Abstract
Coordinated cochaperone interactions with Hsp90 and associated client proteins are crucial for a multitude of signaling pathways in normal physiology, as well as in disease settings. Research on the molecular mechanisms regulated by the Hsp90 multi-protein complexes has demonstrated increasingly diverse roles for cochaperones throughout Hsp90-regulated signaling pathways. Thus, the Hsp90-associated cochaperones have emerged as attractive therapeutic targets in a wide variety of disease settings. The TPR-domain immunophilins FKBP51 and FKBP52 are of special interest among the Hsp90-associated cochaperones given their Hsp90 client protein specificity, ubiquitous expression across tissues, and their increasingly important roles in neuronal signaling, intracellular calcium release, peptide bond isomerization, viral replication, steroid hormone receptor function, and cell proliferation to name a few. This review summarizes the current knowledge of the structure and molecular functions of TPR-domain immunophilins FKBP51 and FKBP52, recent findings implicating these immunophilins in disease, and the therapeutic potential of targeting FKBP51 and FKBP52 for the treatment of disease.
Immunophilins comprise two families of proteins that exhibit peptidyl/prolyl cis-trans isomerase (PPIase) activity that can also bind immunosuppressive drugs. In addition, their distinct binding affinity to specific immunosuppressive drugs is used to further classify immunophilins. For example, cyclophilin proteins have binding affinity to cyclosporin, whereas the FKBPs (FK506 binding proteins) bind FK506 or rapamycin with high affinity (Galat 1993). To date, 24 cyclophilins, and 18 FKBPs have been identified as part of this superfamily in humans (He, Li et al. 2004, Somarelli, Lee et al. 2008). In the case of FKBPs, their calculated molecular weight is used to assign a name to each of the 18 members of this family(Dunyak and Gestwicki 2016).
The first description of an FKBP was documented by Harding in 1989. With a calculated molecular weight of 12kDa, it was termed FKBP12 and is currently the smallest FKBP family member (Harding, Galat et al. 1989, Kolos, Voll et al. 2018). With the use of an FK506 affinity matrix, Peattie and colleagues isolated an immunophilin with an approximate molecular mass of 55kDa that was later named FKBP52. The same studies revealed a consensus sequence with FKBP12, the archetypal member of immunophilins, and other FKBPs at the N-terminus (Peattie, Harding et al. 1992). Their role in basic cellular processes involving protein folding, receptor signaling, and protein trafficking has also been highlighted in a number of previous reviews (Baker, Ozsan et al. 2018, Zgajnar, De Leo et al. 2019) (Kang, Hong et al. 2008). Due to this involvement in an array of physiological processes, FKBPs have emerged as promising therapeutic targets for pathways associated with reproduction, lipid metabolism, regulation of stress response, hormone-dependent cancers including prostate and breast cancer, infertility, and stress-related psychiatric disorders.
In this review we will focus on two larger members of the FKBP family of proteins, FKBP51 and FKBP52. FKBP51 and FKBP52 are encoded by FKBP5 and FKBP4 genes respectively and regulate steroid hormone receptor (SHR) activity via the heat shock protein 90 (Hsp90) heterocomplex (Kolos, Voll et al. 2018). Since their identification, the structural features of FKBPs have been well studied and crystal structures reveal several conserved regions. FKBP51 and FKBP52 are well-established as closely related homologs that share 60% amino acid sequence identity and 70% amino acid sequence similarity with a similar tertiary structure (Figure 1) [4]. The conserved domains consist of an N-terminal FK1 domain with a peptidyl/prolyl cis-trans isomerase (PPIase) active site to which the immunosuppressant drug FK506 binds, a middle FK2 domain that lacks PPIase activity despite structural similarities with FK1, and a C-terminal Hsp90-binding tetratricopeptide repeat (TPR) domain (Storer, Dickey et al. 2011). Although highly similar, three-dimensional crystallographic structures suggest similar domain conformations with slight variations in domain orientations (Sinars, Cheung-Flynn et al. 2003, Wu, Li et al. 2004), although it is important to point out that the differences in domain orientation could be an artifact given that the full length crystallographic structure of FKBP52 that is typically shown is made up of two partial FKBP52 structures. The full-length structure of FKBP52 has not been solved to-date.
Figure 1: FKBP51 and FKBP52 Structure and Sequence Comparisons.
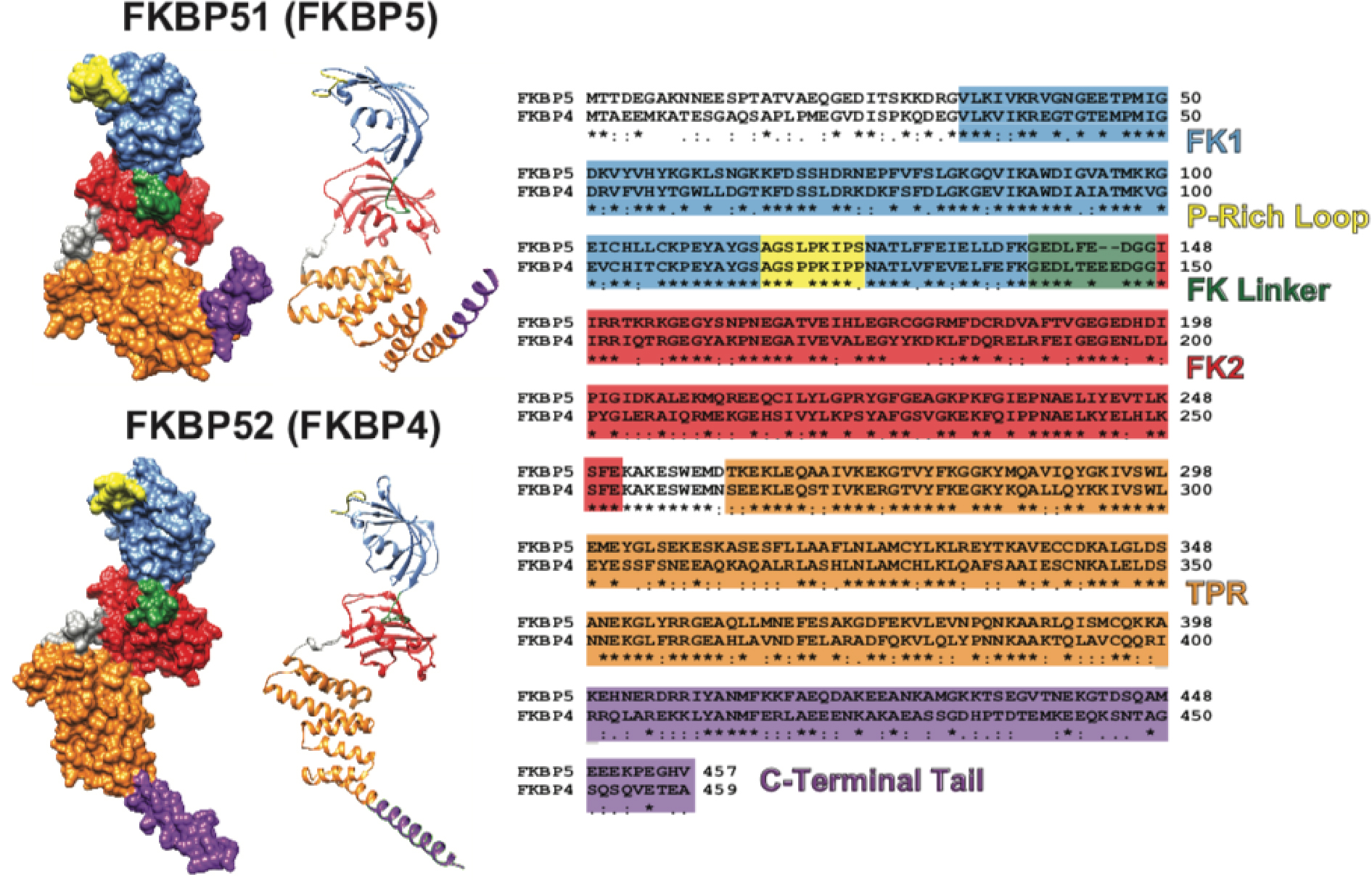
Both the ribbon and molecular surface models of the human FKBP51 crystal structure (FKBP5; PDB ID 1KT0) and overlaid images of two partial human FKBP52 crystal structures (FKBP4; PDB ID 1Q1C and 1P5Q) are shown on the left. The visible difference in the orientation of the TPR (orange) and C-Terminal Tail (purple) between FKBP51 and FKBP52 may be an artifact given that FKBP52 has been only partially crystalized. A multiple sequence alignment comparing the FKBP51 and FKBP52 amino acid sequence is shown on the right with known functional domains and regions colored according to their location on the crystal structures (* denotes identical amino acids, : denotes highly conserved amino acids, and . denotes partially conserved amino acids). Human FKBP51 and FKBP52 are approximately 60% identical and approximately 70% similar. The functional domains and regions highlighted include: the FK506 binding domain 1 (FK1) that contains a functional PPIase active site to which FK506 binds (blue); the proline-rich loop that overhangs the PPIase pocket and serves as a functionally relevant surface for the regulation of steroid hormone receptors (yellow); the linker region that links the two FK domains and contains a casein kinase 2 phosphorylation site (T143) in FKBP52 that may regulate Hsp90 binding (green); the FK2 domain that is structurally similar to FK1, but lacks a functional PPIase active site (red); the tetratricopeptide repeat (TPR) domain that mediates binding to the C-terminal EEVD motif on Hsp90 (orange); and the C-terminal Tail containing the Charge-Y motif that has also been shown to influence Hsp90 binding (purple). It is important to note that FKBP51 and FKBP52 were crystalized without the last 45 C-terminal amino acids and 32 C-terminal amino acids respectively. Thus, the structures shown on the left are truncated within the C-Terminal Tail. UCSF Chimera candidate version 1.12 (build 41600) was used to generate the ribbon and molecular surface images. The multiple sequence alignment was generated by CLUSTALW prior to illustration.
FKBP51 and FKBP52 Structural domains
FK1 domain
Both, FKBP51 and FKBP52, contain a N-terminal FK1 domain which exhibits PPIase activity, characteristic of FKBP family members. PPIases are ubiquitously distributed enzymes that are phylogenetically highly conserved and that accelerate the otherwise slow steps of refolding denatured proteins (Fischer, Bang et al. 1984). Hence, PPIases have been referred to as molecular switches that impact downstream signaling events. While the smallest members of FKBPs are composed almost entirely of a PPIase motif in a single FK domain, the larger FKBPs, such as FKBP51 and FKBP52, possess functionally independent domains (Ghartey-Kwansah, Li et al. 2018).
Being PPIases, FKBP51 and FKBP52 have the enzymatic ability to catalyze conformational interconversions of their client proteins. More specifically, they catalyze a form of isomerization known as cis/trans isomerization. This conformational change consists of altering the spatial arrangement of functional groups across a prolyl bond, a peptide bond preceding proline, from the same side (‘Cis’) to opposite sides (‘trans’) (Rostam, Piva et al. 2015). Therefore, PPIases reduce the rotation barriers, alter the orientation of functional groups, and produce stereoisomers with functional variability. PPIase functions extend beyond their ability to catalyze protein folding. Studies in vitro demonstrated multiple effects by PPIases on client proteins in processes such as protein folding and trafficking(Ghartey-Kwansah, Li et al. 2018), and regulation of the cell cycle (Laplante and Sabatini 2012).
It is important to mention that, while PPIases play crucial roles in multiple cellular processes, their effects are not necessarily dependent on the enzymatic activity, but rather on the domain itself as an interaction and regulatory surface. Riggs et. al. initially suggested a role for FKBP52’s PPIase activity in glucocorticoid receptor (GR) potentiation (Riggs, Roberts et al. 2003). This was concluded after GR potentiation was abrogated as a result of point mutations to the FK1 domain, and FK506 binding to PPIase pocket, both of which inhibit PPIase activity. Interestingly, it was previously established that the large FK506 drug can extend beyond the PPIase pocket, which may physically interfere with other interactions or allosterically inhibit them (Van Duyne, Standaert et al. 1991). Taking these findings into consideration, an important question arose; is enzymatic activity responsible for receptor potentiation or is this effect independent of PPIase activity? Through mutation of additional residues important for PPIase activity, Riggs et. al. later demonstrated that enzymatic activity was, in fact, not necessary for steroid hormone receptor potentiation by FKBP52 (Riggs, Cox et al. 2007).
PPIases FKBP51 has become a promising target for androgen-dependent prostate cancer, since prostate cancer cell proliferation is increased in response to its interaction with the androgen receptor (AR) (Maeda, Habara et al. 2022). Given that AR is a client protein of FKBP51, and that this positive regulation of AR is inhibited by immunosuppressor FK506, it has been suggested that the mechanism of AR upregulation by FKBP51 is the PPIase effect on AR conformation alteration and isomerization of the peptide backbone (Periyasamy, Hinds et al. 2010).
Recently, the ability of FKBP51 to act on Cdk4 via the Hsp90-heterocomplex was identified. In this study, authors propose a possible role for FKBP51 PPIase activity for Cdk4 inhibition. By sequestering the Cdk4 in the Hsp90 complex, FKBP51 prevents the formation of the Cdk4-cyclin D1 complex resulting in proliferation inhibition and myogenesis activation (Ruiz-Estevez, Staats et al. 2018). In terms of PPIase regulation of Cdk4, FKBP51 PPIase promotes cis-trans isomerization of the Thr172-Pro173 peptide bond, which is required for Cdk4 activation. Conversely, FKBP52 does not show isomerization induction of Cdk4 (Ruiz-Estevez, Staats et al. 2018). This observation further highlights the different roles of FKBP51 and FKBP52 despite their highly conserved structural organization and PPIase domain similarities.
The ability of PPIases to exert functionally relevant conformational alterations at a single bond may offer a promising target for cell regulatory networks involved in disease. However, given the structural similarities amongst PPIase family members, inhibitor specificity continues to be a challenge. Due to the structural similarities within the PPIase pocket of FKBP51 and FKBP52, drugs targeting the FKBP52 PPIase pocket will likely target, not only FKBP52, but also the closely related FKBP51 protein simultaneously.
To investigate the mechanism of selectivity against protein subtypes and its potential, Sattler and colleagues analyzed the structural conformation of FKBP51 bound to a class of recently discovered FKBP51 inhibitors called SAFit (Selective Antagonists of FKBP51 by induced fit) (Gaali, Kirschner et al. 2015). Combining NMR spectroscopy, molecular dynamics, and thermodynamics with mutational analysis, they identified minor variations in residue sequences that established selectivity of SAFit molecules against FKBP51. From their data, the authors conclude that SAFit molecules preferentially bind to FKBP51 at a transient pocket that is unavailable in its closely related paralogue FKBP52. However, despite the seemingly successful selectivity for FKBP51 over FKBP52, SAFit molecules were still not able to discriminate against FKBP12 and its isoform FKBP12.6 (Jagtap, Asami et al. 2019).
Hausch and colleagues were able to enhance SAFit molecules by macrocyclization and provide the first ligands able to selectively target FKBP51, but not FKBP12 and FKBP12.6. Macrocycles of SAFit analogs were synthesized and screened for affinity towards FKBP12, FKBP12.6, FKBP51, and FKBP52. In a cell system, the newly synthesized macrocycles were able to engage selectively with FKBP51 and interfere with its cellular functions (Voll, Meyners et al. 2021). It can be hypothesized that the lack of selectivity for FKBP52 when compared to FKBP51, is due to the fact that SAFit molecules target and inhibit PPIase activity of FKBP52, but may not affect the conformation of the proline-rich loop that overhangs the PPIase pocket (discussed below). All things considered, conformational selection is now considered a contributing factor in drug binding mechanisms and specificity.
The FK1 domain also contains a proline-rich loop suspended above the PPIase pocket, which has been characterized as an important surface for SHR potentiation. Given that FKBP51 and FKBP52 are structurally similar, but FKBP51 is unable to potentiate AR like FKBP52 in the cellular systems being used, the goal was to determine if potentiation could be interchanged between both FKBPs through mutations. Riggs et. al. performed gain-of-function random mutagenesis in which two single point mutations (A116V and L119P) in the FKBP51 proline-rich loop resulted in AR potentiation similar to that of FKBP52 (Riggs, Cox et al. 2007). The functional difference that can be attributed to both immunophilins due to these residues suggests a critical role of the proline rich loop for FKBP-mediated regulation of receptor activity. This observation demonstrated that FKBP52-mediated receptor potentiation was, in fact, due to the FK1 domain itself rather than the PPIase activity exerted by this domain. The importance of the proline-rich loop surface was further highlighted by a more recent study in which gain-of-function random mutagenesis was used to identify a single point mutation (A111V) in the zebra fish FKBP52 proline-rich loop that confers full receptor potentiating ability; wild type zebra fish FKBP52 does not potentiate SHR activity (Harris, Garcia et al. 2019). Thus, it is likely that FKBP51 and FKBP52 functionally diverged sometime after the boney fishes in evolution through minor changes in the proline rich loop sequence.
FK linker
Joining the active FK1 domain to the FK2 domain is a flexible hinge region known as the FK linker. Structural differences between FKBP51 and FKBP52 can be seen in the 8 residues linking the two domains with FKBP52 containing an acidic, two residue-region at the first loop of FK2 domain(Sinars, Cheung-Flynn et al. 2003, Bracher, Kozany et al. 2013). The FKBP52 linker sequence also contains a TEEED phosphorylation sequence whose modification can inhibit FKBP52-mediated steroid regulation by interfering with FKBP52-Hsp90 interaction (Miyata, Chambraud et al. 1997, Zgajnar, De Leo et al. 2019). On the other hand, the FK linker in FKBP51 contains an FED sequence not subject to phosphorylation. Structural comparison of the conformational isomers of FKBP52 revealed the FK1 and FK2 domains to be fixed structural domains amongst all isomers, while the linker region demonstrated variations between isomers. Based on these structural analyses, the authors suggest the linker region as a flexible region that allows for alternate organization of the FK1 and FK2 domains in response to different interactions with client proteins (Bracher, Kozany et al. 2013). It has been proposed that modulation of this short region by phosphorylation may offer an explanation for the differential regulatory roles that FKBP51 and FKBP52 have on SHRs.
FK2 domain
Both FKBP51 and FKBP52 contain an FKBP-like domain (FK2) that is similar to FK1 but lacks PPIase activity and is unable to bind immunosuppressive drugs, such as FK506 (Chambraud, Rouviere-Fourmy et al. 1993) (Rouviere, Vincent et al. 1997). Hence, a crucial role for this domain has not been elucidated. However, point mutations in this domain did impair the proper intergration of FKBP51, but not FKBP52, into the Hsp90 heterocomplex with PR, as demonstrated by coimmunoprecipitation (Sinars, Cheung-Flynn et al. 2003). The plasticity offered by the FK-linker may influence the orientation of the, seemingly trivial, FK2 domain that may play a role for FKBP51 interaction with some of the SHRs.
TPR domain
FKBP51 and FKBP52 also contain a C-terminal TPR domain consisting of 34 amino acids each arranged in tandem to form sets of alpha-helices. The TPR confers the ability to form protein associations with the unstructured C-terminal tail of Hsp90 and Hsp70 (Riggs, Cox et al. 2007, Jaaskelainen, Makkonen et al. 2011) (Smith 2004). Because the last four residues of the Hsp70 and Hsp90 peptides are identical (EEVD), specificity is achieved by electrostatic contacts between TPR domains and the EEVD motif, and by hydrophobic contacts with residues N-terminal to this EEVD motif (Scheufler, Brinker et al. 2000). It has been demonstrated via isothermal titration calorimetry studies comparing several PPIases, Cyp40, FKBP51, and FKBP52, that FKBP52 interacts with Hsp90 with the highest affinity of the three related proteins (Pirkl and Buchner 2001). In summary, ir is through these interactions with Hsp90 that FKBP51 and FKBP52 are linked to steroid hormone receptor maturation.
At the C-terminus, FKBP51 and FKBP52 also contain a calmodulin binding consensus, like other members of the FKBP family. For example, the TPR-containing homolog FKBP38, which is otherwise PPIase inactive, can be activated allosterically by binding of calmodulin.
While FKBP38, and perhaps other FKBPs, can be dependent on calmodulin binding to this site, dependence of FKBP51 and FKBP52 has not been established (Callebaut, Renoir et al. 1992) (Shirane-Kitsuji and Nakayama 2014). Since its identification as a protein interaction module for cell division proteins in yeast, the TPR domain has been demonstrated to be ubiquitous (Sikorski, Boguski et al. 1990). Its presence in a variety of unrelated proteins has illustrated the involvement of the TPR domain as a mediator of protein-protein interactions. Thus, the TPR motif is not exclusive to cochaperones, but is rather present in a multitude of proteins with different functions.
Charge-Y domain
Lastly, downstream of the TPR domain in the C-terminal tail, an 11-amino acid motif has been identified as the charge-Y motif and is considered important for Hsp90 binding. Both, FKBP51 and FKBP52 contain a residues that match the consensus sequence of the charge-Y motif (Cheung-Flynn, Roberts et al. 2003). From the 7 helices (H1-H7) composing the TPR domain, the charge-Y motif lies at H7, which protrudes beyond the central portion of the TPR domain. Considering this location, several theories have been postulated on the possible mechanism by which the charge-Y motif mediates Hsp90 binding. One possibility for Hsp90 binding is that the charge-Y motif at H7 can directly contact Hsp90 in its extended site beyond the core TPR domain. A second possibility for binding is that H7 may be re-structured to form an eighth helix (H8) closer to the core TPR domain, and that would enhance TPR interaction with Hsp90. (Cheung-Flynn, Roberts et al. 2003)
Despite the growing knowledge of FKBP structure and their prevalence in disease, their specialized functions in a multitude of pathways remains poorly understood. Perhaps a relatively simple way of gaining insight into these specialized functions is through comparative studies using WT and KO models. Analysis of KO phenotypes of FKBP51 and FKBP52 can help elucidate how cell type specific signaling pathways are regulated in the presence or absence of these immunophilins and what role they play in cooperative protein networks.
FKBP51 and FKBP52 regulation of steroid hormone receptor signaling
FKBP51 and FKBP52 serve as modulators of nuclear receptor function, including the mineralocorticoid, glucocorticoid, androgen, and progesterone receptors. FKBP52 is a known positive regulator of AR, GR and PR activity (Cheung-Flynn, Prapapanich et al. 2005, Tranguch, Cheung-Flynn et al. 2005, Yang, Wolf et al. 2006, Ni, Yang et al. 2010, Cluning, Ward et al. 2013, Maeda, Habara et al. 2022), whereas FKBP51 has been characterized as a negative regulator of GR (Hubler, Denny et al. 2003, Tranguch, Cheung-Flynn et al. 2005, Ni, Yang et al. 2010, Storer, Dickey et al. 2011, Fries, Gassen et al. 2017, Zgajnar, De Leo et al. 2019) and PR (Hubler, Denny et al. 2003, Tranguch, Cheung-Flynn et al. 2005, Storer, Dickey et al. 2011, Maeda, Habara et al. 2022) activity, while positively regulating AR signaling (Ni, Yang et al. 2010, Zgajnar, De Leo et al. 2019, Maeda, Habara et al. 2022). It is important to note that the receptor specificity, as well as the nature of the regulation, displayed by FKBP51 and FKBP52 has been a point of confusion. Our group has reported for years that FKBP52 is a positive regulator of AR (Cheung-Flynn, Prapapanich et al. 2005), GR (Riggs, Roberts et al. 2003), and PR (Tranguch, Cheung-Flynn et al. 2005), while FKBP51 does not regulate these receptors to any appreciable degree, as this is what we have observed in the cell lines and systems in which we have worked. That being said, others have reported divergent evidence that typically centers around FKBP51 including positive regulation of AR in prostate cancer cells as discussed above. Thus, it is likely that the FKBPs display cell and organ-specific regulatory mechanisms.
AR signaling is regulated via the orchestrated assembly of a mature receptor heterocomplex that involves the immunophilins FKBP51 and FKBP52. Recent studies where FKBP51 or FKBP52 were depleted in prostate cancer cells, showed reduced AR dimer formation, chromatin binding, and phosphorylation, suggesting that both proteins are necessary for dimer formation and chromatin binding of AR (Maeda, Habara et al. 2022). Similarly, it has been reported that both FKBP51 and FKBP52 enhance the biological activities of AR by increasing hormone affinity (Ni, Yang et al. 2010, Zgajnar, De Leo et al. 2019, Maeda, Habara et al. 2022).
Evidence suggests that FKBP52 potentiates the function of GR through hormone binding (Riggs, Roberts et al. 2003). In Saccharomyces cerevisiae models for GR activity, FKBP52 can potentiate hormone-dependent GR activity by as much as 20-fold at limiting hormone concentrations, while the co-expression of FKBP51 in the same model blocked FKBP52 mediated potentiation. In accordance with these findings, FKBP51 has been documented as a potent inhibitor of the GR signaling pathway leading to GR resistance (Denny, Valentine et al. 2000, Criado-Marrero, Rein et al. 2018), which has been associated with disorders such as reduced stress coping behavior (Criado-Marrero, Rein et al. 2018) and adipogenesis (Smedlund, Sanchez et al. 2021).
Similar to GR regulation, the antagonistic effects induced by FKBP51 and FKBP52 have been recorded for PR. Studies with 52KO female mice, failed to complete embryo implantation in the uterus due to compromised uterine receptivity (Tranguch, Cheung-Flynn et al. 2005). Similarly, FKBP52 deficient female mice, presented complete sterility due to in utero implantation failure (Cox, Riggs et al. 2007). These results established FKBP52 as an essential regulator of PR activity in vivo. Contrary to FKBP52, FKBP51 is a negative regulator of PR function. Recent studies found that an enhanced FKBP51-PR interaction play a significant role in preterm birth, while making 51 a novel therapeutic target to prevent this disease (Guzeloglu-Kayisli, Semerci et al. 2021). Overall, FKBP51 has been reported to act as a negative regulator of SHR activity, as opposed to FKBP52 that positively regulates AR, GR, and PR.
Role in Nuclear Translocation
Several characteristics of the large immunophilins have been demonstrated to be essential for SHR function, such as Hsp90 interaction, interaction with dynein, and PPIase enzyme activity. Based on the classic model of steroid receptor nuclear translocation, hormone binding to SHR in the Hsp90-heterocomplex was deemed a crucial trigger for receptor dissociation, thus, facilitating nuclear translocation of the un-complexed receptor. This proposed model was partly due to the idea that the nuclear localization signal (NLS) in cytoplasmic steroid receptors remains hidden while bound to the Hsp90 heterocomplex (Stewart 2007, Sivils, Storer et al. 2011). However, studies on the GR- and MR-Hsp90 heterocomplexes demonstrated that cross-linked complexes can pass through the nuclear pore intact and accumulate in the nucleus. The fact that Hsp90 hetercomplexes can exist in the nucleus, may suggest that the entire complex can pass intact through the nuclear pore. The findings that the larger immunophilins, as part of these complexes, can travel across the nuclear membrane suggest that transformation and dissociation of the heterocomplex occurs at the nucleus, instead of the cytoplasm (Echeverria, Mazaira et al. 2009, Galigniana, Erlejman et al. 2010). It has been reported that GR hormone binding and recruitment of the transport protein dynein is increased in the presence of FKBP52, while FKBP51 is known to inhibit hormone binding to GR and decrease nuclear transport (discussed below). These findings led to the idea that hormone binding results, not in the dissociation of the receptor from the heterocomplex, but rather in a switch from FKBP51 to FKBP52 in the heterocomplex (Ebong, Beilsten-Edmands et al. 2016).
The switching of both immunophilins prior to nuclear translocation is coherent with the fact that FKBP51 and FKBP52 compete for binding to the Hsp90 heterocomplex (Nair, Rimerman et al. 1997). Furthermore, FKBP51 and FKBP52 have regulatory roles in steroid hormone receptor interaction with the dynactin complex involved in nuclear translocation. Dynactin is an essential cofactor for dynein, and a multi-subunit protein complex that is required for dynein-driven retrograde transport of vesicles along cytoskeletal microtubules (Schroer 2004). In the context of the Hsp90-SHR heterocomplex, the question was raised regarding the mechanism for the continuous transport of SHR to and from the nucleus (Pratt, Silverstein et al. 1999, Galigniana, Harrell et al. 2002). Using purified proteins, it was demonstrated that FKBP52 and an independent PPIase fragment can bind directly to cytoplasmic dynein and plays a role in tethering SHRs to the retrograde transport machinery (Galigniana, Harrell et al. 2002, Wochnik, Ruegg et al. 2005). On the contrary, FKBP51 displayed the opposite effect on nuclear translocation of GR. The inhibitory effects of GR were demonstrated in mammalian cells when efficient nuclear translocation of GR was delayed in the presence of FKBP51, which makes sense given the limited interaction of FKBP51 with dynein, as opposed to FKBP52 (Davies, Ning et al. 2002). Interestingly, swapping the N-terminal PPIase domains of FKBP51 and FKBP52 reverses their activity in a way that FKBP52 exhibits GR inhibitory effects with reduced dynein association, as is expected for FKBP51. The opposite occurred with FKBP51 whose inhibitory effect on GR was abolished and was able to form an association with dynein (Wochnik, Ruegg et al. 2005). Based on these data, it is clear that FKBP51 and FKBP52 have more diverse roles in the SHR signaling pathways than was originally thought, including continued regulation after hormone binding.
FKBP51 and FKBP52 in reproductive disorders
A role for FKBP51 and FKBP52 in a variety of disorders of the reproductive system have been identified. Female reproductive disorders, such as endometriosis and polycystic ovarian syndrome (PCOS), are associated with opposite hormonal profiles involving steroid hormone receptors (Dinsdale and Crespi 2021). Given that FKBP52 is crucial for progesterone receptor (PR) function, and that the anti-inflammatory effects of PR’s signaling axis are suppressed in endometriosis, FKBP52 has been studied as a potential contributing factor (Bulun, Cheng et al. 2006, Lousse, Van Langendonckt et al. 2012). To study the effects of FKBP52 deficiency on endometriosis, Hirota and colleagues transplanted endometrial tissue into the peritoneum of mice with different expression levels of FKBP52. They found that 52KO mice developed significantly higher number of endometriotic lesions when compared to their WT counterparts. In the context of PR, deletion of FKBP52 reduced PR mediated signaling, which led to increased cell proliferation and inflammation (Hirota, Tranguch et al. 2008). In women and non-human primate models with endometriosis, there is diminished levels of FKBP52 in the ectopic endometrium, which leads to dysregulated progesterone response and upregulation of MicroRNA-29c (miR-29c) expression (Joshi, Miyadahira et al. 2017). MiR are single stranded RNA molecules that function to repress gene expression through messenger RNA (mRNA) and have been implicated in reproductive disease, such as endometriosis. The results of this study suggest that absence or decrease in levels of FKBP52 directly affect levels of miR-29c by causing levels to increase, resulting in poor progesterone signaling. This suggests that FKBP52 may be involved in progesterone resistance often seen in endometriosis. Recently, an endometrial stromal cell (ESC) model was developed to evaluate the relationship between FKBP52 and progesterone receptor (PR) levels. These findings revealed that when downregulation of FKBP52 occurs, PR expression decreases, leading to proliferation of ESC and development of endometriosis. These data are consistent with clinical data for women with endometriosis in that there was a direct correlation between FKBP52 and PR levels (Liu, Cheng et al. 2021). FKBP52 is also implicated in infertility as human endometrial stromal cells treated with FKBP52 and progesterone revealed that FKBP52 expression mediated through the HOXA10 pathway is diminished in endometriosis and leads to disrupted decidualization, progesterone resistance, and infertility in women (Yang, Zhou et al. 2012).
Along these lines, FKBP52 has been shown to impact the development and progression of polycystic ovarian syndrome (PCOS) as an analysis of the influence of multiple genes on PCOS revealed that FKBP52 influences development risk. Two single nucleotide polymorphisms (SNPs) of FKBP52, rs4409904 and rs2968909, were associated with reduced risk of PCOS development. Rs4409904 was associated with lowered odds of PCOS and rs2968909 was associated with lower body mass index (BMI) and diminished adiposity, which are traits known to amplify PCOS symptoms (Ketefian, Jones et al. 2016). In another instance, a recent discovery revealed that rats with PCOS display higher levels of FKBP52 expression in all cell types of the ovary when compared to expression levels observed in control group rats (Song and Tan 2019).
Finally, FKBP52 may be implicated in preeclampsia (PE) and intrauterine growth restriction (IUGR) in pregnant women. A case study revealed that FKBP52 was downregulated in the placentas of PE patients when compared with the control group with normal placentas. Additionally, FKBP52 was upregulated in the placentas of IURG patients (Acar and Ustunel 2015).
Similar to FKBP52, FKBP51 has also been linked to female reproductive disorders relating to the establishment and maintenance of pregnancy. More specifically, a role for FKBP51 in decidualization has been described (Gellersen and Brosens 2014, Wei, Gao et al. 2018). Decidualization refers to the process by which endometrial stromal fibroblasts specialize into secretory decidual cells, which is a crucial step for embryo implantation and placental development (Gellersen, Brosens et al. 2007, Gellersen and Brosens 2014). This transformative event is indispensable for the initiation and maintenance of pregnancy because it provides maternal immunological tolerance against fetal antigens and protects the conceptus against stressors (Leitao, Jones et al. 2010). Given that FKBP51 plays a role in steroid hormone response and in the AKT pathway, and that AKT pathway is closely related to the decidualization progress (Hirota, Acar et al. 2010), Wei et al sought to examine a potential mechanism for FKBP51 in the regulation of decidualization in endometrial stromal cells (ESCs). Interestingly, knockdown of FKBP51-shRNA in ESCs in vitro, resulted in decidualization inhibition, while reintroduction of FKBP51-cDNA was able to rescue this inhibition. These results supported that FKBP51 can promote decidualization perhaps by reducing AKT phosphorylation levels, as suggested by the authors (Wei, Gao et al. 2018).
The role of FKBPs does is not limited to reproductive disorders in females. FKBP52 KO mouse lines have highlighted a role for FKBP52 in the development of the male reproductive system as well. Adult FKBP52-deficient male mice display phenotypes corresponding to partial androgen insensitivity, such as development of hypospadias, and prostate dysgenesis (Yong, Yang et al. 2007), likely due to the role of Fkbp52 as a co-chaperone of the androgen receptor (AR). In these same studies, Yong et al. reported that FKBP51 showed no defects in AR-mediated reproductive function and no hypospadias. Complementing the data that demonstrates FKBP52’s role in male reproductive health, FKBP52 KO animal models have also been reported to have reduced sperm motility and reduced fertilizing capacity (Hong, Kim et al. 2007). All things considered, FKBP51 and FKBP52 seem to play critical roles in normal reproductive health of males and females, while FKBP52 has demonstrated a greater effect on male reproductive health and sexual differentiation.
Neurodegenerative diseases
In recent years, studies have revealed that FKBP51 and FKBP52 are important for neurological function and may be a key factor in the development of neurodegenerative diseases. FKBP52 is widely expressed throughout the nervous system and is critical for signaling, transport of protein, neurite outgrowth and differentiation of neurons (Quintá, Maschi et al. 2010, Giustiniani, Sineus et al. 2012). Given that FKBP52 is important for several neurological functions, we can assume that it also plays a role in disease progression in the brain.
FKBP52 has been implicated in the development of Alzheimer’s disease (AD) through its roles on the regulation and translocation of steroid hormone receptors, including glucocorticoid receptors (GR), into the nucleus of neurons (Chambraud, Byrne et al. 2022). Since the GR is known to regulate tau protein pathology, this potentially provides a mechanism by which AD is influenced by FKBP52 (Blair, Baker et al. 2015). In addition, FKBP52 is thought to exacerbate tau pathology through its direct interaction with Tau-P301L, a Tau mutant known to induce significant tauopathy in humans (Meduri, Guillemeau et al. 2016). In this instance, FKBP52 not only has the ability to interact with the mutant, but it also contributes to protein conformational changes, leading to the assembly of filaments (Giustiniani, Chambraud et al. 2014). In fact, FKBP52 has been proposed as a biomarker for AD since patients with AD exhibit abnormally low expression levels of FKBP52 in the frontal cortex. This observation correlated with pathological levels of tau in the cerebral cortex, which were not attributed to neuronal loss (Giustiniani, Sineus et al. 2012). Interestingly, FKBP52 has also been shown to induce tau proteins that display prion like behavior in vitro. These tau proteins have the capacity to penetrate neurons as well as propagate and migrate to other neurons (Giustiniani, Guillemeau et al. 2015).
Given that FKBP52 is known to play a role in tau accumulation, naturally, it also has been associated to memory deficits. In mice expressing a Tau variant (rTg4510), viral overexpression of FKBP52 resulted in neuronal dysfunction mediated by tau via a caspase-dependent pathway. In turn, this resulted in impaired spatial learning and neuronal loss in the hippocampus at 6-months old when compared to control wild type mice. This suggests that FKBP52 itself is not responsible for memory and learning deficits but that these deficits are the result of tau accumulation in combination with FKBP52. It is still unknown what contribution FKBP52 has at different points of tau accumulation and further studies are needed to better understand this mechanism (Criado-Marrero, Gebru et al. 2021). Regarding the role of FKBP51 in AD, an initial study overexpressing FKBP51 in HeLa cells resulted in a dramatic increase in tau levels. It was proposed that this increase is the result of FKBP51 disabling the ubiquitination of tau, and thereby, preserving its tau levels. FKBP51 may also utilize tau to alter the dynamics of microtubules as shown in western blots by the increase of microtubule complexity when FKBP51 is present as opposed to when it is absent. This suggests that FKBP51 has an impact in both tau levels and microtubule formation and function (Jinwal, Koren et al. 2010). To complement these findings, 51KO mice present low levels of tau in the brain, while human AD patients have increased levels of FKBP52 associated with tau accumulation. This increase in FKBP51 accumulation was found to be correlated with age, as higher levels of FKBP51 are observed in old patients diagnosed with AD (Blair, Nordhues et al. 2013). In this manner, it is possible that some individuals are predisposed to high levels of FKBP51, and therefore, have a higher risk of AD development. Also, AD pathogenesis may drive the increased FKBP51 expression. Both hypotheses account for the high levels of FKBP51 seen in old individuals with AD in comparison to age-matched healthy individuals. Overall, this study showed that the increase of FKBP51 in aged AD brains likely promoted an environment where tau accumulation could occur. It was also shown that FKBP51 synchronizes with Hsp90 to not only preserve tau structure but also promote its formation (Blair, Nordhues et al. 2013). Hsp90 works as a scaffolding protein to bring FKBP51 into proximity to Tau. More specifically, Hsp90 can join FKBP51’s PPIase pocket with Tau’s proline rich region, allowing FKBP51 to perform co-chaperone regulatory action. These dynamics, combined, work to amplify tau oligomerization (Oroz, Chang et al. 2018).
FKBP51 and FKBP52 have also recently been identified as potential therapeutic targets for Huntington disease (HD) and Parkison’s disease (PD). By lowering the levels of FKBP52, Bailus et al, demonstrated that levels of mutant huntingtin (mHtt) were reduced in vitro and in vivo HD models. This is significant as reduced levels of mHtt correlated with reduced HD pathology suggesting that FKBP52 is implicated in HD and offering support for its potential development as a therapeutic target (Bailus, Scheeler et al. 2021). In the case of PD, FKBP51 has been shown to interact with PTEN-induced putative kinase 1 (Pink1), which plays a major role in the development of PD. Furthermore, recent data indicates that FKBP51 negatively regulates PARK2 resulting in a volumetric reduction of hippocampi in Fkbp51 KO mice (Qiu, Zhong et al. 2022). This observation suggests a regulatory mechanism of FKBP51 on PARK2 expression, and potential target to regulate PARK2 expression in the onset of PD. In the same context, a mitochondrial serine/threonine-protein kinase, encoded by the PINK1 gene and associated with the onset of autosomal recessive from of Parkinson’s disease (PD), has been demonstrated to promote neuronal survival via direct inhibition of FKBP51-PHLPP (PH domain leucine-rich repeat protein phosphatase) interaction and subsequent activation of the AKT pathway (Boonying, Joselin et al. 2019). More studies are needed to further explore this interaction and how these FKBPs may be involved in HD and PD (Boonying, Joselin et al. 2019).
Cancer
Over the past decade, significant information about the role of FKBP51 and FKBP52 in human malignancy has highlighted the dysregulated expression of these proteins. Functional attribution of these immunophilins in regulation of different signaling pathways such as steroid receptor signaling, Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (Fries, Gassen et al. 2017)and Protein kinase B- leucine-rich repeat protein phosphatase (AKT – PHLPP) (Pei, Li et al. 2009), determines their role in tumorigenesis and chemoresistance of several cancers. As regulatory components of AR signaling pathways, FKBP51 and FKBP52 play an important role in the etiology of prostate neoplasia. Protein expression analysis from 500 PCa samples revealed that overexpression of FKBP52 was correlated with poor prognosis in hormone naïve PCa patients (Federer-Gsponer, Quintavalle et al. 2018). Furthermore, patient samples with hormone CRPC presented elevated levels of FKBP52 when compared to their hormone naïve counterparts, which authors propose as a potential mechanism for tumor evolution and CRPC development. Considering the integral role of chaperones regulating the multiple steps in AR signaling leading up to CRPC, Maeda et al. sought to investigate the role of FKBP51 and FKBP52 in AR dimer formation (Maeda, Habara et al. 2022). Given that AR dimerization is believed to play a crucial role in ligand-dependent activation of the receptor, the authors analyzed AR dimer formation after the addition of DHT, and in the presence or absence of FKBP51 and FKBP52. It was demonstrated that dimer formation in response to DHT was reduced in cells deficient of FKBP51 or FKBP52 by 0.54-fold and 0.30-fold, respectively. These results suggest that both immunophilins may influence AR signaling by targeting similar receptor functions, and hence both are promising targets for prostate cancer treatment. Interestingly, a separate study of PCa tissues measuring mRNA and protein expression of PPIases Cyp40, FKBP51, and FKBP52, showed upregulation of FKBP51. On the contrary, FKBP52 levels remained unaltered when comparing PCa samples with normal tissue (Periyasamy, Hinds et al. 2010). The opposing roles of both immunophilins has also been recently documented in breast cancer cells. FKBP52 has been suggested to stabilize ER, thus promoting breast cancer cell proliferation, while FKBP51 was shown to reduce stability of ER (Habara, Sato et al. 2022). FKBP52 overexpression is also suggested to be involved in breast cancer progression and invasion considering its significant association with advanced Tumor-Node-Metastasis (TNM) stage and lymph node metastasis (Hong, Li et al. 2017).
While very little work has been done to characterize a role for PR in prostate cancer, data suggests that PR expression is elevated in metastatic disease, and that PR antagonist are also potential treatments for prostate cancer (Check, Dix et al. 2010) (Fischer 1994). Thus, FKBP51 and FKBP52 regulation of PR signaling may also be a relevant therapeutic target in this disease setting.
In several other types of cancer, the influence of FKBP51 on neoangiogenesis, cell proliferation, invasion, motility, and chemosensitivity is modulated through the NF-κB and AKT pathways. Overexpression of FKBP51 triggering NF-κB activation can result in sustained cell proliferation and chemoresistance in different cancer types. For instance, elevated FKBP51 has been reported in all kinds of glioblastoma cells, including GSCs (Glioma stem cells) and vascular endothelial cells (Rotoli, Diaz-Flores et al. 2022), and its expression is inversely correlated with overall glioblastoma patient survival rates (Jiang, Cazacu et al. 2008). In oral squamous cell carcinoma (OSCC), patients with high FKBP51 (>51% of FKBP51 positive tumor cells) have and 88% estimated probability of death within five years from the diagnosis (Russo, Merolla et al. 2017). Analogous outcomes were reported by Xie et al. in ulcerative colitis (UC) associated colorectal cancer (CRC) patients where elevated FKBP51 expression is associated with increased levels of TANs (tumor-associated neutrophils), which in turn regulates the inflammatory microenvironment and is associated with UC-CRC progression and poor prognosis (Xia, Zhang et al. 2021). Here, the regulation of inflammatory microenvironment mediated by FKBP51 may depend on NF-κB. Consistent with this notion, NF-κB constitutive activation by FKBP51 in aggressive malignant melanoma cells, elicits apoptosis resistance by escaping antitumor immune response via various mechanisms (Tufano, Cesaro et al. 2021). Upregulation of FKBP51 inducing NF-κB activation also promotes the progression of castration resistant prostate cancer (Yu, Sun et al. 2019). The underlying mechanism involves physical interaction of FKBP51 with IKK (IkappaB kinase) subunits to facilitate IKK complex assembly and, in this IKK regulatory role, both enzymatic (as an isomerase) as well as scaffold function of FKBP51 are essential (Romano, Xiao et al. 2015). In addition, higher expression of FKBP52 also promotes transcriptional activation and nuclear translocation of NF-κB in lung cancer and causes significantly shorter survival with aggressive cell proliferation, invasion, and metastasis (Zong, Jiao et al. 2021).
On the contrary, it has also been demonstrated that FKBP51 overexpression induces inhibition of glioma cell proliferation, apoptosis ad chemosensitivity, which repudiates the involvement of only NF-κB because FKBP51 hyperexpression should promote the IKK activation and increase the cell proliferation (Li, Jiao et al. 2020). This contradictory action implies the engagement of another regulating mechanism that is cell context dependent. Pei et al. showed that AKT phosphorylation is the responsible mechanism for such response and mechanistically AKT is negatively regulated by FKBP51 (Pei, Li et al. 2009). The mechanism elucidated involves a functional role for FKBP51 as a scaffold protein enhancing PHLPP-AKT interaction and enabling PHLPP mediated dephosphorylation of AKT-Ser473 (Hou and Wang 2012). Increased AKT phosphorylation mediated by downregulation of FKBP51 reduces chemosensitivity, as observed in pancreatic and breast cancer (Li, Lou et al. 2011). Whereas a decrease in AKT phosphorylation mediated by FKBP51 hyperexpression increases chemosensitivity as reported in endometrial adenocarcinoma where high FKBP51 attenuates cell proliferation and progestin resistance by decreasing AKT signaling (Dong, Jiao et al. 2017). FKBP51 overexpression also promotes cellular apoptosis in lung cancer by enhancing p53 signaling pathway activity (Chen, Liu et al. 2020). Although it is known that p53 interacts with nuclear AKT and regulates its activation (Chen, Liu et al. 2020), and may promote cellular autophagy following inhibition of AKT activation (Cordani, Butera et al. 2017), comprehensive elucidation of the p53-AKT mechanism impeding lung malignancy is yet to be determined. However, association between FKBP51 and p53 expression implies that lung cancer progression can be blocked by FKBP51 via this pathway (Chen, Liu et al. 2020). On the other hand, overexpression of FKBP52 in non-small-cell lung cancer, triggers tumorigenesis by potentiating Akt signaling (Meng, Meng et al. 2020), instead of suppressing malignant progress. Identical outcomes were also recorded with a triple negative breast cancer cell model and murine xenograft tumor models which offers an alternative treatment to hormonal therapy for non-responding triple negative breast cancer patients (Mange, Coyaud et al. 2019).
Conclusively, the findings to-date have established TPR-domain immunophilins FKBP51 and FKBP52 as prognosis biomarkers, therapeutic targets, and chemotherapy response indicators which can assist to tailor individualized anticancer treatments. Yet, the roles of FKBP51 and FKBP52 as tumor regulators remain controversial, delineating a complex scenario of various intersections of FKBP51-related molecular pathways among different tumors (Staibano, Mascolo et al. 2011). Hence, the comprehensive elucidation of these metabolic pathways and underlying mechanisms remains elusive and warrants further investigation.
Stress-related disorders
The underlying mechanisms of stress-influenced health conditions involves the role of FKBP51 and FKBP52 as integral components of glucocorticoid receptor complex. FKBP51 and FKB52 are major regulators of glucocorticoid receptor activity and participate in restoring hypothalamic–pituitary–adrenal (HPA) homeostasis post stress induction, which is mediated by various feedback signaling loops. FKBP51 displays negative feedback control of GR sensitivity by decreasing glucocorticoid receptor (GR) affinity for glucocorticoids and nuclear translocation of GR (Riggs, Roberts et al. 2003, Zannas, Wiechmann et al. 2016, Gan, Wang et al. 2022). Malfunction of GR activity owing to FKBP51 overexpression is one of the most prominent characteristics associated with stress-related illnesses and psychiatric disorders (Wang, Chai et al. 2010, Xie, Kranzler et al. 2010, Fani, Gutman et al. 2013). Since FKBP52 is known to positively regulate the glucocorticoid receptor activity, heterozygous fkbp52-deficient mice exhibited induced stress sensitivity mimicking the response to FKBP51, likely owing to reduced GR sensitivity (Hartmann, Wagner et al. 2012). The intricate combinations of reactions like deletion of FKBP52, and downregulation of FKBP51 may provide a possible explanation (Hartmann, Wagner et al. 2012). Furthermore, FKBP52 does not engage in all areas of GR signaling pathways. Instead, regulation of GR transcriptional activity by FKBP52 is gene specific, where FKBP52 may act as a modulatory factor (Wolf, Periyasamy et al. 2009). However, precise mechanisms involved here remain elusive.
Studies on the role of FKBP51 in PTSD and major depression showed that alterations in the epigenetic modifications of fkbp5 can lead to glucocorticoid resistance in the brain, which contributes to the onset of these neuropsychiatric disorders, among others. (Zannas, Wiechmann et al. 2016). Genes regulating HPA homeostasis are often associated with neuronal functions and stress related disorders, and interaction of these genes with stress factors have influence on the disease onset. Fkbp5 genetic variants (single nucleotide polymorphisms) expressing higher FKBP51 is frequently found in multiple stress related anxiety and depression disorders. For example, exposure of a stressor with FKBP5 single nucleotide polymorphisms (SNP) is correlated with increased risk of post-traumatic stress disorder (PTSD) (Sabbagh, Cordova et al. 2018). In line with these findings, combination of FKBP5 SNPs with early life stressor such as childhood adversity can cause maladaptive GR activity and long term HPA homeostasis disequilibrium. Ultimately, this influences adult response to trauma, and increases the risk of PTSD (Binder, Bradley et al. 2008, Sabbagh, Cordova et al. 2018). These findings delineate a role for FKBP51 in neuronal plasticity and identify it as a hallmark for PTSD prediction. There is significant association between FKBP51 and hippocampal volume alterations in PTSD patients (Dannlowski, Grabe et al. 2015, Yun, Jin et al. 2022).
Direct correlation between FKBP51 and anxiety related behavior is supported by the observations of reduced corticosterone secretion with deletion of FKBP51 in mice (Hartmann, Wagner et al. 2012, Hartmann, Wagner et al. 2015). Lack of FKBP51 is found to change the brain structure and connectivity, revealing link among genetic status of FKBP5, stress sensitivity and psychiatric disorders (Engelhardt, Boulat et al. 2021). Site specific deletion of FKBP5 increases GR sensitivity and rescues the stress response, whereas overexpression of the same gene exhibits over-activation of HPA axis (Hausl, Brix et al. 2021). This suggests that FKBP51 is a potential target for advanced treatment of stress related disorders, and that FKBP51 hypoexpression could have a wider role in inflammatory stress-associated mental disturbance (Gan, Wang et al. 2022).
Evidence suggests that GR-FKBP51 complex or FKBP5 genes can be potential therapeutic targets or stress responsive markers for various central nervous system disorders. While new reports corroborating correlation of FKBP51 and FKBP52 with stress related disorders are emerging, there is a lot more to unveil to develop diagnostic tools and disease specific treatments.
Therapeutic targeting
Given the many roles for FKBP51 and FKBP52 discussed above, the FKBPs have emerged as attractive therapeutic targets for a myriad of endocrine and cardiovascular diseases (Sivils, Storer et al. 2011, Guy, Garcia et al. 2015, Ghartey-Kwansah, Li et al. 2018) and are well-documented druggable targets due to their PPIase catalytic site, which is an ideal hydrophobic drug-binding pocket. While the FKBP51 and FKBP52 PPIase pockets have been selectively targeted with the SaFit class of drugs (Gaali, Kirschner et al. 2015, Jagtap, Asami et al. 2019, Bauder, Meyners et al. 2021), as discussed above, this class of drugs are not likely to have utility in the treatment of hormone-dependent diseases given that FKBP regulation of the SHRs is independent of the PPIase catalytic activity. That being said, the FKBP51 and FKBP52 PPIase inhibitors will likely have therapeutic utility in a wide variety of disease settings in which the PPIase activity is critical; the stabilization of abnormal tau protein in AD is a prime example (Jinwal, Koren et al. 2010).
Given the positive regulation of AR by FKBP52 in prostate cancer, FKBP52 and other cochaperones have emerged as potential therapeutic targets for the treatment of androgen-dependent prostate cancer. In line with this idea, we previously characterized a putative FKBP52 regulatory surface on AR termed binding function 3 (BF3) (De Leon, Iwai et al. 2011). In addition, we have demonstrated that FKBP52 interacts directly with β-catenin and promotes β-catenin interaction with AR, likely through the AR BF3 surface, to promote a synergistic upregulation of AR activity. Interestingly, the FKBP52 proline-rich loop, but not FKBP52 binding to Hsp90, is critical for this novel co-regulatory mechanism (Storer 2010). We have also developed a first-in-class drug, termed MJC13, that specifically targets the FKBP52-dependent potentiation of AR activity, likely through binding the AR BF3 surface (De Leon, Iwai et al. 2011). Consequently, MJC13 was also shown to block FKBP52/β-catenin interaction with, and potentiation of, AR (Suh, Chattopadhyay et al. 2015). Thus, MJC13 represents the first drug that is capable of blocking the regulation of AR activity by both FKBP52 and β-catenin, and has displayed promising results in prostate cancer xenograft models (Liang, Bian et al. 2016). While the rationale for the use of MJC13 in the treatment of prostate cancer is obvious, recent findings strongly suggest that these drugs will also have utility in the treatment of subsets of breast cancer (D’Amato, Gordon et al. 2016). AR is expressed in 90% of ER positive breast cancer tumors, and by decreasing AR nuclear localization and genome binding using MJC13 and other antiandrogens, the authors suggest that AR supports ER activity in breast cancer. Additionally, it was demonstrated that inhibiting AR directly, also inhibits ER indirectly in breast cancer (D’Amato, Gordon et al. 2016). While MJC13 shows great promise for the treatment of prostate cancer and specific subtypes of breast cancer, it will not likely have utility outside of these disease settings given that it specifically targets an FKBP52 regulatory surface on the androgen receptor and is specific to FKBP52-regulated AR activity. Thus, targeting FKBP52 directly could potentially have broader utility across many relevant disease settings.
The inhibition of FKBP52 PPIase activity is not synonymous with the inhibition of FKBP52 regulation of receptor activity. As discussed above, the proline-rich loop overhanging the PPIase pocket is the more functionally relevant target. However, the proline-rich loop surface does not represent an ideal small molecule docking site. To overcome this barrier, we more recently used structure-based drug design and in silico screening to identify an early hit molecule termed GMC1 that is not only predicted to target the FKBP52 PPIase pocket, but is also predicted to affect the conformation of the proline-rich loop surface (Ekpenyong, Cooper et al. 2020). Targeting the PPIase pocket with molecules that can simultaneously disrupt interactions on the proline-rich loop surface would be predicted to target a wide variety of factors simultaneously including AR, GR and PR activity, as well as PPIase-dependent functions of the FKBPs. Much work remains to be done to validate this targeting approach. However, combining this approach with the approach used to develop SaFit molecules with the ability to selectively target individual FKBP proteins holds promise for the development of FKBP targeting drugs with broad utility.
Much like FKBP52, FKBP51 has also been the focus of novel therapeutic interventions of diseases that have an aberrant expression of FKBP51. FKBP51 is a negative regulator of GR signaling that can dysregulate the HPA axis through alteration of GR. Thus, Sabbagh et. al sought to determine if disturbing FKBP51 could restore GR activity in Hela cells by treating cells with benztropine mesylate. Unequivocally, an in vitro protein interaction assay demonstrated the disruption of FKBP51 from the GR/Hsp90 complex in response to treatment, which was later verified by co-immunoprecipitation of GR heterocomplex. Furthermore, benztropine reversed FKBP51 inhibition of GR translocation and restored expression of GRE regulated genes (Sabbagh, Cordova et al. 2018).
Concluding remarks
The functions of the TPR-domain immunophilins FKBP51 and FKBP52 in normal physiology and disease are diverse, and new roles, mechanisms and interactors for these FKBPs continue to be identified. FKBP51 and FKBP52 are now known to interact, or at least associate, with a wide variety of other proteins (Figure 2). Among these 50 genes that were identified from the database, PINK1, PARK2, and AR have been discussed previously in this article as interactors of immunophilins and their relationship described. While many of these associated factors are shared among the two FKBPs, many are also unique to FKBP51 or FKBP52. While the fkbp51- and fkbp52-deficient mice display unique phenotypes (Yang, Wolf et al. 2006, Tranguch, Wang et al. 2007), the knockout of both FKBP51 and FKBP52 in mice results in embryonic lethality (Storer, Dickey et al. 2011). Thus, FKBP51 and FKBP52 not only have distinct functions, but they clearly have some redundant functions that are critical for embryonic development. Perhaps the most logical next step towards understanding the critical roles that FKBP51 and FKBP52 have in normal physiology and disease is to compare the known interactomes for FKBP51 and FKBP52 to begin to understand their distinct, as well, as shared functions. This would, in turn, better inform the therapeutic targeting strategies, given that FKBP51 and FKBP52 have emerged as attractive therapeutic targets in a wide variety of disease settings.
Figure 2: Protein-Protein Interaction Network For Known Interactors of both FKBP5 and FKBP4.
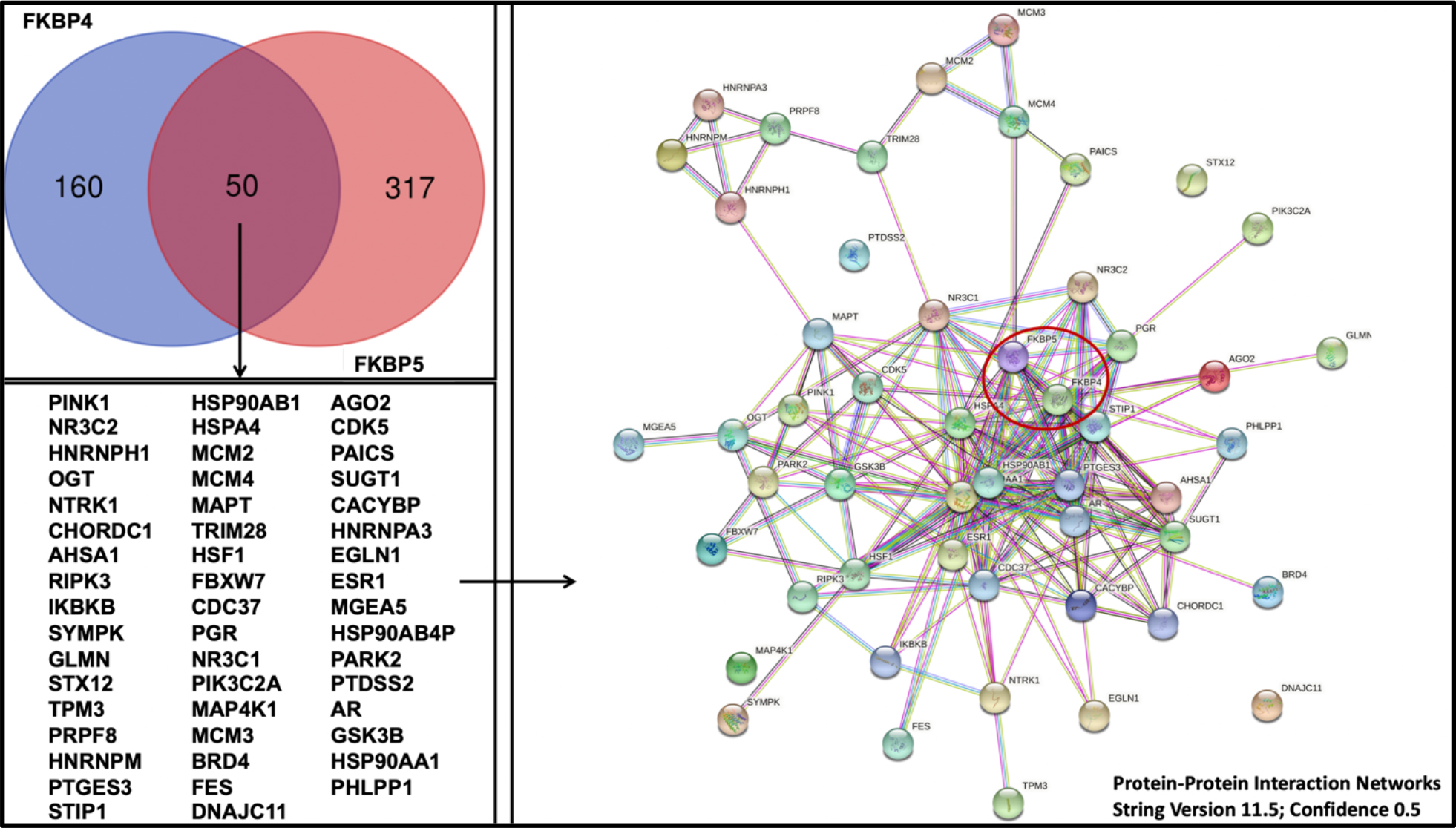
The venn diagram (top left) collates interactors from the BioGRID protein interaction database for FKBP4 and FKBP5, respectively. TheBioGRID database was accessed 25 September 2022 at 2:32 AM UTC. Results were filtered by evidence type “Interactors with ONLY Physical Evidence (LTP or HTP)” and organism (H. sapiens). Interactors identified only in pre-publication datasets were excluded. Shared interactors (bottom left) were used to generate a PPI network using STRING v11.5 (1–ɑ ≥ 0.5). While interactors were filtered based on methodology detecting physical interaction, not all of these methodologies distinguish between direct interaction versus association through protein complexes. Thus, the interactors shown here may be associated with the FKBPs through larger protein complexes in some cases, but the figure highlights the fact that FKBP51 and FKBP52 have shared interactors as well as a significant number of distinct interactors.
Acknowledgements
The authors’ laboratories are partially supported by NIH grant 2G12MD007592 to the Border Biomedical Research Center (BBRC), from the National Institutes on Minority Health and Health Disparities (NIMHD). M.B.C was also supported by the Lizanell and Colbert Coldwell Foundation and the Department of Defense (DOD) Prostate Cancer Research Program (PCRP) through grant number W81XWH-17-1-0435. S.R. is supported by the cancer Prevention and Research Institute of Texas (CPRIT) Texas Regional Excellence in Cancer Research (TREC) program through grant number RP210153. C.R. is supported by U-RISE through grant number T34GM145529 from the National Institute of General Sciences (NIGMS). J.D. is supported by G-RISE through NIGMS grant number T32GM144919.
REFERENCES
- Acar N and Ustunel I (2015). “Expression of 52-kDa FK506-binding protein (FKBP52) in human placenta complicated by preeclampsia and intrauterine growth restriction.” Anal Quant Cytopathol Histpathol 37(2): 87–95. [PubMed] [Google Scholar]
- Bailus BJ, Scheeler SM, Simons J, Sanchez MA, Tshilenge KT, Creus-Muncunill J, Naphade S, Lopez-Ramirez A, Zhang N, Lakshika Madushani K, Moroz S, Loureiro A, Schreiber KH, Hausch F, Kennedy BK, Ehrlich ME and Ellerby LM (2021). “Modulating FKBP5/FKBP51 and autophagy lowers HTT (huntingtin) levels.” Autophagy 17(12): 4119–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JD, Ozsan I, Rodriguez Ospina S, Gulick D and Blair LJ (2018). “Hsp90 Heterocomplexes Regulate Steroid Hormone Receptors: From Stress Response to Psychiatric Disease.” Int J Mol Sci 20(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauder M, Meyners C, Purder PL, Merz S, Sugiarto WO, Voll AM, Heymann T and Hausch F (2021). “Structure-Based Design of High-Affinity Macrocyclic FKBP51 Inhibitors.” J Med Chem 64(6): 3320–3349. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF and Ressler KJ (2008). “Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults.” JAMA 299(11): 1291–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair LJ, Baker JD, Sabbagh JJ and Dickey CA (2015). “The emerging role of peptidyl-prolyl isomerase chaperones in tau oligomerization, amyloid processing, and Alzheimer’s disease.” J Neurochem 133(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair LJ, Nordhues BA, Hill SE, Scaglione KM, O’Leary JC 3rd, Fontaine SN, Breydo L, Zhang B, Li P, Wang L, Cotman C, Paulson HL, Muschol M, Uversky VN, Klengel T, Binder EB, Kayed R, Golde TE, Berchtold N and Dickey CA (2013). “Accelerated neurodegeneration through chaperone-mediated oligomerization of tau.” J Clin Invest 123(10): 4158–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonying W, Joselin A, Huang E, Qu D, Safarpour F, Iyirhiaro GO, Gonzalez YR, Callaghan SM, Slack RS, Figeys D, Chung YH and Park DS (2019). “Pink1 regulates FKBP5 interaction with AKT/PHLPP and protects neurons from neurotoxin stress induced by MPP(.).” J Neurochem 150(3): 312–329. [DOI] [PubMed] [Google Scholar]
- Bracher A, Kozany C, Hahle A, Wild P, Zacharias M and Hausch F (2013). “Crystal structures of the free and ligand-bound FK1-FK2 domain segment of FKBP52 reveal a flexible inter-domain hinge.” J Mol Biol 425(22): 4134–4144. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J and Julie Kim J (2006). “Progesterone resistance in endometriosis: link to failure to metabolize estradiol.” Mol Cell Endocrinol 248(1–2): 94–103. [DOI] [PubMed] [Google Scholar]
- Callebaut I, Renoir JM, Lebeau MC, Massol N, Burny A, Baulieu EE and Mornon JP (1992). “An immunophilin that binds M(r) 90,000 heat shock protein: main structural features of a mammalian p59 protein.” Proc Natl Acad Sci U S A 89(14): 6270–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambraud B, Byrne C, Meduri G, Baulieu EE and Giustiniani J (2022). “FKBP52 in Neuronal Signaling and Neurodegenerative Diseases: A Microtubule Story.” Int J Mol Sci 23(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambraud B, Rouviere-Fourmy N, Radanyi C, Hsiao K, Peattie DA, Livingston DJ and Baulieu EE (1993). “Overexpression of p59-HBI (FKBP59), full length and domains, and characterization of PPlase activity.” Biochem Biophys Res Commun 196(1): 160–166. [DOI] [PubMed] [Google Scholar]
- Check JH, Dix E, Wilson C and Check D (2010). “Progesterone receptor antagonist therapy has therapeutic potential even in cancer restricted to males as evidenced from murine testicular and prostate cancer studies.” Anticancer Res 30(12): 4921–4923. [PubMed] [Google Scholar]
- Chen Y, Liu Z, Wang Y, Zhuang J, Peng Y, Mo X, Chen J, Shi Y, Yu M, Cai W, Li Y, Zhu X, Yuan W, Li Y, Li F, Zhou Z, Dai G, Ye X, Wan Y, Jiang Z, Zhu P, Fan X and Wu X (2020). “FKBP51 induces p53-dependent apoptosis and enhances drug sensitivity of human non-small-cell lung cancer cells.” Exp Ther Med 19(3): 2236–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C and Smith DF (2005). “Physiological Role for the Cochaperone FKBP52 in Androgen Receptor Signaling.” Molecular Endocrinology 19(6): 1654–1666. [DOI] [PubMed] [Google Scholar]
- Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C and Smith DF (2005). “Physiological role for the cochaperone FKBP52 in androgen receptor signaling.” Mol Endocrinol 19(6): 1654–1666. [DOI] [PubMed] [Google Scholar]
- Cheung-Flynn J, Roberts PJ, Riggs DL and Smith DF (2003). “C-terminal Sequences outside the Tetratricopeptide Repeat Domain of FKBP51 and FKBP52 Cause Differential Binding to Hsp90.” J Biol Chem 278(19): 17388–17394. [DOI] [PubMed] [Google Scholar]
- Cluning C, Ward BK, Rea SL, Arulpragasam A, Fuller PJ and Ratajczak T (2013). “The helix 1–3 loop in the glucocorticoid receptor LBD is a regulatory element for FKBP cochaperones.” Mol Endocrinol 27(7): 1020–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordani M, Butera G, Pacchiana R and Donadelli M (2017). “Molecular interplay between mutant p53 proteins and autophagy in cancer cells.” Biochim Biophys Acta Rev Cancer 1867(1): 19–28. [DOI] [PubMed] [Google Scholar]
- Cox MB, Riggs DL, Hessling M, Schumacher F, Buchner J and Smith DF (2007). “FK506-binding protein 52 phosphorylation: a potential mechanism for regulating steroid hormone receptor activity.” Mol Endocrinol. 21(12): 2956–2967. [DOI] [PubMed] [Google Scholar]
- Criado-Marrero M, Gebru NT, Gould LA, Blazier DM, Vidal-Aguiar Y, Smith TM, Abdelmaboud SS, Shelton LB, Wang X, Dahrendorff J, Beaulieu-Abdelahad D, Dickey CA and Blair LJ (2021). “FKBP52 overexpression accelerates hippocampal-dependent memory impairments in a tau transgenic mouse model.” NPJ Aging Mech Dis 7(1): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado-Marrero M, Rein T, Binder EB, Porter JT, Koren J 3rd and Blair LJ (2018). “Hsp90 and FKBP51: complex regulators of psychiatric diseases.” Philos Trans R Soc Lond B Biol Sci 373(1738). [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato NC, Gordon MA, Babbs B, Spoelstra NS, Carson Butterfield KT, Torkko KC, Phan VT, Barton VN, Rogers TJ, Sartorius CA, Elias A, Gertz J, Jacobsen BM and Richer JK (2016). “Cooperative Dynamics of AR and ER Activity in Breast Cancer.” Mol Cancer Res 14(11): 1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Grabe HJ, Wittfeld K, Klaus J, Konrad C, Grotegerd D, Redlich R, Suslow T, Opel N, Ohrmann P, Bauer J, Zwanzger P, Laeger I, Hohoff C, Arolt V, Heindel W, Deppe M, Domschke K, Hegenscheid K, Volzke H, Stacey D, Meyer Zu Schwabedissen H, Kugel H and Baune BT (2015). “Multimodal imaging of a tescalcin (TESC)-regulating polymorphism (rs7294919)-specific effects on hippocampal gray matter structure.” Mol Psychiatry 20(3): 398–404. [DOI] [PubMed] [Google Scholar]
- Davies TH, Ning YM and Sanchez ER (2002). “A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins.” J Biol Chem 277(7): 4597–4600. [DOI] [PubMed] [Google Scholar]
- De Leon JT, Iwai A, Feau C, Garcia Y, Balsiger HA, Storer CL, Suro RM, Garza KM, Lee S, Kim YS, Chen Y, Ning YM, Riggs DL, Fletterick RJ, Guy RK, Trepel JB, Neckers LM and Cox MB (2011). “Targeting the regulation of androgen receptor signaling by the heat shock protein 90 cochaperone FKBP52 in prostate cancer cells.” Proc Natl Acad Sci U S A 108(29): 11878–11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny WB, Valentine DL, Reynolds PD, Smith DF and Scammell JG (2000). “Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding.” Endocrinology 141(11): 4107–4113. [DOI] [PubMed] [Google Scholar]
- Dinsdale NL and Crespi BJ (2021). “Endometriosis and polycystic ovary syndrome are diametric disorders.” Evol Appl 14(7): 1693–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Jiao Y, Mu W, Lu B, Wei M, Sun L, Hu S, Cui B, Liu X, Chen Z and Zhao Y (2017). “FKBP51 decreases cell proliferation and increases progestin sensitivity of human endometrial adenocarcinomas by inhibiting Akt.” Oncotarget 8(46): 80405–80415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunyak BM and Gestwicki JE (2016). “Peptidyl-Proline Isomerases (PPIases): Targets for Natural Products and Natural Product-Inspired Compounds.” J Med Chem 59(21): 9622–9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebong IO, Beilsten-Edmands V, Patel NA, Morgner N and Robinson CV (2016). “The interchange of immunophilins leads to parallel pathways and different intermediates in the assembly of Hsp90 glucocorticoid receptor complexes.” Cell Discov 2: 16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria PC, Mazaira G, Erlejman A, Gomez-Sanchez C, Piwien Pilipuk G and Galigniana MD (2009). “Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta.” Mol Cell Biol 29(17): 4788–4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekpenyong O, Cooper C, Ma J, Guy NC, Payan AN, Ban F, Cherkasov A, Cox MB, Liang D and Xie H (2020). “Bioanalytical Assay Development and Validation for the Pharmacokinetic Study of GMC1, a Novel FKBP52 Co-chaperone Inhibitor for Castration Resistant Prostate Cancer.” Pharmaceuticals (Basel) 13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt C, Boulat B, Czisch M and Schmidt MV (2021). “Lack of FKBP51 Shapes Brain Structure and Connectivity in Male Mice.” J Magn Reson Imaging 53(5): 1358–1365. [DOI] [PubMed] [Google Scholar]
- Fani N, Gutman D, Tone EB, Almli L, Mercer KB, Davis J, Glover E, Jovanovic T, Bradley B, Dinov ID, Zamanyan A, Toga AW, Binder EB and Ressler KJ (2013). “FKBP5 and attention bias for threat: associations with hippocampal function and shape.” JAMA Psychiatry 70(4): 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federer-Gsponer JR, Quintavalle C, Muller DC, Dietsche T, Perrina V, Lorber T, Juskevicius D, Lenkiewicz E, Zellweger T, Gasser T, Barrett MT, Rentsch CA, Bubendorf L and Ruiz C (2018). “Delineation of human prostate cancer evolution identifies chromothripsis as a polyclonal event and FKBP4 as a potential driver of castration resistance.” J Pathol 245(1): 74–84. [DOI] [PubMed] [Google Scholar]
- Fischer G (1994). “Peptidyl-Prolyl &/trans Isomerases and Their Effectors.” 1415–1436 [Google Scholar]
- Fischer G, Bang H and Mech C (1984). “[Determination of enzymatic catalysis for the cis-trans-isomerization of peptide binding in proline-containing peptides].” Biomed Biochim Acta. 43(10): 1101–1111. [PubMed] [Google Scholar]
- Fries GR, Gassen NC and Rein T (2017). “The FKBP51 Glucocorticoid Receptor Co-Chaperone: Regulation, Function, and Implications in Health and Disease.” Int J Mol Sci 18(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaali S, Kirschner A, Cuboni S, Hartmann J, Kozany C, Balsevich G, Namendorf C, Fernandez-Vizarra P, Sippel C, Zannas AS, Draenert R, Binder EB, Almeida OF, Ruhter G, Uhr M, Schmidt MV, Touma C, Bracher A and Hausch F (2015). “Selective inhibitors of the FK506-binding protein 51 by induced fit.” Nat Chem Biol 11(1): 33–37. [DOI] [PubMed] [Google Scholar]
- Galat A (1993). “Peptidylproline cis-trans-isomerases: immunophilins.” Eur J Biochem 216(3): 689–707. [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Erlejman AG, Monte M, Gomez-Sanchez C and Piwien-Pilipuk G (2010). “The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events.” Mol Cell Biol 30(5): 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galigniana MD, Harrell JM, Murphy PJ, Chinkers M, Radanyi C, Renoir JM, Zhang M and Pratt WB (2002). “Binding of hsp90-associated immunophilins to cytoplasmic dynein: direct binding and in vivo evidence that the peptidylprolyl isomerase domain is a dynein interaction domain.” Biochemistry 41(46): 13602–13610. [DOI] [PubMed] [Google Scholar]
- Gan YL, Wang CY, He RH, Hsu PC, Yeh HH, Hsieh TH, Lin HC, Cheng MY, Jeng CJ, Huang MC and Lee YH (2022). “FKBP51 mediates resilience to inflammation-induced anxiety through regulation of glutamic acid decarboxylase 65 expression in mouse hippocampus.” J Neuroinflammation 19(1): 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellersen B, Brosens IA and Brosens JJ (2007). “Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives.” Semin Reprod Med 25(6): 445–453. [DOI] [PubMed] [Google Scholar]
- Gellersen B and Brosens JJ (2014). “Cyclic decidualization of the human endometrium in reproductive health and failure.” Endocr Rev 35(6): 851–905. [DOI] [PubMed] [Google Scholar]
- Ghartey-Kwansah G, Li Z, Feng R, Wang L, Zhou X, Chen FZ, Xu MM, Jones O, Mu Y, Chen S, Bryant J, Isaacs WB, Ma J and Xu X (2018). “Comparative analysis of FKBP family protein: evaluation, structure, and function in mammals and Drosophila melanogaster.” BMC Dev Biol 18(1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustiniani J, Chambraud B, Sardin E, Dounane O, Guillemeau K, Nakatani H, Paquet D, Kamah A, Landrieu I, Lippens G, Baulieu EE and Tawk M (2014). “Immunophilin FKBP52 induces Tau-P301L filamentous assembly in vitro and modulates its activity in a model of tauopathy.” Proc Natl Acad Sci U S A 111(12): 4584–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustiniani J, Guillemeau K, Dounane O, Sardin E, Huvent I, Schmitt A, Hamdane M, Buee L, Landrieu I, Lippens G, Baulieu EE and Chambraud B (2015). “The FK506-binding protein FKBP52 in vitro induces aggregation of truncated Tau forms with prion-like behavior.” FASEB J 29(8): 3171–3181. [DOI] [PubMed] [Google Scholar]
- Giustiniani J, Sineus M, Sardin E, Dounane O, Panchal M, Sazdovitch V, Duyckaerts C, Chambraud B and Baulieu EE (2012). “Decrease of the immunophilin FKBP52 accumulation in human brains of Alzheimer’s disease and FTDP-17.” J Alzheimers Dis 29(2): 471–483. [DOI] [PubMed] [Google Scholar]
- Guy NC, Garcia YA and Cox MB (2015). “Therapeutic Targeting of the FKBP52 Co-Chaperone in Steroid Hormone Receptor-Regulated Physiology and Disease.” Curr Mol Pharmacol 9(2): 109–125. [DOI] [PubMed] [Google Scholar]
- Guzeloglu-Kayisli O, Semerci N, Guo X, Larsen K, Ozmen A, Arlier S, Mutluay D, Nwabuobi C, Sipe B, Buhimschi I, Buhimschi C, Schatz F, Kayisli UA and Lockwood CJ (2021). “Decidual cell FKBP51-progesterone receptor binding mediates maternal stress-induced preterm birth.” Proc Natl Acad Sci U S A 118(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habara M, Sato Y, Goshima T, Sakurai M, Imai H, Shimizu H, Katayama Y, Hanaki S, Masaki T, Morimoto M, Nishikawa S, Toyama T and Shimada M (2022). “FKBP52 and FKBP51 differentially regulate the stability of estrogen receptor in breast cancer.” Proc Natl Acad Sci U S A 119(15): e2110256119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding MW, Galat A, Uehling DE and Schreiber SL (1989). “A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase.” Nature 341(6244): 758–760. [DOI] [PubMed] [Google Scholar]
- Harris DC, Garcia YA, Samaniego CS, Rowlett VW, Ortiz NR, Payan AN, Maehigashi T and Cox MB (2019). “Functional Comparison of Human and Zebra Fish FKBP52 Confirms the Importance of the Proline-Rich Loop for Regulation of Steroid Hormone Receptor Activity.” Int J Mol Sci 20(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Wagner KV, Gaali S, Kirschner A, Kozany C, Ruhter G, Dedic N, Hausl AS, Hoeijmakers L, Westerholz S, Namendorf C, Gerlach T, Uhr M, Chen A, Deussing JM, Holsboer F, Hausch F and Schmidt MV (2015). “Pharmacological Inhibition of the Psychiatric Risk Factor FKBP51 Has Anxiolytic Properties.” J Neurosci 35(24): 9007–9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Wagner KV, Liebl C, Scharf SH, Wang XD, Wolf M, Hausch F, Rein T, Schmidt U, Touma C, Cheung-Flynn J, Cox MB, Smith DF, Holsboer F, Muller MB and Schmidt MV (2012). “The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress.” Neuropharmacology 62(1): 332–339. [DOI] [PubMed] [Google Scholar]
- Hausl AS, Brix LM, Hartmann J, Pohlmann ML, Lopez JP, Menegaz D, Brivio E, Engelhardt C, Roeh S, Bajaj T, Rudolph L, Stoffel R, Hafner K, Goss HM, Reul J, Deussing JM, Eder M, Ressler KJ, Gassen NC, Chen A and Schmidt MV (2021). “The co-chaperone Fkbp5 shapes the acute stress response in the paraventricular nucleus of the hypothalamus of male mice.” Mol Psychiatry 26(7): 3060–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Li L and Luan S (2004). “Immunophilins and parvulins. Superfamily of peptidyl prolyl isomerases in Arabidopsis.” Plant Physiol 134(4): 1248–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y, Acar N, Tranguch S, Burnum KE, Xie H, Kodama A, Osuga Y, Ustunel I, Friedman DB, Caprioli RM, Daikoku T and Dey SK (2010). “Uterine FK506-binding protein 52 (FKBP52)-peroxiredoxin-6 (PRDX6) signaling protects pregnancy from overt oxidative stress.” Proc Natl Acad Sci U S A 107(35): 15577–15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y, Tranguch S, Daikoku T, Hasegawa A, Osuga Y, Taketani Y and Dey SK (2008). “Deficiency of immunophilin FKBP52 promotes endometriosis.” Am J Pathol 173(6): 1747–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C, Li T, Zhang F, Wu X, Chen X, Cui X, Zhang G and Cui Y (2017). “Elevated FKBP52 expression indicates a poor outcome in patients with breast cancer.” Oncol Lett 14(5): 5379–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Kim ST, Tranguch S, Smith DF and Dey SK (2007). “Deficiency of co-chaperone immunophilin FKBP52 compromises sperm fertilizing capacity.” Reproduction. 133(2): 395–403. [DOI] [PubMed] [Google Scholar]
- Hou J and Wang L (2012). “FKBP5 as a selection biomarker for gemcitabine and Akt inhibitors in treatment of pancreatic cancer.” PLoS One 7(5): e36252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF and Scammell JG (2003). “The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness.” Endocrinology 144(6): 2380–2387. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen T, Makkonen H and Palvimo JJ (2011). “Steroid up-regulation of FKBP51 and its role in hormone signaling.” Curr Opin Pharmacol 11(4): 326–331. [DOI] [PubMed] [Google Scholar]
- Jagtap PKA, Asami S, Sippel C, Kaila VRI, Hausch F and Sattler M (2019). “Corrigendum: Selective Inhibitors of FKBP51 Employ Conformational Selection of Dynamic Invisible States.” Angew Chem Int Ed Engl 58(39): 13619. [DOI] [PubMed] [Google Scholar]
- Jiang W, Cazacu S, Xiang C, Zenklusen JC, Fine HA, Berens M, Armstrong B, Brodie C and Mikkelsen T (2008). “FK506 binding protein mediates glioma cell growth and sensitivity to rapamycin treatment by regulating NF-kappaB signaling pathway.” Neoplasia 10(3): 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinwal UK, Koren J III, Borysov SI, Schmid AB, Abisambra JF, Blair LJ, Johnson AG, Jones JR, Shults CL, O’Leary JC III, Jin Y, Buchner J, Cox MB and Dickey CA (2010). “The Hsp90 cochaperone, FKBP51, increases Tau stability and polymerizes microtubules.” J.Neurosci. 30(2): 591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NR, Miyadahira EH, Afshar Y, Jeong JW, Young SL, Lessey BA, Serafini PC and Fazleabas AT (2017). “Progesterone Resistance in Endometriosis Is Modulated by the Altered Expression of MicroRNA-29c and FKBP4.” J Clin Endocrinol Metab 102(1): 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang CB, Hong Y, Dhe-Paganon S and Yoon HS (2008). “FKBP family proteins: immunophilins with versatile biological functions.” Neurosignals 16(4): 318–325. [DOI] [PubMed] [Google Scholar]
- Ketefian A, Jones MR, Krauss RM, Chen YD, Legro RS, Azziz R and Goodarzi MO (2016). “Association study of androgen signaling pathway genes in polycystic ovary syndrome.” Fertil Steril 105(2): 467–473 e464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolos JM, Voll AM, Bauder M and Hausch F (2018). “FKBP Ligands-Where We Are and Where to Go?” Front Pharmacol 9: 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M and Sabatini DM (2012). “mTOR signaling in growth control and disease.” Cell 149(2): 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitao B, Jones MC, Fusi L, Higham J, Lee Y, Takano M, Goto T, Christian M, Lam EW and Brosens JJ (2010). “Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals.” FASEB J 24(5): 1541–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Jiao YL, Zhou RF, Liu S, Cui B, Wang LC, Liu XW and Zhao YR (2020). “FKBP51 acts as a biomarker of early metastasis and is related to carmustine sensitivity in human glioma cells.” Eur Rev Med Pharmacol Sci 24(17): 8918–8930. [DOI] [PubMed] [Google Scholar]
- Li L, Lou Z and Wang L (2011). “The role of FKBP5 in cancer aetiology and chemoresistance.” Br J Cancer 104(1): 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Bian X, Liang D, Sivils JC, Neckers LM, Cox MB and Xie H (2016). “Solution formulation development and efficacy of MJC13 in a preclinical model of castration-resistant prostate cancer.” Pharm Dev Technol 21(1): 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Cheng J, Wei F, Pang L, Zhi Z, Yang W and Tan W (2021). “The Influence Mechanism of Abnormal Immunophilin FKBP52 on the Expression Levels of PR-A and PR-B in Endometriosis Based on Endometrial Stromal Cell Model in Vitro.” Organogenesis 17(1–2): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lousse JC, Van Langendonckt A, Defrere S, Ramos RG, Colette S and Donnez J (2012). “Peritoneal endometriosis is an inflammatory disease.” Front Biosci (Elite Ed) 4(1): 23–40. [DOI] [PubMed] [Google Scholar]
- Maeda K, Habara M, Kawaguchi M, Matsumoto H, Hanaki S, Masaki T, Sato Y, Matsuyama H, Kunieda K, Nakagawa H and Shimada M (2022). “FKBP51 and FKBP52 regulate androgen receptor dimerization and proliferation in prostate cancer cells.” Mol Oncol 16(4): 940–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mange A, Coyaud E, Desmetz C, Laurent E, Beganton B, Coopman P, Raught B and Solassol J (2019). “FKBP4 connects mTORC2 and PI3K to activate the PDK1/Akt-dependent cell proliferation signaling in breast cancer.” Theranostics 9(23): 7003–7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meduri G, Guillemeau K, Dounane O, Sazdovitch V, Duyckaerts C, Chambraud B, Baulieu EE and Giustiniani J (2016). “Caspase-cleaved Tau-D(421) is colocalized with the immunophilin FKBP52 in the autophagy-endolysosomal system of Alzheimer’s disease neurons.” Neurobiol Aging 46: 124–137. [DOI] [PubMed] [Google Scholar]
- Meng W, Meng J, Jiang H, Feng X, Wei D and Ding Q (2020). “FKBP4 Accelerates Malignant Progression of Non-Small-Cell Lung Cancer by Activating the Akt/mTOR Signaling Pathway.” Anal Cell Pathol (Amst) 2020: 6021602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y, Chambraud B, Radanyi C, Leclerc J, Lebeau MC, Renoir JM, Shirai R, Catelli MG, Yahara I and Baulieu EE (1997). “Phosphorylation of the immunosuppressant FK506-binding protein FKBP52 by casein kinase II: regulation of HSP90-binding activity of FKBP52.” Proc Natl Acad Sci U S A 94(26): 14500–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SC, Rimerman RA, Toran EJ, Chen S, Prapapanich V, Butts RN and Smith DF (1997). “Molecular cloning of human FKBP51 and comparisons of immunophilin interactions with Hsp90 and progesterone receptor.” Mol Cell Biol 17(2): 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L, Yang CS, Gioeli D, Frierson H, Toft DO and Paschal BM (2010). “FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells.” Mol Cell Biol 30(5): 1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroz J, Chang BJ, Wysoczanski P, Lee CT, Perez-Lara A, Chakraborty P, Hofele RV, Baker JD, Blair LJ, Biernat J, Urlaub H, Mandelkow E, Dickey CA and Zweckstetter M (2018). “Structure and pro-toxic mechanism of the human Hsp90/PPIase/Tau complex.” Nat Commun 9(1): 4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie DA, Harding MW, Fleming MA, DeCenzo MT, Lippke JA, Livingston DJ and Benasutti M (1992). “Expression and characterization of human FKBP52, an immunophilin that associates with the 90-kDa heat shock protein and is a component of steroid receptor complexes.” Proc Natl Acad Sci U S A 89(22): 10974–10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, Petersen G, Lou Z and Wang L (2009). “FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt.” Cancer Cell 16(3): 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, Petersen G, Lou Z and Wang L (2009). “FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt.” Cancer Cell 16(3): 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periyasamy S, Hinds T Jr., Shemshedini L, Shou W and Sanchez ER (2010). “FKBP51 and Cyp40 are positive regulators of androgen-dependent prostate cancer cell growth and the targets of FK506 and cyclosporin A.” Oncogene 29(11): 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkl F and Buchner J (2001). “Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40.” J Mol Biol 308(4): 795–806. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Silverstein AM and Galigniana MD (1999). “A model for the cytoplasmic trafficking of signalling proteins involving the hsp90-binding immunophilins and p50cdc37.” Cell Signal 11(12): 839–851. [DOI] [PubMed] [Google Scholar]
- Qiu B, Zhong Z, Righter S, Xu Y, Wang J, Deng R, Wang C, Williams KE, Ma YY, Tsechpenakis G, Liang T and Yong W (2022). “FKBP51 modulates hippocampal size and function in post-translational regulation of Parkin.” Cell Mol Life Sci 79(3): 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintá HR, Maschi D, Gomez-Sanchez C, Piwien Pilipuk G and Galigniana MD (2010). “Subcellular rearrangement of hsp90-binding immunophilins accompanies neuronal differentiation and neurite outgrowth.” J Neurochem 115: 716–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs DL, Cox MB, Tardif HL, Hessling M, Buchner J and Smith DF (2007). “Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling.” Mol Cell Biol 27(24): 8658–8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs DL, Cox MB, Tardif HL, Hessling M, Buchner J and Smith DF (2007). “Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling.” Mol Cell Biol. 27(24): 8658–8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, Gaber R, Picard D and Smith DF (2003). “The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo.” EMBO J 22(5): 1158–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano S, Xiao Y, Nakaya M, D’Angelillo A, Chang M, Jin J, Hausch F, Masullo M, Feng X, Romano MF and Sun SC (2015). “FKBP51 employs both scaffold and isomerase functions to promote NF-kappaB activation in melanoma.” Nucleic Acids Res 43(14): 6983–6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostam MA, Piva TJ, Rezaei HB, Kamato D, Little PJ, Zheng W and Osman N (2015). “Peptidyl-prolyl isomerases: functionality and potential therapeutic targets in cardiovascular disease.” Clin Exp Pharmacol Physiol 42(2): 117–124. [DOI] [PubMed] [Google Scholar]
- Rotoli D, Diaz-Flores L, Gutierrez R, Morales M, Avila J and Martin-Vasallo P (2022). “AmotL2, IQGAP1, and FKBP51 Scaffold Proteins in Glioblastoma Stem Cell Niches.” J Histochem Cytochem 70(1): 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouviere N, Vincent M, Craescu CT and Gallay J (1997). “Immunosuppressor binding to the immunophilin FKBP59 affects the local structural dynamics of a surface beta-strand: time-resolved fluorescence study.” Biochemistry 36(24): 7339–7352. [DOI] [PubMed] [Google Scholar]
- Ruiz-Estevez M, Staats J, Paatela E, Munson D, Katoku-Kikyo N, Yuan C, Asakura Y, Hostager R, Kobayashi H, Asakura A and Kikyo N (2018). “Promotion of Myoblast Differentiation by Fkbp5 via Cdk4 Isomerization.” Cell Rep 25(9): 2537–2551 e2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo D, Merolla F, Mascolo M, Ilardi G, Romano S, Varricchio S, Napolitano V, Celetti A, Postiglione L, Di Lorenzo PP, Califano L, Dell’Aversana GO, Astarita F, Romano MF and Staibano S (2017). “FKBP51 Immunohistochemical Expression: A New Prognostic Biomarker for OSCC?” Int J Mol Sci 18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh JJ, Cordova RA, Zheng D, Criado-Marrero M, Lemus A, Li P, Baker JD, Nordhues BA, Darling AL, Martinez-Licha C, Rutz DA, Patel S, Buchner J, Leahy JW, Koren J 3rd, Dickey CA and Blair LJ (2018). “Targeting the FKBP51/GR/Hsp90 Complex to Identify Functionally Relevant Treatments for Depression and PTSD.” ACS Chem Biol 13(8): 2288–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl FU and Moarefi I (2000). “Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine.” Cell 101(2): 199–210. [DOI] [PubMed] [Google Scholar]
- Schroer TA (2004). “Dynactin.” Annu Rev Cell Dev Biol 20: 759–779. [DOI] [PubMed] [Google Scholar]
- Shirane-Kitsuji M and Nakayama KI (2014). “Mitochondria: FKBP38 and mitochondrial degradation.” Int J Biochem Cell Biol 51: 19–22. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Boguski MS, Goebl M and Hieter P (1990). “A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis.” Cell 60(2): 307–317. [DOI] [PubMed] [Google Scholar]
- Sinars CR, Cheung-Flynn J, Rimerman RA, Scammell JG, Smith DF and Clardy J (2003). “Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes.” Proc Natl Acad Sci U S A 100(3): 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivils JC, Storer CL, Galigniana MD and Cox MB (2011). “Regulation of steroid hormone receptor function by the 52-kDa FK506-binding protein (FKBP52).” Curr Opin Pharmacol 19: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedlund KB, Sanchez ER and Hinds TD Jr. (2021). “FKBP51 and the molecular chaperoning of metabolism.” Trends Endocrinol Metab 32(11): 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF (2004). “Tetratricopeptide repeat cochaperones in steroid receptor complexes.” Cell Stress Chap 9(2): 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somarelli JA, Lee SY, Skolnick J and Herrera RJ (2008). “Structure-based classification of 45 FK506-binding proteins.” Proteins 72(1): 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S and Tan Y (2019). “Expression of FKBP52 in the ovaries of PCOS rats.” Int J Mol Med 43(2): 868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staibano S, Mascolo M, Ilardi G, Siano M and De Rosa G (2011). “Immunohistochemical analysis of FKBP51 in human cancers.” Curr Opin Pharmacol 11(4): 338–347. [DOI] [PubMed] [Google Scholar]
- Stewart M (2007). “Molecular mechanism of the nuclear protein import cycle.” Nat Rev Mol Cell Biol 8(3): 195–208. [DOI] [PubMed] [Google Scholar]
- Storer CL (2010). A Novel Synergistic Relationship between FKBP52 and Beta Catenin. El Paso, TX, The University of Texas at El Paso. [Google Scholar]
- Storer CL, Dickey CA, Galigniana MD, Rein T and Cox MB (2011). “FKBP51 and FKBP52 in signaling and disease.” Trends Endocrinol Metab 22(12): 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JH, Chattopadhyay A, Sieglaff DH, Storer Samaniego C, Cox MB and Webb P (2015). “Similarities and Distinctions in Actions of Surface-Directed and Classic Androgen Receptor Antagonists.” PLoS One 10(9): e0137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF and Dey SK (2005). “Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation.” Proc Natl Acad Sci U S A. 102(40): 14326–14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF and Dey SK (2005). “Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation.” Proc Natl Acad Sci U S A 102(40): 14326–14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranguch S, Wang H, Daikoku T, Xie H, Smith DF and Dey SK (2007). “FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific.” J Clin Invest. 117(7): 1824–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufano M, Cesaro E, Martinelli R, Pacelli R, Romano S and Romano MF (2021). “FKBP51 Affects TNF-Related Apoptosis Inducing Ligand Response in Melanoma.” Front Cell Dev Biol 9: 718947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL and Clardy J (1991). “Atomic structure of FKBP-FK506, an immunophilin-immunosuppressant complex.” Science 252(5007): 839–842. [DOI] [PubMed] [Google Scholar]
- Voll AM, Meyners C, Taubert MC, Bajaj T, Heymann T, Merz S, Charalampidou A, Kolos J, Purder PL, Geiger TM, Wessig P, Gassen NC, Bracher A and Hausch F (2021). “Macrocyclic FKBP51 Ligands Define a Transient Binding Mode with Enhanced Selectivity.” Angew Chem Int Ed Engl 60(24): 13257–13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Chai SC and Holahan MR (2010). “Effect of stimulus pre-exposure on inhibitory avoidance retrieval-associated changes in the phosphorylated form of the extracellular signal-regulated kinase-1 and −2 (pERK1/2).” Neurobiol Learn Mem 93(1): 66–76. [DOI] [PubMed] [Google Scholar]
- Wei M, Gao Y, Lu B, Jiao Y, Liu X, Cui B, Hu S, Sun L, Mao S, Dong J, Yan L, Chen Z and Zhao Y (2018). “FKBP51 regulates decidualization through Ser473 dephosphorylation of AKT.” Reproduction 155(3): 283–295. [DOI] [PubMed] [Google Scholar]
- Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F and Rein T (2005). “FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells.” J Biol Chem 280(6): 4609–4616. [DOI] [PubMed] [Google Scholar]
- Wolf IM, Periyasamy S, Hinds T Jr., Yong W, Shou W and Sanchez ER (2009). “Targeted ablation reveals a novel role of FKBP52 in gene-specific regulation of glucocorticoid receptor transcriptional activity.” J Steroid Biochem Mol Biol 113(1–2): 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Li P, Liu Y, Lou Z, Ding Y, Shu C, Ye S, Bartlam M, Shen B and Rao Z (2004). “3D structure of human FK506-binding protein 52: Implications for the assembly of the glucocorticoid receptor/Hsp90/immunophilin heterocomplex.” Proc Natl Acad Sci U S A 101(22): 8348–8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Zhang G, Wang C and Feng Y (2021). “The role of FKBP51 in the prognosis of ulcerative colitis-associated colorectal cancer.” Adv Med Sci 66(1): 89–97. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA and Gelernter J (2010). “Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder.” Neuropsychopharmacology 35(8): 1684–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhou Y, Edelshain B, Schatz F, Lockwood CJ and Taylor HS (2012). “FKBP4 is regulated by HOXA10 during decidualization and in endometriosis.” Reproduction 143(4): 531–538. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, Yong W, Shi S, Zhao W, Xu J, Srivastava A, Sanchez ER and Shou W (2006). “FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform.” Mol Endocrinol. 20(11): 2682–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong W, Yang Z, Periyasamy S, Chen H, Yucel S, Li W, Lin LY, Wolf IM, Cohn MJ, Baskin LS, Sanchez ER and Shou W (2007). “Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology.” J Biol Chem. 282(7): 5026–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Sun L, Hao T, Zhang B, Chen X, Li H, Zhang Z, Zhu S, Quan C, Niu Y and Shang Z (2019). “Restoration of FKBP51 protein promotes the progression of castration resistant prostate cancer.” Ann Transl Med 7(23): 729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun JY, Jin MJ, Kim S and Lee SH (2022). “Stress-related cognitive style is related to volumetric change of the hippocampus and FK506 binding protein 5 polymorphism in post-traumatic stress disorder.” Psychol Med 52(7): 1243–1254. [DOI] [PubMed] [Google Scholar]
- Zannas AS, Wiechmann T, Gassen NC and Binder EB (2016). “Gene-Stress-Epigenetic Regulation of FKBP5: Clinical and Translational Implications.” Neuropsychopharmacology 41(1): 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgajnar NR, De Leo SA, Lotufo CM, Erlejman AG, Piwien-Pilipuk G and Galigniana MD (2019). “Biological Actions of the Hsp90-binding Immunophilins FKBP51 and FKBP52.” Biomolecules 9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong S, Jiao Y, Liu X, Mu W, Yuan X, Qu Y, Xia Y, Liu S, Sun H, Wang L, Cui B, Liu X, Li P and Zhao Y (2021). “FKBP4 integrates FKBP4/Hsp90/IKK with FKBP4/Hsp70/RelA complex to promote lung adenocarcinoma progression via IKK/NF-kappaB signaling.” Cell Death Dis 12(6): 602. [DOI] [PMC free article] [PubMed] [Google Scholar]


