Abstract
This paper provides an overview of the different types of mitochondrial myopathies (MM), associated phenotypes, genotypes as well as a practical clinical approach towards disease diagnosis, surveillance, and management. nDNA-related MM are more common in pediatric-onset disease whilst mtDNA-related MMs are more frequent in adults. Genotype-phenotype correlation in MM is challenging due to clinical and genetic heterogeneity. The multisystemic nature of many MMs adds to the diagnostic challenge. Diagnostic approaches utilizing genetic sequencing with next generation sequencing approaches such as gene panel, exome and genome sequencing are available. This aids molecular diagnosis, heteroplasmy detection in MM patients and furthers knowledge of known mitochondrial genes. Precise disease diagnosis can end the diagnostic odyssey for patients, avoid unnecessary testing, provide prognosis, facilitate anticipatory management, and enable access to available therapies or clinical trials. Adjunctive tests such as functional and exercise testing could aid surveillance of MM patients. Management requires a multi-disciplinary approach, systemic screening for comorbidities, cofactor supplementation, avoidance of substances that inhibit the respiratory chain and exercise training. This update of the current understanding on MMs provides practical perspectives on current diagnostic and management approaches for this complex group of disorders.
Keywords: Mitochondrial myopathy, Mitochondrial disease, Genetic sequencing, Diagnostic approach, Mitochondrial disease treatment
Graphical abstract
Introduction
Mitochondrial myopathies (MM) are a genetically diverse group of mitochondrial diseases that affect skeletal muscles. They are caused by mutations in mitochondrial or nuclear genes encoding proteins related to mitochondrial oxidative metabolism resulting in the inability of mitochondria to sustain cellular energy demand. Body systems relying heavily on oxidative metabolism such as muscle, heart, nervous system, kidneys, and endocrine organs often manifest pathology most frequently. Myopathy is a common manifestation of mitochondrial disorders because the skeletal muscles have a high and constant cellular energy demand. However, individuals with MM often have dysfunction in other organ systems as well and it is relatively rare to present with isolated mitochondrial myopathy. Recognizing MM requires the clinician to be alert to symptoms of progressive worsening of muscle weakness, episodic symptoms of exercise intolerance, cramps, and fatigue as well as presence of other organ dysfunction.
MM has been reported with a frequency of about 5–15/10000 [1]. The estimated prevalence of mitochondrial disorders across populations is approximately 1/5000 [2]. The real prevalence is likely underestimated as mitochondrial disorders are often undiagnosed due to the diagnostic complexity of disease presentation as most mitochondrial disorders present with multisystemic symptoms with variable age of onset ranging from infancy to adulthood.
Mitochondrial DNA (mtDNA) mutations are maternally-inherited whereas nuclear DNA (nDNA) mutations follow Mendelian inheritance principles (autosomal dominant, recessive or X-linked). Each cell contains multiple copies of mtDNA and variable load of wild-type and pathogenic mtDNA (mitochondrial heteroplasmy) and therefore disease manifestation may occur only when the mutation load crosses a threshold for respiratory chain dysfunction to occur. Mitochondrial heteroplasmy often contributes to phenotypic variability and variable disease expression even within the same family. Most mtDNA mutations impact structural protein subunits of the respiratory chain while nDNA mutations impact structural and ancillary protein subunits of the respiratory chain as well as elements of mtDNA maintenance and expression. Ultimately, making sense of the heterogenous presentation of mitochondrial disorders also requires an appreciation of the underlying disease mechanisms as well as identification of specific clues and red flags that may help in the diagnostic approach to narrow down the differential diagnoses and allow more targeted testing. In this review, we discuss a rational diagnostic approach to MM, which is one of the common presentations of mitochondrial diseases.
Pediatric and adult phenotypes
MM can present at any age. Patients with pediatric-onset MM typically have more significant generalized muscle and systemic involvement [2,3]. Isolated myopathy is uncommon in children and the more typical finding is that of myopathy being one of the many features of a multisystemic disease.
Individuals with adult-onset MM often have milder phenotypes, occasionally with presentation confined to specific muscles [3,4]. The most common presentation in adults is Chronic Progressive External Ophthalmoplegia (CPEO) and represents up to 20% of adult-onset mitochondrial disorders [2,5]. Interestingly, Kearns Sayre Syndrome (KSS) may resemble some cases of CPEO that are caused by single large-scale mitochondrial deletions, and is a clinical subtype of CPEO [5]. KSS emerges before the age of 20 years, with similar symptoms of ptosis and progressive external ophthalmoplegia but with additional multisystemic features of pigmentary retinopathy, cardiac conduction block, sensorineural hearing loss, ataxia and endocrine manifestations. This underlines the typically more severe pediatric phenotype for mitochondrial disorders and the greater burden of disease.
MtDNA disorders versus nDNA disorders
Genotype-phenotype correlation in mitochondrial disorders is challenging due to phenotypic variability and genetic heterogeneity. nDNA-related mitochondrial disorders are generally more common in paediatric-onset mitochondrial diseases while mtDNA-related diseases are more frequently observed in adults [6]. Yet it is not uncommon for disorders such as MELAS (Mitochondrial Encephalopathy, Lactic Acidosis and Stroke-like episodes) related to the common A3243G mutation to appear even in the neonatal and infant age group and therefore genetic evaluation in all age groups should include both mtDNA and nDNA testing [7].
Disorders of mtDNA can be classified according to the type of genetic defect: large-scale rearrangements, such as mitochondrial deletions or duplications, or point mutations. Large-scale rearrangements like deletions affecting protein synthesis genes may result in KSS, Pearson syndrome and CPEO and are generally sporadic in inheritance. Point mutations may occur in protein synthesis genes such as the tRNA genes causing MELAS (tRNALeu(UUR) A3243G) or MERRF (Myoclonic Epilepsy with Ragged Red Fibers) (tRNA Lys A8344G) or in protein coding genes like ATP6 subunit T8993G variant causing NARP (Neuropathy, Ataxia, Retinitis Pigmentosa) or MILS (Maternally Inherited Leigh Syndrome) and are maternally-inherited.
nDNA-encoded mitochondrial disorders are often tissue-specific, severe, and frequently fatal.
Underlying pathogenic mechanisms for nDNA disorders include.
-
(1)
nuclear gene mutations resulting in OXPHOS subunit deficiency such as ACAD9 related to complex I deficiency with a clinical spectrum including infantile encephalomyopathy, hypertrophic cardiomyopathy, myopathy with exercise intolerance [8];
-
(2)
nuclear genes involved in mtDNA maintenance and replication such as TK2, RRM2B, MGME1 influencing myopathy related to mitochondrial depletion [[9], [10], [11]];
-
(3)
nuclear genes influencing mitochondrial translation such as YARS2 and PUS1 and its associated mitochondrial myopathy, lactic acidosis and sideroblastic anemia (MLASA) [12,13];
-
(4)
nuclear genes influencing mitochondrial fusion and fission such as MIEF2 [14];
-
(5)
nuclear genes encoding proteins involved in the assembly of iron-sulfur clusters which are important components of respiratory complexes, such as ISCU and associated congenital myopathy and FDX1L and associated childhood onset proximal myopathy [15,16]; and
-
(6)
nuclear genes influencing lipid metabolism and fatty oxidation such as HADHA or HADHB and Long Chain 3-Hydroxyacyl-CoA Dehydrogenase (LCHAD) deficiency) [17], ETFDH and myopathy related to coenzyme Q10 deficiency [18], CPT2 and carnitine transport disorder [19] and ACADVL-associated Very Long Chain Acyl-CoA Dehydrogenase deficiency (VLCAD) [20].
Mitochondrial myopathy syndromes
Many MM disorders are part of general multisystemic mitochondrial disorders with a conglomeration of symptom clusters with recognizable presentations (Table 1). Recognizing these clinical syndromes is helpful to narrow down the possible disorder and, in some cases, may even allow limited and specific testing e.g. mtDNA sequencing or gene panel for MELAS.
Table 1.
Genotype and phenotype of common multisystemic mitochondrial “syndromes” presenting with mitochondrial myopathy.
| Disease | mtDNA variation | nDNA genes | Inheritance | Age of onset | Key clinical symptoms | Distinctive features | Prognosis |
|---|---|---|---|---|---|---|---|
| Chronic Progressive External Ophthalmoplegia (CPEO) | MT-TL1 m.3243A>G or large-scale deletion of mtDNA | POLG (95%), C10orf2, RRM2B, SLC25A4, POLG2, DGUOK, SPG7 | Autosomal dominant, autosomal recessive, mitochondrial maternal inheritance | 20–40s | Ptosis, ophthalmo-plegia, hearing loss, mild muscle weakness, dysphagia, cataracts | Pigmentary retinopathy (“salt and pepper” pigmentation) | Slowly progressive |
| Mitochondrial Encephalomyopathy, Lactic Acidosis and Stroke-like episodes (MELAS) | MT-TL1 m.3243A>G (80%), m.3271T>C, MT-TQ, MT-TH, MT-TK, MT-TS1, MT-ND1, MT-ND5, MT-ND6, MT-TS2 | Mitochondrial maternal inheritance | Childhood to 40s | Stroke-like episodes, hemiparesis, seizures, hearing loss, muscle weakness, vision, renal impairment, mitochondrial diabetes | Lactic acidosis; strokes that do not conform to vascular territories | Prognosis depends on level of organ involvement | |
| Kearn-Sayre syndrome (KSS) | Large-scale deletions of mtDNA | Usually sporadic | Childhood, <20y | Progressive external ophthalmoplegia, pigmentary retinopathy, cardiac conduction block, cerebellar ataxia, deafness, short stature | CSF protein >100mg/dL Severe combined defects of mitochondrial complexes especially cytochrome C oxidase |
Progressive disorder, prognosis depends on level of organ involvement | |
| Myoclonus Epilepsy with Ragged Red Fibers (MERRF) | MT-TK m.8344A>G (80%), m.8356T>C, m,8363G>A, m.8361G>A | Sporadic or mitochondrial maternal inheritance | Childhood, <30y | Myoclonus, epilepsy, ataxia, myopathy, optic atrophy, deafness, peripheral neuropathy, cardiomyopathy with WPW syndrome | Myoclonus | Prognosis depends on level of organ involvement | |
| Primary Coenzyme Q10 Deficiency [21] |
COQ2, COQ7, COQ8A, COQ8B COQ9 |
Autosomal recessive | Infantile, childhood to adult onset | Predominant myopathy associated with encephalopathy, ataxia and retinopathy | Late onset disease shows better response to high dose CoQ10 supplementation | ||
| TK2 Deficiency | TK2 | Autosomal recessive | Infantile, Childhood and adult onset | Muscle weakness, hypotonia, bulbar dysarthria and dysphagia | Elevated creatine kinase (5-10x upper limit of normal), Severe reduction in mtDNA content in affected tissues and organs |
Poor | |
| Reversible Infantile Respiratory Chain Deficiency myopathy (RIRCD) [22] | mt-tRNAGlu m.14674T>C (homoplasmic) |
Associated with nDNA genes interacting with mt-tRNA Glu e.g. EARS2, TRMU | Infancy | Profound muscle weakness, hypotonia, feeding difficulties, Reversble transaminitis during periods of severe metabolic crises | Muscle biopsy in neonatal period shows numerous RRF and COX negative fibres, accumulation of lipids and glycogen which later resolve | Spontaneous improvement by 1 year, mostly asymptomatic by 2–3 years old |
Certain pharmacological agents can contribute towards inhibition of the respiratory chain and are known to aggravate myopathy symptoms. Such medications can sometimes be described to result in iatrogenic MM and should be avoided in patients with MM [23]. Examples include statins which has been linked to mitochondrial complex III inhibition [24], metformin which inhibits mitochondria and can trigger lactic acidosis [25], linezolid which inhibits mitochondrial ribosomal protein synthesis [26], and sodium valproate which is known to inhibit oxidative phosphorylation and beta-oxidation, exacerbating disease especially in individuals with POLG disease [23].
It is imperative to obtain a specific diagnosis for patients to end their diagnostic odyssey, avoid unnecessary testing, provide prognosis, and facilitate anticipatory management of systemic risks. This also enables access to specific therapies (e.g. coenzyme Q10 supplementation for CoQ10 deficiency), novel therapies or clinical trials. Screening of at-risk relatives is also imperative, and their pre-symptomatic diagnosis could facilitate earlier treatment with potentially better outcomes.
Diagnostic algorithm for mitochondrial myopathy
A strong index of suspicion is required in approaching MM - for the very young, delayed motor milestones are common and children are often described to be less athletic than their peers. The most common presentation across the ages is limb weakness and fatigue followed by sudden onset of respiratory failure, ptosis, myalgia, limb swelling and tremor [9]. Creatine kinase and lactic acid levels are often high but could fluctuate in severity between stable state and metabolic crises.
Because of the high energy demands of metabolically active organs, there can be a puzzling multisystemic presentation that can present a challenge for the diagnostician. It is often the combination of seemingly unrelated pathologies in a patient that when put together, suggest a mitochondrial pathology [27]. This emphasizes the importance of a thorough review of the patient's medical history and detailed history taking. Features that should arouse the suspicion of MM include exercise intolerance, acquired ptosis, ophthalmoplegia, pigmentary retinopathy, sideroblastic anemia, stroke-like episodes, epilepsy, multisystemic involvement (e.g. hearing loss, cardiac arrhythmia, cardiomyopathy), elevated plasma or CSF lactate, elevated plasma alanine, and presence of urinary 3-methylglutaconic acid [28].
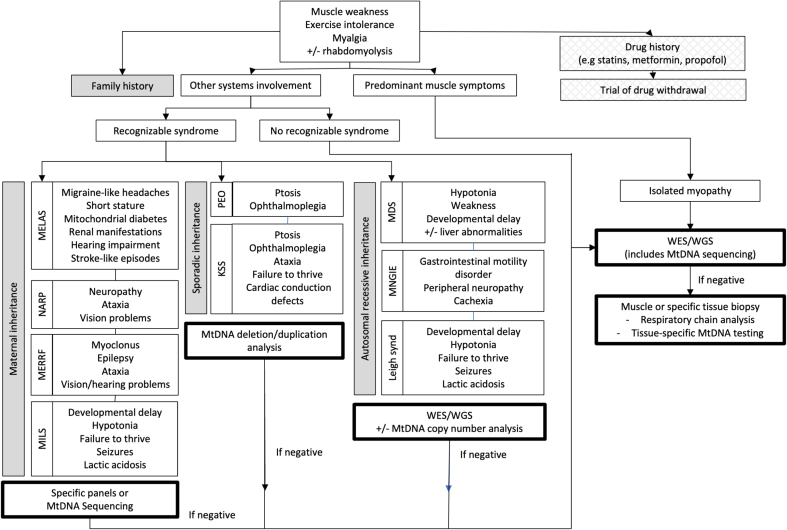
Family history should be reviewed in detail, where features that should arouse interest include a history of conditions like diabetes, epilepsy, myopathy, hearing loss, blindness in the maternal family line. The absence of any suggestive family history should not exclude the possibility of MM, as some conditions can be sporadic or arise de novo in the affected individual. A diagnostic algorithm is suggested (Fig. 1) based on the symptoms, some of which may cluster into a recognizable syndrome.
Fig. 1.
Diagnostic algorithm for mitochondrial myopathies. (KSS - Kearns Sayre Syndrome; MDS - Mitochondrial Depletion Syndrome; MELAS - Mitochondrial Encephalopathy, Lactic Acidosis and Stroke-like episodes; MERFF - Myoclonic Epilepsy and Ragged Red Fibers; MILS - Maternally Inherited Leigh Syndrome; MNGIE - Mitochondrial Neuro-Gastro-Intestinal Encephalopathy; NARP - Neuropathy, Ataxia and Retinitis Pigmentosa, PEO - Progressive External Ophthalmoplegia; WES - Whole Exome Sequencing; WGS - Whole Genome Sequencing).
Testing strategy
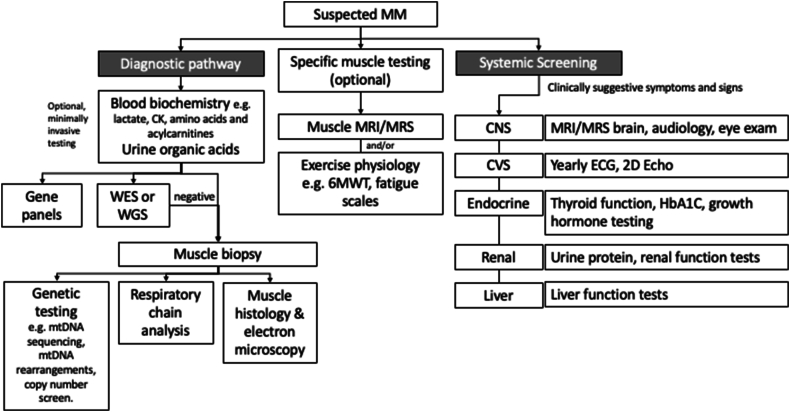
Testing of patients with MM includes both diagnostic testing as well as disease severity evaluation and monitoring (Fig. 2).
Fig. 2.
Testing strategy and systemic screening for mitochondrial myopathies for diagnosis as well as evaluation of disease severity. (CK - creatine kinase; WES - whole exome sequencing; WGS - whole genome sequencing; MRI - magnetic resonance imaging; MRS - magnetic resonance spectroscopy; 6MWT - 6 minute walk test; CNS - central nervous system; CVS - cardiovascular system; ECG - electrocardiogram; 2D Echo - echocardiogram).
Biochemistry
Lactate and creatine kinase (CK) levels have been demonstrated to be elevated in only a proportion of patients with specific genotypes [29,30]. Normal levels do not exclude the diagnosis of MM. For example, only 40% of individuals with the common m.3243A>G patients and MM had elevated lactate levels [31]. Plasma amino acids may be helpful as elevated alanine may be seen especially during periods of metabolic decompensation. Urine organic acids may show increased excretion of TCA cycle intermediates such as ethylmalonic acid and 3-methyl-glutaconic acid or dicarbolic aciduria [32].
Biochemical markers like growth differentiation factor 15 (GDF-15) and fibroblast growth factor 21 (FGF-21) were significantly elevated in patients with mitochondrial myopathies compared with a matched control population, with no correlation with severity of disease [31]. However it is important to note that GDF-15 can also be elevated in individuals with other neuromuscular diseases of non-mitochondrial origin such as muscular dystrophy or spinal muscular atrophy and hence it is not specific [33].
Molecular genetics
Genetic testing plays a crucial role in the diagnosis of MM, and broad coverage approaches using next generation sequencing (NGS) techniques such as whole exome sequencing (WES) or whole genome sequencing (WGS) with mtDNA sequencing coverage is recommended as the first line test in individuals suspected with a mitochondrial disease [[34], [35], [36]]. These could include individuals with a myopathy phenotype and with a personal or family history of multisystem involvement (e.g. diabetes, deafness, developmental delay, seizures). It is important to consider analysis of both nDNA and mtDNA as MM can be caused by both. This approach has multiple advantages, including reducing the need for invasive muscle biopsy, enabling a rapid diagnosis, and parallel evaluation of nDNA and mtDNA variants, as well as for other rare genetic diagnoses with similar phenotypes which can present as phenocopies.
WES has coverage of the exome, or protein-coding regions of the genome. 80% of disease-variants are reported to be found in the exome [37]. WGS covers exonic and intronic regions of the genome and has better sensitivity and identification of exonic single nucleotide variants (SNV) or copy number variants (CNV). WGS is, however, more costly than WES and thus potentially less accessible to patients. Additionally, many, though not all, laboratories include mtDNA analysis with WES/WGS analysis and hence this must be included if MM is suspected.
Multiple options of genetic testing exist. Targeted variant or gene testing has largely been phased out in favor of broad scale analysis due to relative affordability and coverage of NGS panels, WES and WGS. Individuals with a myopathy phenotype may be offered testing with a neuromuscular gene panel due to cost considerations. However, If MM is suspected, it is imperative to ascertain that the test selected has adequate coverage of relevant nDNA genes and mtDNA or to consider more detailed testing using WES or WGS with mtDNA coverage instead.
Analysis of the genetic findings is best undertaken with specialists familiar with clinical interpretation of genomic test results. Adoption of the American College of Medical Genetics and the Association of Molecular Pathology (ACMG-AMP) standards and guidelines for variant interpretation and specifications for mtDNA variant interpretation has aided the standardization of analysis but discrepancies can still occur, particularly in the analysis of novel variants where variant-level information may be limited [38,39].
Genetic testing is usually performed with whole blood DNA with alternatives like hair follicle DNA, saliva/buccal swab DNA. On occasion, the mtDNA variant may not be detectable on blood samples due to tissue-specific heteroplasmy. In addition, mtDNA deletions, depletion and rearrangements are often only detectable in muscle DNA and may be missed on analysis of blood specimens. In such instances, negative results from blood samples require follow up genetic testing on other tissue samples such as muscle (see section on Biopsy).
MtDNA findings are usually identified heteroplasmic although there are disease-causing homoplasmic mtDNA mutations. Measuring the level of heteroplasmy in blood samples has limited efficacy as this often does not correlate with disease severity or prognosis. This could be due to different distribution of heteroplasmy among different tissue types and variable heteroplasmy with age due to mitotic division throughout a person's lifetime [[40], [41], [42]]. One study has suggested that the level of heteroplasmy in the urine correlates better than blood as urinary samples include post-mitotic cells derived from the urinary system [43,44].
In addition, transcriptome analysis via RNA-seq may enable genetic diagnosis in patients with uncertain splice or null variants or who remain unsolved by current DNA sequencing approaches [45,46]. Emerging methods like real-time and long read sequencing can enhance diagnosis with the ease of detection of mtDNA deletions, other structural variants, tandem repeats, epigenetic modifications and cis/trans phasing of compound heterozygote variants for patients suspected with MM [47,48].
Tissue biopsies
Muscle biopsies have traditionally been done as part of MM diagnostic work up and includes mtDNA sequencing, histopathology, and respiratory chain enzyme analysis. However, with the facility of NGS technologies, muscle biopsies now form the next layer of testing when molecular genetic testing is negative or if there is a suspicion of tissue-specific heteroplasmy or mtDNA large scale rearrangements. It has also been shown that atypical MELAS variants encoding mtDNA-encoded complex subunits had mutation loads that were very low in blood but were high in muscle tissues [49]. Similarly, other mtDNA copy number variants may be missed on NGS in blood as well. Such findings are assessed for in muscle tissue specimens via Sanger sequencing, long range PCR, quantitative PCR, digital droplet PCR, or deep sequencing [50,51].
Muscle histopathology findings for MM include 1) ragged-red fibers (RRF) on modified Gomori trichrome stain, 2) proliferation of mitochondria observed with histochemical stain for succinate dehydrogenase (SDH) often described as ragged-blue fibers, 3) cytochrome c oxidase (COX or complex IV) deficient or negative fibers, and on electron microscopy 4) subsarcolemmal accumulation of ultrastructurally abnormal mitochondria, often with paracrystalline inclusions [51]. Such findings help to distinguish MM from other muscle disorders like congenital myopathies, muscular dystrophies, or metabolic myopathies. Certain features such as RRF may however not be seen in young children as there may not have been adequate time for muscle degeneration and subsarcolemmal mitochondrial accumulation to occur.
Muscle respiratory chain (RC) enzyme (complexes I-IV) analysis is done spectrophotometrically or in native gels while western blot, native gel, or two-dimensional gel assays and can assess levels of oxidative phosphorylation proteins on blood, skin, muscle, liver samples [51]. Abnormalities of several complex activities may be seen in disorders of mtDNA maintenance, transcription, translation, or nucleotide synthesis. On the other hand, defects in a single complex could indicate a deficit in a structural subunit or an assembly protein [52]. More recently, this can also be assessed via a quadruple immunofluorescent technique enabling the quantification of key RC subunits of complexes I and IV which gives precise and objective quantification of protein abundance in large numbers of individual muscle fibres [53]. Limitations of RC analysis include the fact that RC defects can be secondary to other metabolic or neuromuscular diseases.
Imaging
There are limited studies on the utility of muscle imaging in MM. Magnetic resonance spectroscopy (MRS) of muscle has detected high lactate in patient with CPEO and Fourier transform infrared spectroscopy (FTIR) has been shown to be able to distinguish CPEO from other muscle disorders but these biomarkers may not be in common clinical use as molecular genetics often suffice for diagnostic purposes [54,55].
MRI brain structural imaging has been used to identify specific mitochondrial syndromes such as MELAS where patients may have strokes that cross vascular territories. In general however, MRI brain findings tend to be non-specific although specific features such as symmetrical signal abnormality of the deep gray structures showing high signal on T2 and FLAIR images and hypointensity on T1 images may be very suggestive of a mitochondrial neurological disorder. MRS changes may also be seen when impaired RC function shifts metabolism from the tricarboxylic acid cycle to glycolysis [56]. 1H-MRS has also been helpful especially if areas of abnormality are compared with normal regions of the brain and demonstrate a double lactate peak and corresponding decreased NAA peak.
Positron emission tomography (PET) imaging measures metabolic flux and is able to directly evaluate subtle biological changes like the redox status of the brain. Several radioisotopically labeled metabolites have been used to study mitochondrial disorders and include 15O, 2-deoxy-2 18F-fluoro-d-glucose (FDG) and 11C pyruvate. PET study of tissue-specific bioenergetics has shown both region and global impairment of cerebral oxygen metabolic rate in patients with mitochondrial disease with neurological phenotype e.g. MELAS A3243G variant. However, patients with predominant muscle phenotype may not demonstrate as robust PET imaging changes as patients with neurological involvement [57].
Functional testing
Functional testing aids with definition of the extent and severity of muscle weakness but is unlikely to provide a specific molecular diagnosis. These tests may be useful in the clinical setting to evaluate disease progression as well as responses to specific interventions.
Exercise tests like the 6-Minute Walk Test (6MWT), Timed Up and Go test (3TUG), Five Times Sit to Stand Test (5XSST) and Test of Masticating and Swallowing Solids (TOMASS) have been demonstrated to display a significant difference in patients with mitochondrial myopathies versus normative controls at time of diagnosis [31]. These scores were not shown to correlate with heteroplasmy levels.
Patient-reported outcome measures such as the Fatigue Severity Scale (FSS) and West Haven-Yale Multidimentional Pain Inventory (WHYMPI) also had differential scores in MM patients with significant statistical differences, and more prominent in females [31].
Mitochondrial disease-specific scales such as the Newcastle Mitochondrial Disease Adult Scale (NMDAS) and the International Pediatric Mitochondrial Disease Scale (IPMDS) are often used for natural disease evaluation of patients with multiple system involvement and may be less useful for patients with an isolated or predominant muscle phenotype [58,59]. Specific muscle scales such as the Primary Mitochondrial Myopathy Symptom Assessment (PMMSA) scale may however be useful in adult patients with predominant myopathy symptoms and may also identify degree of fatigue that may affect daily functioning [60].
Exercise testing
Exercise testing has a limited role in evaluating patients with MM, although it may be helpful in patients who are oligosymptomatic who experience exercise intolerance with fatigue. Challenges to exercise testing would be an individual's exercise capacity due to cardiovascular fitness, intellectual ability to follow commands and presence of other physical disabilities [28].
Whole body maximal exercise testing measuring maximum oxygen uptake (VO2max) has been shown to correlate with mutation load and is reflective of the oxidative capacity of skeletal muscle in MM [61]. Measuring VO2max with cycle ergometry is therefore an attractive non-invasive method of evaluating oxidative capacity of skeletal muscle. However, patients with other forms of myopathy may also have poorer VO2max and so diagnostic utility requires a combination of VO2max testing with other parameters such as ventilation response or serum biomarkers such as serum growth and differentiation factor-15 (GDF-15). A short duration maximal exercise test has been found to show 5-fold increase in GDF-15 levels in patients with MM compared to healthy controls or patients with metabolic myopathy.
Novel biomarkers
Development of biomarkers requires prioritization as new therapies are being evaluated. Biomarkers include.
-
1.
Metabolomic markers measured with nuclear magnetic resonance (NMR) spectrometry or mass spectrometry (MS) methods. Specific diseases such as Leber's Hereditary Optic Neuropathy (LHON) with distinctive signatures such as altered sphingomyelins and phosphatidylcholines may be studied with MS methods [62].
-
2.
Serum markers such as fibroblast growth factor-21 (FGF-21) and growth and differentiation factor-15 (GDF-15) are cytokines that were studied in mitochondrial disease mouse models but have yet to be used clinically as biomarkers in humans. Although FGF-21 is produced in skeletal muscle, and both FGF-21 and GDF-15 have been found to be elevated in patients with mitochondrial diseases, specificity is low as other diseases such as renal failure, hepatic disease, malignancy, diabetes, and obesity may also be associated with elevated levels. Nonetheless, these cytokine biomarkers may be useful in evaluating mitochondrial diseases especially those affecting mitochondrial translation and mtDNA maintenance [63].
-
3.
MicroRNAs are small, highly conserved non-coding RNA regions that regulate gene expression. Distinctive microRNA patterns have been found in A3243G cybrid cell lines and may potentially be useful in cell-based screening of potential mitochondrial disease treatments.
-
4.
Small molecule reporters are tailor-made probes that can be targeted to a substrate of interest and delivered to accumulate within the mitochondria within an organ of interest. This may allow in vivo measurement of mitochondrial function and generation of reactive oxygen species. This is an emerging technology that has not been used in human studies yet.
Treatment of mitochondrial myopathies
General management of patient with MM
Clinical goals of managing a patient with MM are to evaluate and screen for comorbidities, mitigate symptoms and minimize or slow down disease progression [64,65]. Management of a patient with MM requires a multidisciplinary approach, including involvement of the physiotherapist and occupational therapist for optimization of strength and motor function, speech therapist for dysphagia management, dietician for optimization of nutrition and avoidance of rapid weight loss and catabolism, and clinical specialists such as the neurologist, ophthalmologist, geneticist, respiratory specialist and other relevant specialists depending on the patient's symptoms and manifestations [65]. Appropriate post-test genetic counseling and support is imperative after diagnosis to help the patient and family cope with acceptance of a challenging rare disease diagnosis.
Regular and periodic screening for systemic involvement should be considered [65]. This can vary depending on the genotype identified on genetic testing if there are known genotype-phenotype correlations. Such screens can include testing for hearing loss, arrhythmia or cardiomyopathy, endocrinological problems like diabetes or hypothyroidism, pulmonary function, ophthalmic involvement, or renal disease, Medications inhibiting respiratory chain function with the potential to exacerbate clinical symptoms should be avoided as discussed above [65,66]. These include statins, metformin, valproate, linezolid acid, aminoglycosides, and neuromuscular blocking agents. A comprehensive list of medications considered safe and unsafe can be found at https://www.mitopatients.org/mitodisease/list-of-medicines [66]. The patient's drug chart should be reviewed and unsuitable medications adjusted to suitable alternatives, for example statins should be switched to drugs like ezetimibe or alirocumab for treatment of hyperlipidemia in MM patients [67].
Prevention of illnesses which can induce catabolism and metabolic decompensation is important and the patient should be informed accordingly. This should be done by vaccination for preventable infections and typical infection control measures including avoidance of sick individuals, mask-wearing, and hand hygiene. Vaccination safety in MM has not been specifically investigated but expert consensus is that the risk of mitochondrial disease worsening due to an infectious trigger is greater than the theoretical risk of metabolic decompensation directly related to vaccination [66]. Aggressive treatment of fever, seizures, hyperlacticaemia and electrolyte and hormonal abnormalities is necessary to get the patient into a state of balanced homeostasis and decreased risk of metabolic decompensation.
Cofactor supplementation is recommended in MM with established cofactor deficiency known to respond to supplementation such as coenzyme Q10 (CoQ10) in primary or secondary CoQ10 deficiency [64]. The use of mitochondrial medication regimens including carnitine, CoQ10, arginine and others could optimize residual respiratory chain function, reduce oxidative stress, support alternative pathways for energy production and remove toxic metabolites where relevant [64]. Such regimens are colloquially termed as “mito-cocktails”. There is a paucity of randomized controlled trials and difficulty in measuring clinical outcomes to evaluate the efficacy of such regimens. For example, a systematic review of 37 articles on the use of l-arginine in MELAS showed no demonstrable clinical efficacy, but the studies were assessed to be of poor methodologic quality [68]. Nonetheless, such regimens have good safety profiles and reasonable acceptance and relatively easy applicability.
Management of acute metabolic crises
Patients with MM are susceptible to metabolic decompensation and worsening of symptoms during intercurrent illnesses or other catabolic stresses. Upon diagnosis, patients should be well-educated on their risks, emergency measures and to carry a memo stating their diagnosis and risks/precautions [65]. Prompt attention and intervention by a care team familiar with management of MM will aid in mitigating risks. Acute crises include severe lactic acidosis, which may require the use of dichloroacetate which can acutely reduce blood and CSF lactate but is not known to improve overall long term mortality from MM [69], or acute neurological deficits with metabolic stroke in MELAS requiring urgent administration of IV L-arginine [70]. Supportive intensive care for patients with MM should include surveillance for systemic risks related to MM including cardiac arrhythmia, cardiomyopathy, respiratory decline requiring ventilatory support and electrolyte disturbances due to tubulopathy or adrenal dysfunction [65].
Exercise training
In the absence of specific treatments for MM, efforts have been focused on exercise training to improve muscle function for patients with MM. Exercise training increases oxidative capacity of the muscles by increasing mitochondrial mass but may also increase mutation load of the muscles [3]. Nonetheless, exercise training has been shown to be generally beneficial to patients and a gradually progressive program of alternating aerobic and resistance training is recommended, avoiding exercise on days where the patients may have fever, illness, significant muscle pain or fasting [71].
Special considerations
High altitude travel including air travel should be carefully considered in patients with MM due to a theoretical risk of medical worsening. Hypoxia associated with altitude can downregulate respiratory chain capacity and reduce muscle mitochondria content [72]. Early recognition of medical deterioration and appropriate care, as well as need for oxygen saturation monitoring and supplemental oxygen in those with cardiomyopathy or respiratory weakness should be considered [65].
When undergoing general anesthesia, care must be taken to minimize the metabolic stress of surgery to prevent metabolic decompensation [73]. Certain medications should be used with caution such as propofol, known to inhibit the respiratory chain with its use associated with propofol infusion syndrome [74]. Use of depolarizing neuromuscular blockers such as suxamethonium should be avoided due to risk of exaggerated hyperkalemic response [75]. Avoidance of intraoperative hypoglycemia, hypotension, hypoxia and hypothermia, monitoring of intraoperative lactate as a marker of metabolic stress and avoidance of lactate-containing solutions is also recommended [76].
New and experimental treatments
Novel therapies targeted at improving mitochondrial function, patient's functional status and replacing defective genes have been studied in recent years [77]. Recent developments have been summarized in Table 2.
Table 2.
New and experimental treatments.
| Type of therapy | Mechanism of action | Compound/drug | Evidence | References |
|---|---|---|---|---|
| Drug Therapy | Increase cellular concentration of mitochondrial NAD+ | KL1333 | KL1333 has been shown to improve mitochondrial biogenesis and function in fibroblast line derived from a MELAS patient. No in vivo studies yet. | [82] |
| Increase cellular concentration of mitochondria concentration | Omaveloxolone | Well tolerated and improved lowering heart rate and lactate levels during submaximal exercise, did not significantly change peak exercise workload in MM. | [83] | |
| REN001 | PPAR β/δ agonist shown to improve fatigue and function in patients with fatty acid oxidation defects. Phase II trials in MM ongoing. | [84] | ||
| Bezafibrate | Modest improvement in cardiac function and reduction in immunodeficient muscle fibers in MM patients | [85] | ||
| Acipimox | Acipimox has been shown to improve mitochondria expression in vitro. Phase I clinical trials in adult MM patients ongoing. | [86,87] | ||
| Protecting mitochondria from damage | Elamipretide | Shown to be associated with clinical and functional improvements in children and adults with MM. | [[88], [89], [90], [91], [92], [93]] | |
| Restoring mitochondrial homeostasis | Deoxynucleoside therapy | Use in patients with TK2 deficiency showed improved motor and respiratory function | [[94], [95], [96]] | |
| Enzyme replacement | Erythrocyte Encapsulated Thymidine Phosphorylase (EE-TP) | Use of patients with MNGIE showed clinical improvement and reductions in thymidine, and deoxyuridine. | [[97], [98], [99]] | |
| Dietary supplementation | Correct taurine modification defect at the first anticodon nucleotide of mitochondrial tRNALeu(UUR) | High dose taurine | Use in MELAS patients was shown to reduce frequency of stroke-like episodes and improved taurine modification of mitochondrial tRNALeu(UUR) from peripheral blood leukocytes | [100] |
| Improve systemic NAD+ deficiency | Niacin | Oral niacin supplement increased blood NAD+ up to 8-fold and muscle NAD+ up to level of controls | [101,102] | |
| Influencing glutamate-glutamine cycle and glutamine transporters in the blood-brain barrier | High dose glutamine | Significant reduction in CSF glutamate and increment of CSF glutamine level in MELAS patients | [103] | |
| Stimulate mitochondrial function | Resveratrol | In vitro studies suggest improvements in mitochondrial fatty oxidation. However in vivo studies demonstrate lack of improvement in exercise capacity in adults with MM. | [104,105] | |
| Dietary manipulation | Stimulate mitochondrial function | Ketogenic diet | Positive impact on mitochondrial bioenergetics, mitochondrial ROS/redox metabolism and mitochondrial dynamics | [106] |
| Exercise therapy | Improve oxidative capacity and activity tolerance | Aerobic training | Aerobic training improves mitochondrial volume. Uncertain effect on muscle strength, effort tolerance and quality of life. | [61,61,107,108] |
| Device | Reduce oxidative stress | Near-infrared light-emitting diode | In vitro evidence as an effective antioxidant therapy | [109,110] |
| Modulate cortical and subcortical functional abnormalities | Transcranial direct current stimulation | Improved mitochondrial function and attenuated mitochondrial damage in mouse models. Aided improved clinical outcomes in autism, dyslexia and attention deficit. | [111,112] | |
| Surgery | Alleviate symptoms due to ptosis-related impairment of visual axis and head posture | Ptosis surgery in CPEO | Ptosis surgery (levator resection or frontalis silicone sling surgery) in patients with CPEO showed statistically significant improvement in marginal-to-reflex distance (MDRI) and chin-up posture. | [113] |
| Gene therapy | tRNA modification | MTO1 overexpression fully restored 5-taurinomethyluridine frequency and partially increased the aminoacylation efficiency of MELAS tRNA, leading to the upregulation of mitochondrial protein synthesis and respiratory activity in MELAS myoblasts in vitro. | [114] | |
| AAV gene delivery | Administration of human NDUFS4 coding sequence by AAV2/9 and/or AAV-PHP.B vectors improved clinical phenotype and prolonged the lifespan in Leigh syndrome mouse models | [[115], [116], [117]] | ||
| AAV9 delivery of human TK2 cDNA delaying disease onset and extending lifespan in mouse models. | [96] | |||
| Mitochondrial targeting with recombinant oligoribonucleotides | In vitro studies showed improved heteroplasmy proportions of mutant mtDNA in cultured cells with KSS mtDNA deletion and with mtDNA ND5 point mutation. | [118,119] | ||
| CRISPR-Cas9-mediated mitochondrial genome editing | In vitro studies in human cell lines and zebrafish has shown ability for this to target and reduce mtDNA copy number. | [120,121] | ||
| CRISPR-free base editing | In vitro studies have shown application for mitochondrial base editing in human cell lines, mice, zebrafish and plants. | [[122], [123], [124]] | ||
Family planning
Risk of recurrence and inheritance of MM depend on the cause of the condition. nDNA-related MM tend to obey Mendelian inheritance, where risk to offspring depends on the mode of inheritance of the gene in question. Prenatal genetic testing and preimplantation genetic testing for the familial variant. mtDNA-related MM follows a maternal inheritance pattern. Prenatal testing is complex in mtDNA variation as factors such as mutant heteroplasmy levels, threshold levels, phenotypic expression need to be considered and may not accurately predict offspring phenotype [78]. Genetic counseling is indicated to aid such families to understand their risks and options. Mitochondrial replacement therapy and assisted reproductive technology offers promise to such families to have unaffected children but has associated ethical considerations that may hinder implementation [[79], [80], [81]].
MM is a common presentation for mitochondrial disorders and may appear in isolation or in tandem with other organ presentations. Diagnosis and management are challenging because of the heterogeneity of disease presentations but an understanding of disease mechanisms as well as a reasoned approach to recognizing the cluster of disease symptomatology may aid in narrowing down the differential diagnoses. Genetic testing in suspected individuals including parallel analysis of nDNA and mtDNA should be considered to enable rapid diagnosis and reduce the need for other investigations. This facilitates prognostication and management of the MM patient. Strides have been made in the development of novel biomarkers and targeted therapies that show future promise to aid and improve the clinical outcomes of an otherwise potentially devastating diagnosis.
Author contribution statement
H.L.C, L.P.S and S.K.H.T contributed to the conception, design and writing of this manuscript.
Declaration of competing interest
H.L.C. is a shareholder in Alamya Health. The other co-authors do not have any conflicts of interests to declare in relation to this manuscript.
Acknowledgements
No funds were utilized in the preparation of this manuscript.
References
- 1.Mancuso M., Klopstock T., editors. Diagnosis and management of mitochondrial disorders. Springer; Cham: 2019. [Google Scholar]
- 2.Rahman S. Mitochondrial disease in children. J Intern Med. 2020 Jun;287(6):609–633. doi: 10.1111/joim.13054. [DOI] [PubMed] [Google Scholar]
- 3.Mancuso M., McFarland R., Klopstock T., Hirano M., Artuch R., Bertini E., et al. Inter Workshop: Neuromus Disorders. 2017 Dec;27(12):1126–1137. doi: 10.1016/j.nmd.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montano V., Gruosso F., Simoncini C., Siciliano G., Mancuso M. Clinical features of mtDNA-related syndromes in adulthood. Archiv Biochem Biophy. 2021 Jan;697 doi: 10.1016/j.abb.2020.108689. [DOI] [PubMed] [Google Scholar]
- 5.Bottoni P., Gionta G., Scatena R. Remarks on Mitochondrial Myopathies. Int J Mol Sci. 2022 Dec 21;24(1):124. doi: 10.3390/ijms24010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell O.M., Gorman G.S., Lightowlers R.N., Turnbull D.M. Mitochondrial Diseases: Hope for the Future. Cell. 2020 Apr 2;181(1):168–188. doi: 10.1016/j.cell.2020.02.051. [DOI] [PubMed] [Google Scholar]
- 7.Okhuijsen-Kroes E.J., Trijbels J.M., Sengers R.C., Mariman E., van den Heuvel L.P., Wendel U., et al. Infantile presentation of the mtDNA A3243G tRNA(Leu (UUR)) mutation. Neuropediatrics. 2001 Aug;32(4):183–190. doi: 10.1055/s-2001-17372. [DOI] [PubMed] [Google Scholar]
- 8.Schrank B., Schoser B., Klopstock T., Schneiderat P., Horvath R., Abicht A., et al. Lifetime exercise intolerance with lactic acidosis as key manifestation of novel compound heterozygous ACAD9 mutations causing complex I deficiency. Neuromuscul Disord. 2017 May;27(5):473–476. doi: 10.1016/j.nmd.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Wang J., El-Hattab A.W., Wong L.J.C. In: GeneReviews® [Internet] Adam M.P., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J., Gripp K.W., et al., editors. University of Washington, Seattle; Seattle (WA): 1993. TK2-Related Mitochondrial DNA Maintenance Defect, Myopathic Form.http://www.ncbi.nlm.nih.gov/books/NBK114628/ [cited 2023 Jul 24]. Available from: [PubMed] [Google Scholar]
- 10.Lim A.Z., McFarland R., Taylor R.W., Gorman G.S. In: GeneReviews® [Internet] Adam M.P., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J., Gripp K.W., et al., editors. University of Washington, Seattle; Seattle (WA): 1993. RRM2B Mitochondrial DNA Maintenance Defects.http://www.ncbi.nlm.nih.gov/books/NBK195854/ [cited 2023 Jul 24]. Available from: [PubMed] [Google Scholar]
- 11.Hebbar M., Girisha K.M., Srivastava A., Bielas S., Shukla A. Homozygous c.359del variant in MGME1 is associated with early onset cerebellar ataxia. Eur J Med Genet. 2017 Oct;60(10):533–535. doi: 10.1016/j.ejmg.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riley L.G., Cooper S., Hickey P., Rudinger-Thirion J., McKenzie M., Compton A., et al. Mutation of the mitochondrial tyrosyl-tRNA synthetase gene, YARS2, causes myopathy, lactic acidosis, and sideroblastic anemia--MLASA syndrome. Am J Hum Genet. 2010 Jul 9;87(1):52–59. doi: 10.1016/j.ajhg.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oncul U., Unal-Ince E., Kuloglu Z., Teber-Tiras S., Kaygusuz G., Eminoglu F.T. A Novel PUS1 Mutation in 2 Siblings with MLASA Syndrome: A Review of the Literature. J Pediatr Hematol Oncol. 2021 May 1;43(4):e592–e595. doi: 10.1097/MPH.0000000000001806. [DOI] [PubMed] [Google Scholar]
- 14.Bartsakoulia M., Pyle A., Troncoso-Chandía D., Vial-Brizzi J., Paz-Fiblas M.V., Duff J., et al. A novel mechanism causing imbalance of mitochondrial fusion and fission in human myopathies. Hum Mol Genet. 2018 Apr 1;27(7):1186–1195. doi: 10.1093/hmg/ddy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legati A., Reyes A., Ceccatelli Berti C., Stehling O., Marchet S., Lamperti C., et al. A novel de novo dominant mutation in ISCU associated with mitochondrial myopathy. J Med Genet. 2017 Dec;54(12):815–824. doi: 10.1136/jmedgenet-2017-104822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiegel R., Saada A., Halvardson J., Soiferman D., Shaag A., Edvardson S., et al. Deleterious mutation in FDX1L gene is associated with a novel mitochondrial muscle myopathy. Eur J Hum Genet. 2014 Jul;22(7):902–906. doi: 10.1038/ejhg.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diebold I., Schön U., Horvath R., Schwartz O., Holinski-Feder E., Kölbel H., et al. HADHA and HADHB gene associated phenotypes - Identification of rare variants in a patient cohort by Next Generation Sequencing. Mol Cell Probes. 2019 Apr;44:14–20. doi: 10.1016/j.mcp.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Gempel K., Topaloglu H., Talim B., Schneiderat P., Schoser B.G.H., Hans V.H., et al. The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring-flavoprotein dehydrogenase (ETFDH) gene. Brain. 2007 Aug;130(Pt 8):2037–2044. doi: 10.1093/brain/awm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longo N., Amat di San Filippo C., Pasquali M. Disorders of carnitine transport and the carnitine cycle. Am J Med Genet C Semin Med Genet. 2006 May 15;142C(2):77–85. doi: 10.1002/ajmg.c.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leslie N.D., Saenz-Ayala S. In: GeneReviews® [Internet] Adam M.P., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J., Gripp K.W., et al., editors. University of Washington, Seattle; Seattle (WA): 1993. Very Long-Chain Acyl-Coenzyme A Dehydrogenase Deficiency.http://www.ncbi.nlm.nih.gov/books/NBK6816/ [cited 2023 Jul 24]. Available from: [PubMed] [Google Scholar]
- 21.Desbats M.A., Lunardi G., Doimo M., Trevisson E., Salviati L. Genetic bases and clinical manifestations of coenzyme Q10 (CoQ 10) deficiency. J Inherit Metab Dis. 2015 Jan;38(1):145–156. doi: 10.1007/s10545-014-9749-9. [DOI] [PubMed] [Google Scholar]
- 22.Hathazi D., Griffin H., Jennings M.J., Giunta M., Powell C., Pearce S.F., et al. Metabolic shift underlies recovery in reversible infantile respiratory chain deficiency. EMBO J. 2020 Dec 1;39(23) doi: 10.15252/embj.2020105364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mancuso M., Orsucci D., Filosto M., Simoncini C., Siciliano G. Drugs and mitochondrial diseases: 40 queries and answers. Expert Opin Pharmacother. 2012 Mar;13(4):527–543. doi: 10.1517/14656566.2012.657177. [DOI] [PubMed] [Google Scholar]
- 24.Schirris T.J.J., Renkema G.H., Ritschel T., Voermans N.C., Bilos A., van Engelen B.G.M., et al. Statin-Induced Myopathy Is Associated with Mitochondrial Complex III Inhibition. Cell Metab. 2015 Sep 1;22(3):399–407. doi: 10.1016/j.cmet.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Protti A., Russo R., Tagliabue P., Vecchio S., Singer M., Rudiger A., et al. Oxygen consumption is depressed in patients with lactic acidosis due to biguanide intoxication. Crit Care. 2010;14(1):R22. doi: 10.1186/cc8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuccarini N.S., Yousuf T., Wozniczka D., Rauf A.A. Lactic Acidosis Induced by Linezolid Mimics Symptoms of an Acute Intracranial Bleed: A Case Report and Literature Review. J Clin Med Res. 2016 Oct;8(10):753–756. doi: 10.14740/jocmr2687w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munnich A., Rötig A., Chretien D., Cormier V., Bourgeron T., Bonnefont J.P., et al. Clinical presentation of mitochondrial disorders in childhood. J Inherit Metab Dis. 1996;19(4):521–527. doi: 10.1007/BF01799112. [DOI] [PubMed] [Google Scholar]
- 28.Hinojosa J.C., Bhai S. Diagnostic Testing in Suspected Primary Mitochondrial Myopathy. Muscles. 2023 Feb 20;2(1):75–85. [Google Scholar]
- 29.Gorman G.S., Chinnery P.F., DiMauro S., Hirano M., Koga Y., McFarland R., et al. Mitochondrial diseases. Nat Rev Dis Primers. 2016 Oct 20;2 doi: 10.1038/nrdp.2016.80. [DOI] [PubMed] [Google Scholar]
- 30.Kyriakides T., Angelini C., Schaefer J., Sacconi S., Siciliano G., Vilchez J.J., et al. EFNS guidelines on the diagnostic approach to pauci- or asymptomatic hyperCKemia. Eur J Neurol. 2010 Jun 1;17(6):767–773. doi: 10.1111/j.1468-1331.2010.03012.x. [DOI] [PubMed] [Google Scholar]
- 31.Montano V., Gruosso F., Carelli V., Comi G.P., Filosto M., Lamperti C., et al. Primary mitochondrial myopathy: Clinical features and outcome measures in 118 cases from Italy. Neurol Genet. 2020 Dec;6(6):e519. doi: 10.1212/NXG.0000000000000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas R.H., Parikh S., Falk M.J., Saneto R.P., Wolf N.I., Darin N., et al. Mitochondrial Medicine Society’s Committee on Diagnosis The in-depth evaluation of suspected mitochondrial disease. Mol Genet Metab. 2008 May;94(1):16–37. doi: 10.1016/j.ymgme.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morovat A., Weerasinghe G., Nesbitt V., Hofer M., Agnew T., Quaghebeur G., et al. Use of FGF-21 as a Biomarker of Mitochondrial Disease in Clinical Practice. J Clin Med. 2017 Aug 21;6(8):80. doi: 10.3390/jcm6080080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raymond F.L., Horvath R., Chinnery P.F. First-line genomic diagnosis of mitochondrial disorders. Nat Rev Genet. 2018 Jul;19(7):399–400. doi: 10.1038/s41576-018-0022-1. [DOI] [PubMed] [Google Scholar]
- 35.Schon K.R., Ratnaike T., van den Ameele J., Horvath R., Chinnery P.F. Mitochondrial Diseases: A Diagnostic Revolution. Trends Genet. 2020 Sep;36(9):702–717. doi: 10.1016/j.tig.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Arena I.G., Pugliese A., Volta S., Toscano A., Musumeci O. Molecular Genetics Overview of Primary Mitochondrial Myopathies. J Clin Med. 2022 Jan 26;11(3):632. doi: 10.3390/jcm11030632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixon-Salazar T.J., Silhavy J.L., Udpa N., Schroth J., Bielas S., Schaffer A.E., et al. Exome sequencing can improve diagnosis and alter patient management. Sci Transl Med. 2012 Jun 13;4(138):138ra78. doi: 10.1126/scitranslmed.3003544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCormick E.M., Lott M.T., Dulik M.C., Shen L., Attimonelli M., Vitale O., et al. Specifications of the ACMG/AMP standards and guidelines for mitochondrial DNA variant interpretation. Hum Mutat. 2020 Dec;41(12):2028–2057. doi: 10.1002/humu.24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ip E.K.K., Troup M., Xu C., Winlaw D.S., Dunwoodie S.L., Giannoulatou E. Benchmarking the Effectiveness and Accuracy of Multiple Mitochondrial DNA Variant Callers: Practical Implications for Clinical Application. Front Genet. 2022;13 doi: 10.3389/fgene.2022.692257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Laat P., Rodenburg R.R., Roeleveld N., Koene S., Smeitink J.A., Janssen M.C. Six-year prospective follow-up study in 151 carriers of the mitochondrial DNA 3243 A>G variant. J Med Genet. 2021 Jan;58(1):48–55. doi: 10.1136/jmedgenet-2019-106800. [DOI] [PubMed] [Google Scholar]
- 41.Scholle L.M., Zierz S., Mawrin C., Wickenhauser C., Urban D.L. Heteroplasmy and Copy Number in the Common m.3243A>G Mutation-A Post-Mortem Genotype-Phenotype Analysis. Genes (Basel) 2020 Feb 18;11(2):212. doi: 10.3390/genes11020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grady J.P., Pickett S.J., Ng Y.S., Alston C.L., Blakely E.L., Hardy S.A., et al. mtDNA heteroplasmy level and copy number indicate disease burden in m.3243A>G mitochondrial disease. EMBO Mol Med. 2018 Jun;10(6) doi: 10.15252/emmm.201708262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pickett S.J., Grady J.P., Ng Y.S., Gorman G.S., Schaefer A.M., Wilson I.J., et al. Phenotypic heterogeneity in m.3243A>G mitochondrial disease: The role of nuclear factors. Ann Clin Transl Neurol. 2018 Mar;5(3):333–345. doi: 10.1002/acn3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fayssoil A., Laforêt P., Bougouin W., Jardel C., Lombès A., Bécane H.M., et al. Prediction of long-term prognosis by heteroplasmy levels of the m.3243A>G mutation in patients with the mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes syndrome. Eur J Neurol. 2017 Feb;24(2):255–261. doi: 10.1111/ene.13176. [DOI] [PubMed] [Google Scholar]
- 45.Elstner M., Olszewski K., Prokisch H., Klopstock T., Murgia M. Multi-Omics Approach to Mitochondrial DNA Damage in Human Muscle Fibers. Int J Mol Sci. 2021 Oct 14;22(20) doi: 10.3390/ijms222011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macken W.L., Vandrovcova J., Hanna M.G., Pitceathly R.D.S. Applying genomic and transcriptomic advances to mitochondrial medicine. Nat Rev Neurol. 2021 Apr;17(4):215–230. doi: 10.1038/s41582-021-00455-2. [DOI] [PubMed] [Google Scholar]
- 47.Frascarelli C., Zanetti N., Nasca A., Izzo R., Lamperti C., Lamantea E., et al. Nanopore long-read next-generation sequencing for detection of mitochondrial DNA large-scale deletions. Front Genet. 2023 Jun 29;14 doi: 10.3389/fgene.2023.1089956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandiver A.R., Pielstick B., Gilpatrick T., Hoang A.N., Vernon H.J., Wanagat J., et al. Long read mitochondrial genome sequencing using Cas9-guided adaptor ligation. Mitochondrion. 2022 Jul;65:176–183. doi: 10.1016/j.mito.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W., Zhao Y., Xu X., Ma X., Sun Y., Lin Y., et al. A different pattern of clinical, muscle pathology and brain MRI findings in MELAS with mt-ND variants. Ann Clin Transl Neurol. 2023 Jun;10(6):1035–1045. doi: 10.1002/acn3.51787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basu S., Xie X., Uhler J.P., Hedberg-Oldfors C., Milenkovic D., Baris O.R., et al. Accurate mapping of mitochondrial DNA deletions and duplications using deep sequencing. PLoS Genet. 2020 Dec;16(12) doi: 10.1371/journal.pgen.1009242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olimpio C., Tiet M.Y., Horvath R. Primary mitochondrial myopathies in childhood. Neuromuscul Disord. 2021 Oct;31(10):978–987. doi: 10.1016/j.nmd.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Grazina M.M. Mitochondrial respiratory chain: biochemical analysis and criterion for deficiency in diagnosis. Methods Mol Biol. 2012;837:73–91. doi: 10.1007/978-1-61779-504-6_6. [DOI] [PubMed] [Google Scholar]
- 53.Rocha M.C., Grady J.P., Grünewald A., Vincent A., Dobson P.F., Taylor R.W., et al. A novel immunofluorescent assay to investigate oxidative phosphorylation deficiency in mitochondrial myopathy: understanding mechanisms and improving diagnosis. Sci Rep. 2015 Oct 15;5 doi: 10.1038/srep15037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan S.P., Hsueh H.W., Huang H.C., Chang K., Lee N.C., Huang P.H., et al. Lactate peak in muscle disclosed by magnetic resonance spectroscopy in a patient with CPEO-plus syndrome. eNeurologicalSci. 2021 Sep;24 doi: 10.1016/j.ensci.2021.100360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gervasoni J., Primiano A., Marini F., Sabino A., Biancolillo A., Calvani R., et al. Fourier-Transform Infrared Spectroscopy of Skeletal Muscle Tissue: Expanding Biomarkers in Primary Mitochondrial Myopathies. Genes (Basel) 2020 Dec 19;11(12):1522. doi: 10.3390/genes11121522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saneto R.P., Friedman S.D., Shaw D.W.W. Neuroimaging of mitochondrial disease. Mitochondrion. 2008 Dec;8(5–6):396–413. doi: 10.1016/j.mito.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frackowiak R.S., Herold S., Petty R.K., Morgan-Hughes J.A. The cerebral metabolism of glucose and oxygen measured with positron tomography in patients with mitochondrial diseases. Brain. 1988 Oct;111(Pt 5):1009–1024. doi: 10.1093/brain/111.5.1009. [DOI] [PubMed] [Google Scholar]
- 58.Schaefer A.M., Phoenix C., Elson J.L., McFarland R., Chinnery P.F., Turnbull D.M. Mitochondrial disease in adults: a scale to monitor progression and treatment. Neurology. 2006 Jun 27;66(12):1932–1934. doi: 10.1212/01.wnl.0000219759.72195.41. [DOI] [PubMed] [Google Scholar]
- 59.Koene S., Hendriks J.C.M., Dirks I., de Boer L., de Vries M.C., Janssen M.C.H., et al. International Paediatric Mitochondrial Disease Scale. J Inherit Metab Dis. 2016 Sep;39(5):705–712. doi: 10.1007/s10545-016-9948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gwaltney C., Stokes J., Aiudi A., Mazar I., Ollis S., Love E., et al. Psychometric performance of the Primary Mitochondrial Myopathy Symptom Assessment (PMMSA) in a randomized, double-blind, placebo-controlled crossover study in subjects with mitochondrial disease. J Patient Rep Outcomes. 2022 Dec 23;6(1):129. doi: 10.1186/s41687-022-00534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeppesen T.D., Madsen K.L., Poulsen N.S., Løkken N., Vissing J. Exercise Testing, Physical Training and Fatigue in Patients with Mitochondrial Myopathy Related to mtDNA Mutations. J Clin Med. 2021 Apr 20;10(8):1796. doi: 10.3390/jcm10081796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chao de la Barca J.M., Simard G., Amati-Bonneau P., Safiedeen Z., Prunier-Mirebeau D., Chupin S., et al. The metabolomic signature of Leber’s hereditary optic neuropathy reveals endoplasmic reticulum stress. Brain. 2016 Nov 1;139(11):2864–2876. doi: 10.1093/brain/aww222. [DOI] [PubMed] [Google Scholar]
- 63.Finsterer J., Zarrouk-Mahjoub S. Biomarkers for Detecting Mitochondrial Disorders. J Clin Med. 2018 Jan 30;7(2):16. doi: 10.3390/jcm7020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barcelos I., Shadiack E., Ganetzky R.D., Falk M.J. Mitochondrial medicine therapies: rationale, evidence, and dosing guidelines. Curr Opin Pediatr. 2020 Dec;32(6):707–718. doi: 10.1097/MOP.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parikh S., Goldstein A., Karaa A., Koenig M.K., Anselm I., Brunel-Guitton C., et al. Patient care standards for primary mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genetics in Medicine. 2017 Dec;19(12):1380–1397. doi: 10.1038/gim.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Vries M.C., Brown D.A., Allen M.E., Bindoff L., Gorman G.S., Karaa A., et al. Safety of drug use in patients with a primary mitochondrial disease: An international Delphi-based consensus. J Inherit Metab Dis. 2020 Jul;43(4):800–818. doi: 10.1002/jimd.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cicero A.F.G., Fogacci F., Bove M., Borghi C. Successful treatment of a patient with mitochondrial myopathy with alirocumab. J Clin Lipidol. 2020;14(5):646–648. doi: 10.1016/j.jacl.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 68.Stefanetti R.J., Ng Y.S., Errington L., Blain A.P., McFarland R., Gorman G.S. l-Arginine in Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-like Episodes: A Systematic Review. Neurology. 2022 Jun 7;98(23):e2318–e2328. doi: 10.1212/WNL.0000000000200299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schoenmann N., Tannenbaum N., Hodgeman R.M., Raju R.P. Regulating mitochondrial metabolism by targeting pyruvate dehydrogenase with dichloroacetate, a metabolic messenger. Biochim Biophys Acta Mol Basis Dis. 2023 May 30;1869(7) doi: 10.1016/j.bbadis.2023.166769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koga Y., Povalko N., Inoue E., Nakamura H., Ishii A., Suzuki Y., et al. Therapeutic regimen of L-arginine for MELAS: 9-year, prospective, multicenter, clinical research. J Neurol. 2018 Dec;265(12):2861–2874. doi: 10.1007/s00415-018-9057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tarnopolsky M.A. Exercise as a therapeutic strategy for primary mitochondrial cytopathies. J Child Neurol. 2014 Sep;29(9):1225–1234. doi: 10.1177/0883073814538512. [DOI] [PubMed] [Google Scholar]
- 72.Murray A.J., Horscroft J.A. Mitochondrial function at extreme high altitude. J Physiol. 2016 Mar 1;594(5):1137–1149. doi: 10.1113/JP270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van den Bersselaar L.R., Heytens L., Silva H.C.A., Reimann J., Tasca G., Díaz-Cambronero Ó., et al. European Neuromuscular Centre consensus statement on anaesthesia in patients with neuromuscular disorders. Eur J Neurol. 2022 Dec;29(12):3486–3507. doi: 10.1111/ene.15526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hemphill S., McMenamin L., Bellamy M.C., Hopkins P.M. Propofol infusion syndrome: a structured literature review and analysis of published case reports. Br J Anaesth. 2019 Apr;122(4):448–459. doi: 10.1016/j.bja.2018.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsieh V.C., Krane E.J., Morgan P.G. Mitochondrial Disease and Anesthesia. J Inborn Errors Metabol Screen. 2017 Jan;5 [Google Scholar]
- 76.Hoppe K. Mitochondrial disorders. Mitochondrial Disorders. 2017 Apr 10;(6–2017):S125–S133. [Google Scholar]
- 77.Tinker R.J., Lim A.Z., Stefanetti R.J., McFarland R. Current and Emerging Clinical Treatment in Mitochondrial Disease. Mol Diagn Ther. 2021 Mar;25(2):181–206. doi: 10.1007/s40291-020-00510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nesbitt V., Alston C.L., Blakely E.L., Fratter C., Feeney C.L., Poulton J., et al. A national perspective on prenatal testing for mitochondrial disease. Eur J Hum Genet. 2014 Nov;22(11):1255–1259. doi: 10.1038/ejhg.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tachibana M., Kuno T., Yaegashi N. Mitochondrial replacement therapy and assisted reproductive technology: A paradigm shift toward treatment of genetic diseases in gametes or in early embryos. Reprod Med Biol. 2018 Oct;17(4):421–433. doi: 10.1002/rmb2.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan X.Y., Guo L., Chen L.N., Yin S., Wen J., Li S., et al. Reduction of mtDNA heteroplasmy in mitochondrial replacement therapy by inducing forced mitophagy. Nat Biomed Eng. 2022 Apr;6(4):339–350. doi: 10.1038/s41551-022-00881-7. [DOI] [PubMed] [Google Scholar]
- 81.Noohi F., Ravitsky V., Knoppers B.M., Joly Y. Mitochondrial Replacement Therapy: In Whose Interests? J Law Med Ethics. 2022;50(3):597–602. doi: 10.1017/jme.2022.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seo K.S., Kim J.H., Min K.N., Moon J.A., Roh T.C., Lee M.J., et al. KL1333, a Novel NAD+ Modulator, Improves Energy Metabolism and Mitochondrial Dysfunction in MELAS Fibroblasts. Front Neurol. 2018;9:552. doi: 10.3389/fneur.2018.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Madsen K.L., Buch A.E., Cohen B.H., Falk M.J., Goldsberry A., Goldstein A., et al. Safety and efficacy of omaveloxolone in patients with mitochondrial myopathy: MOTOR trial. Neurology. 2020 Feb 18;94(7):e687–e698. doi: 10.1212/WNL.0000000000008861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.D’Annibale O.M., Phua Y.L., Van’t Land C., Karunanidhi A., Dorenbaum A., Mohsen A.W., et al. Treatment of VLCAD-Deficient Patient Fibroblasts with Peroxisome Proliferator-Activated Receptor δ Agonist Improves Cellular Bioenergetics. Cells. 2022 Aug 24;11(17):2635. doi: 10.3390/cells11172635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steele H., Gomez-Duran A., Pyle A., Hopton S., Newman J., Stefanetti R.J., et al. Metabolic effects of bezafibrate in mitochondrial disease. EMBO Mol Med. 2020 Mar 6;12(3) doi: 10.15252/emmm.201911589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van de Weijer T., Phielix E., Bilet L., Williams E.G., Ropelle E.R., Bierwagen A., et al. Evidence for a direct effect of the NAD+ precursor acipimox on muscle mitochondrial function in humans. Diabetes. 2015 Apr;64(4):1193–1201. doi: 10.2337/db14-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abouhajar A., Alcock L., Bigirumurame T., Bradley P., Brown L., Campbell I., et al. AIMM Trial Group Acipimox in Mitochondrial Myopathy (AIMM): study protocol for a randomised, double-blinded, placebo-controlled, adaptive design trial of the efficacy of acipimox in adult patients with mitochondrial myopathy. Trials. 2022 Sep 20;23(1):789. doi: 10.1186/s13063-022-06544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karaa A., Bertini E., Carelli V., Cohen B.H., Enns G.M., Falk M.J., et al. Efficacy and Safety of Elamipretide in Individuals With Primary Mitochondrial Myopathy: The MMPOWER-3 Randomized Clinical Trial. Neurology. 2023 Jul 18;101(3):e238–e252. doi: 10.1212/WNL.0000000000207402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hornby B., Thompson W.R., Almuqbil M., Manuel R., Abbruscato A., Carr J., et al. Natural history comparison study to assess the efficacy of elamipretide in patients with Barth syndrome. Orphanet J Rare Dis. 2022 Sep 2;17(1):336. doi: 10.1186/s13023-022-02469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Barcelos I.P., Emmanuele V., Hirano M. Advances in primary mitochondrial myopathies. Curr Opin Neurol. 2019 Oct;32(5):715–721. doi: 10.1097/WCO.0000000000000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karaa A., Haas R., Goldstein A., Vockley J., Cohen B.H. A randomized crossover trial of elamipretide in adults with primary mitochondrial myopathy. J Cachexia Sarcopenia Muscle. 2020 Aug;11(4):909–918. doi: 10.1002/jcsm.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karaa A., Haas R., Goldstein A., Vockley J., Weaver W.D., Cohen B.H. Randomized dose-escalation trial of elamipretide in adults with primary mitochondrial myopathy. Neurology. 2018 Apr 3;90(14):e1212–e1221. doi: 10.1212/WNL.0000000000005255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koenig M.K., Russo S.N., McBride K.L., Bjornsson H.T., Gunnarsdottir B.B., Goldstein A., et al. Use of Elamipretide in patients assigned treatment in the compassionate use program: Case series in pediatric patients with rare orphan diseases. JIMD Rep. 2023 Jan;64(1):65–70. doi: 10.1002/jmd2.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Domínguez-González C., Madruga-Garrido M., Mavillard F., Garone C., Aguirre-Rodríguez F.J., Donati M.A., et al. Deoxynucleoside Therapy for Thymidine Kinase 2-Deficient Myopathy. Ann Neurol. 2019 Aug;86(2):293–303. doi: 10.1002/ana.25506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hernandez-Voth A., Sayas Catalan J., Corral Blanco M., Castaño Mendez A., Martin M.A., De Fuenmayor Fernandez de la Hoz C., et al. Deoxynucleoside therapy for respiratory involvement in adult patients with thymidine kinase 2-deficient myopathy. BMJ Open Respir Res. 2020 Nov;7(1) doi: 10.1136/bmjresp-2020-000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lopez-Gomez C., Sanchez-Quintero M.J., Lee E.J., Kleiner G., Tadesse S., Xie J., et al. Synergistic Deoxynucleoside and Gene Therapies for Thymidine Kinase 2 Deficiency. Ann Neurol. 2021 Oct;90(4):640–652. doi: 10.1002/ana.26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Levene M., Bain M.D., Moran N.F., Nirmalananthan N., Poulton J., Scarpelli M., et al. Safety and Efficacy of Erythrocyte Encapsulated Thymidine Phosphorylase in Mitochondrial Neurogastrointestinal Encephalomyopathy. J Clin Med. 2019 Apr 5;8(4):457. doi: 10.3390/jcm8040457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bax B.E., Levene M., Bain M.D., Fairbanks L.D., Filosto M., Kalkan Uçar S., et al. Erythrocyte Encapsulated Thymidine Phosphorylase for the Treatment of Patients with Mitochondrial Neurogastrointestinal Encephalomyopathy: Study Protocol for a Multi-Centre, Multiple Dose, Open Label Trial. J Clin Med. 2019 Jul 24;8(8):1096. doi: 10.3390/jcm8081096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kipper K., Hecht M., Antunes N.J., Fairbanks L.D., Levene M., Kalkan Uçar S., et al. Quantification of Plasma and Urine Thymidine and 2’-Deoxyuridine by LC-MS/MS for the Pharmacodynamic Evaluation of Erythrocyte Encapsulated Thymidine Phosphorylase in Patients with Mitochondrial Neurogastrointestinal Encephalomyopathy. J Clin Med. 2020 Mar 13;9(3):788. doi: 10.3390/jcm9030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ohsawa Y., Hagiwara H., Nishimatsu S.I., Hirakawa A., Kamimura N., Ohtsubo H., et al. Taurine supplementation for prevention of stroke-like episodes in MELAS: a multicentre, open-label, 52-week phase III trial. J Neurol Neurosurg Psychiatry. 2019 May;90(5):529–536. doi: 10.1136/jnnp-2018-317964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pirinen E., Auranen M., Khan N.A., Brilhante V., Urho N., Pessia A., et al. Niacin Cures Systemic NAD+ Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy. Cell Metab. 2020 Jun 2;31(6):1078–1090.e5. doi: 10.1016/j.cmet.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 102.Chini E.N. Of Mice and Men: NAD+ Boosting with Niacin Provides Hope for Mitochondrial Myopathy Patients. Cell Metab. 2020 Jun 2;31(6):1041–1043. doi: 10.1016/j.cmet.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 103.Guerrero-Molina M.P., Morales-Conejo M., Delmiro A., Morán M., Domínguez-González C., Arranz-Canales E., et al. High-dose oral glutamine supplementation reduces elevated glutamate levels in cerebrospinal fluid in patients with mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes syndrome. Eur J Neurol. 2023 Feb;30(2):538–547. doi: 10.1111/ene.15626. [DOI] [PubMed] [Google Scholar]
- 104.Løkken N., Khawajazada T., Storgaard J.H., Raaschou-Pedersen D., Christensen M.E., Hornsyld T.M., et al. No effect of resveratrol in patients with mitochondrial myopathy: A cross-over randomized controlled trial. J Inherit Metab Dis. 2021 Sep;44(5):1186–1198. doi: 10.1002/jimd.12393. [DOI] [PubMed] [Google Scholar]
- 105.Storgaard J.H., Løkken N., Madsen K.L., Voermans N.C., Laforêt P., Nadaj-Pakleza A., et al. No effect of resveratrol on fatty acid oxidation or exercise capacity in patients with fatty acid oxidation disorders: A randomized clinical cross-over trial. J Inherit Metab Dis. 2022 May;45(3):517–528. doi: 10.1002/jimd.12479. [DOI] [PubMed] [Google Scholar]
- 106.Qu C., Keijer J., Adjobo-Hermans M.J.W., van de Wal M., Schirris T., van Karnebeek C., et al. The ketogenic diet as a therapeutic intervention strategy in mitochondrial disease. Int J Biochem Cell Biol. 2021 Sep;138 doi: 10.1016/j.biocel.2021.106050. [DOI] [PubMed] [Google Scholar]
- 107.Porcelli S., Marzorati M., Morandi L., Grassi B. Home-based aerobic exercise training improves skeletal muscle oxidative metabolism in patients with metabolic myopathies. J Appl Physiol (1985) 2016 Sep 1;121(3):699–708. doi: 10.1152/japplphysiol.00885.2015. [DOI] [PubMed] [Google Scholar]
- 108.Voet N.B., van der Kooi E.L., van Engelen B.G., Geurts A.C. Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev. 2019 Dec 6;12(12) doi: 10.1002/14651858.CD003907.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ashworth B.E., Stephens E., Bartlett C.A., Serghiou S., Giacci M.K., Williams A., et al. Comparative assessment of phototherapy protocols for reduction of oxidative stress in partially transected spinal cord slices undergoing secondary degeneration. BMC Neurosci. 2016 May 18;17(1):21. doi: 10.1186/s12868-016-0259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Giacci M.K., Hart N.S., Hartz R.V., Harvey A.R., Hodgetts S.I., Fitzgerald M. Method for the assessment of effects of a range of wavelengths and intensities of red/near-infrared light therapy on oxidative stress in vitro. J Vis Exp. 2015 Mar 21;(97) doi: 10.3791/52221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee S.B., Youn J., Jang W., Yang H.O. Neuroprotective effect of anodal transcranial direct current stimulation on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity in mice through modulating mitochondrial dynamics. Neurochem Int. 2019 Oct;129 doi: 10.1016/j.neuint.2019.104491. [DOI] [PubMed] [Google Scholar]
- 112.Salehinejad M.A., Ghanavati E., Glinski B., Hallajian A.H., Azarkolah A. A systematic review of randomized controlled trials on efficacy and safety of transcranial direct current stimulation in major neurodevelopmental disorders: ADHD, autism, and dyslexia. Brain Behav. 2022 Sep;12(9) doi: 10.1002/brb3.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eshaghi M., Arabi A., Eshaghi S. Surgical management of ptosis in chronic progressive external ophthalmoplegia. Eur J Ophthalmol. 2021 Jul;31(4):2064–2068. doi: 10.1177/1120672120952344. [DOI] [PubMed] [Google Scholar]
- 114.Tomoda E., Nagao A., Shirai Y., Asano K., Suzuki T., Battersby B.J., et al. Restoration of mitochondrial function through activation of hypomodified tRNAs with pathogenic mutations associated with mitochondrial diseases. Nucleic Acids Res. 2023 Mar 17 doi: 10.1093/nar/gkad139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Di Meo I., Marchet S., Lamperti C., Zeviani M., Viscomi C. AAV9-based gene therapy partially ameliorates the clinical phenotype of a mouse model of Leigh syndrome. Gene Ther. 2017 Oct;24(10):661–667. doi: 10.1038/gt.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reynaud-Dulaurier R., Benegiamo G., Marrocco E., Al-Tannir R., Surace E.M., Auwerx J., et al. Gene replacement therapy provides benefit in an adult mouse model of Leigh syndrome. Brain. 2020 Jun 1;143(6):1686–1696. doi: 10.1093/brain/awaa105. [DOI] [PubMed] [Google Scholar]
- 117.Silva-Pinheiro P., Cerutti R., Luna-Sanchez M., Zeviani M., Viscomi C. A Single Intravenous Injection of AAV-PHP.B-hNDUFS4 Ameliorates the Phenotype of Ndufs4 -/- Mice. Mol Ther Methods Clin Dev. 2020 Jun 12;17:1071–1078. doi: 10.1016/j.omtm.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tonin Y., Heckel A.M., Vysokikh M., Dovydenko I., Meschaninova M., Rötig A., et al. Modeling of antigenomic therapy of mitochondrial diseases by mitochondrially addressed RNA targeting a pathogenic point mutation in mitochondrial DNA. J Biol Chem. 2014 May 9;289(19):13323–13334. doi: 10.1074/jbc.M113.528968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Comte C., Tonin Y., Heckel-Mager A.M., Boucheham A., Smirnov A., Auré K., et al. Mitochondrial targeting of recombinant RNAs modulates the level of a heteroplasmic mutation in human mitochondrial DNA associated with Kearns Sayre Syndrome. Nucleic Acids Res. 2013 Jan 7;41(1):418–433. doi: 10.1093/nar/gks965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bian W.P., Chen Y.L., Luo J.J., Wang C., Xie S.L., Pei D.S. Knock-In Strategy for Editing Human and Zebrafish Mitochondrial DNA Using Mito-CRISPR/Cas9 System. ACS Synth Biol. 2019 Apr 19;8(4):621–632. doi: 10.1021/acssynbio.8b00411. [DOI] [PubMed] [Google Scholar]
- 121.Bi R., Li Y., Xu M., Zheng Q., Zhang D.F., Li X., et al. Direct evidence of CRISPR-Cas9-mediated mitochondrial genome editing. The Innovation. 2022 Nov;3(6) doi: 10.1016/j.xinn.2022.100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tolle I., Tiranti V., Prigione A. Modeling mitochondrial DNA diseases: from base editing to pluripotent stem-cell-derived organoids. EMBO Reports. 2023 Apr 5;24(4) doi: 10.15252/embr.202255678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mok B.Y., de Moraes M.H., Zeng J., Bosch D.E., Kotrys A.V., Raguram A., et al. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature. 2020 Jul;583(7817):631–637. doi: 10.1038/s41586-020-2477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mok B.Y., Kotrys A.V., Raguram A., Huang T.P., Mootha V.K., Liu D.R. CRISPR-free base editors with enhanced activity and expanded targeting scope in mitochondrial and nuclear DNA. Nat Biotechnol. 2022 Sep;40(9):1378–1387. doi: 10.1038/s41587-022-01256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]