Abstract
Noninvasive brain stimulation (NIBS) techniques have demonstrated their potential for chronic pain management, yet their efficacy exhibits variability across studies. Refining stimulation targets and exploring additional targets offer a possible solution to this challenge. This study aimed to identify potential brain surface targets for NIBS in treating chronic pain disorders by integrating literature review, neuroimaging meta-analysis, and functional connectivity analysis on 90 chronic low back pain patients. Our results showed that the primary motor cortex (M1) (C3/C4, 10–20 EEG system) and prefrontal cortex (F3/F4/Fz) were the most used brain stimulation targets for chronic pain treatment according to the literature review. The bilateral precentral gyrus (M1), supplementary motor area, Rolandic operculum, and temporoparietal junction, were all identified as common potential NIBS targets through both a meta-analysis sourced from Neurosynth and functional connectivity analysis. This study presents a comprehensive summary of the current literature and refines the existing NIBS targets through a combination of imaging meta-analysis and functional connectivity analysis for chronic pain conditions. The derived coordinates (with integration of the international electroencephalography (EEG) 10/20 electrode placement system) within the above brain regions may further facilitate the localization of these targets for NIBS application. Our findings may have the potential to expand NIBS target selection beyond current clinical trials and improve chronic pain treatment.
Keywords: Chronic pain, Functional magnetic resonance imaging, Non-invasive brain stimulation, Neuroimaging, Neuromodulation
Introduction
Chronic pain is a major public health problem that significantly impacts society and individuals worldwide [1]. The prevalence of chronic pain is roughly 20.4 % of the US population, with ∼8.0 % having high-impact chronic pain [2]. Although chronic pain is recognized as a crucial global health problem, its treatments still have tremendous potential for improvement [3]. There is an urgent need to improve existing pain treatments or develop new chronic pain management methods.
Recently, noninvasive brain stimulation (NIBS) techniques, such as transcranial direct current stimulation (tDCS), repetitive transcranial magnetic stimulation (rTMS), and transcranial focused ultrasound (tFUS), have emerged as promising interventions for chronic pain disorders [4]. Based on the 2020 evidence-based guidelines on the therapeutic applications of rTMS and tDCS, formulated by a panel of European experts, high-definition rTMS (HF-rTMS) of the primary motor cortex (M1) can significantly alleviate neuropathic pain (level A evidence). Likewise, level B evidence substantiates the efficacy of interventions such as HF-rTMS of the left M1 or DLPFC, as well as anodal tDCS directed at the left M1, in improving the quality of life and alleviating pain in individuals with fibromyalgia [5,6]. tFUS is another promising neuromodulation method for chronic pain management and has gained FDA approval for applications in thalamotomy for chronic neuropathic pain [7]. Compared to traditional NIBS techniques, such as magnetic or electric stimulations, tFUS stimulates deep brain structures with a higher spatial resolution [8].
Nevertheless, these promising outcomes are confined to a narrow spectrum of chronic pain disorders, namely neuropathic pain and fibromyalgia. The panorama of evidence for NIBS in treating chronic pain is limited by a lack of supported chronic pain conditions and scarcity of identified NIBS targets. Predominantly, most high-quality randomized controlled trials (RCTs) and meta-analyses have centered on NIBS applications over M1 and DLPFC for the management of chronic pain conditions [[9], [10], [11]]. However, the efficacy of these interventions has yielded inconsistent results across studies, particularly in the context of chronic low back pain [[12], [13], [14]], hindering their widespread implementation in clinical practice [15].
The therapeutic effects of NIBS depend on various stimulation variables, with the site of stimulation being a critical factor [16]. However, the variable effectiveness of NIBS in treating chronic pain may stem from the lack of specificity in targeting relevant neural pathways associated with this condition. NIBS studies conventionally administer stimulation to consistent sites, such as M1 (C3/C4) or DLPFC (F3/F4), using the 10/20 EEG system across diverse chronic pain disorders. This approach lacks precise localization and may restrict the selection of stimulation targets, contributing to outcome heterogeneity [5]. To address this issue, possible solutions may be to refine stimulation targets or explore additional potential targets. However, few studies have systematically investigated such brain regions for NIBS methods.
Brain imaging studies have provided compelling evidence of aberrant activity within specific cortical, subcortical, and associative brain regions in individuals with chronic pain. Importantly, these functional abnormalities can be effectively reversed with treatment [17], highlighting the potential of these circuits as interventions targeted. Among such interventions, NIBS interventions such as rTMS and tDCS, have garnered attention due to their capacity to exert neuromodulation effects beyond the targeted cortical area. For instance, studies have suggested that local brain activation induced by stimulation can trigger a transsynaptic spread of action potentials, thereby engaging interconnected subcortical brain regions and entire brain networks [[18], [19], [20]]. To establish a causal link between the neuromodulation of specific brain targets and its observed effects, integrating NIBS techniques with neuroimaging and functional connectivity analysis is imperative [21]. This is particularly crucial given the complex and intricate brain circuitry involved in chronic pain processing. Therefore, directly targeting brain surface areas implicated in pain processing with NIBS techniques may be a promising direction for chronic pain treatments [22]. Despite the potential, these recent advancements from brain neuroimaging studies on chronic pain have not yet been incorporated into existing NIBS treatment protocols.
Seed-based resting-state functional connectivity (rsFC) analysis has been widely used to investigate the intrinsic functional connections among brain regions [23], and has been applied as a primary approach for identifying brain stimulation targets guided by functional connectivity patterns [24,25]. In particular, it allows us to identify surface brain regions that are functionally connected with deep sub-surface brain structures that cannot be easily reached by NIBS and enhances our understanding of how the brain functions at a network level.
Neurosynth is a platform for large-scale, automated synthesis of functional magnetic resonance imaging (fMRI) data [26]. It utilizes machine learning methods on thousands of published articles reporting the results of fMRI studies to perform automated meta-analysis based on the term (key word) provided. Thus, in this study, we also used Neurosynth to identify brain regions involved in chronic pain.
This study integrates literature review, meta-analysis (using Neurosynth), and functional connectivity analysis to investigate brain surface targets for treating chronic pain. The identified brain regions can be used to develop more effective and targeted NIBS-based therapies for chronic pain treatment.
Methods
Literature review of NIBS targets for chronic pain disorders
A comprehensive search of databases, including PubMed (Medline), Embase, Web of Science, and Cochrane Library of Trials, was conducted to identify clinical studies on NIBS for chronic pain published from the inception of the literature to August 22nd, 2023. Free-text keywords and Medical Subject Heading terms were adopted. Furthermore, a manual search of related literature references was done for additional research. The search terms were used as follows: [“chronic pain” OR “widespread chronic pain”] AND [“transcranial magnetic stimulation” OR “transcranial direct current stimulation” OR “transcranial alternating current stimulation” OR “noninvasive brain stimulation”, “reduced impedance noninvasive cortical electrostimulation” OR “cranial electrotherapy stimulation” OR “transcranial ultrasound”] AND [“clinical study”] (see Supplementary Material Table S1 for details).
The eligible studies were screened according to the following inclusion criteria: (1) participants had to present with any chronic pain syndrome; (2) the intervention had to be the therapeutic use of NIBS techniques modulating brain excitability, while invasive forms of brain stimulation involving the use of electrodes implanted within the brain would be excluded; (3) the treatment group applied NIBS tools, while the control group received either sham stimulation or other forms of the intervention; (4) original and peer-reviewed publications would be included. Two trained researchers independently screened the eligible studies and removed duplicate records. Any disagreements among the researchers were resolved with the assistance of a third senior researcher when necessary.
Two reviewers (QK and TTL) independently assessed and cross-checked the risk of bias for each included randomized controlled study utilizing the Cochrane risk-of-bias tool 2.0 (RoB2) [27]. The evaluation covered five essential domains: randomization process, deviations from the intended interventions, missing outcome data, outcome measurements, and selective reporting. The overall risk-of-bias judgement was classified into “low risk of bias”, “some concerns” and “high risk of bias”. Any discrepancies between the reviewers’ assessments were resolved through discussion with a senior investigator to achieve consensus.
Identifying chronic pain-associated brain areas from the meta-analysis
To explore brain areas related to chronic pain, we first used Neurosynth (http://neurosynth.org) to retrieve the brain imaging literature based on the term ‘chronic pain’ by August 22nd, 2023. These studies were associated with various chronic pain conditions, including chronic low back pain (CLBP), fibromyalgia, chronic neuropathic pain, knee osteoarthritis, etc. An automated meta-analysis of 92 studies was used to generate a uniformity test map using a false discovery rate (FDR) criterion of 0.01.
As done in previous studies [28,29] a brain surface mask was applied on the uniformity test map to identify cortically accessible brain stimulation areas. Based on literature review, we predefined the precentral gyrus and dorsolateral prefrontal cortex as regions of interest in the analysis. In addition, 6–8 clusters larger than 30 voxels with the largest peak intensity among all clusters were identified [30,31] using the xjView toolbox (http://www.alivelearn.net/xjview/) as additional potential NIBS targets for chronic pan. The results were then mapped onto a standard brain with the international 10–20 EEG system in MNI space [32] using Surf Ice (www.nitrc.org/projects/surfice/) and a standard head using MRIcroGL (www.mccauslandcenter.sc.edu/mricrogl/). The mapped locations were further visually checked by the study investigators.
For the vital sub-surface structures within the uniformity test map, we first identified the peak coordinates with z-scores of each cluster using the xjView toolbox. Then, 4-mm radius spherical masks centered on the identified peak coordinates were created using MarsBaR v0.45 (https://marsbar-toolbox.github.io). These sub-surface seed regions were further refined by taking the overlap of the original uniformity test map and the masks from MarsBaR to maintain regional specificity for subsequent seed-based rsFC analysis [33].
Identifying chronic low back pain-associated brain surface regions using the resting-state functional connectivity
Modulating deep brain structures related to chronic pain, such as the thalamus and amygdala, can be challenging because they are located beyond the reach of most NIBS tools. We thus explored the connectivity of these deep brain structures, derived from the Neurosynth uniformity test map, to identify brain surface regions functionally connected to these sub-surface structures and thereby facilitate the application of NIBS. By stimulating these surface brain regions that are functionally connected to the sub-surface structures that are involved in chronic pain pathophysiology, we may indirectly influence the function/activity of these deep brain structures.
Participants
The study included 90 chronic low back pain patients (age range, 20–50 years, 38 male). The study protocol was approved by the Partners Institutional Review Board (IRB) of Massachusetts General Hospital, and all participants were provided with written informed consent prior to their involvement in the research study.
The inclusion criteria were: (1) presence of nonspecific low back pain for at least 6 months, as established by a clinical evaluation, including the use of X-ray/MRI reports when available; (2) pain intensity averaging at least 4 on a 0–10 visual analog scale (VAS). Exclusion criteria were: (1) any specific causes of low back pain (e.g., cancer-related/post-surgical/traumatic/neuropathic back pain, rheumatoid arthritis, widespread pain such as fibromyalgia); (2) complicated back problems (e.g., prior back surgery, medicolegal issues); (3) major systemic and/or psychiatric diseases or history of head injury or coma; (4) presence of any contraindications to MRI scanning; (5) history of substance abuse or dependence.
The dataset has been used to explore alterations in mesocorticolimbic functional connectivity [34], the alteration in the amplitude of low-frequency fluctuations (ALFF) [35], and functional connectivity changes in visual networks in patients with CLBP [36]. This study attempts to explore brain stimulation targets using seed-based rsFC in patients with chronic low back pain, which has not been published yet.
MRI data acquisition
All MRI data were acquired with a 32-channel head coil and 3.0 T Siemens (Skyra syngo) scanner at the Martinos Center for Biomedical Imaging. The resting-state functional images were obtained with echo-planar imaging under the following acquisition parameters: repetition time (TR) = 3000 ms, echo time (TE) = 30 ms, flip angle = 90°, slice thickness = 2.6 mm, voxel size = 3 × 3 × 3 mm3, 44 axial slices, 119 image volumes, field of view: 220 × 220 mm2, matrix: 84 × 84 mm2. T1-weighted images were acquired with the Magnetization-Prepared Rapid Gradient Echo (MPRAGE) sequence using the following parameters: TR = 2530 ms, TE = 1,69 ms, flip angle = 7°, slice thickness = 1 mm, voxel size = 1 × 1 × 1 mm3, 176 sagittal slices, field of view: 256 × 256 mm2, matrix: 256 × 256 mm2.
MRI data pre-processing
To identify brain surface regions connected with sub-surface structures related to chronic pain, rsFC analysis was conducted using the CONN toolbox version 21a (http://www.nitrc.org/projects/conn). Pre-processing steps included: the removal of the first five volumes, slice timing correction, head motion correction, outlier detection, co-registration to each subject's high-resolution T1 scan, normalization to the standard Montreal Neurological Institute (MNI) coordinate space (MNI 152 template), smoothing with a Gaussian kernel of FWHM 6 mm, regression of nuisance covariates, including 5 principal components from both the White Matter and CSF ROIs, 12 potential noise components from the estimated subject-motion parameters, as well as head motion scrubbing [37], linear detrending, and filtering with a band-pass frequency window of 0.008–0.09 Hz.
Seed-based functional connectivity analysis
Functional connectivity analysis was computed between each sub-surface seed identified from the meta-analysis and every other voxel in the brain. In the first-level analysis, correlation maps were produced for each subject by extracting the time course of the BOLD signal from the seeds and by computing Pearson's correlation coefficients between the time courses in the seeds and all other brain voxels. Correlation coefficients were transformed into z-scores to increase normality. In the group-level analysis, all subject-level functional connectivity maps were included in a one-sample t-test to obtain a group-level correlation map derived from each seed (positive and negative correlation separately). A voxel-level threshold at p < 0.001 and a cluster-level false discovery rate (FDR) p < 0.05 were applied.
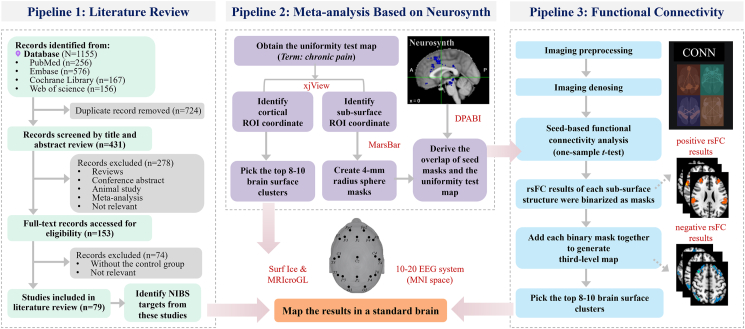
The group-level correlation map of each seed which met the predetermined threshold was then binarized and added up to generate a third-level map (positive and negative correlation maps separately). The intensity of each voxel in the third-level map represents the number of sub-surface seeds correlated with the voxel. Next, a brain surface mask was applied to constrain the results to cortical areas. Then, 8–10 clusters larger than 30 voxels with the largest peak intensity among all clusters were identified as potential brain stimulation regions. These clusters represent the brain surface regions correlated with the largest number of chronic pain-associated sub-surface seeds. The peak MNI coordinates and final brain stimulation protocols were obtained, as described above. Detailed method pipelines can be found in Fig. 1.
Fig. 1.
Three pipelines for identifying potential NIBS targets for chronic pain.
Abbreviations: NIBS, noninvasive brain stimulation.
Results
NIBS stimulation targets for chronic pain from literature review
Chronic low back pain
Six NIBS studies used tDCS, high-definition tDCS (HD-tDCS), rTMS, or transcranial alternating current stimulation (tACS) to treat CLBP [[38], [39], [40], [41], [42], [43]]. For tDCS, the active electrode was typically placed over the primary motor cortex (M1) at C3/C4 (10–20 EEG system) contralateral to the most painful side, while the cathodal electrode was placed over the contralateral supraorbital region [38,39,43]. One HD-tDCS study targeted the medial prefrontal cortex (mPFC), with the anode placed at Fz, four cathodes at F7, Fp1, Fp2, and F8, and the reference electrode on the right earlobe [41]. For rTMS studies, the target was positioned at the left DLPFC (F3) and M1 (C3/C4) contralateral to the most painful side [40,43]. For tACS, the center electrodes were placed at F3 and F4, connected for 10Hz-tACS. The “return” electrode was placed at Pz [42].
Fibromyalgia
A total of nineteen studies used NIBS to treat fibromyalgia (4 rTMS, 13 tDCS, 1 HD-tDCS, 1 Reduced Impedance Noninvasive Cortical Electrostimulation, RINCE, a method that applies electrical currents via scalp electrodes with specific stimulation frequencies hypothesized to reduce electrical impedance from skin and skull tissues, which may facilitate deeper cortical penetration and modulation of lower-frequency cortical activity [44,45]) [19,44,[46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62]]. For rTMS studies, the coil was commonly placed over the left M1 (C3) and right DLPFC (F4) [[56], [57], [58]]. One rTMS study employed the multi-coil for preferential targeting of the dorsal anterior cingulate cortex (dACC), with coils positioned at F3/F4, and anterior and posterior to Fz [59]. The studies of tDCS applied anodal stimulation to the left M1 (C3) and left DLPFC (F3), with the cathodal stimulation applied to the contralateral supraorbital region [19, [46], [47], [48], [49], [50], [51], [52], [53],[60], [61], [62]]. One study utilized a multi-electrode for female fibromyalgia treatment: two-electrode montages were placed over C3/Fp2 and F3/Fp2 to stimulate M1 and the DLPFC, respectively; the multi-electrode was positioned at F3, FC1, F8, FC5, C5, and P3 to stimulate the operculo-insular cortex (OIC) [54]. For the HD-tDCS study, the center electrode was placed over C3 (left M1), and the other four return electrodes were applied to Cz, F3, T7, and P3 [55]. In the RINCE study, the signal delivery lead was placed near the parietal region (PZ), and ground leads were affixed to the subject's ear lobes [44].
Phantom limb pain (PLP)
Four studies focused on the treatment of PLP with NIBS [[63], [64], [65], [66]]. For the three rTMS studies, the coil was applied to the contralateral M1 (C3/C4) of the most painful side[[64], [65], [66]]. One study applied cerebellar transcranial direct current stimulation (ctDCS), the anode was placed on the median line, 2 cm below the inion, with lateral borders about 1 cm medially to the mastoid apophysis, and the cathode was placed over the right shoulder [63].
Neuropathic pain
A total of ten studies used NIBS to treat central neuropathic pain mainly caused by spinal cord lesions (5 rTMS, 5 tDCS) [[67], [68], [69], [70], [71], [72], [73], [74], [75], [76]]. For most rTMS studies, the coil was commonly placed over the M1 at C3/C4 corresponding to the hand area, leg area, and left PFC (Fp1) [[72], [73], [74]]. Two rTMS studies applied a figure-8-shaped coil to target the optimal motor cortex by eliciting motor evoked potentials (MEPs). In the first of the two studies, the coil was positioned at the intersection of the imaginary anteroposterior and mediolateral lines (vertex) of the skull along the midline [76]. In the second study, the intersection of the coil was placed tangentially to the scalp, with the handle pointing backward over the vertex. The term vertex refers to the specific motor cortex area corresponding to the lower extremities [75]. For tDCS studies, M1 (C3/C4) was selected as the location of anodal stimulation, with cathodal stimulation applied to the contralateral supraorbital region [[67], [68], [69], [70], [71]].
Regarding the four NIBS studies to treat peripheral neuropathic pain, the target location of tDCS and rTMS was the same as that of central neuropathic pain [[77], [78], [79], [80]]. Seven studies focused on treating mixed neuropathic pain, including both central and peripheral pain by NIBS [[81], [82], [83], [84], [85], [86], [87]]. For most rTMS studies, M1 (C3/C4) contralateral to the most painful side was commonly selected as the coil location [[83], [84], [85],87]. One rTMS study applied the coil over the motor cortex region corresponding to the leg area through motor evoked potentials (MEPs) [86]. In the tDCS studies, the anodal electrode was placed over the M1 (C3/C4) contralateral to the most painful side, while the cathode was placed over the contralateral supraorbital region [81,82].
Two studies applied transcranial magnetic resonance imaging-guided focused ultrasound (tcMRgFUS) for the treatment of chronic neuropathic pain [88,89]. In these studies, the posterior part of the central lateral thalamic nucleus or the medial thalamus were selected as the precise location for thermal ablations facilitated by real-time patient monitoring, MR imaging, and MR thermometry guidance.
Secondary pain
Nine studies used NIBS to treat secondary pain associated with Parkinson's disease, end-stage renal disease, hepatitis C, multiple sclerosis, stroke, human T-lymphotropic virus type I-infected, and Chikungunya [[90], [91], [92], [93], [94], [95], [96], [97], [98]]. For studies using tDCS, the active electrode was typically placed over the M1 (C3/C4) and left DLPFC (F3) contralateral to the painful side [[90], [91], [92], [93], [94], [95], [96],98]. One study of central post-stroke pain utilized rTMS, with the coil placed over the left DLPFC (F3) [97].
Chronic abdominal pain
Five NIBS studies focused on treating chronic abdominal pain, including inflammatory bowel disease, chronic pancreatitis pain, and chronic pelvic pain [[99], [100], [101], [102], [103]]. Two studies regarding chronic pancreatitis employed rTMS at the secondary somatosensory cortex (SII) [99,100]. For the tDCS studies, the anode was placed at the M1 (C3/C4) contralateral to the most painful side, with the contralateral supraorbital region as the cathodal electrode [[101], [102], [103]].
Knee osteoarthritis pain
Three studies used tDCS to treat knee osteoarthritis pain, with anodal stimulation applied to the M1 (C3/C4) contralateral to the painful side and cathodal stimulation applied to the contralateral supraorbital region [[104], [105], [106]].
Pain syndrome
Five studies used NIBS to treat pain syndromes, including complex regional pain syndrome, subacromial pain syndrome, and burning mouth syndrome [[107], [108], [109], [110], [111]]. For the tDCS studies, the anode was placed on the M1 (C3/C4) contralateral to the painful side, while the cathode was located on the contralateral supraorbital region [107,108]. For the rTMS studies, the target of the coil was positioned at the M1 (C3/C4) contralateral of the painful side, M1 (C3/C4) in the hand area, and the left DLPFC (F3) [[109], [110], [111]].
Chronic myofascial pain
Three studies adopted NIBS in the treatment of chronic myofascial pain [86,112,113]. For the tDCS study, M1 (C3/C4) was selected as the location of anodal stimulation, and cathodal stimulation was applied to the neutral area of the contralateral supraorbital region [112]. Furthermore, left M1 (C3) contralateral to the painful side and motor cortex region corresponding to the leg area were selected for the coil position in the studies of rTMS [86,113].
Trigeminal neuralgia
As for the study of trigeminal neuralgia treated with tDCS, M1(C3/C4) contralateral to the painful side was selected as the location of anodal stimulation, and cathodal stimulation was applied to the neutral area of the contralateral supraorbital region [114].
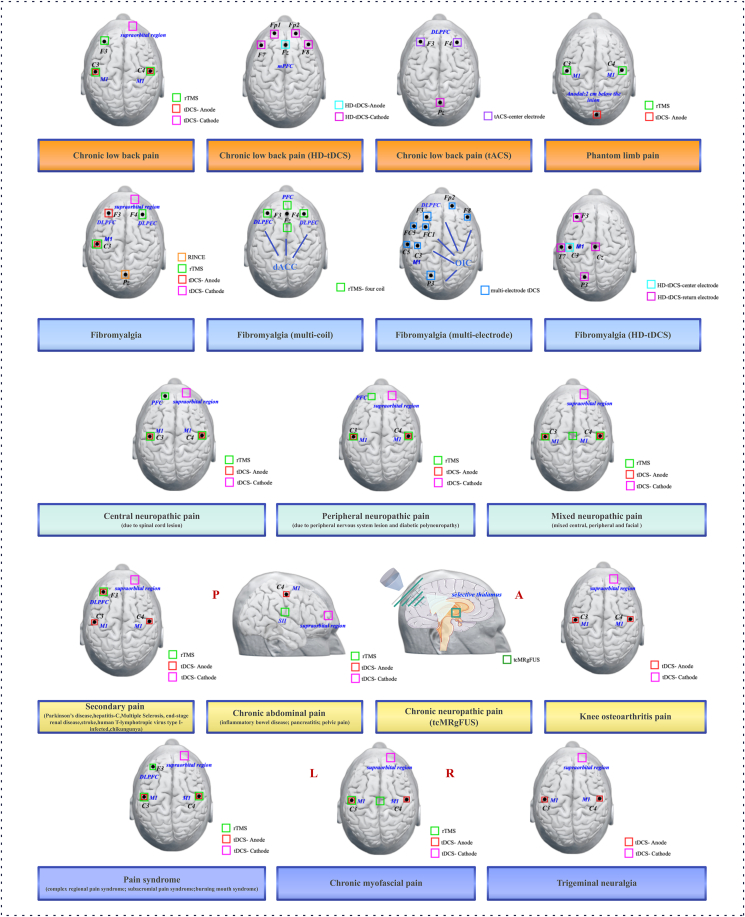
Specific information about NIBS treatments for chronic pain studies mentioned above is provided in Supplementary Material Table S2 and Fig. 2.
Fig. 2.
Stimulation locations of NIBS treatments from chronic pain based on literature review.
Abbreviations: L, Left, R, Right; HD-tDCS, high-definition transcranial direct current stimulation; tACS, transcranial alternating current stimulation; tcMRgFUS, transcranial magnetic resonance imaging-guided focused ultrasound; dACC, dorsal anterior cingulate cortex; OIC, operculo-insular cortex; DLPFC, dorsolateral prefrontal cortex; M1, primary motor cortex; mPFC, medial prefrontal cortex; PFC, prefrontal cortex; SII, secondary somatosensory cortex.
Brain regions implicated in chronic pain identified through meta-analysis
Ten cortical brain regions were identified from the meta-analysis. These regions included the bilateral precentral (M1)/postcentral gyrus (SI), Rolandic operculum (ROL), supramarginal gyrus (SMG), supplementary motor area (SMA), inferior parietal gyrus (IPG), and left inferior frontal gyrus (IFG). These cortical areas hold potential as NIBS candidate targets for chronic pain management (see Table 1, Fig. 3-A).
Table 1.
Brain surface regions identified from brain imaging meta-analysis.
| Cluster ID | Cluster size | Peak T∗ | Peak MNI coordinate |
Identified brain regions | 10-20 EEG system locations | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| 1 | 22 | 5.14 | −56 | 8 | 34 | L Precentral gyrus | ∼3 cm anterior and inferior to C3 |
| 2 | 22 | 5.14 | 52 | −28 | 48 | R Precentral gyrus/postcentral gyrus | ∼2 cm posterior to C4 |
| 3 | 64 | 8.72 | −38 | 24 | 0 | L Inferior frontal gyrus | ∼ close to F7 |
| 4 | 63 | 6.93 | −40 | −18 | 12 | L Rolandic operculum | ∼2 cm superior to T3 |
| 5 | 110 | 6.93 | −56 | −26 | 24 | L Supramarginal gyrus | ∼0.5 cm anterior to the midpoint of C3−T5 |
| 6 | 55 | 6.93 | −44 | −40 | 48 | L Inferior parietal gyrus | ∼0.5 cm inferior to the midpoint of C3–P3 |
| 7 | 102 | 7.82 | −2 | 8 | 44 | Bil Supplementary motor area | ∼ midpoint to Fz−Cz |
| 8 | 68 | 6.03 | 52 | −40 | 48 | R Inferior parietal gyrus | ∼0.5 cm inferior to the midpoint P4–C4 |
| 9 | 200 | 8.72 | 58 | −22 | 22 | R Supramarginal gyrus | ∼2 cm superior to T4 |
| 10 | 63 | 6.03 | 60 | 8 | 8 | R Rolandic operculum | ∼ midpoint to T4−F8 |
∗ FDR correction p < 0.01 from Neurosynth.
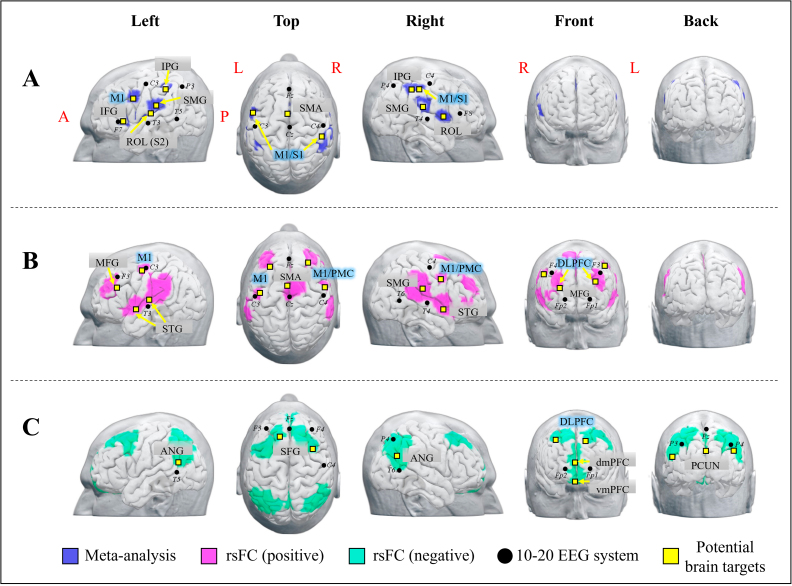
Fig. 3.
Potential brain stimulation targets identified from meta-/rsFC analysis.
(A) Surface brain regions derived from the meta-analysis. (B) Surface brain regions derived from the chronic pain-associated sub-surface ROIs-based positive rsFC. (C) Surface brain regions derived from the chronic pain-associated sub-surface ROIs-based negative rsFC.
Abbreviations, L, Left, R, Right; rsFC, resting-state Functional Connectivity; IFG, Inferior frontal gyrus; ROL, Rolandic operculum; M1, primary motor cortex; S1, primary somatosensory cortex; SMG, Supramarginal gyrus; IPG, Inferior parietal gyrus; SMA, Supplementary motor area; PMC, Premotor cortex; MFG, Middle frontal gyrus; STG, Superior temporal gyrus; ANG, Angular; SFG, Superior frontal gyrus; vmPFC, ventromedial Prefrontal cortex; dmPFC, dorsomedial prefrontal cortex.
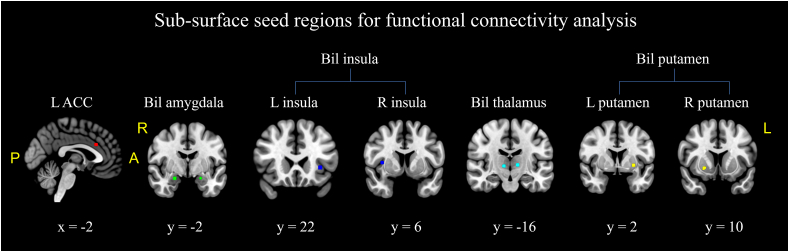
Furthermore, five sub-surface structures associated with chronic pain (the left anterior cingulate cortex (ACC), bilateral amygdala, insula, thalamus, and putamen) from the meta-analysis were also identified, and applied as seed regions for the following rsFC analysis (Fig. 4).
Fig. 4.
The sub-surface seeds used for the functional connectivity analysis in pipeline 3. Sagittal slices are shown in the left hemisphere.
Abbreviations: ACC: anterior cingulate cortex, L, left, R, right, Bil, bilateral. Red = L ACC [−2, 22, 28], green = L amygdala [−22, −2, −18]; R amygdala [22, −4, −18], blue = L insula [−34, 22, 0]; R insula [6, 6, 36]; cyan = L thalamus [−12, −16, 4], R thalamus [10, −16, 2]; yellow = L putamen [−28, 2, −2]; R putamen [22, 10, −6]. Coordinate shown are the Montreal Neurological Institute (MNI) coordinates.
Brain surface regions identified from the rsFC analysis
Sixteen brain surface regions were identified based on the rsFC results derived from the third-level positive and negative correlation maps of the five chronic pain-associated sub-surface ROIs, including the left ACC, bilateral amygdala, insula, thalamus, and putamen. Specifically, the bilateral precentral gyrus, dorsolateral prefrontal cortex (DLPFC) (e.g., middle frontal gyrus, MFG), superior temporal gyrus (STG), supplementary motor area (SMA), and right supramarginal gyrus (SMG) were identified from the third-level positive correlation maps of the chronic pain-associated sub-surface ROIs. The bilateral angular gyrus (ANG), DLPFC (e.g., superior frontal gyrus, SFG), medial prefrontal gyrus (mPFC), such as the ventromedial and dorsomedial prefrontal gyrus, and precuneus were identified from the third-level negative correlation maps of the chronic pain-associated sub-surface ROIs (see Table 2, Fig. 3B, C).
Table 2.
Brain surface regions identified from seed-based functional connectivity analysis.
| rsFC | Cluster ID | Peak MNI coordinate |
Identified brain regions | 10-20 EEG system locations | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Positivea | 1 | −30 | 34 | 28 | L Middle frontal gyrus | ∼2 cm inferior to F3 |
| 2 | −58 | 4 | −6 | L Superior temporal gyrus | ∼2 cm anterior to T3 | |
| 3 | −52 | −16 | 10 | L Superior temporal gyrus | ∼1 cm superior to T3 | |
| 4 | −46 | −6 | 54 | L Precentral gyrus | ∼ close to C3 | |
| 5 | −2 | 6 | 44 | Bil Supplementary motor area | ∼ midpoint to Fz−Cz | |
| 6 | 62 | −24 | 26 | R Supramarginal gyrus | ∼1 cm anterior to the midpoint C4−T6 | |
| 7 | 54 | 8 | −6 | R Superior temporal gyrus | ∼3 cm anterior to T4 | |
| 8 | 30 | 42 | 18 | R Middle frontal gyrus | ∼ midpoint to F4−Fp2 | |
| 9 | 56 | 4 | 40 | R Precentral gyrus/premotor cortex | ∼2 cm anterior and inferior to C4 | |
| Negativeb | 1 | −52 | −60 | 20 | L Angular gyrus | ∼2 cm superior to T5 |
| 2 | −14 | 34 | 46 | L Superior frontal gyrus | ∼1 cm posterior to midpoint F3−Fz | |
| 3 | 38 | 16 | 48 | R Superior frontal gyrus | ∼1 cm left to midpoint F4–C4 | |
| 4 | 50 | −62 | 30 | R Angular gyrus | ∼ midpoint to P4−T6 | |
| 5 | 4 | 56 | −24 | Bil ventromedial prefrontal cortex | ∼1 cm inferior to midpoint Fp2−Fp1 | |
| 6 | 4 | 68 | 8 | Bil dorsomedial prefrontal cortex | ∼1 cm superior to midpoint Fp2−Fp1 | |
| 7 | 4 | −60 | 30 | Bil Precuneus | ∼0.5 cm inferior to midpoint P3–P4 | |
The left precentral gyrus (cluster ID 4) was positively correlated with the bilateral amygdala, ACC, and thalamus, while the right precentral gyrus (cluster ID 9) was positively correlated with the bilateral amygdala and ACC. The other positive rsFC results generated from all five sub-surface seeds based positive rsFC, including the bilateral amygdala, thalamus, insula, putamen and left ACC.
The negative rsFC results generated from the bilateral amygdala, insula and the right ACC based negative rsFC.
For the third-level positive correlation map (Fig. 3B), the left precentral gyrus was positively correlated with the bilateral amygdala, ACC, and thalamus, while the right precentral gyrus/premotor cortex was positively correlated with the bilateral amygdala and ACC. The other rsFC results were compiled from all five sub-surface ROIs. For the third-level negative correlation map (Fig. 3C), only the negative rsFC results from the bilateral amygdala, insula, and left ACC were included. We did not observe significant negative rsFC using the thalamus and putamen as seeds. However, at a less conservative threshold (voxel-level p < 0.005, FDR p < 0.05), we did find that the right cerebellum crus II was negatively correlated with the bilateral thalamus; the right cuneus was negatively correlated with the bilateral putamen.
Discussion
Accurate stimulation targets for NIBS tools are essential for successful treatment outcomes. In this study, we used three different pipelines to identify potential cortical NIBS targets for treating chronic pain. We found that the bilateral M1, DLPFC, mPFC, precuneus, temporoparietal junction areas (IPG/SMG/ANG/STG), Rolandic operculum, SMA, left IFG, right cerebellum, and cuneus may all be considered as NIBS targets for management of chronic pain conditions such as chronic low back pain, fibromyalgia, chronic neuropathic pain, knee osteoarthritis, phantom limb pain, chronic visceral pain, and postoperative pain.
Current NIBS targets for chronic pain compared with neuroimaging-based results
The selection of NIBS targets for treating various chronic pain disorders has varied significantly across studies, suggesting the existence of distinct pain modulation mechanisms depending upon the anatomical location of the chronic pain. Based on our literature review, most NIBS studies for treating chronic pain target the M1 and DLPFC regions.
Motor control dysfunction is common across many chronic pain conditions. Research has identified altered intracortical primary motor cortex excitability in chronic neuropathic pain, musculoskeletal pain, complex regional pain syndrome, and others, making the M1 region a crucial target for chronic pain treatment with NIBS techniques [5,115,116]. Previous animal and human studies have demonstrated that modulating the M1 region could induce significant analgesic effects through the regulation of γ-aminobutyric acid (GABA) neurotransmitter concentrations at the synaptic level and control of cortical excitability [117,118]. Our meta-analysis suggests that the left M1 may lie anterior and inferior to C3, and the right M1 may lie posterior to C4. Additionally, our rsFC results suggest that stimulation site for the left M1 is near C3, and the right M1 stimulation site likely resides anterior and inferior to C4. Furthermore, our findings show that the left M1 target exhibits positive functional connectivity with the bilateral amygdala, ACC, and thalamus; the right M1 target demonstrates positive functional connectivity with the bilateral amygdala and ACC, suggesting potential for NIBS techniques to concurrently modulate the activity and connectivity of these deep brain structures. In addition to M1, we also identified the SMA, an adjacent brain region of M1, whose role in chronic pain modulation will be discussed in subsequent sections.
The DLPFC holds particular prominence as a NIBS target due to its intricate involvement in the cognitive and affective components of the chronic pain [119]. NIBS techniques targeting the DLPFC have demonstrated efficacy in improving memory, executive functions, cognitive control, and emotion regulation in individuals with chronic pain and accompanied cognitive impairments [[120], [121], [122]]. While the therapeutic mechanisms are still not entirely understood, the techniques exhibit the capacity to modulate cortical excitability, exert influence over broader neurocircuitry, and facilitate neuroplastic changes [[123], [124], [125]]. Specifically, a recent meta-analysis study suggested that high frequency (HF) rTMS over the DLPFC can induce a significant mid-term and long-term analgesic effect in various chronic pain conditions such as fibromyalgia, neuropathic pain, migraine, and different pain syndromes [126]. In addition, HF rTMS on the DLPFC can also relieve chronic pain and accompanied depressive symptoms [127]. These results highlight the potential of DLPFC in chronic pain management. In line with these findings, we also identified NIBS targets at the DLPFC. According to the current literature, the locations of DLPFC stimulation sites have been reported roughly at F3/F4. Our study suggests that the stimulation locations for the DLPFC in chronic pain management may lie inferior/anterior or superior/posterior to F3 and F4. Notably, the DLPFC target in our finding exhibits significant functional connection with the left ACC, bilateral amygdala, insula, thalamus, and putamen, offering potential for NIBS techniques to simultaneously modulate the connectivity/activity of these five sub-surface structures.
Key sub-surface structures identified from meta-analysis and their associations with chronic pain
We identified five sub-surface structures using the Neurosynth platform, each of which has been consistently associated with chronic pain. Specifically, the ACC, a component of the medial prefrontal cortex, has been shown to play a significant role in the affective aspects of pain [128]. Similarly, the insula is involved in higher-order pain processing. Both the ACC and insula contribute to regulatory top-down pain processing [129,130]. The amygdala, a key limbic region, also plays an important role in the emotional-affective aspects of pain and pain modulation [131]. Additionally, the thalamocortical circuit has been implicated in the pathophysiology of chronic pain, and NIBS techniques such as transcranial alternating current stimulation (tACS) has been shown to alleviate chronic low back pain by modulating this circuitry [132,133]. The putamen is primarily involved in the sensory-discriminative aspects of pain, as well as motor control and sensory integration [134,135]. These findings provide further support for the roles of these regions in chronic pain and its comorbidities.
Recent investigations have focused on the potential of the ACC and the ventral posterior thalamus, a sensory thalamic area, as effective targets for deep brain stimulation (DBS) in the treatment of chronic pain, including neuropathic pain [[136], [137], [138]]. Despite the significant therapeutic benefit of DBS, the inherent risks and complications associated with the surgical procedure of DBS have driven research toward less-invasive alternatives [139]. Studies suggest that both invasive and noninvasive stimulation techniques can modulate the same brain network and provide therapeutic benefits, thereby facilitating the translation of therapies between different neuromodulation modalities [25]. Furthermore, it has been proposed that NIBS techniques can be applied to cortical targets to effectively modulate deeper brain structures [140]. Consequently, we believe that investigating the functional connectivity of these brain regions may offer valuable insights for selecting appropriate targets for NIBS in the management of chronic pain.
Key brain surface regions in the default mode network area
Previous studies have shown a disrupted default mode network (DMN) connectivity in many chronic pain disorders, including CLBP, fibromyalgia, episodic migraine, and abdominal pain, which may lead to behavioral dysfunctions [[141], [142], [143], [144], [145], [146]]. The DMN consists of the mPFC, including the dorsomedial prefrontal cortex (dmPFC) and ventromedial prefrontal cortex (vmPFC), lateral parietal cortex (spanning the angular and the supramarginal gyrus), precuneus, and the lateral temporal cortex [147]. In this study, we identified many additional brain regions involved in the DMN, primarily from the third-level negative correlation map. Stimulating these regions may modulate activity in the DMN and produce therapeutic effects for chronic pain and its comorbid symptoms.
Medial prefrontal cortex
The mPFC has recently been recognized as a central hub for mental comorbidities associated with chronic pain, exerting important top-down control of the pain sensation [148]. The mPFC is a key node of the DMN, which plays a crucial role in cognitive control and emotional regulation [147]. Abnormal connectivity between the mPFC and other brain regions are associated with various chronic pain conditions, including but not limited to CLBP, fibromyalgia, neuropathic pain, etc [[149], [150], [151]]. The predominant role of the mPFC in pain processing makes it a promising NIBS target for chronic pain treatment.
Several studies have explored the application of NIBS to the mPFC for treating CLBP. For instance, investigators utilized high-definition transcranial direct current stimulation (HD-tDCS) at mPFC (approximately at Fz on the 10–20 EEG system) to enhance affective and attentional modulation in CLBP patients. Although the results were statistically insignificant, these studies suggested a potential relationship between treatment response and baseline pain inhibitory efficacy, with the most promising effects in individuals experiencing severe impairment in descending pain inhibitory mechanisms [152,153].
Additionally, studies have shown that TMS at the mPFC can modulate brain activity in both cortical and subcortical regions, including the DLPFC, ACC, nucleus accumbens, hippocampus, and thalamus [154]. Consistent with prior investigations, we identified two specific mPFC targets: the dmPFC and vmPFC. The target site of the dmPFC is located approximately 1 cm superior to Fpz, while the target site of the vmPFC is located around 1 cm inferior to Fpz. This identification is based on the overlapping negative functional connectivity observed between the mPFC and the bilateral amygdala, insula, and left ACC, which may hold the potential for optimizing mPFC targeting in the modulation of chronic pain. However, further research is needed to elucidate the specific roles of these targets in chronic pain modulation and deepen our understanding of their therapeutic implications.
Precuneus
The precuneus is another crucial hub within the DMN. Although not directly involved in the cortical representation of pain, the precuneus has been implicated in the processing of salient sensory experiences rather than specific nociceptive processing [155,156]. Abnormal activity and disrupted connectivity of the precuneus with other brain regions have been implicated in various chronic pain conditions, including low back pain [35], fibromyalgia [157], and complex regional pain syndrome [158].
To date, limited studies have explored NIBS targeting the precuneus for the treatment of chronic pain. Instead, previous studies have used the posterior parietal cortex (PPC) as the preferred stimulation site. For example, one study found that cathodal tDCS applied to the PPC could modulate nonpainful phantom sensations in chronic phantom limb pain, with the active electrode placed over P3/P4 (10–20 EEG system) contralateral to the amputation [159]. Another study revealed that transcranial alternating current stimulation (tACS) at 10 Hz over the PPC with the electrode positioned over P3/P4 facilitated the processing of bilateral tactile information, which is frequently impaired in individuals with chronic pain [160]. In our study, we identified a promising target within the precuneus, located approximately at the midpoint of P3–P4, highlighting the need for further investigation to determine its clinical efficacy and optimal stimulation parameters.
Notably, the identification of the mPFC and precuneus in our study was based on their negative functional connectivity with the bilateral amygdala, insula, and left ACC. In a recent study, we observed functional and anatomical connections between the mPFC and hippocampus, amygdala, and nucleus accumbens, while the precuneus exhibited connections with the hippocampus and amygdala. These findings designate the mPFC and precuneus as promising targets for NIBS to modulate deep brain structures implicated in psychiatric and neurological disorders, as well as chronic pain [161]. Prior research has demonstrated that hub nodes in the brain tend to have high average controllability within the DMN, indicating that the modulation of hub regions may have significant impacts on brain system functioning [162]. Our results align with this notion, suggesting that the mPFC and precuneus, key nodes of the DMN, can concurrently modulate activity/connectivity within the amygdala, insula, and ACC to treat chronic pain disorders.
Key brain surface regions in the ventral attention network area
The ventral attention network (VAN) is commonly defined as a right-hemisphere-dominant network, involving the temporoparietal junction (TPJ) and the ventral frontal cortex (VFC), that responds to unexpected salient sensory stimuli including pain [163,164]. Abnormal functional relationships between the VAN and other components of the dynamic pain connectome (e.g., DMN, ascending/descending pathways) have been observed in many chronic pain conditions [165,166].
The TPJ is a node of the VAN which plays a crucial role in multisensory integration and processing [167]. Previous literature reviews and meta-analyses have demonstrated the effectiveness of NIBS techniques, such as tDCS and TMS, when targeting the TPJ in various conditions, including depersonalization disorders, tinnitus, auditory hallucinations, and autism spectrum disorder [[167], [168], [169], [170]]. However, the application of NIBS over the TPJ for the treatment of chronic pain remains relatively unexplored. In a previous study, tDCS over the right TPJ led to a reduction in empathic responsiveness to pain experienced by other individuals and decreased late event-related potentials to facial expressions of pain, with the cathodal electrode located at the CP6 (10–20 EEG system) [171].
Moreover, the IFG, a pivotal node within the VAN, plays a crucial role in regulating emotion, interoception, and cognition, and is associated with the perception of pain and encoding of pain-related memories [172,173]. A recent study utilizing a combination of fMRI and TMS demonstrated the involvement of IFG in pain-related empathy, with the TMS coil positioned on the reference coordinate (x = 48, y = 36, z = 6) [174].
Building upon these findings, our study highlights the potential of targeting specific TPJ areas (IPG/SMG/ANG/STG) and IFG identified in this study as promising treatment targets for chronic pain.
Other potential brain surface regions
We also identified brain regions at the left Rolandic operculum (parietal operculum), right Rolandic operculum (frontal operculum), and premotor areas (e.g., the SMA). In our results, the left Rolandic operculum most closely corresponds to SII based on the cytoarchitectonic maps of the human parietal operculum [175]. A recent fMRI study indicated SII as a major brain area involved in the subjective perception of different pain stimulus intensities [176]. Another fMRI study proposed that the parietal operculum serves as an important relay station for the affective-motivational aspects of pain [177]. The Rolandic operculum and SMA identified in our findings are key components of the sensorimotor network (SMN). Literature suggests that changes within regions of the SMN are implicated in the underlying mechanism of chronic pain. Interventions aiming to enhance neuroplasticity in the SMN may hold promise for improving its function (e.g., motor output generation and sensory input encoding), and potentially advancing chronic pain management [178,179].
Previous studies have demonstrated the efficacy of rTMS targeting the opercular somatosensory region (OP) in reducing chronic visceral pain and increasing the threshold of heat pain in healthy participants. The head coil position was guided by individual anatomical landmarks derived from each participant's MRI [180,181]. More recently, another study investigated the neurophysiological effects of tDCS over opercular somatosensory region. Although the pain sensation did not exhibit a significant impact, the study demonstrated modulation of cortical activity, with the stimulation site determined using anatomical brain images and a navigation system [182]. Consistent with these findings, we also identify the opercular somatosensory region as a promising target for the treatment of chronic pain.
The SMA is a brain region located anterior to M1 and is involved in predictive motor planning [183]. Engagement of the motor system (e.g., M1, SMA) has been shown to be an effective source of analgesia, which can produce downstream effects on descending pain modulatory regions and ultimately decrease pain symptoms [184]. A new ALE meta-analysis found that increased activity within the SMA suggests a prominent role for the motor system in responding to pain in the context of sensorimotor integration [185].
A previous study has demonstrated that TMS applied to the premotor area can produce analgesic effects in individuals with postoperative pain. The coil position in this study was located 5 cm anterior to the motor cortex area associated with thumb movement, following the parasagittal line [186]. In another study involving patients with chronic pain and depression, a comparison was made between standard TMS coil positioning and MRI-guided procedures targeting the premotor region. The standard placement was 2–3 cm anterior to the “hand motor hotspot”, while the MRI-guided positioning was even more anterior (approximately 31.7 mm from the hand motor hotspot) [187]. These findings support the use of an individual brain MRI-based strategy for TMS coil placement. Our research aligns with these studies, further corroborating the potential of the SMA as a target for TMS in chronic pain management.
The cerebellum plays a crucial role in pain-related adaptions in motor control and sensorimotor integration [188]. Its rsFC with the thalamus and periaqueductal grey is altered by nociception, and its activity is modulated by perceived pain intensity [189]. Stimulation of the cerebellum has been shown to modulate painful sensations in humans, possibly by interfering with the inhibitory influence exerted by the cerebellum over cortical areas [190,191]. Notably, a previous study demonstrated that cerebellar tDCS can effectively modulate pain perception and its cortical correlates in healthy individuals, offering a promising and safe therapeutic approach for chronic pain conditions [192]. More recently, another study applied anodal cerebellar tDCS on individuals with phantom limb pain, resulting in significant improvements in both paroxysmal pain and non-painful phantom limb sensations. The typical targets for NIBS at the cerebellum are located along the median line, approximately 2 cm below the inion, with lateral boundaries about 1 cm medially to the mastoid apophysis [63,192]. These improvements are likely attributed to maladaptive changes in the sensorimotor network and posterior parietal cortex, respectively [63].
The cuneus is a smaller region in the occipital lobe, which has been related to multisensory integration and cognitive processing [193]. Although the occipital cortex is not traditionally associated with pain processing, studies have shown that painful stimuli can decrease functional connectivity within the DMN and lateral occipital cortex [194]. A recent diffusion tensor imaging study reported weaker structural connectivity from the left cuneus to the occipital cortex in participants with chronic pelvic pain syndrome [195]. However, the exact roles of the cuneus in chronic pain modulation remain undetermined.
Together, the literature above supports the use of the Rolandic operculum, SMA, and IFG, as well as additional areas such as the cerebellum and cuneus, as potential stimulation targets via different pain-related circuits to treat chronic pain (Refer to Table 3 for an overview of pain-related brain functions and previous clinical applications for chronic pain of the identified cortical targets mentioned above).
Table 3.
Pain-related brain functions and previous clinical applications for chronic pain of the identified cortical targets.
| Identified brain regions | Brain network | Pain-related functions | Previous clinical applications for chronic paina | Brain target locations in this study | References |
|---|---|---|---|---|---|
| mPFC (dmPFC/vmPFC) | Default mode network | cognitive control emotional regulation anti-nociceptive |
|
∼1 cm inferior and superior to Fpz | [[147], [148], [152], [153]] |
| Precuneus | Default mode network | self-relevant sensations pain perception |
|
∼midpoint to P3–P4 | [[155], [156], [159], [160]] |
| Temporoparietal junction (IPG/SMG/ANG/STG) | Ventral attention network | multisensory integration and processing empathy and social cognition |
|
See the specific locations of TPG areas (IPG/SMG/ANG/STG) in Tables 1 and 2 | [[163], [164], [167], [171]] |
| VFC (IFG) | Ventral attention network | emotional, interoceptive, cognitive regulation pain-related memories/empathy |
|
∼ close to F7 | [[172], [173], [174]] |
| Rolandic operculum (Parietal operculum-SII) | Sensorimotor network | affective-motivational aspects of pain multisensory integration |
|
L ROL: ∼2 cm superior to T3; R ROL: ∼ midpoint to T4-F8 |
[176,177,180,181] |
| SMA | Sensorimotor network | predictive motor planning sensorimotor integration |
|
∼ midpoint to Fz-Cz | [[183], [184], [185], [186], [187]] |
| Cerebellum | Sensorimotor network | Pain and motor processing sensorimotor integration |
|
N/A | [63,[188], [189], [190], [191], [192]] |
| Cuneus | N/A | multisensory integration cognitive processing | N/A | N/A | [193,194] |
Abbreviations: mPFC, medial prefrontal cortex; HD-tDCS, high-definition transcranial direct current stimulation; tACS, transcranial alternating current stimulation; TMS, transcranial magnetic stimulation; VFC, ventral frontal cortex; SMA: supplementary motor area; N/A, not applicable.
Previous studies for each brain region support its application as NIBS targets in different chronic pain conditions (the list may be not comprehensive).
Limitations and future directions
Our study has several limitations to consider. First, we chose chronic low back pain for the functional connectivity analysis as it is one of the most common types of chronic pain and is often associated with functional disability and work incapacity, possibly affecting quality of life, and requiring treatment [196]. While we believe the relevant targets identified in this manuscript may also be applicable to other chronic pain conditions, further research is needed to investigate this possibility. Secondly, our understanding of the excitatory or inhibitory effects on neural activity remains limited, and different stimulation parameters may produce different effects [197,198]. In addition, the effects produced by NIBS on the target area, surrounding areas, and the connected distal regions may also differ, potentially leading to significant implications for the application of NIBS techniques. Future studies are needed to explore specific stimulation paradigms based on different neuromodulation modalities and individual conditions. Furthermore, our findings are based on group analysis; individualized rsFC analysis may provide more accurate targets for brain stimulation tools (e.g., TMS). Nevertheless, for brain stimulation tools that do not have an accurate spatial resolution (e.g., tDCS and tACS), clinics that do not have MRI data available, or clinicians who do not have the expertise/resources to perform complicated brain imaging data analysis, our findings may provide valuable stimulation guidance. Moreover, a notable advantage of tFUS lies in its superior spatial resolution, which enables precise targeting of virtually any region within central nervous system. Given its exceptional precision compared to conventional NIBS methods like TMS and tDCS, tFUS holds promise as a potential avenue for future research to validate the targets identified in our study. Third, our study only investigated brain surface regions functionally connected to chronic pain-associated ROIs; studies integrating anatomical and functional connectivity may further enhance the brain target generation. Forth, the identified locations may also be applied in other interventions such as scalp acupuncture (stimulating the areas of scalp corresponding to brain regions believed to be involved in the pathphysiology of disorders using acupuncture needles) and transcutaneous electrical nerve stimulation [199]. Finally, clinical trials and additional studies are needed to validate our findings.
The existing literature review suggests that the primary motor cortex (M1) and prefrontal cortex are the most frequently targeted regions for NIBS in chronic pain treatment. However, several other cortical targets, including the mPFC, precuneus, TPJ, IFG, Rolandic operculum, SMA, and cerebellum, may serve as potential NIBS locations for chronic pain treatment. Our findings may extend the NIBS target selection for chronic pain management.
Declaration of competing interest
J.K has a disclosure to report (holding equity in startup companies (MNT, BTT) and a patent on peripheral neuromodulation), but declares no conflict of interest. All other authors declare no conflict of interest.
Acknowledgments
Jian Kong is supported by R33AT009310, R33AT009341, R34DA046635 (through the NIH HEAL Initiative), R01AG063975 and R01NS129059 from the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its NIH HEAL Initiative.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neurot.2023.10.007.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Mansfield K.E., Sim J., Jordan J.L., Jordan K.P. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain. 2016;157:55–64. doi: 10.1097/j.pain.0000000000000314. https://pubmed.ncbi.nlm.nih.gov/26270591/ [Internet] [cited 2023 Jul 26]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahlhamer J., Lucas J., Zelaya C., Nahin R., Mackey S., DeBar L., et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:1001–1006. doi: 10.15585/mmwr.mm6736a2. https://pubmed.ncbi.nlm.nih.gov/30212442/ [Internet] [cited 2023 Feb 20]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knezevic N.N., Candido K.D., Vlaeyen J.W.S., Van Zundert J., Cohen S.P. Low back pain. Lancet. 2021;398:78–92. doi: 10.1016/S0140-6736(21)00733-9. https://pubmed.ncbi.nlm.nih.gov/34115979/ [Internet] [cited 2023 Feb 12]. Available from: [DOI] [PubMed] [Google Scholar]
- 4.Pacheco-Barrios K., Cardenas-Rojas A., Thibaut A., Costa B., Ferreira I., Caumo W., et al. Methods and strategies of tDCS for the treatment of pain: current status and future directions. Expet Rev Med Dev. 2020;17:879–898. doi: 10.1080/17434440.2020.1816168. https://pubmed.ncbi.nlm.nih.gov/32845195/ [Internet] [cited 2023 Feb 28]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefaucheur J.P., Aleman A., Baeken C., Benninger D.H., Brunelin J., Di Lazzaro V., et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018) Clin Neurophysiol. 2020;131:474–528. doi: 10.1016/j.clinph.2019.11.002. https://pubmed.ncbi.nlm.nih.gov/31901449/ [Internet] [cited 2023 Feb 13]. Available from: [DOI] [PubMed] [Google Scholar]
- 6.Lefaucheur J.P., Antal A., Ayache S.S., Benninger D.H., Brunelin J., Cogiamanian F., et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS) Clin Neurophysiol. 2017;128:56–92. doi: 10.1016/j.clinph.2016.10.087. https://pubmed.ncbi.nlm.nih.gov/27866120/ [Internet] [cited 2023 Aug 19]. Available from: [DOI] [PubMed] [Google Scholar]
- 7.Di Biase L., Falato E., Caminiti M.L., Pecoraro P.M., Narducci F., Di Lazzaro V., et al. Focused ultrasound (FUS) for chronic pain management: approved and potential applications. Neurol Res Int. 2021;2021 doi: 10.1155/2021/8438498. https://pubmed.ncbi.nlm.nih.gov/34258062/ [Internet] [cited 2023 Aug 27]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Biase L., Falato E., Di Lazzaro V. Transcranial Focused Ultrasound (tFUS) and Transcranial Unfocused Ultrasound (tUS) neuromodulation: from theoretical principles to stimulation practices. Front Neurol. 2019;10 doi: 10.3389/fneur.2019.00549. https://pubmed.ncbi.nlm.nih.gov/31244747/ [Internet] [cited 2023 Aug 27]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao C., Zhu Q., Gao Z., Zhao J., Jia M., Li T. Can noninvasive brain stimulation improve pain and depressive symptoms in patients with neuropathic pain? A systematic review and meta-analysis. J Pain Symptom Manag. 2022;64:e203–e215. doi: 10.1016/j.jpainsymman.2022.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Giannoni-Luza S., Pacheco-Barrios K., Cardenas-Rojas A., Mejia-Pando P.F., Luna-Cuadros M.A., Barouh J.L., et al. Non-invasive motor cortex stimulation effects on quantitative sensory testing (QST) in healthy and chronic pain subjects: a systematic review and meta-analysis. Pain. 2020;161:1955. doi: 10.1097/j.pain.0000000000001893. https://pmc/articles/PMC7679288/ [Internet] [cited 2023 Aug 19]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardenas-Rojas A., Pacheco-Barrios K., Giannoni-Luza S., Rivera-Torrejon O., Fregni F. Noninvasive brain stimulation combined with exercise in chronic pain: a systematic review and meta-analysis. Expert Rev Neurother. 2020;2020;20:401–412. doi: 10.1080/14737175.2020.1738927. https://www.tandfonline.com/doi/abs/10.1080/14737175.2020.1738927 [Internet] [cited 2023 Aug 19]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patricio P., Roy J.S., Rohel A., Gariépy C., Émond C., Hamel É., et al. The effect of noninvasive brain stimulation to reduce nonspecific low back pain a systematic review and meta-analysis. Clin J Pain. 2021;37:475–485. doi: 10.1097/AJP.0000000000000934. https://journals.lww.com/clinicalpain/fulltext/2021/06000/the_effect_of_noninvasive_brain_stimulation_to.9.aspx [Internet] [cited 2023 Aug 19]. Available from: [DOI] [PubMed] [Google Scholar]
- 13.Chang T.T., Chang Y.H., Du S.H., Chen P.J., Wang X.Q. Non-invasive brain neuromodulation techniques for chronic low back pain. Front Mol Neurosci. 2022;15 doi: 10.3389/fnmol.2022.1032617. https://pubmed.ncbi.nlm.nih.gov/36340685/ [Internet] [cited 2023 Feb 12]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patricio P., Roy J.S., Rohel A., Gariépy C., Émond C., Hamel É., et al. The effect of non-invasive brain stimulation to reduce non-specific low back pain: a systematic review and meta-analysis. Clin J Pain. 2021;37:475–485. doi: 10.1097/AJP.0000000000000934. http://www.ncbi.nlm.nih.gov/pubmed/34009783 [Internet] [cited 2023 Feb 12]. Available from: [DOI] [PubMed] [Google Scholar]
- 15.Knotkova H., Hamani C., Sivanesan E., Le Beuffe M.F.E., Moon J.Y., Cohen S.P., et al. Neuromodulation for chronic pain. Lancet. 2021;397:2111–2124. doi: 10.1016/S0140-6736(21)00794-7. https://pubmed.ncbi.nlm.nih.gov/34062145/ [Internet] [cited 2023 Feb 28] Available from: [DOI] [PubMed] [Google Scholar]
- 16.Jiang X., Yan W., Wan R., Lin Y., Zhu X., Song G., et al. Effects of repetitive transcranial magnetic stimulation on neuropathic pain: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2022;132:130–141. doi: 10.1016/j.neubiorev.2021.11.037. [DOI] [PubMed] [Google Scholar]
- 17.Seminowicz D.A., Wideman T.H., Naso L., Hatami-Khoroushahi Z., Fallatah S., Ware M.A., et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31:7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. https://pubmed.ncbi.nlm.nih.gov/21593339/ [Internet] [cited 2023 Feb 12]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin R.L., Douaud G., Filippini N., Okell T.W., Stagg C.J., Tracey I. Structural connectivity variances underlie functional and behavioral changes during pain relief induced by neuromodulation. Scientific Reports. 2017;7:1–14. doi: 10.1038/srep41603. https://www.nature.com/articles/srep41603 [Internet] [cited 2023 Aug 21]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummiford C.M., Nascimento T.D., Foerster B.R., Clauw D.J., Zubieta J.K., Harris R.E., et al. Changes in resting state functional connectivity after repetitive transcranial direct current stimulation applied to motor cortex in fibromyalgia patients. Arthritis Res Ther. 2016;18 doi: 10.1186/s13075-016-0934-0. https://pmc/articles/PMC4741001/ [Internet] [cited 2023 Aug 21]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergmann T.O., Varatheeswaran R., Hanlon C.A., Madsen K.H., Thielscher A., et al. Concurrent TMS-fMRI for causal network perturbation and proof of target engagement. Neuroimage. 2021;237 doi: 10.1016/j.neuroimage.2021.118093. https://pubmed.ncbi.nlm.nih.gov/33940146/ [Internet] [cited 2023 Feb 28]. Available from: [DOI] [PubMed] [Google Scholar]
- 21.Xiong H.Y., Zheng J.J., Wang X.Q. Non-invasive brain stimulation for chronic pain: state of the art and future directions. Front Mol Neurosci. 2022;15 doi: 10.3389/fnmol.2022.888716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meeker T.J., Jupudi R., Lenz F.A., Greenspan J.D. New developments in non-invasive brain stimulation in chronic pain. Curr Phys Med Rehabil Rep. 2020;8:280. doi: 10.1007/s40141-020-00260-w. https://pmc/articles/PMC7814313/ [Internet] [cited 2023 Feb 12]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers B.P., Morgan V.L., Newton A.T., Gore J.C. Assessing functional connectivity in the human brain by FMRI. Magn Reson Imaging. 2007;25:1347. doi: 10.1016/j.mri.2007.03.007. https://pmc/articles/PMC2169499/ [Internet] [cited 2023 Aug 21]. Available from:/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weigand A., Horn A., Caballero R., Cooke D., Stern A.P., Taylor S.F., et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatr. 2018;84:28–37. doi: 10.1016/j.biopsych.2017.10.028. https://pubmed.ncbi.nlm.nih.gov/29274805/ [Internet] [cited 2023 Feb 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox M.D., Buckner R.L., Liu H., Chakravarty M.M., Lozano A.M., Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci U S A. 2014;111:E4367–E4375. doi: 10.1073/pnas.1405003111. https://pubmed.ncbi.nlm.nih.gov/25267639/ [Internet] [cited 2023 Feb 28]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. https://www.nature.com/articles/nmeth.1635 [Internet] [cited 2023 Feb 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. https://www.bmj.com/content/366/bmj.l4898 [Internet] [cited 2023 Jul 18]. Available from: [DOI] [PubMed] [Google Scholar]
- 28.Liu J., Zhang B., Wilson G., Kong J. New perspective for non-invasive brain stimulation site selection in mild cognitive impairment: based on meta-and functional connectivity analyses. Front Aging Neurosci. 2019;11 doi: 10.3389/fnagi.2019.00228. https://pubmed.ncbi.nlm.nih.gov/31551754/ [Internet] [cited 2023 Feb 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang B., Liu J., Bao T., Wilson G., Park J., Zhao B., et al. Locations for noninvasive brain stimulation in treating depressive disorders: a combination of meta-analysis and resting-state functional connectivity analysis. Aust N Z J Psychiatr. 2020;54:582–590. doi: 10.1177/0004867420920372. https://pubmed.ncbi.nlm.nih.gov/32419470/ [Internet] [cited 2023 Feb 13]. Available from: [DOI] [PubMed] [Google Scholar]
- 30.Cao J., Huang Y., Meshberg N., Hodges S.A., Kong J. Neuroimaging-Based scalp acupuncture locations for dementia. J Clin Med. 2020;9:1–14. doi: 10.3390/jcm9082477. https://pubmed.ncbi.nlm.nih.gov/32752265/ [Internet] [cited 2022 Nov 30]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao J., Chai-Zhang T.C., McDonald C.M., Kong J. Scalp stimulation targets for neurological conditions-evidence from large-scale meta-analyses. J Integr Neurosci. 2022;21:83. doi: 10.31083/j.jin2103083. https://www.imrpress.com/journal/JIN/21/3/10.31083/j.jin2103083/htm [Internet] [cited 2023 Jun 27]. Available from: [DOI] [PubMed] [Google Scholar]
- 32.Cutini S., Scatturin P., Zorzi M. A new method based on ICBM152 head surface for probe placement in multichannel fNIRS. Neuroimage. 2011;54:919–927. doi: 10.1016/j.neuroimage.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y., Zhang B., Cao J., Yu S., Wilson G., Park J., et al. Potential locations for noninvasive brain stimulation in treating autism spectrum disorders—a functional connectivity study. Front Psychiatr. 2020;11 doi: 10.3389/fpsyt.2020.00388. https://pubmed.ncbi.nlm.nih.gov/32457666/ [Internet] [cited 2023 Jun 27]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu S., Li W., Shen W., Edwards R.R., Gollub R.L., Wilson G., et al. Impaired mesocorticolimbic connectivity underlies increased pain sensitivity in chronic low back pain. Neuroimage. 2020;218 doi: 10.1016/j.neuroimage.2020.116969. https://pubmed.ncbi.nlm.nih.gov/32439536/ [Internet] [cited 2023 Feb 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang B., Jung M., Tu Y., Gollub R., Lang C., Ortiz A., et al. Identifying brain regions associated with the neuropathology of chronic low back pain: a resting-state amplitude of low-frequency fluctuation study. Br J Anaesth. 2019;123:e303–e311. doi: 10.1016/j.bja.2019.02.021. https://pubmed.ncbi.nlm.nih.gov/30948036/ [Internet] [cited 2023 Feb 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen W., Tu Y., Gollub R.L., Ortiz A., Napadow V., Yu S. Visual network alterations in brain functional connectivity in chronic low back pain: a resting state functional connectivity and machine learning study. Neuroimage Clin. 2019;22 doi: 10.1016/j.nicl.2019.101775. https://pubmed.ncbi.nlm.nih.gov/30927604/ [Internet] [cited 2023 Feb 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. https://pubmed.ncbi.nlm.nih.gov/17560126/ [Internet] [cited 2023 Jun 20]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hazime F.A., Baptista A.F., de Freitas D.G., Monteiro R.L., Maretto R.L., Hasue R.H., et al. Treating low back pain with combined cerebral and peripheral electrical stimulation: a randomized, double-blind, factorial clinical trial. Eur J Pain. 2017;21:1132–1143. doi: 10.1002/ejp.1037. https://pubmed.ncbi.nlm.nih.gov/28440001/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 39.Luedtke K., Rushton A., Wright C., Jürgens T., Polzer A., Mueller G., et al. Effectiveness of transcranial direct current stimulation preceding cognitive behavioural management for chronic low back pain: sham controlled double blinded randomised controlled trial. BMJ. 2015;350 doi: 10.1136/bmj.h1640. https://pubmed.ncbi.nlm.nih.gov/25883244/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avery D.H., Zarkowski P., Krashin D., Rho W.K., Wajdik C., Joesch J.M., et al. Transcranial magnetic stimulation in the treatment of chronic widespread pain: a randomized controlled study. J ECT. 2015;31:57–66. doi: 10.1097/YCT.0000000000000125. https://pubmed.ncbi.nlm.nih.gov/24755729/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McPhee M.E., Graven-Nielsen T. Medial prefrontal high-definition transcranial direct current stimulation to improve pain modulation in chronic low back pain: a pilot randomized double-blinded placebo-controlled crossover trial. J Pain. 2021;22:952–967. doi: 10.1016/j.jpain.2021.02.012. https://pubmed.ncbi.nlm.nih.gov/33676009/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 42.Ahn S., Prim J.H., Alexander M.L., McCulloch K.L., Fröhlich F.I. Identifying and engaging neuronal oscillations by transcranial alternating current stimulation in patients with chronic low back pain: a randomized, crossover, double-blind, sham-controlled pilot study. J Pain. 2019;20:277.e1–277.e11. doi: 10.1016/j.jpain.2018.09.004. https://pubmed.ncbi.nlm.nih.gov/30268803/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Attal N., Ayache S., Ciampi de Andrade D., Mhalla A., Baudic S., Jazat F., et al. Comparison of the analgesic effects of RTMS and TDCS in painful radiculopathy: a randomized double blind placebo controlled study. J Neurol Sci. 2015;357:e357. http://www.jns-journal.com/article/S0022510X15017748/fulltext [Internet] [cited 2023 Aug 25]. Available from: [Google Scholar]
- 44.Hargrove J.B., Bennett R.M., Simons D.G., Smith S.J., Nagpal S., Deering D.E. A randomized placebo-controlled study of noninvasive cortical electrostimulation in the treatment of fibromyalgia patients. Pain Med. 2012;13:115–124. doi: 10.1111/j.1526-4637.2011.01292.x. https://pubmed.ncbi.nlm.nih.gov/22233397/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 45.O'Connell N.E., Marston L., Spencer S., DeSouza L.H., Wand B.M. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2018;4 doi: 10.1002/14651858.CD008208.pub5. https://pubmed.ncbi.nlm.nih.gov/29652088/ [Internet] [cited 2023 Aug 26]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fregni F., Gimenes R., Valle A.C., Ferreira M.J., Rocha R.R., Natalle L., et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54:3988–3998. doi: 10.1002/art.22195. https://pubmed.ncbi.nlm.nih.gov/17133529/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 47.Valle A., Roizenblatt S., Botte S., Zaghi S., Riberto M., Tufik S. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trial. J Pain Manag. 2009;2:353. https://pmc/articles/PMC3002117/ [Internet] [cited 2023 Aug 24]. Available from:/ [PMC free article] [PubMed] [Google Scholar]
- 48.Riberto M., Marcon Alfieri F., Monteiro de Benedetto Pacheco K., Dini Leite V., Nemoto Kaihami H., Fregni F., et al. Efficacy of transcranial direct current stimulation coupled with a multidisciplinary rehabilitation program for the treatment of fibromyalgia. Open Rheumatol J. 2011;5:45–50. doi: 10.2174/1874312901105010045. https://pubmed.ncbi.nlm.nih.gov/22046206/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fagerlund A.J., Hansen O.A., Aslaksen P.M. Transcranial direct current stimulation as a treatment for patients with fibromyalgia: a randomized controlled trial. Pain. 2015;156:62–71. doi: 10.1016/j.pain.0000000000000006. https://pubmed.ncbi.nlm.nih.gov/25599302/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 50.Foerster B.R., Nascimento T.D., DeBoer M., Bender M.A., Rice I.C., Truong D.Q., et al. Excitatory and inhibitory brain metabolites as targets of motor cortex transcranial direct current stimulation therapy and predictors of its efficacy in fibromyalgia. Arthritis Rheumatol. 2015;67:576–581. doi: 10.1002/art.38945. https://pubmed.ncbi.nlm.nih.gov/25371383/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jales Junior L.H., Costa do M.D.L., Jales Neto L.H., Ribeiro J.P.M., Freitas Wjs do N., Teixeira M.J. Transcranial direct current stimulation in fibromyalgia: effects on pain and quality of life evaluated clinically and by brain perfusion scintigraphy. Revista Dor. 2015;16:37–42. https://www.scielo.br/j/rdor/a/m4FbqdSqNxt9gtbMqDM7zrK/?lang=en [Internet] [cited 2023 Aug 24]. Available from: [Google Scholar]
- 52.Khedr E.M., Omran E.A.H., Ismail N.M., El-Hammady D.H., Goma S.H., Kotb H., et al. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: a double blinded, randomized clinical trial. Brain Stimul. 2017;10:893–901. doi: 10.1016/j.brs.2017.06.006. https://pubmed.ncbi.nlm.nih.gov/28684258/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 53.Mendonca M.E., Santana M.B., Baptista A.F., Datta A., Bikson M., Fregni F., et al. Transcranial DC stimulation in fibromyalgia: optimized cortical target supported by high-resolution computational models. J Pain. 2011;12:610–617. doi: 10.1016/j.jpain.2010.12.015. https://pubmed.ncbi.nlm.nih.gov/21497140/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 54.Samartin-Veiga N., González-Villar A.J., Pidal-Miranda M., Vázquez-Millán A., Carrillo-de-la-Peña M.T. Active and sham transcranial direct current stimulation (tDCS) improved quality of life in female patients with fibromyalgia. Qual Life Res. 2022;31:2519–2534. doi: 10.1007/s11136-022-03106-1. https://pubmed.ncbi.nlm.nih.gov/35229253/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Villamar M.F., Wivatvongvana P., Patumanond J., Bikson M., Truong D.Q., Datta A., et al. Focal modulation of the primary motor cortex in fibromyalgia using 4×1-ring high-definition transcranial direct current stimulation (HD-tDCS): immediate and delayed analgesic effects of cathodal and anodal stimulation. J Pain. 2013;14:371–383. doi: 10.1016/j.jpain.2012.12.007. https://pubmed.ncbi.nlm.nih.gov/23415877/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 56.Boyer L., Dousset A., Roussel P., Dossetto N., Cammilleri S., Piano V., et al. rTMS in fibromyalgia: a randomized trial evaluating QoL and its brain metabolic substrate. Neurology. 2014;82:1231–1238. doi: 10.1212/WNL.0000000000000280. https://pubmed.ncbi.nlm.nih.gov/24670891/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 57.Carretero B., Martín M.J., Juan A., Pradana M.L., Martín B., Carral M., et al. Low-frequency transcranial magnetic stimulation in patients with fibromyalgia and major depression. Pain Med. 2009;10:748–753. doi: 10.1111/j.1526-4637.2009.00625.x. https://pubmed.ncbi.nlm.nih.gov/19460131/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 58.Lee S.J., Kim D.Y., Chun M.H., Kim Y.G. The effect of repetitive transcranial magnetic stimulation on fibromyalgia: a randomized sham-controlled trial with 1-mo follow-up. Am J Phys Med Rehabil. 2012;91:1077–1085. doi: 10.1097/PHM.0b013e3182745a04. https://pubmed.ncbi.nlm.nih.gov/23159954/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 59.Tzabazis A., Aparici C.M., Rowbotham M.C., Schneider M.B., Etkin A., Yeomans D.C. Shaped magnetic field pulses by multi-coil repetitive transcranial magnetic stimulation (rTMS) differentially modulate anterior cingulate cortex responses and pain in volunteers and fibromyalgia patients. Mol Pain. 2013;9 doi: 10.1186/1744-8069-9-33. https://pubmed.ncbi.nlm.nih.gov/23819466/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schein B., Beltran G., França B.R., Sanches P.R.S., Silva D.P., Jr., Torres I.L., et al. Effects of hypnotic analgesia and transcranial direct current stimulation on pain tolerance and corticospinal excitability in individuals with fibromyalgia: a cross-over randomized clinical trial. J Pain Res. 2023;16:187–203. doi: 10.2147/JPR.S384373. https://pubmed.ncbi.nlm.nih.gov/36718400/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loreti E.H., Freire A.M., Alexandre da Silva A., Kakuta E., Martins Neto U.R., Konkiewitz E.C. Effects of anodal transcranial direct current stimulation on the primary motor cortex in women with fibromyalgia: a randomized, triple-blind clinical trial. Neuromodulation. 2023;26 doi: 10.1016/j.neurom.2022.11.007. https://pubmed.ncbi.nlm.nih.gov/36702675/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 62.Paula TMH de, Castro M.S., Medeiros L.F., Paludo R.H., Couto F.F., Costa T.R.D., et al. Association of low-dose naltrexone and transcranial direct current stimulation in fibromyalgia: a randomized, double-blinded, parallel clinical trial. Braz J Anesthesiol. 2023;73 doi: 10.1016/j.bjane.2022.08.003. https://pubmed.ncbi.nlm.nih.gov/35988815/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bocci T., De Carolis G., Ferrucci R., Paroli M., Mansani F., Priori A., et al. Cerebellar transcranial direct current stimulation (ctDCS) ameliorates phantom limb pain and non-painful phantom limb sensations. Cerebellum. 2019;18:527–535. doi: 10.1007/s12311-019-01020-w. https://pubmed.ncbi.nlm.nih.gov/30830672/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 64.Malavera M., Silva F., García R., Quiros J., Dallos M., Pinzón A. Effects of transcranial magnetic stimulation in the treatment of phantom limb pain in landmine/INS;victims: a randomized clinical trial. J Neurol Sci. 2013;333:e534. http://www.jns-journal.com/article/S0022510X13021916/fulltext [Internet] [cited 2023 Aug 24]. Available from: [Google Scholar]
- 65.Irlbacher K., Kuhnert J., Röricht S., Meyer B.U., Brandt S.A. Central and peripheral deafferent pain: therapy with repetitive transcranial magnetic stimulation. Nervenarzt. 2006;77:1196–1203. doi: 10.1007/s00115-006-2148-1. https://pubmed.ncbi.nlm.nih.gov/16955313/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 66.Ahmed M.A., Mohamed S.A., Sayed D. Long-term antalgic effects of repetitive transcranial magnetic stimulation of motor cortex and serum beta-endorphin in patients with phantom pain. Neurol Res. 2011;33:953–958. doi: 10.1179/1743132811Y.0000000045. https://pubmed.ncbi.nlm.nih.gov/22080997/ [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 67.Fregni F., Boggio P.S., Lima M.C., Ferreira M.J., Wagner T., Rigonatti S.P., et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122:197–209. doi: 10.1016/j.pain.2006.02.023. https://pubmed.ncbi.nlm.nih.gov/16564618/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 68.Soler M.D., Kumru H., Pelayo R., Vidal J., Tormos J.M., Fregni F., et al. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain. 2010 Sep;133(9):2565–2577. doi: 10.1093/brain/awq184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wrigley P.J., Gustin S.M., McIndoe L.N., Chakiath R.J., Henderson L.A., Siddall P.J., et al. Longstanding neuropathic pain after spinal cord injury is refractory to transcranial direct current stimulation: a randomized controlled trial. Pain. 2013;154:2178–2184. doi: 10.1016/j.pain.2013.06.045. https://pubmed.ncbi.nlm.nih.gov/23831866/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 70.Yoon E.J., Kim Y.K., Kim H.R., Kim S.E., Lee Y., Shin H.I. Transcranial direct current stimulation to lessen neuropathic pain after spinal cord injury: a mechanistic PET study. Neurorehabil Neural Repair. 2014;28:250–259. doi: 10.1177/1545968313507632. https://pubmed.ncbi.nlm.nih.gov/24213958/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 71.Thibaut A., Carvalho S., Morse L.R., Zafonte R., Fregni F. Delayed pain decrease following M1 tDCS in spinal cord injury: a randomized controlled clinical trial. Neurosci Lett. 2017;658:19–26. doi: 10.1016/j.neulet.2017.08.024. https://pubmed.ncbi.nlm.nih.gov/28822837/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 72.Jetté F., Côté I., Meziane H.B., Mercier C. Effect of single-session repetitive transcranial magnetic stimulation applied over the hand versus leg motor area on pain after spinal cord injury. Neurorehabil Neural Repair. 2013;27:636–643. doi: 10.1177/1545968313484810. https://pubmed.ncbi.nlm.nih.gov/23579183/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 73.Kang B.S., Shin H.I., Bang M.S. Effect of repetitive transcranial magnetic stimulation over the hand motor cortical area on central pain after spinal cord injury. Arch Phys Med Rehabil. 2009;90:1766–1771. doi: 10.1016/j.apmr.2009.04.008. https://pubmed.ncbi.nlm.nih.gov/19801069/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 74.Nardone R., Höller Y., Langthaler P.B., Lochner P., Golaszewski S., Schwenker K., et al. rTMS of the prefrontal cortex has analgesic effects on neuropathic pain in subjects with spinal cord injury. Spinal Cord. 2017;55:20–25. doi: 10.1038/sc.2016.87. https://pubmed.ncbi.nlm.nih.gov/27241450/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 75.Yílmaz B., Kesikburun S., Yaşar E., Tan A.K. The effect of repetitive transcranial magnetic stimulation on refractory neuropathic pain in spinal cord injury. J Spinal Cord Med. 2014;37:397–400. doi: 10.1179/2045772313Y.0000000172. https://pubmed.ncbi.nlm.nih.gov/24621025/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Defrin R., Grunhaus L., Zamir D., Zeilig G. The effect of a series of repetitive transcranial magnetic stimulations of the motor cortex on central pain after spinal cord injury. Arch Phys Med Rehabil. 2007;88:1574–1580. doi: 10.1016/j.apmr.2007.07.025. https://pubmed.ncbi.nlm.nih.gov/18047871/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 77.Kim Y.J., Ku J., Kim H.J., Im D.J., Lee H.S., Han K.A., et al. Randomized, sham controlled trial of transcranial direct current stimulation for painful diabetic polyneuropathy. Ann Rehabil Med. 2013;37:766–776. doi: 10.5535/arm.2013.37.6.766. https://pubmed.ncbi.nlm.nih.gov/24466511/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Attal N., Ayache S.S., Ciampi De Andrade D., Baudic S., Jazat F., Ahdab R., et al. Repetitive transcranial magnetic stimulation and transcranial direct-current stimulation in neuropathic pain due to radiculopathy: a randomized sham-controlled comparative study. Pain. 2016;157:1224–1231. doi: 10.1097/j.pain.0000000000000510. https://pubmed.ncbi.nlm.nih.gov/26845524/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 79.Borckardt J.J., Smith A.R., Reeves S.T., Madan A., Shelley N., Branham R., et al. A pilot study investigating the effects of fast left prefrontal rTMS on chronic neuropathic pain. Pain Med. 2009;10:840–849. doi: 10.1111/j.1526-4637.2009.00657.x. https://pubmed.ncbi.nlm.nih.gov/19594842/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Onesti E., Gabriele M., Cambieri C., Ceccanti M., Raccah R., Di Stefano G., et al. H-coil repetitive transcranial magnetic stimulation for pain relief in patients with diabetic neuropathy. Eur J Pain. 2013;17:1347–1356. doi: 10.1002/j.1532-2149.2013.00320.x. https://pubmed.ncbi.nlm.nih.gov/23629867/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 81.Antal A., Terney D., Kühnl S., Paulus W. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J Pain Symptom Manag. 2010;39:890–903. doi: 10.1016/j.jpainsymman.2009.09.023. https://pubmed.ncbi.nlm.nih.gov/20471549/ [Internet] [cited 2023 Aug 24]. Available from: [DOI] [PubMed] [Google Scholar]
- 82.Harvey M.P., Lorrain D., Martel M., Bergeron-Vezina K., Houde F., Séguin M., et al. Can we improve pain and sleep in elderly individuals with transcranial direct current stimulation? - results from a randomized controlled pilot study. Clin Interv Aging. 2017;12:937–947. doi: 10.2147/CIA.S133423. https://pubmed.ncbi.nlm.nih.gov/28652716/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.André-Obadia N., Magnin M., Garcia-Larrea L. On the importance of placebo timing in rTMS studies for pain relief. Pain. 2011;152:1233–1237. doi: 10.1016/j.pain.2010.12.027. https://pubmed.ncbi.nlm.nih.gov/21342747/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 84.Nurmikko T., MacIver K., Bresnahan R., et al. Motor cortex reorganization and repetitive transcranial magnetic stimulation for pain-A methodological study. Neuromodulation. 2016;19:669–678. doi: 10.1111/ner.12444. https://pubmed.ncbi.nlm.nih.gov/27187056/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 85.Lefaucheur J.P., Drouot X., Ménard-Lefaucheur I., Keravel Y., Nguyen J.P. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology. 2006;67:1568–1574. doi: 10.1212/01.wnl.0000242731.10074.3c. https://pubmed.ncbi.nlm.nih.gov/17101886/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 86.Rollnik J.D., Wüstefeld S., Däuper J., Karst M., Fink M., Kossev A., et al. Repetitive transcranial magnetic stimulation for the treatment of chronic pain - a pilot study. Eur Neurol. 2002;48:6–10. doi: 10.1159/000064950. https://pubmed.ncbi.nlm.nih.gov/12138303/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 87.André-Obadia N., Peyron R., Mertens P., Mauguière F., Laurent B., Garcia-Larrea L. Transcranial magnetic stimulation for pain control. Double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clin Neurophysiol. 2006;117:1536–1544. doi: 10.1016/j.clinph.2006.03.025. https://pubmed.ncbi.nlm.nih.gov/16753335/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 88.Martin E., Jeanmonod D., Morel A., Zadicario E., Werner B., et al. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol. 2009;66:858–861. doi: 10.1002/ana.21801. https://pubmed.ncbi.nlm.nih.gov/20033983/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 89.Jeanmonod D., Werner B., Morel A., Michels L., Zadicario E., Schiff G., et al. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg Focus. 2012;32 doi: 10.3171/2011.10.FOCUS11248. https://pubmed.ncbi.nlm.nih.gov/22208894/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 90.Ayache S.S., Palm U., Chalah M.A., Al-Ani T., Brignol A., Abdellaoui M., et al. Prefrontal tDCS decreases pain in patients with multiple sclerosis. Front Neurosci. 2016;10 doi: 10.3389/fnins.2016.00147. https://pubmed.ncbi.nlm.nih.gov/27092048/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mori F., Codecà C., Kusayanagi H., Monteleone F., Buttari F., Fiore S. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J Pain. 2010;11:436–442. doi: 10.1016/j.jpain.2009.08.011. https://pubmed.ncbi.nlm.nih.gov/20018567/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 92.Souto G., Borges I.C., Goes B.T., de Mendonça M.E., Gonçalves R.G., Garcia L.B., et al. Effects of tDCS-induced motor cortex modulation on pain in HTLV-1: a blind randomized clinical trial. Clin J Pain. 2014;30:809–815. doi: 10.1097/AJP.0000000000000037. https://pubmed.ncbi.nlm.nih.gov/24300224/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 93.Quintiliano A., Bikson M., Oehmen T., Pegado R., Kirsztajn G.M. Transcranial direct current stimulation (tDCS): pain management in end-stage renal disease - report of an early randomized controlled trial. J Pain Symptom Manag. 2022;64:234–243.e1. doi: 10.1016/j.jpainsymman.2022.05.018. https://pubmed.ncbi.nlm.nih.gov/35640767/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 94.De Souza C.G., Pegado R., Costa J., Morya E., Baptista A.F., Unal G., et al. Alternate sessions of transcranial direct current stimulation (tDCS) reduce chronic pain in women affected by chikungunya. A randomized clinical trial. Brain Stimul. 2021;14:541–548. doi: 10.1016/j.brs.2021.02.015. https://pubmed.ncbi.nlm.nih.gov/33667699/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 95.Young J., Zoghi M., Khan F., Galea M.P. The effect of transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis: randomized controlled trial. Pain Med. 2020;21:3451–3457. doi: 10.1093/pm/pnaa128. https://pubmed.ncbi.nlm.nih.gov/32594139/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 96.Bae S.H., Kim Do G., Kim K.Y. Analgesic effect of transcranial direct current stimulation on central post-stroke pain. Tohoku J Exp Med. 2014;234:189–195. doi: 10.1620/tjem.234.189. https://pubmed.ncbi.nlm.nih.gov/25341455/ [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 97.De Oliveira R.A.A., De Andrade D.C., Mendonça M., Barros R., Luvisoto T., Myczkowski M.L., et al. Repetitive transcranial magnetic stimulation of the left premotor/dorsolateral prefrontal cortex does not have analgesic effect on central poststroke pain. J Pain. 2014;15:1271–1281. doi: 10.1016/j.jpain.2014.09.009. https://pubmed.ncbi.nlm.nih.gov/25267523/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 98.Brietzke A.P., Rozisky J.R., Dussan-Sarria J.A., Deitos A., Laste G., Hoppe P.F., et al. Neuroplastic effects of transcranial direct current stimulation on painful symptoms reduction in chronic hepatitis C: a phase II randomized, double blind, sham controlled trial. Front Neurosci. 2016;9 doi: 10.3389/fnins.2015.00498. https://pubmed.ncbi.nlm.nih.gov/26793047/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fregni F., Potvin K., Dasilva D., Wang X., Lenkinski R.E., Freedman S.D., et al. Clinical effects and brain metabolic correlates in non-invasive cortical neuromodulation for visceral pain. Eur J Pain. 2011;15:53–60. doi: 10.1016/j.ejpain.2010.08.002. https://pubmed.ncbi.nlm.nih.gov/20822942/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fregni F., DaSilva D., Potvin K., Ramos-Estebanez C., Cohen D., Pascual-Leone A., et al. Treatment of chronic visceral pain with brain stimulation. Ann Neurol. 2005;58:971–972. doi: 10.1002/ana.20651. https://pubmed.ncbi.nlm.nih.gov/16315281/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 101.Fenton B.W., Palmieri P.A., Boggio P., Fanning J., Fregni F. A preliminary study of transcranial direct current stimulation for the treatment of refractory chronic pelvic pain. Brain Stimul. 2009;2:103–107. doi: 10.1016/j.brs.2008.09.009. https://pubmed.ncbi.nlm.nih.gov/20633407/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 102.Volz M.S., Farmer A., Siegmund B. Reduction of chronic abdominal pain in patients with inflammatory bowel disease through transcranial direct current stimulation: a randomized controlled trial. Pain. 2016;157:429–437. doi: 10.1097/j.pain.0000000000000386. https://pubmed.ncbi.nlm.nih.gov/26469395/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 103.Mechsner S., Grünert J., Wiese J.J., Vormbäumen J., Sehouli J., Siegmund B., et al. Transcranial direct current stimulation to reduce chronic pelvic pain in endometriosis: phase II randomized controlled clinical trial. Pain Med. 2023;24 doi: 10.1093/pm/pnad031. https://pubmed.ncbi.nlm.nih.gov/36882181/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 104.Ahn H., Woods A.J., Choi E., Padhye N., Fillingim R. Transcranial direct current stimulation and mobility functioning in older adults with knee osteoarthritis pain: a double-blind, randomized, sham-controlled pilot clinical study. Brain Stimul. 2017;10:e21. doi: 10.1016/j.brs.2017.05.007. http://www.brainstimjrnl.com/article/S1935861X17306654/fulltext [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chang W.J., Bennell K.L., Hodges P.W., Hinman R.S., Young C.L., Buscemi V., et al. Addition of transcranial direct current stimulation to quadriceps strengthening exercise in knee osteoarthritis: a pilot randomised controlled trial. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180328. https://pubmed.ncbi.nlm.nih.gov/28665989/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tavares D.R.B., Okazaki J.E.F., Santana MV. de A., Pinto A.C.P.N., Tutiya K.K., Gazoni F.M., et al. Motor cortex transcranial direct current stimulation effects on knee osteoarthritis pain in elderly subjects with dysfunctional descending pain inhibitory system: a randomized controlled trial. Brain Stimul. 2021;14:477–487. doi: 10.1016/j.brs.2021.02.018. https://pubmed.ncbi.nlm.nih.gov/33684598/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 107.Lagueux E., Bernier M., Bourgault P., Whittingstall K., Mercier C., Léonard G., et al. The effectiveness of transcranial direct current stimulation as an add-on modality to graded motor imagery for treatment of complex regional pain syndrome: a randomized proof of concept study. Clin J Pain. 2018;34:145–154. doi: 10.1097/AJP.0000000000000522. https://pubmed.ncbi.nlm.nih.gov/28654557/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 108.Larrivée S., Balg F., Léonard G., Bédard S., Tousignant M., Boissy P. Transcranial direct current stimulation (a-tCDS) after subacromial injections in patients with subacromial pain syndrome: a randomized controlled pilot study. BMC Muscoskel Disord. 2021;22 doi: 10.1186/s12891-021-04139-2. https://pubmed.ncbi.nlm.nih.gov/33706729/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Picarelli H., Teixeira M.J., De Andrade D.C., Myczkowski M.L., Luvisotto T.B., Yeng L.T., et al. Repetitive transcranial magnetic stimulation is efficacious as an add-on to pharmacological therapy in complex regional pain syndrome (CRPS) type I. J Pain. 2010;11:1203–1210. doi: 10.1016/j.jpain.2010.02.006. https://pubmed.ncbi.nlm.nih.gov/20430702/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 110.Pleger B., Janssen F., Schwenkreis P., Völker B., Maier C., Tegenthoff M. Repetitive transcranial magnetic stimulation of the motor cortex attenuates pain perception in complex regional pain syndrome type I. Neurosci Lett. 2004;356:87–90. doi: 10.1016/j.neulet.2003.11.037. https://pubmed.ncbi.nlm.nih.gov/14746870/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 111.Umezaki Y., Badran B.W., Devries W.H., Moss J., Gonzales T., George M.S. The efficacy of daily prefrontal repetitive transcranial magnetic stimulation (rTMS) for burning mouth syndrome (BMS): a randomized controlled single-blind study. Brain Stimul. 2016;9:234–242. doi: 10.1016/j.brs.2015.10.005. https://pubmed.ncbi.nlm.nih.gov/26597930/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 112.Sakrajai P., Janyacharoen T., Jensen M.P., Sawanyawisuth K., Auvichayapat N., Tunkamnerdthai O., et al. Pain reduction in myofascial pain syndrome by anodal transcranial direct current stimulation combined with standard treatment: a randomized controlled study. Clin J Pain. 2014;30:1076–1083. doi: 10.1097/AJP.0000000000000069. https://pubmed.ncbi.nlm.nih.gov/25373724/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dall'Agnol L., Medeiros L.F., Torres I.L.S., Deitos A., Brietzke A., Laste G., et al. Repetitive transcranial magnetic stimulation increases the corticospinal inhibition and the brain-derived neurotrophic factor in chronic myofascial pain syndrome: an explanatory double-blinded, randomized, sham-controlled trial. J Pain. 2014;15:845–855. doi: 10.1016/j.jpain.2014.05.001. https://pubmed.ncbi.nlm.nih.gov/24865417/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PubMed] [Google Scholar]
- 114.Hagenacker T., Bude V., Naegel S., Holle D., Katsarava Z., Diener H.C., et al. Patient-conducted anodal transcranial direct current stimulation of the motor cortex alleviates pain in trigeminal neuralgia. J Headache Pain. 2014;15 doi: 10.1186/1129-2377-15-78. https://pubmed.ncbi.nlm.nih.gov/25424567/ [Internet] [cited 2023 Aug 25]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chang W.J., O'Connell N.E., Beckenkamp P.R., Alhassani G., Liston M.B., Schabrun S.M. Altered primary motor cortex structure, organization, and function in chronic pain: a systematic review and meta-analysis. J Pain. 2018;19:341–359. doi: 10.1016/j.jpain.2017.10.007. https://pubmed.ncbi.nlm.nih.gov/29155209/ [Internet] [cited 2023 Feb 28]. Available from: [DOI] [PubMed] [Google Scholar]
- 116.Corti E.J., Marinovic W., Nguyen A.T., Gasson N., Loftus A.M. Motor cortex excitability in chronic low back pain. Exp Brain Res. 2022;240:3249. doi: 10.1007/s00221-022-06492-7. https://pmc/articles/PMC9678990/ [Internet] [cited 2023 Mar 2]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoogendam J.M., Ramakers G.M.J., Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3:95–118. doi: 10.1016/j.brs.2009.10.005. https://pubmed.ncbi.nlm.nih.gov/20633438/ [cited 2023 Mar 1]. Available from: [DOI] [PubMed] [Google Scholar]
- 118.Stagg C.J., Best J.G., Stephenson M.C., O'Shea J., Wylezinska M., Kincses Z.T., et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29:5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. https://pubmed.ncbi.nlm.nih.gov/19386916/ [Internet] [cited 2023 Mar 1]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seminowicz D.A., Moayedi M. The dorsolateral prefrontal cortex in acute and chronic pain. J Pain. 2017;18:1027–1035. doi: 10.1016/j.jpain.2017.03.008. https://pubmed.ncbi.nlm.nih.gov/28400293/ [cited 2023 Jun 25]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brasil-Neto J.P. Learning, memory, and transcranial direct current stimulation. Front Psychiatr. 2012;3 doi: 10.3389/fpsyt.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Silva A.F., Zortea M., Carvalho S., Leite J., Torres I.L., Fregni F., et al. Anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex modulates attention and pain in fibromyalgia: randomized clinical trial. Sci Rep. 2017;7:1–11. doi: 10.1038/s41598-017-00185-w. https://pubmed.ncbi.nlm.nih.gov/28273933/ [Internet] [cited 2023 Aug 26]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alcon C.A., Wang-Price S. Non-invasive brain stimulation and pain neuroscience education in the cognitive-affective treatment of chronic low back pain: evidence and future directions. Front Pain Res. 2022;3 doi: 10.3389/fpain.2022.959609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lefaucheur J.P., Wendling F. Mechanisms of action of tDCS: a brief and practical overview. Neurophysiol Clin. 2019;49:269–275. doi: 10.1016/j.neucli.2019.07.013. https://pubmed.ncbi.nlm.nih.gov/31350060/ [cited 2023 Aug 26]. Available from: [DOI] [PubMed] [Google Scholar]
- 124.Moisset X., De Andrade D.C., Bouhassira D. From pulses to pain relief: an update on the mechanisms of rTMS-induced analgesic effects. Eur J Pain. 2016;20:689–700. doi: 10.1002/ejp.811. https://pubmed.ncbi.nlm.nih.gov/26471248/ [cited 2023 Aug 26]. Available from: [DOI] [PubMed] [Google Scholar]
- 125.Alcon C.A., Wang-Price S. Non-invasive brain stimulation and pain neuroscience education in the cognitive-affective treatment of chronic low back pain: evidence and future directions. Front Pain Res. 2022:3. doi: 10.3389/fpain.2022.959609. https://pmc/articles/PMC9686004/ [cited 2023 Aug 27]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Che X., Cash R.F.H., Luo X., Luo H., Lu X., Xu F., et al. High-frequency rTMS over the dorsolateral prefrontal cortex on chronic and provoked pain: a systematic review and meta-analysis. Brain Stimul. 2021;14:1135–1146. doi: 10.1016/j.brs.2021.07.004. https://pubmed.ncbi.nlm.nih.gov/34280583/ [Internet] [cited 2023 Mar 3]. Available from: [DOI] [PubMed] [Google Scholar]
- 127.Zhu Y., Li D., Zhou Y., Hu Y., Xu Z., Lei L., et al. Systematic review and meta-analysis of high-frequency rTMS over the dorsolateral prefrontal cortex .on chronic pain and chronic-pain-accompanied depression. ACS Chem Neurosci. 2022 doi: 10.1021/acschemneuro.2c00395. https://pubmed.ncbi.nlm.nih.gov/35969469/ [Internet] [cited 2023 Mar 16]. Available from: [DOI] [PubMed] [Google Scholar]
- 128.Lançon K., Qu C., Navratilova E., Porreca F., Séguéla P. Decreased dopaminergic inhibition of pyramidal neurons in anterior cingulate cortex maintains chronic neuropathic pain. Cell Rep. 2021;37:109933. doi: 10.1016/j.celrep.2021.109933. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8728690/ [Internet] [cited 2023 Jul 1]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Urien L., Wang J. Top-down cortical control of acute and chronic pain. Psychosom Med. 2019;81:851–858. doi: 10.1097/PSY.0000000000000744. https://pubmed.ncbi.nlm.nih.gov/31609921/ [cited 2023 Jul 1]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jensen K.B., Regenbogen C., Ohse M.C., Frasnelli J., Freiherr J., Lundström J.N. Brain activations during pain: a neuroimaging meta-analysis of patients with pain and healthy controls. Pain. 2016;157:1279–1286. doi: 10.1097/j.pain.0000000000000517. https://pubmed.ncbi.nlm.nih.gov/26871535/ [Internet] [cited 2023 Jul 1]. Available from: [DOI] [PubMed] [Google Scholar]
- 131.Neugebauer V., Mazzitelli M., Cragg B., Ji G., Navratilova E., Porreca F., et al. Amygdala, neuropeptides, and chronic pain-related affective behaviors. Neuropharmacology. 2020;170:108052. doi: 10.1016/j.neuropharm.2020.108052. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7214122/ [Internet] [cited 2023 Jul 1]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jones E.G. Thalamocortical dysrhythmia and chronic pain. Pain. 2010;150:4–5. doi: 10.1016/j.pain.2010.03.022. https://pubmed.ncbi.nlm.nih.gov/20395046/ [cited 2023 Apr 11]. Available from: [DOI] [PubMed] [Google Scholar]
- 133.Ahn S., Prim J.H., Alexander M.L., McCulloch K.L., Fröhlich F. Identifying and engaging neuronal oscillations by transcranial alternating current stimulation in patients with chronic low back pain: a randomized, crossover, double-blind, sham-controlled pilot study. J Pain. 2019;20:277.e1. doi: 10.1016/j.jpain.2018.09.004. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6382517/ [Internet] [cited 2023 Apr 11]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Borsook D., Upadhyay J., Chudler E.H., Becerra L. A key role of the basal ganglia in pain and analgesia - insights gained through human functional imaging. Mol Pain. 2010;6:27. doi: 10.1186/1744-8069-6-27. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2883978/ [Internet] [cited 2023 Jul 1]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Azqueta-Gavaldon M., Youssef A.M., Storz C., Lemme J., Schulte-Göcking H., Becerra L., et al. Implications of the putamen in pain and motor deficits in complex regional pain syndrome. Pain. 2020;161:595. doi: 10.1097/j.pain.0000000000001745. https://pubmed.ncbi.nlm.nih.gov/31693538/ [Internet] [cited 2023 Jul 1]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alamri A., Pereira E.A.C. Deep brain stimulation for chronic pain. Neurosurg Clin. 2022;33:311–321. doi: 10.1016/j.nec.2022.02.013. https://pubmed.ncbi.nlm.nih.gov/35718401/ [cited 2023 Jul 1]. Available from: [DOI] [PubMed] [Google Scholar]
- 137.Russo J.F., Sheth S.A. Deep brain stimulation of the dorsal anterior cingulate cortex for the treatment of chronic neuropathic pain. Neurosurg Focus. 2015;38 doi: 10.3171/2015.3.FOCUS1543. https://pubmed.ncbi.nlm.nih.gov/26030699/ [cited 2023 Jul 1]. Available from: [DOI] [PubMed] [Google Scholar]
- 138.Boccard S.G.J., Pereira E.A.C., Moir L., Van Hartevelt T.J., Kringelbach M.L., FitzGerald J.J., et al. Deep brain stimulation of the anterior cingulate cortex: targeting the affective component of chronic pain. Neuroreport. 2014;25:83–88. doi: 10.1097/WNR.0000000000000039. https://pubmed.ncbi.nlm.nih.gov/24100411/ [Internet] [cited 2023 Jul 1]. Available from: [DOI] [PubMed] [Google Scholar]
- 139.Lee D.J., Lozano C.S., Dallapiazza R.F., Lozano A.M. Current and future directions of deep brain stimulation for neurological and psychiatric disorders. J Neurosurg. 2019;131:333–342. doi: 10.3171/2019.4.JNS181761. https://pubmed.ncbi.nlm.nih.gov/31370011/ [Internet] [cited 2023 Apr 16]. Available from: [DOI] [PubMed] [Google Scholar]
- 140.Huang Y., Parra L.C. Can transcranial electric stimulation with multiple electrodes reach deep targets? Brain Stimul. 2019;12:30–40. doi: 10.1016/j.brs.2018.09.010. https://pubmed.ncbi.nlm.nih.gov/30297323/ [cited 2023 May 18]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kregel J., Meeus M., Malfliet A., Dolphens M., Danneels L., Nijs J., et al. Structural and functional brain abnormalities in chronic low back pain: a systematic review. Semin Arthritis Rheum. 2015;45:229–237. doi: 10.1016/j.semarthrit.2015.05.002. https://pubmed.ncbi.nlm.nih.gov/26092329/ [Internet] [cited 2023 Feb 18]. Available from: [DOI] [PubMed] [Google Scholar]
- 142.Zhang S.S., Wu W., Huang G.Z., Liu Z., Guo S., Yang J., et al. Resting-state connectivity in the default mode network and insula during experimental low back pain. Neural Regen Res. 2014;9:135–142. doi: 10.4103/1673-5374.125341. https://pubmed.ncbi.nlm.nih.gov/25206794/ [Internet] [cited 2023 Feb 18]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jones S.A., Morales A.M., Holley A.L., Wilson A.C., Nagel B.J. Default mode network connectivity is related to pain frequency and intensity in adolescents. Neuroimage Clin. 2020;27:102326. doi: 10.1016/j.nicl.2020.102326. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7338779/ [Internet] [cited 2023 Mar 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hunt C.A., Letzen J.E., Krimmel S.R., Burrowes S.A.B., Haythornthwaite J.A., Finan P.H., et al. Is mindfulness associated with lower pain reactivity and connectivity of the default mode network? A replication and extension study in healthy and episodic migraine participants. J Pain. 2022;23:2110–2120. doi: 10.1016/j.jpain.2022.07.011. https://pubmed.ncbi.nlm.nih.gov/35934277/ [Internet] [cited 2023 Mar 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cunningham N.R., Nahman-Averbuch H., Lee G.R., King C.D., Coghill R.C. Amygdalar functional connectivity during resting and evoked pain in youth with functional abdominal pain disorders. Pain. 2022;163:2031–2043. doi: 10.1097/j.pain.0000000000002601. https://pubmed.ncbi.nlm.nih.gov/35472070/ [Internet] [cited 2023 Mar 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Čeko M., Frangos E., Gracely J., Richards E., Wang B., Schweinhardt P., et al. Default mode network changes in fibromyalgia patients are largely dependent on current clinical pain. Neuroimage. 2020;216 doi: 10.1016/j.neuroimage.2020.116877. https://pubmed.ncbi.nlm.nih.gov/32344063/ [Internet] [cited 2023 Mar 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Andrews-Hanna J.R., Smallwood J., Spreng R.N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. https://pubmed.ncbi.nlm.nih.gov/24502540/ [cited 2023 Feb 18]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kummer K.K., Mitrić M., Kalpachidou T., Kress M. The medial prefrontal cortex as a central hub for mental comorbidities associated with chronic pain. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21103440. https://pubmed.ncbi.nlm.nih.gov/32414089/ [Internet] [cited 2023 Feb 16]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tu Y., Jung M., Gollub R.L., Napadow V., Gerber J., Ortiz A., et al. Abnormal medial prefrontal cortex functional connectivity and its association with clinical symptoms in chronic low back pain. Pain. 2019;160:1308–1318. doi: 10.1097/j.pain.0000000000001507. https://pubmed.ncbi.nlm.nih.gov/31107712/ [Internet] [cited 2023 Feb 18]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Park S.H., Baker A.K., Krishna V., Mackey S.C., Martucci K.T. Altered resting-state functional connectivity within corticostriatal and subcortical-striatal circuits in chronic pain. Sci Rep. 2022;12 doi: 10.1038/s41598-022-16835-7. https://pubmed.ncbi.nlm.nih.gov/35879602/ [Internet] [cited 2023 Mar 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Descalzi G., Mitsi V., Purushothaman I., Gaspari S., Avrampou K., Loh Y.E., et al. Neuropathic pain promotes adaptive changes in gene expression in brain networks involved in stress and depression. Sci Signal. 2017;10 doi: 10.1126/scisignal.aaj1549. https://pubmed.ncbi.nlm.nih.gov/28325815/ [Internet] [cited 2023 Mar 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McPhee M.E., Graven-Nielsen T. Medial prefrontal transcranial direct current stimulation aimed to improve affective and attentional modulation of pain in chronic low back pain patients. J Clin Med. 2021;10:1–17. doi: 10.3390/jcm10040889. https://pubmed.ncbi.nlm.nih.gov/33671714/ [cited 2023 Feb 18]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McPhee M.E., Graven-Nielsen T. Medial prefrontal high-definition transcranial direct current stimulation to improve pain modulation in chronic low back pain: a pilot randomized double-blinded placebo-controlled crossover trial. J Pain. 2021;22:952–967. doi: 10.1016/j.jpain.2021.02.012. https://pubmed.ncbi.nlm.nih.gov/33676009/ [cited 2023 Jun 15]. Available from: [DOI] [PubMed] [Google Scholar]
- 154.Mylius V., Borckardt J.J., Lefaucheur J.P. Noninvasive cortical modulation of experimental pain. Pain. 2012;153:1350–1363. doi: 10.1016/j.pain.2012.04.009. https://pubmed.ncbi.nlm.nih.gov/22633979/ [cited 2023 Feb 19]. Available from: [DOI] [PubMed] [Google Scholar]
- 155.Legrain V., Iannetti G.D., Plaghki L., Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol. 2011;93:111–124. doi: 10.1016/j.pneurobio.2010.10.005. https://pubmed.ncbi.nlm.nih.gov/21040755/ [Internet] [cited 2023 Feb 19]. Available from: [DOI] [PubMed] [Google Scholar]
- 156.Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. https://pubmed.ncbi.nlm.nih.gov/16399806/ [cited 2023 Feb 19]. Available from: [DOI] [PubMed] [Google Scholar]
- 157.González-Roldán A.M., Bomba I.C., Diesch E., Montoya P., Flor H., Kamping S. Controllability and hippocampal activation during pain expectation in fibromyalgia syndrome. Biol Psychol. 2016;121:39–48. doi: 10.1016/j.biopsycho.2016.09.007. https://pubmed.ncbi.nlm.nih.gov/27678310/ [Internet] [cited 2023 Mar 14]. Available from: [DOI] [PubMed] [Google Scholar]
- 158.Iwatsuki K., Hoshiyama M., Yoshida A., Uemura J.I., Hoshino A., Morikawa I., et al. Chronic pain-related cortical neural activity in patients with complex regional pain syndrome. IBRO Neurosci Rep. 2021;10:208–215. doi: 10.1016/j.ibneur.2021.05.001. https://pubmed.ncbi.nlm.nih.gov/34095892/ [Internet] [cited 2023 Mar 14]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bolognini N., Olgiati E., Maravita A., Ferraro F., Fregni F., et al. Motor and parietal cortex stimulation for phantom limb pain and sensations. Pain. 2013;154:1274–1280. doi: 10.1016/j.pain.2013.03.040. https://pubmed.ncbi.nlm.nih.gov/23707312/ [Internet] [cited 2023 Jun 21]. Available from: [DOI] [PubMed] [Google Scholar]
- 160.Otsuru N., Kamijo K., Otsuki T., Kojima S., Miyaguchi S., Saito K., et al. 10 Hz transcranial alternating current stimulation over posterior parietal cortex facilitates tactile temporal order judgment. Behav Brain Res. 2019;368 doi: 10.1016/j.bbr.2019.111899. [DOI] [PubMed] [Google Scholar]
- 161.Kong Q., Sacca V., Zhu M., Ursitti A.K., Kong J. Anatomical and functional connectivity of critical deep brain structures and their potential clinical application in brain stimulation. J Clin Med. 2023;12:4426. doi: 10.3390/jcm12134426. https://pubmed.ncbi.nlm.nih.gov/37445460/ [Internet] [cited 2023 Jul 4]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gu S., Pasqualetti F., Cieslak M., Telesford Q.K., Yu A.B., Kahn A.E., et al. Controllability of structural brain networks. Nat Commun. 2015;6 doi: 10.1038/ncomms9414. https://pubmed.ncbi.nlm.nih.gov/26423222/ [Internet] [cited 2023 May 17]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kucyi A., Moayedi M., Weissman-Fogel I., Hodaie M., Davis K.D. Hemispheric asymmetry in white matter connectivity of the temporoparietal junction with the insula and prefrontal cortex. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035589. https://pubmed.ncbi.nlm.nih.gov/22536413/ [Internet] [cited 2023 Feb 18]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Farrant K., Uddin L.Q. Asymmetric development of dorsal and ventral attention networks in the human brain [cited 2023 Mar 13] Dev Cogn Neurosci. 2015;12:165. doi: 10.1016/j.dcn.2015.02.001. https://pmc/articles/PMC4396619/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bosma R.L., Kim J.A., Cheng J.C., Rogachov A., Hemington K.S., Osborne N.R., et al. Dynamic pain connectome functional connectivity and oscillations reflect multiple sclerosis pain. Pain. 2018;159:2267–2276. doi: 10.1097/j.pain.0000000000001332. https://pubmed.ncbi.nlm.nih.gov/29994989/ [Internet] [cited 2023 Mar 13]. Available from: [DOI] [PubMed] [Google Scholar]
- 166.Hemington K.S., Wu Q., Kucyi A., Inman R.D., Davis K.D., et al. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct Funct. 2016;221:4203–4219. doi: 10.1007/s00429-015-1161-1. https://pubmed.ncbi.nlm.nih.gov/26669874/ [Internet] [cited 2023 Mar 13]. Available from: [DOI] [PubMed] [Google Scholar]
- 167.Donaldson P.H., Rinehart N.J., Enticott P.G. Noninvasive stimulation of the temporoparietal junction: a systematic review. Neurosci Biobehav Rev. 2015;55:547–572. doi: 10.1016/j.neubiorev.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 168.Christopeit M., Simeon D., Urban N., Gowatsky J., Lisanby S.H., Mantovani A., et al. Effects of repetitive transcranial magnetic stimulation (rTMS) on specific symptom clusters in depersonalization disorder (DPD) Brain Stimul. 2014;7:141–143. doi: 10.1016/j.brs.2013.07.006. https://pubmed.ncbi.nlm.nih.gov/23941986/ [Internet] [cited 2023 Jun 27]. Available from: [DOI] [PubMed] [Google Scholar]
- 169.Song J.J., Vanneste S., Van De Heyning P., De Ridder D. Transcranial direct current stimulation in tinnitus patients: a systemic review and meta-analysis. Sci World J. 2012;2012 doi: 10.1100/2012/427941. https://pubmed.ncbi.nlm.nih.gov/23133339/ [Internet] [cited 2023 Jun 27]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Slotema C.W., Blom J.D., Van Lutterveld R., Hoek H.W., Sommer I.E. Review of the efficacy of transcranial magnetic stimulation for auditory verbal hallucinations. Biol Psychiatr. 2014;76:101–110. doi: 10.1016/j.biopsych.2013.09.038. https://pubmed.ncbi.nlm.nih.gov/24315551/ [Internet] [cited 2023 Jun 27]. Available from: [DOI] [PubMed] [Google Scholar]
- 171.Coll M.P., Tremblay M.P.B., Jackson P.L. The effect of tDCS over the right temporo-parietal junction on pain empathy. Neuropsychologia. 2017;100:110–119. doi: 10.1016/j.neuropsychologia.2017.04.021. https://pubmed.ncbi.nlm.nih.gov/28419862/ [cited 2023 Jun 21]. Available from: [DOI] [PubMed] [Google Scholar]
- 172.Han X., Liu X., Li L., Xie B., Fan B., Qiu Y., et al. Neural activation during tonic pain and interaction between pain and emotion in bipolar disorder: an fMRI study. Front Psychiatr. 2018;9 doi: 10.3389/fpsyt.2018.00555. https://pubmed.ncbi.nlm.nih.gov/30459652/ [Internet] [cited 2023 Feb 20]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vachon-Presseau E., Roy M., Martel M.O., Albouy G., Chen J., Budell L., et al. Neural processing of sensory and emotional-communicative information associated with the perception of vicarious pain. Neuroimage. 2012;63:54–62. doi: 10.1016/j.neuroimage.2012.06.030. https://pubmed.ncbi.nlm.nih.gov/22732556/ [Internet] [cited 2023 Feb 23]. Available from: [DOI] [PubMed] [Google Scholar]
- 174.Li Y., Li W., Zhang T., Zhang J., Jin Z., Li L. Probing the role of the right inferior frontal gyrus during Pain-Related empathy processing: evidence from fMRI and TMS. Hum Brain Mapp. 2021;42:1518–1531. doi: 10.1002/hbm.25310. https://pubmed.ncbi.nlm.nih.gov/33283946/ [Internet] [cited 2023 Jun 23]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Eickhoff S.B., Grefkes C., Zilles K., Fink G.R. The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cereb Cortex. 2007;17:1800–1811. doi: 10.1093/cercor/bhl090. https://pubmed.ncbi.nlm.nih.gov/17032710/ [Internet] [cited 2023 Mar 12]. Available from: [DOI] [PubMed] [Google Scholar]
- 176.Lötsch J., Oertel B.G., Felden L., Nöth U., Deichmann R., Hummel T., et al. Central encoding of the strength of intranasal chemosensory trigeminal stimuli in a human experimental pain setting. Hum Brain Mapp. 2020;41:5240–5254. doi: 10.1002/hbm.25190. https://pubmed.ncbi.nlm.nih.gov/32870583/ [Internet] [cited 2023 Feb 19]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kamping S., Andoh J., Bomba I.C., Diers M., Diesch E., Flor H. Contextual modulation of pain in masochists: involvement of the parietal operculum and insula. Pain. 2016;157:445–455. doi: 10.1097/j.pain.0000000000000390. https://pubmed.ncbi.nlm.nih.gov/26808014/ [Internet] [cited 2023 Feb 19]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li T., Zhang S., Kurata J. Suppressed descending pain modulatory and enhanced sensorimotor networks in patients with chronic low back pain. J Anesth. 2018;32:831–843. doi: 10.1007/s00540-018-2561-1. https://link.springer.com/article/10.1007/s00540-018-2561-1 [cited 2023 Aug 27]. Available from: [DOI] [PubMed] [Google Scholar]
- 179.Brumagne S., Diers M., Danneels L., Moseley G.L., Hodges P.W. Neuroplasticity of sensorimotor control in low back pain. J Orthop Sports Phys Ther. 2019;49:402–414. doi: 10.2519/jospt.2019.8489. https://pubmed.ncbi.nlm.nih.gov/31151373/ [Internet] [cited 2023 Aug 27]. Available from: [DOI] [PubMed] [Google Scholar]
- 180.Fregni F., DaSilva D., Potvin K., Ramos-Estebanez C., Cohen D., Pascual-Leone A., et al. Treatment of chronic visceral pain with brain stimulation. Ann Neurol. 2005;58:971–972. doi: 10.1002/ana.20651. https://pubmed.ncbi.nlm.nih.gov/16315281/ [Internet] [cited 2023 Jun 27]. Available from: [DOI] [PubMed] [Google Scholar]
- 181.Valmunen T., Pertovaara A., Taiminen T., Virtanen A., Parkkola R., Jääskeläinen S.K. Modulation of facial sensitivity by navigated rTMS in healthy subjects. Pain. 2009;142:149–158. doi: 10.1016/j.pain.2008.12.031. https://pubmed.ncbi.nlm.nih.gov/19201092/ [Internet] [cited 2023 Jun 27]. Available from: [DOI] [PubMed] [Google Scholar]
- 182.Nakagawa K., Koyama S., Inui K., Tanaka S., Kakigi R., Sadato N. Polarity-independent effects of transcranial direct current stimulation over the bilateral opercular somatosensory region: a magnetoencephalography study. Neuroreport. 2017;28:838–844. doi: 10.1097/WNR.0000000000000845. https://pubmed.ncbi.nlm.nih.gov/28719422/ [Internet] [cited 2023 Jun 27]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Makoshi Z., Kroliczak G., Van Donkelaar P. Human supplementary motor area contribution to predictive motor planning. J Mot Behav. 2011;43:303–309. doi: 10.1080/00222895.2011.584085. https://pubmed.ncbi.nlm.nih.gov/21732868/ [cited 2023 Feb 20]. Available from: [DOI] [PubMed] [Google Scholar]
- 184.Holmes S.A., Kim A., Borsook D. The brain and behavioral correlates of motor-related analgesia (MRA) Neurobiol Dis. 2021;148 doi: 10.1016/j.nbd.2020.105158. https://pubmed.ncbi.nlm.nih.gov/33157210/ [cited 2023 Feb 20]. Available from: [DOI] [PubMed] [Google Scholar]
- 185.Gombaut C., Holmes S.A. Sensorimotor integration and pain perception: mechanisms integrating nociceptive processing. A systematic review and ALE-meta analysis. Front Integr Neurosci. 2022;16 doi: 10.3389/fnint.2022.931292. https://pubmed.ncbi.nlm.nih.gov/35990591/ [cited 2023 Feb 20]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Borckardt J.J., Reeves S.T., Weinstein M., Smith A.R., Shelley N., Kozel F.A., et al. Significant analgesic effects of one session of postoperative left prefrontal cortex repetitive transcranial magnetic stimulation: a replication study. Brain Stimul. 2008;1:122. doi: 10.1016/j.brs.2008.04.002. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2744083/ [Internet] [cited 2023 Jun 29] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ahdab R., Ayache S.S., Brugières P., Goujon C., Lefaucheur J.P. Comparison of “standard” and “navigated” procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiol Clin. 2010;40:27–36. doi: 10.1016/j.neucli.2010.01.001. https://pubmed.ncbi.nlm.nih.gov/20230933/ [Internet] [cited 2023 Jun 30]. Available from: [DOI] [PubMed] [Google Scholar]
- 188.Coombes S.A., Misra G. Pain and motor processing in the human cerebellum. Pain. 2016;157:117–127. doi: 10.1097/j.pain.0000000000000337. https://journals.lww.com/pain/Fulltext/2016/01000/Pain_and_motor_processing_in_the_human_cerebellum.12.aspx [cited 2023 Jun 30]. Available from: [DOI] [PubMed] [Google Scholar]
- 189.Mehnert J., Schulte L., Timmann D., May A. Activity and connectivity of the cerebellum in trigeminal nociception. Neuroimage. 2017;150:112–118. doi: 10.1016/j.neuroimage.2017.02.023. https://pubmed.ncbi.nlm.nih.gov/28192274/ [Internet] [cited 2023 Mar 14]. Available from: [DOI] [PubMed] [Google Scholar]
- 190.Minks E., Kopickova M., Marecek R., Streitova H., Bares M. Transcranial magnetic stimulation of the cerebellum. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010;154:133–139. doi: 10.5507/bp.2010.020. https://pubmed.ncbi.nlm.nih.gov/20668494/ [Internet] [cited 2023 Feb 23]. Available from: [DOI] [PubMed] [Google Scholar]
- 191.Bocci T., Barloscio D., Parenti L., Sartucci F., Carli G., Santarcangelo E.L. High hypnotizability impairs the cerebellar control of pain. Cerebellum. 2017;16:55–61. doi: 10.1007/s12311-016-0764-2. https://pubmed.ncbi.nlm.nih.gov/26846218/ [Internet] [cited 2023 Jun 30]. Available from: [DOI] [PubMed] [Google Scholar]
- 192.Bocci T., Santarcangelo E., Vannini B., et al. Cerebellar direct current stimulation modulates pain perception in humans. Restor Neurol Neurosci. 2017;33:597–609. doi: 10.3233/RNN-140453. https://air.unimi.it/handle/2434/532739 [Internet] [cited 2023 Jun 30]. Available from: [DOI] [PubMed] [Google Scholar]
- 193.Parise M., Kubo T.T.A., Doring T.M., Tukamoto G., Vincent M., Gasparetto E.L. Cuneus and fusiform cortices thickness is reduced in trigeminal neuralgia. J Headache Pain. 2014;15 doi: 10.1186/1129-2377-15-17. https://pubmed.ncbi.nlm.nih.gov/24661349/ [Internet] [cited 2023 Feb 23]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kong J., Loggia M.L., Zyloney C., Tu P., LaViolette P., Gollub R.L. Exploring the brain in pain: activations, deactivations and their relation. Pain. 2010;148:257. doi: 10.1016/j.pain.2009.11.008. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2815185/ [Internet] [cited 2023 Mar 16]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang C., Kutch J.J., Labus J.S., Yang C.C., Harris R.E., Mayer E.A., et al. Reproducible microstructural changes in the brain associated with the presence and severity of urologic chronic pelvic pain syndrome (UCPPS): a 3-year longitudinal diffusion tensor imaging study from the MAPP network. J Pain. 2022 doi: 10.1016/j.jpain.2022.11.008. https://pubmed.ncbi.nlm.nih.gov/36435486/ [Internet] [cited 2023 Mar 16]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vlaeyen J.W.S., Maher C.G., Wiech K., Van Zundert J., Meloto C.B., Diatchenko L., et al. Low back pain. Nat Rev Dis Prim. 2018;4 doi: 10.1038/s41572-018-0052-1. https://pubmed.ncbi.nlm.nih.gov/30546064/ [Internet] [cited 2023 Feb 11]. Available from: [DOI] [PubMed] [Google Scholar]
- 197.Mueller J.K., Grigsby E.M., Prevosto V., Petraglia F.W., 3rd, Rao H., Deng Z.D., et al. Simultaneous transcranial magnetic stimulation and single-neuron recording in alert non-human primates. Nat Neurosci. 2014;17:1130–1136. doi: 10.1038/nn.3751. https://pubmed.ncbi.nlm.nih.gov/24974797/ [Internet] [cited 2023 Oct 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yu K., Niu X., Krook-Magnuson E., He B. Intrinsic functional neuron-type selectivity of transcranial focused ultrasound neuromodulation. Nat Commun. 2021;12:1–17. doi: 10.1038/s41467-021-22743-7. https://www.nature.com/articles/s41467-021-22743-7 [Internet] [cited 2023 Oct 10]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhou J.W., Li J., Zhao J.J., Xie H.J., Wang M. Meta analysis on ischemic stroke treated with scalp acupuncture. World J Acupuncture-Moxibustion. 2013;23(2):41–47. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.