Abstract
Introduction:
Urinary incontinence (UI) is a common and disruptive symptom of Parkinson’s disease (PD). This study aimed to identify neural correlates associated with UI among PD patients with UI (UI-PD) compared to those PD patients without UI (nonUI-PD) with the expectation of demonstrating increased functional connectivity (FC) between areas in the striatum and limbic system and decreased FC in executive areas.
Methods:
rsfMRI and T1w data (n = 119) were retrieved from the Parkinson’s Progression Markers Initiative (PPMI). Resting-state FC analyses assessed temporal covariance with anterior cingulate gyrus, precuneus, and putamen seed regions.
Results:
The UI-PD group (n = 32, 16 females) showed significantly greater positive FC between the bilateral putamen seed and the right caudate and right thalamus (p < 0.01), relative to individuals with PD but who did not have UI (n = 87, 18 females). The UI-PD group showed greater negative FC between the anterior cingulate seed and right angular gyrus (p < 0.01) relative to nonUI-PD.
Conclusion:
Individuals with PD and UI display stronger FC within neural circuits likely affected by PD such as between the putamen and caudate, as well as within those associated with brain bladder control, compared to persons with PD and without UI. Clinical application based on this study’s results can provide greater discernment of treatment strategies for UI-PD patients.
Keywords: fMRI, functional connectivity, Parkinson’s disease, urinary incontinence
1 |. INTRODUCTION
Urinary incontinence (UI) is a common non-motor symptom experienced by many individuals with Parkinson’s disease (PD) and contributes to poor quality of life.1 Most persons with PD and UI experience urgency UI, which is UI preceded by the sudden, compelling desire to pass urine that is difficult to defer.2 Several studies using functional magnetic resonance imaging (fMRI) have compared functional connectivity (FC) between persons with UI who do not have PD and healthy (without UI) controls.3–5 These studies demonstrate that UI correlates to increased connectivity of a network related to bladder control including, but not limited to, aACC-insula-supplementary motor area, prefrontal cortex, and basal ganglia.3–5 However, less is known about the networks involved in persons living with PD and UI (UI-PD) compared to those with PD who do not have UI (nonUI-PD). To inform novel targets for treatment, the present study aimed to identify resting-state FC differences between these two populations to gain insight into neurological correlates associated with UI in persons with PD by focusing on areas associated with bladder control.
In particular, the putamen, anterior cingulate (AC), and precuneus have a close to relationship with both UI and PD. These three regions work together to regulate micturition and play multiple roles in other neurological networks related to cognition and motor functions. The putamen, a striatal region heavily regulated by dopamine, is associated with voluntary movement, learning, reward, and micturition.6,7 A prominent mechanism of bladder symptoms in PD is a deficiency in dopamine released throughout the striatum (including the putamen) resulting in reduced motor capabilities, cognitive impairment, and possibly causing incontinence via detrusor hyperactivity in persons with UI.5,8
The AC is a neurological region that has been researched extensively when it comes to its role in bladder control and UI.5,9 As an integral part of the interoceptive network and emotion regulation-related circuits, the AC is associated with visceral sensations and the emotional context for those sensations,6 including the urge to void.5 Altered AC engagement with regions involved in emotion regulation and interoception is a central component of theories of neural contributions to urgency UI.3,8,9
The precuneus is a part of the posterior region of the default mode network (DMN) which also includes the medial prefrontal cortex and angular gyrus. The DMN is engaged during resting states and disengaged when individuals are occupied with a task and has been associated with a host of functions including emotion and memory processing and self-monitoring.3 It has been suggested that urge sensations emerge from a process of self-monitoring internal states. In persons with PD, such self-monitoring processes may be affected by neurodegenerative effects inflicted upon aspects of the DMN.3,4
Informed by prior results in non-PD populations, the presented study tested the hypotheses that UI in PD would be linked with (1) greater FC between the putamen with other regions involved in motor function and motivated cognition, including prefrontal (PFC), sensorimotor regions, and caudate; (2) greater FC between the AC, insula, and other regions of the limbic system, and negative FC between AC and PFC; and (3) lower FC between the precuneus and the basal ganglia for the UI-PD group relative to nonUI-PD group.
2 |. MATERIALS AND METHODS
2.1 |. Subjects
Data used in this study were retrieved from the Parkinson’s Progression Markers Initiative’s repository,10 a longitudinal study that collects and stores research and clinical data from persons with early PD with the purpose of understanding the development of the disease. As PPMI is an international multisite study, each site received ethical approval and written consent from all participating subjects from study investigators.
Early PD was defined as a diagnosis of PD through physical exam and a positive dopamine transporter (DAT) scan within 2 years of the screening PPMI evaluation.1,11 Additionally, participants were not expected to require PD medication (dopaminergic, MAO-B inhibitor, or amantadine) within at least 6 months of the baseline evaluation and had not taken PD medication within 60 days of baseline. MRI and demographic information for an initial group of 318 PD persons was downloaded from the PPMI database via the Laboratory of Neuro Imaging.12 We included persons who responded to the Scales for Outcomes in Parkinson’s disease—Autonomic Dysfunction (SCOPA-AUT) assessment question (SCAU9) relating to UI13 (see Supporting Information). Participants who responded with “sometimes,” “regularly,” or “often” were labeled as UI-PD, with those who responded with “never” as nonUI-PD. Also included were the non-motor (Part 1) and motor (Part 3) scores on the Movement Disorder Society-Unified Parkinson Disease Rating Scale (MDS-UPDRS)14 (see Supporting Information). All subjects had one high-resolution anatomical MRI (i.e., T1-weighted) and one resting state fMRI (rsfMRI) blood oxygen level dependent (BOLD) scan that were conducted at the same visit date of a SCOPA-AUT assessment in the PD cohort.
Of the original 318 persons, we excluded individuals from the prodromal and genetic PD cohorts resulting in 175 persons with PD. We also excluded participants who responded to the SCAU9 question with “Use Catheter” as these persons do not have to inhibit the urge to void, reducing the number of participants to 134. Nine participants were excluded due to T1 magnetization equilibrium artifact and five participants were excluded due to excessive head motion identified using MRIQC v21.0.0rc. If >15% of volumes in the rsfMRI time-course contained a framewise displacement (FD) > 1 mm, that participant’s scan was excluded from further analyses. The 1 mm threshold similar to previous studies conducting fMRI analyses with persons with PD.15 The final sample size for the current study was therefore n = 119 participants with PD (see Supporting Information for full exclusion criteria details).
2.2 |. MRI protocol
Based on the PPMI MRI Technical Operations Manual v3.0 (https://www.ppmi-info.org), patients were instructed to keep their eyes open and remain still while in the scanner. The scanners used were either 3-Tesla (3T) Siemens (Siemens Medical Solutions), 3T Philips (Healthcare Imaging Systems), or 3T GE (General Electric Healthcare). The rsfMRI data used in the study followed one of two distinct protocols (2D Gradient-echo T2*-weighted EPI, TR = 2.4 or 2.5 s, TE = 0.025 or 0.03 s, FOV = 224, voxel sizes = 3.29 × 3.29 × 3.3 or 3.5 × 3.5 × 3.5, flip angle = 80°, number of volumes = 210 or 240, duration of scans = 8 or 10 min, primary slice direction = axial; see Supporting Information).
2.3 |. Preprocessing
Preprocessing of both anatomical and functional MRI scans was performed using fMRIPrep 20.2.5 (RRID:SCR_016216), which is based on Nipype 1.6.1 (RRID:SCR_002502). Briefly, for the T1-weighted (T1w) image, preprocessing steps included brain extraction, tissue segmentation, and were normalized into the Montreal Neurological Institute (MNI) template space, specifically, MNI152NLin2009cAsym space. For the rsfMRI time-course, preprocessing steps included BOLD reference image estimation, head-motion estimation, slice timing correction, registration to the T1w for the functional images, and resampled into MNI152N-Lin2009cAsym space (see Supporting Information for full preprocessing pipeline details). Preprocessed T1w and rsfMRI data were used in the FC analyses.
2.4 |. Functional connectivity
FC correlational analyses were conducted in CONN Toolbox v21a16 (RRID:SCR_009550). FC analyses seek to identify correlations in the BOLD time-course between spatially discrete neurological regions across time.3,16 Preprocessed images of the final n = 119 subjects were imported and smoothed with an 8 mm Gaussian FWHM kernel. To remove confounding effects, a linear regression was conducted on the data based on CONN Toolbox’s default denoising pipeline (CONN Toolbox Manual, https://web.conn-toolbox.org/resources/documentation/manual). During denoising, physiological and extraneous noise were accounted for by modeling covariates for the averaged WM and CSF BOLD time-courses, 12 motion parameters (3 translation, 3 rotation, and the first derivatives of those 6), and volumes were scrubbed of motion at 0.09 mm. Additionally, a temporal band-pass filter of 0.008–0.09 Hz was applied based once again on CONN Toolbox’s default denoising pipeline. Cortical and subcortical ROIs were defined using the Harvard-Oxford atlas and cerebellar parcellation from the Automated Anatomical Labeling (AAL) atlas.17–19
2.4.1 |. Seed-to-voxel connectivity analysis
We tested a priori predictions that the UI-PD group would show differences in FC with specific seed regions using one-way ANCOVA for seed-to-voxel connectivity (SVC) analyses. Our three seed regions included the bilateral AC, bilateral precuneus, and a custom bilateral putamen ROI (the ROI mask was created in FSL v6.0.1 using the Harvard-Oxford Structural atlas), based on prior established involvement in brain–bladder control networks and UI in otherwise healthy populations.4,5 To identify group differences in FC between the UI-PD and nonUI-PD groups, Fisher transformed Z-score correlations between seed timeseries and voxel timeseries were computed across the whole brain. To correct for multiple comparisons, random field parametric distributions were utilized to calculate a cluster threshold over the whole brain to reject the null hypothesis at p < 0.05. Voxel-wise statistics were thresholded using an uncorrected level of p < 0.01, followed by a corrected FWE cluster-size/extent (k) threshold for each seed. Age and sex were included in model as covariates to control for any potential significant differences that may occur due to these two variables.
2.4.2 |. Exploratory FC analysis
To identify group differences (UI-PD vs. nonUI-PD) in FC between all networks, we performed a group-level exploratory ROI-to-ROI connectivity analysis. A one-way ANCOVA analysis contrasted UI-PD versus nonUI-PD, controlling for age and sex. Fisher transformed Z-scores were computed to compare each pair of ROI timeseries. Connectivity differences were thresholded at p < 0.005 (uncorrected) at the connection level, and p < 0.05 (uncorrected) at the ROI-level using a multivariate pattern analysis (MVPA) within the CONN toolbox to identify group differences between networks. As with the seed-to-voxel analysis, age and sex were included in model as covariates to control for any potential significant differences that may occur due to these two variables.
3 |. RESULTS
Descriptive statistics to assess group differences (UI-PD vs. nonUI-PD) in demographic sample characteristics were conducted in RStudio v3.6.3.20 We identified significant differences in age and sex between the groups (Table 1). Age and sex were therefore included as covariates in all subsequent analyses of the neuroimaging data. To identify any potential cognitive, motor, and non-motor aspects of PD that may play a role in the differences seen between the UI-PD and nonUI-PD groups, Pearson correlational analyses were conducted on participants’ total scores on the Montreal Cognitive Assessment (MoCA),21 Part 1 (Non-Motor Aspects of Experiences of Daily Living) and Part 3 (Motor Examination) of the MDS-UPDRS (Table 1). Results from the analyses did not show any significant association between UI and MoCA nor MDS-UPDRS scores.
TABLE 1.
Descriptive statistics and group differences among individuals with early-stage Parkinson’s disease according to urinary incontinence status.
| UI-PD | NonUI-PD | ||
|---|---|---|---|
| n = 32 | n = 87 | Group comparison | |
| Age, M (SD) | 67 (8) | 61 (11) | p < .0a |
| Sex, n | 16 female (50%) | 18 female (20.7%) | p < .01b |
| UPDRS Part 1 total, M (SD) | 1.94 (2.05) | 1.77 (2.37) | p = .73a |
| UPDRS Part 3 total, M (SD) | 23.77 (12.00) | 21.73 (9.04) | p = .33a |
| MoCA total, M (SD) | 26.43 (3.46) | 26.43 (4.85) | p= 0.99a |
Note: Correlational analyses comparing UPDRS and MoCA total scores and group differences were nonsignificant at p < 0.05.
Abbreviations: M, mean; MDS-UPDRS, Movement Disorder Society—Unified Parkinson’s Disease Rating Scale; MoCA, Montreal Cognitive Assessment; SD, standard deviation; UI-PD, Parkinson’s disease patients with urinary incontinence; UPDRS, Unified Parkinson Disease Rating Scale.
Independent samples t-test.
Chi-square test.
3.1 |. SVC results
Significant results of the SVC analysis demonstrated FC differences with multiple brain regions associated with bladder control in UI-PD compared to nonUI-PD groups. The UI-PD group showed significantly greater positive FC between the bilateral putamen seed and the right caudate and right thalamus (t(115) = 4.82, k = 489, p < 0.01) (Figure 1A). The UI-PD group showed significantly greater negative FC between the AC and right angular gyrus (AG) (t(115) = −4.12, k = 488, p < 0.01) compared to the nonUI-PD group (Figure 1B). There were no significant differences between the groups for the precuneus seed.
FIGURE 1.
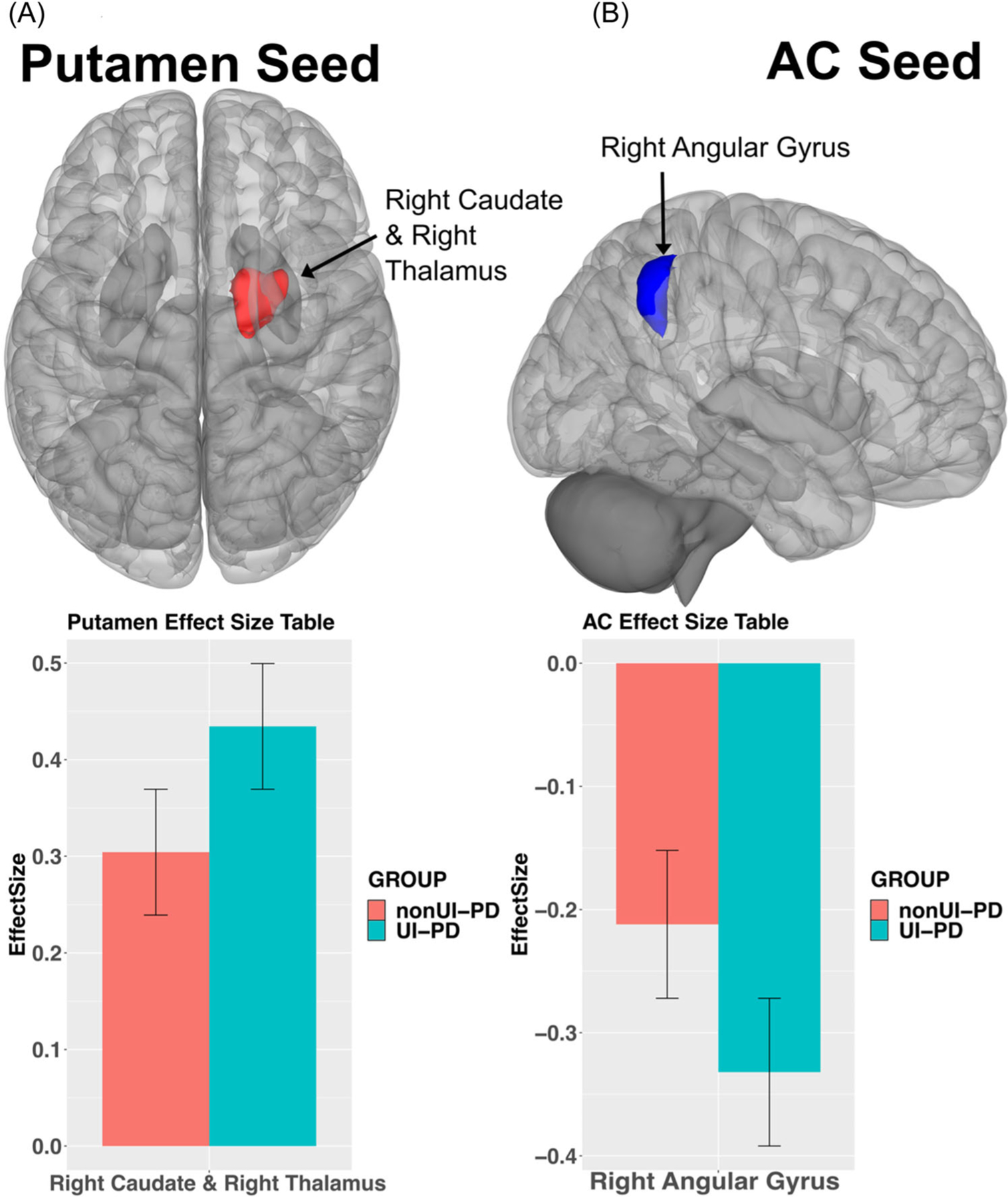
(A) Results for the bilateral putamen seed. UI-PD showed greater positive connectivity with the right caudate and thalamus (x y z = 14 0 16, red), pFWEc < 0.05. (B) Results for the AC seed. The UI-PD group showed less negative connectivity with the right angular gyrus (54 –54 46, blue), pFWEc < 0.05. Bar charts indicate directionality between groups effects (UI-PD = blue, nonUI-PD = red). AC, anterior cingulate; UI-PD, Parkinson’s disease patients with urinary incontinence; pFWEc, probability family-wise error corrected.
3.2 |. Exploratory FC results
Results of the exploratory ROI-to-ROI FC analysis identified 10 pairs of ROIs exceeding the multivariate threshold for FC differences between UI-PD and nonUI-PD groups that displayed significant connectivity between each other. (Figure 2). In follow-up testing of each seed region, UI-PD showed greater positive FC between the left putamen and bilateral caudate, and greater negative FC between the left putamen and right anterior parahippocampal gyrus relative to the nonUI-PD group (t(115) = 2.17, p < 0.05). There was greater negative FC between the anterior cingulate and the right middle temporal gyrus, angular gyrus, and frontoparietal networks (t(115) = −4.15, p < 0.01). Lastly, the anterior left temporal gyrus displayed greater negative FC with multiple medial visual networks, and the cerebellum (t(115) = −4.68, p < 0.01).
FIGURE 2.

Circle plot shows significant multivariate associations between a seed and a number of targets, with red lines indicating significantly greater connectivity in UI-PD (blue bars) versus nonUI-PD (red bars), and blue indicating significantly less connectivity in UI-PD versus nonUI-PD. Results were thresholded at ROI-level p < 0.005, and cluster threshold p < 0.05 using MVPA omnibus test. Bar chart shows directionality of the effects, and error bars represent ±1 SD. AC, anterior cingulate gyrus; AG, angular gyrus; aMTG, anterior middle temporal gyrus; aPaHC, anterior parahippocampal gyrus; Cereb, cerebellum; FP, frontal pole; ICC, intracalcarine cortex; iLOC, inferior lateral occipital cortex; PaCiG, paracingulate gyrus; pITG, posterior inferior temporal gyrus; PostCG, postcentral gyrus; PreCG, precentral gyrus; pTFusC, posterior temporal fusiform cortex; SCC, supracalcarine cortex; SFG, superior frontal gyrus; SMG, supramarginal gyrus; SPL, superior parietal lobe; toMTG, temporoocipital middle temporal gyrus; TP, temporal pole; UI-PD, Parkinson’s disease patients with urinary incontinence; Ver, vermis.
4 |. DISCUSSION
Results from this study show persons with early PD and UI display different FC patterns compared to persons with PD but without UI. Many of these regions are known to be associated with bladder control and play a role maintaining continence. Our results provide support for our hypothesis that UI is associated with disrupted brain–bladder functional circuitry. Specifically, we found persons with UI-PD showed greater positive FC in the striatum and limbic systems, while also showing decreases in FC in areas associated with the default mode network (DMN). To our knowledge, this is the first study utilizing FC to examine neural correlates of urinary symptoms in early-stage PD before significant motor and non-motor symptom burden has occurred. Such results will provide greater understanding of neurological correlates of UI in PD and may provide targets for therapeutic intervention.
The UI-PD group displayed greater positive putamen connectivity with the caudate and thalamus compared to the nonUI-PD group. This is notable given the central role of dopaminergic dysfunction within the striatum in the progression of PD.11 The putamen, caudate, and thalamus all show atrophy at early stages of the disease, putatively related to a failure of dopamine production from the substantia nigra.22 These striatal regions have been associated with voluntary movements, memory, and emotional processing.15 Striatal projections to the thalamus additionally reflect the processing of goal-relevant sensory information.6 The current findings suggest that reduced FC of these regions may contribute to UI early after a PD diagnosis, which fits with the current understanding of these regions’ role in micturition inhibition being engaged during pelvic contractions when the urge to void arises, and during bladder filling.6
Our results showed the AC to have a negative connectivity with the right angular gyrus which was stronger in the UI-PD group. Connections between the AC and angular gyrus are associated with the DMN, memory processing, and attention.23 In contrast to Zuo et al.,5 we did not find decreased FC between the AC and superior frontal gyrus. We also did not see positive correlations between the AC, insula, or PFC, three regions that are typically involved with instances of UI.9 Finally, the results from the current study did not show any significant results from the precuneus seed, another key region of the DMN. Overall, this suggested that UI in Parkinson’s does impact DMN connectivity, particularly in regions important for memory and self-processing, but does not impact AC FC with lateral prefrontal and insula regions as has previously been observed in UI among otherwise-healthy samples.
4.1 |. Limitations
A few limitations exist with the current study. First, the bladder state of participants was not known at the time of the MRI scan. Knowledge of the bladder state of the subjects of this study could have provided further context for the results, a method future studies should undertake. Second, the SCOPA-AUT questionnaire does not identify UI severity or the type of UI subjects had (i.e., stress, urgency). Similar to studies of UI in persons without PD,24 women with early PD were more likely to report UI than men with early PD; however, a previous study leveraging PPMI data demonstrated that the prevalence of UI was three times higher in early PD than the PPMI healthy control cohort that was well-balanced regarding age and sex.1 Lastly, recent research has shown that to improve replication rates of effect sizes of brain-wide association studies should be conducted on a sample size of 1000+.25 The current study is limited based on the available data from the PPMI study. However, it would be interesting for future research to identify FC differences among UI-PD persons with a much greater sample size.
5 |. CONCLUSION
Our results support and add to the growing body of research related to understanding the neurological underpinnings of UI in persons with PD. Results from this study provide greater evidence that the functional circuits associated with bladder control are disrupted in persons with PD and exacerbated in those with both PD and UI. Such insight into the neurological correlates relating to UI-PD could contribute to the development of more targeted clinical treatment of persons with both UI and PD and inform preventive strategies such as awareness of behavioral techniques for suppressing UI to promote bladder health in persons with early PD.
Supplementary Material
ACKNOWLEDGMENTS
The authors of this paper would like to thank the Michael J. Fox Foundation and the PPMI organization for their research in Parkinson’s Disease. We would also like to thank Dr. Megan E. Huibregtse for proof-reading and providing feedback for this manuscript. This research was supported in part by the US Department of Veterans Affairs Rehabilitation Research Center Grant #I01 RX002358, I01 RX003093, I01 RX002293-01A2, IK2 RX000747-01, I01RX002619, and IK2RX000956. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Funding information
U.S. Department of Veterans Affairs
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors of this paper affirm that all subjects provided informed consent before data collection in accordance with the Declaration of Helsinki.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in Image & Data Archive (IDA) at https://ida.loni.usc.edu/login.jsp. These data were derived from the following resources available in the public domain: Parkinson’s Progression Markers Initiative’s (PPMI, https://www.ppmi-info.org).
REFERENCES
- 1.Serra MC, Landry A, Juncos JL, et al. Increased odds of bladder and bowel symptoms in early Parkinson’s disease. Neurourol Urodyn 2018;37(4):1344–1348. doi: 10.1002/nau.23443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams P, Artibani W, Cardozo L, Dmochowski R, van Kerrebroeck P, Sand P. Reviewing the ICS 2002 terminology report: the ongoing debate. Neurourol Urodyn 2009;28(4):287. doi: 10.1002/nau.20737 [DOI] [PubMed] [Google Scholar]
- 3.Ketai LH, Komesu YM, Dodd AB, Rogers RG, Ling JM, Mayer AR. Urgency urinary incontinence and the interoceptive network: a functional magnetic resonance imaging study. Am J Obstet Gynecol 2016;215(4):449.e1–449.e17. doi: 10.1016/j.ajog.2016.04.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nardos R, Karstens L, Carpenter S, et al. Abnormal functional connectivity in women with urgency urinary incontinence: can we predict disease presence and severity in individual women using Rs-fcMRI/. Neurourol Urodyn 2016;35(5): 564–573. doi: 10.1002/nau.22767 [DOI] [PubMed] [Google Scholar]
- 5.Zuo L, Zhou Y, Wang S, Wang B, Gu H, Chen J. Abnormal brain functional connectivity strength in the overactive bladder syndrome: a resting-state fMRI study. Urology 2019;131:64–70. doi: 10.1016/j.urology.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 6.Arya NG, Weissbart SJ. Central control of micturition in women: brain-bladder pathways in continence and urgency urinary incontinence. Clin Anat 2017;30(3):373–384. doi: 10.1002/ca.22840 [DOI] [PubMed] [Google Scholar]
- 7.Graybiel AM, Grafton ST. The striatum: where skills and habits meet. Cold Spring Harbor Perspect Biol 2015;7(8): a021691. doi: 10.1101/cshperspect.a021691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winge K, Fowler CJ. Bladder dysfunction in parkinsonism: mechanisms, prevalence, symptoms, and management. Mov Disord 2006;21(6):737–745. doi: 10.1002/mds.20867 [DOI] [PubMed] [Google Scholar]
- 9.Tam J, Cohen T, Kim J, Weissbart S. Insight into the central control of overactive bladder symptoms by functional brain imaging. Curr Bladder Dysfunct Rep 2018;13(2):31–37. doi: 10.1007/s11884-018-0464-5 [DOI] [Google Scholar]
- 10.Home | Parkinson’s Progression Markers Initiative Accessed June 21, 2022. https://www.ppmi-info.org/
- 11.Marek K, Chowdhury S, Siderowf A, et al. The Parkinson’s progression markers initiative (PPMI)—establishing a PD biomarker cohort. Ann Clin Transl Neurol 2018;5(12): 1460–1477. doi: 10.1002/acn3.644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.[dataset] Crawford. IDA—Image and Data Archive Accessed August 23, 2022. https://ida.loni.usc.edu/login.jsp
- 13.Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ. Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. Mov Disord 2004;19(11):1306–1312. doi: 10.1002/mds.20153 [DOI] [PubMed] [Google Scholar]
- 14.Goetz CG, Fahn S, Martinez-Martin P, et al. The MDS-sponsored Revision of the Unified Parkinson’s Disease Rating Scale 33. Published online: 2019.
- 15.Disbrow EA, Carmichael O, He J, et al. Resting state functional connectivity is associated with cognitive dysfunction in non-demented people with Parkinson’s disease. J Parkinsons Dis 2014;4(3):453–465. doi: 10.3233/JPD-130341 [DOI] [PubMed] [Google Scholar]
- 16.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2(3):125–141. doi: 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- 17.François-Brosseau FE, Martinu K, Strafella AP, Petrides M, Simard F, Monchi O. Basal ganglia and frontal involvement in self-generated and externally-triggered finger movements in the dominant and non-dominant hand. Eur J Neurosci 2009;29(6):1277–1286. doi: 10.1111/j.1460-9568.2009.06671.x [DOI] [PubMed] [Google Scholar]
- 18.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 19.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 20.RStudio Team. RStudio: Integrated Development for R. RStudio. PBC; 2020. http://www.rstudio.com/
- 21.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 22.Zeighami Y, Ulla M, Iturria-Medina Y, et al. Network structure of brain atrophy in de novo Parkinson’s disease. eLife 2015;4:e08440. doi: 10.7554/eLife.08440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 2013;19(1):43–61. doi: 10.1177/1073858412440596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tennstedt SL, Link CL, Steers WD, McKinlay JB. Prevalence of and risk factors for urine leakage in a racially and ethnically diverse population of adults: the Boston Area Community Health (BACH) Survey. Am J Epidemiol 2008;167(4):390–399. doi: 10.1093/aje/kwm356 [DOI] [PubMed] [Google Scholar]
- 25.Marek S, Tervo-Clemmens B, Calabro FJ, et al. Reproducible brain-wide association studies require thousands of individuals. Nature 2022;603(7902):654–660. doi: 10.1038/s41586-022-04492-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available in Image & Data Archive (IDA) at https://ida.loni.usc.edu/login.jsp. These data were derived from the following resources available in the public domain: Parkinson’s Progression Markers Initiative’s (PPMI, https://www.ppmi-info.org).


