Abstract
Background and Aims
Pooideae grasses contain some of the world’s most important crop and forage species. Although much work has been conducted on understanding the genetic basis of trait diversification within a few annual Pooideae, comparative studies at the subfamily level are limited by a lack of perennial models outside ‘core’ Pooideae. We argue for development of the perennial non-core genus Melica as an additional model for Pooideae, and provide foundational data regarding the group’s biogeography and history of character evolution.
Methods
Supplementing available ITS and ndhF sequence data, we built a preliminary Bayesian-based Melica phylogeny, and used it to understand how the genus has diversified in relation to geography, climate and trait variation surveyed from various floras. We also determine biomass accumulation under controlled conditions for Melica species collected across different latitudes and compare inflorescence development across two taxa for which whole genome data are forthcoming.
Key Results
Our phylogenetic analyses reveal three strongly supported geographically structured Melica clades that are distinct from previously hypothesized subtribes. Despite less geographical affinity between clades, the two sister ‘Ciliata’ and ‘Imperfecta’ clades segregate from the more phylogenetically distant ‘Nutans’ clade in thermal climate variables and precipitation seasonality, with the ‘Imperfecta’ clade showing the highest levels of trait variation. Growth rates across Melica are positively correlated with latitude of origin. Variation in inflorescence morphology appears to be explained largely through differences in secondary branch distance, phyllotaxy and number of spikelets per secondary branch.
Conclusions
The data presented here and in previous studies suggest that Melica possesses many of the necessary features to be developed as an additional model for Pooideae grasses, including a relatively fast generation time, perenniality, and interesting variation in physiology and morphology. The next step will be to generate a genome-based phylogeny and transformation tools for functional analyses.
Keywords: Evo-devo, growth rate, inflorescence diversity, Melica, model system, phylogeny, Pooideae grasses, stress tolerance
INTRODUCTION
Plant evolutionary developmental biology (evo-devo) is the study of developmental processes across disparate taxa to infer by what means traits have evolved. Traditionally, evo-devo studies have focused on the evolution of morphological traits, deriving hypotheses about changing form and function based on detailed knowledge from one or two model taxa (e.g. Cubas et al., 1999; Zhao et al., 2004). These hypotheses were then tested in a few morphologically divergent species by combining detailed observations of organogenesis with organ-specific analyses of gene expression (e.g. Busch and Zachgo, 2007; Whipple et al., 2010; Preston et al., 2011). More recently, the establishment of affordable methods to generate ‘omic resources and transgenic lines in non-model taxa has opened the door to richer comparative studies of both morphological and physiological traits across broader phylogenetic distances (e.g. Lemmon et al., 2016; reviewed in Di Stilio et al., 2017; McSteen and Kellogg, 2022). As such, researchers in the field of evo-devo can now double their focus on developing non-model systems to more rigorously test developmental hypotheses of trait evolution, as well as to ask questions about the extent of developmental conservation and the nature of phenotypic convergence.
Evo-devo in grasses (Poaceae) has a rich history, due in large part to the economic importance of this family, and its ecological dominance in many parts of the world (Kellogg, 2001; Strömberg, 2011). Genetically tractable grass taxa, such as rice (Oryza sativa, Oryzoideae), Brachypodium distachyon (purple false brome, Pooideae) and sorghum (Sorghum bicolor, Panicoideae), are dispersed across different subfamilies of grasses, allowing for comparative macroevolutionary studies at the whole family level. However, more focused comparative studies still require the development of additional taxonomic groups in which to understand trait ontogeny and its evolution. This is particularly true for traits and underlying genetic variation that together contribute to increased biomass, reproductive yield and/or agricultural sustainability.
Pooideae, for example, is the largest grass subfamily, comprising ~4000 species, including some of the world’s most important crops: wheat (Triticum sp.), barley (Hordeum vulgare), rye (Secale cereale) and oat (Avena sp.). An exception among the majority of tropical grasses, Pooideae species are generally restricted to temperate or cool montane tropical climates, and many are considered hardy in the face of cold and drought (Preston and Fjellheim, 2020). Given emerging challenges associated with population growth, food security and global warming, understanding how Pooideae have diversified to maximize fitness in diverse environments promises potential avenues for trait introgression and novel sources of perennial-based biofuels (Porensky et al., 2014; Payne et al., 2017). The largely annual crops of Pooideae are found within the so-called ‘core group’ that is sister to the Brachypodieae tribe containing the model annual species B. distachyon (Raissig and Woods, 2022) (Fig. 1A). Successively sister to the core-Brachypodieae clade are seven additional perennial tribes that are relatively understudied, including tribe Meliceae (Schubert et al., 2019a; but see Zhong et al., 2018; Schubert et al., 2019b; Lindberg et al., 2020; Das et al., 2021; Fjellheim et al., 2022) (Fig. 1A). Here we discuss the development of knowledge and resources in a large tribe of Meliceae as a phylogenetic and developmental counterpoint to model core group Pooideae, and espouse the specific attributes of this system as an emerging model for evo-devo studies.
Fig. 1.
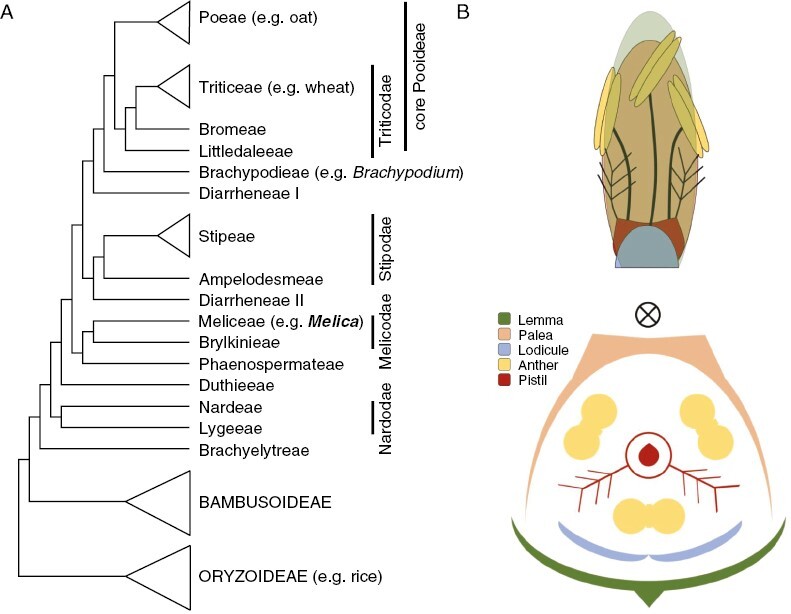
Melica in the context of temperate Pooideae. (A) Simplified Pooideae phylogeny showing the monophyletic ‘core’ clade and the paraphyletic ‘non-core’ clade containing several largely perennial tribes, including Meliceae to which Melica belongs. The tree topology is adapted from Zhang et al. (2022). (B) Basic unit of the grass inflorescence, modelled after an idealized Melica species. Grass spikelets typically comprise two basal glumes subtending one to several florets. Each fertile floret has two bract-like structures (lemma and palea), three lodicules, three stamens, three fused carpels with two feathery stigmas, and one ovule. In Melica there are two fused lodicules which resemble a single organ. Circle with an x marks the axis of growth, with the palea being adaxial and the lemma being abaxial.
An overview of the genus Melica
The largest genus of Meliceae is Melica, comprising ~90 currently recognized long-day flowering perennials that grow in dense clumps or through the production of rhizomes (Kellogg, 2015; Fjellheim et al., 2022). Melica species have a wide distribution, ranging across all continents except Antarctica, and are found in diverse habitats. Members of the genus Melica occur almost exclusively in temperate regions in both the northern and southern hemispheres (Supplementary Data Fig. S1; Chen et al., 2006). In the most current revision of European, Asian and North African Melica species, Hempel (2012) describes two subgenera. The first of these is subgenus Melica, found primarily in Europe, Asia and Africa, but also including a few species from North and South America. The second subgenus is Bulbimelica, named after the bulbous shoots found in a few species, which are described as bulbs or corms. Bulbimelica taxa are mostly from western North America and central Chile, with the exception of two Eastern Hemisphere species. The two subgenera are further divided into nine sections, which are detailed comprehensively in Hempel (2012).
As in most grasses, the basic reproductive unit of Melica is the floret, which contains two feathery stigmas, three anthers, two fused lodicules that fill with water to open the floret at maturity, and protective bract-like structures called the palea and lemma (Fig. 1B) (Schrager-Lavelle et al., 2017). Of those studied, Melica species have short stamens and cleistogamous flowers, leading to a high degree of selfing. When outcrossing does occur it is by wind (Szczepaniak and Cieślak, 2007). Fertilization in general results in one-seeded dry fruits (caryopses) that continue attachment to the palea/lemma to enable wind dispersal (Hensen and Müller, 1997; RBG Kew, 2023).
Biogeographical and evolutionary context for Melica diversification
Recent studies have started to shed light on the evolutionary history of Melica through fossil- and time-calibrated molecular dating techniques (Gallaher et al., 2022; Zhang et al., 2022). Supertribe Melicodae, which includes Meliceae and the monospecific tribe Brylkinieae (Brylkinia caudata), is inferred to have originated in the mid-Eocene around 48–37 million years ago (Mya) (Zhang et al., 2022). This is correlated with both the expansion of tribes across Pooideae and with falling global temperatures (Zhang et al., 2022). Tribe Meliceae probably diverged from its sister group Brylkinieae during the early Oligocene, following a sharp decline in global temperatures at the Eocene–Oligocene boundary 36 Mya (Zhang et al., 2022). Current evidence suggests that Melica diverged from the other six Meliceae genera in the Miocene (Gallaher et al., 2022; Zhang et al., 2022). However, the specific nature of phylogenetic relationships within Meliceae has yet to be fully resolved. Most of the species-level diversity in Melica probably began evolving at the start of the Pleistocene 2–3 Mya (Zhang et al., 2022). While the biogeographical origins of the Meliceae are probably found in the Palaearctic region (Gallaher et al., 2022), the full evolutionary history of Melica through time and space remains obscure.
Ethnobotanical importance of Melica grasses
To our knowledge there have been no attempts to comprehensively characterize the cultural or economic importance of the genus Melica. However, two North American species have been reported as being used as food by Indigenous peoples. The roots of M. bulbosa, a species which often develops corms, are processed as food by the Pomo people of coastal California (Gifford, 1967). The seeds of M. imperfecta are traditionally winnowed and ground before cooking by the Kawaiisu of the Sierra Nevada and Tehachapi Mountains, also in California (Zigmond, 1981). In northeast Asia, M. scabrosa is commonly found and widely used as forage for livestock (Yu et al., 2019). This species is also listed as an ingredient in patents for traditional Chinese medicine (Zhang, 2014; Xinglu and Xiuling, 2016). The current trend of gardening with native plants has also drawn attention to Melica. In Europe, several native species are grown for ornamental purposes, such as M. uniflora, M. altissima and M. ciliata, all native to the continent (RHS, 2023). Also, in western North America several native species are being propagated commercially, with M. imperfecta and M. californica being the most common (CNPLX, 2018).
Specific attributes of Melica as an evo-devo model
Beyond its phylogenetic position between rice and temperate cereal grasses (Fig. 1A), Melica has several attributes of a model taxon. First, most recognized species are diploid (2n = 18) (but see exceptions in Delay, 1950; Baltisberger and Leuchtmann, 1991; Rice et al., 2015) with an estimated nuclear genome size of 4500–5500 Mb (Tyler, 2004; Pustahija et al., 2013; Probatova et al., 2016; Šmarda et al., 2019), and a chloroplast genome of ~135 kb (Yu et al., 2019). The general lack of homeologues allows for easier genome assembly, and at least two Melica genome sequencing projects are in the pipeline for public release by the Joint Genome Institute (JGI; M. ciliata) and the Norwegian University of Life Sciences (Siri Fjellheim, pers. comm.; M. nutans). At least one species, M. scabrosa, also has its entire chloroplast genome sequenced (Yu et al., 2019). Additionally, crosses between Melica species are not only possible, but naturally occurring, with five currently recognized hybrid Melica species. Taken together, a propensity for selfing and a largely stable chromosome number across the genus increases the potential for making successful controlled crosses that will facilitate quantitative trait studies and testing barriers to gene flow.
A second consideration is that Melica plants are generally easy to grow, relatively small (~27 cm in height) at first flowering for perennials, rapid cycling (~6 months seed to first flower in inductive conditions) and produce large amounts of seed that do not require scarification or stratification (our pers. obs.). Perenniality itself is an important trait for investigation since there is a great deal of interest in engineering perennial traits into grass crops to promote more sustainable farming (Snapp et al., 2019; Zhang et al., 2023). That said, the perennial habit comprises a syndrome of traits that should be disentangled if progress is to be made on overcoming potential trade-offs between growth and reproduction. These traits include resource allocation between roots and shoots, length of the juvenile phase, flowering time, development of rhizomes and spatiotemporal regulation of senescence (reviewed in Hjertaas et al., 2023).
Finally, within Melica there is a great deal of variation in morphology, physiology and ecology, much of which is also reflected in other Pooideae genera. In a comprehensive revision of European, Asian and North African Melica, Hempel (2012) described key diagnostic features within the genus. The study found that Melica species exhibit variation in a number of traits, most notably spikelet structure, the position of the abscission zone for mature fruit disarticulation, fertile floret number, lemma morphology, and leaf traits associated with either moist (mesomorphic leaves) or dry (xeromorphic leaves) environments. Interestingly, it was hypothesized that mesomorphic leaves are a more ancestral trait, with xeromorphic leaf characters developing independently a number of times in European, Asian and North African species (Hempel, 2012). Rigorous testing of this hypothesis awaits the generation of a strongly supported Melica phylogeny. Several Melica species also show interesting geophytic modifications such as rhizomes, bulbs and corms (Burns, 1946). Such structures have been linked to increased nutrient uptake and environmental resilience (reviewed in Tribble et. al., 2021), although little is known about their developmental underpinnings in temperate grasses.
A broader context for Melica as an evo-devo model
Combining intrageneric and intraspecific studies across Pooideae tribes has the power to reveal both parallel and divergent mechanisms underlying variation in important traits such as flowering time plasticity, biomass accumulation, drought and freezing resilience, and inflorescence architecture that all contribute to lifetime fitness. For example, available data suggest that whereas the high-latitude Eurasian M. nutans (Tyler, 2002) has an absolute requirement for an extended period of chilling (vernalization) to trigger flowering competency, lower latitude Mediterranean M. ciliata has a weak to absent response to vernalization (McKeown et al., 2016). Furthermore, recent work in Melica and other Pooideae species has shown that accessions from regions with colder winters have increased chilling tolerance, and accessions from regions with higher aridity have increased drought tolerance (Das et al., 2021). Determining what complexes of traits have led to these geographical patterns of stress tolerance will require combining phylogenetically informed studies of various developmental and physiological traits across several branches of the Pooideae tree.
Current barriers to Melica-based comparative studies
Despite the potential for developing Melica as a model evo-devo group, there are several barriers to conducting comparative work in this genus. First is the lack of a phylogenetic framework. Hempel (2012) proposed phylogenies for sections described within the group. However, these relationships have not yet been investigated either at the species level or using molecular data sets. There may also be unexplored species diversity within Melica, which could hinder future work on the ecology and evolution of the group. Melica ciliata, for example, has been described as a species complex, and there is a proposal for species-level recognition of the currently unaccepted name M. magnolii (Castro et al., 2022).
Second, there are inconsistencies between what has been reported in the literature, including floras, as will be described below. Part of this inconsistency might be explained as a result of morphological variation within species, especially across vast ranges such as those in the previously discussed M. ciliata species complex (Castro et al., 2022). The third barrier to comparative work in Melica is the absence of tools for plant transformation. This final issue will need to be resolved by adapting tissue culture techniques from other grasses (e.g. Chen et al., 2019) and will not be discussed further here. Rather, we focus on tackling the first two issues, using available markers to begin inferring the biogeographical structure of the genus, and combining published and newly generated data on biomass accumulation and morphology to develop testable hypotheses of trait evolution within the group. We then discuss avenues for future research within Melica and similar underrepresented groups in Pooideae.
MATERIALS AND METHODS
Phylogenetic analysis
To generate a phylogenetic hypothesis as a backbone to understand biogeography and character evolution in Melica L., we downloaded available nuclear internal transcribed spacer (ITS) and chloroplast NADH dehydrogenase (ndhF) sequences from GenBank (Supplementary Data Table S1). Markers were selected that show the most overlap across taxa and that encompass species for which we have some previous physiological, genomic and morphological data. For the ITS dataset, we aligned nucleotide sequences from 21 Melica species and three Pooideae outgroups [Nassella pubiflora (Stipeae), Nardus stricta (Nardeae) and Brachyeletrum erectum (Brachyelytreae)] using MAFFT (Katoh and Standley, 2013). The data matrix was supplemented with new sequences generated from PCR amplification of DNA from M. argyrea, M. nitens and M. rigida (Tables S1 and S2). DNA was extracted using the Qiagen DNeasy Plant Mini Kit and ITS amplified using the primers ITS.F (5ʹ-GTGACCCTGACCAAAATAGA-3ʹ) and ITS.R (5ʹ-TATGCTTAAAYTCAGCGGGTA-3ʹ). Amplicons were cleaned with ExoSap-IT (ThermoFisher Scientific), cloned into the pGEM-T using the pGEM-T Easy Vector System (Promega), and Sanger sequenced using the universal primers T7 and SP6. After checking the alignment manually in Mesquite (Maddison and Maddison, 2011), a Bayesian analysis was conducted using MrBayes 3.2.2 in XSEDE (Miller et al., 2010) on the Cipres Science Gateway using 10 million generations and discarding 25 % of trees as burn-in. A similar analysis was done separately for the ndhF dataset that comprised nucleotide sequences for 25 Melica species and four Meliceae outgroups (Glyceria notata, Pleuropogon sabinei, Schizachne purpurascens and Triniochloa stipoides). Of all the ingroup taxa, 17 overlapped between datasets. Majority rule consensus trees with posterior probability support values were generated in FigTree (Rambaut, 2010).
Distribution map for Melica
To generate a distribution map, we compiled georeferenced samples of Melica for each species from the Global Biodiversity Information Facility (GBIF, http://www.gbif.org/). The GBIF locations were restricted to collection material in the preserved specimen excluding fossil records. Data were cleaned by excluding invalid coordinates, for example those located in the ocean. We first plotted the localities of all Melica species to visualize their distribution around the globe (Supplementary Data Fig. S1). Fifteen available species that exist in our phylogenetic tree were then selected for distribution mapping to determine the relationship between phylogeny and biogeography. Distribution mapping was conducted in ggplot2 using world map data from Natural Earth (rnaturalearth and rnaturalearthdata) with help from the simple features (sf) package. All the packages were built under R version 4.0.5 (R Core Team, 2021).
Climatic principal component analysis (PCA)
To determine the relationship between phylogeny, biogeography and climate, we downloaded climatic data from the WorldClim database version 2.1 with 2.5 arcminute resolution, which includes a list of 19 bioclimatic variables measured and summarized for 1970–2000. These variables include various axes of temperature (bio1 to bio11) and precipitation (bio12 to bio19) (Fick and Hijmans, 2017). Variables were extracted from WorldClim for all localities of the selected species using R package raster built under R version 4.0.5. Extracted variables were then analysed using PCA by means of the prcomp function in R and visualized using the fviz_pca function in package factoextra.
Biomass accumulation measurements
To determine variation in biomass for several Melica taxa across both warm and cold conditions, we first ordered seeds of 11 species from the United States Department of Agriculture (USDA) Germplasm Resources Information Network (GRIN) (Supplementary Data Table S2). One hundred seeds of each species, one seed per pot, were planted in wet ProMix BX soil (Griffin Greenhouse Supply) and stratified at 4 °C in the dark for 5 d. Pots were then transferred to a Conviron model A1000 Growth Chamber (Conviron, Winnipeg, MB, Canada) at 20 °C 16-h day and 18 °C 8-h night conditions for 6 weeks at the lowest light intensity, after which ten plants per species were subjected to 0, 2, 4 or 6 weeks of 4 °C constant cold (vernalization) under the same light regime. To make sure plants were receiving the same light conditions across treatments, control plants and plants that had received less than 6 weeks of cold were removed to an identical Conviron chamber set to the original 20 °C 16-h day and 18 °C 8-h night and low light conditions until the 6-week mark. After 6 weeks, all plants were transferred to glasshouse conditions under 16-h photoperiods and an average temperature of 20 °C in the day and 18 °C at night. For plants that failed to flower within the first 200 d post-germination (i.e. most individuals), height and tiller number were measured as a proxy of above-ground biomass. Linear regression models were run in R version 4.0.3 using the lm function, first including latitude of accession origin, chilling weeks and their interaction as explanatory variables for height or tiller number. As the interaction term was not significant, we removed this term. Furthermore, since there was no significant effect of temperature on growth (see Results), we finally tested the prediction of a negative effect of latitude on biomass under warm (0 weeks cold) versus cold (combined 2-, 4- and 6-week data) or all temperature (combined 0-, 2-, 4- and 6-week data) conditions. A linear regression model was also used to test the prediction that plant height and tiller number are positively correlated.
Morphological character matrix construction
Morphological data of Melica species were compiled using several online resources such as Plants of the World Online (POWO), the Flora of China, the Flora of Argentina, the Flora of North America and the Jepson Herbarium Flora (eFloras, 2008; Pozner, 2018; Barkworth, 2021; Jepson Flora Project, 2023; RBG Kew, 2023). Selected taxa are those with names accepted by GrassBase – The World Online Grass Flora, including five known hybrids, for a total of 94 species (Clayton et al., 2006 onward). A character matrix was built by first checking the POWO Melica genus description to identify the most variable traits and/or other traits of interest across the group (Supplementary Data Table S3). Of these, particular attention was given to traits with implications for flowering, fruiting, seed dispersal, nutrient uptake and environmental resilience. This includes below-ground structures such as rhizomes, bulbs and corms. Basal shoot modification is hereafter used as a category for characters found at the base of the shoot, that is bulbs, corms, and any features of the basal nodes, internodes or leaf sheaths. Flowering and fruiting characters of note include panicle structure, number of spikelets, fertile floret number per spikelet, sterile floret number per spikelet, spikelet dynamics at maturity, and the presence or absence of lemma awns. Some included characters have previously been identified as diagnostic for groups within the genus. These are the presence or absence of spikelet pedicels, mature spikelet dynamics and fertile floret number per spikelet (Hempel, 2012).
Scanning electron microscopy (SEM) of developing inflorescences
As a start to quantify morphological differences in inflorescence development between species, we selected to study a single representative for two of our resolved clades (see Results): M. ciliata and M. imperfecta. Plants were grown in the University of Vermont glasshouse with supplemental light at 20–22 °C with a 16-h photoperiod. Inflorescence buds were dissected and fixed in 10:50:5 formaldehyde/ethanol/acetic acid (FAA) before being dehydrated in an ethanol series. Samples were then critical point dried and mounted on stubs for sputter-coating in argon. SEM images were produced using a JEOL 6060 scanning electron microscope.
RESULTS
Melica systematics and distribution
A Bayesian analysis of the ITS sequence data resolved three well-supported Melica clades (Fig. 2A). Furthermore, although there was little support for the ndhF topology, probably due to a paucity of phylogenetically informative characters, relationships among overlapping taxa were generally congruent with the ITS tree (Fig. 2B). Based on these data, we hypothesize three working clades: ‘Ciliata’, ‘Imperfecta’ and ‘Nutans’, the former two of which are more closely related to each other than to the third.
Fig. 2.

Bayesian reconstruction of Melica relationships based on a nucleotide alignment of ITS (A) and ndhF (B). Both topologies are based on majority-rule consensus trees, with thick branches indicating posterior probabilities >0.9. Coloured boxes in A delimit well-supported clades with their relative positions in the ndhF tree (B) indicated with dashed lines. Each of the main supported clades was given a name based on a representative species: ‘Ciliata’ (orange), ‘Imperfecta’ (blue) and ‘Nutans’ (pink).
Distribution mapping of taxa within our three working clades revealed a strong geographical pattern. Specifically, taxa within the ‘Ciliata’ clade span the Iberian peninsula, Mediterranean and South Africa, whereas members of the ‘Nutans’ clade are largely in central-eastern Europe, spreading east across Russia and Asia, and the ‘Imperfecta’ clade is mostly in the western continental USA (Fig. 3). Despite its sister relationship with the ‘Imperfecta’ clade (Fig. 2), the ‘Ciliata’ clade is more closely aligned geographically with the ‘Nutans’ clade. Further work will be needed to determine if these general patterns are maintained with more phylogenetic resolution and taxon sampling.
Fig. 3.

Geographical map of known Melica taxa within the ‘Imperfecta’ (blue), ‘Ciliata’ (orange) and ‘Nutans’ (pink) clades as defined in Fig. 2.
Distinct from the geographical data, and more in line with the phylogenetic relationships, a PCA based on temperature and precipitation variables revealed separation of the ‘Nutans’ clade from the ‘Imperfecta’ plus ‘Ciliata’ clades along PC axis 1 (Fig. 4). PC1 explained 38.6 % of the variation across all individuals and was loaded on heavily by variation in the temperature niche (bio1–11) and precipitation seasonality (bio15). Along this axis, ‘Nutans’ species occupied colder environments with high temperature seasonally but low precipitation seasonality, whereas ‘Ciliata’–‘Imperfecta’ species occupied warmer environments with low temperature seasonality and high precipitation seasonality. Variation within the ‘Nutans’ and ‘Imperfecta’ clades accounted for most of the variation along PC axis 2, with the ‘Ciliata’ points being nested within the ‘Imperfecta’ points. As such, species in at least the ‘Nutans’ and ‘Imperfecta’ clades show similar niche breadth in both arid and humid environments (Fig. 4).
Fig. 4.

Principal component analysis (PCA) of the climatic niche for known Melica taxa within the ‘Imperfecta’ (blue), ‘Ciliata’ (orange) and ‘Nutans’ (pink) clades as defined in Fig. 2. Data are based on the 19 Worldclim bioclimatic (bio) variables: 1, annual mean temperature; 2, mean diurnal range; 3, isothermality; 4, temperature seasonality; 5, maximum temperature of the warmest month; 6, minimum temperature of the coldest month; 7, temperature annual range; 8, mean temperature of the wettest quarter; 9, mean temperature of the driest quarter; 10, mean temperature of the warmest quarter; 11, mean temperature of the coldest quarter; 12, annual precipitation; 13, precipitation of the wettest month; 14, precipitation of the driest month; 15, precipitation seasonality; 16, precipitation of the wettest quarter; 17, precipitation of the driest quarter; 18, precipitation of the warmest quarter; and 19, precipitation of the coldest quarter. The direction of loadings for each variable is shown with an arrow and general climates are shown in a green box for each quadrant.
Above-ground biomass and latitude of origin
Given general partitioning of the Melica ‘Ciliata–Imperfecta’ and ‘Nutans’ clades into warm and cold environments, respectively (Fig. 4), we were interested in determining whether members of each clade have inherently different growth rates and/or if relative differences in growth rate change under variable growth temperatures. We used latitude of origin to represent a cline in temperature and its seasonality, predicting that taxa or clades from lower latitudes would accumulate above-ground biomass more quickly under all growing temperatures than those from higher latitudes. This expectation is based on previous observations in several plant groups that demonstrate a life history trade-off between plant growth and cold stress resilience (Lundgren and Des Marais, 2020; Monson et al., 2022). A linear model testing the effects of growing temperature and latitude of origin on plant height or tiller number after 200 d of growth revealed no significant interaction between the two explanatory variables. Thus, subsequent models were simplified to remove the interaction term. Based on plant height, species separated into two main groups, with shorter plants under both cold and warm conditions being members of the ‘Ciliata’ and ‘Imperfecta’ clades and taller plants being members of the ‘Nutans clade’ (Fig. 5A). The fact that plant height positively correlated (P = 0.012) with tiller number suggests that tall plants have higher above-ground biomass overall relative to short plants (Fig. 5B).
Fig. 5.

Melica biomass accumulation in response to temperature and latitude. (A) Variable thermal reaction norms in Melica height 200 d post-germination. Species names are provided only on one side of their reaction norm and are coloured based on the ‘Imperfecta’ (blue), ‘Ciliata’ (orange), ‘Nutans’ (pink) or other (black) clades as defined in Fig. 2. Although the sample size is small, the three members of the ‘Ciliata’–‘Imperfecta’ clade are smaller in stature under both warm and cold conditions relative to the single representative of the ‘Nutans’ clade. (B) Height and tiller number across Melica are positively correlated (P = 0.012), suggesting that height is a good proxy for above-ground biomass. (C) Latitude of origin and plant height 200 d post-germination are significantly positively correlated (P = 0.0002) according to a linear regression model, with no significant difference between warm and cold conditions. (D) Latitude of origin and tiller number 200 d post-germination are marginally positively correlated (P = 0.0576) according to a linear regression model, again with no significant difference between warm and cold conditions. Each filled or open triangle (warm conditions) and circle (cold conditions) represents the average for a Melica species.
Although there was some species variation in the reaction norms of height and tiller number between cold and warm conditions (Fig. 5A; Supplementary Data Fig. S2), the effect of temperature on these responses was not significant (P = 0.393 and P = 0.318, respectively). This suggests that all species are relatively chilling tolerant and able to maintain high levels of growth under cold conditions, and/or that the rate of biomass accumulation of chilled plants increased relative to control plants during some part of the 120+ d post-chilling. In contrast, and contrary to our predictions, height was positively correlated with latitude of origin (P = 0.0002) (Fig. 5C), and tiller number marginally so (P = 0.0576) (Fig. 5D). Interspecific variation in flowering time in response to temperature was not calculated since many species failed to flower within a year of seed planting even with 6 weeks of chilling. This suggests either that some species have a vernalization requirement longer than 6 weeks or that they are not competent to respond to vernalization in their first year.
Morphological variation within Melica
Among the morphological characters evaluated in a phylogenetic context (Supplementary Data Table S3), several exhibited patterns of variation, including the presence/absence of basal shoot modifications such as bulbs or corms, presence/absence of lemma awns, fertile floret number per spikelet and position of the abscission zone for mature fruit disarticulation. Interestingly, although limited in terms of sampling, the variation in these traits was found to be associated with the three main clades identified in our phylogenetic analysis (Fig. 6).
Fig. 6.

Comparison of morphological variation within the genus Melica. A simplified phylogeny based on the three major ‘Imperfecta’ (blue), ‘Ciliata’ (orange) and ‘Nutans’ (pink) clades in Fig. 2 is shown with their corresponding character matrix. Variable characters that were consistently defined in the literature (Clayton et al., 2006; eFloras, 2008; Pozner, 2018; Barkworth, 2021; Jepson Flora Project, 2023; RBG, 2023) include the presence (+) or absence (-) of basal shoot modifications (bulbs, corms or distinguishing features found at the base of the shoot), the presence or absence of lemma awns, the number of fertile florets per spikelet, and the position of the abscission zone for mature fruit disarticulation. A more comprehensive character matrix can be found in Supplementary Data Table S3.
In the ‘Ciliata’ clade, all five species are described as having either one or two fertile florets per spikelet, none are described as having any notable modifications to the basal portion of their shoots or feature lemma awns, and all have spikelets that fall entire or break-up between each fertile floret at maturity (Fig. 6). By contrast, five of the seven members of the ‘Imperfecta’ clade exhibit some sort of growth, swelling or other notable modifications to their basal shoots, with two species having awned lemmas. Compared to the ‘Ciliata’ clade, ‘Imperfecta’ species also show more variation in spikelet structure and dynamics. Six species have at least two, but often five or more fertile florets per spikelet. Three taxa disarticulate above the glumes but not between florets, while another three break-up below each fertile floret, and M. stricta mature spikelets fall entire. Despite the closer phylogenetic and climatic affinity between the ‘Ciliata’ and ‘Imperfecta’ clades (Fig. 2), members of the ‘Nutans’ clade are more similar in our selected character set to ‘Ciliata’ species based on the lack of shoot modification or awned lemmas. However, they still have stronger affinity with the ‘Imperfecta’ clade in terms of their, albeit less variable, number of florets (two or three) per spikelet, and mature spikelets either falling entire or disarticulating above the glumes (Fig. 6).
In contrast to the previously mentioned morphological traits, inflorescence architecture is less consistently and more ambiguously scored across available Melica floras. For example, the terms ‘lax’, ‘rather lax’, ‘dense’ and ‘open’ in relation to the panicle are difficult to interpret (Chen et al., 2006; eFloras, 2008), and it is often unclear whether spikelet number is calculated per branch or for the whole inflorescence. As such, we decided to investigate different stages of inflorescence development for M. ciliata and M. imperfecta (Fig. 7) that show highly divergent morphologies at seed set (Fig. 7G, P). Although similar in several respects, such as having two orders of inflorescence branching (Fig. 7A, J) and several spikelets per secondary branch (Fig. 7B, C, K), differences in overall architecture could be explained by three major variables. First, the internodes between secondary branches are much longer for M. imperfecta (Fig. 7A) relative to M. ciliata (Fig. 7I, J). Second, M. imperfecta has alternate/distichous phyllotaxy (Fig. 7A, C, E, G), whereas M. ciliata phyllotaxy is spiral (Fig. 7I, J, L, M, O, P). Finally, M. imperfecta has fewer spikelets per secondary branch than M. ciliata, being somewhere in the order of six (Fig. 7C) and nine (Fig. 7K), respectively. We also noted at least two (M. ciliata; Fig. 7F) or three (M. imperfecta; Fig. 7M, N) florets per spikelet, although further work will be needed to investigate the maximum number, any intraspecific variation and what ratio are sterile to fertile.
Fig. 7.
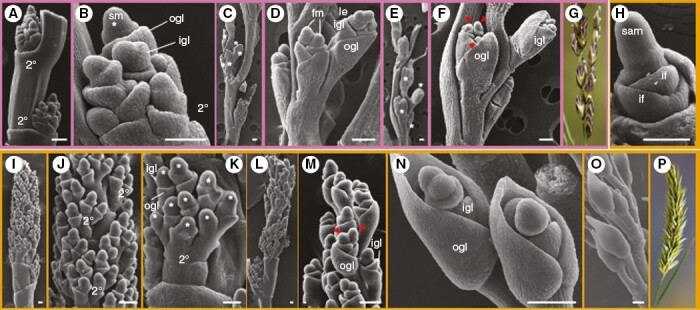
Evolution of Melica inflorescence development. (A–G) Scanning electron (A–F) and light (G) micrographs for M. imperfecta in the ‘Imperfecta’ clade. Secondary branches (2°) develop from nodes spaced out in a distichous arrangement along the primary branch (A), with around six spikelets (white asterisks) developing per secondary branch (B, C). Each spikelet develops two to three floret meristems (fm; red arrowheads), subtended by an inner glume (igl) and outer glume (ogl). Floret meristems are subtended by lemmas (le). (H–P) Scanning electron (H–O) and light (P) micrographs for M. ciliata in the ‘Ciliata’ clade. The shoot apical meristem (sam) develops leaves (lf) during vegetative growth (H) and then transitions to an inflorescence meristem. Secondary branches develop from nodes along the primary branch in a spiral arrangement (I, J, L), with around nine spikelets per secondary branch (K). Each spikelet develops at least two floret primordia again subtended by an inner and outer glume (M, N). At maturity, the outer glume wraps around the florets on the abaxial side (O, P). Scale bars = 100 µm.
DISCUSSION
Research into largely annual cereal crops within the grass subfamily Pooideae has provided excellent insight into the genetic basis of variation in life history traits, such as flowering time and grain yield (reviewed in Sakuma and Schnurbusch, 2020; Fernandez-Calleja et al., 2021). However, to determine how well these findings translate to Pooideae as a whole, and to gain a better understanding of traits not generally associated with conventional crops (e.g. perenniality and abiotic stress resistance), we need to expand our repertoire of ‘model taxa’. The increasing availability of affordable next-generation sequencing and high-throughput phenotyping technologies, combined with ever more precise information on plant distribution and local climate, provides novel opportunities to do just that. In recent years, B. distachyon has emerged as a non-traditional model outgroup to core Pooideae, revealing both conserved and divergent mechanisms of plant development and physiology within this group (reviewed in Raissig and Woods, 2022). Here we argue for the further development of phylogenetic, genomic and gene editing resources in the non-core Pooideae perennial genus Melica as a comparative counterpoint to rice and the core Pooideae + Brachypodieae. Melica has several attributes of a model taxon, including being relatively small and rapid cycling with a large seed yield. More importantly though, this genus shows a great deal of geographical, climate and associated trait variation that provides an untapped resource for breeding more climate change-resilient and sustainable food, fodder and biofuel crops.
A phylogeographical context for Melica evolution
The biggest current impediment to conducting evo-devo studies in Melica is the lack of a robust phylogeny. Using just two markers, we have generated a preliminary phylogenetic hypothesis for Melica that delimits three monophyletic groups, referred to here as the ‘Ciliata’, ‘Imperfecta’ and ‘Nutans’ clades. Analysis of species within these clades suggests a strong biogeographical pattern, with members of ‘Ciliata’ and ‘Nutans’ occupying various parts of Eurasia and Africa, and ‘Imperfecta’ taxa being found in North America. However, despite the geographical closeness of ‘Ciliata’ and ‘Nutans’, analysis of the climatic niche suggests more similarity between the warm Mediterranean habitats of sister clades ‘Ciliata’ and ‘Imperfecta’ relative to the seasonally cold habitats of ‘Nutans’. A previous analysis of cold and drought tolerance in Melica and other Pooideae species found evidence for adaptation to climate of origin after accounting for phylogeny, suggesting that evolution of abiotic stress tolerance has been ongoing within this group (Das et al., 2021). Nonetheless, it will be interesting to determine when and how many times within Melica there has been a major shift in stress tolerance, and to determine the genetic basis of these shifts.
Toward our goal of a fully resolved Melica phylogeny, we recommend an initial focus on taxa within the same geographical regions as our three focal groups, with particular emphasis on the ‘Imperfecta’ group that apparently houses most variation in several traits of economic interest. To fill out the backbone phylogeny, sampling should also be conducted for South American taxa that have generally been overlooked. There are a number of phylogenomic methods available to tackle this endeavour. However, a good candidate is the Angiosperms353 toolkit, which can work effectively to resolve both higher level relationships and relationships at or below the species level when flanking regions are included (Johnson et al., 2019; McDonnell et al., 2021).
Hybridization appears to play a role in Melica diversification among species that have been studied more closely. There are five currently accepted hybrid species names, plus an additional species with a more recent support of hybrid origin (Supplementary Data Table S3; Castro et al., 2022). Of these hybrids, four have M. ciliata parentage (Clayton et al., 2006 onward). This includes the more recently investigated M. amethystina, the only currently known tetraploid (2n = 36) Melica species with a proposed hybrid origin of M. ciliata × M. minuta (Castro et al., 2022). The other two recognized hybrids share M. nutans parentage. This highlights the potential for studies to investigate hybridization as a driver of speciation in understudied Melica taxa. One of the most recently recognized species, M. chatkalika, has also been proposed to be of hybrid origin (M. persica × M. secunda). However, this species currently appears to be known only from the type specimen and warrants further investigation (Lazkov and Usupbaev, 2017).
Our phylogenetic hypothesis of clades within Melica suggests that the current subgenera of Melica and Bulbimelica may be polyphyletic groups. However, there are also similarities of note between our preliminary phylogenetic results and the most recent taxonomic groupings (Fig. 2; Hempel, 1971, 2012). Starting with the ‘Imperfecta’ clade, nearly all species in this group are assigned to subgen. Bulbimelica, with the exception of M. stricta, which is placed in subgen. Melica. The supported grouping of M. bulbosa with M. subulata does correspond with the current taxonomy, as both taxa are placed in the same subsection of the group. In the ‘Ciliata’ clade, the internal groupings with high support correspond closely with the current taxonomy. All species in this clade are grouped into section Dalycum within subgen. Melica. Additionally placing M. persica and M. cupani as sister to the other three taxa corresponds to further nested taxonomic groupings. The two former species make up part of subsect. Pilosae, and the three others (M. ciliata, M. transilvanica and M. racemosa) are found in subsect. Ciliatae. The ‘Nutans’ clade identified in our phylogenetic results showed less resolution for nested groupings of taxa. When comparing those species with the current taxonomy, all are found in subgen. Melica, but split between sect. Melica (M. nutans, M. picta and M. minor) and sect. Altimelica (M. altissima and M. turczaninowiana).
Melica as a model for understanding Pooideae growth, yield and resilience, and their potential trade-offs
Global warming is posing major issues for crop producers, with increased drought, flooding and unseasonal bouts of freezing damaging cereals and other crops in traditional growing areas throughout the world (USGCRP, 2017; Sasidharan et al., 2021). Continued population growth is also placing challenges on food security (Teferra, 2021). Thus, not only do we need more stress-hardy crops, but they need to be both higher yielding and more sustainable, the latter to avoid exacerbating climate change. A major issue with this endeavour is the potential antagonistic relationship or ‘trade-off’ between life history traits, such as growth and resilience or longevity and annual yield (Stearns, 1992). For example, in many temperate trees and herbs, latitude of origin is positively correlated with freezing resilience, but negatively correlated with growth rate (Li et al., 1998; Koehler et al., 2012). In the case of Melica, we found that latitude of origin was actually positively correlated with growth rate, even under cold conditions. This unexpected result could be explained by a further counterintuitive negative correlation between latitude and cold resilience, although our previous work with a different subset of Melica species suggests this is not the case (Das et al., 2021). Another possibility is that a trade-off only shows up when temperatures decrease beyond a certain threshold (Stearns, 1992). Future work integrating results on latitude, resilience, growth rate and yield will be important for determining the nature of relationships between these traits across Melica, and assessing their genetic architecture under different environmental conditions.
The study of other life history traits to which Melica is well suited include flowering time and perenniality. Similar to most Pooideae grasses, all Melica species tested to date are long-day flowering, meaning that they flower faster in photoperiods above than below 12 h of light per day (Fjellheim et al., 2022). However, although tested species are found in each of our three ‘Ciliata’, ‘Imperfecta’ and ‘Nutans’ clades, formal studies have not yet been conducted to our knowledge on species from the neotropics where we might expect a transition to short-day or day-neutral flowering. Unlike photoperiod-regulated flowering, variation for vernalization responsive flowering has been found, with a range of responses from an absolute requirement for cold in a Belgian population of M. nutans to no response in a Chinese population of M. transsilvanica (McKeown et al., 2016). Whether these differences in vernalization responsiveness are found only at the species level, or also vary across populations of the same species as has been found for many domesticated crops (Liu et al., 2019), is a matter for further research. Furthermore, much is still to be learned about how different climates have shaped vernalization temperatures that can range from 1 to 16 °C (Wollenberg and Amasino, 2012), the period of vernalization that is needed to saturate the response (Stinchcombe et al., 2005), and whether the epigenetic ‘memory’ of winter can be reversed with high temperatures as in the case of some winter wheat and barley cultivars (Dixon et al., 2019). In Arabidopsis thaliana, European populations show a cline in vernalization sensitivity, with more southern populations requiring less cold to saturate their response than northern ones (Stinchcombe et al., 2005). However, data also suggest that less predictable winters, such as in coastal regions, have selected for higher vernalization sensitivity to avoid premature flowering during the occasional warm days of winter (Kinmonth-Schultz et al., 2021). Melica represents a useful group in which to study these phenological differences, particularly given our limited knowledge in perennial systems.
Beyond developing a model perennial clade to better understand Pooideae flowering time, where physiological resetting of shoot apical meristems has to occur following each yearly bout of reproduction (Wang et al., 2009; Finnegan et al., 2021), breeding perennial crops is at the forefront of conversations on both novel biofuels and sustainable agriculture (Chapman et al., 2022). In particular, cool- as opposed to warm-season grasses are better biofuel candidates for drought-prone regions, calling for the development of dual purpose biofuel–forage grasses in Pooideae (Porensky et al., 2014; Payne et al., 2017). Pioneering work in the Oryza sativa × O. longistaminata perennial has resulted in hybrid rice that produces more seed per harvest over four harvests than its replanted annual counterpart (Zhang et al., 2023). To accomplish similar goals for Pooideae grasses, and to develop unique options for perennial forage and biofuel crops, research in Melica offers a unique opportunity for perennial trait and gene discovery.
Melica as a model to understand morphological evolution in economically important traits
In addition to traits (e.g. perenniality) that make Melica an important comparative taxon in relation to model species in Pooideae, interspecific variation within the tribe exists for several economically important characters. Of particular interest is the ‘Imperfecta’ group that appears more variable than the other two groups in terms of basal shoot modifications, lemma awn presence/absence and the position of the abscission zone for seed dispersal. This pattern awaits confirmation with a more robust and inclusive phylogeny. However, for now it presents a unique target for understanding the developmental and genetic underpinnings of phenotypic evolution within relatively closely related taxa.
Basal shoot modifications, including rhizomes, bulbs and corms, have evolved independently many times across the angiosperms, with a notably high concentration in monocots (Tribble et al., 2021). These traits are of particular interest when studying adaptations to seasonality and other environmental changes as they protect resting buds at or near the soil level from changes in light, temperature, fires and floods (Khokhar, 2017; Ott et al., 2019). Additionally, they can provide storage or photosynthetic capacity (Tribble et al., 2021). While the genetic basis for bulb/corm development is poorly understood for most taxa, long-day photoperiods are known to upregulate FLOWERING LOCUS T 1 (FT1) and downregulate its antagonist FT4, resulting in edible bulb growth in the monocot onion (Allium cepa) (Lee et al., 2013; Khokhar, 2017). Twelve of the 13 Melica species described as having notable basal shoot modifications are from the Western Hemisphere, and their presence in the ‘Imperfecta’ group is tentatively associated with warmer and potentially drier habitats. Detailed investigations into the anatomy, development and genetics of Melica rhizomes, bulbs and corms are needed to begin understanding the evolution of these apparently homologous structures, and to determine whether the same genetic mechanisms have been recruited in the convergent origins of these geophytic structures more broadly (Tribble et al., 2021).
Another character that is associated with different climates is the presence/absence of lemma awns. Awns are hypothesized to be homologous to leaf blades and are associated with photosynthesis to aid grain filling, protection from animal predation, seed dispersal, and seed burying as an aid against drought and fire (Guo and Schnurbusch, 2016; Petersen and Kellogg, 2022). Extensive work in barley has revealed a number of genes involved in awn development, including LEAFY LEMMA, HOODED LEMMA 1, SIX-ROWED SPIKE 1 and SHORT AWN 1 (Ntakirutimana and Xie, 2019; Huang et al., 2021). Although many crops (e.g. rice) have been selected to lack awns for easier harvest, the reduction in grain number for awnless barley has meant that they have been largely retained during domestication (Rebetzke et al., 2016). Similar to grasses as a whole, Melica species vary, not only in the presence/absence of awns, but in their length, shape and insertion point along the lemma. Thus, they provide an excellent system in which to determine if and how these modifications associate with climate and dispersal biology and if the cost and benefits of possessing awns mirrors that found for other grass taxa (Petersen and Kellogg, 2022).
Many aspects of panicle morphology are probably conserved across grasses, as exemplified by quantitative trait loci (QTLs) that map to the same genes, such as DENSE AND ERECT PANICLE1 (DEP1) that affects meristem activity and grain yield in rice (Huang et al., 2009) and several Triticeae species (Vavilova et al., 2017). However, the different meristem types – secondary branch, spikelet pair, spikelet and floret – provide a number of targets for selection to modify grain number and size (Bommert and Whipple, 2018; Sakuma and Schnurbusch, 2020). Evidence suggests that in rice and maize, grain number is affected mostly by genes that act early in defining inflorescence architecture, whereas in barley and wheat the most important genes are late acting (Sakuma and Schnurbusch, 2020). QTL and broader comparative studies incorporating non-traditional Pooideae models are thus needed to determine whether ‘Triticeae-specific’ inflorescence architecture genes can be extended to the subfamily as a whole. To that end, we found that M. ciliata inflorescences differ from those of M. imperfecta in the distance of secondary branches along the stem, their phyllotaxy and number of spikelets per branch. Furthermore, analysis of floret number per spikelet in our preliminary phylogenetic framework suggests variation in both total number and the ratio of fertile to sterile florets.
Making sterile florets might appear to be a costly maladaptation, but the fact that this trait is quite common and possibly ancestral to Pooideae suggests that it provides some benefit such as in seed dispersal (Arber, 1934; Hensen and Müller 1997). To hold true, this latter hypothesis predicts that the overall biomass of sterile florets within a spikelet will be associated with the dispersal unit, which in turn is determined by the location of the abscission zone (Yu et al., 2020a). Melica varies considerably in abscission zone positioning, developing at the base of the spikelet, above the glumes on the spikelet rachilla or below each fertile floret. As such it provides a means to test the sterile floret-dispersal hypothesis. Variation in abscission zone mechanics and position is also an interesting trait in itself, having been associated with broadened dispersal ranges, improved germination and easier harvest for domesticated crops (Tang et al., 2013; Linder et al., 2018). The ancestral position of the abscission zone in grasses is hypothesized to be above the glumes on the spikelet rachilla and expression of the SHATTERING 1 (SH1) gene contributes to its development in rice, Setaria and wheat (Yu et al., 2020b). Nonetheless Yu et al. (2020b) also found that SH1 expression does not always correspond to the morphology or anatomy of grass abscission zones, suggesting co-option of other mechanisms throughout evolution. What the situation is for Melica remains to be tested, but the high level of variation, specifically in the ‘Imperfecta’ clade that also shows the highest variability of fertile florets, provides an ideal framework for study.
CONCLUSIONS
We hope to have provided a convincing case for the development of Melica as a unique non-traditional model grass that can be studied by the next generation of plant biologists. Not only does Melica present an excellent phylogenetic and perennial counterpoint to the largely annual contingent of core Pooideae grasses (but see Steinwand et al., 2013; Hasterok et al., 2022), it also provides a tractable system for understanding convergent trait evolution within this economically and ecologically important subfamily. In particular, the Melica grasses possess geographically driven variation in flowering time and abiotic stress tolerance, and show potentially counterintuitive differences in growth rates across latitude that should be investigated in relation to the growth–resilience trade-off hypothesis. Variation in shoot modifications related to nutrient acquisition and potentially drought tolerance, as well as differences in fertile floret number and architecture, are also prevalent in the ‘Imperfecta’ clade, making this an excellent target for evo-devo studies. In light of several forthcoming genomic and transcriptomic datasets, the main issues that need to be addressed are the development of a robust phylogeny, transformation and gene editing tools within the group. With the community’s help, these are barriers that can quickly be overcome.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Fig. S1. Global distribution of all Melica species based on locality data from the Global Biodiversity Information Facility (GBIF, http://www.gbif.org/). Fig. S2. Reaction norms of tiller number at 200 d post-germination for selected Melica species. Table S1. GenBank nucleotide sequence accessions for phylogenetic markers ITS and ndhF. Table S2. Accession and locality data for Melica species included in the biomass accumulation study. Table S3. Extended character matrix for Melica species.
ACKNOWLEDGEMENTS
We thank Noah Beckage for generating ITS sequences.
Contributor Information
Masoumeh Khodaverdi, Department of Plant Biology, The University of Vermont, 111 Jeffords Hall, 63 Carrigan Drive, Burlington, VT 05405, USA.
Mark D Mullinger, Department of Plant Biology, The University of Vermont, 111 Jeffords Hall, 63 Carrigan Drive, Burlington, VT 05405, USA.
Hannah R Shafer, Department of Plant Biology, The University of Vermont, 111 Jeffords Hall, 63 Carrigan Drive, Burlington, VT 05405, USA.
Jill C Preston, Department of Plant Biology, The University of Vermont, 111 Jeffords Hall, 63 Carrigan Drive, Burlington, VT 05405, USA.
FUNDING
The work was supported by the National Science Foundation [IOS2120732 to J.C.P.] and the National Institute of Food and Agriculture [VT-H02712 to J.C.P.]. H.S. was funded by a National Science Foundation Quantitative and Evolutionary STEM Traineeship [QuEST; NRT-1735316].
LITERATURE CITED
- Arber A. 1934. The Gramineae: a study of cereal, bamboo, and grass. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511700668. [DOI] [Google Scholar]
- Baltisberger M, Leuchtmann A.. 1991. Investigations on some Gramineae from Albania and Greece (chromosome numbers and endophyte infection). Berichte des Geobotanischen Institutes der Eidgeno¨ssischen Technischen Hochschule Stiftung Ru¨bel 57: 182–192. [Google Scholar]
- Barkworth ME. 2021. Melica. In: Flora of North America Editorial Committee, eds. 1993+. Flora of North America North of Mexico [Online], Vol. 3. 22+ vols. New York and Oxford: Oxford University Press. http://beta.floranorthamerica.org/Melica. (16 March 2023, date last accessed). [Google Scholar]
- Board of Trustees of the Royal Botanic Gardens (RBG), Kew. 2023. Plants of the World Online (POWO). https://powo.science.kew.org/. (02 January 2023, date last accessed).
- Bommert P, Whipple C.. 2018. Grass inflorescence architecture and meristem determinacy. Seminars in Cell and Developmental Biology 79: 37–47. doi: 10.1016/j.semcdb.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Burns W. 1946. Corm and bulb formation in plants, with special reference to the Gramineae. Transactions of the Botanical Society of Edinburgh 34: 316–347. doi: 10.1080/13594864609441403. [DOI] [Google Scholar]
- Busch A, Zachgo S.. 2007. Control of corolla monosymmetry in the Brassicaceae Iberis amara. Proceedings of the National Academy of Science USA 104: 16714–16719. doi: 10.1073/pnas.0705338104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Native Plant Link Exchange (CNPLX). 2018. Plant Search Results. http://www.cnplx.info/nplx/nplx?page=match&noSyn=t&tvhl=all&taxon=Melica&cnames=&family=none&lifeform=none&native=none&countylist=none&out=HTML&cat=none&photo=t. (13 March 2023, date last accessed).
- Castro S, Muratet A, Szczepaniak M, Nguefack J, Hardion L.. 2022. RAD sequencing, morphometry and synecology clarify the taxonomy of the Melica ciliata (Poaceae) complex in France and Poland. Journal of Systematics and Evolution 00: 1–12. doi: 10.1111/jse.12940. [DOI] [Google Scholar]
- Chapman EA, Thomsen HC, Tulloch S, et al. 2022. Perennials as future grain crops: opportunities and challenges. Frontiers in Plant Science 13: 898769. doi: 10.3389/fpls.2022.898769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Li DZ, Zhu GH, et al. 2006. Poaceae. In: Wu ZY, Raven PH. eds. Flora of China, Vol. 22. Beijing: Science Press and Missouri Botanical Garden Press, 18–42. [Google Scholar]
- Chen F, Liu Q, Vogel JP, Wu J.. 2019. Agrobacterium-mediated transformation of Brachypodium distachyon. Current Protocols in Plant Biology 4: e20088. doi: 10.1002/cppb.20088. [DOI] [PubMed] [Google Scholar]
- Clayton WD, Vorontsova MS, Harman KT, Williamson H.. 2006 onward. GrassBase - The Online World Grass Flora. http://www.kew.org/data/grasses-db.html. (08 November 2006 date last accessed).
- Cubas P, Vincent C, Coen E.. 1999. An epigenetic mutation responsible for natural variation in floral symmetry. Nature 401: 157–161. doi: 10.1038/43657. [DOI] [PubMed] [Google Scholar]
- Das A, Prakash A, Dedon N, Doty A, Siddiqui M, Preston JC.. 2021. Variation in climatic tolerance, but not stomatal traits, partially explains Pooideae grass species distributions. Annals of Botany 128: 83–95. doi: 10.1093/aob/mcab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay C. 1950. Nombres chromosomiques chez les Phanerogames. Revue Cytologie et Biologie Vegetales 12: 1–368. [Google Scholar]
- Di Stilio VS, Melzer R, Hall JC.. 2017. Editorial: A broader view for plant evodevo: novel approaches for diverse model species. Frontiers in Plant Science 8: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon LE, Karsai I, Kiss T, et al. 2019. VERNALIZATION1 controls developmental responses of winter wheat under high ambient temperatures. Development 146: 172684. doi: 10.1242/DEV.172684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- eFloras. 2008. Flora of China. St Louis, MO and Cambridge, MA: Missouri Botanical Garden, Harvard University Herbaria, http://www.efloras.org. (16 March 2023, date last accessed). [Google Scholar]
- Fernandez-Calleja M, Casas AM, Igartua E.. 2021. Major flowering time genes of barley: allelic diversity, effects, and comparison with wheat. Theoretical and Applied Genetics 134: 1867–1897. doi: 10.1007/s00122-021-03824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick S, Hijmans RJ.. 2017. WorldClim 2: new 1km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37: 4302–4315. [Google Scholar]
- Finnegan EJ, Robertson M, Helliwell CA.. 2021. Resetting FLOWERING LOCUS C expression after vernalization is just activation in the early embryo by a different name. Frontiers in Plant Science 11: 620155. doi: 10.3389/fpls.2020.620155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjellheim S, Young DA, Paliocha M, Johnsen SS, Schubert M, Preston JC.. 2022. Major niche transitions in Pooideae correlate with variation in photoperiodic flowering and evolution of CCT domain genes. Journal of Experimental Botany 73: 4079–4093. doi: 10.1093/jxb/erac149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher TJ, Peterson PM, Soreng RJ, et al. 2022. Grasses through space and time: An overview of the biogeographical and macroevolutionary history of Poaceae. Journal of Systematics and Evolution 60: 522–569. [Google Scholar]
- Gifford EW. 1967. Ethnographic notes on the Southwestern Pomo. Anthropological Records 25: 10–15. [Google Scholar]
- Guo Z, Schnurbusch T.. 2016. Costs and benefits of awns. Journal of Experimental Botany 67: 2533–2535. doi: 10.1093/jxb/erw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasterok R, Catalan P, Hazen SP, et al. 2022. Brachypodium: 20 years as a grass biology model system: the way forward? Trends in Plant Science 27: 1002–1016. doi: 10.1016/j.tplants.2022.04.008. [DOI] [PubMed] [Google Scholar]
- Hempel W. 1971. Die systematisehe Stellung von Melica unifora Retz. und Melica rectifora Boiss. er Heldr. (Melica L. Subgen. Bulbimelica, subgen. nov.) (Vorarbeiten zu einer Revision der Gattung Melica L. -1). Feddes Repertorium 81: 657–686. doi: 10.1002/fedr.19710811002. [DOI] [Google Scholar]
- Hempel W. 2012. Revision und Phylogenie der Arten der Gattung Melica L. (Poaceae) in Eurasien und Nordafrika. Feddes Repertorium 122: 1–253. doi: 10.1002/fedr.201100029. [DOI] [Google Scholar]
- Hensen I, Müller C.. 1997. Experimental and structural investigations of anemochorous dispersal. Plant Ecology 133: 169–180. [Google Scholar]
- Hjertaas AC, Preston JC, Kainulainen K, Humphreys AM, Fjellheim S.. 2023. Convergent evolution of the annual life history syndrome from perennial ancestors. Frontiers in Plant Science 13: 1048656. doi: 10.3389/fpls.2022.1048656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Qian Q, Liu Z, et al. 2009. Natural variation at the DEP1 locus enhances grain yield in rice. Nature Genetics 41: 494–497. doi: 10.1038/ng.352. [DOI] [PubMed] [Google Scholar]
- Huang B, Wu W, Hong Z.. 2021. Genetic loci underlying awn morphology in barley. Genes 12: 1613. doi: 10.3390/genes12101613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson Flora Project. eds.2023. Jepson eFlora. https://ucjeps.berkeley.edu/eflora/ (16 March 2023, date last accessed).
- Johnson MG, Pokorny L, Dodsworth S, et al. 2019. A universal probe set for targeted sequencing of 353 nuclear genes from any flowering plant designed using k-medoids clustering. Systematic Biology 68: 594–606. doi: 10.1093/sysbio/syy086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM.. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg EA. 2001. Evolutionary history of grasses. Plant Physiology 125: 1198–1205. doi: 10.1104/pp.125.3.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg EA. 2015. Flowering plants, monocots, poaceae. Volume XIII. In: Kubitzki K. ed. The families and genera of vascular plants. Cham: Springer International Publishing, 199–265. [Google Scholar]
- Khokhar KM. 2017. Environmental and genotypic effects on bulb development in onion–a review. Journal of Horticultural Science and Biotechnology 92: 448–454. doi: 10.1080/14620316.2017.1314199. [DOI] [Google Scholar]
- Kinmonth-Schultz H, Lewandowska-Sabat A, Imaizumi T, Ward JK, Rognli OA, Fjellheim S.. 2021. Flowering times of wild Arabidopsis accessions from across Norway correlate with expression levels of FT, CO, and FLC genes. Frontiers in Plant Science 12: 747740. doi: 10.3389/fpls.2021.747740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler K, Center A, Cavender-Bares J.. 2012. Evidence for a freezing tolerance–growth rate tradeoff in the live oaks (Quercus series Virentes) across the tropical–temperate divide. New Phytologist 193: 730–744. doi: 10.1111/j.1469-8137.2011.03992.x. [DOI] [PubMed] [Google Scholar]
- Lazkov GA, Usupbaev AK.. 2017. The synopsis of the genus Melica (Poaceae) in the flora of Kyrgyz Republic. Novitates Systematicae Plantarum Vascularium 48: 26–32. doi: 10.31111/novitates/2017.48.26. [DOI] [Google Scholar]
- Lee R, Baldwin S, Kennel F, McCallum J, Macknight R.. 2013. FLOWERING LOCUS T genes control onion bulb formation and flowering. Nature Communications 4: 2884. doi: 10.1038/ncomms3884. [DOI] [PubMed] [Google Scholar]
- Lemmon ZH, Park SJ, Jiang K, Van Eck J, Schatz MC, Lippman ZB.. 2016. The evolution of inflorescence diversity in the nightshades and heterochrony during meristem maturation. Genome Research 26: 1676–1686. doi: 10.1101/gr.207837.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Suzuki J-I, Hara T.. 1998. Latitudinal variation in plant size and relative growth rate in Arabidopsis thaliana. Oecologia 115: 293–301. doi: 10.1007/s004420050519. [DOI] [PubMed] [Google Scholar]
- Lindberg CL, Hanslin HM, Schubert M, et al. 2020. Increased above-ground resource allocation is a likely precursor for independent evolutionary origins of annuality in the Pooideae grass subfamily. New Phytologist 228: 318–329. doi: 10.1111/nph.16666. [DOI] [PubMed] [Google Scholar]
- Linder HP, Lehmann CER, Archibald S, Osborne CP, Richardson DM.. 2018. Global grass (Poaceae) success underpinned by traits facilitating colonization, persistence and habitat transformation. Biological Reviews 93: 1125–1144. doi: 10.1111/brv.12388. [DOI] [PubMed] [Google Scholar]
- Liu J, Rasheed A, He Z, et al. 2019. Genome-wide variation patterns between landraces and cultivars uncover divergent selection during modern wheat breeding. Theoretical and Applied Genetics 132: 2509–2523. doi: 10.1007/s00122-019-03367-4. [DOI] [PubMed] [Google Scholar]
- Lundgren MR, Des Marai DL.. 2020. Life history variation as a model for understanding trade-offs in plant–environment interactions. Current Biology 30: R180–R189. doi: 10.1016/j.cub.2020.01.003. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR.. 2011. Mesquite: a Modular System for Evolutionary Analysis. Version 3.2. http://mesquiteproject.org
- McDonnell AJ, Baker WJ, Dodsworth S, et al. 2021. Exploring Angiosperm353: developing and applying a universal toolkit for flowering plant phylogenomics. Applied Plant Science 9. doi: 10.1002/aps3.11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M, Schubert M, Marcussen T, Fjellheim S, Preston JC.. 2016. Evidence for an early origin of vernalization responsiveness in temperate Pooideae grasses. Plant Physiology 172: 416–426. doi: 10.1104/pp.16.01023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSteen P, Kellogg EA.. 2022. Molecular, cellular, and developmental foundations for grass diversity. Science 377: 599–602. doi: 10.1126/science.abo5035. [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T.. 2010. Creating the CIPRES science gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), November 14, 2010, New Orleans, LA, 1–8.
- Monson RK, Trowbridge AM, Lindroth RL, Lerdau MT.. 2022. Coordinated resource allocation to plant growth–defense tradeoff. New Phytologist 233: 1051–1066. [DOI] [PubMed] [Google Scholar]
- Ntakirutimana F, Xie W.. 2019. Morphological and genetic mechanisms underlying awn development in monocotyledonous grasses. Genes 10: 573. doi: 10.3390/genes10080573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott JP, Klimešová J, Hartnett DC.. 2019. The ecology and significance of below-ground bud banks in plants. Annals of Botany 123: 1099–1118. doi: 10.1093/aob/mcz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C, Wolfrum EJ, Nagle N, Brummer JE, Hansen N.. 2017. Evaluation of fifteen cultivars of cool-season perennial grasses as biofuel feedstocks using near-infrared. Agronomy Journal 109: 1923–1934. doi: 10.2134/agronj2016.09.0510. [DOI] [Google Scholar]
- Petersen KB, Kellogg EA.. 2022. Diverse ecological functions and the convergent evolution of grass awns. American Journal of Botany 109: 1331–1345. doi: 10.1002/ajb2.16060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porensky LM, Davison J, Leger EA, et al. 2014. Grasses for biofuels: a low water-use alternative for cold desert agriculture? Biomass and Bioenergy 66: 133–142. doi: 10.1016/j.biombioe.2014.01.046. [DOI] [Google Scholar]
- Pozner R. 2018. Poaceae. In: Anton AM, Zuloaga FO. directors. Flora Argentina. Buenos Aires, Argentina: IBODA-IMBIV, CONICET. http://www.floraargentina.edu.ar (16 March 2023, date last accessed). [Google Scholar]
- Preston JC, Fjellheim S.. 2020. Understanding past, and predicting future, niche transitions based on grass flowering time. Plant Physiology 183: 822–839. doi: 10.1104/pp.20.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JC, Martinez CC, Hileman LC.. 2011. Gradual disintegration of the floral symmetry gene network is implicated in the evolution of a wind-pollination syndrome. Proceedings of the National Academy of Sciences USA 108: 2343–2348. doi: 10.1073/pnas.1011361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probatova NS, Seledets VP, Chernyagina OA.. 2016. Chromosome numbers in some species of Poaceae from Russia: further studies. Botanica Pacifica 5: 59–65. [Google Scholar]
- Pustahija F, Brown SC, Bogunic F, et al. 2013. Small genomes dominate in plants growing on serpentine soils in West Balkans, an exhaustive study of 8 habitats covering 308 taxa. Plant and Soil 373: 427–453. [Google Scholar]
- R Core Team. 2021. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- Raissig MT, Woods DP.. 2022. The wild grass Brachypodium distachyon as a developmental model species. Current Topics in Developmental Biology 147: 33–71. doi: 10.1016/bs.ctdb.2021.12.012. [DOI] [PubMed] [Google Scholar]
- Rambaut A. 2010. FigTree v1.3.1. Edinburgh: Institute of Evolutionary Biology, University of Edinburgh, http://tree.bio.ed.ac.uk/software/figtree/ [Google Scholar]
- Rebetzke G, Bonnett D, Reynolds M.. 2016. Awns reduce grain number to increase grain size and harvestable yield in irrigated and rainfed spring wheat. Journal of Experimental Botany 67: 2573–2586. doi: 10.1093/jxb/erw081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A, Glick L, Abadi S, et al. 2015. The Chromosome Counts Database (CCDB) – a community resource of plant chromosome numbers. New Phytologist 206: 19–26. doi: 10.1111/nph.13191. [DOI] [PubMed] [Google Scholar]
- Royal Horticultural Society (RHS). 2023. Find a Plant. https://www.rhs.org.uk/plants/search-results?form-mode=true&query=Melica. (13 March 2023, date last accessed).
- Sakuma S, Schnurbusch T.. 2020. Of floral fortune: tinkering with the grain potential of cereal crops. New Phytologist 225: 1873–1882. doi: 10.1111/nph.16189. [DOI] [PubMed] [Google Scholar]
- Sasidharan R, Voesenek LACJ, Perata P.. 2021. Plant performance and food security in a wetter world. New Phytologist 229: 5–7. doi: 10.1111/nph.17067. [DOI] [PubMed] [Google Scholar]
- Schrager-Lavelle A, Klein H, Fisher A, Bartlett M.. 2017. Grass flowers: an untapped resource for evo-devo. Journal of Systematics and Evolution 55: 525–541. doi: 10.1111/jse.12251. [DOI] [Google Scholar]
- Schubert M, Grønvold L, Sandve SR, Hvidsten TR, Fjellheim S.. 2019a. Evolution of cold acclimation and its role in niche transition in the temperate grass subfamily Pooideae. Plant Physiology 180: 404–419. doi: 10.1104/pp.18.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M, Marcussen T, Meseguer AS, Fjellheim S.. 2019b. The grass subfamily Pooideae: Cretaceous–Palaeocene origin and climate-driven Cenozoic diversification. Global Ecology and Biogeography 28: 1168–1182. [Google Scholar]
- Šmarda P, Knápek O, Březinová A, et al. 2019. Genome size and genomic GC content data for the nearly complete Czech flora with new estimates for 1632 species. Preslia 90: 117–142. [Google Scholar]
- Snapp S, Roge P, Okori P, Chikowo R, Peter B, Messina J.. 2019. Perennial grains for Africa: possibility or pipedream? Experimental Agriculture 55: 251–272. [Google Scholar]
- Stearns SC. 1992. The evolution of life histories. London: Oxford University Press. [Google Scholar]
- Steinwand MA, Young HA, Bragg JN, Tobias CM, Vogel JP.. 2013. Brachypodium sylvaticum, a model for perennial grasses: transformation and inbred line development. PLoS One 8: e75180. doi: 10.1371/journal.pone.0075180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe JR, Caicedo AL, Hopkins R, et al. 2005. Vernalization sensitivity in Arabidopsis thaliana (Brassicaceae): the effects of latitude and FLC variation. American Journal of Botany 92: 1701–1707. doi: 10.3732/ajb.92.10.1701. [DOI] [PubMed] [Google Scholar]
- Strömberg CAE. 2011. Evolution of grasses and grassland ecosystems. Annual Review of Earth and Planetary Science 39: 517–544. [Google Scholar]
- Szczepaniak M, Cieślak E.. 2007. Low level of genetic variation within Melica transsilvanica populations from the Kraków-Częstochowa upland and the Pieniny Mts revealed by AFLPs analysis. Acta Societatis Botanicorum Poloniae 76: 321–331. doi: 10.5586/asbp.2007.036. [DOI] [Google Scholar]
- Tang H, Cuevas HE, Das S, Paterson AH.. 2013. Seed shattering in a wild sorghum is conferred by a locus unrelated to domestication. Proceedings of the National Academy of Sciences USA 110: 15824–15829. doi: 10.1073/pnas.1305213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teferra TF. 2021. Should we still worry about the safety of GMO foods? Why and why not? A review. Food Science & Nutrition 9: 5324–5331. doi: 10.1002/fsn3.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribble CM, Martínez-Gómez J, Howard CC, et al. 2021. Get the shovel: morphological and evolutionary complexities of belowground organs in geophytes. American Journal of Botany 108: 372–387. doi: 10.1002/ajb2.1623. [DOI] [PubMed] [Google Scholar]
- Tyler T. 2002. Large-scale geographic patterns of genetic variation in Melica nutans, a widespread Eurasian woodland grass. Plant Systematics and Evolution 236: 73–87. [Google Scholar]
- Tyler T. 2004. Studies in the Melica ciliata – complex: 1. Distribution of allozyme variation within and among individuals, populations and geographic regions. Plant Systematics and Evolution 248: 1–30. [Google Scholar]
- USGCRP. 2017. Impacts, risks, and adaptation in the United States: Fourth National Climate Assessment, Volume II. Reidmiller DR, Avery CW, Easterling DR, Kunkel KE, Lewis KLM, Maycock TK, Stewart BC (eds.). Washington, DC: U.S. Global Change Research Program. doi: 10.7930/NCA4.2018. [DOI] [Google Scholar]
- Vavilova V, Konopatskaia I, Kuznetsova AE, Blinov A, Goncharov NP.. 2017. DEP1 gene in wheat species with normal, compactoid and compact spikes. BMC Genetics 18: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Farrona S, Vincent C, et al. 2009. PEP1 regulates perennial flowering in Arabis alpina. Nature 459: 423–427. doi: 10.1038/nature07988. [DOI] [PubMed] [Google Scholar]
- Whipple CJ, Hall DH, DeBlasio S, Taguchi-Shiobara F, Schmidt RJ, Jackson DP.. 2010. A conserved mechanism of bract suppression in the grass family. Plant Cell 22: 565–578. doi: 10.1105/tpc.109.073536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberg AC, Amasino RM.. 2012. Natural variation in the temperature range permissive for vernalization in accessions of Arabidopsis thaliana. Plant and Cell Environment 35: 2181–2191. doi: 10.1111/j.1365-3040.2012.02548.x. [DOI] [PubMed] [Google Scholar]
- Xinglu X, Xiuling L.. 2016. Traditional Chinese medicine for treating thyroid adenoma and preparation method thereof. CN105250749A. Google Patents. https://patents.google.com/patent/CN105250749A/en. [Google Scholar]
- Yu J, Zhao F, Li S, Fang Y, Xiang J, Dong H.. 2019. The complete chloroplast genome sequence of Melica scabrosa. Mitochondrial DNA B Resources 4: 2872–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Leyva P, Tavares RL, Kellogg EA.. 2020a. The anatomy of abscission zones is diverse among grass species. American Journal of Botany 107: 549–561. doi: 10.1002/ajb2.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Hu H, Doust AN, Kellogg EA.. 2020b. Divergent gene expression networks underlie morphological diversity of abscission zones in grasses. New Phytologist 225: 1799–1815. doi: 10.1111/nph.16087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M. 2014. Traditional Chinese medicine preparation for treating icteric hepatitis as well as preparation method thereof. CN103495063A. Google Patents. https://patents.google.com/patent/CN103495063A/en.
- Zhang L, Zhu X, Zhao Y, et al. 2022. Phylotranscriptomics resolves the phylogeny of Pooideae and uncovers factors for their adaptive evolution. Molecular Biology and Evolution 39: msac026. doi: 10.1093/molbev/msac026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Huang G, Zhang Y, et al. 2023. Sustained productivity and agronomic potential of perennial rice. Nature Sustainability 6: 28–38. doi: 10.1038/s41893-022-00997-3. [DOI] [Google Scholar]
- Zhao Y, Medrano L, Ohashi K, et al. 2004. HANABA TARANU is a GATA transcription factor that regulates shoot apical meristem and flower development in Arabidopsis. Plant Cell 16: 2586–2600. doi: 10.1105/tpc.104.024869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Robbett M, Poire A, Preston JC.. 2018. Successive evolutionary steps drove Pooideae grasses from tropical to temperate regions. New Phytologist 217: 925–938. doi: 10.1111/nph.14868. [DOI] [PubMed] [Google Scholar]
- Zigmond ML. 1981. Kawaiisu ethnobotany. Salt Lake City, UT: University of Utah Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


