outdoors, while in Wenzhou, urban residents inhaled 3,360 particles/day from outdoor air, and 1,515 particles/day for rural residents [45]. MPs in outdoor air have a considerably smaller impact on human health than in indoor air. However, further investigation of the concentrations of MNPs in the air around sites such as roads, construction sites, or landfills is needed to assess the potential health risks of MNPs to people living or working in these environments.
Based on the above information, people are constantly inhaling MNPs, but the amount of MNPs inhalation into the body remains uncertain. Zhang et al. [48] roughly estimated the annual human inhalation of MPs through indoor and outdoor air to be 1.9 × 103–1.0 × 105 and 0–3.0 × 107 particles, respectively. However, they overlooked the variations in daily respiration rates among different demographic groups, including men and women, as well as adults and children. Cox et al. [42] further subdivided the population, with the highest amount of MPs inhaled annually by adult males at 6.2 × 104 particles and the lowest amount by female children at 3.9 × 104 particles. However, there is still a great gap between the current estimate and the actual amount of MNPs inhaled by human beings. On the one hand, human beings will still exhale some of the MNPs when they breathe out, and on the other hand, the concentration of NPs in the air is still unknown in general.
2.2. Ingestion
2.2.1. Seafood
MNPs have been found in over 690 marine species, including the seafood humans regularly consume, such as fish, mollusks, and crustaceans [[49], [50], [51]]. Nearly half of the 338 fish species investigated contained MPs, with an average abundance of 3.5 ± 0.8 particles/fish [52]. MPs are predominantly detected in fish intestines but rarely in fish meat [52]. In contrast, mollusks such as oysters, mussels, and clams are consumed whole by humans. The highest abundance of MPs in oysters is 99.9 particles/individual in some waters with high MP contamination [53]. Mollusks obtained on the market contain fewer MPs than mollusks caught directly, presumably because the marketed soft-bodied creatures have been cleaned [54]. Crustaceans, such as crabs and shrimp, also have edible and inedible parts. The inedible parts (4.4 particles/animal) primarily consist of the stomach and gills, which contain an average concentration of MPs four times that of the edible parts (1.2 particles/animal) [55]. To minimize exposure, it is advisable not to consume the intestines and stomachs of shrimp or crab. Additionally, when assessing human exposure to MPs through seafood consumption, it is crucial to focus on the edible portion of the seafood for a more accurate evaluation.
2.2.2. Drinking water and beverages
There are significant differences in the abundance of MPs found in tap and bottled water (Table 1). Oβmann et al. [56] detected that the abundance of MPs in bottled water is up to 2,649 ± 2,857 particles/L in single-use PET bottles and 6,292 ± 10,521 particles/L in glass bottles, which is by far the highest abundance of MPs in bottled water [57]. Therefore, in addition to the packaging itself, other sources of contamination must also be considered, such as cleaning, packaging, and transport. The majority of MPs in bottled water have a particle size between 1 and 5 μm [57]. Overall, the abundance of MPs in tap water was lower than in bottled water, with the current maximum abundance of MPs in tap water being 930 particles/L [58]. The effort of boiling tap water before drinking fails to diminish the number of MNPs in the water [59]. Based on the available data, Danopoulos et al. [12] estimated that the maximum annual intake of MPs for adults from consuming tap water and bottled water was 4.58 × 105 and 3.57 × 107 particles, respectively.
Table 1.
Presence of micro- and nano-plastics in the daily human diets.
| Species | Location | Abundance | Size (μm) | Type | Shape | Detection method | Reference |
|---|---|---|---|---|---|---|---|
| Skipjack Tuna (Euthynnus affinis) | Southern Coast of Java, Indonesia | 4 particles/fish | 500–5,000 | / | Filament (84%), angular (11%), | Stereomicroscope | [166] |
| Deep-sea fish | South China Sea | Stomachs: 1.53 ± 1.08 particles/g intestines: 4.82 ± 4.74 particles/g | 0–1,000 (68.9%–76.7%) | / | Film, fiber | Optical microscope | [167] |
| Oysters | South Australia | 0.09 ± 0.01 particles/g wet weight | / | LDPE, PE | Fiber (61.8%), fragment (37.7%) | FTIR | [168] |
| Mussel (Perna Viridis) | Hong Kong | 0.21–1.83 particles/g wet weight | 40–1,000 | PP (56%), PE (25%), PET (10%) | Fragment, fiber | Raman | [51] |
| Brown shrimp (Metapenaeus monocerous) | Bangladesh coast | 3.40 ± 1.23 particles/g digestive tract | 1,000–5,000 (40%) 500–1,000 (17%) |
PA, rayon | Fiber (57%), particle (29%), fragment (14%) | μ-FTIR | [169] |
| Tiger shrimp (Penaeus monodon) | Bangladesh coast | 3.87 ± 1.05 particles/g digestive tract | 1,000–5,000 (70%) 500–1,000 (27%) |
PA, rayon | Fiber (32%), particle (16%), fragment (26%) | μ-FTIR | [169] |
| Whiteleg shrimp (Litopenaeus vannamei) | Malaysia | 20.8 ± 3.57 particles/g wet weight | / | / | Film (93%–97%) | Microscope | [50] |
| Argentine red shrimp (Pleoticus muelleri) | Argentina Southwest Atlantic, FAO 41 | 7,050 ± 4,178 particles/g wet weight | / | / | Sphere (70%) | Microscope | [50] |
| Tap water | China | 440 ± 275 particles/L | 1–50 (31.25%–100%) 50–100 (1.47%–31.25%) 100–300 (1.72%–31.25%) |
PE (26.8%), PP (24.4%), PE + PP (22.0%) |
Fragment: 53.85%–100%, Fiber: 1.18%–30.77% Sphere: 2.27%–36.36% |
μ-Raman | [170] |
| Tap watera | Barcelona Metropolitan Area | 1 ng/L–9 μg/L | 0.7–20 | PE. PP | HPLC–HRMS | [171] | |
| Drinking water | Mexico City | 18 ± 7 particles/L | 100–1,000 (75%) | PTT, EP | Fibers | μ-Raman | [172] |
| Bottled water Mineral water |
Kermanshah, Iran | 8.5 ± 10.2 particles/L | 1,280–4,200 | PET, PS, PP | Fragment (93%), fiber (7%) | FTIR, Raman | [173] |
| Bottled water | China | 2–23 particles/bottle | 25–5,000 | Cellulose (71.16%), PET (6.98%), PE (6.05%) | Fiber, fragment | μ-FTIR | [174] |
| Soft drinks | Mexico | ND–7 particles/L | 100–3,000 | PA | Fiber | Microscope | [60] |
| Cold tea | Mexico | 1–6 particles/L | 100–2,000 | PA | Fiber | Microscope | |
| Beer | Mexico | ND–28 particles/L | 100–3,000 | PA | Fiber, fragment | Microscope | |
| Milk | Switzerland; France | 2,040–10,040 particles/L | ≥5, <20 | PE (31%), PS (27%), PES (23%) | / | μ-Raman | [61] |
| Table salts | Africa | 1.68 ± 1.83 particles/kg | >50, <5,000 | PVA, PP, PE | Fragment, fiber, granule | FTIR | [175] |
| Table salts | India | 115–575 particles/kg | 100–200 (37.7%), 200–500 (31.2%), 500–1,000 (16.2%) >1,000 (15%) |
PE (78%), PE (19%), PVC (3%) | Fibers (88.5%), film (4.9%), pellet (2.9%) | μ-FTIR | [14] |
| Sugar | Bangladeshi | 343.7 ± 32.08 particles/kg | <300 (64%) | ABS (25%), PVC (18%), PET (15%) | Fiber (38.4%), fragment (28.4%), film (25.2%) | FTIR | [65] |
LDPE, low-density polyethylene; PP, polypropylene; EP, epoxy resin; PET, polyethylene terephthalate; PE, polyethylene; PA, polyamide; PES, polyester; PVC, polyvinyl chloride; PVA, polyvinyl alcohol; ABS, acrylonitrile butadiene styrene; FTIR, fourier transform infrared; HPLC–HRMS, high performance liquid chromatography–high-resolution mass spectrometry.
Mass concentrations.
MNPs also have been detected in beer, tea, soft drink, and milk [13,60,61]. The most essential component of beverages is water, and the presence of MNPs in water can lead to beverage contamination. Shruti et al. [60] investigated four beverage categories, and the most contaminated with MPs was beer (less than 28 ± 5.29 particles/L). Li et al. [13] investigated 15 brands of beer from various nations and detected that the abundance of MPs ranged from 1.2 × 104 to 9.7 × 104 particles/L. This is mainly due to the different identification methods used. Shuri et al. [60] did not count MPs <100 μm using microscopy, whereas Li et al. [13] counted all MPs <5 mm using microscopic Raman. In addition, tea also contains MPs, especially when brewed in tea bags. During the brewing process, around 50 plastic particles per tea bag will detach from the tea bags and fall into the tea [62]. Milk and related dairy derivatives are one of the human body’s primary sources of protein and calcium but are currently contaminated with MPs [63,64]. The abundance of MPs in milk varies considerably from country to country, with 3–11 particles/L in Brazil [63], 164–427 particles/L in India [64], and 2.04 × 103–1.0 × 104 particles/L in Switzerland [61]. In the future, continuous monitoring of MNPs in drinking water and beverages will be of utmost importance. Equally vital will be the establishment of appropriate standards for bottled water to effectively regulate the levels of MNPs.
2.2.3. Salt, sugar, and honey
MNPs are present not only in food but also in food spices like salt, sugar, and honey (Table 1) [14,65,66]. MNPs are commonly found in different types of salt, including sea salt, rock salt, and lake salt, with an abundance range from 0 to 39.8 particles/g and predominantly presenting a fibrous and fragmented shape [67,68]. The annual intake of MPs through salt consumption by adults ranges from 35.8 to 36,172 particles [68]. Sugar and honey also have been found to be contaminated with MNPs. In Bangladesh, MPs were detected in all sugar samples, with a mean abundance of 344 ± 32 particles/g [65]. The abundance of MPs in honey was relatively low at 22–114 particles/L [66]. Other food spices (cooking oil, monosodium glutamate, and soy sauce) also need to be examined for the presence of MNPs to ensure food safety.
2.2.4. Crops and livestock
At present, MNP contamination in crops and livestock remains unknown. However, laboratory studies have shown that crops can take up MNPs. In hydroponic experiments, wheat (Triticum aestivum L.), lettuce (Lactuca sativa), and carrots (Kurodagosun) could take up MNPs [69,70]. Moreover, in soil experiments, MNPs were taken up by lettuce (L. sativa), rice (Oryza sativa L.), and peanut (Arachis hypogaea L.) and accumulated in the stems and leaves of lettuce, the seeds of rice and the peanut [71,72]. These results raise concerns about crops in heavily MNP-contaminated soils. Although crops tend to absorb few MNPs under natural exposure, long-term consumption of crops containing MNPs may adversely affect human health. Livestock is not immune to MNP contamination, with about 46 particles/gizzards in chicken in the family yard [73]. MPs were also detected in edible snails (Helix pomatia) [74]. Therefore, it is crucial to pay attention to the risk of human exposure to MNPs through the food chain, particularly to NPs, which are more likely to be transmitted through the food chain and have biomagnification effects.
Furthermore, the rice or meat that we purchase in our daily lives is directly sourced from the market, making them susceptible to MNP contamination during production, packaging, or transportation processes [75,76]. Dessì et al. [75] detected MPs in all 52 rice samples from Australia, and the average concentration of MPs in rice was 67 ± 26 μg/g dry weight. Kedzierski et al. [76] also detected MPs in packaged meat with abundances ranging from 4.0 to 18.7 particles/kg. The researchers also discovered that rinsing rice with water was effective in significantly reducing MP contamination [75]. However, it was observed that MPs on the surface of packaged meat were more difficult to remove through simple rinsing due to their stronger adhesion to the meat [76]. Therefore, washing food before consumption is necessary, especially for packaged meats that need to be carefully cleaned.
The assessment of MNPs ingested via the ingestion exposure pathway is inherently more complex than inhalation, mainly due to the wide range of food types consumed by humans. A recent review has attempted to extrapolate to human exposures, reporting annual ingestion of (0–5.5) × 104 particles (seafood) [77], (0–4.7) × 103 particles (drinking water) [48], (0–7.3) × 104 particles (table salt) [48], and a mean amount of 1.9 × 1010 particles (fruit and vegetables) [78]. Furthermore, the ingestion of MPs through dust should not be overlooked. According to estimates, adults have an annual intake of 4.0 × 102–2.5 × 104 particles, while infants and young children have an annual intake of 7.2 × 102–4.5 × 104 particles [79]. However, there is a lack of investigations on the concentration of MNPs in foods such as vegetables, fruits, rice, wheat or meat. Moreover, these foods are essential components of the human diet, making it challenging to accurately estimate the amount of MNPs ingested through dietary intake at present. Therefore, it is crucial to take into account the dietary composition of the local population and the concentration of MNPs in food when evaluating the MNP intake among individuals.
2.3. Dermal contact
Human skin is directly exposed to MNPs, which can be detected in personal care products, such as toothpaste, hand soap, face wash, and sunscreen [80,81]. Prior to 2018, MNPs were used in large quantities in personal protective equipment to replace natural substances such as pumice, oatmeal, or almonds in order to exfoliate and deep cleanse the skin [82]. Concerns were raised about the presence of <100 nm particles in personal skin care products that might breach the dermal barrier and pose health risks [83]. In addition, plastic components in human protheses generate MNPs as a result of normal wear and tear, thereby putting them into direct contact with the skin [84]. Moreover, airborne MNPs can settle with dust and come into contact with the skin [85]. To date, there are no literature estimates of the amount of MNPs absorbed by dermal contact. However, due to the current lack of investigation of NPs, the effects of NPs on human skin are still not negligible, especially in cases where there are wounds or infections on the skin surface.
3. Tissue accumulation and translocation of MNPs in the human body
In recent years, MNPs have been increasingly detected in various human body fluids and organs, suggesting that they can escape the body’s immune cells and translocate across the biological barriers into the circulatory system, eventually accumulating in organs or tissues (Table 2). However, restricted by ethical considerations and limited detection techniques, the translocation and accumulation of MNPs in humans were scarcely investigated. This review thus refers to existing knowledge of other kinds of particles which should help understand the properties of MNPs that affect accumulation in the human body (Fig. 1).
Table 2.
Presence of micro- and nano-plastics in human tissues or fluids.
| Human body sample | Digestion methods | Detection method | Sample Number | Type | Shape | Abundance | Size (μm) | Reference |
|---|---|---|---|---|---|---|---|---|
| Lung | H2O2 (30%, v/v) | μ-FTIR, Raman | 100 | Cotton, rayon, PE | Fiber (>90%) | Tumor tissues: 38 particles/50 samples, normal tissues: 27 particles/50 samples |
1,450 ± 980 | [86] |
| Lung | Enzymatic | Raman | 20 | PP (35.1%), PE (24.3%), cotton (16.2%) | Fragment (87.5%), fiber (12.5%), | 0.56 particles/g of lung tissue | Fragment: 1.60–5.56 fiber: 8.12–16.80 | [30] |
| Lung | 30% H2O2 | μ-FTIR | 13 | PP (23%), PET (18%), resin (15%) | Fragment (67%), fiber (22%) | 1.42 ± 1.50 particles/g | 12–2,475 | [120] |
| Bronchoalveolar lavage fluid | No | μ-FTIR | 44 | Rayon (40.48%), PE (19.05%), cellulose (16.67%) | Fiber (97.06%) | 9.18 ± 2.45 particles/100 mL | 1,730 ± 150 | [87] |
| Sputum | HNO3, NaOH | FTIR | 22 | PU, PE, PVC | – | 18.75−91.75 particles/10 mL | 20–500 | [29] |
| Sputum | 30% KOH | Polarized light microscopy | 16 | Couriers: PC (24.2%), PVC (23.0%); office staff: PVC (39.1%), PA (24.8%) | Couriers: fiber (94.3%), office staff: fiber (83.3%) | Couriers: 26.9−161.5 particles/g Office staff: 0.4−1.4 particles/g |
– | [88] |
| Nasal lavage fluid | 30% KOH | FTIR | 16 | Couriers: PA (25.3%), PE (22.9%); office staff: PVC (41.1%), PA (31.6%) | Couriers: Fiber (83.8%); office staff: fiber (87%) | Couriers:17.6−728.6 particles/g; office staff: 0.9−3.3 particles/g | – | [88] |
| Human blooda | Proteinase K | Py-GC/MS | 22 | PET, PE | – | 1.6 μg/mL | ≥0.7 | [32] |
| Thrombi | 30% KOH | Raman | 26 | LDPE | Fragment | 1 particle/26 sample | ∼5 | [33] |
| Liver, spleen, kidney | KOH | Raman | Liver (11), spleen (3), kidney (3) | PS, PVC, PET | – | 1.4 particles/g | 4–30 | [35] |
| Placenta | 10% KOH | Raman | 43 | PE, PS | Film, fiber, fragment | Normal: 6 particles/30 sample IUGR: 302 particles/13 sample |
Normal:7.3−27.6 IUGR: 2.9−34.5 |
[138] |
| Placenta | 10% KOH | Raman | 6 | PP | Fragment | 4 particles/6 placentas | ∼5–10 | [36] |
| Breast milk | 10% KOH | Raman | 34 | PE (38%), PVC (21%), PP (17%) | Fragment | 26 particles/34 samples | 2–12 | [176] |
| Human colectomy | 10% KOH | FTIR | 11 | PC, PA, PP | Fiber (96.1%), | 28.1 ± 15.4 particles/g | 800−1,600 | [31] |
| Adult stoola | H2O2 30%, | FTIR | 26 | PET, PP, PS | – | 1−36 particles/g | 20–800 | [97] |
| Infant stoola | KOH | LC–MS/MS | 6 | PET, PC | – | PET: 5,700−82,000 ng/g PC: 49−2,100 ng/g |
– | [27] |
| Meconiuma | KOH | LC–MS/MS | 3 | PET, PC | – | PET: 3,200−12,000 ng/g PC: 110 ng/g |
– | |
| Adult stool | FTIR | 8 | PP (62.8%), PET (17.0%), PS (11.2%) | 20 particles/10 g | 50–500 | [96] | ||
| Adult stoola | KOH | LC–MS/MS | 10 | PET, PC | – | PET: <16,000 ng/g PC: 37−620 ng/g |
– | [27] |
| Urine | KOH | Raman | 6 | PP, PE, PVC, PVA | Fragment | – | 4–15 | [34] |
PU, polyether urethane; PC, polycarbonate; Py-GC/MS, pyrolysis gas chromatography–mass spectrometry; LC–MS/MS, liquid chromatography–tandem masa spectrometry; IUGR, intrauterine growth restriction.
Mass concentrations.
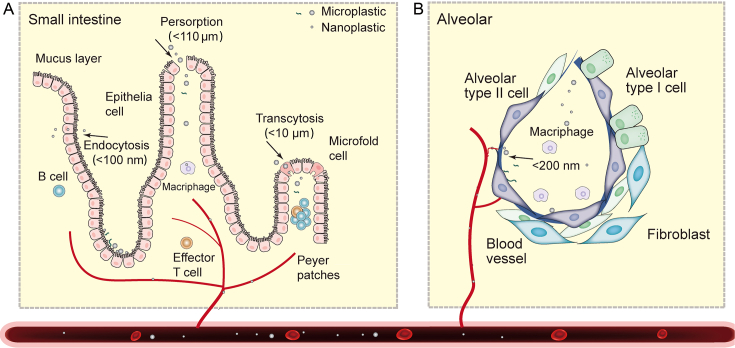
Fig. 1.
The possible absorption, transfer and accumulation mechanisms of micro- and nano-plastics in human intestines (A) and lungs (B).
3.1. Accumulation and translocation of MNPs in the lung
Inhalation might be the most likely pathway to the human body for MNPs [48]. Various types and shapes of MNPs have been found in the respiratory system, including those in the alveoli, with abundance ranging from 0.56 to 1.42 particles/g [30,86]. The mean size of these particles was reported to be 1,730 ± 150 μm [87]. These findings were obtained despite the fact that MNPs can be eliminated by nasal hair blockage, mucus cilia adhesion, or macrophage phagocytosis, and subsequently cleared out by coughing or sputum. However, some MNPs are still able to evade these clearance mechanisms and can adhere or embed themselves, eventually accumulating in the respiratory system and even translocating into circulation [29,88,89]. Particle size plays a significant role in the clearance, accumulation, and translocation of MNPs. Particles with sizes ranging from 0.5 to 5 μm can be easily cleared through alveolar macrophages and mucus villi, while larger fibers and fragments (15–20 μm) are more difficult to clear [84,90]. As a result, larger fibers and fragments tend to accumulate in lung tissue, and the researchers detected MPs in human lung tissue in the particle size range of 1.6–1,450 μm [30,86]. Additionally, the thin and lengthy natures of fibers enable their decreased mobility and increased adhesion to the lungs, consequently causing lung accumulation. Respirable particles typically have a size smaller than 10 μm, whereas fibers that enter the lungs can reach lengths of thousands of microns, posing a long-term health hazard that cannot be overlooked.
A small fraction of MNPs may be able to cross the alveolar wall, enter the capillaries, and ultimately the bloodstream. The translocation of particles could be size-dependent. For instance, studies in mice have shown that nanoparticles with sizes of up to 200 nm can pass through the air–blood barrier (ABB) [91]. Similarly, human exposure experiments have found that carbon particles with sizes below 100 nm can penetrate across the ABB [92]. In addition, aging can affect the translocation of nanoparticles across the ABB. In neonates, the transport of gold nanoparticles is not size-dependent, while in adult animals, smaller nanoparticles (5 nm) can cross the ABB more efficiently than larger nanoparticles (100 nm) [93]. The ABB also can be influenced by the surface charge of nanoparticles and negatively charged particles easier cross the barrier [91]. NPs might cross the ABB through the large gaps formed between alveolar epithelial cells or through the endocytosis of cells (Fig. 1B) [94]. Further investigation is needed to determine whether MPs can cross the ABB. The mechanisms by which MNPs in the environment cross the ABB are more complex due to their different forms, particle size range, and surface charge.
3.2. Accumulation and translocation of MNPs in the intestine
MNPs that enter the digestive system are subject to a similar pathway as those entering the respiratory system. When water and food containing MNPs enter the human digestive tract, the intestinal tract is exposed to MNPs directly or indirectly. However, MNPs are difficult to digest and degrade in the body [95]. The majority of MNPs in the human digestive tract could be excreted by the body. Schwabl et al. [96] reported nine distinct types of MPs in human feces with sizes ranging from 20 to 500 μm. The abundance of MPs in human feces ranged from 1 to 36 particles/g [96,97]. Surprisingly, the concentration of MNPs in infant feces was higher than in adults [27], suggesting that infants and children are exposed more to MNPs than adults.
The researchers further found that MNPs accumulate in the human intestine and can even translocate into the circulatory system. The abundance of MPs in the dead human colon was 28 ± 15 particles/g, indicating accumulation of MPs in the intestine over a long period of time [31]. MNPs in the intestine could be translocated into the circulatory system through three main channels (Fig. 1 A) [98]. The first channel involves the endocytosis of epithelial cells, which is mainly capable of translocating nanoscale particles. In vitro experiments have demonstrated that plastic particles <100 nm can permeate the barrier of Caco-2 (human colon cancer)/HT29 (human cell line) + Raji-B (lymphoblast-like cell line) cells and even traverse the intestinal barrier [99]. The ecological corona generated by particles of plastic exposed to the environment is more conducive to the passage of NPs across the intestinal barrier [100]. The second channel involves the transcytosis transport of microfold (M) cells in the Peyer’s patches of the ileum, which is thought to be the main mode of translocation of MNPs [84,98]. M cells can translocate particles smaller than <10 μm to the mucosal lymphoid tissue and concentrate them on the plasma membrane side of the Peyer’s patch [84,101]. In in vitro experiments, M cells co-cultured with Caco-2 cells were more likely to take up fluorescent MPs than a single Caco-2 culture [102]. The third channel is the persorption process, which involves the shedding of intestinal epithelial cells from their villi-like tips, and generates pores that allow big particles to pass through. The experiment showed that polyvinyl chloride (PVC) particles of 5–110 μm can pass through the intestinal barrier via persorption [103].
However, a gap still exists between current in vitro cellular experiments and the actual human intestinal absorption mechanism of MNPs. For instance, it is uncertain whether MNPs in the gut are completely detached from food and irregular MNPs have the same fate in the gut as spherical particles. Additionally, further research is needed to determine the distribution of MNPs in different regions of the gastrointestinal tract, such as the small intestine, colon, duodenum, jejunum, and ileum. The translocation rate of MNPs in the intestine should be further estimated through in vivo models.
3.3. Accumulation and translocation of MNPs in the skin
The skin, being the largest organ of the human body, serves as a barrier that prevents the penetration of particulate matter. However, there is currently a lack of research on the accumulation and transfer of MNPs on the skin. Alvarez-Roman et al. [104] observed that PS microspheres (20 and 200 nm) preferentially accumulate in the follicular openings of porcine skin and increase over time. Whereas the mechanism by which these NPs penetrate the skin barrier remains unclear. Based on previous advances in nanoparticle research, NPs have the potential pathways to penetrate the skin barrier [105,106]. Currently, there are three pathways by which NPs are transferred from the outer skin to the body: (i) via cellular bypass (<1–4 nm), (ii) via sweat glands and hair follicles (4–20 nm), and (iii) damaged skin (21–45 nm) [105]. Indeed, when the skin is severely damaged, there is a possibility that larger-sized NPs can penetrate through.
3.4. MNPs accumulation in organs
After crossing the intestinal barrier and the ABB, MNPs enter the circulation system. The largest blood vessel in the human body, the aorta, has a diameter of about 25,000 μm and the smallest capillary is about 8 μm [107], which allows convenient transfer of different sizes of MNPs through the bloodstream circulation in the human body and eventually accumulation in organs, tissues, and body fluids (Table 2). Researchers detected the presence of PET and PC-type plastics in human blood for the first time [32], although their size was not determined. More investigations are needed to reveal the kinetics of MNPs in blood. Recently, MPs also have been found in human thrombosis (∼5 μm), the liver (4–30 μm), and even the placenta (5–10 μm) [33,35,36] using Raman or infrared spectroscopy. In the future, it is necessary to observe MNPs in vivo by labeling them with radioisotopes or upconversion fluorescence, and in situ image their location using positron emission computed tomography or photoacoustic imaging techniques.
4. Potential effects of MNPs on human organ systems
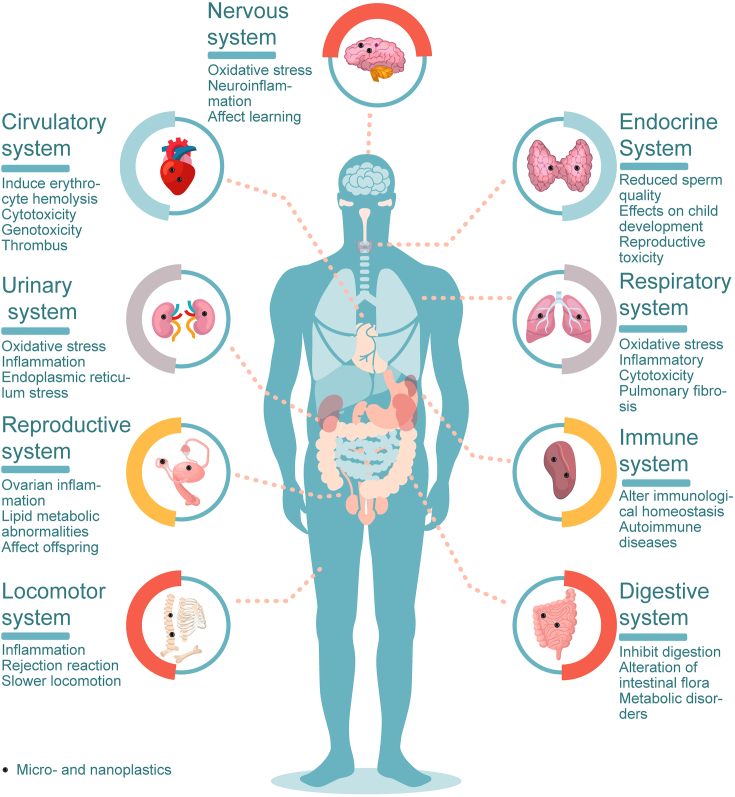
The potential impact of MNPs on health is a major concern. The human body mainly consists of nine organ systems, namely the digestive, respiratory, circulatory, reproductive, nervous, immune, endocrine, urinary, and locomotor systems, whose functional balances are required for human well-being. In this paper, the effects of MNPs on these nine organ systems are summarized through knowledge derived from in vivo and in vitro toxicological studies (Fig. 2).
Fig. 2.
Potential health risks of micro- and nano-plastics to nine human organ systems.
4.1. Digestive system
The digestive system plays a vital role in breaking down food, absorbing nutrients, and eliminating waste [108]. However, MNPs might have adverse effects on the intestinal tract. MNPs can potentially impact nutrient absorption in the human intestine, disrupt intestinal homeostasis, and ultimately lead to intestinal diseases [[109], [110], [111], [112], [113], [114], [115], [116]].
Preliminary experiments have shown that MNPs inhibit lipid digestion and reduce the absorption of vitamin D3 [109,110], causing nutritional imbalances. The main reason is that MNPs can agglomerate nutrients and reduce their bioavailability or affect the activity of the corresponding enzymes. Additionally, fibrous MPs featured honeycomb-like pores that competitively absorb nutrients [111].
A stable intestinal microbiota is essential for human health. MNPs altered the human intestinal microbiota and caused an imbalance in intestinal microecology. In vivo exposure experiments in model animals showed that MPs alter bacterial abundance in the intestine of mice, feeding polyethylene (PE) MPs showed significant increased abundances in Staphylococcus alongside with a decrease in Parabacteroides [112], and feeding polystyrene (PS) MPs decreased Actinobacteria abundance [113]. The concentration of MPs also affected the intestinal microbiota, and a high concentrations of PE MPs (600 μg/day) increased intestinal microbial species, bacterial abundance, and flora diversity in mice [112]. Considering the possible access of environmental MNPs that carry microorganisms and even pathogenic bacteria to the human digestive system, their impacts on the stabilization of the intestinal flora deserve carefully examination.
MNPs negatively affect human intestinal cells. In human colonic epithelial cells CCD841CoN and small intestinal epithelial cells HIEC-6, 0.1 μm PS microspheres caused cellular oxidative stress and 5 μm PS exposure resulted in higher levels of mitochondrial depolarization [114]. Therefore, MNPs exposure in the intestine causes intestinal barrier dysfunction, metabolic disorders, immune response, inflammation, and ultimately to the development of related diseases [[112], [113], [114], [115], [116]].
Risks from co-interactions of MNPs and other contaminants such as heavy metals also should be cautiously considered. Once these contaminants have entered the human body through MNPs as the carrier, their release could greatly impair human health. In an in vitro human digestive model, both lead (Pb) and chromium (Cr) could be desorbed from MPs into simulated gastric and intestinal fluids. This is indicative of increased risk of the metals to human health [117,118]. On the other hand, no significant desorption of benzophenone-3 from MNPs occurred in the simulated human gastrointestinal fluid [119]. Desorption behaviors of pollutants from MNPs in the human gastrointestinal system should be differential, based on the type/size of MNPs, as well as pH and the presence of surfactants in the surrounding intestinal environment.
4.2. Respiratory system
MPs have been detected in both the upper respiratory tract (sputum, nasal cavity) [88] and lower respiratory tract (alveoli, lung tissue) [87,120] of humans, which raises concerns about their potential health effects on the respiratory system. Although there is still no direct link between MNPs and human respiratory disease, recent research suggests that MNPs may alter endogenous surfactants of human lungs, impair lung cells, and increase their susceptibility to lung disorders such as pulmonary fibrosis, pulmonary frosted glass nodules, and asthma [[121], [122], [123], [124], [125], [126]].
Lung surfactants play an important role in reducing alveolar surface tension and preventing invasion by exogenous particles [121]. Researchers discovered an abundance of 9.18 particles/100 mL in alveolar lavage fluid, 97.06% of which were fibers [87]. Shi et al. [122] found that MPs modify the phase behavior, surface tension, and membrane structure of simulated lung surfactants, as well as increase the amount of reactive oxygen species (ROS) in lung surfactants by in vitro simulations. MNPs were more accessible to lung cells by altering the composition of the pulmonary surfactants, and ROS damaged DNA and caused lung damage [122]. The toxicity of MPs to human lung cells could be correlated to the concentration and size of MPs [123,124]. Low doses of PS particles (10 μg/cm2) cause cytotoxic, inflammatory effects in lung epithelial cells (BEAS-2B) and disrupt lung barrier function, while high concentrations (1,000 μg/cm2) increase the risk of occurrence of chronic obstructive pulmonary disease [124]. The smaller sized MNPs are more toxic to human lung cells, which could be attributed to the higher bioactivity and greater intracellular accumulation of smaller NPs [123].
MNPs might be able to cause lung diseases. In mice, 5 μm PS MPs were found to persist in lungs, initiated oxidative stress and chronically damaged epithelial tissues, elicited inflammation and consequently activated the Wnt/β-catenin signaling that led to lung fibrosis. In addition, inhaled tire wear plastic (<1 μm) induced pulmonary fibrosis injury [125]. Moreover, fibrous MPs may be associated with the formation of ground glass nodules in the lung. By comparison with human lung tumors and normal tissue, fibrous MPs were more frequently detected in tumor tissues (58%) than in normal tissues (42%). In people more exposed to MPs in their living or working environment, fibrous MPs were detected in 72% of tumor tissues [126]. To date, the toxicity of MNPs on the respiratory system is not well understood and requires further investigations.
4.3. Circulatory system
The circulatory system supplies oxygen and nutrients to the various tissues in the body and removes waste products. A recent study has identified the presence of PET, PS, PE, and poly (methyl methacrylate) plastics (>700 nm) in the blood of 22 healthy individuals, with an average concentration of 1.6 μg/mL of MNPs [32]. Moreover, MNPs have been identified in human thrombus [127], raising concerns about their potential impact on the human circulatory system. Current evidence suggests that MNPs may be harmful to red blood cells and could potentially affect angiogenesis and platelet function, even leading to thrombosis in humans [[128], [129], [130], [131], [132]].
Once entering the circulation, MNPs interact with different components of the blood such as plasma proteins, red blood cells, platelets, and peripheral blood lymphocytes. On the one hand, MNPs absorb plasma proteins to form a multilayer corona on the exterior, resulting in an aggregation effect [128]. On the other hand, MNPs adsorb to the surface of blood erythrocytes, and certain NPs (amino-modified) induce erythrocyte hemolysis [129]. Particulate matter also causes the aggregation and activation of platelets and ultimately the formation of blood thrombosis [130]. In addition, MNPs cause cytotoxicity and genotoxicity in human peripheral blood lymphocytes [131]. Moreover, PS MPs reduce the biological activity of endothelial cells, which in turn inhibits angiogenic and wound-healing signaling pathways, thus impacting the development of new blood vessels and wound healing [132]. When blood vessels are injured, endothelial cells promote coagulation and thrombosis by synthesizing and secreting a variety of coagulation-related molecules. These effects are also related to the particle size, shape, and surface charge of MNPs.
Current research on the effects of MNPs on the human circulatory system is still limited, and there are several research deficiencies to be addressed. First, most studies have been conducted in vitro, and there is a lack of in vivo studies that can provide more conclusive evidence. Moreover, most studies have focused on the acute effects of MNPs, while chronic exposure studies are needed to understand the long-term effects of MNPs on the circulatory system. Another research deficiency is the lack of standardized protocols for such studies. There is a need for standardized methods for the characterization and quantification of MNPs in blood samples to facilitate comparison between studies. In addition, there is a need for standardized protocols for assessing the effects of MNPs on blood components, including red blood cells, platelets, and peripheral blood lymphocytes.
4.4. Reproductive system
Researchers have recently paid increasing attention to the potential impact of MNPs on the human reproductive system since MPs were discovered in the human placenta [36]. One of the concerns is the potential threat to future generations posed by MNP effects on reproductive health. In vivo animal research has demonstrated that MNPs can cause reproductive toxicity and may also have health effects on the offspring [[133], [134], [135], [136], [137], [138]].
Studies on mice and rats have shown that MNPs can cause reproductive toxicity in both males and females [[133], [134], [135]]. PS particle exposure at high levels (30 mg/kg body weight) produced ovarian inflammation and decreased oocyte quality in mice [134]. PS particles similarly induced testicular inflammation, decreased sperm quality, and damaged the blood-testis barrier in mice [133]. In addition, the reproductive system of female rats is more susceptible to MNPs than that of males [135]. Moreover, Deng et al. [136] demonstrated that exposure to MNPs at a concentration of 50 mg/kg of food also influenced the testicular and sperm quality of mice. The underlying mechanisms behind the reproductive toxicity of MNPs are not yet fully understood, but inflammation and lipid metabolic abnormalities may play a significant role [136]. Future research could focus on identifying the specific pathways through which MNPs exert their effects on reproductive health, such as changes in gene expression or disruption of hormonal signaling.
To further investigate the effects of MNPs on mouse offspring, Deng et al. [136] found that prolonged exposure of male mice to MNPs decreased body weight and liver mass in the offspring, as well as causing disorders of lipid metabolism. In addition, prolonged maternal exposure to MNPs can cause impaired energy metabolism in the offspring [137]. Recent research detected significantly higher MPs in the placentas of pregnant women with intrauterine growth restriction (302 particles/13 placentas) than in normal placentas (6 particles/13 placentas) [138]. As mentioned earlier, MNPs have been shown to have transgenerational effects on reproductive health in animal models. Future research could investigate whether these effects are also present in humans, and whether they are passed down through multiple generations.
4.5. Nervous system
The nervous system is a complex network of neurons that regulates the body’s physiological activities [139,140]. However, there is a paucity of research on the effects of MNPs on the human nervous system. Currently, in vivo animal tests have demonstrated that 2 μm PS particles can cross the blood–brain barrier and aggregate in the brain of mice [141]. Qi et al. [142] also discovered exogenous fine particles (such as malayaite and anatase TiO2) in human cerebrospinal fluids, but no micron-level particles have been identified in the human brain. This suggests that NPs have likely permeated the human brain.
Lee et al. [141] further found that MPs affected learning and memory in the brains of mice continuously fed with 2 μm of PS MPs (0.016 mg/g) for eight weeks. This may be mainly due to the fact that PS particles entering the brain cause neuroinflammation in the hippocampus, which in turn alters genes and proteins that contribute to synaptic plasticity [141]. In addition, Wang et al. [143] also found that PS particles (5 μm) induced oxidative stress and reduced acetylcholine levels in mice, resulting in learning and memory impairment. The neurotoxicity of MPs was also dependent on dose, size, composition, and shape [143].
Based on current research advances, future in vivo and epidemiological research is needed to investigate the potential long-term effects of long-term exposure to MNPs on the human nervous system, including cognitive function and behavior. Additionally, there is a need to explore the effects of MNPs on neural networks and specialized neurons in the human brain by examining changes in neurotransmitter levels, gene expression, and synaptic plasticity following exposure to MNPs and to study the effects of MNPs on specific populations, such as children, the elderly and individuals with pre-existing neurological disorders. This will help to identify potential vulnerabilities and inform targeted interventions to protect these populations.
4.6. Immune system
The immune system is a network of lymphoid organs, tissues, cells, humoral substances, and cytokines that work together to defend the body [144]. An important function of the immune system is to eliminate invading bacteria, foreign cells, macromolecular compounds (antigens), and extraneous particles. MNPs trigger a local or systemic immunological response when entering an organism, and some MNPs generate a protein corona on their surface that enables them to escape the immune system [128]. Experiments on animals or cells have shown that MNPs can lead to increased secretion of pro-inflammatory cytokines, disrupting immune homeostasis and ultimately leading to immune system disorders such as autoimmune diseases [[145], [146], [147], [148], [149], [150], [151]].
Secretion of pro-inflammatory cytokines is essential for maintaining homeostasis of the immune system. In in vitro human peripheral blood mononuclear cell experiments, Han et al. found that both acrylonitrile–butadiene–styrene (ABS) and PVC particles induced the release of interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) and that the particle size and concentration of plastic particles affected the release of IL-6 and TNF-α [146]. Larger PVC (75–200 μm) tended to induce the release of IL-6 and TNF-α; smaller ABS (25–75 μm) particles resulted in the elevated release of IL-6 at higher concentrations (1,000 μg/mL). Conversely, larger ABS (75–200 μm) particles showed a tendency to induce the release of TNF-α across all concentrations (10–1,000 μg/mL) [146]. In addition, mice were exposed to PE particles through the diet and the PE particles were found to induce an intestinal immune response. This resulted in increased levels of interleukin-1α (IL-1α) and decreased levels of interleukin 2 (IL-2) [112]. Moreover, PS particles cause a maternal–fetal immunological imbalance in mice, which might ultimately result in abortion due to reduced immune cells and the proportion of macrophages [147]. An immune system overreacting to MNPs can lead to massive inflammation, resulting in an imbalance in the homeostasis of the immune response.
Autoimmune diseases are a group of diseases in which an immune response to an autoantigen leads to damage or dysfunction of the tissues and organs. In addition to genetic factors, autoimmune diseases can be triggered by environmental factors [148]. Numerous studies have demonstrated that air pollution might exacerbate autoimmune disorders. Particularly, ambient fine particulate matter may raise the incidence of systemic lupus erythematosus [149], type 1 diabetes [150], and rheumatoid arthritis [151]. Similarly, environmental MNPs have a strong propensity to induce autoimmune disorders.
The immune system is present in various organs throughout the body, including the spleen, lymph nodes, and bone marrow, among others. Future research should investigate the impact of MNP exposure on immune system function in different organs and even overall immune system health. Research is also needed to investigate the effects of MNP exposure on different immune cell populations, including T cells, B cells, macrophages, and dendritic cells.
4.7. Endocrine system
The endocrine system is responsible for regulating the normal physiological activities of the body through hormones. Despite limited toxicity to the endocrine system, MNPs can carry and desorb some endocrine-disrupting chemicals (EDCs), such as bisphenol A, phthalates, or steroid hormones [26,152]. There is growing evidence from laboratory animal studies and epidemiological studies that EDCs can interfere with the development of the endocrine system and affect the function of organs that respond to hormonal signals [152], leading to a variety of health problems such as reduced sperm quality and sex hormone concentrations, effects on child development, type 2 diabetes, obesity, and so on [153,154]. Furthermore, Deng et al. [155] showed that the presence of MNPs significantly increased the absorption of EDCs in the intestine and increased their reproductive toxicity. It is, therefore, necessary to focus on the interaction between MNPs and EDCs in organisms and to further investigate their combined toxicity to organisms. In addition, other endocrine disruptors such as polychlorinated biphenyls and dioxins are also present in the environment and can enter the body through adsorption on the surface of MNPs, and their release in the body and combined toxicity with MNPs require more attention.
4.8. Urinary system
The urinary system is the main metabolic pathway of the body and maintains stability within the organism. Unquestionably, MNPs can also penetrate the urinary system and harm the kidneys and bladder. At the cellular level, exposure of human embryonic kidney cells (HEK 293) to PS particles drastically reduces cell proliferation and causes cellular oxidative stress [156]. In addition, PS particles cause mitochondrial dysfunction, endoplasmic reticulum stress, inflammation, and autophagy in kidney cells [157]. At the organ level, PS particles (50–400 μm) can accumulate in the kidney, with 600 nm PS particles aggregating while 4 μm PS particles appearing as single particles [158]. MNPs also cause significant kidney quality decrease, histopathological lesions, kidney inflammation, and endoplasmic reticulum stress [157,158]. In addition, PS particles produce bladder epithelial necrosis and inflammation, with 1–10 μm particles causing the most severe necrosis and 50–100 μm particles causing the most severe inflammatory damage [159].
Animal studies have shown that MNPs (100 nm, 3 μm) are excreted in mouse urine [160], and recent studies have shown that MPs are also present in human urine [28]. Pironti et al. detected seven irregular MPs with particle sizes of approximately 4–15 μm in urine samples from six individuals, the main types being PP, PE, polyvinyl acetate, and PVC [28]. Based on the aforementioned studies, future research needs to focus on improving the detection methods for the concentration and particle size range of MNPs in human urine to accurately assess the glomerular filtration rate. Furthermore, future studies should investigate the potential effects of MNP exposure on urinary protein filtration and reabsorption.
4.9. Locomotor system
MNPs have been shown to inhibit the mobility of fish, soil animals, and birds [[161], [162], [163]], but their effect on the human locomotor system is negligible. However, individuals who use prostheses need to be aware that wear and tear can produce MNPs that may cause inflammation and possible rejection, thereby affecting mobility [164]. MNPs may also affect movement by influencing the central nervous system. For example, one study found that feeding mice food containing MPs resulted in shorter walking distances and slower locomotion [165].
5. Future research recommendations
Based on the above discussion, research on the effects of MNPs on mammalian and, in particular, human health is still in its early stages. There are significant gaps regarding the quantification of the concentrations of MNPs in different foods, the intake of MNPs by different routes of exposure in humans, the absorption and transfer of MNPs in the human body, and the mechanisms of the health effects of MNPs on humans after ingestion. Therefore, systematic and in-depth studies on the effects of MNPs on human health are needed. Recommendations for future research are as follows.
i. Standardize MNPs detection methods and establish quality control and quality assurance systems to avoid contamination and facilitate comparison between studies. Based on the nature of MNPs, a standard formula for converting MNPs from abundance to mass concentration needs to be established, which allows for a more realistic and comparable assessment of daily human exposure.
ii. Develop in vitro models that simulate the complexity of human tissues and organs to better understand the accumulation and transfer of MNP in the human body. There is a further need to develop innovative techniques for characterizing and studying MNPs, including advanced imaging techniques and novel analytical tools for better in situ imaging and characterization.
iii. Standardize in vitro cellular and model animal experiments for dose–response studies of MNPs. Evaluate and compare the toxicity and mechanism of action of different types and particle sizes of MNPs. And, conduct long-term epidemiological studies to assess the chronic effects of MNPs on human health. Particularly, vulnerable populations, including pregnant women, children, and the elderly, may be more susceptible to the toxic effects of MNPs.
iv. Based on the one health framework, the issue of MNPs requires interdisciplinary collaboration and scientific and technological innovation [161]. There is a need to strengthen communication and cooperation between professionals in different fields, explore new research methods and technologies, and promote the solution to MNPs problems.
Author contributions
Y.D.F.: conceptualization, investigation, writing–original draft, writing–original revise. C.T.: conceptualization, investigation, writing–original draft, supervision, project administration, writing–review & editing. R.J.L., D.W., J.Y.: writing–review & editing. Y.K.X., W.J.G.M.P.: supervision, writing–review & editing. Y.M.L.: conceptualization, supervision, writing–review & editing.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (22241602, 41991330, and 42177039). Funding by the European Commission within the Horizon Europe funded project Plasticsfate (grant agreement number 965367) is kindly acknowledged.
References
- 1.Prata J.C., da Costa J.P., Lopes I., Duarte A.C., Rocha-Santos T. Environmental exposure to microplastics: an overview on possible human health effects. Sci. Total Environ. 2020;702:134455. doi: 10.1016/j.scitotenv.2019.134455. [DOI] [PubMed] [Google Scholar]
- 2.Lau W.W.Y., Shiran Y., Bailey R.M., Cook E., Stuchtey M.R., Koskella J., Velis C.A., Godfrey L., et al. Evaluating scenarios toward zero plastic pollution. Science. 2020;369:1455–1461. doi: 10.1126/science.aba9475. [DOI] [PubMed] [Google Scholar]
- 3.Lei K., Qiao F., Liu Q., Wei Z., Qi H., Cui S., Yue X., Deng Y., An L. Microplastics releasing from personal care and cosmetic products in China. Mar. Pollut. Bull. 2017;123:122–126. doi: 10.1016/j.marpolbul.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Waldman W.R., Rillig M.C. Microplastic research should embrace the complexity of secondary particles. Environ. Sci. Technol. 2020;54:7751–7753. doi: 10.1021/acs.est.0c02194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avio C.G., Gorbi S., Regoli F. Plastics and microplastics in the oceans: from emerging pollutants to emerged threat. Mar. Environ. Res. 2017;128:2–11. doi: 10.1016/j.marenvres.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Dusaucy J., Gateuille D., Perrette Y., Naffrechoux E. Microplastic pollution of worldwide lakes. Environ. Pollut. 2021;284:117075. doi: 10.1016/j.envpol.2021.117075. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z., Deng C., Dong L., Liu L., Li H., Wu J., Ye C. Microplastic pollution in the Yangtze River Basin: heterogeneity of abundances and characteristics in different environments. Environ. Pollut. 2021;287:117580. doi: 10.1016/j.envpol.2021.117580. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q., Zhao Y., Du F., Cai H., Wang G., Shi H. Microplastic fallout in different indoor environments. Environ. Sci. Technol. 2020;54:6530–6539. doi: 10.1021/acs.est.0c00087. [DOI] [PubMed] [Google Scholar]
- 9.Dris R., Gasperi J., Mirande C., Mandin C., Guerrouache M., Langlois V., Tassin B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017;221:453–458. doi: 10.1016/j.envpol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Hu J., He D., Zhang X., Li X., Chen Y., Wei G., Zhang Y., Ok Y.S., et al. National-scale distribution of micro(meso)plastics in farmland soils across China: implications for environmental impacts. J. Hazard. Mater. 2022;424:127283. doi: 10.1016/j.jhazmat.2021.127283. [DOI] [PubMed] [Google Scholar]
- 11.Kibria G. Impacts of microplastic on fisheries and seafood security—Global analysis and synthesis. Sci. Total Environ. 2023;904:166652. doi: 10.1016/j.scitotenv.2023.166652. [DOI] [PubMed] [Google Scholar]
- 12.Danopoulos E., Twiddy M., Rotchell J.M. Microplastic contamination of drinking water: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y., Peng L., Fu J., Dai X., Wang G. A microscopic survey on microplastics in beverages: the case of beer, mineral water and tea. Analyst. 2022;147:1099–1105. doi: 10.1039/D2AN00083K. [DOI] [PubMed] [Google Scholar]
- 14.Vidyasakar A., Krishnakumar S., Kumar K.S., Neelavannan K., Anbalagan S., Kasilingam K., Srinivasalu S., Saravanan P., et al. Microplastic contamination in edible sea salt from the largest salt-producing states of India. Mar. Pollut. Bull. 2021;171:112728. doi: 10.1016/j.marpolbul.2021.112728. [DOI] [PubMed] [Google Scholar]
- 15.Vethaak A.D., Legler J. Microplastics and human health. Science. 2021;371:672–674. doi: 10.1126/science.abe5041. [DOI] [PubMed] [Google Scholar]
- 16.Revel M., Châtel A., Mouneyrac C. Micro(nano)plastics: a threat to human health? Curr. Opin. Enviro. Sci. Health. 2018;1:17–23. doi: 10.1016/j.coesh.2017.10.003. [DOI] [Google Scholar]
- 17.Chang X.R., Xue Y.R., Li J.Y., Zou L.Y., Teng M. Potential health impact of environmental micro- and nanoplastics pollution. J. Appl. Toxicol. 2020;40:4–15. doi: 10.1002/jat.3915. [DOI] [PubMed] [Google Scholar]
- 18.Zarus G.M., Muianga C., Hunter C.M., Steven P.R. A review of data for quantifying human exposures to micro and nanoplastics and potential health risks. Sci. Total Environ. 2021;756:144010. doi: 10.1016/j.scitotenv.2020.144010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elizalde-Velázquez G.A., Gómez-Oliván L.M. Microplastics in aquatic environments: a review on occurrence, distribution, toxic effects, and implications for human health. Sci. Total Environ. 2021;780:146551. doi: 10.1016/j.scitotenv.2021.146551. [DOI] [PubMed] [Google Scholar]
- 20.Koelmans A.A., Redondo-Hasselerharm P.E., Nor N.H.M., de Ruijter V.N., Mintenig S.M., Kooi M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022;7:138–152. doi: 10.1038/s41578-021-00411-y. [DOI] [Google Scholar]
- 21.Ebrahimi P., Abbasi S., Pashaei R., Bogusz A., Olsezczuk P. Investigating impact of physicochemical properties of microplastics on human health: a short bibliometric analysis and review. Chemosphere. 2022;289:133146. doi: 10.1016/j.chemosphere.2021.133146. [DOI] [PubMed] [Google Scholar]
- 22.Xing Y.F., Xu Y.H., Shi M.H., Lian Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016;8:E69–E74. doi: 10.3978/j.issn.2072-1439.2016.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang S., Wang M., Germ K.E., Du H.M., Sun W.J., Gao W.M., Mayer G.D. Health implications of engineered nanoparticles in infants and children. World J. Pediatr. 2015;11:197–206. doi: 10.1007/s12519-015-0028-0. [DOI] [PubMed] [Google Scholar]
- 24.Li X., Zhang T., Lv W., Wang H., Chen H., Xu Q., Cai H., Dai J. Intratracheal administration of polystyrene microplastics induces pulmonary fibrosis by activating oxidative stress and Wnt/β-catenin signaling pathway in mice. Ecotoxicol. Environ. Saf. 2022;232:113238. doi: 10.1016/j.ecoenv.2022.113238. [DOI] [PubMed] [Google Scholar]
- 25.Khan A., Jia Z. Recent insights into uptake, toxicity, and molecular targets of microplastics and nanoplastics relevant to human health impacts. iScience. 2023;26(2) doi: 10.1016/j.isci.2023.106061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campanale C., Massarelli C., Savino I., Locaputo V., Uricchio V.F. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health. 2020;17:1212. doi: 10.3390/ijerph17041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J., Wang L., Trasande L., Kannan K. Occurrence of polyethylene terephthalate and polycarbonate microplastics in infant and adult feces. Environ. Sci. Technol. Lett. 2021;8:989–994. doi: 10.1021/acs.estlett.1c00559. [DOI] [Google Scholar]
- 28.Pironti C., Notarstefano V., Ricciardi M., Motta O., Giorgini E., Montano L. First evidence of microplastics in human urine, a preliminary study of intake in the human body. Toxics. 2023;11(1):40. doi: 10.3390/toxics11010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang S., Huang X., Bi R., Guo Q., Yu X., Zeng Q., Huang Z., Liu T., et al. Detection and analysis of microplastics in human sputum. Environ. Sci. Technol. 2022 doi: 10.1021/acs.est.1c03859. [DOI] [PubMed] [Google Scholar]
- 30.Amato-Lourenço L.F., Carvalho-Oliveira R., Júnior G.R., dos Santos Galvão L., Ando R.A., Mauad T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021;416:126124. doi: 10.1021/acs.est.1c03859. [DOI] [PubMed] [Google Scholar]
- 31.Ibrahim Y.S., Tuan Anuar S., Azmi A.A., Wan Mohd Khalik W.M.A., Lehata S., Hamzah S.R., Ismail D., Ma Z.F., et al. Detection of microplastics in human colectomy specimens. JGH Open. 2021;5:116–121. doi: 10.1002/jgh3.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leslie H.A., van Velzen M.J., Brandsma S.H., Vethaak D., Garcia-Vallejo J.J., Lamoree M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022:107199. doi: 10.1016/j.envint.2022.107199. [DOI] [PubMed] [Google Scholar]
- 33.Wu D., Feng Y., Wang R., Jiang J., Guan Q., Yang X., Wei H., Xia Y., et al. Pigment microparticles and microplastics found in human thrombi based on Raman spectral evidence. J. Adv. Res. 2022 doi: 10.1016/j.jare.2022.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan Q., Jiang J., Huang Y., Wang Q., Liu Z., Ma X., Yang X., Li Y., et al. The landscape of micron-scale particles including microplastics in human enclosed body fluids. J. Hazard. Mater. 2023;442:130138. doi: 10.1016/j.jhazmat.2022.130138. [DOI] [PubMed] [Google Scholar]
- 35.Horvatits T., Tamminga M., Liu B., Sebode M., Carambia A., Fischer L., Püschel K., Huber S., et al. Microplastics detected in cirrhotic liver tissue. EBioMedicine. 2022;82:104147. doi: 10.1016/j.ebiom.2022.104147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ragusa A., Svelato A., Santacroce C., Catalano P., Notarstefano V., Carnevali O., Papa F., Rongioletti M.C.A., et al. Plasticenta: first evidence of microplastics in human placenta. Environ. Int. 2021;146:106274. doi: 10.1016/j.envint.2020.106274. [DOI] [PubMed] [Google Scholar]
- 37.Chen E.Y., Lin K.T., Jung C.C., Chang C.L., Chen C.Y. Characteristics and influencing factors of airborne microplastics in nail salons. Sci. Total Environ. 2022;806:151472. doi: 10.1016/j.scitotenv.2021.151472. [DOI] [PubMed] [Google Scholar]
- 38.Yao Y., Glamoclija M., Murphy A., Gao Y. Characterization of microplastics in indoor and ambient air in northern New Jersey. Environ. Res. 2022;207:112142. doi: 10.1016/j.envres.2021.112142. [DOI] [PubMed] [Google Scholar]
- 39.Zhan L., Zhang Q., Bulati A., Wang R., Xu Z. Characteristics of microplastics and the role for complex pollution in e-waste recycling base of Shanghai, China. Environ. Int. 2022;169:107515. doi: 10.1016/j.envint.2022.107515. [DOI] [PubMed] [Google Scholar]
- 40.Akanyange S.N., Lyu X., Zhao X., Li X., Zhang Y., Crittrnden J.C., Anning C., Chen T., et al. Does microplastic really represent a threat? A review of the atmospheric contamination sources and potential impacts. Sci. Total Environ. 2021;777:146020. doi: 10.1016/j.scitotenv.2021.146020. [DOI] [PubMed] [Google Scholar]
- 41.Kacprzak S., Tijing L.D. Microplastics in indoor environment: sources, mitigation and fate. J. Environ. Chem. Eng. 2022;10:107359. doi: 10.1016/j.jece.2022.107359. [DOI] [Google Scholar]
- 42.Cox K.D., Covernton G.A., Davies H.L., Dower J.F., Juanes F., Dudas S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019;53:7068–7074. doi: 10.1021/acs.est.9b01517. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J., Wang L., Kannan K. Microplastics in house dust from 12 countries and associated human exposure. Environ. Int. 2020;134:105314. doi: 10.1016/j.envint.2019.105314. [DOI] [PubMed] [Google Scholar]
- 44.Nematollahi M.J., Zarei F., Keshavarzi B., Zarei M., Moore F., Busquets R., Kelly F.J. Microplastic occurrence in settled indoor dust in schools. Sci. Total Environ. 2022;807:150984. doi: 10.1016/j.scitotenv.2021.150984. [DOI] [PubMed] [Google Scholar]
- 45.Liao Z., Ji X., Ma Y., Lv B., Huang W., Zhu X., Fang M., Wang Q., et al. Airborne microplastics in indoor and outdoor environments of a coastal city in Eastern China. J. Hazard. Mater. 2021;417:126007. doi: 10.1016/j.jhazmat.2021.126007. [DOI] [PubMed] [Google Scholar]
- 46.Zhu X., Huang W., Fang M., Liao Z., Wang Y., Xu L., Mu Q., Shi C., et al. Airborne microplastic concentrations in five megacities of northern and southeast China. Environ. Sci. Technol. 2021;55:12871–12881. doi: 10.1021/acs.est.1c03618. [DOI] [PubMed] [Google Scholar]
- 47.Liu K., Wang X., Fang T., Xu P., Zhu L., Li D. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci. Total Environ. 2019;675:462–471. doi: 10.1016/j.scitotenv.2019.04.110. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Q., Xu E.G., Li J., Chen Q., Ma L., Zeng E.Y., Shi H. A review of microplastics in table salt, drinking water, and air: direct human exposure. Environ. Sci. Technol. 2020;54:3740–3751. doi: 10.1021/acs.est.9b04535. [DOI] [PubMed] [Google Scholar]
- 49.Jâms I.B., Windsor F.M., Poudevigne-Durance T., Ormerod S.J., Durance I. Estimating the size distribution of plastics ingested by animals. Nat. Commun. 2020;11:1594. doi: 10.1038/s41467-020-15406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curren E., Leaw C.P., Lim P.T., Leong S.C.Y. Evidence of marine microplastics in commercially harvested seafood. Front. Bioeng. Biotechnol. 2020;8:562760. doi: 10.3389/fbioe.2020.562760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leung M.M.L., Ho Y.W., Maboloc E.A., Lee C.H., Wang Y., Hu M., Cheung S.G., Fang J.K.H. Determination of microplastics in the edible green-lipped mussel Perna viridis using an automated mapping technique of Raman microspectroscopy. J. Hazard. Mater. 2021;420:126541. doi: 10.1016/j.jhazmat.2021.126541. [DOI] [PubMed] [Google Scholar]
- 52.Wootton N., Reis-Santos P., Gillanders B.M. Microplastic in fish – a global synthesis. Rev. Fish Biol. Fish. 2021;31:753–771. doi: 10.1007/s11160-021-09684-6. [DOI] [Google Scholar]
- 53.Expósito N., Rovira J., Sierra J., Gimenez G., Domingo J.L., Schuhmacher M. Levels of microplastics and their characteristics in molluscs from North-West Mediterranean Sea: human intake. Mar. Pollut. Bull. 2022;181:113843. doi: 10.1016/j.marpolbul.2022.113843. [DOI] [PubMed] [Google Scholar]
- 54.Nicole W. Microplastics in seafood: how much are people eating? Environ. Health Perspect. 2021;129:34001. doi: 10.1289/EHP8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin J., Li J.-Y., Craig N.J., Su L. Microplastic pollution in wild populations of decapod crustaceans: a review. Chemosphere. 2022;291:132985. doi: 10.1016/j.chemosphere.2021.132985. [DOI] [PubMed] [Google Scholar]
- 56.Oßmann B.E., Sarau G., Holtmannspötter H., Pischetsrieder M., Christiansen S.H., Dicke W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018;141:307–316. doi: 10.1016/j.watres.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 57.Akhbarizadeh R., Dobaradaran S., Schmidt T.C., Nabipour I., Spitz J. Worldwide bottled water occurrence of emerging contaminants: a review of the recent scientific literature. J. Hazard. Mater. 2020;392:122271. doi: 10.1016/j.jhazmat.2020.122271. [DOI] [PubMed] [Google Scholar]
- 58.Kirstein I.V., Gomiero A., Vollertsen J. Microplastic pollution in drinking water. Curr. Opin. Toxicol. 2021;28:70–75. doi: 10.1016/j.cotox.2021.09.003. [DOI] [Google Scholar]
- 59.Munno K., Helm P., Jackson D.A., Rochman C.M., Sims A. Impacts of temperature and selected chemical digestion methods on microplastic particles. Environ. Toxicol. Chem. 2018;37:91–98. doi: 10.1002/etc.3935. [DOI] [PubMed] [Google Scholar]
- 60.Shruti V.C., Pérez-Guevara F., Elizalde-Martínez I., Kutralam-Muniasamy G. First study of its kind on the microplastic contamination of soft drinks, cold tea and energy drinks – future research and environmental considerations. Sci. Total Environ. 2020;726:138580. doi: 10.1016/j.scitotenv.2020.138580. [DOI] [PubMed] [Google Scholar]
- 61.Da Costa Filho P.A., Andrey D., Eriksen B., Peixoto R.P., Carreres B.M., Ambühl M.E., Descarrega J.B., Dubascoux S., et al. Detection and characterization of small-sized microplastics (≥5 μm) in milk products. Sci. Rep. 2021;11:24046. doi: 10.1038/s41598-021-03458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Afrin S., Rahman M.M., Akbor M.A., Siddique M.A.B., Uddin M.K., Malafaia G. Is there tea complemented with the appealing flavor of microplastics? A pioneering study on plastic pollution in commercially available tea bags in Bangladesh. Sci. Total Environ. 2022;837:155833. doi: 10.1016/j.scitotenv.2022.155833. [DOI] [PubMed] [Google Scholar]
- 63.Kutralam-Muniasamy G., Pérez-Guevara F., Elizalde-Martínez I., Shruti V.C. Branded milks – are they immune from microplastics contamination? Sci. Total Environ. 2020;714:136823. doi: 10.1016/j.scitotenv.2020.136823. [DOI] [PubMed] [Google Scholar]
- 64.Kiruba R., Preethi M., Aganasteen R., Rithick Raj M., Hannah Thabitha C., Monica P., Sakthivel J., Levince C., et al. Identification of microplastics as emerging contaminant in branded milk of Tamil Nadu State, India. Asian J. Biol. Life Sci. 2022;11:181. doi: 10.5530/ajbls.2022.11.25. [DOI] [Google Scholar]
- 65.Afrin S., Rahman M.M., Hossain M.N., Uddin M.K., Malafaia G. Are there plastic particles in my sugar? A pioneering study on the characterization of microplastics in commercial sugars and risk assessment. Sci. Total Environ. 2022;837:155849. doi: 10.1016/j.scitotenv.2022.155849. [DOI] [PubMed] [Google Scholar]
- 66.Diaz-Basantes M.F., Conesa J.A., Fullana A. Microplastics in honey, beer, milk and refreshments in Ecuador as emerging contaminants. Sustainability. 2020;12:5514. doi: 10.3390/su12145514. [DOI] [Google Scholar]
- 67.Peixoto D., Pinheiro C., Amorim J., Oliva-Teles L., Guilhermino L., Vieira M.N. Microplastic pollution in commercial salt for human consumption: a review. Estuar. Coast Shelf Sci. 2019;219:161–168. doi: 10.1016/j.ecss.2019.02.018. [DOI] [Google Scholar]
- 68.Lee H.J., Song N.S., Kim J.S., Kim S.K. Variation and uncertainty of microplastics in commercial table salts: critical review and validation. J. Hazard. Mater. 2021;402:123743. doi: 10.1016/j.jhazmat.2020.123743. [DOI] [PubMed] [Google Scholar]
- 69.Li L., Luo Y., Li R., Zhou Q., Peijnenburg W.J.G.M., Yin N., Yang J., Tu C., et al. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020;3:929–937. doi: 10.1038/s41893-020-0567-9. [DOI] [Google Scholar]
- 70.Dong Y., Gao M., Qiu W., Song Z. Uptake of microplastics by carrots in presence of as (III): combined toxic effects. J. Hazard. Mater. 2021;411:125055. doi: 10.1016/j.jhazmat.2021.125055. [DOI] [PubMed] [Google Scholar]
- 71.Luo Y., Li L., Feng Y., Li R., Yang J., Peijnenburg W.J., Tu C. Quantitative tracing of uptake and transport of submicrometre plastics in crop plants using lanthanide chelates as a dual-functional tracer. Nat. Nanotechnol. 2022:1–8. doi: 10.1038/s41565-021-01063-3. [DOI] [PubMed] [Google Scholar]
- 72.Jiang M., Wang B., Ye R., Yu N., Xie Z., Hua Y., Zhou R., Tian B., et al. Evidence and impacts of nanoplastic accumulation on crop grains. Adv. Sci. 2022:2202336. doi: 10.1002/advs.202202336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huerta Lwanga E., Mendoza Vega J., Ku Quej V., Chi J.d.l.A., Sanchez del Cid L., Chi C., Escalona Segura G., Gertsen H., et al. Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep. 2017;7:1–7. doi: 10.1038/s41598-017-14588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Panebianco A., Nalbone L., Giarratana F., Ziino G. First discoveries of microplastics in terrestrial snails. Food Control. 2019;106:106722. doi: 10.1016/j.foodcont.2019.106722. [DOI] [Google Scholar]
- 75.Dessì C., Okoffo E.D., O'Brien J.W., Gallen M., Samanipour S., Kaserzon S., Rauert C., Wang X., et al. Plastics contamination of store-bought rice. J. Hazard. Mater. 2021;416:125778. doi: 10.1016/j.jhazmat.2021.125778. [DOI] [PubMed] [Google Scholar]
- 76.Kedzierski M., Lechat B., Sire O., Le Maguer G., Le Tilly V., Bruzaud S. Microplastic contamination of packaged meat: occurrence and associated risks. Food Packag. Shelf Life. 2020;24:100489. doi: 10.1016/j.fpsl.2020.100489. [DOI] [Google Scholar]
- 77.Danopoulos E., Jenner L.C., Twiddy M., Rotchell J.M. Microplastic contamination of seafood intended for human consumption: a systematic review and meta-analysis. Environ. Health Perspect. 2020;128:126002. doi: 10.1289/EHP7171. https://ehp.niehs.nih.gov/doi/abs/10.1289/EHP7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Domenech J., Marcos R. Pathways of human exposure to microplastics, and estimation of the total burden. Curr. Opin. Food Sci. 2021;39:144–151. doi: 10.1016/j.cofs.2021.01.004. [DOI] [Google Scholar]
- 79.Zhu J., Zhang X., Liao K., Wu P., Jin H. Microplastics in dust from different indoor environments. Sci. Total Environ. 2022;833:155256. doi: 10.1016/j.scitotenv.2022.155256. [DOI] [PubMed] [Google Scholar]
- 80.Rahman A., Sarkar A., Yadav O.P., Achari G., Slobodnik J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: a scoping review. Sci. Total Environ. 2021;757:143872. doi: 10.1016/j.scitotenv.2020.143872. [DOI] [PubMed] [Google Scholar]
- 81.Lett Z., Hall A., Skidmore S., Alves N.J. Environmental microplastic and nanoplastic: exposure routes and effects on coagulation and the cardiovascular system. Environ. Pollut. 2021;291:118190. doi: 10.1016/j.envpol.2021.118190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fendall L.S., Sewell M.A. Contributing to marine pollution by washing your face: microplastics in facial cleansers. Mar. Pollut. Bull. 2009;58:1225–1228. doi: 10.1016/j.marpolbul.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 83.Sykes E.A., Dai Q., Tsoi K.M., Hwang D.M., Chan W.C. Nanoparticle exposure in animals can be visualized in the skin and analysed via skin biopsy. Nat. Commun. 2014;5:1–8. doi: 10.1038/ncomms4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wright S.L., Kelly F.J. Plastic and human health: a micro issue? Environ. Sci. Technol. 2017;51:6634–6647. doi: 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- 85.Abafe O.A., Harrad S., Abdallah M.A.-E. Novel insights into the dermal bioaccessibility and human exposure to brominated flame retardant additives in microplastics. Environ. Sci. Technol. 2023 doi: 10.1021/acs.est.3c01894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Q., Gao J., Yu H., Su H., Yang Y., Cao Y., Zhang Q., Ren Y., et al. An emerging role of microplastics in the etiology of lung ground glass nodules. Environ. Sci. Eur. 2022;34:1–15. doi: 10.1186/s12302-022-00605-3. [DOI] [Google Scholar]
- 87.Baeza-Martínez C., Olmos S., González-Pleiter M., López-Castellanos J., García-Pachón E., Masiá-Canuto M., Hernández-Blasco L., Bayo J. First evidence of microplastics isolated in European citizens' lower airway. J. Hazard. Mater. 2022;438:129439. doi: 10.1016/j.jhazmat.2022.129439. [DOI] [PubMed] [Google Scholar]
- 88.Jiang Y., Han J., Na J., Fang J., Qi C., Lu J., Liu X., Zhou C., et al. Exposure to microplastics in the upper respiratory tract of indoor and outdoor workers. Chemosphere. 2022;307:136067. doi: 10.1016/j.chemosphere.2022.136067. [DOI] [PubMed] [Google Scholar]
- 89.Geiser M. Morphological aspects of particle uptake by lung phagocytes. Microsc. Res. Tech. 2002;57:512–522. doi: 10.1002/jemt.10105. [DOI] [PubMed] [Google Scholar]
- 90.Ruge C.A., Kirch J., Lehr C.-M. Pulmonary drug delivery: from generating aerosols to overcoming biological barriers—therapeutic possibilities and technological challenges. Lancet Respir. Med. 2013;1:402–413. doi: 10.1016/S2213-2600(13)70072-9. [DOI] [PubMed] [Google Scholar]
- 91.Kreyling W.G., Hirn S., Möller W., Schleh C., Wenk A., Celik G., Lipka J., Schäffler M., et al. Air–blood barrier translocation of tracheally instilled gold nanoparticles inversely depends on particle size. ACS Nano. 2014;8:222–233. doi: 10.1021/nn403256v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nemmar A., Hoet P.M., Vanquickenborne B., Dinsdale D., Thomeer M., Hoylaerts M., Vanbilloen H., Mortelmans L., et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 93.Tsuda A., Donaghey T.C., Konduru N.V., Pyrgiotakis G., Van Winkle L.S., Zhang Z., Edwards P., Bustamante J.M., et al. Age-dependent translocation of gold nanoparticles across the air–blood barrier. ACS Nano. 2019;13:10095–10102. doi: 10.1021/acsnano.9b03019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shimada A., Kawamura N., Okajima M., Kaewamatawong T., Inoue H., Morita T. Translocation pathway of the intratracheally instilled ultrafine particles from the lung into the blood circulation in the mouse. Toxicol. Pathol. 2006;34:949–957. doi: 10.1080/01926230601080502. [DOI] [PubMed] [Google Scholar]
- 95.Stock V., Fahrenson C., Thuenemann A., Dönmez M.H., Voss L., Böhmert L., Braeuning A., Lampen A., et al. Impact of artificial digestion on the sizes and shapes of microplastic particles. Food Chem. Toxicol. 2020;135:111010. doi: 10.1016/j.fct.2019.111010. [DOI] [PubMed] [Google Scholar]
- 96.Schwabl P., Köppel S., Königshofer P., Bucsics T., Trauner M., Reiberger T., Liebmann B. Detection of various microplastics in human stool: a prospective case series. Ann. Intern. Med. 2019;171:453–457. doi: 10.7326/M19-0618. [DOI] [PubMed] [Google Scholar]
- 97.Zhang N., Li Y.B., He H.R., Zhang J.F., Ma G.S. You are what you eat: microplastics in the feces of young men living in Beijing. Sci. Total Environ. 2021;767:144345. doi: 10.1016/j.scitotenv.2020.144345. [DOI] [PubMed] [Google Scholar]
- 98.Powell J.J., Faria N., Thomas-McKay E., Pele L.C. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J. Autoimmun. 2010;34:J226–J233. doi: 10.1016/j.jaut.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 99.Domenech J., Hernández A., Rubio L., Marcos R., Cortés C. Interactions of polystyrene nanoplastics with in vitro models of the human intestinal barrier. Arch. Toxicol. 2020;94:2997–3012. doi: 10.1007/s00204-020-02805-3. [DOI] [PubMed] [Google Scholar]
- 100.Ramsperger A.F.R.M., Narayana V.K.B., Gross W., Mohanraj J., Thelakkat M., Greiner A., Schmalz H., Kress H., et al. Environmental exposure enhances the internalization of microplastic particles into cells. Sci. Adv. 2020;6:eabd1211. doi: 10.1126/sciadv.abd1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jani P., Florence A., McCarthy D. Further histological evidence of the gastrointestinal absorption of polystyrene nanospheres in the rat. Int. J. Pharm. 1992;84:245–252. doi: 10.1016/0378-5173(92)90162-U. [DOI] [Google Scholar]
- 102.Stock V., Böhmert L., Lisicki E., Block R., Cara-Carmona J., Pack L.K., Selb R., Lichtenstein D., et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 2019;93:1817–1833. doi: 10.1007/s00204-019-02478-7. [DOI] [PubMed] [Google Scholar]
- 103.Volkheimer G. Hematogenous dissemination of ingested polyvinyl chloride particles. Ann. N. Y. Acad. Sci. 1975;246:164–171. doi: 10.1111/j.1749-6632.1975.tb51092.x. [DOI] [PubMed] [Google Scholar]
- 104.Alvarez-Román R., Naik A., Kalia Y.N., Guy R.H., Fessi H. Skin penetration and distribution of polymeric nanoparticles. J. Control Release. 2004;99:53–62. doi: 10.1016/j.jconrel.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 105.Larese Filon F., Bello D., Cherrie J.W., Sleeuwenhoek A., Spaan S., Brouwer D.H. Occupational dermal exposure to nanoparticles and nano-enabled products: part I—factors affecting skin absorption. Int. J. Hyg. Environ. Health. 2016;219:536–544. doi: 10.1016/j.ijheh.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 106.Tachaprutinun A., Meinke M.C., Richter H., Pan-in P., Wanichwecharungruang S., Knorr F., Lademann J., Patzelt A. Comparison of the skin penetration of Garcinia mangostana extract in particulate and non-particulate form. Eur. J. Pharm. Biopharm. 2014;86:307–313. doi: 10.1016/j.ejpb.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 107.Müller B., Lang S., Dominietto M., Rudin M., Schulz G., Deyhle H., Germann M., Pfeiffer F., et al. vol. 7078. SPIE; 2008. (High-resolution Tomographic Imaging of Microvessels, Optical Engineering + Applications). [DOI] [Google Scholar]
- 108.Svihus B. Function of the digestive system. J. Appl. Poult. Res. 2014;23:306–314. doi: 10.3382/japr.2014-00937. [DOI] [Google Scholar]
- 109.Tan H., Yue T., Xu Y., Zhao J., Xing B. Microplastics reduce lipid digestion in simulated human gastrointestinal system. Environ. Sci. Technol. 2020;54:12285–12294. doi: 10.1021/acs.est.0c02608. [DOI] [PubMed] [Google Scholar]
- 110.Li C., Zhang R., Ma C., Shang H., McClements D.J., White J.C., Xing B. Food-grade titanium dioxide particles decreased the bioaccessibility of vitamin D3 in the simulated human gastrointestinal tract. J. Agric. Food Chem. 2021;69:2855–2863. doi: 10.1021/acs.jafc.0c06644. [DOI] [PubMed] [Google Scholar]
- 111.DeLoid G.M., Sohal I.S., Lorente L.R., Molina R.M., Pyrgiotakis G., Stevanovic A., Zhang R., McClements D.J., et al. Reducing intestinal digestion and absorption of fat using a nature-derived biopolymer: interference of triglyceride hydrolysis by nanocellulose. ACS Nano. 2018;12:6469–6479. doi: 10.1021/acsnano.8b03074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li B., Ding Y., Cheng X., Sheng D., Xu Z., Rong Q., Wu Y., Zhao H., et al. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere. 2020;244:125492. doi: 10.1016/j.chemosphere.2019.125492. [DOI] [PubMed] [Google Scholar]
- 113.Jin Y., Lu L., Tu W., Luo T., Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019;649:308–317. doi: 10.1016/j.scitotenv.2018.08.353. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Y., Wang S., Olga V., Xue Y., Lv S., Diao X., Zhang Y., Han Q., et al. The potential effects of microplastic pollution on human digestive tract cells. Chemosphere. 2022;291:132714. doi: 10.1016/j.chemosphere.2021.132714. [DOI] [PubMed] [Google Scholar]
- 115.Sun H., Chen N., Yang X., Xia Y., Wu D. Effects induced by polyethylene microplastics oral exposure on colon mucin release, inflammation, gut microflora composition and metabolism in mice. Ecotoxicol. Environ. Saf. 2021;220:112340. doi: 10.1016/j.ecoenv.2021.112340. [DOI] [PubMed] [Google Scholar]
- 116.Thaiss C.A., Zmora N., Levy M., Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 117.Liao Y.L., Yang J.Y. Microplastic serves as a potential vector for Cr in an in-vitro human digestive model. Sci. Total Environ. 2020;703:134805. doi: 10.1016/j.scitotenv.2019.134805. [DOI] [PubMed] [Google Scholar]
- 118.Godoy V., Martínez-Férez A., Martín-Lara M.Á., Vellido-Pérez J.A., Calero M., Blázquez G. Microplastics as vectors of chromium and lead during dynamic simulation of the human gastrointestinal tract. Sustainability. 2020;12:4792. doi: 10.3390/su12114792. [DOI] [Google Scholar]
- 119.Cui R., Jong M.C., You L., Mao F., Yao D., Gin K.Y.H., He Y. Size-dependent adsorption of waterborne benzophenone-3 on microplastics and its desorption under simulated gastrointestinal conditions. Chemosphere. 2022;286:131735. doi: 10.1016/j.chemosphere.2021.131735. [DOI] [PubMed] [Google Scholar]
- 120.Jenner L.C., Rotchell J.M., Bennett R.T., Cowen M., Tentzeris V., Sadofsky L.R. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 2022;831:154907. doi: 10.1016/j.scitotenv.2022.154907. [DOI] [PubMed] [Google Scholar]
- 121.Zuo Y.Y., Veldhuizen R.A.W., Neumann A.W., Petersen N.O., Possmayer F. Current perspectives in pulmonary surfactant — inhibition, enhancement and evaluation. Biochim. Biophys. Acta. 2008;1778:1947–1977. doi: 10.1016/j.bbamem.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 122.Shi W., Cao Y., Chai X., Zhao Q., Geng Y., Liu D., Tian S. Potential health risks of the interaction of microplastics and lung surfactant. J. Hazard. Mater. 2022;429:128109. doi: 10.1016/j.jhazmat.2021.128109. [DOI] [PubMed] [Google Scholar]
- 123.Shi X., Huang R., Wang X., Tang C., Hu C., Wang F. 2021. Cytotoxicity and Genotoxicity of Polystyrene Microplastics with Different Size and Surface Modification in A549 Human Lung Cells. Available at: SSRN 3935593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dong C.D., Chen C.W., Chen Y.C., Chen H.H., Lee J.S., Lin C.H. Polystyrene microplastic particles: in vitro pulmonary toxicity assessment. J. Hazard. Mater. 2020;385:121575. doi: 10.1016/j.jhazmat.2019.121575. [DOI] [PubMed] [Google Scholar]
- 125.Li Y., Shi T., Li X., Sun H., Xia X., Ji X., Zhang J., Liu M., et al. Inhaled tire-wear microplastic particles induced pulmonary fibrotic injury via epithelial cytoskeleton rearrangement. Environ. Int. 2022;164:107257. doi: 10.1016/j.envint.2022.107257. [DOI] [PubMed] [Google Scholar]
- 126.Chen Q., Gao J., Yu H., Su H., Yang Y., Cao Y., Zhang Q., Ren Y., et al. An emerging role of microplastics in the etiology of lung ground glass nodules. medRxiv. 2021 doi: 10.1186/s12302-022-00605-3. [DOI] [Google Scholar]
- 127.Wu D., Feng Y., Wang R., Jiang J., Guan Q., Yang X., Wei H., Xia Y., et al. Pigment microparticles and microplastics found in human thrombi. J. Adv. Res. 2022 doi: 10.1016/j.jare.2022.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gopinath P.M., Saranya V., Vijayakumar S., Mythili Meera M., Ruprekha S., Kunal R., Pranay A., Thomas J., et al. Assessment on interactive prospectives of nanoplastics with plasma proteins and the toxicological impacts of virgin, coronated and environmentally released-nanoplastics. Sci. Rep. 2019;9:1–15. doi: 10.1038/s41598-019-45139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim E.-H., Choi S., Kim D., Park H.J., Bian Y., Choi S.H., Chung H.Y., Bae O.-N. Amine-modified nanoplastics promote the procoagulant activation of isolated human red blood cells and thrombus formation in rats. Part. Fibre Toxicol. 2022;19:1–15. doi: 10.1186/s12989-022-00500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bihari P., Holzer M., Praetner M., Fent J., Lerchenberger M., Reichel C.A., Rehberg M., Lakatos S., et al. Single-walled carbon nanotubes activate platelets and accelerate thrombus formation in the microcirculation. Toxicology. 2010;269:148–154. doi: 10.1016/j.tox.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 131.Çobanoğlu H., Belivermiş M., Sıkdokur E., Kılıç Ö., Çayır A. Genotoxic and cytotoxic effects of polyethylene microplastics on human peripheral blood lymphocytes. Chemosphere. 2021;272:129805. doi: 10.1016/j.chemosphere.2021.129805. [DOI] [PubMed] [Google Scholar]
- 132.Lee H.S., Amarakoon D., Wei C.i., Choi K.Y., Smolensky D., Lee S.H. Adverse effect of polystyrene microplastics (PS-MPs) on tube formation and viability of human umbilical vein endothelial cells. Food Chem. Toxicol. 2021;154:112356. doi: 10.1016/j.fct.2021.112356. [DOI] [PubMed] [Google Scholar]
- 133.Jin H., Ma T., Sha X., Liu Z., Zhou Y., Meng X., Chen Y., Han X., et al. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021;401:123430. doi: 10.1016/j.jhazmat.2020.123430. [DOI] [PubMed] [Google Scholar]
- 134.Liu Z., Zhuan Q., Zhang L., Meng L., Fu X., Hou Y. Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mater. 2022;424:127629. doi: 10.1016/j.jhazmat.2021.127629. [DOI] [PubMed] [Google Scholar]
- 135.Wei Z., Wang Y., Wang S., Xie J., Han Q., Chen M. Comparing the effects of polystyrene microplastics exposure on reproduction and fertility in male and female mice. Toxicology. 2022;465:153059. doi: 10.1016/j.tox.2021.153059. [DOI] [PubMed] [Google Scholar]
- 136.Deng Y., Chen H., Huang Y., Wang Q., Chen W., Chen D. Polystyrene microplastics affect the reproductive performance of male mice and lipid homeostasis in their offspring. Environ. Sci. Technol. Lett. 2022;9:752–757. doi: 10.1021/acs.estlett.2c00262. [DOI] [Google Scholar]
- 137.Luo T., Wang C., Pan Z., Jin C., Fu Z., Jin Y. Maternal polystyrene microplastic exposure during gestation and lactation altered metabolic homeostasis in the dams and their F1 and F2 offspring. Environ. Sci. Technol. 2019;53:10978–10992. doi: 10.1021/acs.est.9b03191. [DOI] [PubMed] [Google Scholar]
- 138.Amereh F., Amjadi N., Mohseni-Bandpei A., Isazadeh S., Mehrabi Y., Eslami A., Naeiji Z., Rafiee M. Placental plastics in young women from general population correlate with reduced foetal growth in IUGR pregnancies. Environ. Pollut. 2022;314:120174. doi: 10.1016/j.envpol.2022.120174. [DOI] [PubMed] [Google Scholar]
- 139.Sousa A.M.M., Meyer K.A., Santpere G., Gulden F.O., Sestan N. Evolution of the human nervous system function, structure, and development. Cell. 2017;170:226–247. doi: 10.1016/j.cell.2017.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu P., Lin S., Cao G., Wu J., Jin H., Wang C., Wong M.H., Yang Z., et al. Absorption, distribution, metabolism, excretion and toxicity of microplastics in the human body and health implications. J. Hazard. Mater. 2022;437:129361. doi: 10.1016/j.jhazmat.2022.129361. [DOI] [PubMed] [Google Scholar]
- 141.Lee C.W., Hsu L.F., Wu I.L., Wang Y.L., Chen W.C., Liu Y.J., Yang L.T., Tan C.L., et al. Exposure to polystyrene microplastics impairs hippocampus-dependent learning and memory in mice. J. Hazard. Mater. 2022;430:128431. doi: 10.1016/j.jhazmat.2022.128431. [DOI] [PubMed] [Google Scholar]
- 142.Qi Y., Wei S., Xin T., Huang C., Pu Y., Ma J., Zhang C., Liu Y., et al. Passage of exogeneous fine particles from the lung into the brain in humans and animals. Proc. Natl. Acad. Sci. U. S. A. 2022;119 doi: 10.1073/pnas.2117083119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang S., Han Q., Wei Z., Wang Y., Xie J., Chen M. Polystyrene microplastics affect learning and memory in mice by inducing oxidative stress and decreasing the level of acetylcholine. Food Chem. Toxicol. 2022;162:112904. doi: 10.1016/j.fct.2022.112904. [DOI] [PubMed] [Google Scholar]
- 144.Parkin J., Cohen B. An overview of the immune system. Lancet. 2001;357:1777–1789. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- 145.Remmelts M. 2021. Effect of Nano-And Microplastics on the Human Immune System and Their Influence on Inflammatory Bowel Disease.https://fse.studenttheses.ub.rug.nl/id/eprint/25026 Diss. [Google Scholar]
- 146.Han S., Bang J., Choi D., Hwang J., Kim T., Oh Y., Hwang Y., Choi J., et al. Surface pattern analysis of microplastics and their impact on human-derived cells. ACS Appl. Polym. Mater. 2020;2:4541–4550. doi: 10.1021/acsapm.0c00645. [DOI] [Google Scholar]
- 147.Hu J., Qin X., Zhang J., Zhu Y., Zeng W., Lin Y., Liu X. Polystyrene microplastics disturb maternal-fetal immune balance and cause reproductive toxicity in pregnant mice. Reprod. Toxicol. 2021;106:42–50. doi: 10.1016/j.reprotox.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 148.Davidson A., Diamond B. Autoimmune diseases. N. Engl. J. Med. 2001;345:340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 149.Bernatsky S., Fournier M., Pineau C.A., Clarke A.E., Vinet E., Smargiassi A. Associations between ambient fine particulate levels and disease activity in patients with systemic lupus erythematosus (SLE) Environ. Health Perspect. 2011;119:45–49. doi: 10.1289/ehp.1002123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Beyerlein A., Krasmann M., Thiering E., Kusian D., Markevych I., D'Orlando O., Warncke K., Jochner S., et al. Ambient air pollution and early manifestation of type 1 diabetes. Epidemiology. 2015;26:e31–e32. doi: 10.1097/EDE.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 151.Chang K.H., Hsu C.C., Muo C.H., Hsu C.Y., Liu H.C., Kao C.H., Chen C.Y., Chang M.Y., et al. Air pollution exposure increases the risk of rheumatoid arthritis: a longitudinal and nationwide study. Environ. Int. 2016;94:495–499. doi: 10.1016/j.envint.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 152.Guedes-Alonso R., Sosa-Ferrera Z., Santana-Rodríguez J.J. Analysis of microplastics-sorbed endocrine-disrupting compounds in pellets and microplastic fragments from beaches. Microchem. J. 2021;171:106834. doi: 10.1016/j.microc.2021.106834. [DOI] [Google Scholar]
- 153.Rochester J.R. Bisphenol A and human health: a review of the literature. Reprod. Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 154.Matsumoto M., Hirata-Koizumi M., Ema M. Potential adverse effects of phthalic acid esters on human health: a review of recent studies on reproduction. Regul. Toxicol. Pharmacol. 2008;50:37–49. doi: 10.1016/j.yrtph.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 155.Deng Y., Yan Z., Shen R., Huang Y., Ren H., Zhang Y. Enhanced reproductive toxicities induced by phthalates contaminated microplastics in male mice (Mus musculus) J. Hazard. Mater. 2021;406:124644. doi: 10.1016/j.jhazmat.2020.124644. [DOI] [PubMed] [Google Scholar]
- 156.Goodman K.E., Hua T., Sang Q.X.A. Effects of polystyrene microplastics on human kidney and liver cell morphology, cellular proliferation, and metabolism. ACS Omega. 2022;7:34136–34153. doi: 10.1021/acsomega.2c03453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang Y.L., Lee Y.H., Hsu Y.H., Chiu I.J., Huang C.C.Y., Huang C.C., Chia Z.C., Lee C.P., et al. The kidney-related effects of polystyrene microplastics on human kidney proximal tubular epithelial cells HK-2 and male C57BL/6 mice. Environ. Health Perspect. 2021;129:57003. doi: 10.1289/EHP7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meng X., Zhang J., Wang W., Gonzalez-Gil G., Vrouwenvelder J.S., Li Z. Effects of nano- and microplastics on kidney: physicochemical properties, bioaccumulation, oxidative stress and immunoreaction. Chemosphere. 2022;288:132631. doi: 10.1016/j.chemosphere.2021.132631. [DOI] [PubMed] [Google Scholar]
- 159.Wang Y., Wang S., Xu T., Cui W., Shi X., Xu S. A new discovery of polystyrene microplastics toxicity: the injury difference on bladder epithelium of mice is correlated with the size of exposed particles. Sci. Total Environ. 2022;821:153413. doi: 10.1016/j.scitotenv.2022.153413. [DOI] [PubMed] [Google Scholar]
- 160.Sun W., Jin C., Bai Y., Ma R., Deng Y., Gao Y., Pan G., Yang Z., et al. Blood uptake and urine excretion of nano- and micro-plastics after a single exposure. Sci. Total Environ. 2022;848:157639. doi: 10.1016/j.scitotenv.2022.157639. [DOI] [PubMed] [Google Scholar]
- 161.Sun T., Zhan J., Li F., Ji C., Wu H. Environmentally relevant concentrations of microplastics influence the locomotor activity of aquatic biota. J. Hazard. Mater. 2021;414:125581. doi: 10.1016/j.jhazmat.2021.125581. [DOI] [PubMed] [Google Scholar]
- 162.Matthews S., Xu E.G., Dumont E.R., Meola V., Pikuda O., Cheong R.S., Guo M., Tahara R., et al. Polystyrene micro-and nanoplastics affect locomotion and daily activity of Drosophila melanogaster. Environ. Sci. Nano. 2021;8:110–121. doi: 10.1039/D0EN00942C. [DOI] [Google Scholar]
- 163.da Costa Araújo A.P., de Andrade Vieira J.E., Malafaia G. Toxicity and trophic transfer of polyethylene microplastics from Poecilia reticulata to Danio rerio. Sci. Total Environ. 2020;742:140217. doi: 10.1016/j.scitotenv.2020.140217. [DOI] [PubMed] [Google Scholar]
- 164.Halappanavar S., Mallach G. Adverse outcome pathways and in vitro toxicology strategies for microplastics hazard testing. Curr. Opin. Toxicol. 2021;28:52–61. doi: 10.1016/j.cotox.2021.09.002. [DOI] [Google Scholar]
- 165.da Costa Araújo A.P., Malafaia G. Microplastic ingestion induces behavioral disorders in mice: a preliminary study on the trophic transfer effects via tadpoles and fish. J. Hazard. Mater. 2021;401:123263. doi: 10.1016/j.jhazmat.2020.123263. [DOI] [PubMed] [Google Scholar]
- 166.Andreas T. Hadibarata, Sathishkumar P., Prasetia H., Hikmat, Pusfitasari E.D., Tasfiyati A.N., Muzdalifah D., et al. Microplastic contamination in the skipjack tuna (Euthynnus affinis) collected from southern coast of Java, Indonesia. Chemosphere. 2021;276:130185. doi: 10.1016/j.chemosphere.2021.130185. [DOI] [PubMed] [Google Scholar]
- 167.Zhu L., Wang H., Chen B., Sun X., Qu K., Xia B. Microplastic ingestion in deep-sea fish from the South China Sea. Sci. Total Environ. 2019;677:493–501. doi: 10.1016/j.scitotenv.2019.04.380. [DOI] [PubMed] [Google Scholar]
- 168.Wootton N., Sarakinis K., Varea R., Reis-Santos P., Gillanders B.M. Microplastic in oysters: a review of global trends and comparison to southern Australia. Chemosphere. 2022;307:136065. doi: 10.1016/j.chemosphere.2022.136065. [DOI] [PubMed] [Google Scholar]
- 169.Hossain M.S., Rahman M.S., Uddin M.N., Sharifuzzaman S.M., Chowdhury S.R., Sarker S., Nawaz Chowdhury M.S. Microplastic contamination in penaeid shrimp from the northern bay of Bengal. Chemosphere. 2020;238:124688. doi: 10.1016/j.chemosphere.2019.124688. [DOI] [PubMed] [Google Scholar]
- 170.Tong H., Jiang Q., Hu X., Zhong X. Occurrence and identification of microplastics in tap water from China. Chemosphere. 2020;252:126493. doi: 10.1016/j.chemosphere.2020.126493. [DOI] [PubMed] [Google Scholar]
- 171.Vega-Herrera A., Llorca M., Borrell-Diaz X., Redondo-Hasselerharm P.E., Abad E., Villanueva C.M., Farré M. Polymers of micro(nano) plastic in household tap water of the Barcelona Metropolitan Area. Water Res. 2022;220:118645. doi: 10.1016/j.watres.2022.118645. [DOI] [PubMed] [Google Scholar]
- 172.Shruti V.C., Pérez-Guevara F., Kutralam-Muniasamy G. Metro station free drinking water fountain – a potential “microplastics hotspot” for human consumption. Environ. Pollut. 2020;261:114227. doi: 10.1016/j.envpol.2020.114227. [DOI] [PubMed] [Google Scholar]
- 173.Makhdoumi P., Amin A.A., Karimi H., Pirsaheb M., Kim H., Hossini H. Occurrence of microplastic particles in the most popular Iranian bottled mineral water brands and an assessment of human exposure. J. Water Process Eng. 2021;39:101708. doi: 10.1016/j.jwpe.2020.101708. [DOI] [Google Scholar]
- 174.Zhou X.J., Wang J., Li H.Y., Zhang H.M., Hua J., Zhang D.L. Microplastic pollution of bottled water in China. J. Water Process Eng. 2021;40:101884. doi: 10.1016/j.jwpe.2020.101884. [DOI] [Google Scholar]
- 175.Fadare O.O., Okoffo E.D., Olasehinde E.F. Microparticles and microplastics contamination in African table salts. Mar. Pollut. Bull. 2021;164:112006. doi: 10.1016/j.marpolbul.2021.112006. [DOI] [PubMed] [Google Scholar]
- 176.Ragusa A., Notarstefano V., Svelato A., Belloni A., Gioacchini G., Blondeel C., Zucchelli E., De Luca C., et al. Raman microspectroscopy detection and characterisation of microplastics in human breastmilk. Polymers. 2022;14:2700. doi: 10.3390/polym14132700. [DOI] [PMC free article] [PubMed] [Google Scholar]