Abstract
Mitochondrial diseases, a diverse and intricate group of disorders, result from both nuclear DNA and mitochondrial DNA malfunctions, leading to a decrease in cellular energy (ATP) production. The increasing understanding of molecular, biochemical, and genetic irregularities associated with mitochondrial dysfunction has led to a wider recognition of varying mitochondrial disease phenotypes. This broadening landscape has led to a diverse array of neuroimaging findings, posing a challenge to radiologists in identifying the extensive range of possible patterns. This review meticulously describes the central imaging features of mitochondrial diseases in children, as revealed by neuroimaging. It spans from traditional imaging findings to more recent and intricate diagnoses, offering insights and highlighting advancements in neuroimaging technology that can potentially guide a more efficient and accurate diagnostic approach.
Keywords: Mitochondrial syndromes, Mitochondrial dysfunction, Mitochondrial neuroimaging
Background
Primary mitochondrial disorders encompass a diverse range of genetically inherited diseases that can arise from mutations in either the nuclear genome (nDNA) or the mitochondrial genome (mtDNA). These mutations disrupt the oxidative phosphorylation (OXPHOS) in the inner membrane of the mitochondria, which is a crucial process for the normal production of cellular energy. Additionally, these disorders can affect various metabolic pathways within cells, giving rise to dysregulation of multiple and complex metabolisms such as calcium and iron, as well as affecting the cascades of cellular apoptosis [1] and disturbing the normal cellular mediated inflammatory responses [2]. The multifaceted nature of these disorders underscores the complexity and wide-ranging impact they can have on cellular function and the overall normal function of multiple organs and systems which consequently results in a large variety of clinical phenotypes.
In the last decades with the advances in personalized medicine, the understanding of the molecular, biochemical, and genetic background as well as the clinical presentation of those affected patients has substantially expanded. Consequently, a thorough evaluation of clinical and family history, biochemical markers, histopathological analysis, and molecular diagnostic testing has been considered a prerequisite to an accurate diagnosis. Concurrently, a significant development has also occurred in how those conditions are assessed by imaging, where magnetic resonance imaging (MRI) has taken place as the gold-standard imaging technique for diagnostic access and for evaluation of disease progression. The imaging pattern recognition approach in these disorders is a continually evolving field and still represents a diagnostic challenge for all neuroradiologists. However, nowadays neuroimaging experts have paramount importance in the early diagnosis and delineation of the natural history of these disorders, offering a better evaluation of the most common syndromes, and their potential overlaps, as well as giving support in the recognition and research of novel genes underlying most of the mitochondrial conditions.
Along with neuroimaging pattern recognition, radiologists must learn to recognize the most common demographics, clinical symptoms, and abnormal laboratory results as the appropriate clinical context improves diagnostic specificity during initial suspicion for these disorders. Mitochondrial diseases most often arise in children although the onset can occur at any age. Differing age groups and clinical presentations are related to multiple factors including differences in genomes affected (nDNA mutations most frequently present with earlier onset when compared with mtDNA) [3,4], the type, size, and location of genetic mutation, as well as the degrees of heteroplasmy in the mitochondrial mutations. Several poorly understood and currently under investigation epigenetic and environmental factors additionally contribute to enhancing or reducing the final overall impairment of mitochondrial dysfunction [5]. The most common clinical symptoms include failure to thrive, hypotonia, muscle weakness, and lactic acidosis; these are symptoms particularly noted in the neonatal to early childhood period, however, many times difficult to recognize. More characteristic clinical phenotypes such as stroke-like episodes, particular forms of epilepsy, hearing and visual loss, systemic (gastrointestinal and endocrinological) symptoms along with developmental delay tend to occur or become more evident outside the neonatal period [6].
First neuroimaging steps for characterizing mitochondrial disorders
As a common statement, mitochondrial disorders are progressive diseases with multiple relapses separated by intermittent periods of activity and latency. These relapses are often identified during clinical analysis, along with laboratory work-up and neuroimaging evaluation. However, it is still challenging for researchers and expert clinicians to identify those biomarkers that accurately indicate the severity or expected timeline interval of disease progression.
Although lesions in the context of mitochondrial disorders are classically described to have a predilection for the cortex, deep gray matter, and brainstem, recent advances in novel genotype-phenotype correlations have expanded this conventional understanding of the disease presentation [7]. It is now evident that brain lesions can occur in any location of the brain or spinal cord, and the specific distribution of lesions is mostly determined by the affected gene or molecular pathway [8]; C. A. P. F. [9,10]. Changes in the central nervous system (CNS) are frequently symmetrical and bilateral, although it should be noted that an asymmetric pattern can also be observed, particularly in some classical syndromes like Mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) and Polymerase gamma related disorders (POLG-RD [10,11]).
The neuroimaging appearance of mitochondrial lesions on MRI often differs significantly between the activity and latency periods and may also vary based on their location in the brain. Active lesions, for example, in the striatum typically exhibit heterogeneous signals on T2 weighted imaging (WI) and Diffusion weighted imaging (DWI), including mixed areas of reduced and increased diffusivity (Fig. 1A and B), indicating varying degrees of cytotoxic, vasogenic, and intramyelinic edema. On the other hand, lesions in the globus palladium and along brainstem nuclei and small tracts tend to have a more uniform appearance, with reduced diffusivity and hyperintense signal on T2WI. The presence of hemorrhagic lesions is not expected within acute lesions, although concurrent treatment and underlying clinical conditions may increase the risk of hemorrhage. Chronic residual lesions often present with atrophy and hyperintensity on T2-FLAIR. Although uncommon, residual lesions with large fluid/necrotic components may exhibit signal saturation on T2-FLAIR, resembling a “T2-FLAIR mismatch-like” feature (Fig. 1C–and D).
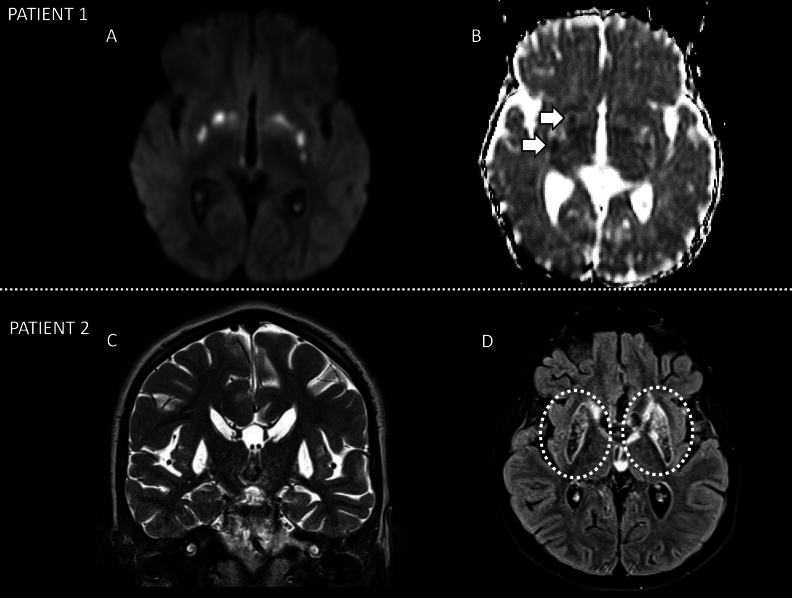
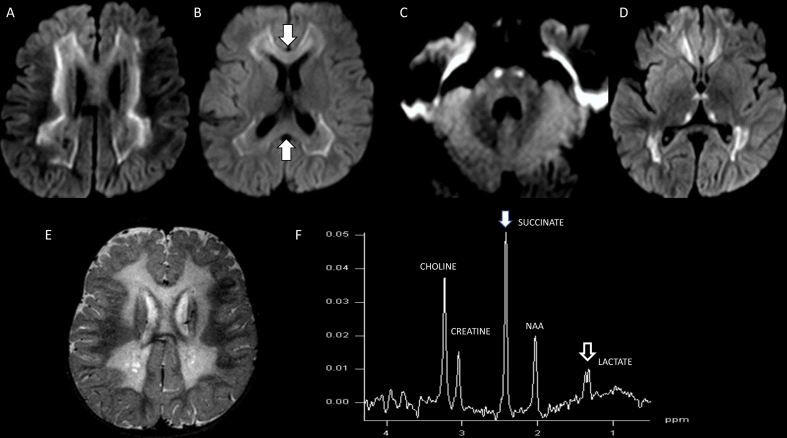
Fig. 1.
Two different patients with Leigh syndrome, Patient 1 (A and B) and Patient 2 (B and C) both with characteristic MR imaging findings of Leigh syndrome. Patient 1 shows active metabolic lesions with heterogeneous components of reduced diffusivity on DWI involving the striatum bilaterally (arrow, B). Patient 2 shows lesions with increased signal on T2WI (C) and signal suppression on T2 FLAIR (D), involving the striatum bilaterally suggestive of increased liquid/necrotic components due prior severe metabolic insult.
Advanced techniques
Although advanced techniques should not be considered as single features to rely on for the diagnosis of mitochondrial disorders, some techniques such as proton magnetic resonance spectroscopy (1H-MRS) or arterial spin labeling (ASL) may be extremely useful to support the mitochondrial diagnosis and assess disease severity, and may also offer important hints in some particular pathogenic variants and mitochondrial pathways [[12], [13], [14]].
-
•
Proton Magnetic Resonance Spectroscopy (1H-MRS)
1H-MR Spectroscopy represents a valuable tool in the diagnosis of diverse brain diseases, including neurometabolic disorders [15]. When considering the diagnosis of mitochondrial disorders, the importance of 1H MRS becomes even more evident due to the common occurrence of severe cellular anaerobiosis, which is reflected in increased lactate levels. Abnormal lactate levels can generally be easily detected on short (TE </= 35 msec) and intermediate (TE = 135–144 msec) time echos as a doublet peak on 1H-MRS studies resonating at 1.33 ppm, with peak inversion at intermediate echo times. The presence of elevated lactate levels indicates severe cellular anaerobiosis, which is the hallmark of mitochondrial disorders [16]. However, it is important to be mindful that anaerobiosis can also occur in other severe conditions affecting brain metabolisms, such as intractable seizures, vascular strokes, hypoxic-ischemic injuries, or even other metabolic disorders. On the other hand, the absence of lactate cannot exclude a mitochondrial disorder. To help distinguish mitochondrial disorders from other entities, the authors suggest 1H-MRS voxel placement not only within the lesions or the lateral ventricles but also in areas in “normal-appearing” parenchyma, as large lactate peaks in these regions are compelling evidence of an underlying mitochondrial condition [17]. In addition to lactate, 1H-MRS can also detect other characteristic peaks, such as pyruvate (2.37 ppm) or succinate at (2.4 ppm), which can provide further insights into specific metabolic defects such as those associated with pyruvate and succinate dehydrogenase [18]. 1H-MRS can also reveal ketoacid buildup including alanine (doublet peak at 1.5 ppm; a byproduct of lactate) and glycine (3.6 ppm; serine-glycine pathway), which may support cellular energy disruption. Further, elevated citrate (2.6 ppm), a Kreb's cycle intermediate, is produced when the glycolytic rate exceeds Kreb's cycle activity.
-
•
Arterial Spin Labeling (ASL)
ASL is another technique that can be helpful in the context of neuroimaging for mitochondrial disorders. ASL is a non-invasive method that allows for the measurement of cerebral blood flow. It involves the labeling of arterial blood water protons before they enter the brain, which can be detected using magnetic resonance imaging (MRI). Although ASL is not currently utilized to differentiate specific genes or pathways, it can aid in the interpretation of lesions and help determine the stage of the lesion (lesion activity). ASL often shows increased perfusion in active lesions, particularly in regions such as the basal ganglia and cortex [14,19]. As the lesion progresses, perfusion may decrease, and areas of lack of perfusion may be observed, indicating tissue necrosis or fluid replacement. In the future, the authors believe that ASL as well as different tools that may support the analysis of disease activity will have crucial importance in assessing treatment effects and evaluating the impact of potential drugs on lesion progression during clinical trials.
Imaging-based genotype-phenotype correlations
The differentiation of the neuroimaging findings of mitochondrial disorders can be approached in various and complementary ways. General statements for narrowing the diagnosis can be based on pattern-recognition of classic syndromes such as Leigh syndrome, MELAS, LHON (Leber hereditary optic neuropathy), POLG-RD, MGIE (mitochondrial gastrointestinal encephalopathy), “Kearns Sayre” syndrome, and others. This characterization is particularly helpful in bringing to clinicians’ information on what clinical symptoms to correlate, what may be the potential expectations of disease progression, and for checking the most common genes that may underlie those syndromes. However, classic syndromes may not be present in neuroimaging, may overlap each other, or maybe difficult to recognize. Moreover, some may also present with large neuroimaging variability along with multiple genes related to them such as Leigh syndrome (Cesar A. P. F. [7,20]. In an attempt to differentiate these disorders based on underlying genetic and molecular dysfunctions, the authors suggest:
Initially, one can attempt to differentiate mitochondrial dysfunction based on which of the two genomes may be affected (mitochondrial versus nuclear). This approach may help clinicians and geneticists to consider where pathogenic variant(s) are most likely to be present and where they should be promptly investigated. As a guideline, nuclear genes when affected, particularly nuclear genes directly related to actions in the complex (I–V) units, (often present in an earlier onset compared to those from the mitochondrial genome), have increased rates of supra- and infratentorial white matter involvement, often with a typical pattern characterized by multiple areas of cavitation in the deep white matter, surrounded by components of reduced diffusivity, that may be associated with areas of contrast enhancement indicating an acute inflammatory process on those regions. These white matter lesions frequently extend through the corpus callosum sparing the outlets, and also involve the corticospinal tracts (Fig. 2). Although there is no clear predilection for specific areas or lobes, these lesions tend to extend progressively over time from the deep to the superficial white matter and form new areas of cavitation and/or increasingly more extensive components of reduced diffusivity as they evolve. Involvement of other brain structures is also not uncommon. Overlap of common mitochondrial phenotypes, particularly Leigh syndrome has been currently accepted and can be present in over 20 % of the cases [7]. Extensive areas of encephalomalacia and diffuse brain volume loss are often observed in those few patients who survive after disease activity.
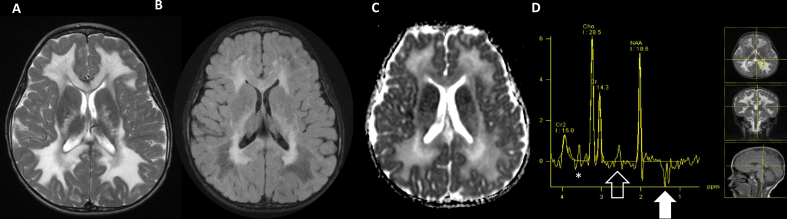
Fig. 2.
Male, 12 years TOMM40, nuclear encoded on chromosome 3. Brain MRI (A–D). Axial T2WI shows confluent white matter hyperintensity, with areas of signal saturation on T2 FLAIR (arrow, B) suggestive of white matter rarefaction/increased water content. Areas of reduced diffusivity are present in the corpus callosum and small segments of the white matter mixed with more extensive facilitated diffusion. MRS 135 ms TE shows lactate (1.33 ppm arrow), citrate (2.6 ppm open arrow) and glycine (3.6 ppm, asterisk), together, indicated TCA cycle disturbance.
Mitochondrial tRNA mutations and disease
Disorders related to mutations in the mitochondrial tRNA (MTT) genes correspond to a broad spectrum of pathologies that may include one specific organ such as myopathy or hearing impairment, as well as multisystem and more severe disorders often leading to encephalopathy, gastrointestinal dysmotility, and potentially fatal cardiomyopathy [21]. Nevertheless, the exact mechanism by which MTT mutations lead to disease is not fully understood, and our knowledge is further complicated by the intricate relationship between genotype and phenotype.
This complexity is evident in the occurrence of vastly differing phenotypes in patients carrying the same mutation, or the manifestation of clinically identical syndromes in genetically diverse patients. Additionally, the highly changeable nature of mitochondrial DNA (mtDNA) adds another layer of complexity, cautioning us against immediate assumptions of pathogenicity for new MTT substitutions. Despite these challenges, significant strides are being made. This article briefly explores the clinical phenotype associated with MTTL1 mutation at m.3243A > G which is strongly related to the MELAS syndrome phenotype and the major phenotype with neurological involvement among the MTT.
The classic form of MELAS represents a well-established mitochondrial syndrome with a generally recognizable clinical presentation and imaging findings.
Clinical symptoms including headaches with vomiting, seizures, hemiplegia, and cortical blindness along with laboratory results showing increased plasma or CSF lactate; mitochondrial abnormalities on muscle biopsy; MELAS-related pathogenic variant on genetic testing are the major established features of the final diagnosis [22].
Neuroimaging findings with corresponding imaging symptoms also play a critical role in the definition of MELAS, often revealing a characteristic pattern of disappearing and relapsing large cortical lesions in the brain with components of restricted diffusivity characteristically located in the occipital, posterior parietal, or temporal lobes at the onset, and not respecting vascular territories (Fig. 3). Atypical patterns of cortical lesion at the onset, including smaller size, absence of posterolateral distribution, and higher frequency of cerebellar involvement, although also described for patients with MELAS these are not expected to be observed in the context of MTTL1 mutations, but in different mitochondrial pathogenic variants [10], such as mtND3 (Fig. 4). It is worth mentioning, however, that the distribution and appearance of the lesions may become variable during the disease progression and the imaging distinction between atypical and classic forms of MELAS becomes challenging in more advanced stages. Additionally, the expected evolution of the lesions may be noted with different degrees of atrophy and/or cortical necrosis [23] as well as more diffuse volumetric changes including cerebellar atrophy along with calcifications in the basal ganglia. Similar to other forms of mitochondrial disorders, 1H-MRS can help demonstrate large abnormal peaks of lactate at 1.33 ppm, which is particularly evident in younger patients and during disease activity.
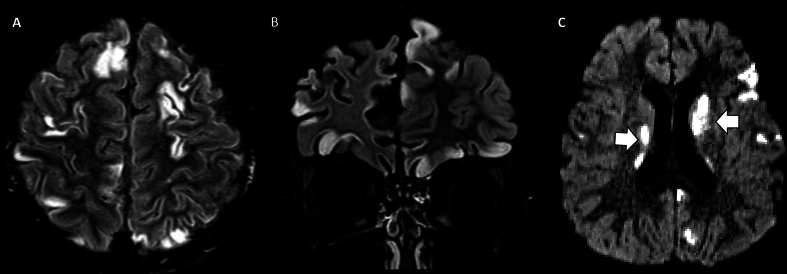
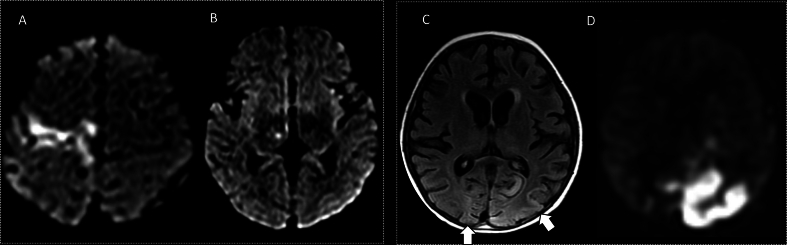
Fig. 3.
Male, 12 years old with classic MELAS. Brain MRI, A-D and 1H-MRS, E. Axial T2 FLAIR, DWI and ADC map shows abnormal hyperintensity (A) and restricted diffusion in the right occipital and temporal lobes (B and C), along with diffuse regional hyperperfusion on arterial spin labeling (ASL) images (dotted circle, D). 1H-1-MRS (echo time 144 msec) with voxel placed on the right occipital lobe (not shown) demonstrates an elevated lactate peak (solid arrow, E) consistent with acute exacerbation of MELAS.
Fig. 4.
Male, 5 years old with atypical MELAS features, ND3 pathogenic variant. Brain MRI study. Axial and coronal T2 FLAIR (A and B) and axial DWI (C) show multiple small cortical lesions with hyperintensity and restricted diffusion (ADC map not shown) mostly in the frontoparietal regions in association with involvement of the striatum bilaterally (arrows, C).
Primary Complex Deficiencies
In summary, complex deficiencies disrupt the normal functioning of one or more of the five complexes, including their subunits and assembly factors that are involved in electron transfer and ATP synthesis. In these “sequences” of generating energy, complex I is responsible for transferring electrons from NADH to ubiquinone while complex II transfers electrons from succinate to ubiquinone, complex III transfers electrons from ubiquinol to cytochrome c, and complex IV transfers electrons from cytochrome c to oxygen followed by a final ATP synthesis in the complex V which uses the proton gradient generated by the previous steps. These complexes work in perfect harmony to generate ATP, and disruptions in their structure or assembly factors can lead to variable degrees of impaired OXPHOS function as well as various clinical and neuroimaging manifestations. The authors provide a table (Table 1) that summarizes the most common genes associated with each complex, including the expected clinical presentation and neuroimaging findings during disease activity.
-
•
Complex I
Table 1.
Primary complex deficiency: Phenotype genotype correlation.
| Respiratory Chain | Genes with common neuroimaging | Neuroimaging Findings | Summary of often Demographics and Clinical Symptoms |
|---|---|---|---|
| Complex I mtDNA | ND1–ND6; ND4L | Leigh syndrome features (striatal and brainstem lesions) Stroke-like lesions and MELAS overlap (↑ND3) LHON homoplasmia (↑ND1 and ND4) Overlap with leigh syndrome |
Leigh syndrome: Childhood (typical onset < 3 years-old) Developmental delay, encephalopathy, seizures, hypotonia, dystonia, ataxia, ocular abnormalities, and hearing loss LHON: Adolescent and young adults Male>Female Unilateral vision loss FU: Contra- lateral ∗Harding syndrome |
| Complex I nDNA |
NDUFS1-4; NDUFS6-8; NDUFV1-2; NDUFA1-2; NDUFA9-11; NDUFB 3,9 NUBPL |
Necrotizing leukoencephalopathy cavitation. Brainstem involvement Diffuse cerebellum (↑NUBPL) |
Most often during first years of life (0–2 years old) Developmental delay Encephalopathy Seizures Hypotonia Ataxia Infants and childhood |
| Complex II | SDHA; SDHB; SDHC; SDHD; SDHAF1; SDHAF2 | Necrotizing leukoencephalopathy cavitation ↑Corticospinal tracts ↑Pyramids ↑Transverse pontine fibers ↑Middle cerebellar peduncles 1H-MRS: 2.4 ppm (succinate peak) |
Most often during first years of life Encephalopathy Developmental delay Hypotonia Some genes with associated predisposition tumors (i.e, paraganglioma and gastrointestinal stromal tumor) |
| Complex III | Mitochondrial DNA in the MTCYB gene Nuclear DNA- BCS1L, UQCRB and UQCRQ; LYRM7 |
Leigh syndrome (mt-DNAs) MTCYB - ↑MELAS (uncommon but potential phenotype) LYRM7 - ↑Necrotizing leukoencephalopathy cavitation |
Symptoms varies widely among affected individuals. Severe: Onset at birth of lactic acidosis, hypotonia, hypoglycemia, failure to thrive, encephalopathy. Multisystemic involvement Liver, kidney, and heart disease Mild: Onset at childhood (fatigue) |
| COX (complex IV) |
SURF1 APOPT1 |
Brainstem Basal ganglia (spared at onset - ↑SURF1). Thalamus and subthalamic nucleus. Dentate nucleus and inferior olivary nuclei Necrotizing leukoencephalopathy cavitation (↑ APOPT1) |
Onset: Early infancy to childhood Developmental delay, encephalopathy, hypertrichosis, brainstem signs, myopathy, short stature, hypotonia, and ataxia |
| Complex V | MT-ATP6 | Basal ganglia Cerebellar atrophy Atypical stroke-like lesions (↑ cerebellar involvement) |
Childhood, adolescence, and adults Ataxia, hypotonia and developmental delay |
| Additional assembly factors |
ISCA2- IBA57 Iron-sulfur clusters |
Necrotizing leukoencephalopathy cavitation ↑Corticospinal tracts ↑Transverse pontine fibers ↑Middle cerebellar peduncles ↑Cortical malformative features |
Onset: Birth and infants Lactic acidosis, hypotonia, hypoglycemia, failure to thrive, and encephalopathy. |
Note. All brain lesions may present with components of reduced diffusivity (white matter or gray matter) and 1H-MR spectroscopy with lactate peak in 1.3 ppm. ↑ = more commonly involved. ∗ Harding syndrome: LHON superimposed to neuroinflammatory process indicative of Multiple Sclerosis.
Complex I (NADH: ubiquinone oxidoreductase) is the largest and most complex component of the respiratory chain, consisting of 45 subunits. Among these subunits, 7 are `encoded by mtDNA (ND1–ND6 and ND4L), while 38 are encoded by nDNA. Additionally, there are 6 genes responsible for the formation of assembly proteins, which play a role in the assembly and stability of Complex I. Pathogenic variants have been identified in 19 core subunits, involving 16 nDNA genes (NDUFS1,2,3,4,6,7, and 8), (NDUFV1 and 2), (NDUFA 1,2,9,10,11), (NDUFB3,9), as well as all 7 mtDNA genes and assembly factors.
Complex I disorders can lead to various clinical and neuroimaging features. These include Leigh syndrome, atypical forms of MELAS, and LHON. While Leigh syndrome has been associated with different nuclear and mitochondrial pathogenic variants, atypical forms of MELAS and LHON are primarily linked to genes of mitochondrial subunits, mostly ND3 and ND5 for atypical forms of MELAS which may also overlap with Leigh features [10] and ND1, ND4 and ND6 for LHON [24].
In the case of Leigh syndrome, which is the most common manifestation due to disarrangement in the Complex I unit related to mitochondrial pathogenic variants, the expected neuroimaging presentation includes lesions involving selective areas of the brainstem such as oculomotor nuclei, inferior colliculi, and inferior vestibular nuclei. Additionally, periaqueductal gray matter, substantia nigra, subthalamic nuclei, bilateral striatal lesions, and areas involving the mediodorsal nuclei of the thalami are commonly observed as the main neuroimaging manifestation of Leigh syndrome.
Leukoencephalopathy, on the other hand, commonly presents with the presence of cavitation surrounded by areas of reduced diffusivity in the context of nuclear genes such as NDUFV1, NDUFV2, NDUFS1, NDUFS3, NDUFS4, NDUFS6, NDUFS7, NDUFS8, NDUFAF5, and the Complex I assembly factor NUBPL. Cases with NUBPL mutations may differ in presentation from other nuclear genes of Complex I due to the particularly high frequency of diffuse cerebellar involvement, also known as “bright cerebellum” [8].
-
•
Complex II
Complex II, also known as succinate dehydrogenase, is the smallest among all respiratory chain complexes. It is made up of subunits encoded by specific genes (SDHA, SDHB, SDHD, and SDHC), along with four assembly factor proteins (SDHAF1-4).
Complex II deficiency is typically characterized by a leukodystrophy pattern with extensive white matter involvement with a severe cavitating leukodystrophy pattern, at baseline mostly affecting the deep white matter evolving with diffuse extension including the corpus callosum. The corpus callosum changes are often characterized by extensive edema of the middle layer of the axonal tracts initially sparing the outlier fibers. White matter lesions also extend through different projections and association axonal tracts, often involving the corticospinal and the central tegmental tracts, along with the transverse pontine fibers (particularly frequent in complex II deficiency compared to other complex unit disorders), middle cerebellar peduncles, and cerebellar hemispheres. As discussed before in this article, 1H-MRS has a particular importance in the diagnosis of complex II deficiency since it may reveal an abnormally prominent singlet peak around 2.4 ppm, indicating the presence of increased succinate in the brain and CSF (Fig. 5). Classic syndromes are not commonly associated with complex II deficiency, with Leigh syndrome findings accounting for approximately 2–7% of cases [25].
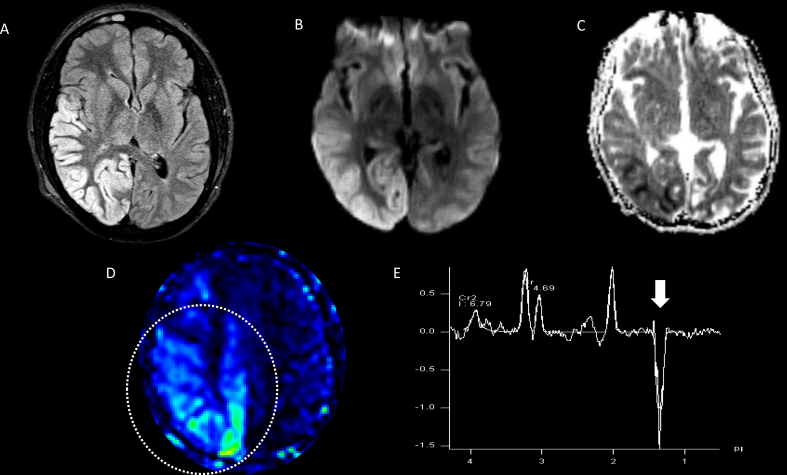
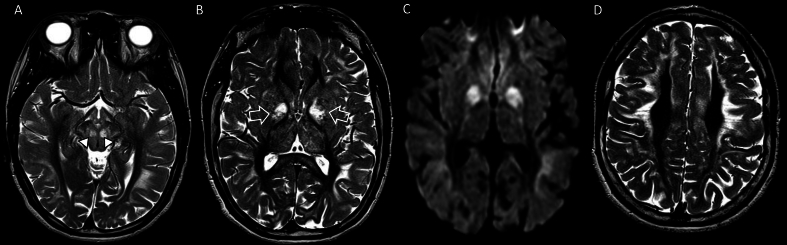
Fig. 5.
Male, 3 years old with succinate dehydrogenase-related leukoencephalopathy (complex II). Brain MRI. DWI. (A–D) demonstrate bilateral areas of restricted diffusion in the deep white matter with involvement of the corpus callosum (arrow, B) base of the pons, including the corticospinal tracts (C), medial and dorsal segments of the thalami (D). Focal areas of cavitation are noted on axial T2WI (arrow, E). F. 1H-MRS (echo time 144 msec) with voxel placed on the right nucleocapsular structures (not shown) demonstrate an elevated succinate peak at 2.4 ppm (solid arrow) and elevated lactate peak (open arrow).
It's worth mentioning that SDH tumorigenesis has been also implicated in some SDH subunits encoding genes including SDHB, SDHC, and SDHD. These genes have been strongly associated with predisposition tumors particularly paragangliomas and adrenal or extra-adrenal pheochromocytomas. This unexpected connection between mitochondrial dysfunction, tumor suppressor genes, and cancers, including neural crest-derived tumors, is currently an ongoing and strong research field [26].
-
•
Complex III
Complex III also known as Ubiquinol cytochrome C oxidoreductase deficiency is associated with mutations in both mitochondrial DNA and nuclear genes including pathogenic variants which include the mitochondrial gene CYB, as well as nuclear genes directly related to the complex subunit's proteins UQCRB, UQCRQ, UQCRC2, CYC1, and the assembly factors BCS1L, TTC19, LYRM7, UQCC2, and UQCC3.
Clinical manifestations may present in four major forms. These include:
-
1.
Fatal infantile encephalomyopathy, which is characterized by congenital lactic acidosis, hypotonia, dystrophic posturing, seizures, and coma.
-
2.
Encephalomyopathies of later onset, from childhood to adult life, with various combinations of symptoms such as weakness, short stature, ataxia, dementia, hearing loss, sensory neuropathy, pigmentary retinopathy, and pyramidal signs.
-
3.
Myopathy, which is characterized by exercise intolerance that evolves into a fixed weakness.
-
4.
Infantile histiocytoid cardiomyopathy.
Brain MRI may present with atypical forms of MELAS, a phenotype particularly noted in association with CYB mutations, although Leigh syndrome association or Leigh syndrome/MELAS overlap features can also present [27].
LYRM7 pathogenic variants have been often described in the context of extensive leukodystrophies with cavitations surrounded by areas of restricted diffusion, overall indistinctive of the other leukodystrophies related to complex deficiencies [28].
-
•
Complex IV
Complex IV, also known as Cytochrome c oxidase, is the second most common isolated deficiency in the respiratory chain complex, following complex I deficiency. The most common genes associated with the disease are the nuclear COX assembly factors SURF1, COX10, COX15, SCO2, and SCO1, although the mtDNA encoded subunits (MTCO1, MTCO2, and MTCO3) may be also causative of disease and patients may develop stroke-like episodes and ataxia [7].
Brain MRI features in complex IV deficiency are often reflected by lesions in specific regions of the brainstem, such as the substantia nigra, subthalamic nuclei, and cerebellar dentate nuclei.
Among those associated genes, there is a well-known correlation between SURF1 mutations and Leigh syndrome phenotype, often presenting with distinctive features that need to be recognized by neuroradiologists. This pattern is characterized by the involvement of the inferior olivary nuclei and subthalamic nuclei while sparing the striatum [29]. Although striatal sparing is considered a characteristic feature of SURF1-related Leigh syndrome (Fig. 6), it is important to note that the absence of striatal lesions is less frequently observed in follow-up studies compared to baseline MRIs. This suggests that striatal lesions may develop later in the natural progression of the disease.
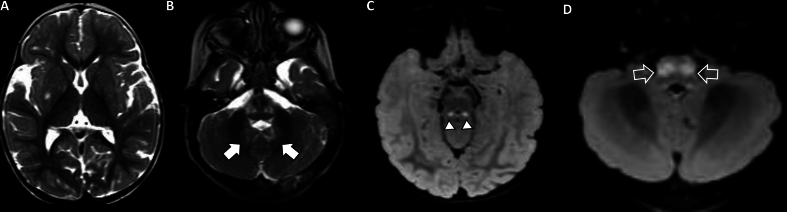
Fig. 6.
Female, 4 years old with Leigh syndrome and SURF1 pathogenic variant, Brain MRI (A–D). Axials T2WI (A and B) show hyperintense lesions involving the brainstem and dentate nuclei (arrow, B), with relative sparing of the striatum bilaterally, except for a diminutive hyperintense focus on the right putamen (A). Axials DWI show lesions with increased signal involving the inferior colliculi (arrowheads, C) and inferior olivary nuclei (open arrows, D).
Additional patterns including cavitation leukodystrophy can be noted although less often and have been described for SURF1 and APOPT1 genes [30].
-
•
Complex V
Complex V, the complex responsible for the synthesis ATP, is composed of 14 subunits encoded by nDNA and 2 mtDNA genes, where the majority of established genetic variants causative of disease are related to mtDNA.
Mutations in the ATP6 are responsible for the majority of the complex V dysfunctions resulting in a heterogeneous and broad variety of clinical symptoms particularly associated with neurological impairment in the context of Leigh syndrome although oligosymptomatic patients with ataxia and cerebellar atrophy and/or neuropathy or asymptomatic individuals are also described [31]. The neurodegenerative disorder characterized by psychomotor regression, epilepsy, and variable liver involvement (NARP) is less frequently noted and most often observed during childhood. MRI findings of lesions in the striatum with components of restricted diffusion (Leigh syndrome pattern) may be associated with multifocal cortical lesions extending through the cerebral and cerebellar cortex in the context of stroke-like episodes. There is questionable reliability of the correlation between rates of heteroplasmy and clinical presentation and severity in these patients.
In addition to the complex assembly factors described, IBA57 and ISCA2 (two iron/sulfur cluster assemblies), should be also mentioned here since there is strong evidence between pathogenic variants in these genes and abnormal brain MRI findings, including most frequently extensive cavitating leukodystrophy, involving also the brainstem white matter tracts, middle cerebellar peduncles, and cervical segment of the spinal cord [32] as well as cortical malformations [33].
Single large-scale mitochondrial deletions
Single large-scale deletions in the mtDNA (SLSMD) are rare and devastating disorders encompassing three major syndromes: Kearns-Sayre (KSS), Pearson, and Congenital Progressive Ophthalmoplegia (CPEO). These syndromes are defined by a range of systemic clinical features that vary in severity and age of onset among patients [34,35]. The most common symptoms include pigmentary retinopathy, progressive external ophthalmoplegia (CPEO), cardiac conduction abnormalities, cerebellar ataxia, sensorineural hearing loss, dementia, short stature, hypotonia, dysphagia, and endocrine and hematological problems [34,35]. Some intrinsic variables of the disease including the location and size of the deletion in the mitochondrial genome, and the levels of heteroplasmy, are considered potential markers to help define severity and disease progression [36].
Classic neuroimaging findings include the involvement of brainstem nuclei and tegmental tracts, basal ganglia lesions with discrete involvement of the globus pallidus with or without striatal association, and supratentorial white matter changes displaying selective involvement of the subcortical regions and commissural tracts, sparing the periventricular and deep white matter (Fig. 7). Neuroimaging features of patients with isolated Pearson syndrome are still poorly described, mostly descriptions including normal brain MRIs or diffuse leukodystrophy without significant involvement of the brainstem.
Fig. 7.
Male, 16 years old with KSS. Brain MRI study. A, B and D - Axials T2WI show hyperintensity bilaterally in the red nuclei (arrowheads, A), substantia nigra, thalami and globus pallidi (open arrow, B) and hyperintensity of the subcortical white matter and sparing the deep and periventricular regions (D). C - Axial DWI shows increased signal in the globus pallidi.
Mitochondrial DNA maintenance and replication
Mitochondrial DNA (mtDNA) maintenance and replication depend on specific proteins all encoded by nuclear genes. When maintenance is compromised, it leads to mtDNA depletion and multiple deletions, ultimately resulting in impaired protein synthesis and organ dysfunction. Key genes like POLG, TWNK, and TFAM contribute significantly to maintenance, while others such as TK2 and DGUOK play major roles in maintaining a balanced nucleotide pool. Genes including OPA1 and FBXL4 are directly implicated in mitochondrial fusion.
Among all the genes associated with mtDNA maintenance and replication, POLG stands out for its significant role in associated neurological diseases. POLG is the only DNA polymerase active during mtDNA transcription. Mutations in this nuclear gene can be either autosomal dominant or autosomal recessive, both leading to a continuum of overlapping clinical phenotypes [37,38] known as POLG-related disorders (POLG-RDs). Six major clinical disorders have been previously identified in the context of POLG mutations, including Alpers–Huttenlocher syndrome, one of the most common and severe phenotypes, childhood myocerebrohepatopathy spectrum, myoclonic epilepsy myopathy sensory ataxia, ataxia neuropathy spectrum, autosomal recessive progressive external ophthalmoplegia, and autosomal dominant progressive external ophthalmoplegia.
POLG-RD are typically multisystemic and may present over an extended period with symptoms accumulating over years. The onset of symptoms can occur at any age, from infancy to late adulthood, often with some consistency within families. Diagnosis is confirmed by the identification of two (biallelic) pathogenic variants in trans (proven compound heterozygote) unless the disorder is autosomal dominant, in which case only one pathogenic variant is required.
The distinguishing clinical features of this disorder include intractable seizures, developmental regression, and liver dysfunction. Liver involvement, though typical, varies in onset and may precede seizure onset in some patients or only manifest at the terminal stages of the disease in others. Seizures are present in over 50 % of cases and can rapidly progress to medical intractability, with frequent episodes of epilepsia partialis continua (EPC) or status epilepticus leading to severe encephalopathy. Typically, pre-existing developmental delay with episodic regression is often preceded by intercurrent infection. Febrile seizures are also common, with EEGs frequently showing occipital lobe predominance. Other clinical features include ataxia, sensory neuropathy, gastrointestinal (GI) dysmotility, impaired swallowing, and liver dysfunction. Cardiomyopathy and diabetes are not common findings in POLG-RDs.
Early during POLG-RD, an MRI of the brain may be normal. However, active lesions involving the perirolandic and/or occipital regions of the brain, in an uni- or bilateral fashion, tend to be the initial neuroimaging features (Fig. 8) [11]. Subsequently, as the disease progresses, the cerebellum, basal ganglia, thalamus, and brainstem become involved. Neuroimaging may also reveal progressive cerebral volume loss (central > cortical) with ventriculomegaly. The cerebellum may be atrophic, or it may progressively become atrophic over the course of the disease. These neuroimaging characteristics often overlap with those of other mitochondrial disorders that involve cortical gray matter, particularly MELAS. 1H-MRS typically shows reduced NAA, normal creatine, and an elevated peak of lactate, findings associated with anaerobiosis.
Fig. 8.
Two different patients with confirmed POLG-RD. Patient 1: male, 2 years old, brain MRI axials DWI (A and B). Patient 2: male patient 4 years old, axial T2 FLAIR (C) and axial ASL (D). Selective hyperintense signal of the right perirolandic region and right thalamus (A and B). Abnormal hyperintensity of the cortex in the occipital regions, more evident on the left (arrow) with diffuse increased hyperperfusion (D).
Features of chronic hepatic encephalopathy, such as increased signal intensity on T1WI sequences in the globus pallidi and subthalamic regions, or acute hepatic encephalopathy, may be associated. Patients with POLG-RDs must exercise caution with certain medications, particularly sodium valproate, a drug used for epilepsy control. This medication must be avoided due to its hepatotoxicity, which can result in a rapid decline in liver function in patients predisposed to liver failure, such as those with Alpers-Huttenlocher syndrome.
Additional POLG-RD clinical phenotypes may include progressive external ophthalmoplegia (PEO) where autosomal recessive PEO may be followed by other manifestations over years or decades and include depression, premature ovarian failure, parkinsonism, ataxia, and an MNGIE-like with severe gastrointestinal dysmotility and autosomal dominant PEO presenting with variable multisystemic symptoms including generalized myopathy, sensorineural hearing loss, axonal neuropathy, ataxia, depression, parkinsonism, hypogonadism, cataracts, psychosis, and dementia.
Mitochondrial tRNA synthetase disorders
This article does not cover a detailed review of mt-tRNA synthetase disorders, which goes beyond the scope and deserves a separate diagnostic approach. As a brief overview, mt-tRNA synthetase defects most often lead to different degrees of leukoencephalopathy. The most common and first described mutation in this context was DARS2. Pathogenic variants of DARS2 are related to abnormal synthesis of the mitochondrial enzyme aspartyl-tRNA synthetase. These mutations are more often presented in late childhood/young adults with leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation (LBSL). Clinically, there is a variable progression of neurological impairment in association with cerebellar ataxia and spasticity. Selective brain lesions distributed along the dorsal column and lateral corticospinal tracts of the spinal cord, pyramids and decussation of the medial lemniscus, and along with involvement of the cerebellar white matter, corpus callosum, posterior limb of the internal capsules, trigeminal nerve tracts, medial lemniscus, and middle cerebellar peduncles makes this phenotype particularly important to be recognized by radiologists [39].
Pyruvate Dehydrogenase Complex Deficiency
The Pyruvate dehydrogenase complex (PDHc), a vital multienzyme complex within the mitochondrial matrix, plays a crucial role in connecting glycolysis and the tricarboxylic cycle. It facilitates the oxidative decarboxylation of pyruvate to acetyl-CoA, making it a central player in carbohydrate metabolism and energy production. PDHc consists of three distinct enzymes, with a majority of Pyruvate dehydrogenase (PDH) deficiencies, a prevalent cause of congenital lactic acidosis, being associated with defects in the E1α subunit gene (PDHA1) located on Xp21.3.
Primary PDH deficiency, or PDCD, commonly manifests as a neurologic syndrome and is typically diagnosed before two years of life [40]; [41]. It may present as early as the prenatal stage, with routine prenatal ultrasound revealing intrauterine growth restriction, microcephaly, structural brain anomalies, and lactic acidosis, or in older children exhibiting features of Leigh syndrome [42]. Along with other potential chronic neurologic symptoms. The classical biochemical indicators of PDH deficiency include elevated blood lactate and pyruvate levels alongside a normal or mildly high lactate-to-pyruvate ratio [43].
Neuroimaging features of PDH deficiency are divided into two most common phenotypes with the early onset of the disease [40] (fetal period) including asymmetrical supratentorial ventricular dilatation with cerebral atrophy, partial or complete agenesis of the corpus callosum, agenesis of the pyramids, irregular and ectopic inferior olivary nuclei, periventricular cystic lesions (Fig. 9), and patients with childhood-onset are characterized by Leigh syndrome features including basal ganglia with increased rates of globus pallidus involvement, brainstem, and cerebellum [7]. 1H-MRS may be increased lactate at 1.33 ppm, supporting the laboratory lactic acidosis classically observed, pyruvate elevation at 2.37 ppm, and alanine at 1.5 ppm.
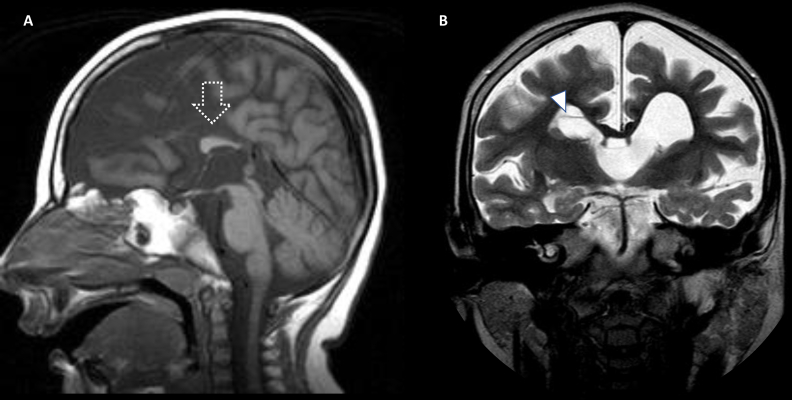
Fig. 9.
Male, 4 years old with PDH. Brain MRI study. A, B, Sagittal T1WI and Coronal T2WI show severe dysgenesis of the corpus callosum (open arrow, A) and asymmetric enlargement of the lateral ventricles with internal septations (arrowhead, B).
In conclusion, mitochondrial disorders present a complex and diverse set of diseases, making their diagnosis challenging due to the multitude of possible diagnoses and overlapping clinical and neuroimaging findings. However, neuroimaging plays a crucial role in unraveling characteristic patterns associated with these disorders. Not only can neuroimaging aid in identifying broad syndromic phenotypes, but it can also serve as a valuable tool for pinpointing specific genetic nuclear or mitochondrial DNA pathogenic variants. This article provides an in-depth review of the neuroimaging aspects of mitochondrial disorders, highlighting their correlation with key clinical data, as well as the most frequent mutations observed. By shedding light on the advancements in neuroimaging techniques and their integration with clinical and genetic information, this review aims to contribute to a better understanding and improved diagnostic approach for mitochondrial disorders.
Author contribution
Cesar Alves and Matthew Whitehead conceived of the presented reviewed manuscript. Cesar Alves wrote the manuscript and Matthew Whitehead reviewed the entire material.
Funding source
No funding was secured for this study.
Declaration of competing interest
The authors have no conflicts of interest to disclose.
All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Contributor Information
César Augusto P.F. Alves, Email: cesar.alves@childrens.harvard.edu.
Matthew T. Whitehead, Email: whiteheadm@chop.edu.
References
- 1.Kwong Jennifer Q., Henning Matthew S., Starkov Anatoly A., Manfredi Giovanni. The mitochondrial respiratory chain is a modulator of apoptosis. J Cell Biol. 2007;179(6):1163–1177. doi: 10.1083/jcb.200704059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Missiroli Sonia, Genovese Ilaria, Perrone Mariasole, Vezzani Bianca, Veronica A., Vitto M., et al. The role of mitochondria in inflammation: from cancer to neurodegenerative disorders. J Clin Med Res. 2020;9(3) doi: 10.3390/jcm9030740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinnery Patrick F. In: GeneReviews®. Adam Margaret P., Feldman Jerry, Mirzaa Ghayda M., Pagon Roberta A., Wallace Stephanie E., Bean Lora J.H., Gripp Karen W., Amemiya Anne., editors. University of Washington; Seattle (WA): 2000. Primary mitochondrial disorders overview. Seattle. [Google Scholar]
- 4.Leonard J.V., Schapira A.H. Mitochondrial respiratory chain disorders I: mitochondrial DNA defects. Lancet. 2000;355(9200):299–304. doi: 10.1016/s0140-6736(99)05225-3. [DOI] [PubMed] [Google Scholar]
- 5.Sharma Nidhi, Pasala Monica S., Prakash Aishwarya. Mitochondrial DNA: epigenetics and environment. Environ Mol Mutagen. 2019;60(8):668–682. doi: 10.1002/em.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eom Soyong, Lee Young-Mock. Long-term developmental trends of pediatric mitochondrial diseases: the five stages of developmental decline. Front Neurol. 2017;8(May):208. doi: 10.3389/fneur.2017.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alves Cesar A.P. F., Alves Cesar A.P., Teixeira Sara R., Martin-Saavedra Juan S., Guimarães Gonçalves Fabrício, Russo Francesco Lo, et al. Pediatric Leigh syndrome: neuroimaging features and genetic correlations. Ann Neurol. 2020 doi: 10.1002/ana.25789. [DOI] [PubMed] [Google Scholar]
- 8.Roosendaal S.D., van de Brug T., Alves C.A.P.F., Blaser S., Vanderver A., Wolf N.I., et al. Imaging patterns characterizing mitochondrial leukodystrophies. AJNR. American Journal of Neuroradiology. 2021;42(7):1334–1340. doi: 10.3174/ajnr.A7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alves C.A.P.F., Goldstein A., Teixeira S.R., Martin-Saavedra J.S., de Barcelos I.P., Fadda G., et al. Involvement of the spinal cord in primary mitochondrial disorders: a neuroimaging mimicker of inflammation and ischemia in children. AJNR. American Journal of Neuroradiology. 2021;42(2):389–396. doi: 10.3174/ajnr.A6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alves C.A.P.F., Zandifar A., Peterson J.T., Tara S.Z., Ganetzky R., Viaene A.N., et al. MELAS: phenotype classification into classic-versus-atypical presentations. AJNR. American Journal of Neuroradiology. 2023;44(5):602–610. doi: 10.3174/ajnr.A7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonçalves F.G., Hill B., Guo Y., Muraresku C.C., McCormick E., Alves C.A.P.F., et al. The perirolandic sign: a unique imaging finding observed in association with polymerase γ-related disorders. AJNR. American Journal of Neuroradiology. 2020;41(5):917–922. doi: 10.3174/ajnr.A6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gramegna Laura L., Evangelisti Stefania, Di Vito Lidia, La Morgia Chiara, Maresca Alessandra, Caporali Leonardo, et al. Brain MRS correlates with mitochondrial dysfunction biomarkers in MELAS-associated mtDNA mutations. Annals of Clinical and Translational Neurology. 2021;8(6):1200–1211. doi: 10.1002/acn3.51329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loiselet Klervie, Ruzzenente Benedetta, Roux Charles-Joris, Barcia Giulia, Pennisi Alessandra, Desguerre Isabelle, et al. Metabolics Group Cerebral blood flow and acute episodes of Leigh syndrome in neurometabolic disorders. Dev Med Child Neurol. 2021;63(6):705–711. doi: 10.1111/dmcn.14814. [DOI] [PubMed] [Google Scholar]
- 14.Whitehead Matthew T., Lee Bonmyong, Gropman Andrea. Lesional perfusion abnormalities in Leigh disease demonstrated by arterial spin labeling correlate with disease activity. Pediatr Radiol. 2016;46(9):1309–1316. doi: 10.1007/s00247-016-3616-9. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead Matthew T., Lai Lillian M., Blüml Stefan. Clinical H MRS in childhood neurometabolic diseases-Part 1: technique and age-related normal spectra. Neuroradiology. 2022;64(6):1101–1110. doi: 10.1007/s00234-022-02917-w. [DOI] [PubMed] [Google Scholar]
- 16.Bianchi M. Cristina, Tosetti Michela, Battini Roberta, Manca Maria L., Mancuso Michelangelo, Cioni Giovanni, et al. Proton MR spectroscopy of mitochondrial diseases: analysis of brain metabolic abnormalities and their possible diagnostic relevance. AJNR. American Journal of Neuroradiology. 2003;24(10):1958–1966. [PMC free article] [PubMed] [Google Scholar]
- 17.ElBeheiry Ahmed A., Abougabal Ahmed M., Omar Tarek I., Etaby Ashraf N. Role of brain magnetic resonance spectroscopy in the evaluation of suspected mitochondrial diseases in children: experience in 30 pediatric cases. The Egyptian Journal of Radiology and Nuclear Medicine. 2014;45(2):523–533. [Google Scholar]
- 18.Helman Guy, Caldovic Ljubica, Whitehead Matthew T., Simons Cas, Brockmann Knut, Simon Edvardson, et al. Magnetic resonance imaging spectrum of succinate dehydrogenase-related infantile leukoencephalopathy. Ann Neurol. 2016;79(3):379–386. doi: 10.1002/ana.24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Weiqin, Zhang Yuting, He Ling. MRI features of stroke-like episodes in mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes. Front Neurol. 2022;13(February) doi: 10.3389/fneur.2022.843386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCormick Elizabeth M., Kierstin Keller Julie P. Taylor, Alison J. Coffey, Shen Lishuang, Krotoski Danuta, Harding Brian, et al. Expert panel curation of 113 primary mitochondrial disease genes for the Leigh syndrome spectrum. Ann Neurol. 2023;94(4):696–712. doi: 10.1002/ana.26716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuppen Helen A.L., Naess Karin, Kennaway Nancy G., Al-Dosary Mazhor, Lesko Nicole, Yarham John W., et al. Mutations in the mitochondrial tRNA ser(AGY) gene are associated with deafness, retinal degeneration, myopathy and epilepsy. Eur J Hum Genet: EJHG (Eur J Hum Genet) 2012;20(8):897–904. doi: 10.1038/ejhg.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khasminsky Vadim, Auriel Eitan, Luckman Judith, Eliahou Ruth, Inbar Edna, Pardo Keshet, et al. Clinicoradiologic criteria for the diagnosis of stroke-like episodes in MELAS. Neurology. Genetics. 2023;9(4) doi: 10.1212/NXG.0000000000200082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitehead Matthew T., Wien Michael, Lee Bonmyong, Bass Nancy, Gropman Andrea. Black toenail sign in MELAS syndrome. Pediatr Neurol. 2017;75(October):61–65. doi: 10.1016/j.pediatrneurol.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Manickam Agaath Hedina, Michael Minu Jenifer, Ramasamy Sivasamy. Mitochondrial genetics and therapeutic overview of leber's hereditary optic neuropathy. Indian J Ophthalmol. 2017;65(11):1087–1092. doi: 10.4103/ijo.IJO_358_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fullerton Millie, McFarland Robert, Taylor Robert W., Alston Charlotte L. The genetic basis of isolated mitochondrial complex II deficiency. Mol Genet Metabol. 2020;131(1-2):53–65. doi: 10.1016/j.ymgme.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasini B., Stratakis C.A. SDH mutations in tumorigenesis and inherited endocrine tumours: lesson from the phaeochromocytoma-paraganglioma syndromes. J Intern Med. 2009;266(1):19–42. doi: 10.1111/j.1365-2796.2009.02111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei Yanping, Huang Yan, Yang Yingmai, Qian Min. MELAS/LS overlap syndrome associated with mitochondrial DNA mutations: clinical, genetic, and radiological studies. Front Neurol. 2021;12(May) doi: 10.3389/fneur.2021.648740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dallabona Cristina, Abbink Truus E.M., Carrozzo Rosalba, Torraco Alessandra, Legati Andrea, Carola G., et al. LYRM7 mutations cause a multifocal cavitating leukoencephalopathy with distinct MRI appearance. Brain: J Neurol. 2016;139(Pt 3):782–794. doi: 10.1093/brain/awv392. [DOI] [PubMed] [Google Scholar]
- 29.Rossi Andrea, Biancheri Roberta, Bruno Claudio, Di Rocco Maja, Angela Calvi, Pessagno Alice, et al. Leigh syndrome with COX deficiency and SURF1 gene mutations: MR imaging findings. AJNR. American Journal of Neuroradiology. 2003;24(6):1188–1191. [PMC free article] [PubMed] [Google Scholar]
- 30.Melchionda Laura, Haack Tobias B., Hardy Steven, Truus E., Abbink M., Fernandez-Vizarra Erika, et al. Mutations in APOPT1, encoding a mitochondrial protein, cause cavitating leukoencephalopathy with cytochrome c oxidase deficiency. Am J Hum Genet. 2014;95(3):315–325. doi: 10.1016/j.ajhg.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stendel Claudia, Neuhofer Christiane, Floride Elisa, Shi Yuqing, Ganetzky Rebecca D., Park Joohyun, et al. Delineating MT-ATP6-associated disease: from isolated neuropathy to early onset neurodegeneration. Neurology. Genetics. 2020;6(1):e393. doi: 10.1212/NXG.0000000000000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishiyama Akihiko, Sakai Chika, Matsushima Yuichi, Noguchi Satoru, Mitsuhashi Satomi, Endo Yukari, et al. IBA57 mutations abrogate iron-sulfur cluster assembly leading to cavitating leukoencephalopathy. Neurology. Genetics. 2017;3(5):e184. doi: 10.1212/NXG.0000000000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wongkittichote Parith, Pantano Cassandra, Bogush Emily, Alves Cesar Augusto P., Hong Xinying, He Miao, et al. Molecular Genetics and Metabolism; October: 2023. Clinical, radiological, biochemical and molecular characterization of a new case with multiple mitochondrial dysfunction syndrome due to IBA57: lysine and tryptophan metabolites as potential biomarkers. [DOI] [PubMed] [Google Scholar]
- 34.Björkman Kristoffer, Vissing John, Østergaard Elsebet, Bindoff Laurence A., Irenaeus F.M., de Coo, et al. Phenotypic spectrum and clinical course of single large-scale mitochondrial DNA deletion disease in the paediatric population: a multicentre study. J Med Genet. 2023;60(1):65–73. doi: 10.1136/jmedgenet-2021-108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds Elizabeth, Byrne Matthew, Ganetzky Rebecca, Parikh Sumit. Pediatric single large-scale mtDNA deletion syndromes: the power of patient reported outcomes. Mol Genet Metabol. 2021;134(4):301–308. doi: 10.1016/j.ymgme.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Grady John P., Campbell Georgia, Ratnaike Thiloka, Blakely Emma L., Gavin Falkous, Nesbitt Victoria, et al. Disease progression in patients with single, large-scale mitochondrial DNA deletions. Brain: J Neurol. 2014;137(Pt 2):323–334. doi: 10.1093/brain/awt321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hikmat Omar, Naess Karin, Engvall Martin, Klingenberg Claus, Rasmussen Magnhild, Tallaksen Chantal Me, et al. Simplifying the clinical classification of polymerase gamma (POLG) disease based on age of onset; studies using a cohort of 155 cases. J Inherit Metab Dis. 2020;43(4):726–736. doi: 10.1002/jimd.12211. [DOI] [PubMed] [Google Scholar]
- 38.Rahman Shamima, Copeland William C. POLG-related disorders and their neurological manifestations. Nat Rev Neurol. 2019;15(1):40–52. doi: 10.1038/s41582-018-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berge Laura van, Hamilton Eline M., Linnankivi Tarja, Uziel Graziella, Steenweg Marjan E., Isohanni Pirjo, et al. Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation: clinical and genetic characterization and target for therapy. Brain: J Neurol. 2014;137(Pt 4):1019–1029. doi: 10.1093/brain/awu026. [DOI] [PubMed] [Google Scholar]
- 40.Sofou Kalliopi, Dahlin Maria, Hallböök Tove, Lindefeldt Marie, Viggedal Gerd, Darin Niklas. Ketogenic diet in pyruvate dehydrogenase complex deficiency: short- and long-term outcomes. J Inherit Metab Dis. 2017;40(2):237–245. doi: 10.1007/s10545-016-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeBrosse Suzanne D., Okajima Kazuki, Zhang Shulin, Nakouzi Ghunwa, Schmotzer Christine L., Lusk-Kopp Marilyn, et al. Spectrum of neurological and survival outcomes in pyruvate dehydrogenase complex (PDC) deficiency: lack of correlation with genotype. Mol Genet Metabol. 2012;107(3):394–402. doi: 10.1016/j.ymgme.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Schubert Baldo Manuela, Vilarinho Laura. Molecular basis of Leigh syndrome: a current look. Orphanet J Rare Dis. 2020;15(1):31. doi: 10.1186/s13023-020-1297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel Kavi P., O'Brien Thomas W., Subramony Sankarasubramon H., Shuster Jonathan, Stacpoole Peter W. The spectrum of pyruvate dehydrogenase complex deficiency: clinical, biochemical and genetic features in 371 patients. Mol Genet Metabol. 2012;106(3):385–394. doi: 10.1016/j.ymgme.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]