Abstract
Methods
We searched MEDLINE, Embase, Global Health, CINAHL, China National Knowledge Infrastructure, Wanfang, CQvip, and the World Health Organization (WHO) COVID-19 global literature databases for primary studies recruiting children aged ≤18 years with a diagnosis of SARS-CoV-2 infection confirmed either by molecular or antigen tests. We used the Joanna Briggs Institute critical appraisal tools to appraise the study quality and conducted meta-analyses using the random effects model for all outcomes except for race/ethnicity as risk factors of SARS-CoV-2 infection.
Results
We included 237 studies, each reporting at least one of the study outcomes. Based on data from 117 studies, the pooled SARS-CoV-2 positivity rate was 9.30% (95% confidence interval (CI) = 7.15–11.73). Having a comorbidity was identified as a risk factor for SARS-CoV-2 infection (risk ratio (RR) = 1.33; 95% CI = 1.04–1.71) based on data from 49 studies. Most cases in this review presented with mild disease (n = 50; 52.47% (95% CI = 44.03–60.84)). However, 20.70% of paediatric SARS-CoV-2 infections were hospitalised (67 studies), 7.19% required oxygen support (57 studies), 4.26% required intensive care (93 studies), and 2.92% required assisted ventilation (63 studies). The case fatality ratio (n = 119) was 0.87% (95% CI = 0.54–1.28), which included in-hospital and out-of-hospital deaths.
Conclusions
Our data showed that children were at risk for SARS-CoV-2 infections and severe outcomes in the pre-Omicron era. These findings underscore the need for effective vaccination strategies for the paediatric population to protect against the acute and long-term sequelae of COVID-19.
Registration
PROSPERO: CRD42022327680.
The World Health Organization (WHO) declared the coronavirus disease 2019 (COVID-19) outbreak a global pandemic on 11 March 2020 [1]. WHO estimates suggest that there have been over 663 million confirmed COVID-19 cases, including at least 6 million reported deaths as of 19 January 2023 [2]. Since the emergence of the first case in Wuhan, China in late 2019, numerous SARS-CoV-2 variants have emerged and have been circulating globally. The Omicron variant, (also called variant B.1.1.529), was first reported to WHO on 24 November 2021 and has been circulating ever since [3]. There is a large volume of data on the epidemiology and economic impact of SARS-CoV-2 infections; unfortunately, comprehensive data on the burden of SARS-CoV-2 infections in children and adolescents are limited, particularly in the pre-Omicron era.
The United Nations (UN) defines children as persons under the age of 18 years [4] and adolescents as persons between the ages of 10 and 19 years [5]. Evidence suggests that individuals under 20 years of age represented 33% of the 2020 global population and contributed to 21% of all reported COVID-19 cases since the beginning of the pandemic until the end of 2022 [6]. Although these numbers indicate that the incidence of COVID-19 is low in this compared to other age groups, a lack of data from this population has been identified as a challenge for developing precise estimates [6]. Moreover, the case numbers represent just one aspect of the disease burden. A comprehensive determination of the burden of COVID-19 is incomplete without estimating the disease severity and highest level of management or care required by cases. Furthermore, it is important to identify vulnerable groups within this population to prioritise protection and prevention measures, access to health care, or even treatment.
Therefore, we aimed to estimate the burden of SARS-CoV-2 infections (in terms of test positivity, severity of clinical presentation, and the level of care required) in children and adolescents aged ≤18 years from published literature reporting data from the pre-Omicron era, and to report data on risk factors for SARS-CoV-2 infections and related mortality in this population. This systematic review of existing evidence and synthesis of knowledge will help improve our understanding of the burden of SARS-CoV-2 infections in children and adolescents globally and help shape and inform protection and prevention policies and management guidelines.
METHODS
We registered the protocol for this systematic review in PROSPERO (CRD42022327680) and followed the PRISMA-P 2020 guidelines in reporting our findings [7] (Table S12–13 in the Online Supplementary Document). We did not seek formal ethical approval as this review was based on data from open-access published primary studies.
Literature search
We searched MEDLINE, Embase, Global Health, CINAHL, and the WHO COVID-19 global literature databases on 27 February 2022 using pre-developed search strategies to identify studies reporting incidence, risk factors, severity, and outcomes of COVID-19 infection in children and adolescents aged ≤18 years. We also searched three Chinese literature databases (CNKI, Wanfang, and CQvip) on 21 March 2022 (Text S2 in the in the Online Supplementary Document).
Literature selection
Following deduplication, we uploaded the references retrieved from the searches in English language databases into Covidence (Veritas Health Innovation, Melbourne, Australia). A single reviewer (DK) then screened their titles and abstracts. During the screening, we observed that many studies defined children as those aged ≤18 years rather than those aged <18 years. Therefore, the review population included children and adolescents, i.e. those aged ≤18 years, as data were very commonly reported for this age group. A single reviewer screened the full text of the studies based on pre-defined inclusion criteria, depending on the study language (English: DK, Chinese: FZ or XW, Portuguese: JS, Spanish: KA). We included observational studies with a sample size of at least 100 children; presenting data on people aged ≤18 years; reporting on SARS-CoV-2 infection confirmed by polymerase chain reaction (PCR) or antigen tests; and reporting test positivity, severity, risk factors, or mortality due to SARS-CoV-2 infection or the differential impacts of SARS-CoV-2 variants. We set no criteria regarding settings (community, educational institutions, health care settings, etc.) (Text S1 in the Online Supplementary Document).
Data extraction
We designed a data extraction form in Microsoft Access to capture characteristics and outcomes for studies included in this systematic review and meta-analysis (see variables in Text S3 in the Online Supplementary Document). A single reviewer extracted the data into Microsoft Access (DK, JS, or KA) or Microsoft Excel (FZ), while a second reviewer cross-checked the extractions in MS Excel (English-language studies: NFI, Chinese-language studies: XW). Any disagreements between the two reviewers were resolved through discussion or by consulting a third reviewer (HN) if necessary.
Quality assessment
A single reviewer (DK, KA, or FZ) assessed the quality of the included studies using the modified Joanna Briggs Institute quality assessment checklists, according to each study’s design [8] (Text S4 in the Online Supplementary Document). English language studies marked as good quality or poor quality by the first reviewer were re-assessed by the second reviewer (NFI). Studies were deemed to be of good quality if they had a score of 7–8 (cross-sectional studies), 8–10 (case-control studies and quasi-experimental studies), 9–11 (cohort studies), or 8–10 (diagnostic test accuracy studies); of fair quality if they had a score of 4–6 (cross-sectional studies), 4–7 (case-control studies and quasi-experimental studies), 6 and 8 (cohort studies), or 4–7 (diagnostic test accuracy studies); and of poor quality if they had a score of 0–3 (cross-sectional, case-control, quasi-experimental, and diagnostic test accuracy studies) or 0–5 (cohort studies). Any discrepancies were resolved by discussion or by consulting a third reviewer (HN). We used the quality scores to conduct sensitivity analysis.
Statistical analysis
We conducted all statistical analyses in R, version 4.2.1 (R Core Team, Vienna, Austria). Since we anticipated significant heterogeneity, we adopted the DerSimonian and Laird random effects model [9]. We applied the Freeman-Tukey double arcsine transformation for all analyses except for the analysis on race/ethnicity as a risk factor for SARS-CoV-2 infections, which we synthesised narratively due to a lack of standardised data. The Freeman-Tukey double arcsine transformation enabled the inclusion of zero event estimates. The transformation also standardises the variance, which was appropriate to our analyses, as there was substantial heterogeneity across study estimates. We derived the pooled proportion as back-transformed values as the weighted mean of the transformed proportions to report all the pooled estimates with their 95% confidence intervals (CIs). We assessed heterogeneity by calculating the I2 and τ2 values. For risk factors, we calculated the pooled risk ratio (RR) with their 95% CIs. We developed forest plots to display results from individual studies along with the pooled estimates.
Test positivity estimates
We generated a pooled estimate for the proportion of children and adolescents testing positive for SARS-CoV-2 either by antigen tests or PCR among all children and adolescents that were tested in each study. All nucleic acid amplification tests were coded as PCR, as we estimated this to be the most widely used nucleic acid test across settings. We then performed a leave-one-out meta-analysis to investigate the influence of each study on the overall estimate and to identify influential studies, and also conducted a sensitivity analysis to determine the pooled estimate for the proportion of positive cases by including only good-quality studies. We conducted subgroup analyses to report the test positivity by setting (community, health care, educational institution, or other), age groups, type of diagnostic test, and geographical location.
By age group
We also performed subgroup analyses by two age groups (<5 and 5 to ≤18 years), in which we included any studies that reported age-stratified data for these two groups. We then further split these age groups into two narrower age bands (<5-year-old group into those aged <1 and 1 to <5 years; 5 to ≤18-year-old group into those aged 5 to <11 and 11 to 18 years). If a study reported data for groups that overlapped over subgroups within a group, we classified the data in a way that they fell in the group where most of their sample can be expected to fall in (for example, those <3 years old were included in the 1 to <5-year-old group) to maximise the inclusion of data in the analysis. Age groups that could not be classified into either of our groups (because they fell exactly in between) were placed in the higher age group (for example, the <2-year-old group was placed in the 1 to <5-year-old group).
By regions
To assess regional differences, we conducted subgroup analysis by WHO regions [10] and World Bank income levels [11].
By SARS-CoV-2 variants
To investigate the differential impact of SARS-CoV-2 variants on the proportion of positive cases, we utilised open-access data [12]. These data represented the proportion of the total number of sequences (not cases), over time, that fell into the defined variant groups. Countries were displayed if they had at least 70 sequences for any variant being tracked, over at least 4 weeks. We allotted the most dominant variant, i.e. the one with the highest proportion of the total number of sequences occurring at the mid-study period of each study (month and year), which we calculated in Microsoft Excel.
By study settings
We classified settings as community, health care institution, educational institution, or other. Health facility setting included all studies reporting data from primary, secondary, or tertiary health care facilities including hospitals, COVID-19 isolation/quarantine centres, general practitioner clinics, paediatric clinics including well child visits, intensive care units (ICUs), paediatric intensive care units (PICUs), and health centres delivering specialised care (such as burns centres, oncology centres, etc.). If the setting was mixed or unclear, we classified it as ‘other.’
By testing method
We conducted a subgroup analysis by testing method, i.e. PCR (including any nucleic acid tests), PCR or antigen tests, and antigen tests.
Risk factor analyses
We performed pooled analyses using risk factor data if at least two studies reported data on a particular risk factor. The pooled RRs were calculated with the 95% CI for each factor. Available data enabled us to conduct pooled analysis for age, sex, ethnicity, comorbidities, and pregnancy as risk factors for COVID-19 in people aged ≤18 years. We reported risk ratios for SARS-CoV-2 infection in four age groups (>1 year, 1 to <5 years, 5 to <10 years, and 10 to ≤18 years). However, the data reported in the studies were unstandardised; due to this heterogeneity, we classified the data from overlapping age bands so that they fell in the group where most of their sample can be expected to fall in. We compared the risk of SARS-CoV-2 infection in each of the groups to the other ages in the ≤18 years group. Some included studies also conducted adjusted analyses for several risk factors; in such cases, we were unable to pool the estimates of these adjusted analyses due to a lack of standardised data. Moreover, four studies reported on race or ethnicity; as we were unable to conduct a meta-analysis owing to the nature of these data (i.e. different groups of ethnicities/races reported in each study depending on their populations), we undertook a narrative synthesis for race or ethnicity as a risk for SARS-CoV-2 infection. We calculated the risk of SARS-CoV-2 infection in people having any of the following nine comorbidities: obesity, diabetes, immunosuppression, cardiovascular disease, asthma, epilepsy, renal disease, neurological condition, and congenital cardiac conditions.
Severity of disease
We assessed the severity of COVID-19 in children in two ways. We estimated the clinical management requirements (hospitalisation, ICU admissions, supplemental oxygen, and assisted ventilation) and provided a pooled estimate for the proportion of children (among those testing positive for SARS-CoV-2) requiring each of these. ICU admissions included ICU, PICU, and high dependency unit (HDU) admissions. Oxygen supplementation included all types of supplemental oxygen, including assisted ventilation, if details were unavailable. Assisted ventilation included all types of non-invasive and invasive mechanical ventilation, including continuous positive airway pressure, bilevel positive airway pressure, and tracheal intubation. If studies reported only on assisted ventilation without data on other types of supplemental oxygenation, we included them only for the analysis of assisted ventilation and not in the oxygen supplementation analysis. We included only studies that followed up at least 100 SARS-CoV-2 positive children and adolescents. We excluded studies that recruited only hospitalised children from the analysis of the proportion of children requiring hospitalisation. We could not assess the criteria for hospitalisation, ICU admission, and other requirements in individual studies, which is why we did not conduct further investigations and subgroup analyses based on WHO region or country income levels. As there were limited data on dominant variants and therefore, we did not conduct any statistical tests to explore differences between the effects on severity by variants.
We also assessed severity based on the clinical presentation (asymptomatic, mild, moderate, and severe/ critical disease). When studies combined two groups, we included the children from the combined group in the higher level of severity of the two groups (for example, moderate-severe disease cases were included in severe disease). We combined severe and critical COVID-19 disease as a single group due to overlapping definitions across studies and limited data on critical disease. The definitions of asymptomatic SARS-CoV-2 infections (without any clinical signs or symptoms), mild COVID-19 disease (upper respiratory infections), and moderate COVID-19 disease (non-severe pneumonia) were reasonably consistent across studies. If study authors did not report on the definitions used, these definitions were assumed.
Case fatality ratio
Lastly, we assessed the mortality outcomes in SARS-CoV-2 positive children. We calculated a pooled estimate of the proportion of SARS-CoV-2 positive children with fatal outcomes. The estimate likely included in-hospital and out-of-hospital deaths, as these details were rarely available in individual studies. To increase the confidence in our estimates, we only included studies that followed-up at least 100 SARS-CoV-2 children until the end of the study period or discharge or death (whichever occurred first). If studies differentiated between deaths caused due to COVID-19 and deaths due to other medical reasons in children and adolescents with SARS-CoV-2 infections, we extracted the data for deaths attributable to COVID-19 disease.
RESULTS
We identified 19 050 records through our searches in eight (including three Chinese) academic literature databases. Following the screening process, we included 237 studies in this systematic review [13-249] (Figure 1; Table S11 in the Online Supplementary Document). Our searches did not yield any studies that were entirely conducted in the Omicron era or after Omicron became the dominant variant. (Table S9 in the Online Supplementary Document).
Figure 1.
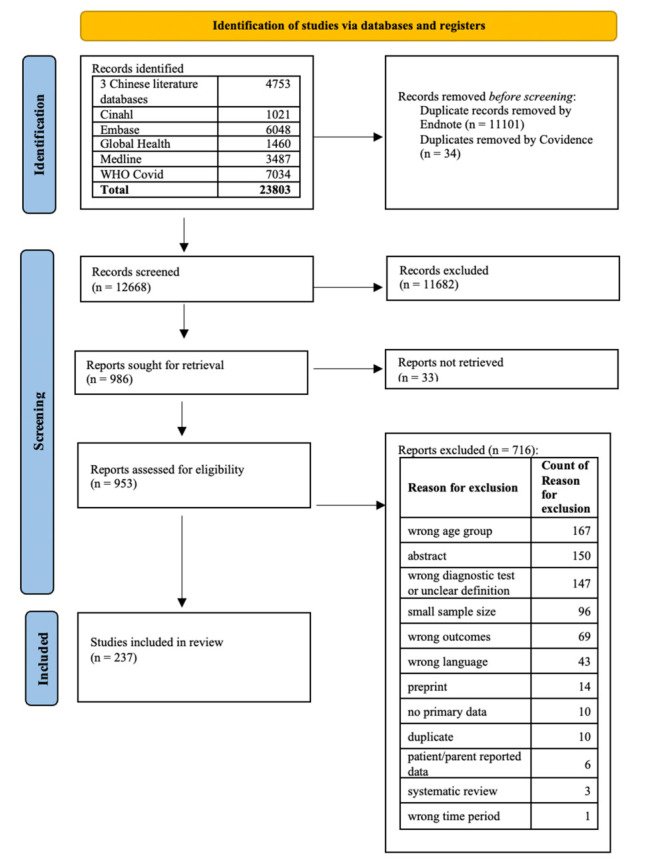
PRISMA flowchart.
Test positivity estimates
Based on 117 studies, we found the test positivity of SARS-CoV-2 to be 9.30% (95% CI = 7.15−11.73). The I2 and τ2 values suggested there was significant heterogeneity across the included study estimates. Three studies [55,88,241] were found to be influential in the estimates. The pooled SARS-CoV-2 test positivity after the exclusion of these studies was calculated as 8.13% (95% CI = 6.45−9.98, I2 = 100%, τ2 = 0.0300). When the analysis was restricted to good-quality studies only, the pooled SARS-CoV-2 test positivity was 9.04% (95% CI = 4.46−15.01, I2 = 99%; τ2 = 0.0172) (Figure S2 and Table S10 in the Online Supplementary Document).
Subgroup analysis of proportion positive estimates
Figure 2 illustrates the findings of the subgroup analyses according to age groups. The results of the subgroup analysis of SARS-CoV-2 proportion positive in children and adolescents by the WHO regions, country income groups, study setting, and testing method are summarised in Table 1 and the plots available in Figures S3−6 in the supplementary materials Online Supplementary Document. The estimates were highest in the African region, middle income countries, health facility settings and in studies employing PCR. The estimates were lowest in the Western Pacific Region, high income countries, educational institutions and studies employing antigen testing.
Figure 2.
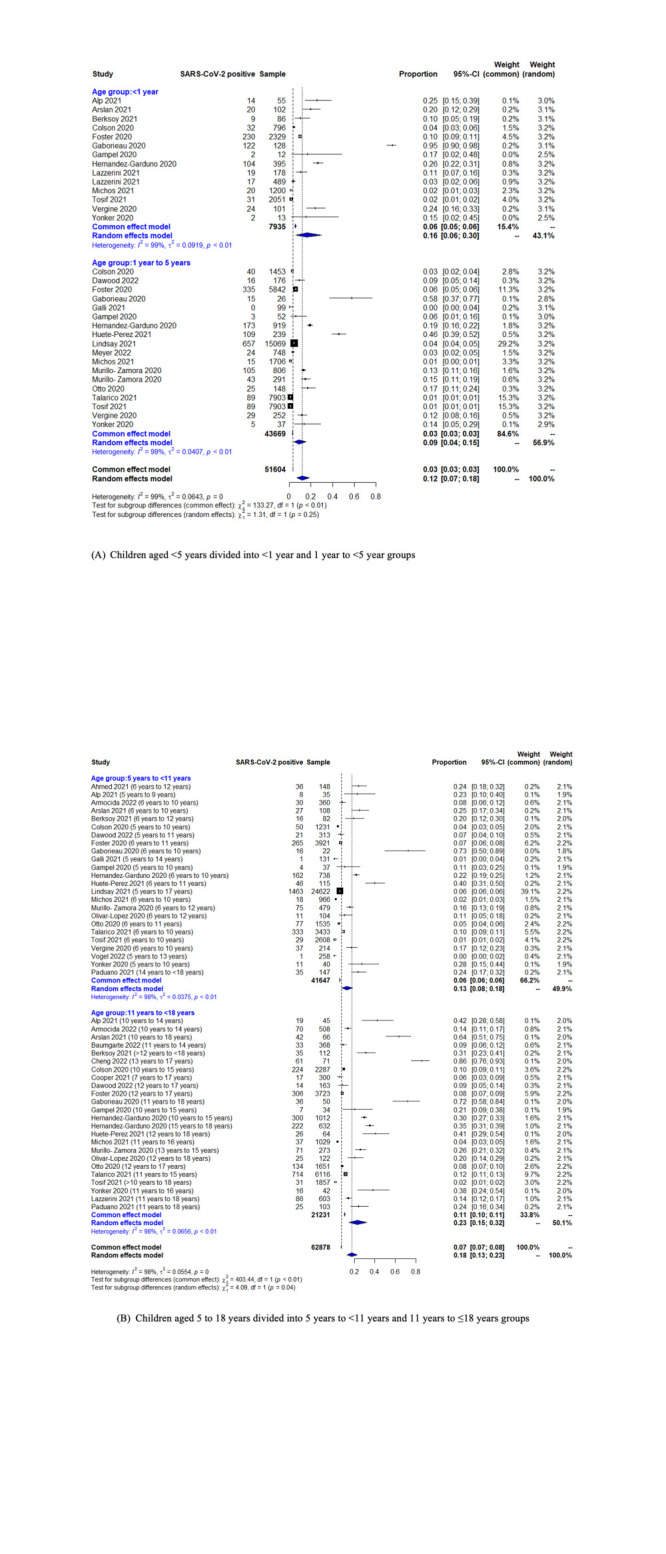
Forest plot for subgroup analysis of SARS-CoV-2 proportion positive in people aged ≤18 years. Panel A. Children aged <5 years divided into <1-year and 1 to <5-year-old groups. Panel B. Children aged 5 to 18 years divided into 5 to <11-year and 11 to ≤18-year-old groups.
Table 1.
Summary of results of subgroup analysis of SARS-CoV-2 test positivity in people aged ≤18 y
| Number of studies | Pooled estimate, (95% CI) | τ2 | |
|---|---|---|---|
|
WHO regions
|
|
|
|
| European region |
62 |
7.83 (5.14−11.01) |
0.0468 |
| Western Pacific region |
11 |
4.57 (0.62−11.71) |
0.0499 |
| Region of the Americas |
28 |
13.28 (7.92−19.76) |
0.0551 |
| Eastern Mediterranean region |
4 |
9.04 (3.08−17.57) |
0.0192 |
| African region |
4 |
14.39 (14.27−14.51) |
0.0000 |
| Southeast Asian region |
6 |
12.67 (7.64−18.73) |
0.0104 |
| Mixed* |
1 |
31.03 (30.15−31.93) |
- |
|
Country income groups
|
|
|
|
| High income |
77 |
5.95 (3.89−8.41) |
0.0443 |
| Upper middle-income |
28 |
17.15 (12.53−22.31) |
0.0300 |
| Lower middle-income |
10 |
15.84 (9.70−23.13) |
0. 0213 |
| Mixed† |
2 |
32.72 (28.94−36.61) |
0.0007 |
|
Study setting
|
|
|
|
| Health facility |
86 |
10.01 (7.24−13.17) |
0.0532 |
| Other setting‡ |
20 |
9.58 (5.85−14.11) |
0.0255 |
| Community |
2 |
9.76 (6.17−14.07) |
0.0019 |
| Educational institution |
9 |
3.38 (0.57−8.20) |
0.0246 |
|
Testing method
|
|
|
|
| PCR |
106 |
9.67 (7.30−12.34)* |
0.0490 |
| PCR or antigen test |
10 |
6.57 (3.02−11.33) |
0.0182 |
| Antigen test | 1 | 3.38 (2.75−4.08) | - |
CI – confidence interval, PCR – polymerase chain reaction, WHO – World Health Organization
*There was one multicentre study [87] which was conducted across more than one WHO region. It was put in the ‘mixed’ group.
†There were two studies [87,232] reporting findings from more than one country and those were from different income groups. They formed a separate ‘mixed’ group.
‡Other setting included those in which children were testing was unclear or it could be expected to have data from more than one setting (health facility, educational institution, and community) This included studies that reported surveillance data or data from testing laboratories. However, the setting in which the children were tested was, however, unclear. Such studies were classified as conducted in other settings [64,65,68,75,89,97,110,129,137,162,171,175,192,196,197,215,218,220,225].
We were unable to assess the effect of different SARS-CoV-2 variants, as there were limited data on the dominant variant in each country at the mid-study period. Most of the studies included in this review were conducted during the early pandemic period. Consequently, we could not provide reliable proportion positive estimates by SARS-CoV-2 variant subgroups (Figure S7 in the Online Supplementary Document.
Risk factors of SARS-CoV-2 infection in children aged ≤18 years
Age
We found the risk of SARS-CoV-2 infection to be lowest in the 1 to <5-year-old group (RR = 0.64; 95% CI = 0.49−0.83) compared with the remaining age groups. This was followed by the 5 to <10-year-old age group (RR = 0.82; 95% CI = 0.69−0.98) and the <1 year age group (RR = 0.88; 95% CI = 0.59−1.31). The risk was highest in those aged between 10 years and ≤18 years (RR = 1.60; 95% CI = 1.23−2.08). Eleven studies [14,22,34,38,50,103,121,143,152,155,200] reported the mean or median age or age range in SARS-CoV-2 positive and SARS-CoV-2 negative children and adolescents. However, the P-value was not reported in all studies. Five studies [33,38,79,134,159] controlled for confounders and reported adjusted risk ratios (Tables S1−3 in the Online Supplementary Document).
Sex
Twenty-one studies [14,22,33,34,50,63,79,103,107,109,121,130,134,143,152,154,155,159,166,175,200] reported data on sex as a risk factor for SARS-CoV-2 infection in children. The results of this meta-analysis showed no evidence of an association between sex and SARS-CoV-2 infection in children aged ≤18 years. Of these 21 studies, four [33,34,79,159] conducted multivariate analyses to estimate the association between SARS-CoV-2 infection and sex after adjusting for other factors (Figure S8 and Table S4 in the Online Supplementary Document).
Ethnicity or race
Six studies [38,63,143,152,200,246] reported data on ethnicity or race as a risk factor for SARS-CoV-2 infection in the ≤18 years population. The findings for non-Hispanic White, Black, and Hispanic groups were variable across studies. The association between being Asian and the risk of SARS-CoV-2 infection was found to be statistically insignificant in all studies reporting data on Asians. However, the RR estimated varied across studies and ranged between 1.15 and 5.10. Some race/ethnicity groups (Secular Jews, Arabs, Ultraorthodox Jews, multiracial non-Latino) were reported by single studies. Three studies were conducted in the United States [38,200,246], and one in Israel [63], Brazil [143] and Spain [152] each (Table S5 in the Online Supplementary Document).
Comorbidities
The findings of the meta-analysis of comorbidities as a risk factor for SARS-CoV-2 infection are presented in Table 2. The results of another analysis that included combined data on at least one of the following comorbidities – obesity, diabetes, immunosuppression, cardiovascular disease, asthma, epilepsy, renal disease, neurologic condition, or congenital heart disease – showed that having any of these comorbidities was a risk factor for SARS-CoV-2 infection (RR = 1.33; 95% CI = 1. −1.71, P < 0.001).
Table 2.
Comorbidities as a risk for SARS-CoV-2 infection
| Comorbidity | Case definitions used | Number of studies included | Total sample size | RR (95% CI) | Direction of association |
|---|---|---|---|---|---|
| Obesity |
Obesity as a comorbidity or obesity based on BMI |
7 |
13 325 |
2.91 (1.68−5.04) |
Risk increase. |
| Diabetes |
Diabetes, diabetes mellitus type 1 |
6 |
13 416 |
2.30 (1.23−4.28) |
Risk increase. |
| Immunosuppression |
Immunosuppressed, immunodeficiency, primary immunodeficiencies, immunosuppressant use, immune thrombocytopenia, and common variable immune deficiency |
6 |
13 509 |
1.14 (0.57−2.30) |
No evidence of association. |
| Cardiovascular disease |
Disease, cardiovascular conditions, and cardiac disease |
5 |
13 538 |
1.07 (0.54−2.13) |
No evidence of association. |
| Asthma |
Includes asthma, asthma or reactive airway disease, and asthma or chronic lung disease |
7 |
300 402 |
0.73 (0.46−1.17) |
No evidence of association. |
| Epilepsy |
|
3 |
608 |
1.90 (1.14−3.17) |
Risk increase. |
| Renal disease |
Chronic renal insufficiency, chronic renal failure, end-stage renal disease, renal disease, kidney-related comorbidity |
4 |
11 285 |
1.04 (0.77−1.40) |
No evidence of association. |
| Neurologic condition |
Malformation, disabilities, neuromuscular diseases, neurological diseases except for epilepsy, neurologic condition, neuromuscular damage, neurological comorbidity |
6 |
3885 |
0.79 (0.41−1.52) |
No evidence of association. |
| Congenital cardiac disease | Any congenital cardiac disease | 3 | 704 | 0.71 (0.34−1.49) | No evidence of association. |
BMI – body mass index, CI – confidence interval, RR – risk ratio
The results of adjusted analyses for some comorbidities were reported by six studies [63,79,103,134,159,200] (Table S6 in the Online Supplementary Document).
Pregnancy
Based on two studies conducted in Mexico with a total sample size of 8297 [31,103] meta-analysis did not show an association of pregnancy with SARS-CoV-2 infection in people aged ≤18 years (RR = 1.19; 95% CI = 0.73−1.92; P = 0.4807).
Severity of SARS-CoV-2 infection in children and adolescents
Severity according to clinical presentation
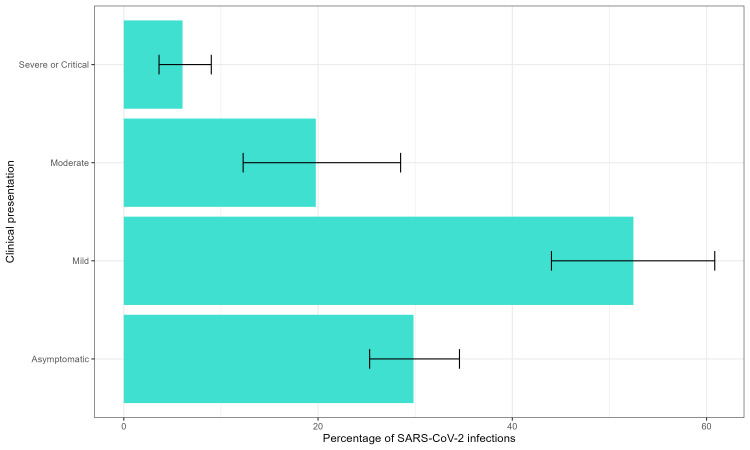
Mild COVID-19 disease was the most common presentation of SARS-CoV-2 infection in those aged ≤18 years (52.47%). Meanwhile, 29.83% children with SARS-CoV-2 infection remained asymptomatic, followed by those presenting with moderate disease (19.77%) and lastly with severe/critical disease (6.05%) (Figure 3). Not all studies reported number of cases with each of these four presentations (asymptomatic, mild, moderate, and severe/critical disease). Therefore, the denominator populations for each of these outcomes are not identical (Figures S9−12, Tables S7−8 in the Online Supplementary Document).
Figure 3.
Severity of SARS-CoV-2 infection in children aged ≤18 years.
Severity according to management requirements
Among those testing positive, the percentage of children requiring hospitalisation was 20.70% (95% CI = 15.04−26.99). In view of oxygen supplementation, our analysis showed that about 7.19% (95% CI = 4.66−10.20) of those testing positive for SARS-CoV-2 required some kind of oxygen supplementation for the management of clinical symptoms. For ICU admission, 4.26% (95% CI = 2.90−5.58) of children and adolescents with SARS-CoV-2 infection required ICU or PICU or HDU admission. Finally, the pooled percentage of children and adolescents with SARS-CoV-2 infections requiring assisted ventilation was 2.92% (95% CI = 1.79−4.30) (Figures S13−16 in the Online Supplementary Document).
Mortality outcomes
The pooled case fatality ratio was 0.87% (95% CI = 0.54−1.28) (Figure S17 in the Online Supplementary Document). Although most of these deaths can be considered as in-hospital deaths, these details, including the follow-up period, were unclear in most studies.
DISCUSSION
We undertook this systematic review and meta-analysis to assess the burden and epidemiology of SARS-CoV-2 infections in people aged ≤18 years to help guide prevention policies and treatment recommendations. Our findings suggest that about 9.30% of children and adolescents tested positive for SARS-CoV-2 infection by PCR or antigen tests in the pre-Omicron era, while around 29.83% of infections were asymptomatic. Likewise, 21 in 100 people who were aged ≤18 years and tested positive for SARS-CoV-2 required hospitalisation, 4 in 100 required ICU admission, and 1 in 100 resulted in death.
Our study showed that the pooled proportion positive estimates were slightly higher in studies only using PCR for diagnosis (9.67%) when compared with all included studies (9.30%). Well-designed large studies and surveillance systems are needed to better understand the true burden of COVID-19 in the paediatric population. Additionally, laboratory testing of SARS-CoV-2 should not be limited to those with symptoms of COVID-19 since at least about one-third of SARS-CoV-2 infected persons remain asymptomatic [250,251]. Without robust data on a representative sample for each country, we will not understand the true magnitude of COVID-19 and transmission patterns at the local and global levels. Importantly, early diagnosis and confirmation of infection will allow individuals to seek health care, therefore reducing disease severity and death.
Variability in sampling strategy and testing policies may have contributed to significant heterogeneity across studies. For example, three influential studies providing unusually high proportions of SARS-CoV-2 positives enrolled individuals within two weeks of exposure to a laboratory-confirmed COVID-19 household contact into the Household Exposure and Respiratory Virus Transmission and Immunity Study (HEARTS) with a convenient recruitment strategy [55] and evaluated a sample of children with a suspicion [241] or high suspicion of COVID-19, respectively [88]. Most studies conducted testing of all children visiting the health centre or educational institution, or individuals having symptoms, or a known contact with a COVID-19 case, or having residence in a geographical area of high COVID-19 incidence.
Our meta-analysis showed that the RR of SARS-CoV-2 positive test was higher in those aged 10 to ≤18 years compared to those <10 years. The number of COVID-19 cases and population by age-groups reported by the UN reflects a similar trend [6]. Globally, males have been shown to be at higher risk of severe COVID-19 disease and adverse outcomes compared to females, yet there does not seem to be strong evidence that suggests that test positivity is significantly higher in males compared to females in individuals of all ages [252]. We also did not detect an association of sex with SARS-CoV-2 test positivity (RR = 0.96; 95% CI = 0.87−1.05) in children and adolescents. Evidence on race/ethnicity as a risk factor for SARS-CoV-2 infections in this population was limited, with wide CIs, while the studies were reported from different countries and thus may not be directly comparable. Moreover, the findings must be interpreted with a caveat, since we did not adjust for demographic factors such as age, sex, deprivation, household size, and underlying health conditions in this analysis. Existing evidence, not limited to the paediatric and adolescent population, indicates that some ethnic communities are disproportionately affected by higher SARS-CoV-2 infection rates and adverse outcomes, and these disparities can often be attributed to pre-existing social inequalities [253]. Adults and children with certain underlying medical conditions are at higher risk of COVID-19 [254]. We found that having at least one of the following comorbidities – obesity, diabetes, immunosuppression, cardiovascular disease, asthma, epilepsy, renal disease, neurologic condition, or congenital heart disease – increased SARS-CoV-2 infection risk by 33% in people aged ≤18 years. In our meta-analysis, we did not observe a statistically significant association between immunosuppression and the risk of SARS-CoV-2 infection. It is plausible that this patient population was likely to have taken more precautions and preventive measures such as shielding, which may have confounded this association. Pregnancy and other comorbidities, like neurological disease, congenital cardiac disease, and renal disease, did not show a statistically significant association with SARS-CoV-2 infection. Like those immunosuppressed patients, pregnant women and patients living with these conditions can be expected to have had a heightened degree of precaution and better compliance to non-pharmacological interventions, particularly in the early pandemic period, when most of the data were collected. Thus, population behaviour factors that were beyond the scope of this systematic review may have confounded this association.
We estimated that about 20.70% of people aged ≤18 years who were positive for SARS-CoV-2 required admission to the hospital, but only 6.05% had severe or critical disease. This suggests that the criteria for hospitalisation were likely influenced by several other factors apart from the severity of COVID-19 disease. Importantly, criteria for hospitalisation were highly heterogeneous in different countries and changed over time. This discrepancy could be attributed to the fact that most countries adopted a very cautious approach at the beginning of the pandemic and set guidelines to hospitalise patients for monitoring and control of infection (isolation) rather than as a requirement for the management of the disease. Besides, we did not explore if the necessity for hospitalisation, ICU admission, oxygen supplementation, and mechanical ventilation was primarily driven by COVID-19 disease or other factors such as underlying health conditions. Therefore, these estimates should not be interpreted as hospitalisation, ICU admission, oxygen supplementation, and mechanical ventilation attributable to COVID-19 disease severity and are likely overestimates. Our findings suggest that about 0.87% of SARS-CoV-2 positive children had fatal outcomes. Children and adolescents with severe and critical disease are more likely to die than those with asymptomatic infection or mild and moderate COVID-19 disease. Only 35 studies reported the number of children and adolescents progressing to critical disease, and it may be expected that these studies were conducted in tertiary health facilities that have equipment and manpower to manage critically ill patients. For studies conducted in other settings, like communities, educational institutions, or primary health facilities, the case fatality ratio, requirement of oxygen supplementation, ICU admission, and assisted ventilation can be expected to be lower, as it is unlikely to have people with critical COVID-19 in these settings. It remains to be seen how the case fatality ratio changes according to disease severity and it would be of particular interest to see the disease severity estimates and death in children with specific underlying conditions. Again, most studies reported the case fatality ratios rather than the disease-attributable case fatality ratios. The estimate is, therefore, likely an overestimate of the true mortality estimate.
Our test-positive estimate of 9.30% is higher than the estimate provided by Jain et al. [255] for influenza in the same population (7.00%) between 2010 and 2012. Their estimates for respiratory syncytial virus, meanwhile, were higher (28.00%) compared to our estimates for SARS-Cov-2 in children aged ≤18 years. However, their study population was restricted to hospitalised children with a specific case definition of acute respiratory infections with radiologic evidence of pneumonia. One would expect higher chances of detection of respiratory virus in such populations presenting with acute respiratory illnesses and evidence of pneumonia as compared to our study population, which also included asymptomatic people who may or may not have had contact with a SARS-CoV-2 positive individual. Our study population was not restricted to those with acute respiratory illnesses, as we also wanted to estimate the proportion of cases with asymptomatic and mild disease presentation. The pooled proportion positive for influenza among hospitalised children and adolescents aged ≤18 years with respiratory illness was reported to be 9.50% (95% CI = 8.10−11.00) between 1982 and 2012 in another study [256]. Although we did not conduct a subgroup analysis for hospitalised patients, our subgroup analysis by study setting showed comparable estimates (10.01%; 95% CI = 7.24−13.17) in studies conducted in health facility settings (which also included outpatients). Considering the risk of COVID-19 is comparable to or higher than influenza, routine COVID-19 vaccination may need to be considered in children and adolescents, at risk of severe illness, as has been done in the case of influenza; moreover, COVID-19 vaccines such as mRNA vaccines have been shown to reduce both infection and severe outcomes such as hospitalisation and death in the paediatric population and have been demonstrated to be safe [257].
This is, to the best of our knowledge, the first systematic review to assess the global data on SARS-CoV-2 infections in children and adolescents. The study was guided by PRISMA-P guidelines and followed a predetermined protocol. We adopted a sensitive search strategy to retrieve relevant publications. However, our review has some limitations. We adopted a single reviewer screening approach that can lead to bias and increase the chance of human error. The differences in testing criteria and selection for inclusion of participants in individual studies led to very high heterogeneity in the included study estimates (I2 = 100%, τ2 = 0 · 0460 for the test positivity estimates). We were unable to provide reliable estimates for subgroup analysis by SARS-CoV-2 variant, as most studies were conducted before reliable and large-scale genomic sequencing data became available. We did not explore the effect of non-pharmaceutical interventions or COVID-19 vaccination in children on our estimates. The vaccination policies were, again, highly variable across countries, including the type of vaccine, age criteria and the time of start of vaccination programmes. Lastly, we included no studies conducted in low-income countries in the proportion positive analysis. The two studies that were conducted across multiple countries also reported data either from high- or middle-income countries. This paucity of data also translates into vaccine coverage in low-income countries, as 69.20% of the global population had received at least one dose of the COVID-19 vaccine vs only 25.90% of people living in low-income countries as of 18 January 2023 [258]. Investment in research and surveillance activities in low-income countries is, therefore, warranted to help inform vaccination and other prevention policies and management strategies.
There were limited and unstandardised data on the long-term sequelae or complications of COVID-19 disease, so we did not undertake a synthesis for these outcomes. The portrayal of the burden of disease is incomplete without estimating the long-term sequelae or the incidence of long COVID-19 in this age group which seems to be more common than previously believed. This is a critical factor in comparing the risks and benefits of vaccinations, particularly because presentation with mild disease is very common in this age group [259]. Our analysis does not consider the impact of vaccination policies or non-pharmacological interventions and other preventive measures that were implemented or relaxed by different countries at different times and that had a likely impact on the burden of disease.
CONCLUSIONS
The detection of SARS-CoV-2 in children is not uncommon. Although many of the paediatric cases presented with asymptomatic or mild forms of disease with complete recovery, there is a considerable proportion of those requiring hospitalisation, oxygen support, and ICU admission. Moreover, it is essential to systematically investigate the long-term sequelae or incidence of long COVID-19 in this population and assess the differential impact of SARS-CoV-2 variants. Given the importance of COVID-19 in this population, more investment in research, diagnostics, treatment, and vaccines for this population is warranted.
Additional material
Acknowledgements
We thank Karen Hartnup (University of Edinburgh) for her feedback and assistance in developing and executing the searches in the English language literature databases.
Data availability: The study data will be made freely available on Edinburgh DataShare at the time of submission of the manuscript.
Footnotes
Funding: This research was funded by Pfizer.
Authorship contributions: MHK and HN conceived the study idea and supervised the study. KA, JS, AS, MHK, HN, DK, and ER contributed to the study protocol. DK, FZ, KA, and JS screened literature for inclusion in the review. DK, FZ, NFI, KA, and JS conducted data extraction and quality appraisal of included studies. DK conducted the statistical analysis with significant contributions from all team members. DK wrote the first draft of the manuscript. All authors provided critical revisions to the manuscript and approved the final version.
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and disclose the following activities and/or relationships: DK reports consulting fees from Pfizer, related to the submitted work and outside the submitted work. XW received research grants from GlaxoSmithKline, Wellcome Trust, and Nanjing Medical University, outside the submitted work, and consultancy fees from Pfizer, related to the submitted work. AS, JS, and MHK are employees of Pfizer. AS was a Pfizer employee during study conduct and manuscript development and may hold stock options; he is currently employed by Orbital Therapeutics. HN reports consulting fees from Pfizer, related to the submitted work; and grants from the Innovative Medicines Initiative outside the submitted work; consulting fees from the Gates Foundation, Pfizer, and Sanofi; honoraria from AbbVie; support from Sanofi for attending meetings; and participation on advisory boards from GSK, Merck, Pfizer, Sanofi, Icosavax, Janssen, Novavax, Reviral, Resvinet, and WHO outside the submitted work.
REFERENCES
- 1.Cucinotta D, Vanelli M.WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2021. Available: https://covid19.who.int/. Accessed: 5 February 2024.
- 3.World Health Organization. Coronavirus disease (COVID-19): Variants of SARS-COV-2. 2021. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-%28covid-19%29-variants-of-sars-cov-2?gclid=EAIaIQobChMI7LeX1Y_P_AIVR4BQBh02QA1VEAAYASAAEgI2rfD_BwE. Accessed: 5 February 2024.
- 4.United Nations Children’s Fund. Convention on the Rights of the Child. 1989. Available: https://www.ohchr.org/en/instruments-mechanisms/instruments/convention-rights-child#:~:text=PART%20I-,Article%201,child%2C%20majority%20is%20attained%20earlier. Accessed: 5 February 2024.
- 5.United Nations Children’s Fund. Investing in a safe, healthy and productive transition from childhood to adulthood is critical. 2022. Available: https://data.unicef.org/topic/adolescents/overview/. Accessed: 5 February 2024.
- 6.United Nations Children’s Fund. COVID-19 confirmed cases and deaths, age- and sex-disaggregated data. 2022. Available: https://data.unicef.org/resources/covid-19-confirmed-cases-and-deaths-dashboard/. Accessed: 5 February 2024.
- 7.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joanna Briggs Institute. Critical appraisal tools. Available: https://jbi.global/critical-appraisal-tools. Accessed: 5 February 2024.
- 9.DerSimonian R, Kacker R.Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–14. 10.1016/j.cct.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Countries. 2023. Available: https://www.who.int/countries. Accessed: 5 February 2024.
- 11.The World Bank. World Bank Country and Lending Groups. 2022. Available: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed: 5 February 2024.
- 12.Hodcroft EB. Overview of Variants in Countries. 2021. Available: https://covariants.org/per-country. Accessed: 5 February 2024.
- 13.Abo YN, Clifford V, Lee LY, Costa AM, Crawford N, Wurzel D, et al. COVID-19 public health measures and respiratory viruses in children in Melbourne. J Paediatr Child Health. 2021;57:1886–92. 10.1111/jpc.15601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed GK, Elbeh K, Gomaa HM, Soliman S.Does COVID-19 infection have an impact on children’s psychological problems? Middle East Current Psychiatry. 2021;28:77. 10.1186/s43045-021-00155-z [DOI] [Google Scholar]
- 15.Aizawa Y, Shobugawa Y, Tomiyama N, Nakayama H, Takahashi M, Yanagiya J, et al. Coronavirus Disease 2019 Cluster Originating in a Primary School Teachers’ Room in Japan. Pediatr Infect Dis J. 2021;40:e418. 10.1097/INF.0000000000003292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akkoç G, Akgün Ö, Kızılırmak C, Yıldız F, Duru HNS, Elevli M.Demographic and Clinical Characteristics of COVID-19 in Children and the Effect of Household Tobacco Smoke Exposure on COVID-19. Turk Arch Pediatr. 2021;56:322. 10.5152/TurkArchPediatr.2021.20226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alattas B, Azaz A, Rawat D, Miqdady M, Bitar R.Clinical manifestations and outcome in children with COVID-19 infection in Abu Dhabi: a retrospective single-centre study. BMJ Paediatr Open. 2021;5:e001219. 10.1136/bmjpo-2021-001219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AlGhamdi A, Al Talhi Y, Al Najjar A, Sobhi A, Al Juaid A, Ibrahim A, et al. Epidemiology, clinical characteristics and risk factors of COVID-19 among children in Saudi Arabia: a multicenter chart review study. BMC Pediatr. 2022;22:86. 10.1186/s12887-021-02959-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alharbi M, Kazzaz YM, Hameed T, Alqanatish J, Alkhalaf H, Alsadoon A, et al. SARS-CoV-2 infection in children, clinical characteristics, diagnostic findings and therapeutic interventions at a tertiary care center in Riyadh, Saudi Arabia. J Infect Public Health. 2021;14:446–53. 10.1016/j.jiph.2020.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almuzaini Y, Alsohime F, Al Subaie S, Temsah MH, Alsofayan Y, Alamri F, et al. Clinical profiles associated with SARS-CoV-2 infection and complications from coronavirus disease-2019 in children from a national registry in Saudi Arabia. Ann Thorac Med. 2021;16:280. 10.4103/atm.atm_709_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alonso GT, Ebekozien O, Gallagher MP, Rompicherla S, Lyons SK, Choudhary A, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13:681–7. 10.1111/1753-0407.13184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alp EE, Dalgic N, Yilmaz V, Altuntas Y, Ozdemir HM.Evaluation of patients with suspicion of COVID-19 in pediatric emergency department. The Medical Bulletin of Sisli Etfal Hospital. 2021;55:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alqayoudhi A, Al Manji A, Al khalili S, Al Maani A, Alkindi H, Alyaquobi F, et al. The role of children and adolescents in the transmission of SARS-CoV-2 virus within family clusters: A large population study from Oman. J Infect Public Health. 2021;14:1590–4. 10.1016/j.jiph.2021.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alsharrah D, Alhaddad F, Alyaseen M, Aljamaan S, Almutairi N, Ayed M, et al. Clinical characteristics of pediatric SARS-CoV-2 infection and coronavirus disease 2019 (COVID-19) in Kuwait. J Med Virol. 2021;93:3246–50. 10.1002/jmv.26684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alshengeti A, Alahmadi H, Barnawi A, Alfuraydi N, Alawfi A, Al-Ahmadi A, et al. Epidemiology, clinical features, and outcomes of coronavirus disease among children in Al-Madinah, Saudi Arabia: A retrospective study. Int J Pediatr Adolesc Med. 2022;9:136–42. 10.1016/j.ijpam.2021.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alshukairi AN, Doar H, Al-Sagheir A, Bahasan MA, Sultan AA, Al Hroub MK, et al. Outcome of COVID19 in patients with Osteogenesis Imperfecta: A retrospective multicenter study in Saudi Arabia. Front Endocrinol (Lausanne). 2022;12:800376. 10.3389/fendo.2021.800376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ana Laura GO, Abraham Josué NR, Briceida LM, Israel PO, Tania AF, Nancy MR, et al. Sensitivity of the Molecular Test in Saliva for Detection of COVID-19 in Pediatric Patients With Concurrent Conditions. Front Pediatr. 2021;9:642781. 10.3389/fped.2021.642781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antúnez-Montes OY, Escamilla MI, Figueroa-Uribe AF, Arteaga-Menchaca E, Lavariega-Saráchaga M, Salcedo-Lozada P, et al. COVID-19 and multisystem inflammatory syndrome in Latin American children: a multinational study. Pediatr Infect Dis J. 2021;40:e1–6. 10.1097/INF.0000000000002949 [DOI] [PubMed] [Google Scholar]
- 29.Anugulruengkitt S, Teeraananchai S, Chantasrisawad N, Promsena P, Jantarabenjakul W, Puthanakit T.Clinical outcomes of pediatric COVID-19 in a tertiary care center in Bangkok, Thailand. IJID Reg. 2021;1:159–62. 10.1016/j.ijregi.2021.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aprea V, Debaisi GE, Guedes V, Guglielmo MC, Miño L, Stabilito LA, et al. Out-of-hospital care setting in a Febrile Emergency Unit of suspected Covid 19 patients. Andes Pediatr. 2021;92:677–82. 10.32641/andespediatr.v92i5.3422 [DOI] [PubMed] [Google Scholar]
- 31.Arellano-Llamas AA, Hernández-Caballero Á.[COVID-19 in Mexican children and adolescents until May 10th, 2020. A focus on patients with diabetes]. Rev Mex Endocrinol Metab Nutr. 2020;7:80–6. Spanish. [Google Scholar]
- 32.Arivoli Kaliyan SP, Prasanna S, Thirumalaikumarasamy S, Ethirajan N, Thandavarayan M, Jeganathan S, et al. Clinical and Epidemiological Profile of Children with COVID-19 in a Tertiary Care Centre in Tamil Nadu, India. J Clin Diagn Res. 2021;15:SC10–4. [Google Scholar]
- 33.Armocida B, Zamagni G, Magni E, Monasta L, Comar M, Zanotta N, et al. Clinical, anamnestic, and sociodemographic predictors of positive SARS-CoV-2 testing in children: A cross sectional study in a tertiary hospital in Italy. PLoS One. 2022;17:e0262923. 10.1371/journal.pone.0262923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arslan G, Aktürk H, Duman M.Clinical characteristics of pediatric coronavirus disease 2019 and predictors of polymerase chain reaction positivity. Pediatr Int. 2021;63:1055–61. 10.1111/ped.14602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asseri AA, Alzaydani I, Al-Jarie A, Albishri A, Alsabaani A, Almaghrabi MK, et al. Clinical characteristics and laboratory abnormalities of hospitalized and critically ill children with coronavirus disease 2019: a Retrospective Study from Saudi Arabia. Int J Gen Med. 2021;14:1949. 10.2147/IJGM.S311831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayed A, Embaireeg A, Benawadh A, Al-Fouzan W, Hammoud M, Al-Hathal M, et al. Maternal and perinatal characteristics and outcomes of pregnancies complicated with COVID-19 in Kuwait. BMC Pregnancy Childbirth. 2020;20:754. 10.1186/s12884-020-03461-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai Y, Gao L, Wang X, Zhong L, Li J, Ding S, et al. Epidemiological characteristics and clinical manifestations of pediatric patients with COVID-19 in China: A multicenter retrospective study. Pediatr Investig. 2021;5:203–10. 10.1002/ped4.12282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bandi S, Nevid MZ, Mahdavinia M.African American children are at higher risk of COVID-19 infection. Pediatr Allergy Immunol. 2020;31:861–4. 10.1111/pai.13298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrera CM, Hazell M, Chamberlain AT, Gandhi NR, Onwubiko U, Liu CY, et al. Retrospective cohort study of COVID-19 among children in Fulton County, Georgia, March 2020–June 2021. BMJ Paediatr Open. 2021;5:e001223. 10.1136/bmjpo-2021-001223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumgarte S, Hartkopf F, Hölzer M, von Kleist M, Neitz S, Kriegel M, et al. Investigation of a Limited but Explosive COVID-19 Outbreak in a German Secondary School. Viruses. 2022;14:87. 10.3390/v14010087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bayesheva D, Boranbayeva R, Turdalina B, Fakhradiyev I, Saliev T, Tanabayeva S, et al. COVID-19 in the paediatric population of Kazakhstan. Paediatr Int Child Health. 2021;41:76–82. 10.1080/20469047.2020.1857101 [DOI] [PubMed] [Google Scholar]
- 42.Bellino S, Punzo O, Rota MC, Del Manso M, Urdiales AM, Andrianou X, et al. COVID-19 disease severity risk factors for pediatric patients in Italy. Pediatrics. 2020;146:e2020009399. 10.1542/peds.2020-009399 [DOI] [PubMed] [Google Scholar]
- 43.Berksoy E, Kanik A, Cicek A, Bardak Ş, Elibol P, Demir G, et al. Clinical and laboratory characteristics of children with SARS-CoV-2 infection. Pediatr Pulmonol. 2021;56:3674–81. 10.1002/ppul.25654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Besli GE, Demir SÖ, Girit S, Arman T, Duyu M, Arslanoglu S.COVID-19 in children: a single center experience from Istanbul, Turkey. Medical Journal of Bakirkoy. 2021;17:64–71. [Google Scholar]
- 45.Biko DM, Ramirez-Suarez KI, Barrera CA, Banerjee A, Matsubara D, Kaplan SL, et al. Imaging of children with COVID-19: experience from a tertiary children’s hospital in the United States. Pediatr Radiol. 2021;51:239–47. 10.1007/s00247-020-04830-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolaños-Almeida CE, Espitia Segura OM.Clinical and Epidemiologic Analysis of COVID-19 Children Cases in Colombia PEDIACOVID. Pediatr Infect Dis J. 2021;40:e7–11. 10.1097/INF.0000000000002952 [DOI] [PubMed] [Google Scholar]
- 47.Brotons P, Jordan I, Bassat Q, Henares D, Fernandez de Sevilla M, Ajanovic S, et al. The positive rhinovirus/enterovirus detection and SARS-CoV-2 persistence beyond the acute infection phase: an intra-household surveillance study. Viruses. 2021;13:1598. 10.3390/v13081598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buonsenso D, Munblit D, De Rose C, Sinatti D, Ricchiuto A, Carfi A, et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110:2208–11. 10.1111/apa.15870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buta G, Cojocaru S, Costru T, Puia R, Abdusa-Ganea D, Ungureanu A. Clinical-Epidemiological Characteristics of Children Hospitalized with COVID-19 in the Republic of Moldova. In: Tignyanu I, Sontea V, Railean S, editors. 5th International Conference on Nanotechnologies and Biomedical Engineering. Proceedings of ICNBME-2021; 2021 November 3-5. Chisinau, Moldova. Cham: Springer International Publishing; 2022. p. 706-711. [Google Scholar]
- 50.Calvani M, Cantiello G, Cavani M, Lacorte E, Mariani B, Panetta V, et al. Reasons for SARS-CoV-2 infection in children and their role in the transmission of infection according to age: a case-control study. Ital J Pediatr. 2021;47:193. 10.1186/s13052-021-01141-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Capozza M, Salvatore S, Baldassarre ME, Inting S, Panza R, Fanelli M, et al. Perinatal Transmission and Outcome of Neonates Born to SARS-CoV-2-Positive Mothers: The Experience of 2 Highly Endemic Italian Regions. Neonatology. 2021;118:665–71. 10.1159/000518060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Capra DR, Ucha G, Antoni E, Malfetano A, Wolfsteiner N, Arias P, et al. Experience at the Department of Pediatrics of a private facility in the Metropolitan Area of Buenos Aires during the COVID-19 pandemic. Arch Argent Pediatr. 2021;119:310–6. [DOI] [PubMed] [Google Scholar]
- 53.Carrasco I, Muñoz-Chapuli M, Vigil-Vázquez S, Aguilera-Alonso D, Hernández C, Sánchez-Sánchez C, et al. SARS-COV-2 infection in pregnant women and newborns in a Spanish cohort (GESNEO-COVID) during the first wave. BMC Pregnancy Childbirth. 2021;21:326. 10.1186/s12884-021-03784-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Çelik B, Doğan M, İnan DB, Sunkak S, Saatçi E, Tubaş F.Demographic and laboratory findings of symptomatic and asymptomatic COVID-19 in children. Guncel Pediatri. 2021;19:280–4. 10.4274/jcp.2021.47640 [DOI] [Google Scholar]
- 55.Cheng WA, Turner L, Marentes Ruiz CJ, Tanaka ML, Congrave-Wilson Z, Lee Y, et al. Clinical manifestations of COVID-19 differ by age and obesity status. Influenza Other Respir Viruses. 2022;16:255–64. 10.1111/irv.12918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiang C-Y, Ellwood P, Ellwood E, García-Marcos L, Masekela R, Asher I, et al. Infection with SARS-CoV-2 among children with asthma: evidence from Global Asthma Network. Pediatr Allergy Immunol. 2022;33:e13709. 10.1111/pai.13709 [DOI] [PubMed] [Google Scholar]
- 57.Chopra S, Saha A, Kumar V, Thakur A, Pemde H, Kapoor D, et al. Acute Kidney Injury in Hospitalized Children with COVID19. J Trop Pediatr. 2021;67:fmab037. 10.1093/tropej/fmab037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chowdhoury SR, Biswas HS, Raut S, Bhakta S, Roy A, Banerjee S, et al. Pediatric Oncology Patients and COVID-19: An Experience from the Tertiary COVID Care Facility in Eastern India: A Prospective Observational Study. Indian J Med Paediatr Oncol. 2021;42:130–4. 10.1055/s-0041-1732814 [DOI] [Google Scholar]
- 59.Chua GT, Wong JSC, Lam I, Ho PPK, Chan WH, Yau FYS, et al. Clinical Characteristics and Transmission of COVID-19 in Children and Youths During 3 Waves of Outbreaks in Hong Kong. JAMA Netw Open. 2021;4:e218824. 10.1001/jamanetworkopen.2021.8824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ciofi Degli Atti ML, Campana A, Muda AO, Concato C, Ravà L, Ricotta L, et al. Facing SARS-CoV-2 Pandemic at a COVID-19 Regional Children’s Hospital in Italy. Pediatr Infect Dis J. 2020;39:e221–5. 10.1097/INF.0000000000002811 [DOI] [PubMed] [Google Scholar]
- 61.Cloete J, Kruger A, Masha M, du Plessis NM, Mawela D, Tshukudu M, et al. Paediatric hospitalisations due to COVID-19 during the first SARS-CoV-2 omicron (B. 1.1. 529) variant wave in South Africa: a multicentre observational study. Lancet Child Adolesc Health. 2022;6:294–302. 10.1016/S2352-4642(22)00027-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cofré F, Mackenney J, Poli C, Riquelme M, Carvajal C, Álvarez P, et al. [Clinical manifestations of SARS-CoV-2 infection in children in the middle of pandemic season in a pediatric tertiary center. Report of local COVID Clinical Committee, Hospital de Niños Roberto del Río, Santiago Chile]. Rev Chilena Infectol. 2020;37:756–61. Spanish. [DOI] [PubMed] [Google Scholar]
- 63.Cohen HA, Gerstein M, Yaniv N, Richenberg Y, Jacobson E, Marton S, et al. Attention-Deficit/Hyperactivity Disorder as a Risk Factor for COVID-19 Infection. J Atten Disord. 2022;26:985–90. 10.1177/10870547211044217 [DOI] [PubMed] [Google Scholar]
- 64.Colson P, Esteves-Vieira V, Giraud-Gatineau A, Zandotti C, Filosa V, Chaudet H, et al. Temporal and age distributions of SARS-CoV-2 and other coronaviruses, southeastern France. Int J Infect Dis. 2020;101:121–5. 10.1016/j.ijid.2020.09.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colson P, Tissot-Dupont H, Morand A, Boschi C, Ninove L, Esteves-Vieira V, et al. Children account for a small proportion of diagnoses of SARS-CoV-2 infection and do not exhibit greater viral loads than adults. Eur J Clin Microbiol Infect Dis. 2020;39:1983–7. 10.1007/s10096-020-03900-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cooper DM, Zulu MZ, Jankeel A, Ibraim IC, Ardo J, Kasper K, et al. SARS-CoV-2 acquisition and immune pathogenesis among school-aged learners in four diverse schools. Pediatr Res. 2021;90:1073–80. 10.1038/s41390-021-01660-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corso MCM, Soares VJ, Amorim AMP, Cipolotti R, Magalhães IMQ, Lins MM, et al. SARS-CoV-2 in children with cancer in Brazil: Results of a multicenter national registry. Pediatr Blood Cancer. 2021;68:e29223. 10.1002/pbc.29223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dash GC, Subhadra S, Turuk J, Parai D, Rout UK, Rath S, et al. COVID-19 in children in Odisha state, India: a retrospective review. BMJ Paediatr Open. 2021;5:e001284. 10.1136/bmjpo-2021-001284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dawood FS, Porucznik CA, Veguilla V, Stanford JB, Duque J, Rolfes MA, et al. Incidence Rates, Household Infection Risk, and Clinical Characteristics of SARS-CoV-2 Infection Among Children and Adults in Utah and New York City, New York. JAMA Pediatr. 2022;176:59–67. 10.1001/jamapediatrics.2021.4217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Lusignan S, Dorward J, Correa A, Jones N, Akinyemi O, Amirthalingam G, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20:1034–42. 10.1016/S1473-3099(20)30371-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Devrim İ, Böncüoğlu E, Kıymet E, Şahinkaya Ş, Çelebi MY, Cem E, et al. Comparison of the pediatric hospitalizations due to COVID-19 and H1N1pdm09 virus infections during the pandemic period. J Med Virol. 2022;94:2055–9. 10.1002/jmv.27589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dilber B, Aydın ZGG, Yeşilbaş O, Sağ E, Aksoy NK, Gündoğmuş F, et al. Neurological Manifestations of Pediatric Acute COVID Infections: A Single Center Experience. J Trop Pediatr. 2021;67:fmab062. 10.1093/tropej/fmab062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Domínguez Rojas J, Estupiñan Vigil M, Garcés-Ghilardi R, Alvarado-Gamarra G, Del Águila O, Lope Tenorio AF, et al. [Cross-sectional study of the clinical characteristics and outcomes of children hospitalized with COVID-19 in Lima, Peru]. Medwave. 2021;21:e8107. Spanish. 10.5867/medwave.2021.01.8107 [DOI] [PubMed] [Google Scholar]
- 74.Domínguez-Rodríguez S, Villaverde S, Sanz-Santaeufemia FJ, Grasa C, Soriano-Arandes A, Saavedra-Lozano J, et al. A Bayesian Model to Predict COVID-19 Severity in Children. Pediatr Infect Dis J. 2021;40:e287–93. 10.1097/INF.0000000000003204 [DOI] [PubMed] [Google Scholar]
- 75.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020;145:e20200702. 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 76.Du H, Dong X, Zhang J-j, Cao Y-y, Akdis M, Huang P-q, et al. Clinical characteristics of 182 pediatric COVID-19 patients with different severities and allergic status. Allergy. 2021;76:510–32. 10.1111/all.14452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eleftheriou I, Dasoula F, Dimopoulou D, Lebessi E, Serafi E, Spyridis N, et al. Real-life evaluation of a COVID-19 rapid antigen detection test in hospitalized children. J Med Virol. 2021;93:6040–4. 10.1002/jmv.27149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elghoudi A, Aldhanhani H, Ghatasheh G, Sharif E, Narchi H.Covid-19 in Children and Young Adolescents in Al Ain, United Arab Emirates- a Retrospective Cross-Sectional Study. Front Pediatr. 2021;8:603741. 10.3389/fped.2020.603741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Söbü E, Karaaslan A, Çetin C, Yasemin A.Vitamin D levels of COVID-19 positive sypmtomatic pediatric cases. Güncel Pediatri. 2021;19:9–14. 10.4274/jcp.2021.0002 [DOI] [Google Scholar]
- 80.Engels G, Sack J, Weissbrich B, Hartmann K, Knies K, Härtel C, et al. Very Low Incidence of SARS-CoV-2, Influenza and RSV but High Incidence of Rhino-, Adeno- and Endemic Coronaviruses in Children With Acute Respiratory Infection in Primary Care Pediatric Practices During the Second and Third Wave of the SARS-CoV-2 Pandemic. Pediatr Infect Dis J. 2022;41:e146–8. 10.1097/INF.0000000000003460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ennab F, ElSaban M, Khalaf E, Tabatabaei H, Khamis AH, Devi BR, et al. Clinical Characteristics of Children With COVID-19 in the United Arab Emirates: Cross-sectional Multicenter Study. JMIR Pediatr Parent. 2021;4:e29049. 10.2196/29049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ergenc Z, Kepenekli E, Çetin E, Ersoy A, Korkmaz B, Selçik R, et al. Incidence of multisystem inflammatory syndrome in children and the comorbidity scores in pediatric coronavirus disease 2019 cases. Pediatr Int. 2022;64:e15084. 10.1111/ped.15084 [DOI] [PubMed] [Google Scholar]
- 83.Erturk A, Demir S, Oztorun Cİ, Erten EE, Guney D, Bostanci SA, et al. Management of a Pediatric Burn Center During the Covid-19 Pandemic. J Burn Care Res. 2022;43:468–73. 10.1093/jbcr/irab137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferraro D, Arias AP, Pérez G, Gómez S, Deschutter V, Highton E, et al. [Epidemiological characteristics according to the progress of SARS-CoV-2 pandemic in a highly complex pediatric hospital in Argentina: a descriptive study]. Rev Chilena Infectol. 2021;38:506–11. Spanish. 10.4067/S0716-10182021000400506 [DOI] [PubMed] [Google Scholar]
- 85.Forster J, Streng A, Rudolph P, Rücker V, Wallstabe J, Timme S, et al. Feasibility of SARS-CoV-2 Surveillance Testing Among Children and Childcare Workers at German Day Care Centers: A Nonrandomized Controlled Trial. JAMA Netw Open. 2022;5:e2142057. 10.1001/jamanetworkopen.2021.42057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Foster CE, Marquez L, Davis AL, Tocco E, Koy TH, Dunn J, et al. A Surge in Pediatric Coronavirus Disease 2019 Cases: The Experience of Texas Children’s Hospital From March to June 2020. J Pediatric Infect Dis Soc. 2021;10:593–8. 10.1093/jpids/piaa164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Funk AL, Florin TA, Kuppermann N, Tancredi DJ, Xie J, Kim K, et al. Outcomes of SARS-CoV-2–Positive Youths Tested in Emergency Departments: The Global PERN–COVID-19 Study. JAMA Netw Open. 2022;5:e2142322. 10.1001/jamanetworkopen.2021.42322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gaborieau L, Delestrain C, Bensaid P, Vizeneux A, Blanc P, Garraffo A, et al. Epidemiology and Clinical Presentation of Children Hospitalized with SARS-CoV-2 Infection in Suburbs of Paris. J Clin Med. 2020;9:2227. 10.3390/jcm9072227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Galli C, Pellegrinelli L, Bubba L, Primache V, Anselmi G, Delbue S, et al. When the COVID-19 Pandemic Surges during Influenza Season: Lessons Learnt from the Sentinel Laboratory-Based Surveillance of Influenza-Like Illness in Lombardy during the 2019–2020 Season. Viruses. 2021;13:695. 10.3390/v13040695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gampel B, Troullioud Lucas AG, Broglie L, Gartrell-Corrado RD, Lee MT, Levine J, et al. COVID-19 disease in New York City pediatric hematology and oncology patients. Pediatr Blood Cancer. 2020;67:e28420. 10.1002/pbc.28420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garazzino S, Montagnani C, Donà D, Meini A, Felici E, Vergine G, et al. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as at 10 April 2020. Euro Surveill. 2020;25:2000600. 10.2807/1560-7917.ES.2020.25.18.2000600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gavriliu L-C, Murariu C, Potop V, Spătaru R.Characteristics of the pediatric patients diagnosed with SARS-CoV-2 infection in a Romanian children’s hospital: a retrospective study. PeerJ. 2021;9:e11560. 10.7717/peerj.11560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ghosh UK, Sultana A, Ghosh NK, Akram A, Ahmed E, Rana IH, et al. Clinico-demographic profile of Coronavirus infection among Bangladeshi children: A tertiary care hospital study. Bangladesh Journal of Infectious Diseases. 2020;7:S16–S21. 10.3329/bjid.v7i00.50157 [DOI] [Google Scholar]
- 94.Göktuğ A, Güngör A, Öz FN, Akelma Z, Güneylioğlu MM, Yaradılmış RM, et al. Evaluation of Epidemiological, Demographic, Clinical Characteristics and Laboratory Findings of COVID-19 in the Pediatric Emergency Department. J Trop Pediatr. 2021;67:fmab066. 10.1093/tropej/fmab066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gomes NTN, Haslett MIC, Percio J, Duarte MMS, Malta JMAS, Carvalho FC, et al. Retrospective cohort of children and adolescents hospitalized by COVID-19 in Brazil from the beginning of the pandemic to August 1st, 2020. Rev Bras Epidemiol. 2021;24:e210026. 10.1590/1980-549720200026 [DOI] [PubMed] [Google Scholar]
- 96.Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Carducci FIC, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–61. 10.1016/S2352-4642(20)30177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382:2302–15. 10.1056/NEJMoa2006100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gujar N, Tambe M, Parande M, Salunke N, Jagdale G, Anderson SG, et al. A case control study to assess effectiveness of measles containing vaccines in preventing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children. Hum Vaccin Immunother. 2021;17:3316–21. 10.1080/21645515.2021.1930471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gumus H, Ozcan Y, Kazanasmaz H, Demir A, Guzelcicek A.Clinical Characteristics of COVID-19 Infection in the Pediatric Age Group. Electron J Gen Med. 2021;18:em308. 10.29333/ejgm/11019 [DOI] [Google Scholar]
- 100.Guzman BV, Elbel B, Jay M, Messito MJ, Curado S.Age-dependent association of obesity with COVID-19 severity in paediatric patients. Pediatr Obes. 2022;17:e12856. 10.1111/ijpo.12856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hedberg P, Karlsson Valik J, van der Werff S, Tanushi H, Requena Mendez A, Granath F, et al. Clinical phenotypes and outcomes of SARS-CoV-2, influenza, RSV and seven other respiratory viruses: a retrospective study using complete hospital data. Thorax. 2022;77:154. 10.1136/thoraxjnl-2021-216949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hendler JV, do Lago PM, Müller GC, Santana JC, Piva JP, Daudt LE.Risk factors for severe COVID-19 infection in Brazilian children. Braz J Infect Dis. 2021;25:101650. 10.1016/j.bjid.2021.101650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hernández-Garduño E.Comorbidities that predict acute respiratory syndrome coronavirus 2 test positivity in Mexican Children: A case-control study. Pediatr Obes. 2021;16:e12740. 10.1111/ijpo.12740 [DOI] [PubMed] [Google Scholar]
- 104.Hijazi LO, Alaraifi AK, Alsaab F.Otolaryngology manifestations of COVID-19 in pediatric patients. Int J Pediatr Otorhinolaryngol. 2021;144:110701. 10.1016/j.ijporl.2021.110701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hobbs CV, Woodworth K, Young CC, Jackson AM, Newhams MM, Dapul H, et al. Frequency, Characteristics and Complications of COVID-19 in Hospitalized Infants. Pediatr Infect Dis J. 2022;41:e81–6. 10.1097/INF.0000000000003435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Howard LM, Garguilo K, Gillon J, LeBlanc K, Seegmiller AC, Schmitz JE, et al. The first 1000 symptomatic pediatric SARS-CoV-2 infections in an integrated health care system: a prospective cohort study. BMC Pediatr. 2021;21:403. 10.1186/s12887-021-02863-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huete-Pérez JA, Ernst KC, Cabezas-Robelo C, Páiz-Medina L, Silva S, Huete A.Prevalence and risk factors for SARS-CoV-2 infection in children with and without symptoms seeking care in Managua, Nicaragua: results of a cross-sectional survey. BMJ Open. 2021;11:e051836. 10.1136/bmjopen-2021-051836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ibrahim LF, Tham D, Chong V, Corden M, Craig S, Buntine P, et al. The characteristics of SARS-CoV-2-positive children who presented to Australian hospitals during 2020: a PREDICT network study. Med J Aust. 2021;215:217–21. 10.5694/mja2.51207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ibrahim LF, Tosif S, McNab S, Hall S, Lee HJ, Lewena S, et al. SARS-CoV-2 testing and outcomes in the first 30 days after the first case of COVID-19 at an Australian children’s hospital. Emerg Med Australas. 2020;32:801–8. 10.1111/1742-6723.13550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Imamura T, Saito M, Ko YK, Imamura T, Otani K, Akaba H, et al. Roles of children and adolescents in COVID-19 transmission in the community: a retrospective analysis of nationwide data in Japan. Front Pediatr. 2021;9:705882. 10.3389/fped.2021.705882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Indriyani SAK, Dewi NE, Kartasasmita CB.Characteristics and Outcomes of Children With COVID-19: Evidence From West Nusa Tenggara Province, Indonesia. Archives of Pediatric Infectionus Diseases. 2021;9:e111762. 10.5812/pedinfect.111762 [DOI] [Google Scholar]
- 112.Isoldi S, Mallardo S, Marcellino A, Bloise S, Dilillo A, Iorfida D, et al. The comprehensive clinic, laboratory, and instrumental evaluation of children with COVID-19: A 6-months prospective study. J Med Virol. 2021;93:3122–32. 10.1002/jmv.26871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jang J, Hwang MJ, Kim YY, Park SY, Yoo M, Kim SS, et al. Epidemiological Characteristics and Transmission Patterns of COVID-19 Cases Among Children and Adolescents Aged 0-18 Years in South Korea. Risk Manag Healthc Policy. 2022;15:219–27. 10.2147/RMHP.S338121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ji SQ, Zhang M, Zhang Y, Xia K, Chen Y, Chu Q, et al. Characteristics of immune and inflammatory responses among different age groups of pediatric patients with COVID-19 in China. World J Pediatr. 2021;17:375–84. 10.1007/s12519-021-00440-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jimenez-García R, Nogueira J, Retuerta-Oliva A, Sainz T, Cano-Fernández J, Flores-Pérez P, et al. Pneumonia in Hospitalized Children During SARS-CoV-2 Pandemic. Is it All COVID-19? Comparison Between COVID and Non-COVID Pneumonia. Pediatr Infect Dis J. 2021;40:e111–3. 10.1097/INF.0000000000003008 [DOI] [PubMed] [Google Scholar]
- 116.Özge K, Yanartaş MS, Törün SH, Alakbarova K, Bayramoğlu Z, Mustafa Ö, et al. Experience in children in the COVID-19 pandemic of a tertiary center, in Istanbul. Journal of Istanbul Faculty of Medicine. 2021;84:293–300. [Google Scholar]
- 117.Kanthimathinathan HK, Dhesi A, Hartshorn S, Ali SH, Kirk J, Nagakumar P, et al. COVID-19: a UK children’s hospital experience. Hosp Pediatr. 2020;10:802–5. 10.1542/hpeds.2020-000208 [DOI] [PubMed] [Google Scholar]
- 118.Kapoor D, Kumar V, Pemde H, Singh P.Impact of Comorbidities on Outcome in Children With COVID-19 at a Tertiary Care Pediatric Hospital. Indian Pediatr. 2021;58:572–5. 10.1007/s13312-021-2244-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kara AA, Böncüoğlu E, Kıymet E, Arıkan KÖ, Şahinkaya Ş, Düzgöl M, et al. Evaluation of predictors of severe-moderate COVID-19 infections at children: A review of 292 children. J Med Virol. 2021;93:6634–40. 10.1002/jmv.27237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Karaaslan A, Çetin C, Akın Y, Tekol SD, Söbü E, Demirhan R.Coinfection in SARS-CoV-2 infected children patients. J Infect Dev Ctries. 2021;15:761–5. 10.3855/jidc.14314 [DOI] [PubMed] [Google Scholar]
- 121.Karaci M, Güven Ş, Boğa A, Varol F, Çalışkan S, Sayman EN, et al. Should We Perform Laboratory and Radiographic Evaluations for All Children with COVID-19?: A Single-Center Experience. Journal of Child Science. 2021;11:e93–9. 10.1055/s-0041-1729630 [DOI] [Google Scholar]
- 122.Karbuz A, Akkoc G, Bedir Demirdag T, Yilmaz Ciftdogan D, Ozer A, Cakir D, et al. Epidemiological, Clinical, and Laboratory Features of Children With COVID-19 in Turkey. Front Pediatr. 2021;9:631547. 10.3389/fped.2021.631547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kavanagh FG, James DL, Brinkman D, Cornyn S, Murphy C, O’Neill S, et al. Safety of elective paediatric surgery during the coronavirus disease 2019 pandemic. Int J Pediatr Otorhinolaryngol. 2021;150:110861. 10.1016/j.ijporl.2021.110861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kepenekli E, Yakut N, Ergenc Z, Aydıner Ö, Sarınoğlu RC, Karahasan A, et al. COVID-19 disease characteristics in different pediatric age groups. J Infect Dev Ctries. 2022;16:16–24. 10.3855/jidc.15353 [DOI] [PubMed] [Google Scholar]
- 125.Kim L, Whitaker M, O’Halloran A, Kambhampati A, Chai SJ, Reingold A, et al. Hospitalization rates and characteristics of children aged< 18 years hospitalized with laboratory-confirmed COVID-19—COVID-NET, 14 states, March 1–July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1081. 10.15585/mmwr.mm6932e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Krithika A, Vindhiya K, Prithviraj S, Manasvini S.An observational study of clinical presentation of covid 19 among children in India. Current Pediatric Research. 2021;25:674–6. [Google Scholar]
- 127.Kuchar E, Załęski A, Wronowski M, Krankowska D, Podsiadły E, Brodaczewska K, et al. Children were less frequently infected with SARS-CoV-2 than adults during 2020 COVID-19 pandemic in Warsaw, Poland. Eur J Clin Microbiol Infect Dis. 2021;40:541–7. 10.1007/s10096-020-04038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kuczborska K, Książyk J.Prevalence and Course of SARS-CoV-2 Infection among Immunocompromised Children Hospitalised in the Tertiary Referral Hospital in Poland. J Clin Med. 2021;10:4556. 10.3390/jcm10194556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kufa T, Jassat W, Cohen C, Tempia S, Masha M, Wolter N, et al. Epidemiology of SARS-CoV-2 infection and SARS-CoV-2 positive hospital admissions among children in South Africa. Influenza Other Respir Viruses. 2022;16:34–47. 10.1111/irv.12916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.More K, Chawla D, Murki S, Tandur B, Deorari AK, Kumar P, et al. Outcomes of neonates born to mothers with coronavirus disease 2019 (COVID-19)—National Neonatology Forum (NNF) India COVID-19 Registry. Indian Pediatr. 2021;58:525–31. 10.1007/s13312-021-2234-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kushner LE, Schroeder AR, Kim J, Mathew R.“For COVID” or “With COVID”: Classification of SARS-CoV-2 Hospitalizations in Children. Hosp Pediatr. 2021;11:e151–6. 10.1542/hpeds.2021-006001 [DOI] [PubMed] [Google Scholar]
- 132.Ladhani SN, Ireland G, Baawuah F, Beckmann J, Okike IO, Ahmad S, et al. SARS-CoV-2 infection, antibody positivity and seroconversion rates in staff and students following full reopening of secondary schools in England: A prospective cohort study, September–December 2020. EClinicalMedicine. 2021;37:100948. 10.1016/j.eclinm.2021.100948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lanari M, Biserni GB, Pavoni M, Borgatti EC, Leone M, Corsini I, et al. Feasibility and Effectiveness Assessment of SARS-CoV-2 Antigenic Tests in Mass Screening of a Pediatric Population and Correlation with the Kinetics of Viral Loads. Viruses. 2021;13:2071. 10.3390/v13102071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lazzerini M, Sforzi I, Trapani S, Biban P, Silvagni D, Villa G, et al. Characteristics and risk factors for SARS-CoV-2 in children tested in the early phase of the pandemic: a cross-sectional study, Italy, 23 February to 24 May 2020. Euro Surveill. 2021;26:2001248. 10.2807/1560-7917.ES.2021.26.14.2001248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee H, Choi S, Park JY, Jo DS, Choi UY, Lee H, et al. Analysis of Critical COVID-19 Cases Among Children in Korea. J Korean Med Sci. 2022;37:e13. 10.3346/jkms.2022.37.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Leidman E, Duca LM, Omura JD, Proia K, Stephens JW, Sauber-Schatz EK.COVID-19 trends among persons aged 0–24 years—United States, March 1–December 12, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:88. 10.15585/mmwr.mm7003e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Levy C, Basmaci R, Bensaid P, Bru CB, Coinde E, Dessioux E, et al. Changes in Reverse Transcription Polymerase Chain Reaction–positive Severe Acute Respiratory Syndrome Coronavirus 2 Rates in Adults and Children According to the Epidemic Stages. Pediatr Infect Dis J. 2020;39:e369–72. 10.1097/INF.0000000000002861 [DOI] [PubMed] [Google Scholar]
- 138.Lindsay L, Secrest MH, Rizzo S, Keebler DS, Yang F, Tsai L.Factors associated with COVID-19 viral and antibody test positivity and assessment of test concordance: a retrospective cohort study using electronic health records from the USA. BMJ Open. 2021;11:e051707. 10.1136/bmjopen-2021-051707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu T, Liang W, Zhong H, He J, Chen Z, He G, et al. Risk factors associated with COVID-19 infection: a retrospective cohort study based on contacts tracing. Emerg Microbes Infect. 2020;9:1546–53. 10.1080/22221751.2020.1787799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu W, Zhang Q, Chen J, Xiang R, Song H, Shu S, et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020;382:1370–1. 10.1056/NEJMc2003717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Loconsole D, Caselli D, Centrone F, Morcavallo C, Campanella S, Aricò M, et al. SARS-CoV-2 Infection in Children in Southern Italy: A Descriptive Case Series. Int J Environ Res Public Health. 2020;17:6080. 10.3390/ijerph17176080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.López-Aguilar E, Cárdenas-Navarrete R, Simental-Toba A, Pacheco-Rosas D, Thomé-Ortiz P, Soto-Pérez G, et al. Children with cancer during COVID-19 pandemic: Early experience in Mexico. Pediatr Blood Cancer. 2021;68:e28660. 10.1002/pbc.28660 [DOI] [PubMed] [Google Scholar]
- 143.Lorenzo VB, Nascimento-Carvalho CM.Differences between children with severe acute lower respiratory infection with or without SARS-Cov-2 infection. J Infect. 2021;83:e1–3. 10.1016/j.jinf.2021.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–5. 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lu Y, Li Y, Deng W, Liu M, He Y, Huang L, et al. Symptomatic Infection is Associated with Prolonged Duration of Viral Shedding in Mild Coronavirus Disease 2019: A Retrospective Study of 110 Children in Wuhan. Pediatr Infect Dis J. 2020;39:e95–9. 10.1097/INF.0000000000002729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lynam K, Twomey J, Mahony M, O'Mahony E, Ahmed I, Murphy A, et al. Low prevalence of SARS-CoV-2 detected in symptomatic children admitted to hospital. Irish Medical Journal. 2021;114:1. [Google Scholar]
- 147.Madani S, Shahin S, Yoosefi M, Ahmadi N, Ghasemi E, Koolaji S, et al. Red flags of poor prognosis in pediatric cases of COVID-19: the first 6610 hospitalized children in Iran. BMC Pediatr. 2021;21:563. 10.1186/s12887-021-03030-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maltezou HC, Magaziotou I, Dedoukou X, Eleftheriou E, Raftopoulos V, Michos A, et al. Children and Adolescents With SARS-CoV-2 Infection: Epidemiology, Clinical Course and Viral Loads. Pediatr Infect Dis J. 2020;39:e388–92. 10.1097/INF.0000000000002899 [DOI] [PubMed] [Google Scholar]
- 149.Mania A, Mazur-Melewska K, Lubarski K, Kuczma-Napierała J, Mazurek J, Jończyk-Potoczna K, et al. Wide spectrum of clinical picture of COVID-19 in children—From mild to severe disease. J Infect Public Health. 2021;14:374–9. 10.1016/j.jiph.2020.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mania A, Pokorska-Śpiewak M, Figlerowicz M, Pawłowska M, Mazur-Melewska K, Faltin K, et al. Pneumonia, gastrointestinal symptoms, comorbidities, and coinfections as factors related to a lengthier hospital stay in children with COVID-19—analysis of a paediatric part of Polish register SARSTer. Infect Dis (Lond). 2022;54:196–204. 10.1080/23744235.2021.1995628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Matteudi T, Luciani L, Fabre A, Minodier P, Boucekine M, Bosdure E, et al. Clinical characteristics of paediatric COVID-19 patients followed for up to 13 months. Acta Paediatr. 2021;110:3331–3. 10.1111/apa.16071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Melé M, Henares D, Pino R, Asenjo S, Matamoros R, Fumadó V, et al. Low impact of SARS-CoV-2 infection among paediatric acute respiratory disease hospitalizations. J Infect. 2021;82:414–51. 10.1016/j.jinf.2020.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Messiah SE, Xie L, Mathew MS, Delclos GL, Kohl HW, Kahn JS.Results of COVID-19 Surveillance in a Large United States Pediatric Healthcare System over One Year. Children (Basel). 2021;8:752. 10.3390/children8090752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Meyer M, Ruebsteck E, Eifinger F, Klein F, Oberthuer A, van Koningsbruggen-Rietschel S, et al. Morbidity of Respiratory Syncytial Virus (RSV) Infections: RSV Compared With Severe Acute Respiratory Syndrome Coronavirus 2 Infections in Children Aged 0-4 Years in Cologne, Germany. J Infect Dis. 2022;226:2050–3. 10.1093/infdis/jiac052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Meyer M, Ruebsteck E, Gruell H, Klein F, Lehmann C, Wendt S, et al. COVID-19 study found that 0.4% of 5730 asymptomatic children aged 0–18 years tested positive for virus before hospital procedures or admission. Acta Paediatr. 2021;110:2584–5. 10.1111/apa.15884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Michos A, Savvidou P, Syridou G, Eleftheriou E, Iosifidis E, Grivea I, et al. SARS-CoV-2 molecular testing in Greek hospital paediatric departments: a nationwide study. Epidemiol Infect. 2021;149:e70. 10.1017/S0950268821000455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Morban DAH, Hidalgo MEC, Jorge MM, Antonio EP.Clinical and epidemiological characteristics of COVID-19 in Pediatrics in Dominican Republic. Rev Cubana Pediatr. 2021;93:1–19. [Google Scholar]
- 158.Moreno-Noguez M, Rivas-Ruiz R, Roy-García IA, Pacheco-Rosas DO, Moreno-Espinosa S, Flores-Pulido AA.Risk factors associated with SARS-CoV-2 pneumonia in the pediatric population. Bol Med Hosp Infant Mex. 2021;78:251–8. 10.24875/BMHIM.20000263 [DOI] [PubMed] [Google Scholar]
- 159.Murillo-Zamora E, Aguilar-Sollano F, Delgado-Enciso I, Hernandez-Suarez CM.Predictors of laboratory-positive COVID-19 in children and teenagers. Public Health. 2020;189:153–7. 10.1016/j.puhe.2020.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Musa OAH, Chivese T, Bansal D, Abdulmajeed J, Ameen O, Islam N, et al. Prevalence and determinants of symptomatic COVID-19 infection among children and adolescents in Qatar: a cross-sectional analysis of 11 445 individuals. Epidemiol Infect. 2021;149:e193. 10.1017/S0950268821001515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mveang Nzoghe A, Padzys GS, Maloupazoa Siawaya AC, Kandet Yattara M, Leboueny M, Avome Houechenou RM, et al. Dynamic and features of SARS-CoV-2 infection in Gabon. Sci Rep. 2021;11:9672. 10.1038/s41598-021-87043-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Navarro-Olivos E, Padilla-Raygoza N, Flores-Vargas G, Gallardo-Luna MJ, León-Verdín MG, Lara-Lona E, et al. COVID-19-Associated Case Fatality Rate in Subjects Under 18 Years Old in Mexico, up to December 31, 2020. Front Pediatr. 2021;9:696425. 10.3389/fped.2021.696425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ng DC, Tan KK, Chin L, Ali MM, Lee ML, Mahmood FM, et al. Clinical and epidemiological characteristics of children with COVID-19 in Negeri Sembilan, Malaysia. Int J Infect Dis. 2021;108:347–52. 10.1016/j.ijid.2021.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Odeleye E, Friar S, Bate J.Successful Implementation of Routine SARS-CoV-2 Screening in Children With Cancer and Their Parents During the Pandemic in the United Kingdom. J Pediatr Hematol Oncol. 2021;43:e1046–7. 10.1097/MPH.0000000000002145 [DOI] [PubMed] [Google Scholar]
- 165.Okur DS.Clinical impact of COVID-19 on Turkish children with neurological and neuromuscular diseases: One center experience. Medicine (Baltimore). 2021;100:e28401. 10.1097/MD.0000000000028401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Olivar-López V, Leyva-Barrera A, López-Martínez B, Parra-Ortega I, Márquez-González H.Clinical risk profile associated with SARS-CoV-2 infection and complications in the emergency area of a pediatric COVID-19 center. Bol Med Hosp Infant Mex. 2020;77:221–7. 10.24875/BMHIM.20000198 [DOI] [PubMed] [Google Scholar]
- 167.Oliveira EA, Colosimo EA, Simões e Silva AC, Mak RH, Martelli DB, Silva LR, et al. Clinical characteristics and risk factors for death among hospitalised children and adolescents with COVID-19 in Brazil: an analysis of a nationwide database. Lancet Child Adolesc Health. 2021;5:559–68. 10.1016/S2352-4642(21)00134-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ollier Q, Pillet S, Mory O, Gagnaire J, Thuiller C, Annino N, et al. Prospective evaluation of the point-of-care use of a rapid antigenic SARS-CoV-2 immunochromatographic test in a paediatric emergency department. Clin Microbiol Infect. 2022;28:734.e1–6. 10.1016/j.cmi.2021.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Olson SM, Newhams MM, Halasa NB, Price AM, Boom JA, Sahni LC, et al. Effectiveness of BNT162b2 vaccine against critical Covid-19 in adolescents. N Engl J Med. 2022;386:713–23. 10.1056/NEJMoa2117995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Omrani AS, Almaslamani MA, Daghfal J, Alattar RA, Elgara M, Shaar SH, et al. The first consecutive 5000 patients with Coronavirus Disease 2019 from Qatar; a nation-wide cohort study. BMC Infect Dis. 2020;20:777. 10.1186/s12879-020-05511-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ortiz-Pinto MA, de Miguel-García S, Ortiz-Marrón H, Ortega-Torres A, Cabañas G, Gutiérrez–Torres LF, et al. Childhood obesity and risk of SARS-CoV-2 infection. Int J Obes (Lond). 2022;46:1155–9. 10.1038/s41366-022-01094-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Osmanov IM, Spiridonova E, Bobkova P, Gamirova A, Shikhaleva A, Andreeva M, et al. Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: a prospective cohort study. Eur Respir J. 2022;59:2101341. 10.1183/13993003.01341-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Otto WR, Geoghegan S, Posch LC, Bell LM, Coffin SE, Sammons JS, et al. The Epidemiology of Severe Acute Respiratory Syndrome Coronavirus 2 in a Pediatric Healthcare Network in the United States. J Pediatric Infect Dis Soc. 2020;9:523–9. 10.1093/jpids/piaa074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Özlü SG, Aydın Z, Bozelli BN, Avcı B, İnözü M, Çaycı FŞ, et al. Can microalbuminuria be an ındicator of renal ınvolvement in pediatric Covid 19 patients? Infection. 2022;50:719–24. 10.1007/s15010-021-01745-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Paduano S, Facchini MC, Greco A, Borsari L, Mingrone VM, Tancredi S, et al. Characteristics and risk factors of isolated and quarantined children and adolescents during the first wave of SARS-CoV-2 pandemic: A cross-sectional study in Modena, Northern Italy: SARS-CoV-2 in Modena children. Acta Biomed. 2021;92:e2021449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pande N, Save S, Kondekar A, Sawant V, Rathi S, Malik S.Clinical profile of children with sars-cov-2 infection from a dedicated covid-19 hospital in India. Current Pediatric Research. 2021;25:697–703. [Google Scholar]
- 177.Pandey M, Sisodia S, Bandi S, Roland D.Incidence of spread of clinically relevant SARS-CoV2 infection between children in a tertiary emergency department: An evaluation. J Infect. 2020;81:979–97. 10.1016/j.jinf.2020.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Parambil BC, Moulik NR, Dhamne C, Dhariwal N, Narula G, Vora T, et al. COVID-19 in Children with Cancer and Continuation of Cancer-Directed Therapy During the Infection. Indian J Pediatr. 2022;89:445–51. 10.1007/s12098-021-03894-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Parcha V, Booker KS, Kalra R, Kuranz S, Berra L, Arora G, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. 10.1038/s41598-021-89553-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Parri N, Lenge M, Cantoni B, Arrighini A, Romanengo M, Urbino A, et al. COVID-19 in 17 Italian Pediatric Emergency Departments. Pediatrics. 2020;146:e20201235. 10.1542/peds.2020-1235 [DOI] [PubMed] [Google Scholar]
- 181.Peaper DR, Murdzek C, Oliveira CR, Murray TS.Severe Acute Respiratory Syndrome Coronavirus 2 Testing in Children in a Large Regional US Health System During the Coronavirus Disease 2019 Pandemic. Pediatr Infect Dis J. 2021;40:175–81. 10.1097/INF.0000000000003024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Peng X, Guo Y, Xiao H, Xia W, Zhai A, Zhu B, et al. Overview of chest involvement at computed tomography in children with coronavirus disease 2019 (COVID-19). Pediatr Radiol. 2021;51:222–30. 10.1007/s00247-020-04826-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Perramon A, Soriano-Arandes A, Pino D, Lazcano U, Andrés C, Català M, et al. Schools as a Framework for COVID-19 Epidemiological Surveillance of Children in Catalonia, Spain: A Population-Based Study. Front Pediatr. 2021;9:754744. 10.3389/fped.2021.754744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pokorska-Śpiewak M, Talarek E, Mania A, Pawłowska M, Popielska J, Zawadka K, et al. Clinical and Epidemiological Characteristics of 1283 Pediatric Patients with Coronavirus Disease 2019 during the First and Second Waves of the Pandemic—Results of the Pediatric Part of a Multicenter Polish Register SARSTer. J Clin Med. 2021;10:5098. 10.3390/jcm10215098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pokorska-Śpiewak M, Talarek E, Popielska J, Nowicka K, Ołdakowska A, Zawadka K, et al. Comparison of clinical severity and epidemiological spectrum between coronavirus disease 2019 and influenza in children. Sci Rep. 2021;11:5760. 10.1038/s41598-021-85340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pudjiadi AH, Putri ND, Sjakti HA, Yanuarso PB, Gunardi H, Roeslani RD, et al. Pediatric COVID-19: Report From Indonesian Pediatric Society Data Registry. Front Pediatr. 2021;9:716898. 10.3389/fped.2021.716898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Qian G, Zhang Y, Xu Y, Hu W, Hall IP, Yue J, et al. Reduced inflammatory responses to SARS-CoV-2 infection in children presenting to hospital with COVID-19 in China. EClinicalMedicine. 2021;34:100831. 10.1016/j.eclinm.2021.100831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rabha AC, Fernandes FR, Solé D, Bacharier LB, Wandalsen GF.Asthma is associated with lower respiratory tract involvement and worse clinical score in children with COVID-19. Pediatr Allergy Immunol. 2021;32:1577–80. 10.1111/pai.13536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rabha AC, Oliveira Junior FI, Oliveira TA, Cesar RG, Fongaro G, Mariano RF, et al. Clinical manifestations of children and adolescents with COVID-19: report of the first 115 cases from Sabará Hospital Infantil. Rev Paul Pediatr. 2020;39:e2020305. 10.1590/1984-0462/2021/39/2020305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Reis BY, Barda N, Leshchinsky M, Kepten E, Hernán MA, Lipsitch M, et al. Effectiveness of BNT162b2 vaccine against delta variant in adolescents. N Engl J Med. 2021;385:2101–3. 10.1056/NEJMc2114290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rha B, Lively JY, Englund JA, Staat MA, Weinberg GA, Selvarangan R, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Infections in Children: Multicenter Surveillance, United States, January–March 2020. J Pediatric Infect Dis Soc. 2020;9:609–12. 10.1093/jpids/piaa075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rivas-Ruiz R, Roy-García IA, Ureña-Wong KR, Aguilar-Ituarte F, Vázquez-de Anda GF, Gutiérrez-Castrellón P.Factors associated with death in children with COVID-19 in Mexico. Gac Med Mex. 2020;156:516–22. [DOI] [PubMed] [Google Scholar]
- 193.Rizzo C, Loconsole D, Pandolfi E, Ciofi Degli Atti ML, Van Summeren J, Paget J, et al. Sars-Cov2 not detected in a pediatric population with acute respiratory infection in primary care in Central and Southern Italy from November 2019 to Early March 2020. Front Pediatr. 2021;9:620598. 10.3389/fped.2021.620598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rodriguez Velásquez S, Jacques L, Dalal J, Sestito P, Habibi Z, Venkatasubramanian A, et al. The toll of COVID-19 on African children: a descriptive analysis on COVID-19-related morbidity and mortality among the pediatric population in sub-Saharan Africa. Int J Infect Dis. 2021;110:457–65. 10.1016/j.ijid.2021.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.De Rose DU, Auriti C, Landolfo F, Capolupo I, Salvatori G, Ranno S, et al. Reshaping neonatal intensive care units (NICUs) to avoid the spread of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) to high-risk infants. Infect Control Hosp Epidemiol. 2021;42:632–3. 10.1017/ice.2020.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sahni LC, Avadhanula V, Ortiz CS, Feliz KE, John RE, Brown CA, et al. Comparison of Mid-Turbinate and Nasopharyngeal Specimens for Molecular Detection of SARS-CoV-2 Among Symptomatic Outpatients at a Pediatric Drive-Through Testing Site. J Pediatric Infect Dis Soc. 2021;10:872–9. 10.1093/jpids/piab046 [DOI] [PubMed] [Google Scholar]
- 197.Salako A, Odubela O, Musari-Martins T, Ezemelue P, Gbaja-Biamila T, Opaneye B, et al. Prevalence and Presentation of Paediatric Coronavirus Disease 2019 in Lagos, Nigeria. Int J Pediatr. 2021;2021:2185161. 10.1155/2021/2185161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Saleh NY, Aboelghar HM, Salem SS, Ibrahem RA, Khalil FO, Abdelgawad AS, et al. The severity and atypical presentations of COVID-19 infection in pediatrics. BMC Pediatr. 2021;21:144. 10.1186/s12887-021-02614-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sananez I, Raiden SC, Algieri SC, Uranga M, Grisolía NA, Filippo D, et al. A poor and delayed anti-SARS-CoV2 IgG response is associated to severe COVID-19 in children. EBioMedicine. 2021;72:103615. 10.1016/j.ebiom.2021.103615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schneider JG, Relich RF, Datta D, Bond C, Goings M, Hall D, et al. Identifying Risk Factors That Distinguish Symptomatic Severe Acute Respiratory Syndrome Coronavirus 2 Infection From Common Upper Respiratory Infections in Children. Cureus. 2021;13:e13266. 10.7759/cureus.13266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sedighi I, Fahimzad A, Pak N, Khalili M, Shokrollahi MR, Heydari H, et al. A multicenter retrospective study of clinical features, laboratory characteristics, and outcomes of 166 hospitalized children with coronavirus disease 2019 (COVID-19): A preliminary report from Iranian Network for Research in Viral Diseases (INRVD). Pediatr Pulmonol. 2022;57:498–507. 10.1002/ppul.25756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.See KC, Tan LP, Ong LT, Lee PY.Clinical and epidemiological characteristics of children with COVID-19 in Selangor, Malaysia. IJID Reg. 2022;2:63–9. 10.1016/j.ijregi.2021.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sena GR, Lima TPF, Vidal SA, Duarte MDCMB, Bezerra PGM, Fonseca Lima EJ, et al. Clinical Characteristics and Mortality Profile of COVID-19 Patients Aged less than 20 years Old in Pernambuco – Brazil. Am J Trop Med Hyg. 2021;104:1507–12. 10.4269/ajtmh.20-1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shahid S, Raza M, Junejo S, Maqsood S.Clinical features and outcome of COVID-19 positive children from a tertiary healthcare hospital in Karachi. J Med Virol. 2021;93:5988–97. 10.1002/jmv.27178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shapiro Ben David S, Rahamim-Cohen D, Tasher D, Geva A, Azuri J, Ash N.COVID-19 in children and the effect of schools reopening on potential transmission to household members. Acta Paediatr. 2021;110:2567–73. 10.1111/apa.15962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sharma AG, Kumar V, Sodani R, Sapre A, Singh P, Saha A, et al. Predictors of mortality in children admitted with SARS-CoV-2 infection to a tertiary care hospital in North India. J Paediatr Child Health. 2022;58:432–9. 10.1111/jpc.15737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sharma AK, Chapagain RH, Bista KP, Bohara R, Chand B, Chaudhary NK, et al. Epidemiological and clinical profile of COVID-19 in Nepali children: An initial experience. J Nepal Paediatr Soc. 2020;40:202–9. 10.3126/jnps.v40i3.32438 [DOI] [Google Scholar]
- 208.Shi T, Pan J, Katikireddi SV, McCowan C, Kerr S, Agrawal U, et al. Risk of COVID-19 hospital admission among children aged 5–17 years with asthma in Scotland: a national incident cohort study. Lancet Respir Med. 2022;10:191–8. 10.1016/S2213-2600(21)00491-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shoji K, Akiyama T, Tsuzuki S, Matsunaga N, Asai Y, Suzuki S, et al. Clinical Characteristics of Hospitalized COVID-19 in Children: Report From the COVID-19 Registry in Japan. J Pediatric Infect Dis Soc. 2021;10:1097–100. 10.1093/jpids/piab085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shoji K, Akiyama T, Tsuzuki S, Matsunaga N, Asai Y, Suzuki S, et al. Comparison of the clinical characteristics and outcomes of COVID-19 in children before and after the emergence of Delta variant of concern in Japan. J Infect Chemother. 2022;28:591–4. 10.1016/j.jiac.2022.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Singh P, Attri K, Mahto D, Kumar V, Kapoor D, Seth A, et al. Clinical Profile of COVID-19 Illness in Children—Experience from a Tertiary Care Hospital. Indian J Pediatr. 2022;89:45–51. 10.1007/s12098-021-03822-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sola AM, David AP, Rosbe KW, Baba A, Ramirez-Avila L, Chan DK.Prevalence of SARS-CoV-2 Infection in Children Without Symptoms of Coronavirus Disease 2019. JAMA Pediatr. 2021;175:198–201. 10.1001/jamapediatrics.2020.4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Somekh I, Stein M, Karakis I, Simões EAF, Somekh E.Characteristics of SARS-CoV-2 Infections in Israeli Children During the Circulation of Different SARS-CoV-2 Variants. JAMA Netw Open. 2021;4:e2124343. 10.1001/jamanetworkopen.2021.24343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Song X, Delaney M, Shah RK, Campos JM, Wessel DL, DeBiasi RL.Common seasonal respiratory viral infections in children before and during the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2022;43:1454–8. 10.1017/ice.2021.430 [DOI] [PubMed] [Google Scholar]
- 215.Soriano-Arandes A, Gatell A, Serrano P, Biosca M, Campillo F, Capdevila R, et al. Household Severe Acute Respiratory Syndrome Coronavirus 2 Transmission and Children: A Network Prospective Study. Clin Infect Dis. 2021;73:e1261–9. 10.1093/cid/ciab228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sousa BLA, Brentani A, Costa Ribeiro CC, Dolhnikoff M, Grisi SJFE, Ferrer APS, et al. Non-communicable diseases, sociodemographic vulnerability and the risk of mortality in hospitalised children and adolescents with COVID-19 in Brazil: a cross-sectional observational study. BMJ Open. 2021;11:e050724. 10.1136/bmjopen-2021-050724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, et al. Coronavirus Disease 2019 Case Surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–65. 10.15585/mmwr.mm6924e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Talarico V, Roppa K, Alcaro T, Vinci M, Minchella P, Raiola G.Epidemiological analysis on children and adolescents with COVID-19 in a central area of Calabria region: one year of pandemia. Acta Biomed. 2021;92:e2021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tang F, Luo W, Wang X, Li H, Mei H, Shao J, et al. Clinical features and follow-up of pediatric patients hospitalized for COVID-19. Pediatr Pulmonol. 2021;56:1967–75. 10.1002/ppul.25407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tosif S, Ibrahim LF, Hughes RR, Cheng D, Wurzel D, Overmars I, et al. Characteristics and outcomes of SARS-CoV-2 infection in Victorian children at a tertiary paediatric hospital. J Paediatr Child Health. 2022;58:618–23. 10.1111/jpc.15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Uka A, Buettcher M, Bernhard-Stirnemann S, Fougère Y, Moussaoui D, Kottanattu L, et al. Factors associated with hospital and intensive care admission in paediatric SARS-CoV-2 infection: a prospective nationwide observational cohort study. Eur J Pediatr. 2022;181:1245–55. 10.1007/s00431-021-04276-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ustundag G, Yilmaz-Ciftdogan D, Kara-Aksay A, Sahin A, Ekemen-Keles Y, Orsdemir-Hortu H, et al. Coronavirus disease 2019 in healthy children: What is the effect of household contact? Pediatr Int. 2022;64:e14890. 10.1111/ped.14890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.van der Zalm MM, Lishman J, Verhagen LM, Redfern A, Smit L, Barday M, et al. Clinical Experience With Severe Acute Respiratory Syndrome Coronavirus 2–Related Illness in Children: Hospital Experience in Cape Town, South Africa. Clin Infect Dis. 2021;72:e938–44. 10.1093/cid/ciaa1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Verd S, Ramakers J, Vinuela I, Martin-Delgado MI, Prohens A, Díez R.Does breastfeeding protect children from COVID-19? An observational study from pediatric services in Majorca, Spain. Int Breastfeed J. 2021;16:83. 10.1186/s13006-021-00430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Vergine G, Fantini M, Marchetti F, Stella M, Valletta E, Biasucci G, et al. Home management of children with COVID-19 in the Emilia-Romagna Region, Italy. Front Pediatr. 2020;8:575290. 10.3389/fped.2020.575290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Vogel S, von Both U, Nowak E, Ludwig J, Köhler A, Lee N, et al. SARS-CoV-2 Saliva Mass Screening in Primary Schools: A 10-Week Sentinel Surveillance Study in Munich, Germany. Diagnostics (Basel). 2022;12:162. 10.3390/diagnostics12010162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wang SM, Tao F, Hou Y, Zhang A, Xiong H, Sun JJ, et al. Screening of SARS-CoV-2 in 299 Hospitalized Children with Hemato-oncological Diseases: A Multicenter Survey in Hubei, China. Curr Med Sci. 2020;40:642–5. 10.1007/s11596-020-2228-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang X, Chen X, Tang F, Luo W, Fang J, Qi C, et al. Be aware of acute kidney injury in critically ill children with COVID-19. Pediatr Nephrol. 2021;36:163–9. 10.1007/s00467-020-04715-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wanga V, Gerdes ME, Shi DS, Choudhary R, Dulski TM, Hsu S, et al. Characteristics and clinical outcomes of children and adolescents aged< 18 years hospitalized with COVID-19—six hospitals, United States, July–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1766. 10.15585/mmwr.mm705152a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ward JL, Harwood R, Smith C, Kenny S, Clark M, Davis PJ, et al. Risk factors for PICU admission and death among children and young people hospitalized with COVID-19 and PIMS-TS in England during the first pandemic year. Nat Med. 2022;28:193–200. 10.1038/s41591-021-01627-9 [DOI] [PubMed] [Google Scholar]
- 231.Węcławek-Tompol J, Zakrzewska Z, Gryniewicz-Kwiatkowska O, Pierlejewski F, Bień E, Zaucha-Prażmo A, et al. COVID-19 in pediatric cancer patients is associated with treatment interruptions but not with short-term mortality: a Polish national study. J Hematol Oncol. 2021;14:163. 10.1186/s13045-021-01181-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wong JJM, Gan CS, Kaushal SH, Chuah SL, Sultana R, Tan NWH, et al. Pediatric COVID-19 Risk Factors in Southeast Asia-Singapore and Malaysia: A Test-Negative Case–Control Study. Am J Trop Med Hyg. 2022;106:1113. 10.4269/ajtmh.21-1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wong-Chew RM, Noyola DE, Villa AR.Características clínicas y factores de riesgo de mortalidad en menores de 18 años con COVID-19 en México y Ciudad de México [Clinical characteristics and mortality risk factors in patients aged less than 18 years with COVID-19 in Mexico and Mexico City]. An Pediatr (Barc). 2022;97:119–28. 10.1016/j.anpedi.2021.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Xiong X, Chua GT, Chi S, Kwan MYW, Sang Wong WH, Zhou A, et al. A Comparison Between Chinese Children Infected with Coronavirus Disease-2019 and with Severe Acute Respiratory Syndrome 2003. J Pediatr. 2020;224:30–6. 10.1016/j.jpeds.2020.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cura Yayla BC, Özsürekçi Y, Aykaç K, Oygar PD, Gürlevik SL, Ilbay S, et al. Characteristics and management of children with COVID-19 in Turkey. Balkan Med J. 2020;37:341. 10.4274/balkanmedj.galenos.2020.2020.7.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yılmaz Çelebi M, Kıymet E, Böncüoğlu E, Şahinkaya Ş, Cem E, Düzgöl M, et al. Evaluation of childhood hospitalization rates and degree of severity of SARS-CoV-2 variants, including B.1.1.7 (Alpha), B.1.315/P.1 (Beta/Gamma), and B.1.617.2 (Delta). J Med Virol. 2022;94:2050–4. 10.1002/jmv.27587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yılmaz K, Gozupirinççioğlu A, Aktar F, Akın A, Karabel M, Yolbas I, et al. Evaluation of the novel coronavirus disease in Turkish children: Preliminary outcomes. Pediatr Pulmonol. 2020;55:3587–94. 10.1002/ppul.25095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yılmaz K, Şen V, Aktar F, Onder C, Yılmaz ED, Yılmaz Z.Does Covid-19 in children have a milder course than Influenza? Int J Clin Pract. 2021;75:e14466. 10.1111/ijcp.14466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yonker LM, Neilan AM, Bartsch Y, Patel AB, Regan J, Arya P, et al. Pediatric Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Clinical Presentation, Infectivity, and Immune Responses. J Pediatr. 2020;227:45–52.e5. 10.1016/j.jpeds.2020.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yoon Y, Choi G-J, Kim JY, Kim K-R, Park H, Chun JK, et al. Childcare exposure to severe acute respiratory syndrome coronavirus 2 for 4-year-old presymptomatic child, South Korea. Emerg Infect Dis. 2021;27:341. 10.3201/eid2702.203189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Nunziata F, Bruzzese E, Poeta M, Pierri L, Catzola A, Ciccarelli GP, et al. Health-care organization for the management and surveillance of SARS-CoV-2 infection in children during pandemic in Campania region, Italy. Ital J Pediatr. 2020;46:170. 10.1186/s13052-020-00928-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Delahoy MJ, Ujamaa D, Whitaker M, O’Halloran A, Anglin O, Burns E, et al. Hospitalizations Associated with COVID-19 Among Children and Adolescents - COVID-NET, 14 States, March 1, 2020-August 14, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1255–60. 10.15585/mmwr.mm7036e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chua GT, Xiong X, Choi EH, Han MS, Chang SH, Jin BL, et al. COVID-19 in children across three Asian cosmopolitan regions. Emerg Microbes Infect. 2020;9:2588–96. 10.1080/22221751.2020.1846462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Haeusler GM, Ammann RA, Carlesse F, Groll AH, Averbuch D, Castagnola E, et al. SARS-CoV-2 in children with cancer or after haematopoietic stem cell transplant: an analysis of 131 patients. Eur J Cancer. 2021;159:78–86. 10.1016/j.ejca.2021.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Okonkwo IN, Howie A, Parry C, Shelton C, Cobley S, Craig R, et al. The safety of paediatric surgery between COVID-19 surges: an observational study. Anaesthesia. 2020;75:1605–13. 10.1111/anae.15264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Otto WR, Grundmeier RW, Montoya-Williams D, Njoroge WF, Wallis KE, Gerber JS, et al. Association between Preferred Language and Risk of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Children in the United States. Am J Trop Med Hyg. 2021;105:1261. 10.4269/ajtmh.21-0779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Shayganmehr A, Dorosti AA, Saboktakin L, Abbasi M, Khaiatzadeh S, Khoshmaram N, et al. Clinical pediatric screening for COVID-19. Iran J Pediatr. 2021;31:e107780. 10.5812/ijp.107780 [DOI] [Google Scholar]
- 248.DU ZS, Xu H, Liu MC.[A retrospective analysis of medication in children with SARS-CoV-2 infection in Wuhan, China]. Zhongguo Dang Dai Er Ke Za Zhi. 2021;23:61–6. Chinese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Daoju J, Jun S, Haomei Y, Yan H, Jing S, Peiqing L.[Clinical Characteristics Analysis of 129 Suspected Cases of 2019 Novel Coronavirus Infection in Children in the Guangzhou Region]. Guangzhou Med J (Ft Sam Houst, Tex). 2022;53:12–9. Chinese. [Google Scholar]
- 250.Oran DP, Topol EJ.The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Ann Intern Med. 2021;174:655–62. 10.7326/M20-6976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Syangtan G, Bista S, Dawadi P, Rayamajhee B, Shrestha LB, Tuladhar R, et al. Asymptomatic SARS-CoV-2 carriers: a systematic review and meta-analysis. Front Public Health. 2021;8:587374. 10.3389/fpubh.2020.587374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Global Health-50/50. The COVID-19 sex-disaggregated data tracker. 2022. Available: https://globalhealth5050.org/the-sex-gender-and-covid-19-project/the-data-tracker/. Accessed: 5 February 2024.
- 253.Yaya S, Yeboah H, Charles CH, Otu A, Labonte R.Ethnic and racial disparities in COVID-19-related deaths: counting the trees, hiding the forest. BMJ Glob Health. 2020;5:e002913. 10.1136/bmjgh-2020-002913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.National Health Service. Who is at high risk from coronavirus (COVID-19). 2022. Available: https://www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/who-is-at-high-risk-from-coronavirus/. Accessed: 5 February 2024.
- 255.Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Children. N Engl J Med. 2015;372:835–45. 10.1056/NEJMoa1405870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lafond KE, Nair H, Rasooly MH, Valente F, Booy R, Rahman M, et al. Global Role and Burden of Influenza in Pediatric Respiratory Hospitalizations, 1982-2012: A Systematic Analysis. PLoS Med. 2016;13:e1001977. 10.1371/journal.pmed.1001977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tian F, Yang R, Chen Z.Safety and efficacy of COVID-19 vaccines in children and adolescents: A systematic review of randomized controlled trials. J Med Virol. 2022;94:4644–53. 10.1002/jmv.27940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mathieu E, Ritche H, Rodés-Guirao L, Appel C, Davrilov D, Giattino C, et al. Coronavirus Pandemic (COVID-19). 2020. Available: https://ourworldindata.org/coronavirus. Accessed: 5 February 2024.
- 259.Zimmermann P, Pittet LF, Curtis N.Long covid in children and adolescents. BMJ. 2022;376:o143. 10.1136/bmj.o143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.