Abstract
Phytochemicals from functional foods are common ingredients in dietary supplements and cosmetic products for anti-skin aging effects due to their antioxidant activities. A proprietary red maple (Acer rubrum) leaf extract (Maplifa™) and its major phenolic compound, ginnalin A (GA), have been reported to show antioxidant, anti-melanogenesis, and anti-glycation effects but their protective effects against oxidative stress in human skin cells remain unknown. Herein, we investigated the cytoprotective effects of Maplifa™ and GA against hydrogen peroxide (H2O2) and methylglyoxal (MGO)-induced oxidative stress in human keratinocytes (HaCaT cells). H2O2 and MGO (both at 400 μM) induced toxicity in HaCaT cells and reduced their viability to 59.2 and 61.6 %, respectively. Treatment of Maplifa™ (50 μg/mL) and GA (50 μM) increased the viability of H2O2− and MGO-treated cells by 22.0 and 15.5 %, respectively. Maplifa™ and GA also showed cytoprotective effects by reducing H2O2-induced apoptosis in HaCaT cells by 8.0 and 7.2 %, respectively. The anti-apoptotic effect of Maplifa™ was further supported by the decreased levels of apoptosis associated enzymes including caspases-3/7 and −8 in HaCaT cells by 49.5 and 19.0 %, respectively. In addition, Maplifa™ (50 μg/mL) and GA (50 μM) reduced H2O2− and MGO-induced reactive oxygen species (ROS) by 84.1 and 56.8 %, respectively. Furthermore, flow cytometry analysis showed that Maplifa™ and GA reduced MGO-induced total cellular ROS production while increasing mitochondria-derived ROS production in HaCaT cells. The cytoprotective effects of Maplifa™ and GA in human keratinocytes support their potential utilization for cosmetic and/or dermatological applications.
Keywords: Red maple (Acer rubrum), ginnalin A, keratinocytes, methylglyoxal, antioxidant, skin protection
Graphical Abstract
1. Introduction
Botanical extracts from functional foods are common ingredients found in cosmeceutical products. These botanical ingredients are used for topical applications, for e.g. as bioactives in cosmetics, as well as for consumable products, such as dietary beauty supplements, i.e. as capsules or tinctures.1 It has been reported that several botanical extracts from functional foods are included in the top ten list of botanical ingredients in anti-aging creams in 2010.1 Numerous published studies have also reported that phytochemicals in botanical extracts show a wide range of biological activities including antioxidant, anti-microbial, anti-inflammation, and anti-glycation effects,2, 3 which contribute to their overall skin beneficial effects.
Skin, the largest organ of the human body, is subjected to exposure of intrinsic oxidative stress from several intra- and extra-cellular biochemical reactions including oxidation and glycation.5 Both extrinsic and intrinsic oxidative stress lead to excessive production of cellular reactive oxygen species (ROS), which further lead to the impairment of skin cells by triggering cell survival signaling pathways including cell necrosis and apoptosis.4 Another contributing factor for the induction of skin cellular oxidative stress is the process of glycation, a non-enzymatic reaction involving the metabolism of glucose. In the glycolysis pathway, glucose is converted into pyruvate and a group of dicarbonyl compounds, for e.g. glyoxal and methylglyoxal (MGO), which are formed as side products.5 These dicarbonyl compounds are highly reactive and can interact with biomacro-molecules including protein, lipids, and DNA, to form a class of complex and heterogeneous compounds known as advanced glycation endproducts (AGEs). Both AGEs and its precursor, MGO, can exacerbate the production of ROS and largely contribute to the occurrence and development of many skin disorders including skin aging and inflammation.6 Thus, inhibitors of AGEs and scavengers of reactive dicarbonyl species, especially natural antioxidants, such as several polyphenols from botanical extracts, are considered as promising management strategies for AGEs associated skin complications.6, 7
Our laboratory has had a long interest in investigating the biological effects of phytochemicals in functional foods and botanical extracts. During the course of our studies, using a combination of in vitro and in vivo assays, we developed an algorithm to screen the anti-aging effects of over thirty botanical extracts, several of which showed promising neuroprotective effects including antioxidant, MGO scavenging capacity, anti-glycation, and anti-inflammatory activity.8 In addition, some of the pure constituents from these botanical extracts, for e.g. punicalagin and ellagic acid from a pomegranate extract (Punica granatum; commercially available as Pomella®), showed anti-glycation effects and protected human keratinocytes against ROS-induced cytotoxicity.9 Our group has also reported on the development of a proprietary phenolic-enriched botanical extract from red maple (Acer rubrum) leaves (known as Maplifa™)10 as a botanical ingredient for dietary supplement and cosmeceutical applications. Maplifa™ showed several skin beneficial effects including antioxidant, anti-glycation effects, and anti-melanogenic activity.10, 11 However, the protective effects of Maplifa™ and its major phenolic compound, ginnalin A (GA), against oxidative stress in human keratinocytes remain unexplored. Herein, we aimed to investigate the cytoprotective effects of Maplifa™ and GA against hydrogen peroxide (H2O2) and MGO-induced oxidative stress in human keratinocytes HaCaT cells.
2. Materials and Methods
2.1. Chemicals and reagents
Maplifa™, a proprietary phenolic-enriched red maple leaf extract, was prepared with protocols developed in our laboratory.10 The phytochemical composition of Maplifa™ has been extensively studied by our laboratory with over 100 phenolics identified.12 Maplifa™ was standardized to ginnalin A (GA) content (c.a. 45%), which is the major phenolic compound present in the extract.10 Methylglyoxal (MGO), hydrogen peroxide (H2O2), crystal violet powder, Hoechst 33342 staining agent, dimethyl sulfoxide (DMSO), and 2’,7’-dichlorofluorescin diacetate (DCFDA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). MitoTracker™ Red CMXRos, and Annexin and propidium iodide (PI) staining kits were purchased from Thermo Fisher Scientific (Waltham, MA, USA). MCellTiter-Glo® (CTG) 2.0 and caspase-Glo® assay kits (caspase- 3/7, −8, and −9) were purchased from Promega (Fitchburg, WI, USA).
2.2. Cell culture and sample preparation
Human keratinocytes (HaCaT cells) were purchased from the American Type Culture Collection (ATCC, Rockville, USA). HaCaT cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS; GIBCO™, Grand Island, NY, USA) at 37 °C in the presence of 5% CO2. Stock solution of test samples were prepared in DMSO and diluted with cell culture medium without FBS to desired concentrations (6.25–200 μg/mL for Maplifa™ and 6.25–200 μM for GA). The final percentage of DMSO in the treatments groups was less than 0.2% and HaCaT cells in all of the control groups were treated with medium containing 0.2% of DMSO.
2.3. Measurement of cell viability
The effects of Maplifa™ and GA on the viability of HaCaT cells were determined by the CTG 2.0 assay.13 The model for the H2O2−induced cell damage was according to our previously reported method with minor modifications.9 In brief, HaCaT cells were seeded in 96-well plates at 1×104 cells per well and allowed to attach for 12 h. Media was then removed and cells were incubated with test samples for 12 h, following treatment of H2O2 (400 μM) for 24 h. CTG 2.0 reagent was then added in each well and the plate was kept at room temperature for 10 min. Luminescence intensity was recorded using a Spectramax M2 plate reader (Molecular Devices, Sunnyvale, CA, USA). The MGO-induced cell damage model was constructed similarly with minor modifications. HaCaT cells were seeded in 96-well plates at 5×103 cells per well and allowed to attach for 12 h. After incubation, the cells were treated with test samples for 2 h. The cells were washed with PBS twice and treated with MGO (400 μM) for 24 h, and then cell viability was measured using the CTG 2.0 assay.
2.4. Measurement of reactive oxygen species (ROS)
HaCaT cells were seeded in 96-well plates at 1×104 cells or 5×103 cells per well for H2O2-induced or MGO-induced cell damage model, respectively, for 12 h. Next, cells were treated with test samples for 12 h (in H2O2-induced model) or 2 h (in MGO-induced model). Then cell culture media were removed and cells were washed twice with phosphate-buffered saline (PBS). Media containing a fluorescent agent (DCFDA; 20 μM) were added to the cells and incubated for 20 mins. Next, HaCaT cells were washed with PBS to remove excessive exogenous ROS and cells were treated with H2O2 or MGO (both at 400 μM) for 1 h or 24 h, respectively. Cellular fluorescence intensity was measured with excitation and emission wavelengths of 485 and 525 nm, respectively, using a Spectramax M2 plate reader (Molecular Devices, Sunnyvale, CA, USA).
2.5. Detection of cell apoptosis
Flow cytometric assays for the measurements of cell apoptosis were conducted as previously reported.9 HaCaT cells were seeded in 6-well plates at 3×105 cells per well and allowed to attach for 12 h, followed by the treatment with test samples for 6 h. Cell culture media were then removed, and cells were washed twice with PBS. Next, H2O2 (400 μM) was added to cells and incubated for 24 h, then cells were harvested and stained with binding buffer containing Annexin and PI agents for 15 mins in the dark. Cell suspensions were quantified using flow cytometry and data were analyzed using FlowJo software.
2.6. Measurements of caspases-3/7, −8, and −9
Levels of caspases −3/7, −8, and −9 were measured as previously reported.9 HaCaT cells were seeded in 96-well plates at 5×103 cells per well and allowed to attach for 12 h, followed by treatment of test samples for 6 h. Media were then removed and cells were washed twice with PBS. Next, H2O2 (400 μM) was added and incubated for 24 h followed by adding caspase-Glo kit reagents. Plates were then incubated at room temperature for 30 min and the luminescence intensity of each well was read using a Spectramax M2 plate reader.
2.7. Detection of total and mitochondria-derived ROS
In the MGO-induced cell damage model, HaCaT cells were seeded in 6-well plates at 3×105 cells per well and allowed to attach for 12 h. Cells were then treated with test samples for 2 h and washed twice with PBS, before adding MGO (400 μM) for 24 h. Cells were then harvested and stained with MitoTracker™ Red agents for 15 mins in dark. Cell suspensions were quantified using flow cytometry (BD FACSCalibur, San Jose, CA, USA) and data were analyzed using FlowJo software (FlowJo, Ashland, OR, USA).
2.8. Statistical analysis
Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). Data are expressed as the mean value ± standard deviation (S.D.) obtained from triplicates of experiments. The significance of differences was determined using a two-way analysis of variance (ANOVA) followed by a post hoc Student-Newman–Keuls multiple comparison test (SNK). P < 0.05, P < 0.01, or P < 0.001 was determined as significant.
3. Results and discussion
3.1. Maplifa™ and GA reduced H2O2− and MGO-induced cytotoxicity in HaCaT
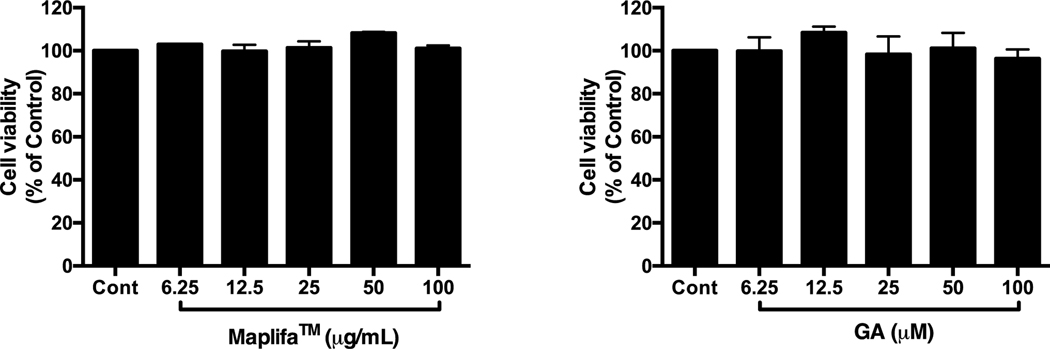
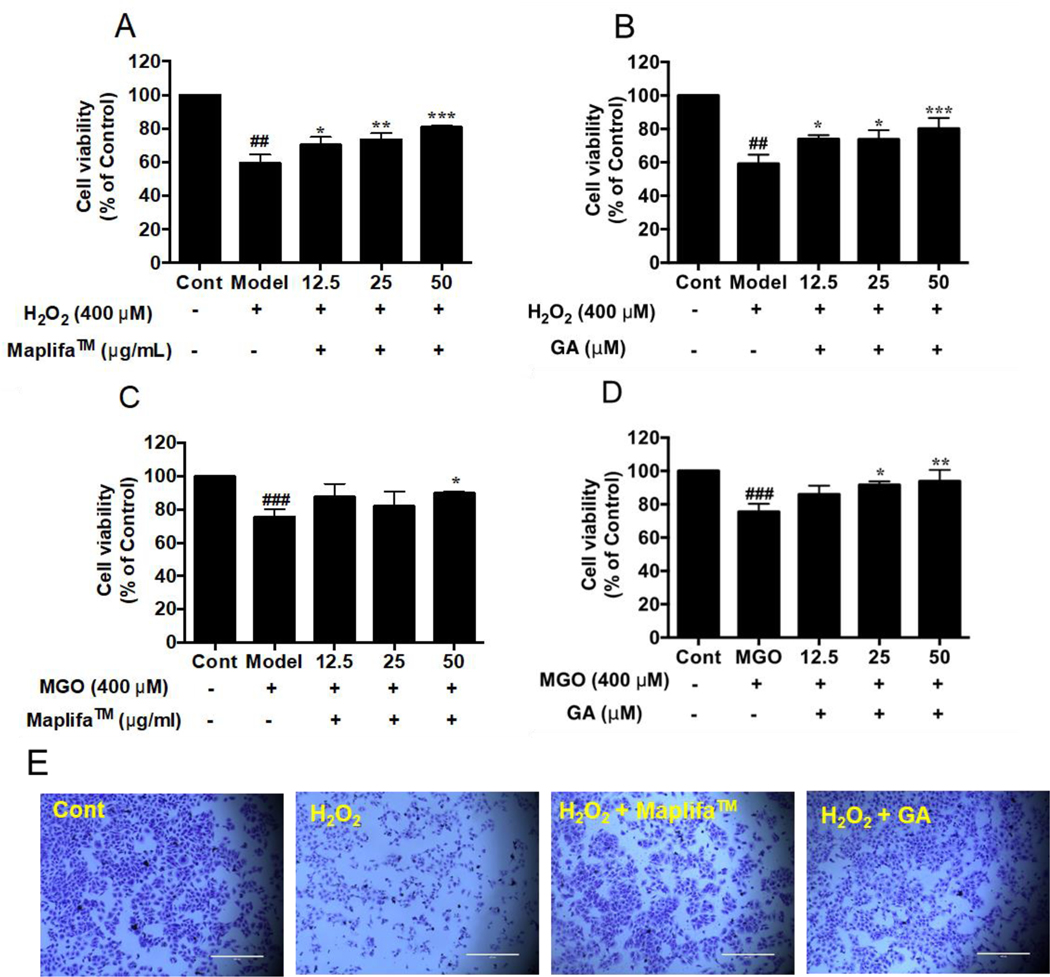
Prior to evaluating the protective effects of Maplifa™ and GA, their range of non-cytotoxic concentrations in HaCaT cells were determined by the CTG 2.0 cell viability assay. Maplifa™ and GA, at concentrations ranging from 6.25–100 μg/mL and 6.25–100 μM, respectively, did not induce cytotoxicity in HaCaT cells (cell viability >96.0%; Fig. 1). Next, concentrations of 12.5, 25, and 50 μg/mL (for Maplifa™) and 12.5, 25, and 50 μM (for GA) were selected for further evaluation. Hydrogen peroxide (H2O2; reactive oxygen species) and methylglyoxal (MGO; reactive carbonyl species) were used as oxidative inducers and their cytotoxic effects were evaluated in HaCaT cells. H2O2 (at 400 μM) induced cytotoxicity by decreasing viability of HaCaT cells to 59.2% (Fig. 2A). Similarly, treatment of MGO (400 μM) reduced viability of HaCaT cells to 61.6% (Fig. 2B). Maplifa™ and GA showed cytoprotective effects by increasing the viability of HaCaT cells exposed to H2O2 (Fig. 2A and B) and MGO (Fig. 2C and D). Both Maplifa™ (12.5, 25, and 50 μg/mL) and GA (12.5, 25, and 50 μM) increased the viability of H2O2 challenged cells by 11.1–21.5% and 13.8–21.0%, respectively. Maplifa™, at the highest test concentration (50 μg/mL), maintained 89.7% viable cells as compared to the control group, while GA at 12.5, 25, and 50 μM, maintained cell viability at 85.9%, 91.7%, and 93.9%, respectively. The ameliorative effect of Maplifa™ and GA in H2O2-treated HaCaT cells was further supported by morphological analysis with crystal violet staining methods (Fig. 2E). Exposure to H2O2 impaired the integrity of cell nuclei which was shown as deformable shapes of stained nuclei whilst the treatment of Maplifa™ (50 μg/mL) and GA (50 μM) redeemed the normal shape of cell nuclei. Similarly, exposure to MGO (at 400 μM) led to significant morphological changes of HaCaT cells. However, Maplifa™ (50 μg/mL) and GA (50 μM) did not change the shape of cell nuclei as compared to the MGO-treated group.
Fig. 1.
Effect of Maplifa™ and GA on viability of HaCaT cells. HaCaT cells were treated with (A) Maplifa™ (12.5, 25, 50, and 100 μg/mL) or (B) GA (12.5, 25, 50, and 100 μM) for 24 h. Cell viabilities were measured using CTG2.0 assay.
Fig. 2.
Effects of Maplifa™ and GA on the viability of HaCaT cells insulted with H2O2 or MGO. (A and C) Maplifa™ (12.5, 25, and 50 μg/mL) or (B and D) GA (12.5, 25, and 50 μM) was incubated with HaCaT cells for 12 h. Then cells were insulted with H2O2 (400 μM) or MGO (400 μM) and further incubated for 24 h. (E) Representative microscopic images of H2O2-insulted HaCaT cells treated with Maplifa™ or GA. HaCaT cells were stained with crystal violet. ##p < 0.01 and ###p < 0.001 as compared with control group; *p <0.05, **p <0.01, and ***p <0.001 as compared with model group.
Hydrogen peroxide, one of the most common form of ROS, has been utilized as an inducer of oxidative stress and cytotoxicity in experimental models.14 Previously reported studies support that H2O2-induced cell death can be ameliorated by dietary hydrolyzable tannins including ellagitannins (e.g. punicalagin) and gallotannins (e.g. penta-O-galloyl-β-D-glucose) in human skin cells.9, 15 MGO, a byproduct formed during the oxidation of glucose and a precursor of AGEs, is also considered as a detrimental factor for skin cells as it exacerbates oxidative stress induced skin cytotoxicity, which further leads to many diabetes related dermatological complications.5 To date, a few studies have shown that MGO-induced cell death in human keratinocytes can be alleviated by some synthetic compounds,16, 17 but there have been no prior reports to show that natural maple-derived phenolics exert protective effects in HaCaT cells. It is possible that Maplifa™ and GA counteracted the MGO-induced toxicity in HaCaT cells by their antioxidant capacity. This is supported by our previous report that the inhibitory effects of Maplifa™ and GA against the formation of MGO-induced glycation were attributed to their antioxidant capacity rather than their ability to directly scavenge MGO.11 Therefore, we further evaluated the antioxidant activity of Maplifa™ and GA in the HaCaT cells.
3.2. Maplifa™ and GA reduced H2O2− and MGO-induced production of ROS in HaCaT
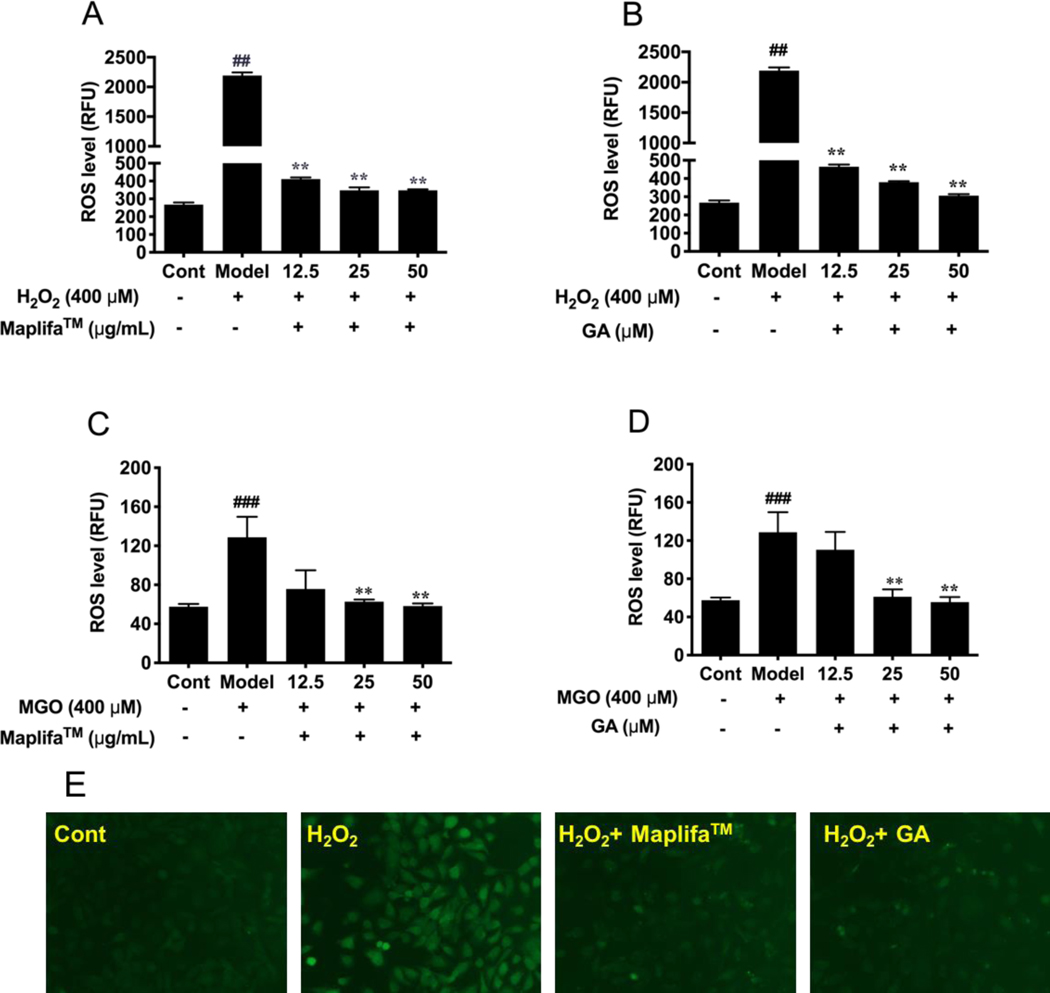
To further understand the cytoprotective of Maplifa™ and GA in HaCaT cells against cellular oxidative stress, we evaluated whether Maplifa™ and GA can diminish H2O2− and MGO-induced production of ROS. HaCaT cells responded to the oxidative stimulation of H2O2 by producing cellular ROS by 8.2-fold as compared to the control group. Maplifa™ (at 12.5, 25, and 50 μg/mL) showed cytoprotective effect in the HaCaT cells by reducing the production of H2O2-induced ROS by 81.2, 84.1, and 84.1%, respectively. Similarly, treatment of GA (12.5, 25, and 50 μM) effectively reduced the level of ROS in HaCaT cells by 78.8, 82.7, and 86.0%, respectively, compared to the H2O2-treated group. In addition, the protective effects of Maplifa™ and GA against MGO-induced oxidative stress in HaCaT cells were evaluated. MGO (400 μM) elevated the production of ROS in HaCaT cells by 2.2-fold as compared to the control group (Fig. 3C and D). Treatment with Maplifa™ (12.5, 25, and 50 μg/mL) alleviated MGO-induced oxidative stress by reducing the level of ROS in HaCaT cells by 41.2, 51.3, and 54.7%, respectively, while treatment with GA (12.5, 25, and 50 μM) also reduced ROS production by 14.2, 52.4, and 56.8%, respectively. This effect was further supported by the morphological analysis of confocal images of cells stained with DCFDA agent (Fig. 3E).
Fig. 3.
Effects of Maplifa™ and GA on the H2O2− and MGO-induced ROS production. Cells were treated with (A and C) Maplifa™ (12.5, 25, and 50 μg/mL) for 12 h or (B and D) GA (12.5, 25, and 50 μM) for 2 h. Then cells were incubated with DMEM containing DCFDA (20 μM) for 20 mins, followed by exposure of H2O2 or MGO (both at 400 μM) for 1 h or 24 h, respectively. ROS level was measured by cellular fluorescence intensity with excitation and emission wavelengths of 485 and 525 nm, respectively. (E) Representative fluorescent images of H2O2− and MGO-insulted HaCaT cells treated with Maplifa™ or GA. ##p < 0.01 and ###p < 0.001 as compared with control group; **p <0.01 as compared with model group.
Although our laboratory has reported that GA reduced the production of H2O2-induced ROS in murine melanoma B16F10 cells,10 this is the first study to show GA’s antioxidant effects against ROS-induced stress in human keratinocytes. The cytoprotective effects of red maple phenolics including GA have been associated with its modulation of antioxidant related genes and proteins.19 For instance, GA was reported to have chemopreventive effects mediated by its activation of antioxidant related signaling pathways including [NAD(P)H quinone dehydrogenase 1; (NQO1)], heme oxygenase-1 (HO-1), and nuclear factor erythroid 2-related factor 2 (Nrf2) in colon cancer cells.20 Notably, the transcription factor, Nrf2, has been reported to play a pivotal role in the protective effects of several phenolic compounds against oxidative stress21. In addition, Nrf2 has been reported to regulate the antioxidant effects of phenolic compounds by the mediation of redox-sensitive anti-inflammatory signaling pathways including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)22, 23. Therefore, it is possible that GA’s antioxidant activity is attributed to its mediation of antioxidant and anti-inflammatory transcription factors including Nrf2 and NF-κB. However, further studies are warranted to confirm this.
3.3. Maplifa™ and GA alleviated H2O2-induced apoptosis in HaCaT cells
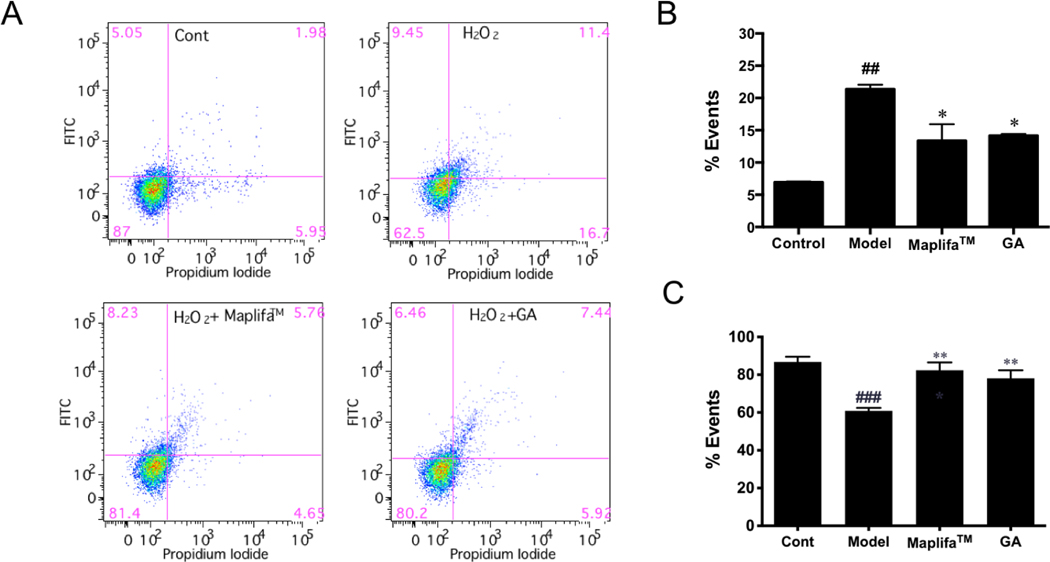
The cytoprotective effects of Maplifa™ and GA in HaCaT cells against H2O2-induced apoptosis were evaluated by flow cytometry analysis. The rate of apoptotic cells in the control group was 6.9 %, which was elevated to 21.4 % after exposure to H2O2 (400 μM; Fig. 4). Maplifa™ (50 μm/mL) and GA (50 μM) showed anti-apoptosis effects by reducing the population of apoptotic cells by 8.0 and 7.2%, respectively, as compared to the H2O2-treated group (Fig. 4B). The population of viable cells in the control group was reduced from 86.8% to 60.8% after exposure to H2O2 (400 μM), and both Maplifa™ (50 μm/mL) and GA (50 μM) showed protective effects by increasing the population of viable cells to 82.4 and 78.1%, respectively (Fig. 4C). In contrast to the pro-apoptotic effect induced by the H2O2 stimulation, no significant increase of apoptotic cells was observed in the MGO-treated group (Supplementary Materials; Fig. S1), suggesting that the keratinocytes responded to H2O2− and MGO-induced oxidative stress via different pathways. It has been observed that MGO can cause apoptosis in HaCaT cells with a longer incubation time (48 h),16 thus, further studies on the mechanisms of MGO-induced cell death, and whether Maplifa™ and GA can mitigate these detrimental effects were explored.
Fig. 4.
Effects of Maplifa™ and GA on H2O2-induced apoptosis in HaCaT cells. (A) Flow cytometry graphs showing apoptotic cell populations (annexin V+/PI− and annexin V+/PI+) and viable cell populations (annexin V−/PI− ) of HaCaT cells with or without treatments of Maplifa™ and GA were quantified by gated patterns in double stains. (B-C) HaCaT cells stained with annexin V-FITC/PI and assayed by flow cytometry. The flow cytometry graphs show the population of cells from one representative values of three separate experiments. ##p < 0.01 as compared with control group; *p <0.05 as compared with model group.
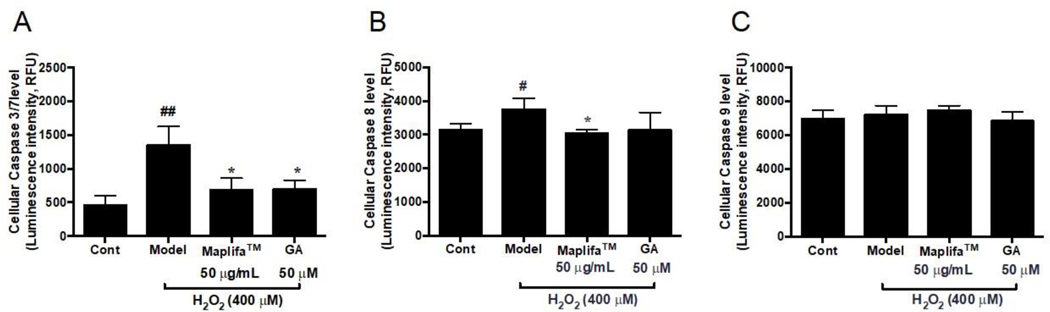
3.4. Maplifa™ down-regulated activity of enzymes caspase-3/7 and −8 in HaCaT cells
The anti-apoptotic effect of Maplifa™ and GA were further investigated by measuring their effects on the level of apoptosis-related enzymes, caspases-3/7, −8, and −9 in HaCaT cells. Stimulation with H2O2 significantly increased the level of caspases-3/7 (by 1.92-fold) and −8 (by 19.5%) as compared to the control group, while not affecting caspase 9 (Fig. 5). Maplifa™ counteracted H2O2-induced upregulation of caspases-3/7 and −8 level by 49.5 and 19.0%, respectively, while GA only reduced caspase-3/7 (by 48.1%). No significant changes of caspase-9 were observed in the Maplifa™ and GA treated groups (Fig. 5).
Fig. 5.
Effects of Maplifa™ and GA on (A) cellular caspase-3/7, (B) caspase-8, and (C) caspase-9 in HaCaT cells exposed to H2O2. HaCaT cells were incubated with Maplifa™ (50 μg/mL) and GA (50 μM) for 12 h before H2O2 induction. #p < 0.05 and ##p < 0.01 as compared with control group; *p < 0.05 as compared with model group.
Caspases are checkpoint proteases responsible for the initiation of cell death (apoptosis) and their activation can be triggered by H2O2 and other oxidative stress inducers.24 Results from our study show that caspases-3/7 and −8, caspase isoforms which mediate cell death via the extrinsic pathway of apoptosis, were elevated by the stimulation of H2O2 in HaCaT cells, and diminished by the treatment of Maplifa™ and GA. This suggested that Maplifa™ and GA may protect HaCaT cells against apoptosis triggered by extracellular ligands binding to cell-surface death receptors.25 This is in agreement with our previously reported study showing that punicalagin, the major polyphenol in a pomegranate fruit extract, protected HaCaT cells from H2O2-induced apoptosis though the modulation of caspases-3/7 and −8 level.9 Conversely, caspase-9, the initiator of apoptosis that senses endogenous oxidative stress, was not affected by stimulation with H2O2 in the HaCaT cells. Since studies have shown that caspase-9 activation is involved in the UV- and ROS-induced apoptosis of human keratinocytes,26, 27 further studies are warranted to delineate the signaling pathways of oxidative stress induced apoptosis in human keratinocytes.
3.5. Maplifa™ and GA decreased total ROS production but increased mitochondria-derived ROS production in MGO-stimulated HaCaT cells
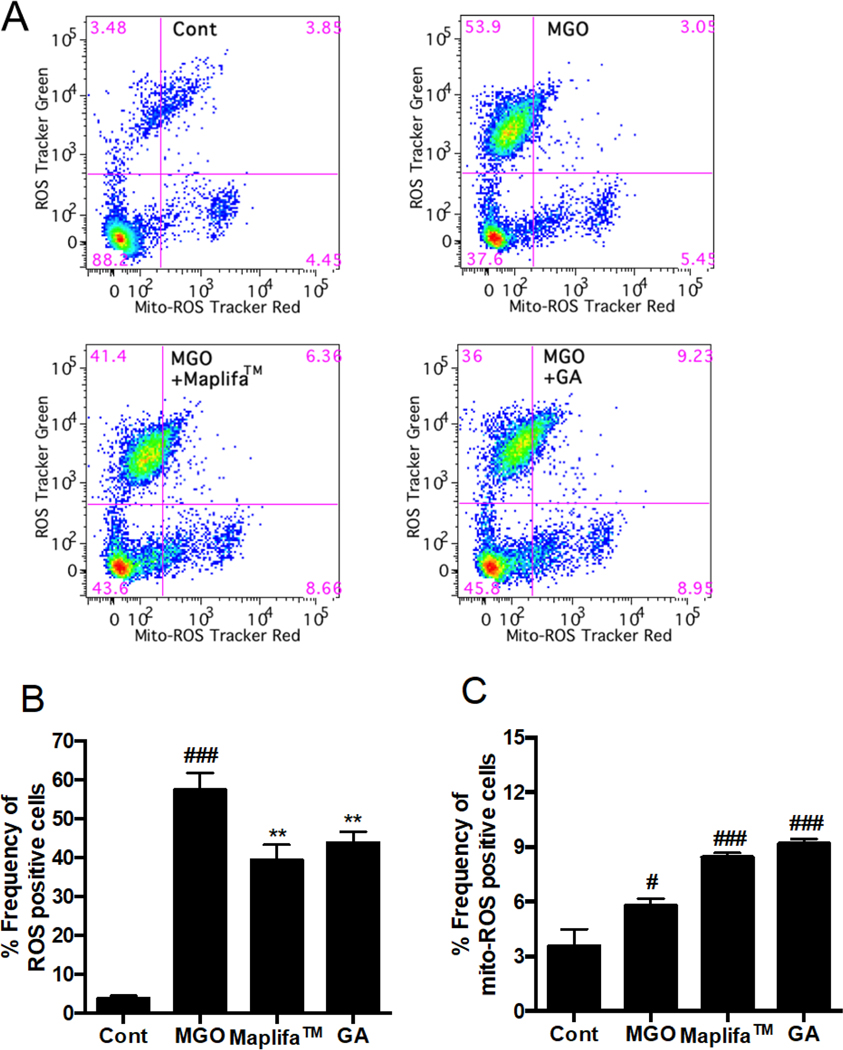
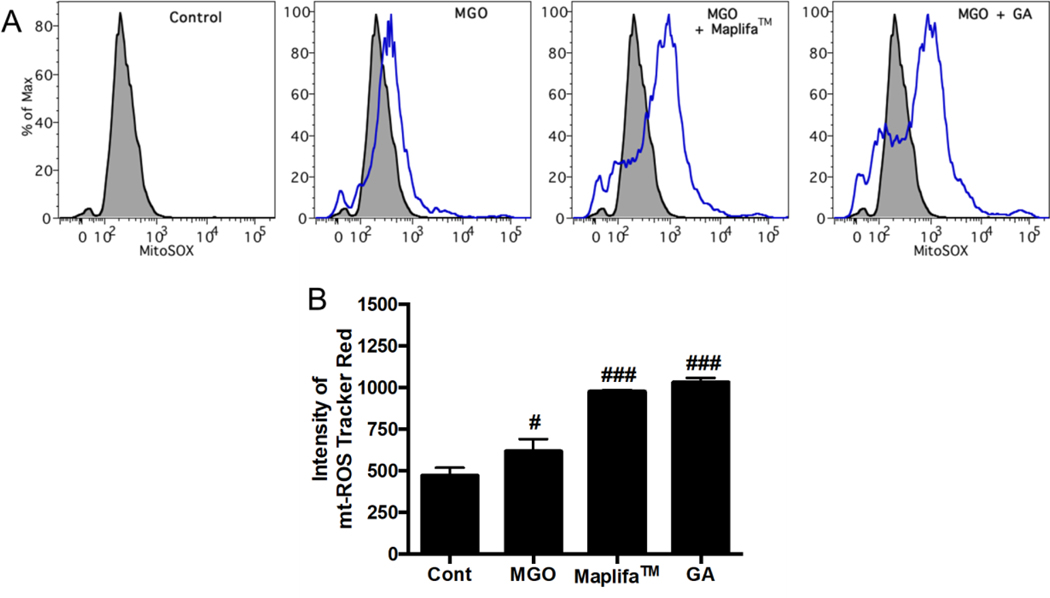
It is possible that Maplifa™ and GA protected HaCaT cells against MGO-induced cell death via their antioxidant activity, rather than through anti-apoptotic effects. This is because MGO did not induce apoptosis in HaCaT cells suggesting that ROS may play a critical role in MGO’s cytotoxic effects. Although DCFDA is a common chemical probe used for the measurement of ROS as shown in the aforementioned section (Fig. 3), it only provides qualitative information on the production of cellular oxidants in the fluorescent based assay18. Therefore, we further characterized the antioxidant effects of Maplifa™ and GA on MGO-induced production of both total ROS and mitochondria-derived ROS in HaCaT cells with quantitative flow cytometry assays. The total cellular ROS was measured with fluorescent dye DCFDA and stimulation by MGO (400 μM) significantly increased fluorescent intensity by 13.5-fold compared to the control group. Treatment of Maplifa™ (50 μg/mL) and GA (50 μM) counteracted MGO-induced total ROS production by 31.2 and 23.6%, respectively (Fig. 6). In addition, MGO increased specific fluorescent intensity for mitochondria-derived ROS by 30.9%, compared to control group. However, Maplifa™ and GA did not prevent MGO-stimulated production of mitochondria-derived ROS (Fig. 6C). In order to confirm this effect, a mitochondria-specific probe, namely, MitoSOX™, was applied to assess the levels of mitochondria-derived ROS in HaCaT cells (Fig. 7). ROS level, generated from mitochondria, increased by 30.9% in the MGO-treated group and was further increased by the treatment of Maplifa™ and GA (by 1.1- and 1.2-fold, respectively). Results from detection by both ROS tracker Green and MitoSOX™ methods confirmed that Maplifa™ and GA enhanced the production of mitochondria-derived ROS in cells stimulated with MGO.
Fig. 6.
Effects of Maplifa™ and GA on MGO-induced production of mitochondria-derived ROS. HaCaT cells were treated with Maplifa™ (50 μg/mL) or GA (50 μM) for 2 h, followed by exposure of MGO (400 μM) for 24 h. Next, the cells were incubated with 20 μM DCFDA for 20 mins. After the cells were washed, 5 μM MitoSOX™ reagent working solution was added and incubated for 10 mins and the cells were assayed by flow cytometry (A). The frequency of ROS positive cells (B) and frequency of mitochondria-derived ROS positive cells were quantified (C) with FlowJo. #p < 0.05 and ###p < 0.001 as compared with control; **p < 0.01 as compared with model goup.
Fig. 7.
Effects of Maplifa™ and GA on MGO-induced production of mitochondria-derived ROS. HaCaT cells were treated with Maplifa™ (50 μg/mL) or GA (50 μM) for 2 h, followed by exposure of MGO (400 μM) for 24 h. Next, the cells were incubated with 5 μM MitoSOX™ reagent working solution for 10 mins and assayed by flow cytometry (A). Intensity of mitochondria-derived ROS measured by Tracker Red were quantified with FlowJo (B). #p < 0.05 and ###p < 0.001 as compared with control group.
ROS-induced intracellular and extracellular oxidative stress is a key factor for skin cellular damage, which leads to skin cell death and greatly contributes to skin aging process.28 Evidence from extensive studies have demonstrated that antioxidants including many dietary natural products exert skin protective effects by reducing ROS production in skin cells.29, 30 Our previously reported study was in agreement with this proposition as phenolics from pomegranate extract alleviated H2O2-induced cytotoxicity in HaCaT cells through reducing the production of cellular ROS.9 Similarly, Maplifa™ and GA reduced the production of MGO-induced total ROS, which may contribute to their overall cytoprotective effects in HaCaT cells. Maplifa™ and GA also increased the levels of specific mitochondria-derived ROS in MGO-treated cells, which seemed contradictory to the results from the measurement of total production of ROS. However, this effect may be justified by the fact that ROS plays complex dual roles in prooxidant and antioxidant pathways. Studies have shown that ROS can also serve as a stress signal that triggers various redox-sensitive signaling pathways, which may lead to protective functions against oxidative stress in microorganism.31 For instance, mitochondria produced ROS is crucial for Caenorhabditis elegans to maintain normal lifespan and increased ROS production even prolonged their lifespan.32, 33 Therefore, it is possible that treatment of Maplifa™ and GA may boost mitochondria oxidative phosphorylation, and consequently trigger signaling pathways as a response to simulation by MGO in HaCaT cells. However, further investigations on the mechanisms of Maplifa™ and GA’s protective effects against MGO-induced oxidative stress are warranted.
In summary, the cytoprotective effects of Maplifa™ and GA against H2O2-and MGO-induced oxidative stress in human keratinocytes were evaluated. Maplifa™ and GA ameliorated H2O2-and MGO-induced cell death and cellular ROS production. Furthermore, the cytoprotective effects Maplifa™ and GA were attributed to their anti-apoptotic activity by reducing the population of apoptotic cells and downregulating the levels of apoptosis related enzymes including caspases-3/7 and −8 in the H2O2-challenged cells. Maplifa™ and GA displayed distinct effects in the MGO-induced oxidative stress model as they diminished the total ROS production whilst increasing the level of mitochondria-derived ROS. Findings from this study support the potential skin beneficial effects of Maplifa™ and GA. To develop Maplifa™ and GA as bioactive ingredients for cosmeceutical and/or dermatological applications, further investigations on their mechanisms of action are warranted.
Supplementary Material
Acknowledgements
Data were acquired from instruments located at the University of Rhode Island in the RI-INBRE core facility obtained from Grant P20GM103430 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). C.L. was supported by a teaching assistantship from College of Pharmacy, the University of Rhode Island.
HM and NPS are co-inventors on a patent application (International Publication Number: WO 2015/154074 A9) on the skin-whitening applications of maple gallotannins.
Footnotes
Declarations of interest:
The other authors declare no conflicts of interest.
REFERENCES
- 1.Cronin H. and Draelos ZD, Top 10 botanical ingredients in 2010 anti-aging creams, J Cosmet Dermatol, 2010, 9, 218–225. [DOI] [PubMed] [Google Scholar]
- 2.Iriti M. and Faoro F, Grape phytochemicals: a bouquet of old and new nutraceuticals for human health, Med Hypotheses, 2006, 67, 833–838. [DOI] [PubMed] [Google Scholar]
- 3.Liu RH, Dietary bioactive compounds and their health implications, Journal of food science, 2013, 78, A18–A25. [DOI] [PubMed] [Google Scholar]
- 4.Farage MA, Miller KW, Elsner P. and Maibach HI, Intrinsic and extrinsic factors in skin ageing: a review, Int J Cosmet Sci, 2008, 30, 87–95. [DOI] [PubMed] [Google Scholar]
- 5.Radjei S, Friguet B, Nizard C. and Petropoulos I, Prevention of dicarbonyl-mediated advanced glycation by glyoxalases: implication in skin aging, Biochem Soc Trans, 2014, 42, 518–522. [DOI] [PubMed] [Google Scholar]
- 6.Gkogkolou P. and Bohm M, Advanced glycation end products: Key players in skin aging?, Dermatoendocrinol, 2012, 4, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elosta A, Ghous T. and Ahmed N, Natural products as anti-glycation agents: possible therapeutic potential for diabetic complications, Current diabetes reviews, 2012, 8, 92–108. [DOI] [PubMed] [Google Scholar]
- 8.Liu W, Ma H, DaSilva NA, Rose KN, Johnson SL, Zhang L, Wan C, Dain JA and Seeram NP, Development of a neuroprotective potential algorithm for medicinal plants, Neurochem Int, 2016, 100, 164–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Guo H, DaSilva NA, Li D, Zhang K, Wan Y, Gao X-H, Chen H-D, Seeram NP and Ma H, Pomegranate (Punica granatum) phenolics ameliorate hydrogen peroxide-induced oxidative stress and cytotoxicity in human keratinocytes, Journal of functional foods, 2019, 54, 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma H, Xu J, DaSilva NA, Wang L, Wei Z, Guo L, Johnson SL, Lu W, Xu J, Gu Q. and Seeram NP, Cosmetic applications of glucitol-core containing gallotannins from a proprietary phenolic-enriched red maple (Acer rubrum) leaves extract: inhibition of melanogenesis via down-regulation of tyrosinase and melanogenic gene expression in B16F10 melanoma cells, Arch Dermatol Res, 2017, 309, 265–274. [DOI] [PubMed] [Google Scholar]
- 11.Ma H, Liu W, Frost L, Kirschenbaum LJ, Dain JA and Seeram NP, Glucitol-core containing gallotannins inhibit the formation of advanced glycation end-products mediated by their antioxidant potential, Food Funct, 2016, 7, 2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C. and Seeram NP, Ultra‐fast liquid chromatography coupled with electrospray ionization time‐of‐flight mass spectrometry for the rapid phenolic profiling of red maple (Acer rubrum) leaves, Journal of separation science, 2018, 41, 2331–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DaSilva NA, Nahar PP, Ma H, Eid A, Wei Z, Meschwitz S, Zawia NH, Slitt AL and Seeram NP, Pomegranate ellagitannin-gut microbial-derived metabolites, urolithins, inhibit neuroinflammation in vitro, Nutr Neurosci, 2019, 22, 185–195. [DOI] [PubMed] [Google Scholar]
- 14.Gille JJ and Joenje H, Cell culture models for oxidative stress: superoxide and hydrogen peroxide versus normobaric hyperoxia, Mutat Res, 1992, 275, 405–414. [DOI] [PubMed] [Google Scholar]
- 15.Kim BH, Choi MS, Lee HG, Lee SH, Noh KH, Kwon S, Jeong AJ, Lee H, Yi EH, Park JY, Lee J, Joo EY and Ye SK, Photoprotective potential of penta-o-galloyl-beta-dglucose by targeting NF-kappaB and MAPK signaling in UVB radiation-induced human dermal fibroblasts and mouse skin, Mol Cells, 2015, 38, 982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang CT, Zhao Y, Xian M, Li JH, Dong Q, Bai HB, Xu JD and Zhang MF, A novel controllable hydrogen sulfide-releasing molecule protects human skin keratinocytes against methylglyoxal-induced injury and dysfunction, Cell Physiol Biochem, 2014, 34, 1304–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang CT, Meng FH, Chen L, Li X, Cen LJ, Wen YH, Li CC and Zhang H, Inhibition of methylglyoxal-induced AGEs/RAGE expression contributes to dermal protection by N-Acetyl-L-Cysteine, Cell Physiol Biochem, 2017, 41, 742–754. [DOI] [PubMed] [Google Scholar]
- 18.Hardy M, Zielonka J, Karoui H, Sikora A, Michalski R, Podsiadly R, Lopez M, Vasquez-Vivar J, Kalyanaraman B. and Ouari O, Detection and characterization of reactive oxygen and nitrogen species in biological systems by monitoring species-specific products, Antioxid Redox Signal, 2018, 28, 1416–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bi W, Gao Y, Shen J, He C, Liu H, Peng Y, Zhang C. and Xiao P, Traditional uses, phytochemistry, and pharmacology of the genus Acer (maple): A review, J Ethnopharmacol, 2016, 189, 31–60. [DOI] [PubMed] [Google Scholar]
- 20.Bi W, He CN, Li XX, Zhou LY, Liu RJ, Zhang S, Li GQ, Chen ZC and Zhang PF, Ginnalin A from Kujin tea (Acer tataricum subsp. ginnala) exhibits a colorectal cancer chemoprevention effect via activation of the Nrf2/HO-1 signaling pathway, Food Funct, 2018, 9, 2809–2819. [DOI] [PubMed] [Google Scholar]
- 21.Valenzuela R, Illesca P, Echeverria F, Espinosa A, Rincon-Cervera MA, Ortiz M, Hernandez-Rodas MC, Valenzuela A. and Videla LA, Molecular adaptations underlying the beneficial effects of hydroxytyrosol in the pathogenic alterations induced by a high-fat diet in mouse liver: PPAR-alpha and Nrf2 activation, and NF-kappaB down-regulation, Food Funct, 2017, 8, 1526–1537. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez-Rodas MC, Valenzuela R, Echeverria F, Rincon-Cervera MA, Espinosa A, Illesca P, Munoz P, Corbari A, Romero N, Gonzalez-Manan D. and Videla LA, Supplementation with docosahexaenoic acid and extra virgin olive oil prevents liver steatosis induced by a high-fat diet in mice through PPAR-alpha and Nrf2 upregulation with concomitant SREBP-1c and NF-kB downregulation, Mol Nutr Food Res, 2017, 61. [DOI] [PubMed]
- 23.Echeverria F, Valenzuela R, Bustamante A, Alvarez D, Ortiz M, Espinosa A, Illesca P, Gonzalez-Manan D. and Videla LA, High-fat diet induces mouse liver steatosis with a concomitant decline in energy metabolism: attenuation by eicosapentaenoic acid (EPA) or hydroxytyrosol (HT) supplementation and the additive effects upon EPA and HT co-administration, Food Funct, 2019, 10, 6170–6183. [DOI] [PubMed] [Google Scholar]
- 24.Li J. and Yuan J, Caspases in apoptosis and beyond, Oncogene, 2008, 27, 6194–6206. [DOI] [PubMed] [Google Scholar]
- 25.Schneider P. and Tschopp J, in Pharmacochemistry Library, Elsevier, 2000, vol. 31, pp. 281–286. [Google Scholar]
- 26.Sitailo LA, Tibudan SS and Denning MF, Activation of caspase-9 is required for UV-induced apoptosis of human keratinocytes, Journal of Biological Chemistry, 2002, 277, 19346–19352. [DOI] [PubMed] [Google Scholar]
- 27.Rezvani HR, Mazurier F, Cario-Andre M, Pain C, Ged C, Taieb A. and de Verneuil H, Protective effects of catalase overexpression on UVB-induced apoptosis in normal human keratinocytes, J Biol Chem, 2006, 281, 17999–18007. [DOI] [PubMed] [Google Scholar]
- 28.Masaki H, Role of antioxidants in the skin: anti-aging effects, J Dermatol Sci, 2010, 58, 85–90. [DOI] [PubMed] [Google Scholar]
- 29.Pacheco-Palencia LA, Noratto G, Hingorani L, Talcott ST and Mertens-Talcott SU, Protective effects of standardized pomegranate (Punica granatum L.) polyphenolic extract in ultraviolet-irradiated human skin fibroblasts, J Agric Food Chem, 2008, 56, 8434–8441. [DOI] [PubMed] [Google Scholar]
- 30.Svobodova A, Psotova J. and Walterova D, Natural phenolics in the prevention of UV-induced skin damage. A review, Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, 2003, 147, 137–145. [PubMed] [Google Scholar]
- 31.Santos AL, Sinha S. and Lindner AB, The good, the bad, and the ugly of ROS: New insights on aging and aging-related diseases from eukaryotic and prokaryotic model organisms, Oxid Med Cell Longev, 2018, 2018, 1941285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doonan R, McElwee JJ, Matthijssens F, Walker GA, Houthoofd K, Back P, Matscheski A, Vanfleteren JR and Gems D, Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans, Genes & development, 2008, 22, 3236–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H. and Kenyon C, Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans, Nature, 2003, 424, 277–283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.