Abstract
Background
Studies on the relationship between insulin resistance (IR) surrogates and long-term all-cause mortality in patients with coronary heart disease (CHD) and hypertension are lacking. This study aimed to explore the relationship between different IR surrogates and all-cause mortality and identify valuable predictors of survival status in this population.
Methods
The data came from the National Health and Nutrition Examination Survey (NHANES 2001–2018) and National Death Index (NDI). Multivariate Cox regression and restricted cubic splines (RCS) were performed to evaluate the relationship between homeostatic model assessment of IR (HOMA-IR), triglyceride glucose index (TyG index), triglyceride glucose-body mass index (TyG-BMI index) and all-cause mortality. The recursive algorithm was conducted to calculate inflection points when segmenting effects were found. Then, segmented Kaplan–Meier analysis, LogRank tests, and multivariable Cox regression were carried out. Receiver operating characteristic (ROC) and calibration curves were drawn to evaluate the differentiation and accuracy of IR surrogates in predicting the all-cause mortality. Stratified analysis and interaction tests were conducted according to age, gender, diabetes, cancer, hypoglycemic and lipid-lowering drug use.
Results
1126 participants were included in the study. During the median follow-up of 76 months, 455 participants died. RCS showed that HOMA-IR had a segmented effect on all-cause mortality. 3.59 was a statistically significant inflection point. When the HOMA-IR was less than 3.59, it was negatively associated with all-cause mortality [HR = 0.87,95%CI (0.78, 0.97)]. Conversely, when the HOMA-IR was greater than 3.59, it was positively associated with all-cause mortality [HR = 1.03,95%CI (1.00, 1.05)]. ROC and calibration curves indicated that HOMA-IR was a reliable predictor of survival status (area under curve = 0,812). No interactions between HOMA-IR and stratified variables were found.
Conclusion
The relationship between HOMA-IR and all-cause mortality was U-shaped in patients with CHD and hypertension. HOMA-IR was a reliable predictor of all-cause mortality in this population.
Keywords: Coronary heart disease, Hypertension, Insulin resistance, All-cause mortality
Background
Coronary heart disease (CHD) is a significant challenge facing global public health. According to the American Heart Association, more than 350,000 people die of CHD in the United States each year [1]. Hypertension is the leading cause of major cardiovascular adverse events [2]. For patients with both CHD and hypertension, early identification and intervention of risk factors that affect prognosis are crucial for reducing the global burden of cardiovascular diseases [3].
Insulin resistance (IR) is a prominent feature of metabolic syndrome, referring to a decrease in the efficiency of insulin in promoting glucose utilization [4]. IR is considered a risk factor for microvascular and macrovascular lesions [5]. The hyperinsulinemic-normal glucose clamp test is the gold standard for IR measurement, but it is a complex and invasive examination that is not suitable for clinical research [6]. A validated alternative evaluation index is the homeostatic model assessment of insulin resistance (HOMA-IR), which is calculated from fasting blood glucose and insulin concentrations [7]. However, circulating insulin concentrations are not routinely measured in primary care, so various simple and feasible alternative evaluation indices for IR have emerged, such as the triglyceride glucose index (TyG index), triglyceride glucose-body mass index (TyG-BMI index) [8, 9]. Some studies have found that the TyG index is associated with in-hospital all-cause mortality in patients with severe CHD [10]. However, few studies have evaluated the correlation between these IR surrogates and all-cause mortality in patients with CHD and hypertension. Only a few related studies conducted in China have found that the TyG index is associated with short-term adverse CVD outcomes in patients with CHD and hypertension [11]. There is still a lack of evidence on which IR surrogates can serve as long-term predictors of all-cause mortality risk in patients with CHD and hypertension.
This study linked the National Health and Nutrition Examination Survey (NHANES) and National Death Index (NDI) data to investigate the relationship between different IR surrogates and long-term all-cause mortality in patients with CHD and hypertension. The aim is to identify valuable predictors of survival status in this population.
Methods
Study design
The baseline data was obtained from NHANES. NHANES is a continuous cross-sectional survey with national representation and complex multi-stage sampling, aiming to assess the nutritional and health status of the non-institutionalized US population. NHANES contains a large amount of data on demographics, dietary nutrition, physical examination, laboratory examination, and medical history. Detailed information about NHANES has been described in other studies [12]. We collected the information of participants who were first interviewed between 2001 and 2018. Then we linked the National Death Index (NDI) of the National Center for Health Statistics (NCHS) to obtain the survival status of the participants, and constructed a NHANES longitudinal follow-up cohort. The NCHS Ethical Review Board approved the study. Informed consent was obtained from all study participants [13]. Therefore, no additional informed consent and ethical review were required for our research.
Study population
Participants who were surveyed between 2001 and 2018 and had both CHD and hypertension were included in this study. Those missing IR surrogates and survival status were excluded. The history of CHD and hypertension was obtained through interviews. CHD information was obtained by asking participants:”Has a doctor or other health professional ever told you that you had coronary heart disease?”” Has a doctor or other health professional ever told you that you had angina, also called angina pectoris?” or “Has a doctor or other health professional ever told you that you had a heart attack (also called myocardial infarction?” If they answered "Yes" to any of the above questions, they were diagnosed CHD. Similarly, the information on hypertension was obtained through self-report of having been diagnosed with hypertension by a doctor or currently taking antihypertensive prescription drugs. Participants who had both CHD and hypertension were included in this study.
IR surrogates
The IR surrogates in this study include the HOMA-IR, TyG index, and TyG-BMI index. The HOMA-IR was calculated as follows: HOMA-IR = fasting glucose (mmol/L) × fasting insulin (µU/mL)/22.5 [14]. The TyG index was calculated as follows: TyG = Ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2] [15]. The TyG-BMI index was calculated as follows: TyG-BMI = TyG index × BMI (kg/m2) [16]. Trained laboratory personnel collected fasting blood from the participants. The blood samples were frozen at -20 °C and transported to the laboratory for testing. Fasting insulin was measured using the AIA-PACK IRI. The AIA-PACK IRI is a two-site immunoenzymometric assay, which is performed on Tosoh AIA System analyzer. The measurement of triglycerides and fasting glucose were measured through enzymatic assays on Roche Modular P and Roche Cobas 6000 chemistry analyzers, respectively. BMI was calculated using a formula that takes into account the participant's standing height and weight.
Survival status
Using a series of identifiers such as social security number and date of birth, NCHS used probabilistic matching to link NHANES with NDI data to obtain survival status data for participants. The follow-up of participants was terminated on December 31, 2019. If there was no match with the NDI, it was assumed that the person was alive [17]. This study considered both survival outcomes and survival time.
Covariates
Demographic, medical history, and laboratory blood test data of participants were collected. Demographic data included age, gender, race, education level, marital status, and income -poverty ratio (PIR). Medical history information included diabetes, cancer, heart failure, stroke, chronic obstructive pulmonary disease (COPD), use of hypoglycemic and lipid-lowering prescription drugs, tobacco use, BMI, waist circumference, and hip circumference. Laboratory blood test data included low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), total cholesterol (TC), alanine aminotransferase (ALT), albumin, alkaline phosphatase (ALP), aspartate aminotransferase (AST), urea nitrogen, creatine kinase (CK), creatinine (Cr), gamma-glutamyltransferase (GGT), lactate dehydrogenase (LDH), iron, phosphorus, potassium, sodium, calcium, total bilirubin, uric acid, glycosylated hemoglobin (HbA1c), hemoglobin (Hb), platelet count, and white blood cell count (WBC). The demographic and medical history information was obtained through interviews. Smoking more than 100 cigarettes in a lifetime was defined as a tobacco user. BMI, waist circumference, and hip circumference were obtained through measurement. The definition of diabetes was self-reported diagnosis, use of insulin or oral hypoglycemic agents, fasting glucose ≥ 7 mmol/L, or HbA1c ≥ 6.5% [18].
Statistic analysis
Participants were divided into two groups according to survival status to describe the characteristics of the study population. Continuous variables were expressed as mean and standard deviation or median and quartile, and the t-test or Kruskal–Wallis rank sum test was selected for hypothesis testing according to applicable conditions. Classified variables were expressed as absolute numbers and percentages, and the chi-square test was used for hypothesis testing.
Multivariable Cox regression models were used to evaluate of the linear relationship between different IR surrogates and survival status. We constructed three regression models by adjusting different covariates to control for confounding biases. The selection of covariates was driven both theoretically and statistically. Some covariates theoretically associated with survival status were fixed in the model, such as age, gender, race, diabetes, and cancer. Other variables were selected using statistical methods. First, variables with variance inflation factors greater than 5 were excluded to avoid multicollinearity. Then, a two-way effect change method was used to screen variables that had an impact on the effect size of the independent variables greater than 10%. Adjust I adjusted for age, gender, race, diabetes, and cancer, and Adjust II was a fully adjusted model. Multiple chain interpolation was used to fill in missing data.
Multivariable restricted cubic splines (RCS) were used to identify the nonlinear relationship between different IR surrogates and survival status. We aimed to identify potentially valuable predictors of survival status based on the shape of the RCS curves. Recursive algorithms were used to calculate potential cut-off points. Based on these cut-off points, we conducted segmented Kaplan–Meier analysis, LogRank tests, and multivariable Cox regression.
In addition, we evaluated the discrimination ability and accuracy of the fully adjusted model using receiver operating characteristic (ROC) curves, area under the curve (AUC), and calibration curves. We compared the differences in AUC using the Z-test. Finally, we conducted stratified analyses and interaction tests based on age, gender, diabetes, cancer, and the use of hypoglycemic and lipid-lowering prescription drugs.
Data analysis was completed by software IBM SPSS Statistics,(version 26.0) and R software (version 4.2.1). P < 0.05 on both sides was considered statistically significant. Taking into account the complex sampling design of NHANES, the minimum subsample weights, clustering, and stratification were included in the analysis [19].
Results
General characteristics of participants
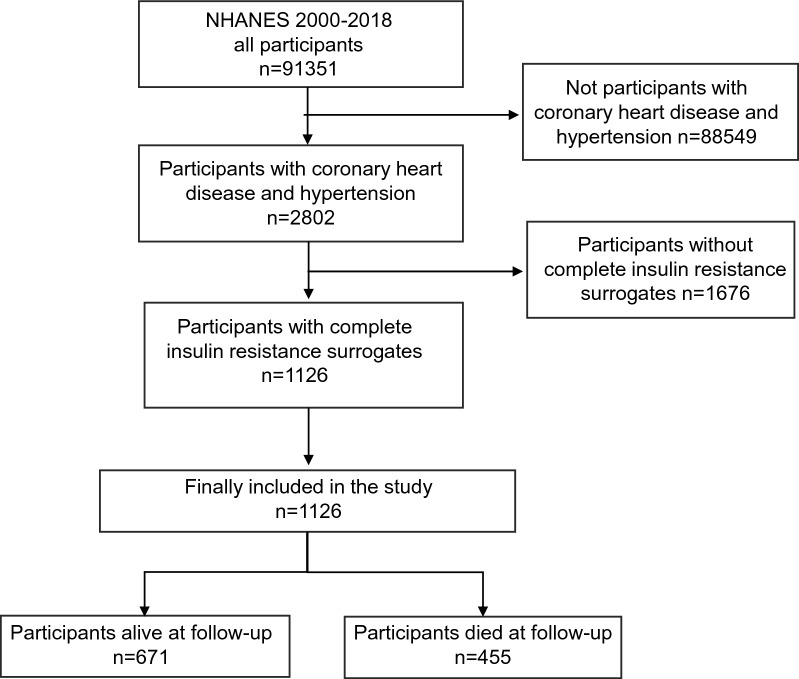
The screening process for the study population is presented in Fig. 1. 1126 participants were included in the analysis. 1126 participants provided follow-up data for a total of 96,048 person-months. And during the median follow-up of 76 months, 455 participants died. They had lower HOMA-IR and TyG-BMI index at baseline, but the difference in TyG index was not statistically significant. In addition, they were older, had a higher proportion of males, and had higher prevalences of diabetes and cancer. Detailed information on the demographics, medical history, laboratory tests at baseline, and the results of univariate analysis, are presented in Tables 1 and 2.
Fig. 1.
Study population screening flow chart
Table 1.
Demographic and medical history baseline characteristics
| Variables | Surviving participants (n = 671) | Dead participants (n = 455) | P-value |
|---|---|---|---|
| Age.years | 64.36 (11.38) | 72.48 (9.63) | < 0.001 |
| Gender | 0.031 | ||
| Male | 380 (56.63%) | 287 (63.08%) | |
| Female | 291 (43.37%) | 168 (36.92%) | |
| Race | < 0.001 | ||
| Mexican American | 75 (11.18%) | 31 (6.81%) | |
| Other Hispanic | 66 (9.84%) | 21 (4.62%) | |
| Non-Hispanic White | 331 (49.33%) | 308 (67.69%) | |
| Non-Hispanic Black | 145 (21.61%) | 78 (17.14%) | |
| Other Race | 54 (8.05%) | 17 (3.74%) | |
| Marriage | < 0.001 | ||
| Having a partner | 416 (62.00%) | 228 (50.11%) | |
| Without partner | 255 (38.00%) | 227 (49.89%) | |
| Education | 0.034 | ||
| High school and below | 224 (33.38%) | 180 (39.56%) | |
| Above high school | 447 (66.62%) | 275 (60.44%) | |
| PIR | 2.32 (1.58) | 2.13 (1.41) | 0.047 |
| BMI | 30.71 (6.39) | 29.19 (6.49) | < 0.001 |
| Hip circumference.cm | 34.16 (4.90) | 32.56 (5.28) | < 0.001 |
| Waist circumference.cm | 106.32 (14.67) | 104.56 (15.65) | 0.062 |
| Diabetics | < 0.001 | ||
| Yes | 609 (90.76%) | 441 (96.92%) | |
| No | 62 (9.24%) | 14 (3.08%) | |
| Cancer patients | < 0.001 | ||
| Yes | 119 (17.73%) | 126 (27.69%) | |
| No | 552 (82.27%) | 329 (72.31%) | |
| Heart failure patients | < 0.001 | ||
| Yes | 163 (24.62%) | 160 (35.71%) | |
| No | 499 (75.38%) | 288 (64.29%) | |
| Stroke patients | 0.016 | ||
| Yes | 104 (15.50%) | 96 (21.10%) | |
| No | 567 (84.50%) | 359 (78.90%) | |
| COPD patients | 0.481 | ||
| Yes | 58 (8.64%) | 34 (7.47%) | |
| No | 613 (91.36%) | 421 (92.53%) | |
| Hypoglycemic drugs or insulin Users | 0.012 | ||
| Yes | 548 (81.67%) | 397 (87.25%) | |
| No | 123 (18.33%) | 58 (12.75%) | |
| Lipid-lowering drug users | 0.574 | ||
| Yes | 624 (93.00%) | 427 (93.85%) | |
| No | 47 (7.00%) | 28 (6.15%) | |
| Tobacco users | 0.023 | ||
| Yes | 399 (59.46%) | 301 (66.15%) | |
| No | 272 (40.54%) | 154 (33.85%) | |
Mean(SD) | Median (Q1-Q3) | N(%). COPD: chronic obstructive pulmonary disease, PIR: income-poverty ratio, BMI: body mass index
Table 2.
Baseline characteristics of laboratory blood test
| Variables | Surviving participants (n = 671) | Dead participants (n = 455) | P-value |
|---|---|---|---|
| LDL-C.mmol/L | 2.61 (0.97) | 2.50 (0.95) | 0.047 |
| HDL-C.mmol/L | 1.29 (0.39) | 1.34 (0.44) | 0.081 |
| TG.mg/dL | 123.00 (87.50–179.50) | 119.00 (84.00–169.00) | 0.261 |
| TC.mmol/L | 4.45 (3.80–5.23) | 4.37 (3.71–5.20) | 0.393 |
| Fasting blood glucose.mmol/L | 6.49 (2.51) | 6.53 (2.17) | 0.795 |
| Albumen.g/L | 41.36 (3.20) | 40.63 (3.77) | < 0.001 |
| ALP. IU/L | 72.00 (59.00–87.14) | 74.38 (62.36–89.00) | 0.011 |
| AST.U/L | 23.00 (19.00–28.00) | 23.00 (19.50–28.00) | 0.318 |
| ALT.U/L | 21.00 (16.00–28.00) | 19.00 (15.00–25.00) | 0.048 |
| CK.IU/L | 126.00 (85.54–178.90) | 128.34 (94.15–166.85) | 0.049 |
| Creatinine.umol/L | 84.86 (69.84–102.54) | 97.24 (79.56–123.76) | < 0.001 |
| GGT.IU/L | 23.00 (17.00–34.00) | 22.00 (16.00–37.00) | 0.061 |
| LDH.IU/L | 138.00 (122.00–156.00) | 139.00 (125.50–161.00) | 0.449 |
| Serum urea nitrogen.mmol/L | 5.36 (4.28–7.14) | 6.43 (4.64–8.93) | < 0.001 |
| Total bilirubin.umol/L | 10.26 (8.55–13.68) | 11.97 (10.26–15.39) | < 0.001 |
| Serum uric acid.umol/L | 354.84 (87.80) | 384.08 (110.50) | < 0.001 |
| Iron.umol/L | 14.30 (11.10–18.10) | 13.43 (10.20–17.70) | 0.074 |
| Calcium.mmol/L | 2.35 (0.09) | 2.35 (0.11) | 0.359 |
| Phosphorus.mmol/L | 1.18 (0.18) | 1.21 (0.21) | 0.008 |
| Potassium.mmol/L | 4.08 (0.39) | 4.17 (0.43) | 0.001 |
| Sodium.mmol/L | 139.79 (2.61) | 138.86 (2.89) | < 0.001 |
| Hemoglobin.g/dL | 14.09 (1.51) | 13.78 (1.79) | 0.002 |
| Platelet count.1000 Cells /uL | 216.00 (182.00–261.50) | 216.00 (182.00–263.50) | 0.939 |
| White blood cell count.1000 Cells /uL | 6.70 (5.70–8.10) | 7.20 (5.70–8.80) | 0.006 |
| Fasting insulin.μU/mL | 12.44 (7.85–20.05) | 10.36 (6.73–18.30) | 0.055 |
| HbA1c.% | 6.25 (1.31) | 6.28 (1.34) | 0.631 |
| HOMA-IR | 3.47 (1.99–5.69) | 2.82 (1.65–5.24) | 0.446 |
| TyG | 8.82 (8.39–9.26) | 8.80 (8.37–9.22) | 0.477 |
| TyG-BMI | 266.16 (226.28–310.56) | 251.23 (214.75–294.37) | < 0.001 |
Mean(SD) | Median (Q1-Q3)
LDL-C low density lipoprotein cholesterol, HDL-C high density lipoprotein cholesterol, TG triglycerides, TC total cholesterol, ALP alkaline phosphatase, AST aspartate aminotransferase, ALT alanine aminotransferase, CK creatine kinase, GGT gamma-glutamyltransferase, LDH lactate dehydrogenase, HbA1c glycosylated hemoglobin, HOMA-IR homeostatic model assessment of insulin resistance, TyG triglyceride glucose index, TyG-BMI triglyceride glucose- body mass index
Association between different IR surrogates and survival status
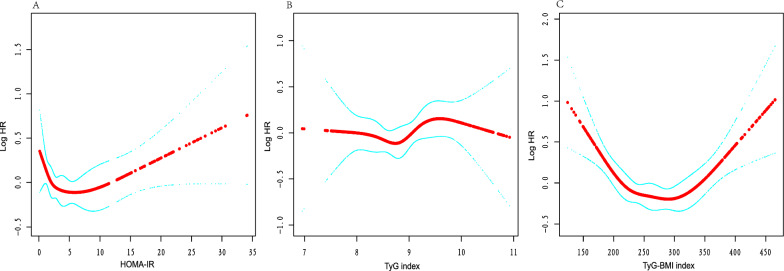
The following variables were adjusted in Adjust II model: age, gender, race, diabetes, cancer, marriage, education, LDL-C, HDL-C, albumen, ALP, AST, CK, creatinine, GGT, LDH, iron, phosphorus, sodium, potassium, calcium, total bilirubin, uric acid, urea nitrogen, hemoglobin, platelet count, white blood cell count, stroke, COPD, hypoglycemic drugs or insulin use, lipid-lowering drug use. The results of the multivariable Cox regression and RCS are presented in Table 3 and Fig. 2, respectively. In the fully adjusted Cox regression model, when IR surrogates were included as continuous variables, their relationship with survival status was not statistically significant. The RCS curve demonstrated the nonlinear relationship between IR surrogates and survival status. Figure 2A showed that the HOMA-IR may have a piecewise effect on survival status with a distinct inflection point, indicating that it may be a predictor of survival status.
Table 3.
Relationship between different IR surrogates and all-cause mortality
| Exposure | Non-adjusted | Adjust I | Adjust II |
|---|---|---|---|
| HOMA-IR | 0.99 (0.97, 1.01) 0.1757 | 1.01 (0.98, 1.03) 0.6197 | 1.01 (0.99, 1.03) 0.3484 |
| TyG | 0.92 (0.79, 1.06) 0.2444 | 0.99 (0.84, 1.16) 0.8762 | 1.05 (0.88, 1.25) 0.5931 |
| TyG-BMI | 1.00 (1.00, 1.00) < 0.0001 | 1.00 (1.00, 1.00) 0.2280 | 1.00 (1.00, 1.00) 0.2329 |
hazard ratio(HR), 95% confidence interval(CI),and P-value. Adjust I: Age, sex, race, diabetes, and cancer. Adjust II: Age, sex, race, diabetes, cancer, and variables obtained by using the two-way effect change method
Fig. 2.
Nonlinear relationship between different IR surrogates and all-cause mortality. The red line in the figure represents Log(HR), and the blue line represents the 95% confidence interva. A HOMA-IR, B TyG index, and C TyG-BMI index
The relationship between HOMA-IR and survival status
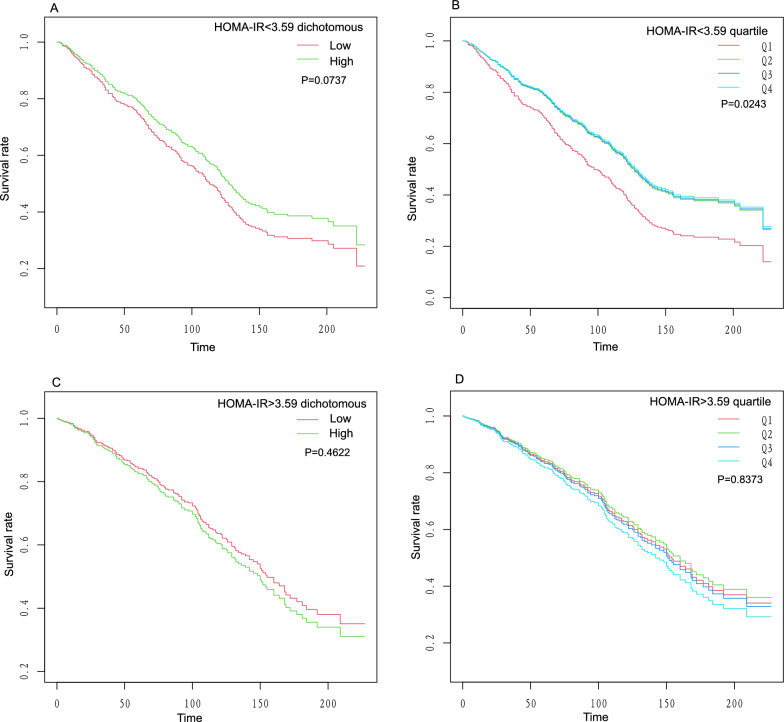
Using recursive partitioning analysis, we found a statistically significant breakpoint (breakpoint = 3.59, P = 0.005) in the relationship between the HOMA-IR and survival status. When the HOMA-IR is less than 3.59, it was negatively associated with survival status [HR = 0.87,95%CI (0.78, 0.97)]. Conversely, when the HOMA-IR was greater than 3.59, it was positively associated with survival status [HR = 1.03,95%CI (1.00, 1.05)]. These results are presented in Table 4. Taking 3.59 as the cut-off point, we further studied the relationship between HOMA-IR and survival status in segments. The results of the segmented Kaplan–Meier analysis are shown in Fig. 3. When the HOMA-IR was less than 3.59, both binary and quartile classification of HOMA-IR were associated with low survival rates in individuals with low levels of HOMA-IR (Fig. 3A, B). However, when the HOMA-IR was greater than 3.59, individuals with high levels of HOMA-IR were associated with low survival rates (Fig. 3C, D). In addition, we also convert HOMA-IR into categorical variables for piecewise multivariate Cox regression. The results showed that when the HOMA-IR is less than 3.59, in the Adjust I, the higher HOMA-IR was associated with a lower all-cause mortality rate [HR = 0.69,95%CI (0.54, 0.87)], and the trend test was statistically significant (P = 0.0004). When the HOMA-IR was greater than 3.59, in the Adjust I, the higher HOMA-IR was associated with an increased all-cause mortality rate [HR = 1.44,95%CI (1.07, 1.95)]. These results are presented in Table 5.
Table 4.
Cut point and segmentation effects of HOMA-IR
| Items | Outcome: |
|---|---|
| Linear effect | 1.01 (0.99, 1.03) 0.3484 |
| Segmentation effect | |
| Cut point (K) | 3.59 |
| < K segment effect | 0.87 (0.78, 0.97) 0.0091 |
| > K segment effect | 1.03 (1.00, 1.05) 0.0177 |
| Effect difference | 1.19 (1.06, 1.34) 0.0044 |
| Logarithmic likelihood ratio test | 0.005 |
| 95%CI of the Cut point | 2.86, 4.34 |
hazard ratio(HR), 95% confidence interval(CI),and P-value
Fig. 3.
Segmented HOMA-IR survival curve. A HOMA-IR < 3.59 dichotomous, B HOMA-IR < 3.59 quartile, C HOMA-IR > 3.59 dichotomous, and D HOMA-IR > 3.59 quartile
Table 5.
Segmented Cox regression analysis and trend test of HOMA-IR
| Non-adjusted | Adjust I | Adjust II | |
|---|---|---|---|
| HOMA-IR < 3.59 | |||
| HOMA-IR dichotomous | |||
| Low | Reference | Reference | Reference |
| High | 0.81 (0.63, 1.02) 0.0751 | 0.69 (0.54, 0.87) 0.0023 | 0.96 (0.73, 1.26) 0.7673 |
| HOMA-IR quartile | |||
| Q1 | Reference | Reference | Reference |
| Q2 | 0.68 (0.49, 0.94) 0.0193 | 0.69 (0.49, 0.96) 0.0275 | 0.68 (0.47, 0.99) 0.0460 |
| Q3 | 0.67 (0.48, 0.93) 0.0159 | 0.59 (0.43, 0.83) 0.0020 | 0.82 (0.56, 1.20) 0.3153 |
| Q4 | 0.65 (0.47, 0.91) 0.0127 | 0.54 (0.39, 0.76) 0.0004 | 0.73 (0.49, 1.07) 0.1042 |
| P trend | 0.0185 | 0.0004 | 0.2615 |
| HOMA-IR > 3.59 | |||
| HOMA-IR dichotomous | |||
| Low | Reference | Reference | Reference |
| High | 1.11 (0.83, 1.49) 0.4635 | 1.44 (1.07, 1.95) 0.0166 | 1.16 (0.84, 1.60) 0.3774 |
| HOMA-IR quartile | |||
| Q1 | Reference | Reference | Reference |
| Q2 | 0.95 (0.62, 1.44) 0.8005 | 0.93 (0.61, 1.43) 0.7427 | 0.93 (0.59, 1.45) 0.7436 |
| Q3 | 1.03 (0.68, 1.56) 0.8769 | 1.37 (0.90, 2.10) 0.1411 | 1.00 (0.63, 1.60) 0.9870 |
| Q4 | 1.14 (0.75, 1.73) 0.5312 | 1.41 (0.92, 2.15) 0.1122 | 1.22 (0.78, 1.91) 0.3784 |
| P trend | 0.3996 | 0.0618 | 0.6046 |
hazard ratio(HR), 95% confidence interval(CI),and P-value. Adjust I: Age, sex, race, diabetes, and cancer. Adjust II: Age, sex, race, diabetes, cancer, and variables obtained by using the two-way effect change method
Subgroup analysis and model evaluation
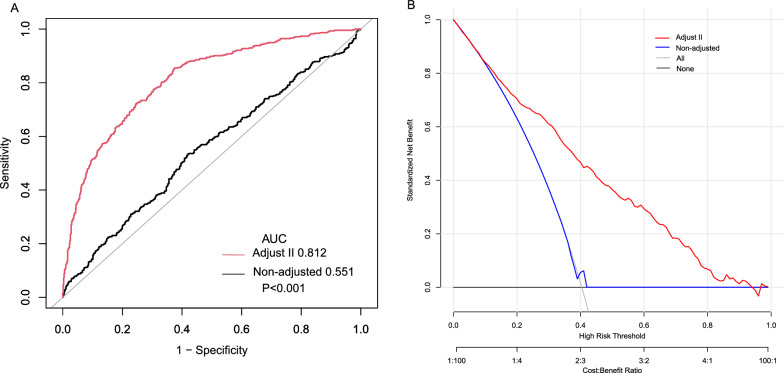
Table 6 presents the results of the segmented subgroup analysis and interaction tests between the HOMA-IR and survival status. Age, gender, diabetes, cancer, hypoglycemic and lipid-lowering prescription drugs did not have significant interactions with the HOMA-IR. The ROC curve and calibration curve in Fig. 4 indicated that when using the HOMA-IR to evaluate survival status, the fully adjusted model we constructed had better discriminatory and accuracy compared to the univariate Cox regression model. The AUC value for the fully adjusted model was 0.812, which was significantly higher than unadjusted model and the difference was statistically significant (P < 0.001).
Table 6.
HOMA-IR segmented subgroup analysis
| Non-adjusted | Adjust I | Adjust II | P for interaction | |
|---|---|---|---|---|
| HOMA-IR < 3.59 | ||||
| Age dichotomous | 0.4039 | |||
| Low | 0.73 (0.55, 0.99) 0.0398 | 0.73 (0.54, 0.98) 0.0339 | 0.81 (0.59, 1.10) 0.1727 | |
| High | 0.75 (0.63, 0.89) 0.0012 | 0.74 (0.62, 0.88) 0.0008 | 0.94 (0.77, 1.14) 0.5309 | |
| Gender | 0.7908 | |||
| Male | 0.79 (0.66, 0.93) 0.0055 | 0.72 (0.61, 0.86) 0.0002 | 0.88 (0.72, 1.06) 0.1758 | |
| Female | 0.91 (0.69, 1.19) 0.4804 | 0.87 (0.66, 1.13) 0.2932 | 0.92 (0.68, 1.24) 0.5745 | |
| Diabetics | 0.6544 | |||
| Yes | 0.82 (0.71, 0.95) 0.0089 | 0.76 (0.65, 0.88) 0.0002 | 0.88 (0.74, 1.04) 0.1424 | |
| No | 1.10 (0.46, 2.59) 0.8329 | 0.95 (0.37, 2.48) 0.9211 | 1.09 (0.45, 2.65) 0.8545 | |
| Cancer | 0.297 | |||
| Yes | 0.65 (0.49, 0.86) 0.0027 | 0.63 (0.47, 0.85) 0.0024 | 0.77 (0.55, 1.06) 0.1065 | |
| No | 0.87 (0.73, 1.04) 0.1230 | 0.81 (0.68, 0.95) 0.0115 | 0.93 (0.77, 1.12) 0.4529 | |
| Hypoglycemic drugs or insulin Users | 0.8085 | |||
| Yes | 0.86 (0.73, 1.01) 0.0583 | 0.73 (0.62, 0.86) 0.0002 | 0.88 (0.73, 1.05) 0.1642 | |
| No | 0.73 (0.50, 1.07) 0.1105 | 0.81 (0.56, 1.18) 0.2788 | 0.93 (0.63, 1.37) 0.7047 | |
| Lipid-lowering drug users | 0.96 | |||
| Yes | 0.83 (0.72, 0.97) 0.0162 | 0.76 (0.66, 0.88) 0.0004 | 0.89 (0.75, 1.05) 0.1637 | |
| No | 0.85 (0.41, 1.77) 0.6620 | 0.77 (0.36, 1.65) 0.5061 | 0.90 (0.42, 1.95) 0.7988 | |
| HOMA-IR > 3.59 | ||||
| Age dichotomous | 0.2771 | |||
| Low | 1.01 (0.97, 1.05) 0.5769 | 1.01 (0.98, 1.05) 0.4820 | 1.00 (0.96, 1.05) 0.8460 | |
| High | 1.03 (1.00, 1.06) 0.0873 | 1.04 (1.01, 1.07) 0.0094 | 1.03 (1.00, 1.07) 0.0657 | |
| Gender | 0.05 | |||
| Male | 0.99 (0.96, 1.03) 0.7300 | 1.02 (0.99, 1.06) 0.2589 | 1.00 (0.97, 1.04) 0.9058 | |
| Female | 1.05 (1.01, 1.08) 0.0053 | 1.05 (1.02, 1.09) 0.0038 | 1.06 (1.02, 1.10) 0.0040 | |
| Diabetics | 0.7656 | |||
| Yes | 1.02 (0.99, 1.04) 0.1842 | 1.03 (1.01, 1.06) 0.0053 | 1.03 (1.00, 1.05) 0.0565 | |
| No | 0.99 (0.67, 1.44) 0.9381 | 1.01 (0.67, 1.54) 0.9530 | 1.10 (0.70, 1.73) 0.6737 | |
| Cancer | 0.0349 | |||
| Yes | 1.09 (1.04, 1.15) 0.0010 | 1.12 (1.05, 1.18) 0.0002 | 1.10 (1.03, 1.17) 0.0043 | |
| No | 1.01 (0.98, 1.04) 0.5390 | 1.02 (0.99, 1.05) 0.1266 | 1.01 (0.98, 1.04) 0.3709 | |
| Hypoglycemic drugs or insulin Users | 0.0716 | |||
| Yes | 1.01 (0.99, 1.04) 0.2559 | 1.03 (1.01, 1.06) 0.0109 | 1.02 (1.00, 1.05) 0.0977 | |
| No | 1.09 (0.96, 1.24) 0.1717 | 1.17 (1.03, 1.32) 0.0131 | 1.17 (1.03, 1.33) 0.0189 | |
| Lipid-lowering drug users | 0.3338 | |||
| Yes | 1.01 (0.99, 1.04) 0.2313 | 1.03 (1.01, 1.06) 0.0095 | 1.02 (1.00, 1.05) 0.0741 | |
| No | 1.09 (0.96, 1.23) 0.1837 | 1.11 (0.98, 1.27) 0.1089 | 1.12 (0.95, 1.32) 0.1699 | |
hazard ratio(HR), 95% confidence interval(CI),and P-value. Adjust I: Age, sex, race, diabetes, and cancer. Adjust II: Age, sex, race, diabetes, cancer, and variables obtained by using the two-way effect change method. Variables used as stratification criteria were not adjusted
Fig. 4.
Discrimination and accuracy of HOMA-IR in evaluating all-cause mortality in the fully adjusted model. A ROC and AUC, B calibration curve
Discussion
This study explored the relationship between different IR surrogates and all-cause mortality in a community-based population with CHD and hypertension in the United States. Using multivariate Cox regression and RCS analysis, we found that HOMA-IR was a reliable predictor of all-cause mortality risk in patients with CHD and hypertension. HOMA-IR was associated with all-cause mortality risk in a U-shaped manner, and high or low HOMA-IR increased the risk of all-cause mortality in this population.
Baseline characteristics of the study population indicated that the control of blood sugar, blood lipids and weight in patients with CHD and hypertension did not reach optimal standards. Participants who died during follow-up were more likely to be male, older, with lower education level and income, and more smokers, diabetes, heart failure, stroke, and cancer patients. In segmented multivariate Cox regression analysis, when HOMA-IR was modeled as a continuous variable, the effect values were statistically significant. When HOMA-IR was modeled as categorical variables, the effect values were not statistically significant, which may due to a decrease in testing efficiency resulting from insufficient sample size for each group.
IR is an important feature of the metabolic syndrome, and in addition to diabetes, obesity, and dyslipidemia, it is also a risk factor for the development of CVD, and may be associated with adverse outcomes in patients with CVD [20]. Some studies have found that IR is positively associated with the development of CVD in patients with prediabetes [21]. A Korean cohort study with a median follow-up time of 9.83 years found that IR increased the risk of all-cause mortality, cardiovascular mortality, and adverse cardiovascular events in CVD patients by 87%, 133%, and 267%, respectively [22]. The hyperinsulinemic-normal glucose clamp technique is the gold standard for the diagnosis of IR, but due to its limitations, it is difficult to use in large-scale clinical studies. Therefore, various different IR surrogates are widely used in clinical research. HOMA-IR is the most widely used surrogate marker, but its calculation requires the measurement of fasting insulin concentration [23]. The TyG index has also received attention due to its simplicity and ease of use. Previous studies have shown that the TyG index has good sensitivity (96.5%) and specificity (85.0%) for the diagnosis of IR compared to the hyperinsulinemic-normal glucose clamp technique [24]. In addition, the TyG-BMI index is also commonly used in clinical research [25].
Previous studies on the relationship between IR surrogates and CVD outcomes have been extensively conducted, but research in community-based populations with CHD and hypertension is lack. Current studies in patients with CHD and hypertension focus on short-term outcomes in hospitalized patients. For example, two cohort studies conducted in China found that in hospitalized patients with CHD and hypertension, the TyG index was positively associated with adverse outcomes, including all-cause mortality, during one-year follow-up [2, 11]. However, our study did not find an association between the TyG index and all-cause mortality, which may be due to differences in study population and follow-up duration. A study has found that in elderly and female patients with CHD who undergo percutaneous coronary intervention, the TyG-BMI index was positively associated with adverse cardiovascular outcomes [26]. However, there is no research on the correlation between the TyG-BMI index and prognosis in patients with CHD and hypertension. Our study did not find a significant correlation between the two. As for HOMA-IR, we found that it was a good predictor of all-cause mortality in patients with CHD and hypertension. In our study, when the HOMA-IR was less than 3.59, it was negatively associated with the risk of death; while when the HOMA-IR was greater than 3.59, it was positively associated with all-cause mortality. The HOMA-IR was U-shapedly associated with all-cause mortality in patients with CHD and hypertension. The results of model evaluation also indicated that the HOMA-IR had good predictive ability for all-cause mortality. Our results are similar to the conclusions of several previous studies.A 20-year follow-up study found that in non-diabetic Finnish men, those with the highest HOMA-IR had a 69% increased risk of CHD mortality [27]. Another study conducted in Chinese with CHD and diabetes found a positive correlation between the HOMA-IR and the risk score for acute coronary events [28]. Other studies have found that when the HOMA-IR is greater than 3.49, CHD patients have an increased risk of plaque progression in the coronary arteries, which is associated with adverse outcomes in coronary heart disease [29]. Above results indicate that the HOMA-IR is associated with the prognosis of CHD. However, this relationship has not been evaluated in patients with CHD and hypertension, and the nonlinear relationship has not been assessed either. Our study fills these gaps.
Hypertension and atherosclerosis processes interact with each other, which can exacerbate the adverse prognosis of CHD [30]. Therefore, CHD patients with comorbid hypertension should receive greater attention. IR is associated with endothelial dysfunction, abnormal lipid metabolism, excessive sympathetic activation, and systemic inflammatory response. These factors are closely related to the development and poor prognosis of CHD and hypertension [7]. Our study found that the HOMA-IR can serve as a predictor of all-cause mortality risk in patients with CHD and hypertension. High and low HOMA-IR indices were associated with an increased risk of all-cause mortality. This suggests that clinicians can use the HOMA-IR to assess the risk of all-cause mortality in patients with CHD and hypertension and take appropriate measures.
The aforementioned studies have indicated an association between IR and its surrogates with the occurrence and prognosis of CVD. However, further quantitative research is warranted in distinct CVD subpopulations to enhance the precision of utilizing IR surrogates in predicting prognosis among CVD patients by clinicians. CHD and hypertension are atherosclerosis-related conditions that frequently coexist. Currently, little studies exam the relationship between IR surrogates and long-term prognosis in individuals with CHD and hypertension. It remains uncertain whether findings from studies focused on short-term outcomes in this population can be extrapolated to predict long-term prognosis. To address the gap, this study was conducted. Our findings revealed a U-shaped association between HOMA-IR and long-term prognosis in patients with CHD and hypertension, exhibiting a segmented effect. This contrasts with the previously reported linear relationship between IR surrogates and short-term prognosis in this population. These results can aid clinicians in more accurately evaluating prognosis using IR surrogates among individuals with CHD and hypertension.
Our research employed cohort study design, an important method for real world study [31]. The cohort study design has perfect external validity, which means that the research conclusions can be more accurately generalized and applied to the real world [31, 32]. However, as an observational study, potential imbalances in the study population may introduce confounding bias [31]. To mitigate this, we collected a comprehensive array of covariates and utilized rigorous statistical techniques, including multivariable Cox regression analysis, to enhance the robustness of our findings. Subsequent researchers can continue to conduct relevant studies to validate their generalizability and applicability in clinical practice.
The strengths and limitations of this study include the following. First, this study fills some of the current research gaps. It evaluates the relationship between different IR surrogates and long-term all-cause mortality in patients with CHD and hypertension from a community-based perspective, and finds that the HOMA-IR can serve as a good prognostic evaluation index. Second, this study simultaneously assesses both linear and nonlinear relationships between variables, identifies segmented effects between variables, and calculates cut-off points. However, there are also some limitations. First, the diagnosis of CHD and hypertension in this study was obtained through self-reporting by participants. Although certain measures were taken during data collection to avoid systematic errors [33], there may still be information bias. Second, this study converts the HOMA-IR into a categorical variable for analysis, and also performs segmented stratified analysis. These operations may reduce the sample size in each group, leading to a decrease in test efficiency. Future studies could expand the sample size or focus on a specific subgroup for separate research.
Conclusion
This study found that HOMA-IR was a reliable predictor of all-cause mortality in patients with CHD and hypertension. The relationship between HOMA-IR and all-cause mortality was U-shaped in this population. Both high or low HOMA-IR were associated with an increase in all-cause mortality.
Acknowledgements
We would like to acknowledge the following financial support: the General Project of National Natural Science Foundation of China (No.82374421), the General Project of Natural Science Foundation of Beijing (No.23G40521) and Science and technology innovation project of China Academy of Chinese Medical Sciences (No.C12021A00921). We thank the NHANES database for sharing the data.
Abbreviations
- IR
Insulin resistance
- CHD
Coronary heart disease
- NHANES
National Health and Nutrition Examination Survey
- NDI
National Death Index
- NCHS
National Center for Health Statistics
- HOMA-IR
Homeostatic model assessment of IR
- TyG index
Triglyceride glucose index
- TyG-BMI index
Triglyceride glucose-body mass index
- ROC
Receiver operating characteristic
- AUC
Area under the curve
- PIR
Income -poverty ratio
- COPD
Chronic obstructive pulmonary disease
- LDL-C
Low density lipoprotein cholesterol
- HDL-C
High density lipoprotein cholesterol
- TC
Total cholesterol
- ALT
Alanine aminotransferase
- ALP
Alkaline phosphatase
- AST
Aspartate aminotransferase
- CK
Creatine kinase
- Cr
Creatinine
- GGT
Gamma-glutamyltransferase
- LDH
Lactate dehydrogenase
- HbA1c
Glycosylated hemoglobin
- Hb
Hemoglobin
- WBC
White blood cell count
Author contributions
Conceptualization, WSH and HXZ; Methodology, LYF; Software, LYS; Validation, WQ; Formal Analysis, LYF; Resources, WSH; Data Curation, YXJ; Writing – Original Draft Preparation, HXZ; Visualization, LQY, YYT, and LLL; Supervision, YCY, WMS, and CLL; Project Administration, WSH and HXZ; Funding Acquisition, WSH and WQ.
Funding
This study was supported by grants from the General Project of National Natural Science Foundation of China (No.82374421), the General Project of Natural Science Foundation of Beijing (No.23G40521) and Science and technology innovation project of China Academy of Chinese Medical Sciences (No.C12021A00921). This research received no external funding.
Availability of data and materials
Data can be found at https://www.cdc.gov/nchs/nhanes/.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have reviewed and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Tao S, Yu L, Li J, Huang L, Huang X, Zhang W, Xie Z, Tan Y, Yang D. Association between the triglyceride-glucose index and 1-year major adverse cardiovascular events in patients with coronary heart disease and hypertension. CARDIOVASC DIABETOL. 2023;22(1):305. doi: 10.1186/s12933-023-02018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2019 Risk Factors Collaborators Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Ding X, Hua B, Liu Q, Gao H, Chen H, Zhao XQ, Li W, Li H. High triglyceride-glucose index is associated with adverse cardiovascular outcomes in patients with acute myocardial infarction. Nutr Metab Cardiovas. 2020;30(12):2351–2362. doi: 10.1016/j.numecd.2020.07.041. [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 6.Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68. doi: 10.1186/s12933-022-01511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirjalili SR, Soltani S, Heidari MZ, Marques-Vidal P, Kraemer A, Sarebanhassanabadi M. An innovative model for predicting coronary heart disease using triglyceride-glucose index: a machine learning-based cohort study. Cardiovasc Diabetol. 2023;22(1):200. doi: 10.1186/s12933-023-01939-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang A, Tian X, Zuo Y, Chen S, Meng X, Wu S, Wang Y. Change in triglyceride-glucose index predicts the risk of cardiovascular disease in the general population: a prospective cohort study. Cardiovasc Diabetol. 2021;20(1):113. doi: 10.1186/s12933-021-01305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N, Xu Y, Xu C, Duan J, Zhou Y, Jin M, Xia H, Yuan W, Chen R. Effects of triglyceride glucose (TyG) and TyG-body mass index on sex-based differences in the early-onset heart failure of ST-elevation myocardial infarction. Nutr Metab Cardiovas. 2023 doi: 10.1016/j.numecd.2023.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Zhang R, Shi S, Chen W, Wang Y, Lin X, Zhao Y, Liao L, Guo Q, Zhang X, Li W, et al. Independent effects of the triglyceride-glucose index on all-cause mortality in critically ill patients with coronary heart disease: analysis of the MIMIC-III database. Cardiovasc Diabetol. 2023;22(1):10. doi: 10.1186/s12933-023-01737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Zhu B, Zhou W, Du Y, Qi D, Wang C, Cheng Q, Zhang Y, Wang S, Gao C. Triglyceride-glucose index as a marker of adverse cardiovascular prognosis in patients with coronary heart disease and hypertension. Cardiovasc Diabetol. 2023;22(1):133. doi: 10.1186/s12933-023-01866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong T, Dang K, Ladhani S, Singal AK, Wong RJ. Prevalence of Alcoholic Fatty Liver Disease Among Adults in the United States, 2001–2016. JAMA-J Am Med Assoc. 2019;321(17):1723–1725. doi: 10.1001/jama.2019.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou XZ, Liu EQ, Liu SQ, Lv H, Cui HF, Han J. The negative association between serum albumin levels and coronary heart disease risk in adults over 45 years old: a cross-sectional survey. Sci Rep-UK. 2023;13(1):672. doi: 10.1038/s41598-023-27974-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozmen MM, Guldogan CE, Gundogdu E. Changes in HOMA-IR index levels after bariatric surgery: comparison of single anastomosis duodenal switch-proximal approach (SADS-p) and one anastomosis gastric bypass-mini gastric bypass (OAGB-MGB) Int J Surg. 2020;78:36–41. doi: 10.1016/j.ijsu.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, Kim JH, Park JS. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–1573. doi: 10.2337/dc18-1920. [DOI] [PubMed] [Google Scholar]
- 16.Peng N, Kuang M, Peng Y, Yu H, Zhang S, Xie G, Sheng G, Zou Y. Associations between TyG-BMI and normal-high blood pressure values and hypertension: cross-sectional evidence from a non-diabetic population. Front Cardiovasc Med. 2023;10:1129112. doi: 10.3389/fcvm.2023.1129112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen X, Wang M, Xu X, Li T. Exposure to per- and polyfluoroalkyl substances and mortality in us adults: a population-based cohort study. Environ Health Persp. 2022;130(6):67007. doi: 10.1289/EHP10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diagnosis and Classification of Diabetes Standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S20–S42. doi: 10.2337/dc24-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q, Xiao S, Jiao X, Shen Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol. 2023;22(1):279. doi: 10.1186/s12933-023-02030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, Sowers JR. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766. doi: 10.1016/j.metabol.2021.154766. [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Li M, Zeng T, Hu R, Xu Y, Xu M, Zhao Z, Chen Y, Wang S, Lin H, et al. Association between insulin resistance and cardiovascular disease risk varies according to glucose tolerance status: a nationwide prospective cohort study. Diabetes Care. 2022;45(8):1863–1872. doi: 10.2337/dc22-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JH, Jeon S, Joung B, Lee HS, Kwon YJ. Associations of homeostatic model assessment for insulin resistance trajectories with cardiovascular disease incidence and mortality. Arterioscl Throm Vas. 2023;43(9):1719–1728. doi: 10.1161/ATVBAHA.123.319200. [DOI] [PubMed] [Google Scholar]
- 23.Tobin AM, Hackett CB, Rogers S, Collins P, Richards HL, O'Shea D, Kirby B. Body mass index, waist circumference and HOMA-IR correlate with the Psoriasis Area and Severity Index in patients with psoriasis receiving phototherapy. Brit J Dermatol. 2014;171(2):436–438. doi: 10.1111/bjd.12914. [DOI] [PubMed] [Google Scholar]
- 24.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocr Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 25.Huang X, He J, Wu G, Peng Z, Yang B, Ye L. TyG-BMI and hypertension in normoglycemia subjects in Japan: a cross-sectional study. Diabetes Vasc Dis Re. 2023;20(3):1497016305. doi: 10.1177/14791641231173617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y, Fang Z, Zhang X, Wen Y, Lu J, He S, Xu B. Association between triglyceride glucose-body mass index and cardiovascular outcomes in patients undergoing percutaneous coronary intervention: a retrospective study. Cardiovasc Diabetol. 2023;22(1):75. doi: 10.1186/s12933-023-01794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurl S, Zaccardi F, Onaemo VN, Jae SY, Kauhanen J, Ronkainen K, Laukkanen JA. Association between HOMA-IR, fasting insulin and fasting glucose with coronary heart disease mortality in nondiabetic men: a 20-year observational study. Acta Diabetol. 2015;52(1):183–186. doi: 10.1007/s00592-014-0615-x. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Li F, Guo J, Li C, Xu D, Wang B. Insulin resistance, blood glucose and inflammatory cytokine levels are risk factors for cardiovascular events in diabetic patients complicated with coronary heart disease. Exp Ther Med. 2018;15(2):1515–1519. doi: 10.3892/etm.2017.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.An X, Yu D, Zhang R, Zhu J, Du R, Shi Y, Xiong X. Insulin resistance predicts progression of de novo atherosclerotic plaques in patients with coronary heart disease: a one-year follow-up study. Cardiovasc Diabetol. 2012;11:71. doi: 10.1186/1475-2840-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen Y, Dai Y, Wang XQ, Zhang RY, Lu L, Ding FH, Shen WF. Searching for optimal blood pressure targets in type 2 diabetic patients with coronary artery disease. Cardiovasc Diabetol. 2019;18(1):160. doi: 10.1186/s12933-019-0959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gokhale M, Stürmer T, Buse JB. Real-world evidence: the devil is in the detail. Diabetologia. 2020;63(9):1694–1705. doi: 10.1007/s00125-020-05217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. 2010;126(6):2234–2242. doi: 10.1097/PRS.0b013e3181f44abc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinderknecht K, Harris C, Jones-Smith J. Association of the healthy, hunger-free kids act with dietary quality among children in the US national school lunch program. JAMA-J Am Med Assoc. 2020;324(4):359–368. doi: 10.1001/jama.2020.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be found at https://www.cdc.gov/nchs/nhanes/.