Key Points
Question
Can inhibitory transcranial magnetic stimulation ameliorate psychomotor slowing in psychosis?
Findings
In this 4-arm randomized clinical trial including 88 patients, 15 sessions of 1-Hz repetitive transcranial magnetic stimulation (rTMS) on the supplementary motor area led to response in significantly more patients than intermittent theta burst stimulation (iTBS), sham, or no treatment. Most of the patients in the waiting group responded to delayed-onset 1-Hz rTMS.
Meaning
The findings indicate that add-on inhibitory rTMS may be an effective treatment for patients with psychosis and psychomotor slowing; further studies are needed to assess neural changes associated with this treatment.
This randomized clinical trial compares repetitive transcranial magnetic stimulation with intermittent theta burst stimulation, sham, and no treatment for reducing psychomotor slowing in individuals with psychosis.
Abstract
Importance
Psychomotor slowing is a frequent symptom of psychosis, impairing gross and fine motor behavior. It is associated with poor outcomes and functioning, and no treatment is available.
Objective
To investigate whether 15 sessions of inhibitory repetitive transcranial magnetic stimulation (rTMS) may reduce psychomotor slowing.
Design, Setting, and Participants
This was a 4-arm, double-blind, randomized, sham-controlled trial at a university hospital in Switzerland. Enrollment took place from March 2019 to August 2022. Adults aged 18 to 60 years with schizophrenia spectrum disorders and severe psychomotor slowing were eligible. All patients continued existing medications, including antipsychotics and benzodiazepines. Those with substance misuse (other than nicotine), conditions associated with impaired or aberrant movement, convulsions, history of hearing problems, other conditions typically excluded from magnetic resonance imaging or TMS, any TMS treatment in the past 3 months, or those who were pregnant or breastfeeding were excluded. Of 615 patients screened for eligibility, 103 were randomized and 88 received at least 1 session of rTMS: 22 were assigned to 1-Hz rTMS, 22 to iTBS, 22 to sham, and 22 to the waiting group. Follow-up was conducted at 6 weeks and 24 weeks following the week 3 assessments including clinical, functional, and motor measures.
Interventions
Fifteen sessions of rTMS in 3 weeks over the supplementary motor area: 1-Hz rTMS, iTBS, sham, or no treatment (waiting). After 3 weeks, the waiting group received 15 sessions of 1-Hz rTMS over the supplementary motor area.
Main Outcomes and Measures
The main outcome was the proportion of responders at week 3 in the Salpêtrière Retardation Rating Scale (SRRS) defined as a 30% or greater reduction from baseline (last-observation-carried-forward). The SRRS has 15 items and a maximum total score of 60.
Results
Of the 88 participants analyzed, 45 were men and 43 were women. The mean (SD) age was 36.3 (12.4) years and the mean (SD) SRRS score was 24.0 (5.9). A total of 69 participants completed the study. At week 3, response rates differed between groups: 15 of 22 (68%) in the 1-Hz rTMS group, 8 of 22 (36%) in the iTBS group, 7 of 22 (32%) in the sham group, and 4 of 22 (18%) in the waiting group (χ23 = 12.1; P = .007). The 1-Hz rTMS group had more responders than sham (odds ratio [OR], 0.13; 95% CI, 0.02-0.65; P = .03), iTBS (OR, 0.12; 95% CI, 0.02-0.61; P = .02), and waiting (OR, 0.04; 95% CI, 0.01-0.22; P = .003). In the waiting group, 10 of 16 participants (63%) responded after receiving 15 sessions of 1-Hz rTMS. No serious adverse events occurred.
Conclusions and Relevance
In this study, inhibitory add-on rTMS safely alleviated psychomotor slowing in psychosis compared with iTBS, sham, and no treatment. The treatment was also effective with delayed onset. Future studies need to explore the neural changes associated with supplementary motor area rTMS in psychosis.
Trial Registration
ClinicalTrials.gov Identifier: NCT03921450
Introduction
Schizophrenia is a severe mental disorder affecting 1% of the population and leading to adverse outcomes and poor quality of life.1 Core schizophrenia symptom dimensions include hallucinations, delusions, disorganized speech, negative symptoms, impaired cognition, and abnormal psychomotor behavior.2 Motor abnormalities have been reported across all stages of schizophrenia spectrum disorders, including untreated patients experiencing a first episode, and indicate poor clinical and functional outcomes.3,4,5,6 One of these motor abnormalities is psychomotor slowing that impairs both fine and gross motor behavior, facial expression, and speech. Psychomotor slowing impacts movement initiation, quantity, and velocity; furthermore, it is associated with lower cognitive processing speed.7,8 Hypokinetic catatonia episodes are considered an extreme form of psychomotor slowing.9,10 Psychomotor slowing often comes with multiple disadvantages, such as cognitive impairment, sedentary behavior, cardiometabolic risks, poor quality of life, lower subjective well-being, and impaired functioning.7,11,12,13,14 No specific treatment is available to target psychomotor slowing.
Psychomotor slowing in schizophrenia can be captured using clinical rating scales and instrumental measures, such as wrist actigraphy, gait analysis, or fine motor tasks.15,16 Multiple neural alterations in the motor circuit are thought to induce psychomotor slowing, a particularly aberrant function of the premotor cortex.8,17 For example, the supplementary motor area was shown to have increased neural activity and connectivity at rest in individuals with catatonia and psychomotor slowing.18,19 Therefore, the modulation of supplementary motor area activity with repetitive transcranial magnetic stimulation (rTMS) has been suggested as a potential treatment for psychomotor slowing, given that stimulation of the premotor cortex would likely also exert distant effects within the motor network.20
Protocols for rTMS have distinct effects on neural activity. For example, low-frequency rTMS (1 Hz) acts inhibitory, while intermittent theta burst stimulation (iTBS) has facilitatory effects.21 Our randomized, double-blind, sham-controlled trial of rTMS for psychomotor slowing in major depressive disorder and schizophrenia suggested that 15 sessions of 1-Hz rTMS may ameliorate psychomotor slowing.22 However, to understand this positive effect of 1-Hz rTMS on psychomotor slowing, we aimed at testing the intervention in a larger sample of patients with schizophrenia, including a comprehensive behavioral battery, 1 group with facilitatory stimulation (iTBS), and 1 group without rTMS treatment, to disentangle unspecific effects of the TMS procedure from treatment as usual. Therefore, we tested in a randomized, double-blind, sham-controlled 4-arm trial whether 3 weeks of add-on rTMS would ameliorate psychomotor slowing in psychosis. We hypothesized that inhibitory 1-Hz rTMS would be superior to facilitatory iTBS, sham-rTMS, or no add-on rTMS.
Methods
Trial Design
This 4-arm, double-blind, randomized, placebo-controlled clinical trial of add-on rTMS was conducted at the University Hospital of Psychiatry and Psychotherapy, Bern, Switzerland. The protocol (Supplement 1) adhered to the Declaration of Helsinki and was approved by the cantonal ethics committee of Bern. Written informed consent was provided by all participants. Sample size estimations are given in eMethods 1 in Supplement 2. There were no relevant changes to the protocol after the trial commencement. The trial was registered on April 16, 2019, when 3 patients were receiving treatment. Trial registration included 2 primary outcomes instead of 1 in the study protocol (eMethods 2 in Supplement 2).
Participants
From March 22, 2019, to August 29, 2022, we screened 615 patients for eligibility. Of these, 103 patients were randomized: 26 to 1-Hz rTMS, 25 to iTBS, 28 to sham, and 24 to the waiting group. We included patients aged between 18 and 60 years who were diagnosed with schizophrenia spectrum disorders according to DSM-5 criteria and had psychomotor slowing as per the Salpêtrière Retardation Rating Scale23 (SRRS score ≥15). Exclusions included substance misuse (other than nicotine), conditions associated with impaired or aberrant movement, convulsions, history of hearing problems, other conditions typically excluded from magnetic resonance imaging or TMS, any TMS treatment in the past 3 months, or those who were pregnant or breastfeeding. The full list of inclusion and exclusion criteria are provided in eTable 1 in Supplement 2. Patients continued preexisting medication, including antipsychotics and benzodiazepines. A total of 88 patients received at least 1 rTMS session or completed the waiting period (intention-to-treat population). Follow-up was completed October 31, 2022.
TMS Protocols
All stimulations were delivered using either MagPro X100, including MagOption or MagPro R30 with theta burst option, both manufactured by Tonica Electronik and distributed by MagVenture. We used the MCF-B70 coil for the real TMS stimulations and the MCF-P-B65 coil for sham stimulations. rTMS application followed published guidelines.24,25 Before each session, resting motor thresholds were acquired.25 All protocols were delivered in 15 daily sessions over 3 weeks targeting the left supplementary motor area by moving the coil 3 cm anterior from the leg motor area along the midline, which stimulates the bilateral supplementary motor area.26,27,28 The coil handle was pointing backward along the midline. Each protocol had identical looking coils and identical duration (eMethods 3 in Supplement 2).
One-hertz rTMS included 960 pulses at an intensity of 110% resting motor thresholds (16:00-minute duration). This protocol is similar to our previous study22 and a study in Parkinson disease.27 The sham control used the identical stimulation protocol as 1-Hz rTMS for 16 minutes with a placebo coil that looks and sounds identical to the real coil but has no magnetic emissions. The active control with iTBS included 2 series of 600 pulses at 50 Hz (stimulation in 2-second trains every 10 seconds for a total of 190 seconds)29 at 80% resting motor threshold separated by a 10-minute pause between the series, totaling 16 minutes, 20 seconds, and 1200 pulses. We applied these changes to the iTBS protocol to harmonize treatment duration.
Outcomes
The primary outcome was the proportion of responders per treatment arm at week 3, defined as 30% or greater reduction from baseline in SRRS total score (higher values indicating more slowing), as in the previous trial.22 The change in SRRS scores from baseline to week 3 was another primary outcome.
Secondary outcomes included responder rates and the course of SRRS in the waiting group following the rTMS treatment phase. We also computed changes from baseline to week 3 in SRRS scores and expert ratings covering general illness severity (Positive And Negative Syndrome Scale [PANSS]),30 negative symptoms (Brief Negative Symptom Scale [BNSS]),31 catatonia (Bush-Francis Catatonia Rating Scale [BFCRS]),32 parkinsonism (Unified Parkinson’s Disease Rating Scale Part III [UPDRS]),33 dyskinesia (Abnormal Involuntary Movement Scale [AIMS]),34 global functioning (Global Assessment of Functioning [GAF]),35 social functioning (Social and Occupational Functioning Assessment Scale [SOFAS]),36 and functional capacity (University of California San Diego Performance-Based Skills Assessment [UPSA brief])37 (eMethods 4 in Supplement 2). In addition, we acquired self-reported negative symptoms (Self-evaluation of Negative Symptoms [SNS])38 and physical activity (International Physical Activity Questionnaire [IPAQ]).39 Moreover, the change in total physical activity during waking hours was measured with wrist actigraphy on the nondominant arm (Move4 [movisens])40 (eMethods 5 in Supplement 2). Manual dexterity of both hands was tested using the coin rotation task41 (eMethods 6 in Supplement 2).
Procedures
After baseline assessments, the 1-Hz rTMS, iTBS, and sham groups received their allocated interventions for 3 weeks with weekly assessments of SRRS scores and safety. Safety outcomes included adverse stimulation effects after each rTMS session and adverse effect rating scale after 5, 10, and 15 sessions (ie, weeks 1, 2, and 3). The waiting group did not receive any rTMS intervention in the first 3 weeks, but after completing a second baseline assessment, these participants received the 1-Hz rTMS protocol daily until week 6. At baseline, week 3, and week 6 (waiting group only), patients were assessed with clinical and motor rating scales, tasks assessing fine and gross motor behavior, and a test of functional capacity. Daily medication was summarized as mean olanzapine equivalents42 for antipsychotics or mean diazepam equivalents43 for benzodiazepines. Follow-up assessments including clinical, functional, and motor measures were conducted at 6 weeks and 24 weeks following the week 3 assessments.
Randomization
After providing informed consent and before baseline measurements, patients were randomized 1:1:1:1 to 1 of the 4 treatment arms. Permutated block randomization lists were generated by research randomizer software (Social Psychology Network) and kept secured, only accessible to one person (S.W.). Treatment allocation was communicated only to the person administering rTMS (N.N.). Allocation was kept in writing in a sealed envelope for each patient (eMethods 7 in Supplement 2).
Blinding
Outcome assessors, clinical staff, and patients were blind to treatment, except for the waiting group, who expected to receive 1-Hz rTMS after the waiting period. Treatment duration, setting, and TMS machinery were identical for all patients. In 41 patients, we assessed the suspected type of stimulation received at week 3.
Statistical Analysis
Primary and secondary outcomes were analyzed by 2 researchers (S.W. and N.N.) with SPSS Statistics version 28.0.0.0(190) (IBM). All analyses were run in the intention-to-treat sample (N = 88 with at least 1 rTMS session44,45,46,47,48) using the last observation carried forward method to account for missing data. Response rates between treatment arms were compared using a χ2 test. Logistic regressions were calculated to obtain odds ratios for responder rates with bootstrapping for CIs using 1000 iterations and Bonferroni correction for post hoc tests. We compared improvement in SRRS scores over 3 weeks between treatment arms using repeated-measures analysis of covariance (ANCOVA) covaried for sex, baseline antipsychotics, baseline benzodiazepines, and the mean dosages of antipsychotics and benzodiazepines during the 3-week intervention. For the 2 primary outcome analyses, 2-sided P < .025 was considered significant (0.05 / 2). We corrected ANCOVA post hoc tests for multiple comparisons using Sidak tests. In addition, we explored post hoc tests with least significant difference correction. To test rTMS effects on secondary outcomes (PANSS, BNSS, BFCRS, AIMS, UPDRS, SOFAS, GAF, UPSA brief, IPAQ, and SNS scores; actigraphy; and coin rotation), we calculated repeated measures ANCOVAs with factors group and time, including the covariates sex, baseline antipsychotics, baseline benzodiazepines, and the mean dosages of antipsychotics and benzodiazepines. Frequencies of adverse events and blinding evaluation were calculated using χ2 tests with 2-sided P ≤ .05 as significance threshold.
Results
Dates of Recruitment
Of the 88 patients analyzed, 45 were men and 43 were women. The mean (SD) age was 36.3 (12.4) years. A total of 69 patients completed the intervention period as well as the week 3 assessments (16 in the 1-Hz rTMS group, 18 in the iTBS group, 19 in sham, and 16 in the waiting group). Reasons for discontinuation were withdrawal of consent (n = 15), treating psychiatrist’s decision (n = 2), lost to follow-up (n = 1), and adverse effects (n = 1) (Figure 1; eTable 2 in Supplement 2).
Figure 1. CONSORT Flow Diagram.
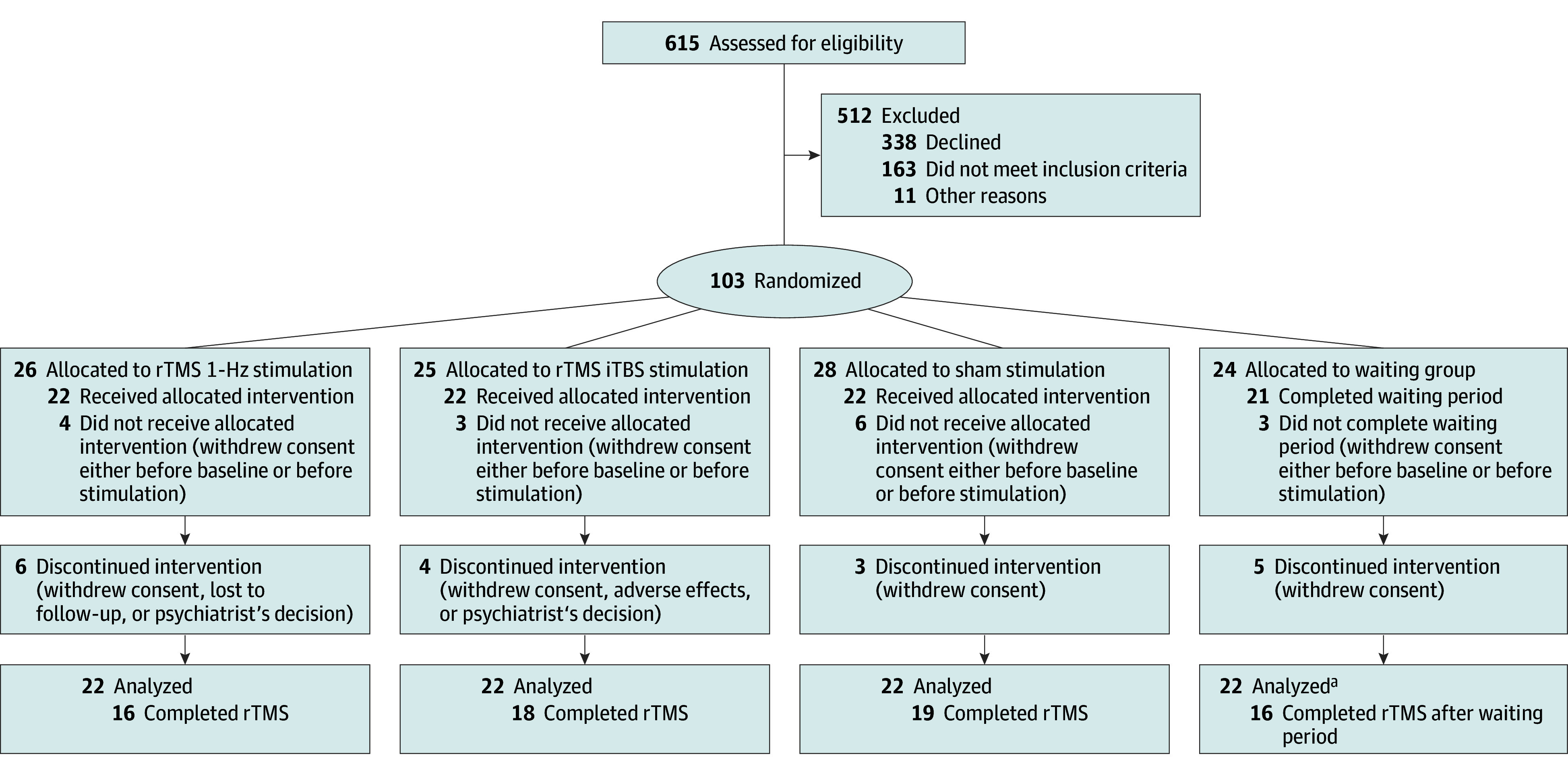
Numbers for dropout reasons were summarized within groups to ensure data privacy. iTBS indicates intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation.
aIn the TMS groups, last observation carried forward analyses were conducted for all participants with at least 1 stimulation. In the waiting group, we did last observation carried forward analyses in all participants with baseline data. Thus, 22 were analyzed in this group.
Baseline demographic and clinical data are given in Table 1. Differences between treatment arms included sex, baseline antipsychotics, baseline benzodiazepines, and the mean dosages of antipsychotics and benzodiazepines.
Table 1. Clinical and Demographic Characteristics.
| Characteristic | Mean (SD) | |||
|---|---|---|---|---|
| 1-Hz rTMS | iTBS | Sham | Waiting | |
| No. | 22 | 22 | 22 | 22 |
| Age, y | 39.5 (13.2) | 33.5 (11.5) | 38.2 (12.2) | 33.8 (12.4) |
| Sex, No. (%)a | ||||
| Male | 16 (73) | 12 (55) | 9 (41) | 8 (36) |
| Female | 6 (27) | 10 (45) | 13 (59) | 14 (64) |
| Education, y | 13.0 (3.0) | 13.1 (2.2) | 12.7 (2.2) | 13.2 (2.0) |
| Duration of illness, y | 12.0 (11.9) | 7.0 (8.4) | 12.9 (12.4) | 9.7 (7.0) |
| No. of episodes | 4.5 (4.3) | 3.4 (2.9) | 5.9 (5.7) | 4.7 (3.9) |
| BMI | 26.3 (4.0) | 24.2 (4.7) | 25.9 (6.6) | 24.3 (5.0) |
| Medicationb | ||||
| Baseline olanzapine equivalents, mg/dc | 13.3 (10.3) | 13.4 (10.6) | 22.6 (12.9) | 15.8 (11.0) |
| Baseline diazepam equivalents, mg/dd | 6.1 (14.6) | 5.1 (8.3) | 0.5 (1.6) | 0.5 (1.5) |
| Mean olanzapine equivalents, mg/de | 14.5 (9.9) | 12.0 (7.9) | 22.9 (12.9) | 15.4 (9.8) |
| Mean diazepam equivalents, mg/df | 3.6 (8.5) | 2.3 (4.5) | 0.7 (1.4) | 0.1 (0.2) |
| Monotherapy, No. (%) | 11 (50) | 15 (68) | 10 (45) | 14 (64) |
| First-generation antipsychotics, No. (%) | 5 (23) | 5 (23) | 4 (18) | 3 (14) |
| Clozapine, No. (%) | 5 (23) | 7 (32) | 3 (14) | 6 (27) |
| Clinical rating scales | ||||
| SRRS total score | 24.7 (5.8) | 23.3 (7.1) | 24.2 (5.7) | 23.7 (5.0) |
| PANSS total score | 76.3 (17.1) | 81.8 (19.9) | 77.3 (14.1) | 84.6 (17.8) |
| PANSS positive score | 14.8 (5.1) | 16.9 (6.4) | 15.0 (4.2) | 16.9 (5.8) |
| PANSS negative score | 22.9 (5.4) | 23.2 (6.1) | 24.7 (6.8) | 24.5 (6.0) |
| BNSS total score | 42.0 (14.4) | 39.3 (12.2) | 45.7 (11.8) | 42.2 (12.4) |
| BFCRS total score | 4.9 (3.7) | 5.9 (4.9) | 7.1 (3.7) | 4.2 (3.0) |
| NCRS total score | 9.9 (4.8) | 10.6 (5.8) | 10.3 (3.3) | 9.0 (4.4) |
| UPDRS total score | 18.7 (8.7) | 21.3 (13.8) | 23.4 (11.4) | 20.0 (9.7) |
| AIMS total score | 1.4 (3.1) | 1.1 (2.0) | 0.9 (2.2) | 0.1 (0.4) |
| NES total score | 18.4 (11.6) | 16.4 (11.4) | 15.0 (7.6) | 12.3 (6.5) |
| GAF | 43.7 (11.7) | 39.1 (14.1) | 39.0 (9.9) | 42.1 (12.7) |
| SOFAS | 41.0 (14.9) | 40.3 (12.6) | 38.0 (10.7) | 42.2 (11.7) |
| UPSA brief | 70.6 (13.9) | 71.2 (17.9) | 74.9 (14.6) | 75.3 (12.0) |
| SNS total score | 18.9 (9.3) | 21.1 (8.0) | 22.0 (8.0) | 19.7 (7.8) |
| IPAQ total score | 1008 (1283) | 815 (940) | 2191 (3732) | 875 (1304) |
| Activity level, counts/h | 12 978 (4795) | 13 548 (4961) | 12 028 (3493) | 11 921 (4924) |
| CR dominant | 10.8 (3.2) | 10.9 (3.5) | 12.2 (4.4) | 10.9 (3.4) |
| CR nondominant | 9.6 (3.8) | 9.8 (3.3) | 10.1 (4.3) | 9.4 (2.3) |
| Cortical excitability, RMT | 42 (7) | 45 (9) | 42 (10) | 43 (10) |
Abbreviations: AIMS, Abnormal Involuntary Movement Scale; BFCRS, Bush-Francis Catatonia Rating Scale; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BNSS, Brief Negative Symptom Scale; CR, coin rotations; GAF, Global Assessment of Functioning Scale; IPAQ, International Physical Activity Questionnaire; iTBS, intermittent theta burst stimulation; NCRS, Northoff Catatonia Rating Scale; NES, Neurological Evaluation Scale; PANSS, Positive and Negative Syndrome Scale; RMT, resting motor threshold; rTMS, repetitive transcranial magnetic stimulation; SNS, Self-evaluation of Negative Symptoms; SOFAS, Social and Occupational Functioning Assessment Scale; SRRS, Salpêtrière Retardation Rating Scale; UPDRS, Unified Parkinson Disease Rating Scale Part III; UPSA brief, University of California San Diego Performance-Based Skills Assessment.
Group difference: P = .07.
None of the participants received anticholinergics.
Group difference: P = .02.
Group difference: P = .04.
Group difference: P = .005.
Group difference: P = .07.
Primary Outcomes
In the intention-to-treat analysis with last observation carried forward, we found a significant difference in the proportion of responders following 3 weeks of rTMS (1-Hz rTMS: 15 of 22 [68%], iTBS: 8 of 22 [36%], sham: 7 of 22 [32%]; waiting: 4 of 22 [18%]; χ23 = 12.1; P = .007) (Figure 2A). The 1-Hz rTMS group had more responders than sham (odds ratio [OR], 0.13; 95% CI, 0.02-0.65; P = .03), iTBS (OR, 0.12; 95% CI, 0.02-0.61; P = .02), and waiting (OR, 0.04; 95% CI, 0.01-0.22; P = .003). One-hertz stimulation was superior to all other protocols (Table 2).
Figure 2. Responder Rates and Course of Psychomotor Slowing.
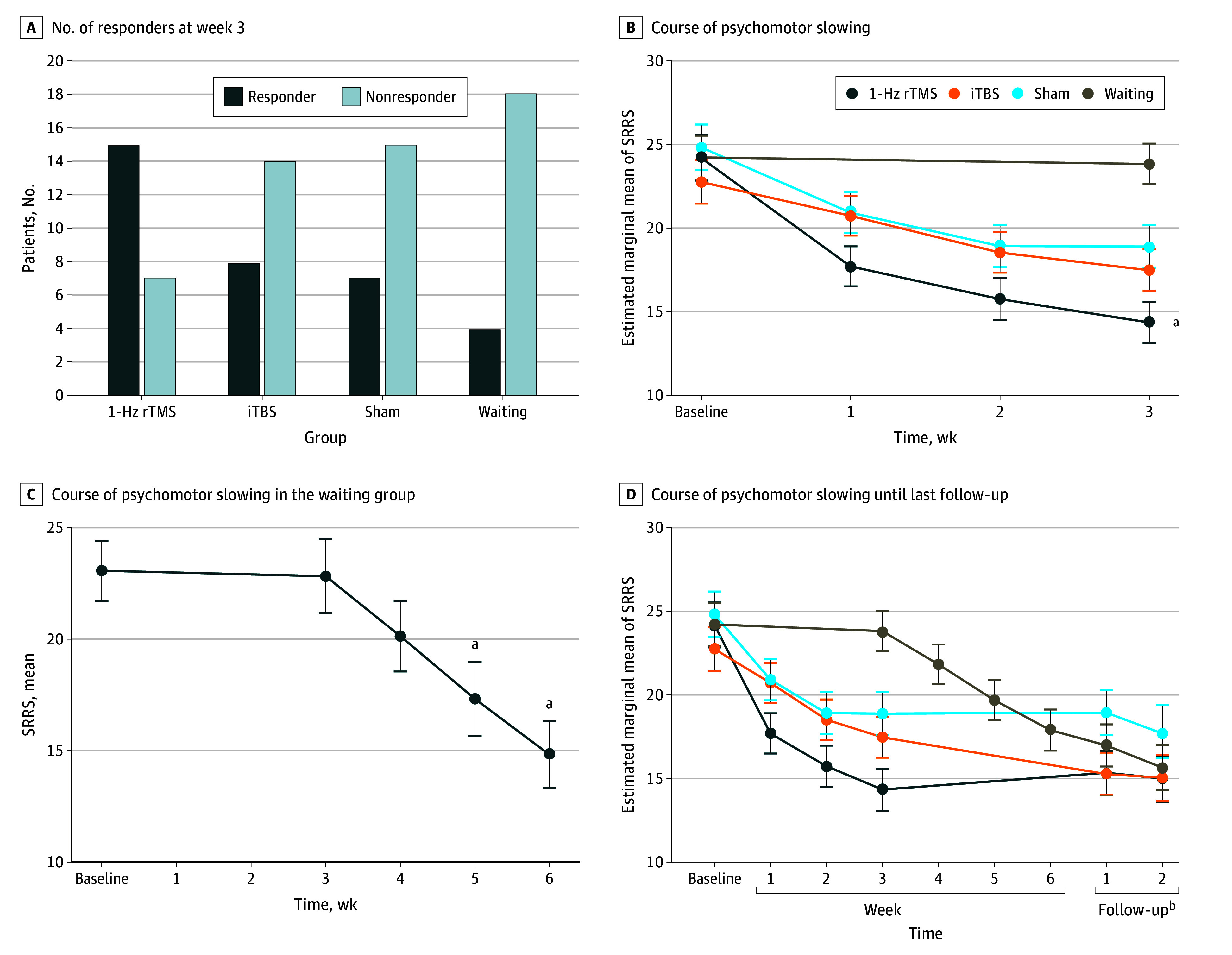
Treatment arm covariates (B and D) included age and medication (baseline antipsychotics, baseline benzodiazepines, and mean dosages of antipsychotics and benzodiazepines), using last observation carried forward for missing values. The waiting sample consisted of 16 patients completing the repetitive transcranial magnetic stimulation (rTMS) treatment phase after the 3-week waiting period (C). Error bars represent SEMs. iTBS indicates intermittent theta burst stimulation.
aSignificant at P < .05.
bFollow-up 1 took place 6 weeks after end of treatment; follow-up 2, 24 weeks after end of treatment.
Table 2. Raw and Adjusted Odds Ratios (ORs) for Treatment Response at Week 3 (N = 88).
| Treatment group comparison | Raw OR (95% CI)a,b | Raw P valuec | Adjusted OR (95% CI)b,d,e | Adjusted P valuec,d |
|---|---|---|---|---|
| 1-Hz rTMS vs sham | 0.22 (0.06-0.08) | .03 | 0.13 (0.02-0.65) | .03 |
| 1-Hz rTMS vs iTBS | 0.27 (0.08-0.93) | .09 | 0.12 (0.02-0.61) | .02 |
| 1-Hz rTMS vs waiting | 0.10 (0.03-0.42) | .009 | 0.04 (0.01-0.22) | .003 |
Abbreviations: iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation.
χ23 = 11.17; P = .01. Omnibus test raw: χ23 = 12.66; P = .005.
Confidence intervals have been bootstrapped with 1000 iterations.
P values are Bonferroni corrected.
Adjustment covariates include baseline sex, baseline antipsychotics, baseline benzodiazepines, and mean dosages of antipsychotics and benzodiazepines.
χ23 = 13.55; P = .004. Omnibus test adjusted: χ28 = 26.51; P = .001.
Repeated-measures ANCOVA of SRRS scores between baseline and week 3 comparing the 4 treatment arms indicated significant effects of time (F1,79 = 26.6; , η2 = 0.25; P < .001) and a time-by–treatment arm interaction (F3,79 = 8.5; η2 = 0.25; P < .001). Post hoc comparisons demonstrated a pronounced decrease in SRRS scores in the 1-Hz rTMS group vs the waiting group. In addition, least significant difference tests indicated superior performance of 1-Hz rTMS vs sham (Figure 2B; eTables 5 and 6 in Supplement 2).
Secondary Outcomes
A repeated-measures ANCOVA with weekly SRRS ratings revealed an effect of time (F3,237 = 16.5; η2 = 0.17; P < .001) and a time-by–treatment arm interaction (F9,237 = 6.3; η2 = 0.19; P < .001). In post hoc tests with Sidak correction, 1-Hz rTMS reduced SRRS scores more compared with the waiting group and sham at trend level. With least significant difference correction, 1-Hz rTMS was superior to sham (eTables 3-6 in Supplement 2). In the waiting group, 10 of 16 participants (63%) responded following 3 weeks of 1-Hz rTMS after the waiting period. In addition, ANOVA with repeated measures of SRRS score demonstrated improvement over time (F4,60 = 9.9; η2 = 0.40; P < .001) (Figure 2C).
Follow-up assessments were acquired in 54 patients after 6 weeks and 32 patients after 6 months. A repeated-measures ANCOVA with SRRS last observation carried forward including follow-up assessments still resulted in significant effects of time (F1,237 = 17.4; η2 = 0.18; P < .001) and a time-by–treatment arm interaction (F9,237 = 5.0; η2 = 0.16; P < .001) (Figure 2D; eTable 7 in Supplement 2). However, Sidak-corrected post hoc tests indicated no differences between treatment arms.
Repeated-measures ANCOVAs of the secondary outcomes demonstrated effects of time in PANSS total score, PANSS positive score, PANSS negative score, PANSS general score, BNSS total score, BNSS asocial score, BFCRS total score, BFCRS abnormal score, BFCRS decreased score, GAF, UPSA brief, and IPAQ. However, time-by–treatment arm interactions were limited to BNSS anhedonia score, BNSS distress score, BFCRS total score, BFCRS abnormal score, and BFCRS decreased score (for last observation carried forward, see eTables 3-7 in Supplement 2; for completers see eTables 8-10 in Supplement 2). The post hoc tests identified a greater decrease in catatonia severity (BFCRS total score) for 1-Hz rTMS vs waiting. Similarly, reductions in BFCRS decreased score were noted in 1-Hz rTMS vs waiting and iTBS vs waiting with Sidak correction, but 1-Hz rTMS was superior to sham with least significant difference correction. iTBS outperformed sham and waiting in decreasing BNSS anhedonia score at week 3 with least significant difference but not with Sidak correction (eTables 3 and 6 in Supplement 2).
Blinding Efficacy
Patients receiving rTMS from baseline to week 3 (1-Hz rTMS, iTBS, and sham) were unable to identify the assigned treatment (n = 41; χ24 = 1.5; P = .82). Thirteen patients (31.7%) correctly guessed that they had received real rTMS or sham.
Safety
There were no severe adverse events during the study period and follow-up. Furthermore, no differences appeared in the number of reported adverse effects per treatment (χ22 = 0.50; P = .78) (Table 3). Adverse effects experienced by participants in the waiting group during rTMS treatment phase are presented in eTable 11 in Supplement 2.
Table 3. Adverse Effects During Intervention Period (Baseline to Week 3).
| Variable | No. (%) | ||
|---|---|---|---|
| 1-Hz rTMS (n = 22) | iTBS (n = 22) | Sham (n = 22) | |
| No adverse effects | 6 (27) | 10 (45.5) | 10 (45.5) |
| Dizziness | 6 (27) | 4 (18) | 3 (13.5) |
| Headache or neck pain | 6 (27) | 10 (45.5) | 4 (18) |
| Fatigue | 10 (45.5) | 5 (23) | 9 (41) |
| Other | <10 (45.5)a | <10 (45.5)b | <10 (45.5)c |
Abbreviations: iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation.
Including fatigue and flash of light, combined to avoid compromising data identifiability requirements.
Including inner restlessness, pressure on head, burning sensation on skin of head, and memory difficulties, combined to avoid compromising data identifiability requirements.
Including eye blinking, bizarre thoughts, suicidal ideation, restlessness, and difficulties swallowing, combined to avoid compromising data identifiability requirements.
Discussion
Psychomotor slowing is a frequent and troubling symptom of psychosis, associated with poor social functioning and minimally responsive to standard treatment. Neuroimaging work suggests higher neural activity in the supplementary motor area in psychomotor slowing.18,19,20 In this randomized clinical trial, we tested whether 15 sessions of add-on daily inhibitory 1-Hz rTMS over the supplementary motor area would reduce psychomotor slowing in psychosis. As hypothesized, 1-Hz stimulation ameliorated psychomotor slowing. This study corroborates the previous transdiagnostic randomized clinical trial on add-on daily 1-Hz rTMS in a new sample of patients with schizophrenia spectrum disorders.22 Within 3 weeks of 1-Hz rTMS, 68% of participants achieved response, compared with patients receiving facilitatory iTBS (36%), sham (32%), or no add-on treatment (18%). Furthermore, 63% of patients responded when the 1-Hz rTMS was commenced after a 3-week waiting period. SRRS scores declined in all groups receiving rTMS, but only the 1-Hz stimulation achieved more decrease than sham. The data suggest beneficial effects with rTMS treatment to last until 6 months’ follow-up, although these findings must be interpreted with caution given the low rate of individuals with 6-month follow-up assessments (36%).
While all participants experienced severe psychomotor slowing and received standard care, including medication, daily add-on rTMS had beneficial effects on psychomotor slowing compared to the waiting group. The reason for this general rTMS effect could be the daily routines involved, such as being taken to the rTMS facilities, enjoying extra social interaction with the study team, and treatment expectations.49,50 Clearly, the data speak to specifically beneficial effects of the inhibitory supplementary motor area stimulation, which is expected to reduce or modulate resting-state hyperactivity in this region.18,19 Still, the effects of the iTBS and sham groups were larger than those of the waiting group. As in the pilot study, approximately one-third of the patients on sham stimulation achieved response.22 However, the iTBS effect was much larger than in the previous randomized clinical trial, when we administered only 1 train of 600 pulses per session.22 iTBS should have opposite effects (facilitation) than the inhibitory 1-Hz stimulation.21 But doubling the iTBS pulses to 1200 on the motor cortex had inhibitory effects in healthy individuals.51 In the current study, we repeated iTBS after 10 minutes to harmonize groups regarding the duration and number of stimuli per session. This may have increased individual variability of iTBS effects with few achieving response. While the neural changes associated with rTMS are out of the scope of this report, we need to explore the probability that repeated iTBS with a pause of 10 minutes may exert inhibitory effects in some participants.51,52,53,54,55
Changes in the severity of catatonia or negative symptoms were noted between treatment arms. BFCRS total scores and BFCRS subscores indicating decreased motor activity were ameliorated with 1-Hz rTMS, whereas BNSS anhedonia scores improved with iTBS stimulation (eFigure 1 in Supplement 2). This rather unexpected finding speaks to recent work suggesting that iTBS might reduce negative symptoms of schizophrenia when applied on the cerebellar vermis56,57 or the dorsolateral prefrontal cortex.58 However, we failed to see this effect on any other BNSS subscore or the PANSS negative score.
In general, specific treatment effects on motor rating scales and actigraphy were lacking. While this could argue for a specific effect of the supplementary motor area stimulation on psychomotor slowing, it might be explained by insufficient statistical power for secondary outcomes. The sample size per group was moderate and the last observation carried forward method is very conservative.
rTMS was well tolerated without any severe adverse events. Mild and transient adverse effects were noted in all treatment arms. This study suggests great potential of noninvasive brain stimulation interventions in the motor system.4,20 Multiple other target regions and symptoms could be tested in this regard, including transdiagnostic studies.22,59,60,61,62
Ideally, the current findings would be replicated in large multicenter trials. Future studies may also test accelerated rTMS protocols with more sessions per day and more stimuli.63,64 Continuous theta burst could deliver larger numbers of inhibitory stimuli on the supplementary motor area. Furthermore, future studies should also test whether cognitive slowing could be improved with inhibitory supplementary motor area stimulation.
Limitations
Using a 4-arm parallel design, we were able to test multiple stimulation types for psychomotor slowing in psychosis. Particularly the inclusion of a waiting group in addition to sham offered valuable insights.49 However, some limitations require consideration when interpreting the results. First, the choice of 2 primary outcomes was made during clinical trial registration and is a deviation from the study protocol. Second, blinding is challenging in rTMS trials. Duration, machinery, and setup were identical across study arms. Most participants were unable to identify the rTMS protocol received at week 3. However, the waiting group was aware of their treatment regimen. Third, randomization was conducted before baseline assessments and thus the intention-to-treat population included all patients with at least 1 rTMS session. This is in line with rTMS studies in psychiatry but slightly different from drug trials.44,45,46,47,48 Results might indicate greater effects than analyses including individuals who had never received the assigned treatment (eTables 12 and 13 in Supplement 2). Fourth, randomization skewed the distribution of medication regimens and sex between the 4 groups, therefore these variables were included as covariates. Fifth, the sample size was calculated for the continuous primary outcomes and fell short in achieving sufficient power for the secondary outcomes. Sixth, randomization also led to some variance in the levels of catatonia severity suggesting floor effects of the treatment in some groups. Seventh, 14 individuals (16% of the intention-to-treat population) dropped out in the first 3 weeks, which is comparable to other trials.44 Last observation carried forward analysis accounted for dropouts.
Conclusions
In this randomized clinical trial, inhibitory rTMS on the supplementary motor area was safe and effective in reducing psychomotor slowing in patients with psychosis. The exact mechanism of this clinical effect remains to be understood. Larger studies will be needed to disentangle secondary effects in the future.
Trial protocol
eMethods 1. Sample size estimation
eMethods 2. Trial registration
eTable 1. Inclusion/Exclusion criteria
eMethods 3. Coils
eMethods 4. Secondary outcome rating scale subscores
eMethods 5. Actigraphy
eMethods 6. Coin rotation task
eMethods 7. Randomization and allocation concealment
eResults
eTable 2. Dropout reasons
eTable 3. Primary and secondary outcomes according to treatment arm for ITT with LOCF and covariates
eTable 4. Primary and secondary outcomes according to treatment arms with LOCF (raw)
eTable 5. Post-hoc-p-values of Time with SIDAK correction
eTable 6. Post-hoc-p-values the interaction between Time & Treatment arm
eTable 7. Follow-ups with LOCF and covariates
eTable 8. Primary and secondary outcomes according to treatment arm of completers with covariates
eTable 9. Primary and secondary outcomes according to treatment arm with completers (raw)
eTable 10. Follow ups of completers with covariates
eTable 11. Side-effects in the waiting group
eFigure 1. Course of anhedonia
eFigure 2. Course of global functioning
eTable 12. Baseline variables in the three sample versions
eTable 13. Results of the primary outcomes in n=88 (ITT) vs. n=97 (with baseline), covaried for sex, baseline and treatment medication
eReferences
Data sharing statement
References
- 1.Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. 2022;399(10323):473-486. doi: 10.1016/S0140-6736(21)01730-X [DOI] [PubMed] [Google Scholar]
- 2.Heckers S, Barch DM, Bustillo J, et al. Structure of the psychotic disorders classification in DSM-5. Schizophr Res. 2013;150(1):11-14. doi: 10.1016/j.schres.2013.04.039 [DOI] [PubMed] [Google Scholar]
- 3.Pieters LE, Nadesalingam N, Walther S, van Harten PN. A systematic review of the prognostic value of motor abnormalities on clinical outcome in psychosis. Neurosci Biobehav Rev. 2022;132:691-705. doi: 10.1016/j.neubiorev.2021.11.027 [DOI] [PubMed] [Google Scholar]
- 4.Walther S, van Harten PN, Waddington JL, et al. Movement disorder and sensorimotor abnormalities in schizophrenia and other psychoses—European consensus on assessment and perspectives. Eur Neuropsychopharmacol. 2020;38:25-39. doi: 10.1016/j.euroneuro.2020.07.003 [DOI] [PubMed] [Google Scholar]
- 5.Pappa S, Dazzan P. Spontaneous movement disorders in antipsychotic-naive patients with first-episode psychoses: a systematic review. Psychol Med. 2009;39(7):1065-1076. doi: 10.1017/S0033291708004716 [DOI] [PubMed] [Google Scholar]
- 6.Peralta V, Campos MS, De Jalón EG, Cuesta MJ. Motor behavior abnormalities in drug-naïve patients with schizophrenia spectrum disorders. Mov Disord. 2010;25(8):1068-1076. doi: 10.1002/mds.23050 [DOI] [PubMed] [Google Scholar]
- 7.Morrens M, Hulstijn W, Sabbe B. Psychomotor slowing in schizophrenia. Schizophr Bull. 2007;33(4):1038-1053. doi: 10.1093/schbul/sbl051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osborne KJ, Walther S, Shankman SA, Mittal VA. Psychomotor slowing in schizophrenia: implications for endophenotype and biomarker development. Biomark Neuropsychiatry. 2020;2:100016. doi: 10.1016/j.bionps.2020.100016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walther S, Stegmayer K, Wilson JE, Heckers S. Structure and neural mechanisms of catatonia. Lancet Psychiatry. 2019;6(7):610-619. doi: 10.1016/S2215-0366(18)30474-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heckers S, Walther S. Catatonia. N Engl J Med. 2023;389(19):1797-1802. doi: 10.1056/NEJMra2116304 [DOI] [PubMed] [Google Scholar]
- 11.Nadesalingam N, Chapellier V, Lefebvre S, et al. Motor abnormalities are associated with poor social and functional outcomes in schizophrenia. Compr Psychiatry. 2022;115:152307. doi: 10.1016/j.comppsych.2022.152307 [DOI] [PubMed] [Google Scholar]
- 12.Stubbs B, Ku PW, Chung MS, Chen LJ. Relationship between objectively measured sedentary behavior and cognitive performance in patients with schizophrenia vs controls. Schizophr Bull. 2017;43(3):566-574. doi: 10.1093/schbul/sbw126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stubbs B, Chen LJ, Chung MS, Ku PW. Physical activity ameliorates the association between sedentary behavior and cardiometabolic risk among inpatients with schizophrenia: a comparison versus controls using accelerometry. Compr Psychiatry. 2017;74:144-150. doi: 10.1016/j.comppsych.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 14.Putzhammer A, Perfahl M, Pfeiff L, Hajak G. Correlation of subjective well-being in schizophrenic patients with gait parameters, expert-rated motor disturbances, and psychopathological status. Pharmacopsychiatry. 2005;38(3):132-138. doi: 10.1055/s-2005-864125 [DOI] [PubMed] [Google Scholar]
- 15.Nadesalingam N, Lefebvre S, Alexaki D, et al. The behavioral mapping of psychomotor slowing in psychosis demonstrates heterogeneity among patients suggesting distinct pathobiology. Schizophr Bull. 2023;49(2):507-517. doi: 10.1093/schbul/sbac170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Docx L, Morrens M, Bervoets C, et al. Parsing the components of the psychomotor syndrome in schizophrenia. Acta Psychiatr Scand. 2012;126(4):256-265. doi: 10.1111/j.1600-0447.2012.01846.x [DOI] [PubMed] [Google Scholar]
- 17.Walther S. Psychomotor symptoms of schizophrenia map on the cerebral motor circuit. Psychiatry Res. 2015;233(3):293-298. doi: 10.1016/j.pscychresns.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 18.Walther S, Schäppi L, Federspiel A, et al. Resting-state hyperperfusion of the supplementary motor area in catatonia. Schizophr Bull. 2017;43(5):972-981. doi: 10.1093/schbul/sbw140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV. Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia spectrum disorders. Schizophr Bull. 2017;43(5):982-992. doi: 10.1093/schbul/sbx091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lefebvre S, Pavlidou A, Walther S. What is the potential of neurostimulation in the treatment of motor symptoms in schizophrenia? Expert Rev Neurother. 2020;20(7):697-706. doi: 10.1080/14737175.2020.1775586 [DOI] [PubMed] [Google Scholar]
- 21.Jannati A, Oberman LM, Rotenberg A, Pascual-Leone A. Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacology. 2023;48(1):191-208. doi: 10.1038/s41386-022-01453-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walther S, Alexaki D, Schoretsanitis G, et al. Inhibitory repetitive transcranial magnetic stimulation to treat psychomotor slowing: a transdiagnostic, mechanism-based randomized double-blind controlled trial. Schizophr Bull Open. 2020;1(1). doi: 10.1093/schizbullopen/sgaa020 [DOI] [Google Scholar]
- 23.Dantchev N, Widlöcher DJ. The measurement of retardation in depression. J Clin Psychiatry. 1998;59(suppl 14):19-25. [PubMed] [Google Scholar]
- 24.Rossi S, Hallett M, Rossini PM, Pascual-Leone A; Safety of TMS Consensus Group . Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008-2039. doi: 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefaucheur JP, André-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125(11):2150-2206. doi: 10.1016/j.clinph.2014.05.021 [DOI] [PubMed] [Google Scholar]
- 26.Hamada M, Ugawa Y, Tsuji S; Effectiveness of rTMS on Parkinson’s Disease Study Group, Japan . High-frequency rTMS over the supplementary motor area for treatment of Parkinson’s disease. Mov Disord. 2008;23(11):1524-1531. doi: 10.1002/mds.22168 [DOI] [PubMed] [Google Scholar]
- 27.Shirota Y, Ohtsu H, Hamada M, Enomoto H, Ugawa Y; Research Committee on rTMS Treatment of Parkinson’s Disease . Supplementary motor area stimulation for Parkinson disease: a randomized controlled study. Neurology. 2013;80(15):1400-1405. doi: 10.1212/WNL.0b013e31828c2f66 [DOI] [PubMed] [Google Scholar]
- 28.Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain. 1997;120(Pt 9):1587-1602. doi: 10.1093/brain/120.9.1587 [DOI] [PubMed] [Google Scholar]
- 29.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201-206. doi: 10.1016/j.neuron.2004.12.033 [DOI] [PubMed] [Google Scholar]
- 30.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. doi: 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 31.Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37(2):300-305. doi: 10.1093/schbul/sbq059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136. doi: 10.1111/j.1600-0447.1996.tb09814.x [DOI] [PubMed] [Google Scholar]
- 33.Fahn S, Elton RL, Members UP. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, eds. Recent Developments in Parkinson’s Disease. Macmillan Healthcare Information; 1987. [Google Scholar]
- 34.Guy W. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education and Welfare; 1976:534-537. [Google Scholar]
- 35.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33(6):766-771. doi: 10.1001/archpsyc.1976.01770060086012 [DOI] [PubMed] [Google Scholar]
- 36.Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry. 1992;149(9):1148-1156. doi: 10.1176/ajp.149.9.1148 [DOI] [PubMed] [Google Scholar]
- 37.Mausbach BT, Harvey PD, Goldman SR, Jeste DV, Patterson TL. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr Bull. 2007;33(6):1364-1372. doi: 10.1093/schbul/sbm014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dollfus S, Mach C, Morello R. Self-evaluation of negative symptoms: a novel tool to assess negative symptoms. Schizophr Bull. 2016;42(3):571-578. doi: 10.1093/schbul/sbv161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 40.Walther S, Ramseyer F, Horn H, Strik W, Tschacher W. Less structured movement patterns predict severity of positive syndrome, excitement, and disorganization. Schizophr Bull. 2014;40(3):585-591. doi: 10.1093/schbul/sbt038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gebhardt A, Vanbellingen T, Baronti F, Kersten B, Bohlhalter S. Poor dopaminergic response of impaired dexterity in Parkinson’s disease: Bradykinesia or limb kinetic apraxia? Mov Disord. 2008;23(12):1701-1706. doi: 10.1002/mds.22199 [DOI] [PubMed] [Google Scholar]
- 42.Leucht S, Samara M, Heres S, et al. Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull. 2015;41(6):1397-1402. doi: 10.1093/schbul/sbv037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatric Annals. 1995;25(3):158-165. doi: 10.3928/0048-5713-19950301-09 [DOI] [Google Scholar]
- 44.Wobrock T, Guse B, Cordes J, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77(11):979-988. doi: 10.1016/j.biopsych.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 45.Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931-938. doi: 10.1176/appi.ajp.2019.18101180 [DOI] [PubMed] [Google Scholar]
- 46.Kaster TS, Daskalakis ZJ, Noda Y, et al. Efficacy, tolerability, and cognitive effects of deep transcranial magnetic stimulation for late-life depression: a prospective randomized controlled trial. Neuropsychopharmacology. 2018;43(11):2231-2238. doi: 10.1038/s41386-018-0121-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67(5):507-516. doi: 10.1001/archgenpsychiatry.2010.46 [DOI] [PubMed] [Google Scholar]
- 48.Kumar N, Vishnubhatla S, Wadhawan AN, Minhas S, Gupta P. A randomized, double blind, sham-controlled trial of repetitive transcranial magnetic stimulation (rTMS) in the treatment of negative symptoms in schizophrenia. Brain Stimul. 2020;13(3):840-849. doi: 10.1016/j.brs.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 49.Burke MJ, Kaptchuk TJ, Pascual-Leone A. Challenges of differential placebo effects in contemporary medicine: the example of brain stimulation. Ann Neurol. 2019;85(1):12-20. doi: 10.1002/ana.25387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yesavage JA, Fairchild JK, Mi Z, et al. ; VA Cooperative Studies Program Study Team . Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893. doi: 10.1001/jamapsychiatry.2018.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gamboa OL, Antal A, Moliadze V, Paulus W. Simply longer is not better: reversal of theta burst after-effect with prolonged stimulation. Exp Brain Res. 2010;204(2):181-187. doi: 10.1007/s00221-010-2293-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirkovski M, Donaldson PH, Do M, et al. A systematic review of the neurobiological effects of theta-burst stimulation (TBS) as measured using functional magnetic resonance imaging (fMRI). Brain Struct Funct. 2023;228(3-4):717-749. doi: 10.1007/s00429-023-02634-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li CT, Huang YZ, Bai YM, Tsai SJ, Su TP, Cheng CM. Critical role of glutamatergic and GABAergic neurotransmission in the central mechanisms of theta-burst stimulation. Hum Brain Mapp. 2019;40(6):2001-2009. doi: 10.1002/hbm.24485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cárdenas-Morales L, Volz LJ, Michely J, et al. Network connectivity and individual responses to brain stimulation in the human motor system. Cereb Cortex. 2014;24(7):1697-1707. doi: 10.1093/cercor/bht023 [DOI] [PubMed] [Google Scholar]
- 55.Nettekoven C, Volz LJ, Kutscha M, et al. Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J Neurosci. 2014;34(20):6849-6859. doi: 10.1523/JNEUROSCI.4993-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brady RO Jr, Gonsalvez I, Lee I, et al. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am J Psychiatry. 2019;176(7):512-520. doi: 10.1176/appi.ajp.2018.18040429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Basavaraju R, Ithal D, Thanki MV, et al. Intermittent theta burst stimulation of cerebellar vermis enhances fronto-cerebellar resting state functional connectivity in schizophrenia with predominant negative symptoms: a randomized controlled trial. Schizophr Res. 2021;238:108-120. doi: 10.1016/j.schres.2021.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bation R, Magnin C, Poulet E, Mondino M, Brunelin J. Intermittent theta burst stimulation for negative symptoms of schizophrenia—a double-blind, sham-controlled pilot study. NPJ Schizophr. 2021;7(1):10. doi: 10.1038/s41537-021-00138-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walther S, Heckers S. Mapping psychomotor behavior in the brain. JAMA Psychiatry. 2024;81(1):7-8. doi: 10.1001/jamapsychiatry.2023.4290 [DOI] [PubMed] [Google Scholar]
- 60.Morris SE, Sanislow CA, Pacheco J, Vaidyanathan U, Gordon JA, Cuthbert BN. Revisiting the seven pillars of RDoC. BMC Med. 2022;20(1):220. doi: 10.1186/s12916-022-02414-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walther S, Bernard JA, Mittal VA, Shankman SA. The utility of an RDoC motor domain to understand psychomotor symptoms in depression. Psychol Med. 2019;49(2):212-216. doi: 10.1017/S0033291718003033 [DOI] [PubMed] [Google Scholar]
- 62.Wüthrich F, Lefebvre S, Mittal VA, et al. The neural signature of psychomotor disturbance in depression. Mol Psychiatry. Published online December 1, 2023. doi: 10.1038/s41380-023-02327-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cole EJ, Phillips AL, Bentzley BS, et al. Stanford neuromodulation therapy (snt): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179(2):132-141. doi: 10.1176/appi.ajp.2021.20101429 [DOI] [PubMed] [Google Scholar]
- 64.Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177(8):716-726. doi: 10.1176/appi.ajp.2019.19070720 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods 1. Sample size estimation
eMethods 2. Trial registration
eTable 1. Inclusion/Exclusion criteria
eMethods 3. Coils
eMethods 4. Secondary outcome rating scale subscores
eMethods 5. Actigraphy
eMethods 6. Coin rotation task
eMethods 7. Randomization and allocation concealment
eResults
eTable 2. Dropout reasons
eTable 3. Primary and secondary outcomes according to treatment arm for ITT with LOCF and covariates
eTable 4. Primary and secondary outcomes according to treatment arms with LOCF (raw)
eTable 5. Post-hoc-p-values of Time with SIDAK correction
eTable 6. Post-hoc-p-values the interaction between Time & Treatment arm
eTable 7. Follow-ups with LOCF and covariates
eTable 8. Primary and secondary outcomes according to treatment arm of completers with covariates
eTable 9. Primary and secondary outcomes according to treatment arm with completers (raw)
eTable 10. Follow ups of completers with covariates
eTable 11. Side-effects in the waiting group
eFigure 1. Course of anhedonia
eFigure 2. Course of global functioning
eTable 12. Baseline variables in the three sample versions
eTable 13. Results of the primary outcomes in n=88 (ITT) vs. n=97 (with baseline), covaried for sex, baseline and treatment medication
eReferences
Data sharing statement


