Abstract
Purpose: Robot-assisted thoracic surgery (RATS) has become popular because of its minimally invasive nature and reduced burden on surgeons. The anterior approach (AA) is beneficial because it utilizes the same field of view and procedures as thoracotomy and video-assisted thoracic surgery, although the disadvantages are less well-known.
Methods: We retrospectively examined 35 consecutive patients who underwent RATS lobectomy via the AA, focusing on clinical factors and postoperative complications.
Results: The study included 12 males and 23 females with a median console time of 177 (120–346) min, median blood loss of 0 (0–100) mL, and median stapler usage of 5 (2–10) units. Postoperative complications, classified as Clavien–Dindo grade ≥III, included three cases of grade IIIa (prolonged air leakage) and one case each of grade IIIb and grade IVa (middle lobe torsion and ventricular arrhythmia). The influence of stapling device operation cannot be ruled out in prolonged air leakage and middle lobe torsion. A moderate correlation (correlation coefficient = 0.492, p = 0.003) was observed between console time and the number of staplers used.
Conclusion: Although no severe incidence of vascular injury was observed with the AA, complications related to the use of stapling devices were noted.
Keywords: robot-assisted thoracic surgery, minimally invasive surgery, postoperative complications, lung cancer, robotic lobectomy
Introduction
Robot-assisted thoracic surgery (RATS) has become widely popular because of its minimally invasive nature and reduced burden on surgeons. In RATS, surgical procedures can be performed precisely owing to motion scaling that eliminates physiological tremors using a 10-fold magnified three-dimensional (3D) camera, and the utilization of a third arm renders assistance at the patient’s side almost unnecessary.1) However, because the da Vinci Si (Intuitive Surgical, Sunnyvale, CA, USA) is not equipped with staplers for its arms, an assistant surgeon needs to introduce staplers safely in cooperation with the console surgeon, especially in lobectomies, where the use of surgical staplers is essential for resecting the pulmonary vessels, bronchi, and lung parenchyma. We adopted an anterior approach (AA) to safely perform RATS using the da Vinci Si system,2) which allows us to perform surgery using the same field of view and procedures as thoracotomy and video-assisted thoracic surgery (VATS).
The AA to RATS was first introduced by Yamazaki et al.2) There are several advantages to using the AA. The first involves patient accessibility. The arrangement of the operating table, anesthesia equipment, and other items in the operating room is the same as that with thoracotomy or VATS. This facilitates airway management by the anesthesiologist, as patients are usually placed in the lateral decubitus position and undergo isolated lung ventilation. The second involves consistency in surgical procedures. Each step in the surgical procedure is consistent among open thoracotomy, video-assisted thoracotomy, and robotic lobectomy. The anterior–posterior view of the thoracoscope, with a confronting monitor for the assistants, enables the surgeon and assistants to share the same vision without being affected by misalignment in hand–eye coordination.3) With the camera placed through the upper middle intercostal space, it is also easier for the surgeon to visualize and handle the hilum structure with comfort, as in robotic lung lobectomy, vascular injury is a catastrophic event that often leads to open conversion.4)
We therefore retrospectively reviewed the procedures of robotic lung lobectomy at our institution and analyzed the problems associated with the anterior approach for robotic lung lobectomy with the da Vinci Si system.
Materials and Methods
Patients
Consecutive patients who underwent robotic lobectomy at the Oita University Hospital between November 2020 and March 2023 were analyzed. There were 34 patients with primary lung cancer and one with a metastatic lung tumor. The stage was defined based on the 8th edition of the Union for International Cancer Control Tumor-Node-Metastasis (TNM) staging for lung cancer.5) A total of 34% of the patients were male and the median age was 66 years (range: 39–84 years). The median pack-year index was 0 (range: 0–120). The clinical stage was IA in 32 patients (94%) and IIB and IIIA in one patient each. Clinicopathological features, surgical outcomes, mortality, and morbidity were also analyzed.
This study was conducted in accordance with the principles of the Declaration of Helsinki. It was approved by the Institutional Review Board of the Oita University Faculty of Medicine (IRB No. 2558), and informed consent was obtained from each patient.
Surgical procedures
Under general anesthesia, the patient was placed in the lateral decubitus position. There is minimal necessity to flex the operating table; however, it is essential to avoid interference between the arm and the patient’s upper extremity on the upper side by flexing the elbow joint and performing adduction and external rotation at the shoulder joint (e.g., boxing guard position). The da Vinci Si was rolled in from the dorsal side of the patient with ports placed horizontally along the longitudinal axis of the trunk, spanning multiple intercostal spaces. Generally, 8-mm ports were placed in the 2nd and the 6th intercostal space on the anterior axillary line and in the 8th intercostal space on the posterior axillary line. A camera port with a 30° downward oblique camera was placed in the 4th intercostal space on the anterior axillary line. Carbon dioxide (CO2) insufflation and stapling device operations were performed through an assist port placed in the 8th intercostal space on the anterior axillary line. We conducted several multidisciplinary simulations for emergent open conversion. We chose to use an axillary incision by connecting and extending port incisions or a posterolateral incision for open conversion.
Postoperative follow-up
As a core hospital in the region, it is possible for patients to remain admitted until they recover from early postoperative complications and reach a state where they can live their daily lives without issue. Inpatient management follows the same critical path as VATS and open surgery, employing strategies for pain management that include epidural anesthesia before surgery, intravenous administration of acetaminophen postsurgery, and non-steroidal anti-inflammatory drugs once oral administration is possible the following morning. The thoracic drain uses a 20- or 24-fr plastic tube and is removed after confirmation that there is no air leakage, bleeding, or chylothorax after the commencement of meals and when the drainage volume has decreased to <200 mL.
Statistical analyses
The statistical significance of differences between groups was determined using the chi-squared test. Statistical significance was set at p < 0.05. All patients were retrospectively analyzed for their age and sex, smoking history, clinical and pathological stage, blood loss, number of staplers used for lobectomy, intra- and postoperative complications, and duration until chest tube removal and discharge. Continuous variables were compared using Pearson’s correlation coefficient.
All statistical analyses were performed using EZR version 1.55 (Jichi Medical University Saitama Medical Center, Saitama, Japan).6)
Results
Surgical outcomes
Of the 35 patients, upper right lobe resection was performed in a large proportion (28 patients). The median surgical time was 250 (range: 182–499) min, and the median console time was 177 (range: 120–346) min. The surgical time includes the time for lung segmentectomy performed for diagnostic purposes in some cases and the time spent repairing air leakages after roll-out. The console time was therefore adopted for the subsequent analyses. Hemostasis (excluding the port site) and air leak repair were primarily performed with robot assistance. The median amount of blood loss was 0 mL, and even in the worst cases, it did not exceed 100 mL; no cases required blood transfusion. Excluding the number of staplers used for partial resection performed for diagnostic purposes, the median number was 5 (range: 2–10) (Table 1).
Table 1. Operative outcomes and complications.
| Factors | Median (range) | |
| Resected lobe (cases) | ||
| RU/RM/RL | 28/1/5 | |
| LU/LL | 0/1 | |
| Operation time (min) | 250 (182–499) | |
| Console time (min) | 177 (120–346) | |
| Blood loss (mL) | 0 (0–100) | |
| Stapler usage (unit) | 5 (2–10) | |
| Pathological stage* | ||
| O/IA/IB/IIA | 1/29/0/1 | |
| IIIA/IIIB | 2/1 | |
| Drainage days | 1 (1–9) | |
| Postoperative stay | 8 (5–14) | |
| Complication (cases)** | ||
| Yes/No | 5/30 | |
| Details of the 5 cases with complications | ||
| Air leak/torsion | 3/1 | |
| VF/VT, chylothorax*** | 1 |
*Metastatic lung tumor in one case.
**Clavien–Dindo grade ≥III.
***VF/VT and chylothorax occurred in one patient.
LL: left lower; LU: left upper; RL: right lower; RM: right middle; RU: right upper; VF: ventricular flutter; VT: ventricular tachycardia
Postoperative course
In 20 patients, the drain was removed in the morning after surgery. There were two cases in which drainage was performed for more than 5 days, both of which were cases of prolonged air leaks. The median length of hospital stay was 8 days (range: 5–14 days). The required tests one week after the procedure, according to the clinical pathway, are considered to have influenced the relatively long length of hospital stay. All patients were discharged to their homes, but one was readmitted due to postdischarge complications.
Postoperative complications of Clavien–Dindo classification ≥III were observed in five cases: three air leaks, one middle lobe torsion requiring reoperation the next day, and one chylothorax recurrence leading to cardiopulmonary arrest due to ventricular flutter/ventricular tachycardia (Table 1). Despite the high number of pulmonary complications, no cases required sputum suctioning by bronchoscopy, and no cases of postoperative pneumonia were noted. All patients were alive at the time of this report, and there were no cases in which postoperative adjuvant therapy was not possible or was delayed due to postoperative complications.
Factors for postoperative complications
An analysis was conducted to determine any correlations between background factors and the presence of postoperative complications, but no significant differences were found according to background factors. Although this is an obvious observation, there were positive relationships between postoperative complications and the number of drainage days or length of hospital stay (Table 2).
Table 2. Relationships between postoperative complications and clinical factors.
| Factors | Postoperative complications | p-value | |
| Present (n = 5, 14%) | Absent (n = 30, 86%) | ||
| Sex | 1.00 | ||
| Male | 2 (17%) | 10 (83%) | |
| Female | 3 (13%) | 20 (87%) | |
| Age (years), median (range) | 71 (66–78) | 66 (39–84) | 0.310 |
| PYI, median (range) | 0 (0–46) | 0 (0–120) | 0.676 |
| Blood loss (mL), median (range) | 0 (0–50) | 0 (0–100) | 0.745 |
| Operation time (min), median (range) | 309 (220–375) | 239.5 (182–499) | 0.125 |
| Console time (min), median (range) | 170 (145–251) | 177.5 (120–346) | 0.850 |
| Stapler used (units), median (range) | 5 (4–9) | 5 (2–10) | 0.616 |
| Pathological stages | 1.000 | ||
| 0–IIA | 5 (16%) | 26 (84%) | |
| IIB–III | 0 (0%) | 3 (100%) | |
| Other | 0 (0%) | 1 (100%) | |
| Drainage day, median (range) | 4 (2–9) | 1 (1–4) | 0.001 |
| Hospital stay (days), median (range) | 13 (10–14) | 8 (5–13) | 0.001 |
PYI: pack-year index
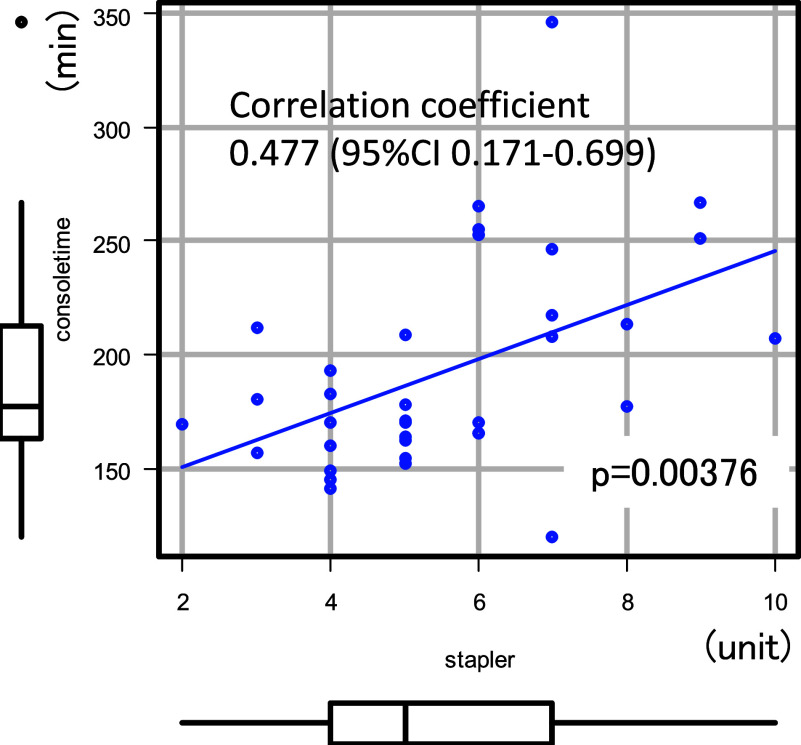
Upon a closer examination of the complications, it was noted that the two patients with prolonged console time and a high number of staplers used developed postoperative pulmonary complications. Conversely, of the four cases of pulmonary complications, one case of prolonged air leakage exceeding one-week postsurgery and another of middle lobe torsion had a console time of over four hours and used nine staplers (Table 3). A moderate correlation was found between console time and the number of staplers used (Fig. 1).
Table 3. Relationships between postoperative complication and operative factors.
| Factors | Postoperative complications | p-value | |
| Present (n = 5, 14%) | Absent (n = 30, 86%) | ||
| Console time | 0.546 | ||
| ≤4 h | 3 (11%) | 25 (89%) | |
| >4 h | 2 (29%) | 5 (71%) | |
| Stapler usage | 0.939 | ||
| ≤6 units | 3 (12%) | 22 (88%) | |
| >6 units | 2 (20%) | 8 (80%) | |
Fig. 1. A positive correlation was observed between console time and the number of staplers used (Pearson’s correlation coefficient = 0.477, 95% confidence interval: 0.171–0.699, p = 0.00376). CI: confidence interval.
Discussion
Robotic thoracic surgery was first performed by Yoshino et al.7) and the approach was modified from robot-assisted surgery to complete portal robotic lobectomy (CPRL).8) CPRL has been widely adopted for the da Vinci Si system and later devices, with ports placed vertically in the same intercostal space, usually in the 6th–8th intercostal space, to provide adequate working space for the robotic arms.8) Theoretically, this approach can minimize intercostal neuralgia, but with the arms lying low, especially at the proximal portion of the rib, it can result in rib fracture, hemorrhaging from the intercostal artery, and intercostal neuralgia.9)
It has also been reported that catastrophic events may be encountered, which require open conversion,4) as the craniocaudal view is limited by the camera inserted from the lower intercostal space; therefore, complete visualization of important vessels, especially the pulmonary artery, cannot usually be accomplished. Furthermore, it is more difficult for the assistant to advance the stapling device to resect pulmonary vessels or to apply an energy device to dissect mediastinal lymph nodes than VATS. A real-world registry study comparing RATS and VATS lobectomy procedures demonstrated a lower rate of conversion to open thoracotomy but more frequent conversions for vascular reasons in RATS lobectomy than in VATS lobectomy.10,11)
In the anterior approach, no bleeding events occurred. However, we encountered difficulties in stapling the lung parenchyma with a narrow working space and narrow vision. This may have led to pulmonary complications in some cases. To improve working space and vision, we utilized CO2 instillation using AirSeal (Conmed, Utica, NY, USA). There were no complications (e.g., air embolism or cardiovascular events) related to CO2 instillation at 5 cmH2O. A severe cardiovascular event was observed in one case (ventricular flatter/cardiopulmonary arrest), but it did not result from intraoperative CO2 instillation, instead being due to undiagnosed stenosis in the coronary arteries triggered by recurrent chylothorax. Chylothorax has been reported to occur more often in RATS than in VATS,12,13) probably because more aggressive lymph node dissection with electrocautery is performed in RATS using a concise 3D camera.
The anterior approach is not unique to robotic surgeries. A similar approach has been adopted for thoracoscopic esophagectomy and mediastinal tumors with ports spanning multiple intercostal spaces.14) The new da Vinci systems, namely X/Xi, have the capacity for camera swapping and stapling (EndoWrist; Intuitive Surgical, Tokyo, Japan). These improvements make the anterior approach more feasible by minimizing the disadvantages observed in previous systems.15) Another CPRL approach with ports placed vertically in the 5th to 6th intercostal spaces could be an option,16) as we are now able to settle the patient cart without disturbing the anesthesiologist with the innovative below-table-mounted robotic arms equipped in the Xi system.
Conclusion
In conclusion, we report the advantages and disadvantages of the anterior approach using the da Vinci Si system. Although there have been several reports regarding port placement in robotic lobectomy, this is the first concerning the disadvantages observed with the anterior approach. Further development is required to improve the outcomes of robotic lobectomies.
Acknowledgments
The authors would like to thank Dr. Brian Quinn for his critical comments on this manuscript.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki. This study was approved by the Institutional Review Board of the Oita University Faculty of Medicine (IRB No. 2558), and informed consent was obtained from each patient.
Consent for publication
Not applicable.
Funding
Not applicable.
Conflicts of interest
The authors have no conflict of interest to disclose.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Author contributions
Conceptualization: Atsushi Osoegawa; Data curation: Atsushi Osoegawa, Yohei Takumi, Takashi Karashima, and Miyuki Abe; Formal analysis: Atsushi Osoegawa; Funding acquisition: Atsushi Osoegawa and Kenji Sugio; Investigation: Atsushi Osoegawa and Takashi Karashima; Methodology: Atsushi Osoegawa and Yohei Takumi; Project administration: Atsushi Osoegawa; Resources: Atsushi Osoegawa and Michiyo Miyawaki; Software: Atsushi Osoegawa; Supervision: Kenji Sugio; Validation: Atsushi Osoegawa and Kenji Sugio; Visualization: Atsushi Osoegawa; Roles/Writing—original draft: Atsushi Osoegawa; Writing—review and editing: Atsushi Osoegawa and Kenji Sugio.
References
- 1).Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006; 131: 54–9. [DOI] [PubMed] [Google Scholar]
- 2).Yamazaki K, Toyokawa G, Shoji F, et al. A novel technique for robotic-assisted lobectomy for lung cancer: the anterior approach. Interact Cardiovasc Thorac Surg 2020; 30: 328. [DOI] [PubMed] [Google Scholar]
- 3).Mun M, Ichinose J, Matsuura Y, et al. Video-assisted thoracoscopic surgery lobectomy via confronting upside-down monitor setting. J Vis Surg 2017; 3: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Cao C, Cerfolio RJ, Louie BE, et al. Incidence, management, and outcomes of intraoperative catastrophes during robotic pulmonary resection. Ann Thorac Surg 2019; 108: 1498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).UICC International Union Against Cancer . TNM Classification of Malignant Tumours. 8th ed. New York: WileyBlackwell; 2016. [Google Scholar]
- 6).Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Yoshino I, Hashizume M, Shimada M, et al. Video-assisted thoracoscopic extirpation of a posterior mediastinal mass using the da Vinci computer enhanced surgical system. Ann Thorac Surg 2002; 74: 1235–7. [DOI] [PubMed] [Google Scholar]
- 8).Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011; 142: 740–6. [DOI] [PubMed] [Google Scholar]
- 9).Sakakura N, Eguchi T. Port placement variations for robotic lung resection: Focusing on their history, conventional look-up-view and horizontal open-thoracotomy-view techniques, and more. J Pers Med 2023; 13: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Herrera LJ, Schumacher LY, Hartwig MG, et al. Pulmonary Open, Robotic, and Thoracoscopic Lobectomy study: Outcomes and risk factors of conversion during minimally invasive lobectomy. J Thorac Cardiovasc Surg 2023; 166: 251–262.e3. [DOI] [PubMed] [Google Scholar]
- 11).Kent MS, Hartwig MG, Vallieres E, et al. Pulmonary Open, Robotic, and Thoracoscopic Lobectomy (PORTaL) Study: An analysis of 5721 cases. Ann Surg 2023; 277: 528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Bryant AS, Minnich DJ, Wei B, et al. The incidence and management of postoperative chylothorax after pulmonary resection and thoracic mediastinal lymph node dissection. Ann Thorac Surg 2014; 98: 232–5; discussion, 235–7. [DOI] [PubMed] [Google Scholar]
- 13).Sarkaria IS, Finley DJ, Bains MS, et al. Chylothorax and recurrent laryngeal nerve injury associated with robotic video-assisted mediastinal lymph node dissection. Innovations (Phila) 2015; 10: 170–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Watanabe M, Kuriyama K, Terayama M, et al. Robotic-assisted esophagectomy: Current situation and future perspectives. Ann Thorac Cardiovasc Surg 2023; 29: 168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Ichinose J, Hashimoto K, Matsuura Y, et al. Initial perioperative outcomes of robot-assisted thoracoscopic lobectomy using a confronting setting. Surg Today 2023; 53: 1073–80. [DOI] [PubMed] [Google Scholar]
- 16).Ninan M, Dylewski MR. Total port-access robot-assisted pulmonary lobectomy without utility thoracotomy. Eur J Cardiothorac Surg 2010; 38: 231–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.